Coordination Chemistry of Solvated Metal Ions in Soft Donor Solvents
Abstract
1. Introduction
2. Results and Discussion
2.1. EXAFS on the Ammine Solvated Indium and Thallium(III) Ions in Liquid Ammonia
2.2. 205Tl NMR Characterization of the Ammine Solvated Thallium(III) Ion in Liquid Ammonia
2.3. Structure Relationship Between Hydrated and Ammine Solvated Metal Ions
2.4. Overview of Solvate Structures of d10 Metal Ions with Nitrogen Solvents
2.4.1. Ammine, Acetonitrile and Pyridine Solvated Copper(I) Ions
2.4.2. Ammine, Acetonitrile and Pyridine Solvated Silver(I) Ions
2.4.3. Ammine, Acetonitrile and Pyridine Solvated Gold(I) Ions
2.4.4. Ammine and Acetonitrile Solvated Zinc(II) Ions
2.4.5. Ammine Solvated Cadmium(II) Ions
2.4.6. Ammine and Pyridine Solvated Mercury(II) Ions
2.4.7. Summary of Coordination Chemistry of Solvated Metal Ions with Nitrogen Donor Solvents
2.5. Structure Relationship Between Hydrated and N,N-Dimethylthioformamide Solvated Metal Ions
2.6. Summary of Solvate Structures of Monovalent d10 Metal Ions with Phosphorus Donor Solvents
2.7. Structural Effects of Soft Solvents on the Coordination Chemistry of Soft Metal Ions
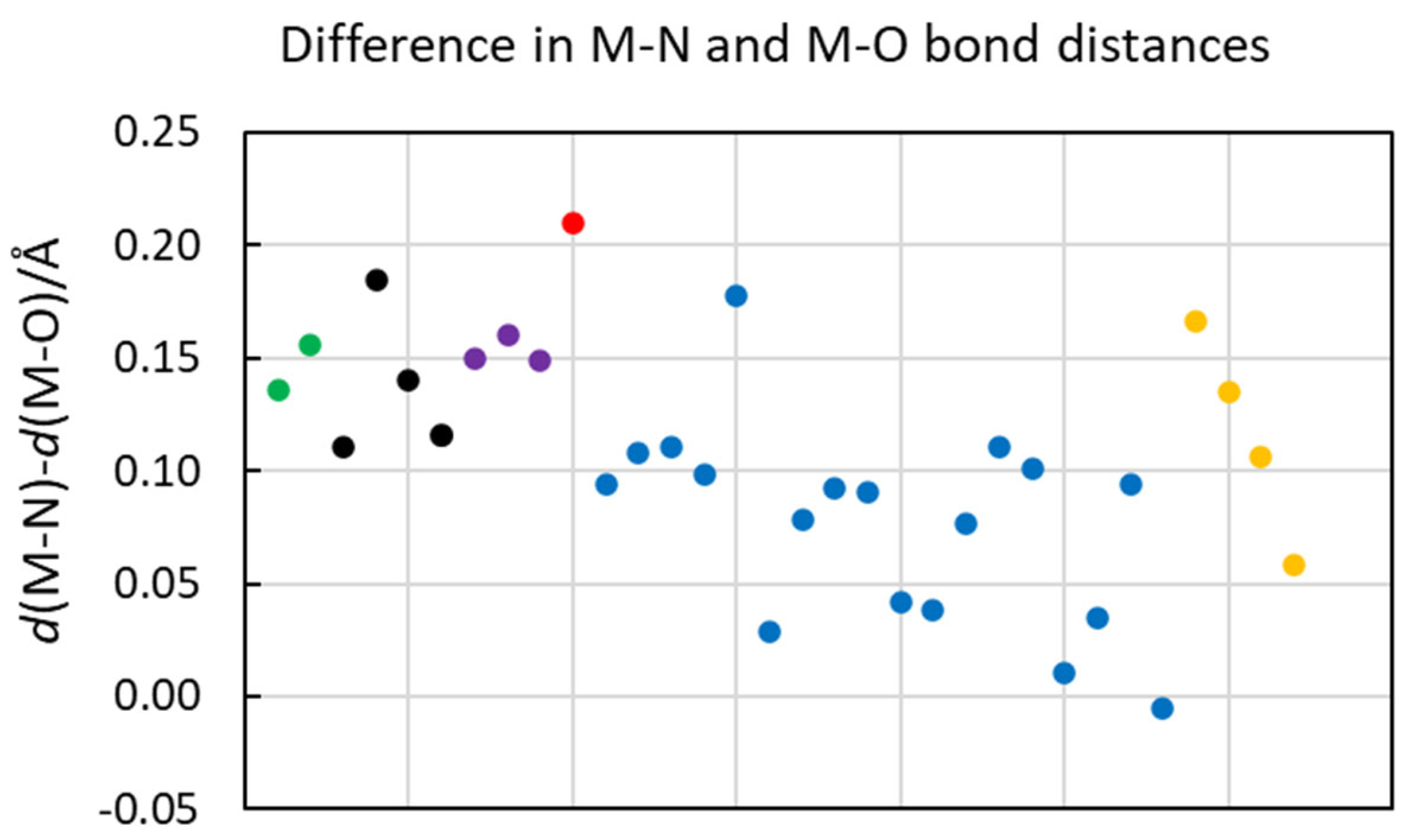
2.8. Correlation Between Difference in Hydrate and Ammine Solvate Metal Ion Solvate Bond Distacnes and Covalent Bonding Index
3. Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Crystallogr. Sect. B 2002, 58, 380–388, Cambridge Structure Database (Conquest 2024.3.0). [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016; pp. 9–97. [Google Scholar]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Calcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Beattie, J.K.; Best, S.P.; Skelton, B.W.; White, A.H. Structural Studies on the Caesium Alums, CsMIII[SO4]2·12H2O. J. Chem. Soc. Dalton Trans. 1981, 10, 2105–2111. [Google Scholar] [CrossRef]
- Persson, I.; D’Angelo, P.; De Panfilis, S.; Sandström, M.; Eriksson, L. Hydration of the Lanthanoid(III) Ions in Aqueous Solution and Crystalline Hydrates Studied by EXAFS Spectroscopy and Crystallography. The Myth of the “Gadolinium Break”. Chem.–Eur. J. 2008, 14, 3056–3066. [Google Scholar] [CrossRef]
- Persson, I.; Damian-Risberg, E.; D’Angelo, P.; De Panfilis, S.; Sandström, M.; Abbasi, A. X-Ray Absorption Fine Structure Spectroscopic Studies of Octakis(dimethylsulfoxide)lanthanoid(III) Complexes in Solution and in the Solid Iodides. Inorg. Chem. 2007, 46, 7742–7748. [Google Scholar] [CrossRef]
- Lundberg, D.; Lindqvist-Reis, P.; Łyczko, K.; Eriksson, L.; Persson, I. The Coordination Chemistry Effects of the Space-demanding Solvent Molecule N,N’-Dimethylpropyleneurea. Dalton Trans. 2024, 53, 1817–1832. [Google Scholar] [CrossRef]
- Ahrland, S.; Chatt, J.; Davies, N.R. The relative affinities of ligand atoms for acceptor molecules and ions. Quart. Rev. 1958, 12, 265–276. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases, HSAB, Part I: Fundamental principles. J. Chem. Edc. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases, HSAB, Part II: Underlying theories. J. Chem. Edc. 1968, 45, 643–648. [Google Scholar] [CrossRef]
- Sandström, M.; Persson, I.; Persson, P. A Study of Solvent Electron-Pair Donor Ability and Lewis Basicity Scales. Acta Chem. Scand. 1990, 44, 653–675. [Google Scholar] [CrossRef]
- Gutmann, V.; Wychera, E. Coordination Reactions in Non-Aqueous Solutions—The Role of the Donor Strength. Inorg. Nucl. Chem. Lett. 1966, 2, 257–260. [Google Scholar] [CrossRef]
- Hinton, J.F.; Metz, K.R.; Briggs, R.W. Thallium NMR Spectroscopy. Prog. NMR Spectrosc. 1998, 20, 423–513. [Google Scholar] [CrossRef]
- Glaser, J. Advances in Thallium Aqueous Solution Chemistry. Adv. Inorg. Chem. 1995, 43, 1–78. [Google Scholar]
- Ma, G.; Ilyukhin, A.; Glaswer, J.; Tóth, I.; Zékány, L. Equilibrium and Structure of Thallium(III)-Ethylenediamine Complexes in Pyridine Solution and Solid. Inorg. Chim. Acta 2001, 320, 92–100. [Google Scholar] [CrossRef]
- Kritikos, M.; Ma, G.; Bodor, A.; Glaser, J. Novel Bis(diethylenetriamine)thallium(III) Complex. Synthesis and Characterization in Pyridine Solution and in Solid. Inorg. Chim. Acta 2002, 331, 224–231. [Google Scholar] [CrossRef]
- Braun, S.; Kalinowski, H.-O.; Berger, S. 100 and More Basic NMR Experiments—A Pratical Course; VCH: Weinheim, Germany, 1996; ISBN 978-3527290918. [Google Scholar]
- Bodor, A.; Bányai, I.; Kowalewski, J.; Glaser, J. Thallium(III) Coordination Compounds: Chemical Information from 205Tl NMR Longitudinal Relaxation Times. Magn. Reon. Chem. 2002, 40, 716–722. [Google Scholar] [CrossRef]
- Persson, I. Structure and Size of Complete Hydration Shells of Metal Ions and Inorganic Anions in Aqueous Solution. Dalton Trans. 2024, 53, 15517–15538. [Google Scholar] [CrossRef]
- Persson, I.; Penner-Hahn, J.E.; Hodgson, K.O. An EXAFS Spectroscopic Study of Solvates of Copper(I) and Copper(II) in Acetonitrile, Dimethyl Sulfoxide, Pyridine and Tetrahydrothiophene Solution and a Large Angle X-Ray Scattering Study of the Copper(II) Acetonitrile Solvate in Solution. Inorg. Chem. 1993, 32, 2497–2501. [Google Scholar] [CrossRef]
- Royappa, A.T.; Royappa, A.D.; Moral, R.F.; Rheingold, A.L.; Papoular, R.J.; Blum, D.M.; Duong, T.Q.; Stepherson, J.R.; Vu, O.D.; Chen, B.; et al. Copper(I) oxalate complexes: Synthesis, structures and surprises. Polyhedron 2016, 119, 563–574. [Google Scholar] [CrossRef]
- Nilsson, K.; Oskarsson, Å. The Crystal Structure of Tetrapyridine Copper(I) Perchlorate and Tetrapyridine Silver Perchlorate at 260 K. Acta Chem. Scand. 1982, 36, 605–610. [Google Scholar] [CrossRef]
- Coles, S.J.; Hursthouse, M.B.; Sengul, A.; Altin, S.; Kurt, O. University of Southampton Crystal Structure Report Archive; University of Southampton: Southampton, UK, 2008. [Google Scholar] [CrossRef]
- Horvat, G.; Portada, T.; Stilinovic, V.; Tomisic, V. Tetrapyridinecopper(I) hexafluoridophosphate(V). Acta Crystallogr. Sect. E 2007, 63, m1734. [Google Scholar] [CrossRef]
- The IUPAC Stability Constants Database; Academic Software: Yorks, UK. Available online: https://www.acadsoft.co.uk/scdbase/scdbase.htm (accessed on 15 July 2025).
- Nilsson, K.B.; Persson, I. The Coordination Chemistry of Copper(I) in Liquid Ammonia, Trialkyl and Triphenyl Phosphite, and Tri-n-butylphosphine Solution. Dalton Trans. 2004, 9, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Woidy, P.; Karttunen, A.J.; Widenmeyer, M.; Niewa, R.; Kraus, F. On copper(I) fluorides, the cuprophilic interaction, the preparation of copper nitride at room temperature, and the formation mechanism at elevated temperatures. Chem.–Eur. J. 2015, 21, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.; Persson, I. The Structure of Silver(I) Solvate and Sodium Diiodoargentate(I) in Acetonitrile Solution. Acta Chem. Scand Ser. A 1987, 41, 139–145. [Google Scholar] [CrossRef]
- Hultén, F.; Persson, I. The Structure of the Tetrakis(pyridine)silver(I) and Bis(tributylstibine)silver(I) Complexes in Pyridine Solution. Acta Chem. Scand Ser. A 1987, 41, 87–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Santos, A.M.; Herdtweck, E.; Mink, J.; Kuhn, F.E. Organonitrile ligated silver complexes with perfluorinated weakly coordinating anions and their catalytic application for coupling reactions. New J. Chem. 2005, 29, 366–370. [Google Scholar] [CrossRef]
- Monticelli, M.; Baron, M.; Tubaro, C.; Bellemin-Laponnaz, S.; Graiff, C.; Bottaro, G.; Armelao, L.; Orian, L. Structural and Luminescent Properties of Homoleptic Silver(I), Gold(I), and Palladium(II) Complexes with nNHC-tzNHC Heteroditopic Carbene Ligands. ACS Omega 2019, 4, 4192–4205. [Google Scholar] [CrossRef]
- Pliquett, D.; Schulz, P.S.; Heinemann, F.W.; Bause, A.; Wasserscheid, P. Liquid silver tris(perfluoroethyl)trifluorophosphate salts as new media for propene/propane separation. Dalton Trans. 2016, 18, 28242–28253. [Google Scholar] [CrossRef]
- Tang, Y.; Yu, B. Coinage Metal (Bisfluorosulfonyl)imide Complexes: Preparation, Characterization, and Catalytic Applications. Eur. J. Inorg. Chem. 2020, 2020, 107–118. [Google Scholar] [CrossRef]
- Raducan, M.; Rodriquez-Escrich, C.; Cambeiro, X.C.; Escudero-Adan, E.C.; Pericas, M.A.; Echavarren, A.M. A multipurpose gold(I) precatalyst. Chem. Commun. 2011, 47, 4893–4895. [Google Scholar] [CrossRef]
- Louise, M.; Heinekey, G.D.M. Crystal Structure of Tetrakis (Acetonitrile) Silver(I) Tetrafluoroborate; Deposition Number 1882471; Cambridge Crystallographic Data Center: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Ahmad, R.; Hardie, M.J. Variable Ag(I) Coordination Modes in Silver Cobalt(III) Bis(dicarbollide) Supramolecular Assemblies with Cyclotriveratrylene Host Molecules. Cryst. Growth Des. 2003, 3, 493–499. [Google Scholar] [CrossRef]
- McGlone, T.; Streb, C.; Busquets-Fite, M.; Yan, J.; Gabb, D.; Long, D.-L.; Cronin, L. Silver Linked Polyoxometalate Open Frameworks (Ag-POMOFs) for the Directed Fabrication of Silver Nanomaterials. Cryst. Growth Des. 2011, 11, 2471–2478. [Google Scholar] [CrossRef]
- Karpova, E.V.; Boltalin, A.I.; Korenev, Y.M.; Zakharov, M.A.; Troyanov, S.I. Synthesis and Structure of Acetonitrile Solvates of Copper(II) Monofluoroacetate and Silver(I) Trifluoroacetate, [Cu2(CH2FCOO)4·2CH3CN](CH3CN) and Ag3(CF3COO)3-(CH3CN)2. Russ. J. Coord. Chem. 2001, 28, 286–291. [Google Scholar] [CrossRef]
- Dyason, J.C.; Healy, P.C.; Engelhardt, L.M.; White, A.H. Lewis-Base Adducts of Group 1B Metal(I) Compounds. XXII. Crystal Structure of ‘Bis(pyridine)silver(I) Perchlorate. Aust. J. Chem. 1985, 38, 1325–1328. [Google Scholar] [CrossRef]
- Kovacs, G.B.; May, N.V.; Bombicz, P.A.; Klebert, S.; Nemeth, P.; Menyhard, A.; Novodarszki, G.; Petrusevski, V.; Franguelli, F.P.; Magyari, J.; et al. An unknown component of a selective and mild oxidant: Structure and oxidative ability of a double salt-type complex having κ1O-coordinated permanganate anions and three- and four-fold coordinated silver cations. RSC Adv. 2019, 9, 28387–28398. [Google Scholar] [CrossRef] [PubMed]
- Hailmann, M.; Wolf, N.; Renner, R.; Schafer, T.; Hupp, B.; Steffen, A.; Finze, M. Unprecedented Efficient Structure Controlled Phosphorescence of Silver(I) Clusters Stabilized by Carba-closo-dodecaboranylethynyl Ligands. Angew. Chem. Int. Ed. 2016, 55, 10507–10511. [Google Scholar] [CrossRef]
- Nilsson, K.; Oskarsson, Å. The crystal structures of tetrapyridine copper(I) perchlorate and tetrapyridine silver(I) perchlorate at 250 K. Acta Crystallogr. Sect. A 1981, 37, C227b. [Google Scholar] [CrossRef]
- McNelly, A.G.; Christensen, K.E.; Thompson, A.L. Variable temperature studies of tetra pyridine silver(I) hexa fluoro phosphate and tetra pyridine silver(I) hexa fluoro anti monate. Acta Crystallogr. Sect. E 2024, 80, 1326–1330. [Google Scholar] [CrossRef]
- Jenne, C.; Wegenerm, B. Silver Salts of the Weakly Coordinating Anion [Me3NB12Cl11]−. Z. Anorg. Allg. Chem. 2018, 644, 1123–1132. [Google Scholar] [CrossRef]
- Nilsson, K.B.; Kessler, V.G.; Persson, I. Coordination Chemistry of the Solvated AgI and AuI Ion in Liquid and Aqueous Ammonia, Trialkyl and Triphenyl Phosphite, and Tri-n-butylphosphine Solutions. Inorg. Chem. 2006, 45, 6912–6921. [Google Scholar] [CrossRef]
- Willner, H.; Schaebs, J.; Hwang, G.; Mistry, F.; Jones, R.; Trotter, J.; Aubke, F. Bis(carbonyl)gold(I) undecafluorodiantimonate(V), [Au(CO)2][Sb2F11]: Synthesis, vibrational, and 13C NMR study and the molecular structure of bis(acetonitrile)gold(I) hexafluoroantimonate(V), [Au(NCCH3)2][SbF6]. J. Am. Chem. Soc. 1992, 114, 8972–8980. [Google Scholar] [CrossRef]
- El Naggara, M.; Shehadia, I.; Reibenspiesb, J.H.; Mohamed, A.A. Deposition Number CCDC 1029092; Cambridge Crystallographic Data Center: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Engesser, T.A.; Friedmann, C.; Martens, A.; Kratzert, D.; Malinowski, P.J.; Krossing, I. Homoleptic Gold Acetonitrile Complexes with Medium to Very Weakly Coordinating Counterions: Effect on Aurophilicity? Chem.–Eur. J. 2016, 22, 15085–15094. [Google Scholar] [CrossRef]
- Corbo, R.; Ryan, G.F.; Haghighatbin, M.A.; Hogan, C.F.; Wilson, D.J.D.; Hulett, M.D.; Barnard, P.J.; Dutton, J.L. Access to the Parent Tetrakis(pyridine)gold(III) Trication, Facile Formation of Rare Au(III) Terminal Hydroxides, and Preliminary Studies of Biological Properties. Inorg. Chem. 2016, 55, 2830–2839. [Google Scholar] [CrossRef]
- Doring, C.; Jones, P.G. Amine Complexes of Gold, Part 8: Two Pyridine Derivatives of Gold(I) Thiocyanate. Z. Naturforsch. Sect. B 2014, 69, 1315–1320. [Google Scholar] [CrossRef]
- Mingos, D.M.P.; Yau, J.; Menzer, S.; Williams, D.J. Synthesis of [Au(NH3)2]+ Salts and the Crystal Structure of [Au(NH3)2]Br. J. Chem. Soc. Dalton Trans. 1995, 2, 319–320. [Google Scholar] [CrossRef]
- Scherf, L.M.; Baer, S.A.; Kraus, F.; Bawaked, S.M.; Schmidbaur, H. Implications of the crystal structure of the ammonia solvate [Au(NH3)2]Cl*4NH3. Inorg. Chem. 2013, 52, 2157–2161. [Google Scholar] [CrossRef] [PubMed]
- Strey, M.; Doring, C.; Jones, P.G. Aminkomplexe des Goldes, Teil 10: Gold(I)-thiocyanat-Komplexe mit Tetrahydrothiophen, Dimethylsulfid, Ammoniak, Aminen und Azaaromaten. Z. Naturforsch. Teil B 2018, 73, 125–147. [Google Scholar] [CrossRef]
- Zheng, S.; Nygren, C.L.; Messerschmidt, M.; Coppens, P. Ligand unsupported Au(I) chains with short Au(I)∙∙∙Au(I) contacts. Chem. Commun. 2006, 35, 3711–3713. [Google Scholar] [CrossRef]
- Ahrland, S.; Nilsson, K.; Persson, I.; Yuchi, A.; Penner-Hahn, J.E. Gold(I) Halide and Thiocyanate Complexes in Pyridine and Acetonitrile, and the Structures of Gold(I) Solvates in These Solvents. A Thermodynamic and EXAFS Spectroscopic Study. Inorg. Chem. 1989, 28, 1833–1838. [Google Scholar] [CrossRef]
- Rach, S.F.; Herdtweck, E.; Kuhn, F.E. A Straightforward Synthesis of Cationic Nitrile Ligated Transition Metal Complexes with the [B(C6F5)4]− Anion. J. Organomet. Chem. 2011, 696, 1817–1823. [Google Scholar] [CrossRef]
- Yang, H.-L.; Yang, F.; Zhu, H.-L. Crystal structure of tetrakis(acetonitrile)zinc(II) sulfate, Zn(C2H3N)4SO4. Z. Kristallogr.-New Struct. 2004, 219, 349–350. [Google Scholar] [CrossRef]
- Groutchik, K.; Jaiswal, K.; Dobrovetsky, R. An Air-Stable, Zn2+-Based Catalyst for Hydrosilylation of Alkenes and Alkynes. Org. Biomol. Chem. 2021, 19, 5544–5550. [Google Scholar] [CrossRef]
- Akkus, O.N.; Decken, A.; Knapp, C.; Passmore, J. Complexes formed by the reactions of fluorinated and non-fluorinated organonitriles with [Zn(SO2)2][AsF6]2: A structural study. J. Chem. Cryst. 2006, 36, 321–329. [Google Scholar] [CrossRef]
- Nilsson, K.B.; Eriksson, L.; Kessler, V.G.; Persson, I. The Coordination Chemistry of the Copper(II), Zinc(II) and Cadmium(II) Ions in Liquid and Aqueous Ammonia Solution, and the Crystal Structrues of Hexaamminecopper(II) Perchlorate and Chloride, and Hexaamminecadmium(II) Chloride. J. Mol. Liq. 2007, 131, 113–120. [Google Scholar] [CrossRef]
- Kalinina, I.V.; Pervukhina, N.V.; Podberezskaya, N.V.; Fedin, V.P. Cubane Tellurido Clusters [Zn(NH3)4]3[Mo4Te4(CN)12] and [Cd(NH3)4]3[W4Te4(CN)12]: Syntheses and Crystal Structures. Russ. J. Coord. Chem. 2002, 29, 389–393. [Google Scholar] [CrossRef]
- Ledneva, A.Y.; Smolentsev, A.I.; Naumov, N.G. Ionic coordination complexes based on [Re6S8(CN)4L2]n− (L = OH−, NH3; n = 2, 3) cluster anions, and Ni(II) and Cd(II) ammine cations. J. Coord. Chem. 2013, 66, 4363–4370. [Google Scholar] [CrossRef]
- Himmel, K.; Jansen, M. On the geometry of the fulleride dianion C602− in crystalline fullerides-Syntheses and crystal structures of [M(NH3)6]C60∙6NH3 (M = Mn2+, Cd2+). Eur. J. Inorg. Chem. 1998, 1998, 1183–1186. [Google Scholar] [CrossRef]
- Paul, B.; Nather, C.; Walfort, B.; Fromm, K.M.; Zimmermann, B.; Lang, H.; Janiak, C. Molecular paneling of rac-1,1′-bi-2-naphthol/∼ate (BINOL/BINOLAT): Hydrogen-bonded assembly of [M(NH3)4 or 6]2+ complexes (M = Ni, Zn, Cd) in cavities of {[BINOLAT]2−(BINOL)2}-strands. CrystEngComm 2004, 6, 293–297. [Google Scholar] [CrossRef]
- Kraus, F. Dissolving the insoluble: CdF2 and moist ammonia form cadmium(II) difluoride monohydrate-synthesis and crystal structure of [Cd(NH3)6]F2∙H2O. Monatsh. Chem. 2012, 143, 1097–1100. [Google Scholar] [CrossRef]
- Åkesson, R.; Sandström, M.; Stålhandske, C.I.; Persson, I. Crystal and Molecular Structure at 298 and 183 K of Hexakis(pyridine)mercury(II) Trifluoromethylsulfonate, [Hg(NC5H5)6](CF3SO3)2, and Thermal Analyses of Hexapyridine Solvates of M,ercury(II). Acta Chem. Scand. 1991, 45, 165–171. [Google Scholar] [CrossRef]
- Nilsson, K.B.; Maliarik, M.; Persson, I.; Fischer, A.; Ullström, A.-S.; Eriksson, L.; Sandström, M. Coordination Chemistry of Mercury(II) in Liquid and Aqueous Ammonia Solution, and the Crystal Structure of Tetraamminemercury(II) Perchlorate. Inorg. Chem. 2008, 47, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.; Ullström, A.-S.; D’Angelo, P.; Persson, I. A Structural Study of the Hydrated and the Dimethylsulfoxide, N,N’-dimethylpropyleneurea, and N,N-dimethylthioformamide Solvated Iron(II) and Iron(III) Ions in Solution and Solid State. Inorg. Chim. Acta 2007, 360, 1809–1818. [Google Scholar] [CrossRef]
- Baumgartner, O. Struktur von Hexakis(N,N-dimethylthioformamid)eisen(II) Diperchlorat. Acta Crystallogr., Sect. C 1986, 42, 1723–1725. [Google Scholar] [CrossRef]
- Kristiansson, O.; Persson, I.; Bobicz, D.; Xu, D. A Structural Study of the Hydrated and the Dimethylsulfoxide, N,N’-Dimethylpropyleneurea, Acetonitrile, Pyridine and N,N-Dimethylthioformamide Solvated Nickel(II) Ion Solution and Solid State. Inorg. Chim. Acta 2003, 344, 15–27. [Google Scholar] [CrossRef]
- Stålhandske, C.M.V.; Stålhandske, C.I.; Persson, I.; Sandström, M.; Jalilehvand, F. Crystal and Solution Structures of N,N-Dimethylthioformamide Solvated Copper(I), Silver(I), and Gold(I) Ions Studied by X-Ray Diffraction, X-Ray Absorption, and Vibrational Spectroscopy. Inorg. Chem. 2001, 40, 6684–6693. [Google Scholar] [CrossRef]
- Stålhandske, C.M.V.; Stålhandske, C.I.; Sandström, M.; Persson, I. Crystal Structure of N,N-dimethylthioformamide Solvates of the Divalent Group 12 Ions with Linear Coordination Geometry for Mercury(II), Tetrahedral for Zinc and Octahedral for Cadmium. Inorg. Chem. 1997, 36, 3167–3173. [Google Scholar] [CrossRef]
- Stålhandske, C.M.V.; Persson, I.; Sandström, M.; Kamienska-Piotrowicz, E. Structure of the Solvated Zinc(II), Cadmium(II) and Mercury(II) Ions in N,N-Dimethylthioformamide Solution. Inorg. Chem. 1997, 36, 3174–3182. [Google Scholar] [CrossRef]
- Topel, Ö.; Persson, I.; Lundberg, D.; Ullström, A.-S. Reactions and Structures in the Gallium(III)/Indium(III)-N,N-dimethylthioformamide Systems. Inorg. Chim. Acta 2011, 365, 220–224. [Google Scholar] [CrossRef]
- Näslund, J.; Persson, I.; Sandström, M. Solvation of the Bismuth(III) Ion by Water, Dimethyl Sulfoxide, N,N’-Dimethylpropyleneurea, and N,N-Dimethylthioformamide. An EXAFS, Large-Angle X-Ray Scattering, and Crystallographic Structural Study. Inorg. Chem. 2000, 39, 4012–4021. [Google Scholar] [CrossRef]
- Peters, K.; Bock, J.; Jacobs, H. Hexaaminaluminiumiodidmonoammoniakat-[Al(NH3)6]I3NH3-Darstellung und Kristallstruktur. J. Less-Common Metal. 1989, 154, 243–250. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Zhou, W.; Yu, X. Dehydrogenation Tuning of Ammine Borohydrides Using Double-Metal Cations. J. Am. Chem. Soc. 2011, 133, 4690–4693. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Tan, Y.; Wu, H.; Gu, Q.; Zhou, W.; Jensen, C.M.; Yu, X. Metal cation-promoted hydrogen generation in activated aluminium borohydride ammoniates. Acta Mater. 2013, 61, 4787–4796. [Google Scholar] [CrossRef]
- Nieboer, E.; Richardson, D.H.S. The Replacement of the Nondescript Term Heavy Metals by a Biologically and Chemically Significant Classification of Metal Ions. Environ. Pollut. Ser. B 1980, 1, 3–26. [Google Scholar] [CrossRef]
- Nieboer, E.; Fletcher, G.G.; Thomassen, Y. Relevance of Reactivity Determinants to Exposure Assessment and Biological Monitoring of the Elements. J. Environ. Monit. 1999, 1, 1–14. [Google Scholar] [CrossRef]
- Allen, L.C. Electronegativity Is the Average One-Electron Energy of the Valence-Shell Electrons in Ground-State Free Atoms. J. Am. Chem. Soc. 1989, 111, 9003–9014. [Google Scholar] [CrossRef]
- Ma, G.; Molla-Abbassi, A.; Kritikos, M.; Ilyukhin, A.; Jalilehvand, F.; Kessler, V.G.; Skripkin, M.; Sandström, M.; Glaser, J.; Näslund, J.; et al. Structure of the Dimethyl Sulfoxide Solvated Thallium(III) Ion in Solution and in the Solid State. Inorg. Chem. 2001, 40, 6432–6438. [Google Scholar] [CrossRef]
- Thompson, A.; Attwood, D.; Gullikson, E.; Howells, M.; Kim, H.-J.; Kirz, J.; Kortright, J.; Lindau, I.; Pianatta, P.; Robinson, A.; et al. X-Ray Data Booklet LBNL/PUB-490 Rev. 3; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2009. [Google Scholar]
- George, G.N.; Pickering, I.J. EXAFSPAK—A Suite of Computer Programs for Analysis of X-Ray Absorption Spectra; SSRL: Stanford, CA, USA, 1993; Available online: https://www-ssrl.slac.stanford.edu/exafspak.html (accessed on 15 July 2025).
- Zabinsky, S.I.; Rehr, J.J.; Ankudinov, A.; Albers, R.C.; Eller, M. Multiple-Scattering Calculations of X-Ray Absorption Spectra. J. Phys. Rev. B 1995, 52, 2995–3009. [Google Scholar] [CrossRef]
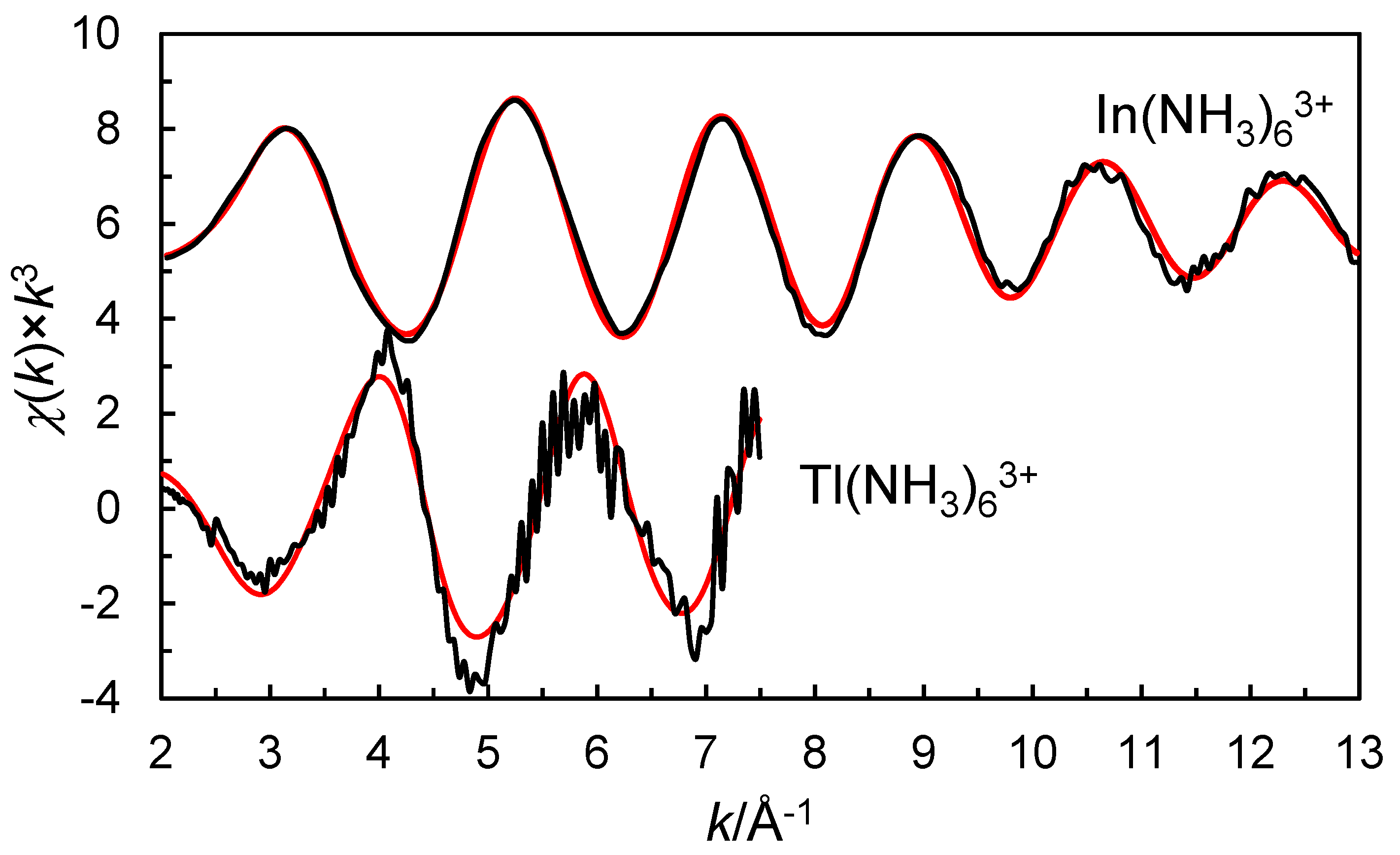
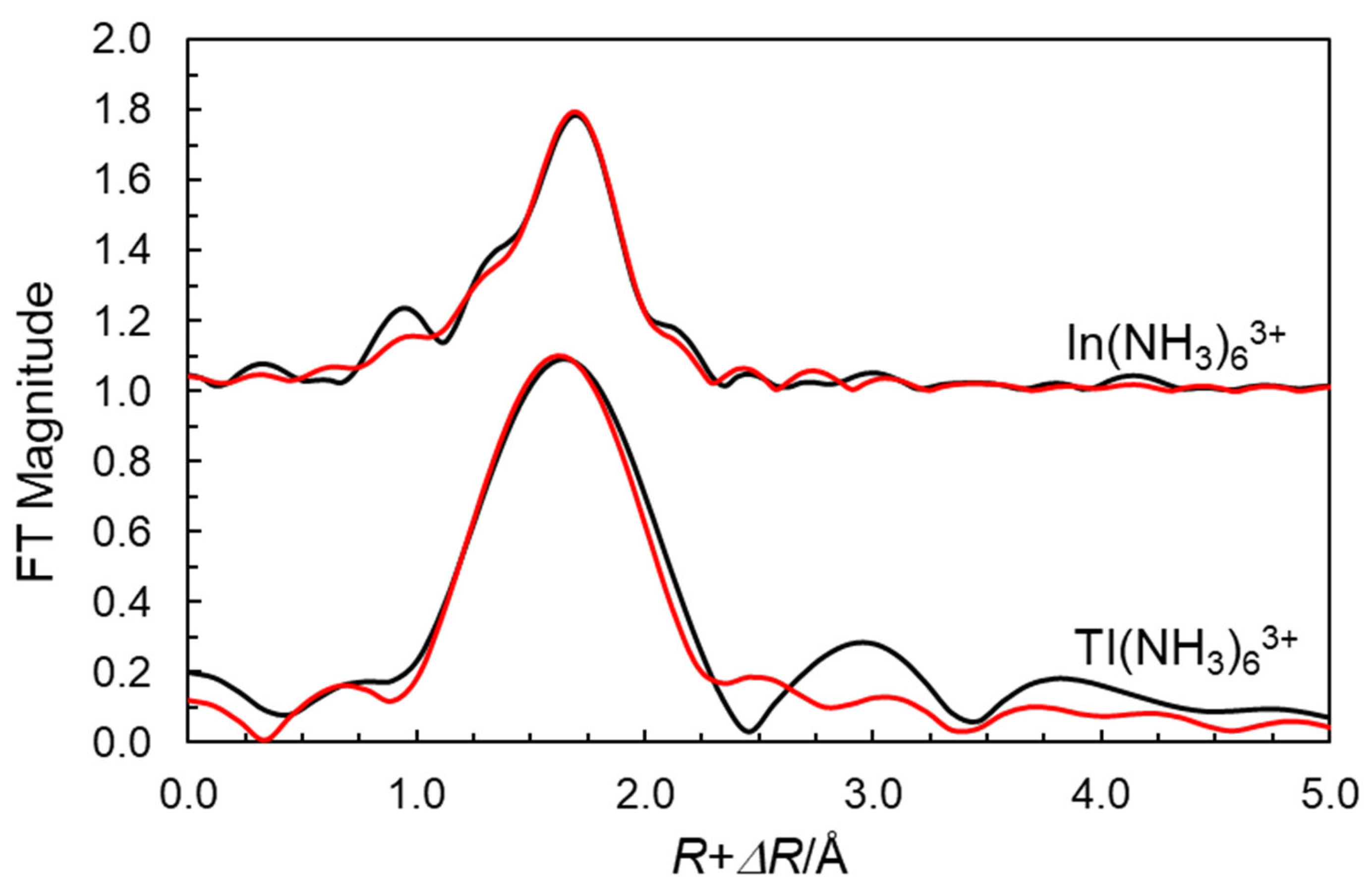


| Sample/Species | Scattering Path | N | d | σ2 | Eo | So2 |
|---|---|---|---|---|---|---|
| [In(H2O)6](ClO4)3 in NH3(l) | ||||||
| [In(NH3)6]3+ | In-N | 6 | 2.232(3) | 0.0054(3) | 27,923.4(4) | 0.95(3) |
| MS(InN6) | 3 × 6 | 4.45(6) | 0.0145(12) | |||
| [Tl(OS(CH3)2)6](ClO4)3 in NH3(l) | ||||||
| [Tl(NH3)6]3+ | Tl-N | 6 | 2.288(8) | 0.008414) | 12,663.1(9) | 1.19(11) |
| MS(TlN6) | 3 × 6 | 4.60(4) | 0.009(5) | |||
| Sample/Species | CTl (mol∙dm−3) | δ (ppm) | Line Width (Hz) |
|---|---|---|---|
| [Tl(OS(CH3)2)6](ClO4)3 in NH3(l) | |||
| [Tl(NH3)6]3+ | 0.046 | 2769 | 35 |
| TlNO3 in NH3(l) | |||
| [Tl(NH3)n]+ | 0.037 | 1768 | 70 |
| TlNO3 in NH3(aq) | |||
| [Tl(NH3)n]+ | 0.044 | 750 | 30 |
| Ion | d(M-N)/Å | CN | N | d(M-S)/Å | CN | N | d(M-O)/Å | CN | N | Diff (NH3-aq) | Diff (DMTF-aq) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Li+ | 2.078 | 4 | 23 | 1.942 | 4 | 58 | 0.136 | ||||
| Na+ | 2.497 | 5 | 3 | 2.341 | 5 | 2 | 0.156 | ||||
| K+ | 2.891 | 6 | 1 | ||||||||
| Mg2+ | 2.177 | 6 | 5 | 2.066 | 6 | 541 | 0.111 | ||||
| Ca2+ | 2.509 | 6 | 2 | 2.324 | 6 | 16 | 0.185 | ||||
| 2.553 | 7 | 1 | 2.400 | 7 | 19 | 0.153 | |||||
| 2.619 | 8 | 2 | 2.474 | 8 | 11 | 0.145 | |||||
| Sr2+ | 2.753 | 8 | 4 | 2.613 | 8 | 20 | 0.140 | ||||
| Ba2+ | 2.892 | 8 | 1 | 2.777 | 8 | 4 | 0.115 | ||||
| 2.962 | 9 | 1 | 2.832 | 9 | 4 | 0.130 | |||||
| Y3+ | 2.474 | 6 | 1 | ||||||||
| 2.487 | 7 | 1 | |||||||||
| La3+ | 2.678 | 6 | 1 | ||||||||
| 2.706 | 9 | 1 | 2.556 | 9 | 10 | 0.150 | |||||
| Ce3+ | 2.654 | 6 | 1 | ||||||||
| Sm3+ | 2.632 | 9 | 1 | 2.472 | 9 | 4 | 0.160 | ||||
| Yb3+ | 2.475. | 6 | 1 | 2.326 | 8 | 29 | 0.149 | ||||
| Zr4+ | 2.407 | 8 | 1 | 2.197 | 8 | 1 | 0.210 | ||||
| V2+ | 2.225 | 6 | 1 | 2.131 | 6 | 4 | 0.094 | ||||
| Cr2+ | 2.224 | 6 | 1 | 2.166 | 6 | 2 | 0.058 | ||||
| Cr3+ | 2.073 | 6 | 20 | 1.965 | 6 | 24 | 0.108 | ||||
| Mn2+ | 2.285 | 6 | 18 | 2.174 | 6 | 169 | 0.111 | ||||
| Fe2+ | 2.218 | 6 | 10 | 2.541 | 6 | 1 | 2.120 | 6 | 95 | 0.098 | 0.421 |
| Fe3+ | 2.206 | 4 | 1 | ||||||||
| 2.173 | 6 | 1 | 1.995 | 6 | 21 | 0.178 | |||||
| Ru2+ | 2.140 | 6 | 1 | 2.111 | 6 | 3 | 0.029 | ||||
| Ru3+ | 2.099 | 6 | 11 | 2.021 | 6 | 2 | 0.078 | ||||
| Os3+ | 1.964 | 6 | 1 | ||||||||
| Co2+ | 2.179 | 6 | 12 | 2.087 | 6 | 471 | 0.092 | ||||
| Co3+ (LS) | 1.964 | 6 | 228 | 1.873 | 6 | 1 | 0.091 | ||||
| Rh3+ | 2.064 | 6 | 6 | 2.022 | 6 | 4 | 0.042 | ||||
| Ir3+ | 2.080 | 6 | 7 | 2.042 | 6 | 1 | 0.038 | ||||
| Ni2+ | 2.132 | 6 | 21 | 2.459 | 6 | 1 | 2.055 | 6 | 399 | 0.077 | 0.404 |
| Pd2+ | 2.039 | 4 | 33 | 2.029 | 6 | 3 | 0.010 | ||||
| Pt2+ | 2.047 | 4 | 45 | 2.012 | 6 | 1 | 0.035 | ||||
| Cu+ | 2.004 | 3 | 1 | ||||||||
| 2.336 | 4 | 1 | |||||||||
| Cu2+ | 2.008 | 4 | 8 | 2.36 | 4 | 1 | 1.927 | 4 | 4 | 0.081 | 0.43 |
| 2.072 | 5 | 6 | 2.009 | 5 | 5 | 0.063 | |||||
| 2.191 | 6 | 1 | 2.097 | 6 | 117 | 0.094 | |||||
| Ag+ | 2.124 | 2 | 54 | 2.129 | 2 | 6 | −0.005 | ||||
| 2.280 | 3 | 4 | |||||||||
| 2.348 | 4 | 1 | 2.58 | 4 | 1 | ||||||
| Au+ | 2.038 | 2 | 5 | 2.283 | 2 | 2 | |||||
| Au3+ | 2.024 | 4 | 1 | 2.097 | 4 | 1 | −0.073 | ||||
| Zn2+ | 2.022 | 4 | 18 | 2.340 | 4 | 1 | 1.977 | 4 | 3 | 0.045 | 0.363 |
| 2.117 | 5 | 1 | 2.017 | 5 | 4 | 0.100 | |||||
| 2.199 | 6 | 6 | 2.088 | 6 | 274 | 0.111 | |||||
| Cd2+ | 2.289 | 4 | 1 | ||||||||
| 2.367 | 6 | 4 | 2.682 | 6 | 2 | 2.266 | 6 | 58 | 0.101 | 0.416 | |
| Hg2+ | 2.074 | 2 | 1 | 2.351 | 2 | 1 | |||||
| 2.306 | 4 | 2 | |||||||||
| 2.527 | 6 | 1 | |||||||||
| Al3+ | 2.048 | 6 | 3 | 1.882 | 6 | 99 | 0.166 | ||||
| Ga3+ | 2.333 | 4 | 1 | ||||||||
| 2.081 | 6 | 1 | 1.946 | 6 | 13 | 0.135 | |||||
| In3+ | 2.232 | 6 | 1 | 2.126 | 6 | 11 | 0.106 | ||||
| Tl3+ | 2.288 | 6 | 1 | 2.230 | 6 | 1 | 0.058 | ||||
| Bi3+ | 2.794 | 6 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilsson, K.B.; Maliarik, M.; Persson, I. Coordination Chemistry of Solvated Metal Ions in Soft Donor Solvents. Molecules 2025, 30, 3063. https://doi.org/10.3390/molecules30153063
Nilsson KB, Maliarik M, Persson I. Coordination Chemistry of Solvated Metal Ions in Soft Donor Solvents. Molecules. 2025; 30(15):3063. https://doi.org/10.3390/molecules30153063
Chicago/Turabian StyleNilsson, Kersti B., Mikhail Maliarik, and Ingmar Persson. 2025. "Coordination Chemistry of Solvated Metal Ions in Soft Donor Solvents" Molecules 30, no. 15: 3063. https://doi.org/10.3390/molecules30153063
APA StyleNilsson, K. B., Maliarik, M., & Persson, I. (2025). Coordination Chemistry of Solvated Metal Ions in Soft Donor Solvents. Molecules, 30(15), 3063. https://doi.org/10.3390/molecules30153063







