Abstract
The determination of multiple energy minima on complex potential energy surfaces is challenging. A systematic desymmetrization procedure was employed to find stationary points on the copper(I) + chloride + water potential energy surface using HF, MP2, and B3LYP methods in conjunction with the 6-31G*, 6-31+G*, and 6-311+G* basis sets. Comparison with experimental results demonstrated that the speciation of copper(I) in the presence of chloride and water may be formulated as [CuCl(H2O)]0, [CuCl2]−, and [CuCl3]2−. Our results indicate that the combination of the MP2 method along with basis sets containing diffuse functions gives excellent agreement with experimental Cu-Cl distances and vibrational frequencies. Poorer results were obtained at the HF levels and/or using the 6-31G* basis set.
1. Introduction
Speciation in chemistry refers to the distribution of an element amongst defined chemical species in a system in some medium. In water, this is called aqueous speciation. For example, solid sodium chloride, NaCl(s), will dissolve in water to give NaCl(aq). Above a certain concentration, however, not all of the sodium chloride will dissolve, and some solid will remain as NaCl(s) above the freezing point of pure water. At lower temperatures, the solid can exist as the dihydrate NaCl•2H2O(s) [1]. In this structure, each sodium ion is surrounded by four water molecules and two chloride ions. Recently, other hydrates of sodium chloride, potentially relevant to Jupiter’s moons, were identified at low temperatures and high pressures: NaCl•8.5H2O(s) and NaCl•13H2O(s) [2]. In both phases, the sodium exists as a hexaaqua ion, [Na(H2O)6]+. Suppose we restrict ourselves to the aqueous phase. In that case, it is known that NaCl(aq) at near-ambient conditions can be described as an equimolar mixture of Na+(aq) and Cl−(aq) ions. It is believed that the sodium ion can also be described as a hexaaqua ion, with the water molecules forming a hydration shell around the sodium ion. In both the crystal and aqueous solution, the environment around the aqua ion may influence the bond distances and vibrational frequencies [3].
An alternate way to study speciation for ionic compounds is to fix the amount of cation and vary the amount of anion. The speciation of copper(I) in aqueous solution in the presence of excess chloride ion was recently investigated by some of the authors [4]. The Cu+(aq) ion is difficult to study experimentally, as it tends to disproportionate into Cu2+(aq) and Cu0(s) in aqueous solution and can be easily oxidized by adventitious oxygen. However, the presence of halide ions stabilizes the +1 oxidation state of copper. Therein, it was shown using a combination of Raman spectroscopy and ab initio calculations that the two bands observed at 297 cm−1 and 247 cm−1 were the totally symmetric Cu-Cl stretching motions of the linear D∞h CuCl2−(aq) and trigonal planar D3h CuCl32−(aq) species, respectively. A less intense band at 350 cm−1 was assigned to the neutral CuCl(H2O)0(aq) species. A systematic desymmetrization procedure was developed and applied by the authors to the possible aquacopper(I) species Cu(H2O)n+, n = 1–6 [5]. Therein, it was shown that the most likely structure should be the diaquacopper(I) ion, Cu(H2O)2+.
Desymmetrization, as first developed in Ref. [5], uses group theory to systematically lower the symmetry of a high symmetry structure, which is a stationary point on the potential energy surface, to another lower-energy stationary point. A stationary point is a structure in which the potential energy gradient vector is zero. Stationary points may be characterized by the number of negative eigenvalues of the energy second-derivative matrix with respect to the nuclear motions. If all eigenvalues are positive, then the structure is a minimum on the potential energy surface. If one eigenvalue is negative, then the structure is called a transition structure. If N eigenvalues are negative, the structure is called an N-th order saddle point. The vibrational frequencies of a stationary point, being related to these eigenvalues, are typically used to characterize the stationary point because they can be related to infrared and Raman spectra. Locally stable structures have all positive vibrational frequencies, whereas a transition state would have one imaginary vibrational frequency corresponding to the maximum along the reaction coordinate (corresponding eigenvector). Distortion along this reaction coordinate from the maximum would result in a lowering of the energy, which sometimes coincides with a lowering of symmetry. Lowering the symmetry (or descent in symmetry) is the reduction in the order of the point group symmetry. For example, the C2v point group has order 4 (there are 4 symmetry operations), whereas its subgroups Cs and C2 are both of order 2. An illustrative example well-known to students of organic chemistry via Newman projections would be the eclipsed form of ethane, which has D3h symmetry (order 12). This structure has an imaginary frequency corresponding to the internal rotation of the irreducible representation A1”. In this form, the dihedral angle H-C-C-H is 0°. A slight distortion along this vibrational mode results in a D3 structure (order 6) and a lowering of energy. Once this angle reaches 60°, a potential energy minimum is reached, called the staggered form, and we have an ascent in symmetry to D3d (order 12). For structures with multiple imaginary frequencies, the advantages of a systematic procedure for desymmetrization become evident.
In this paper, we will further explore chlorocopper(I) complexes, examining the effect of the waters of solvation on the coordination chemistry of chlorocopper complexes, in particular, the Cu-Cl distances and vibrational frequencies, using the techniques developed in Ref. [5].
2. Results
In the text, tables, and figures below, the structures are described by stoichiometry and charge, point group symmetry and numerical label (if more than one exists) corresponding to the stoichiometry, and in cases where not all ligands are bound, the coordination pattern [m + n], where m refers to the number of ligands directly coordinated to the copper(I) ion and n refers to those not directly coordinated to the copper(I) ion but interacting with the ligands. In some cases, n may be appended by Cl if a chloride ligand dissociates.
2.1. Structures of Monochloroaquacopper(I) Complexes, CuCl(H2O)n, n = 1–5, 7
The simplest chlorocopper(I) complex, CuCl0, is of C∞v symmetry (see Figure 1). When a water molecule is added to the copper ion opposite to the chloride to give CuCl(H2O)0, the symmetry reduces to C2v. This structure is usually unstable (except at HF/6-311+G*) and possesses an imaginary B1 mode. Desymmetrization along this mode gives the stable Cs #3 structure, in which a slight pyramidalization of the oxygen atom has occurred. Excited configuration structures corresponding to high-energy Cs #1 and #2 were also found in which the Cl-Cu-O angle was approximately 90 degrees.
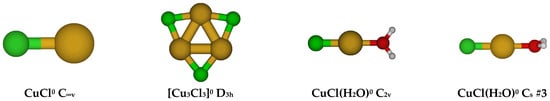
Figure 1.
Chlorocopper(I) structures, CuCl(H2O)n0, n = 0, 1, and Cu3Cl3 (to be discussed later). In this and later figures, a bold label indicates a local minimum of potential energy at some level of theory. The atom color legend is as follows: copper is yellow, chlorine is green, oxygen is red, and hydrogen is white.
For CuCl(H2O)20, we first considered two different C2v structures in which the water molecules are either in the ClCuO2 plane or are bisected by it (Figure 2). These are close in energy but usually possess imaginary A2, B1, and B2 modes, suggesting desymmetrization to either a C2 or one of two Cs structures (#2, #3). The C2 #1 structure has an imaginary B mode at all levels of theory. The Cs #2 structure (close in structure to C2v #3) has two imaginary A” frequencies. The Cs #3 structure only exists at a couple of levels of theory and dissociates a water molecule to become Cs #4 [2 + 1]. Most of these Cs structures contain an imaginary mode and become a [2 + 1] C1 #1 or #2 structure upon desymmetrization. These differ in that the ClCuO angle bends so that one of the hydrogen atoms of the water may interact with the CuCl part of the molecule at some levels of theory. These results suggest that CuCl(aq) is dicoordinate [CuCl(H2O)]0 in aqueous solution and that attempts to place a second water molecule in the first coordination shell of copper result in migration to the second coordination shell.
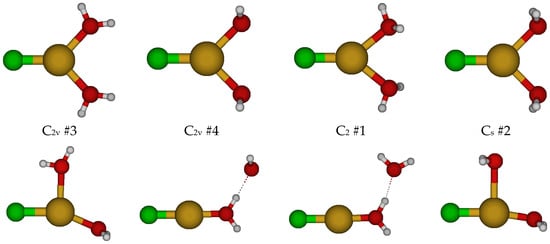
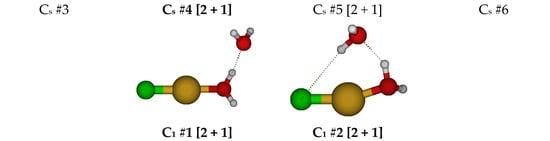
Figure 2.
Chlorocopper(I) structures, CuCl(H2O)n0, n = 2. Several excited configuration structures were also found.
For CuCl(H2O)30, we first considered two different C3v structures in which the water molecules are either in the three σv planes or are bisected by them (Figure 3). Both of these structures have one imaginary A2 mode and at least one imaginary E mode. Along the A2 mode, they desymmetrize to the common C3 #2 structure. This structure is a local minimum at some levels of theory (HF; B3LYP/6-311+G*) but contains an imaginary E mode at others (MP2; B3LYP/6-31G* and 6-31+G*). Desymmetrization of C3v #1 and #2 along the E mode would give one of two Cs structures (#1 and #2, respectively). At some levels of theory, Cs #1 becomes [3 + 1], whereas Cs #2 becomes [2 + 2] at nearly all levels of theory except HF/6-31G* and B3LYP/6-31+G*. The C3 structure can desymmetrize along the E mode to give a C1 #3 [2 + 2] structure, which exists at all levels of theory.
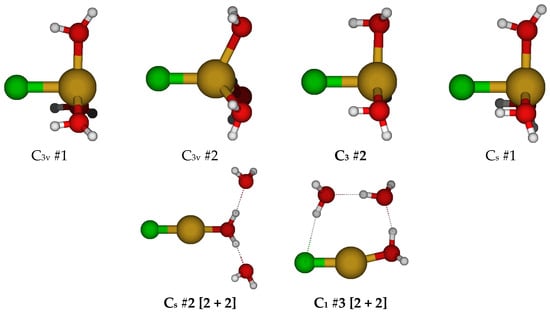
Figure 3.
Chlorocopper(I) structures, CuCl(H2O)n0, n = 3.
For CuCl(H2O)40, we first considered two different C4v structures in which the water molecules are either in the three σv planes or are bisected by them (Figure 4). Both of these had imaginary A2, E, and B1 (and sometimes B2) modes. Along the A2 mode, these desymmetrize to form one of two C4 structures, #1 and #2, respectively, with some coalescence between them. They would desymmetrize along the B1 and B2 modes to form C2v structures #1–#4, and along the E mode, the C2v #5 structures (via Cs). For C4 #2, the waters form a cyclic water tetramer. The C4 #1 structure always has an imaginary B and sometimes an E mode, whereas the C4 #2 structure, when it exists, usually has both. The C2v structures all have an imaginary A2 mode. Most of the C2v structures also have imaginary B1 and B2 modes. Usually, the C2v #3 structure coalesces into the C2v #1 structure. Desymmetrization of the C4 and C2v structures leads to a plethora of C2 and Cs structures, many of which have imaginary modes and would desymmetrize to one of the many C1 structures. With one exception at one level, none of the C2 and Cs structures are 5-coordinated and lose at least one water to form 2-, 3-, and 4-coordinated structures. For the 6-31G* basis set (having no diffuse functions), in some cases, the chloride ion dissociates for some of the C1 structures, or one of the hydrogen atoms of the water molecules interacts with the copper atom instead of the chlorine atom. Notable local minimum energy structures (apart from the obvious C1 structures) include the C2 #2 [3 + 2], C2 #4 [3 + 2], Cs #5 [4 + 1], and Cs #7 [2 + 3].
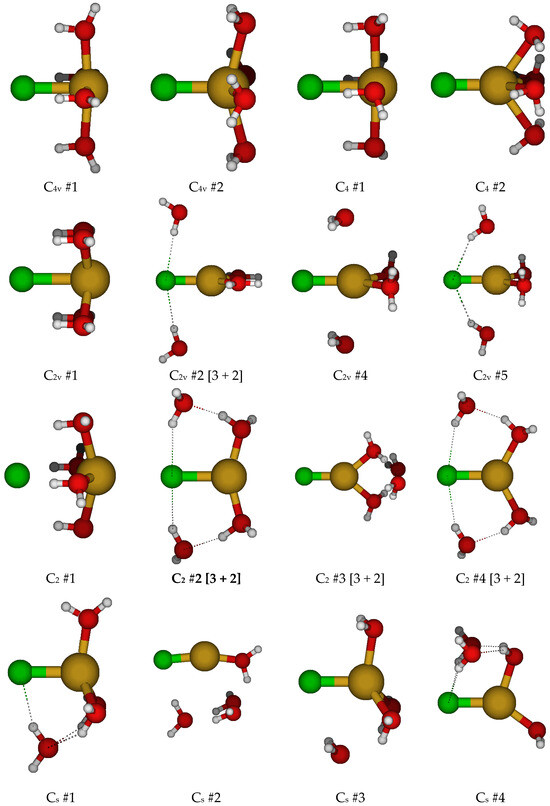
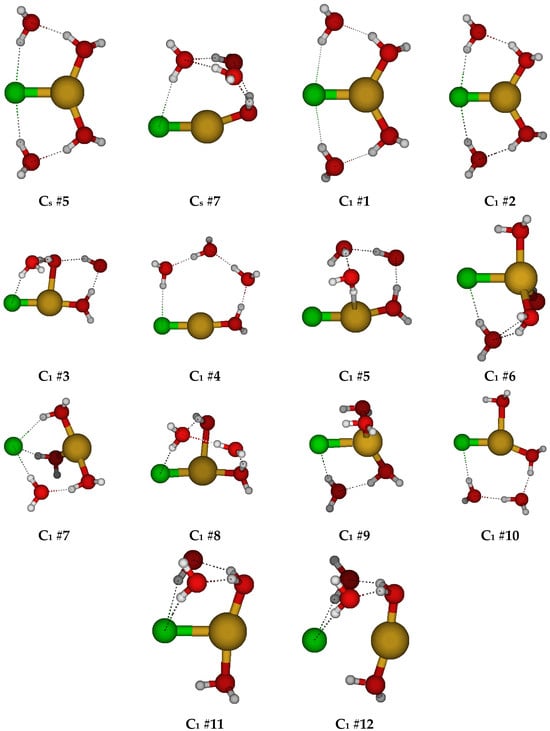
Figure 4.
Chlorocopper(I) structures, CuCl(H2O)n0, n = 4.
For CuCl(H2O)50, we first considered six different C2v structures in which the water molecules and chloride ion are directly bound to the copper(I) ion, initially either in the three σv planes or bisected by them (Figure 5). In every case, at least one ligand underwent dissociation from the copper(I) ion, and none of the resulting structures were local minima on the potential energy surface. Results using the 6-31G* basis set tended to undergo chloride dissociation, whereas those using basis sets with diffuse functions tended to undergo water dissociation.
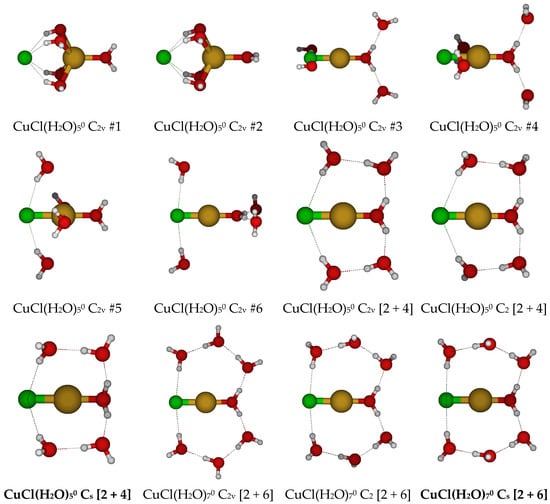
Figure 5.
Chlorocopper(I) structures, CuCl(H2O)n0, n = 5, 7.
We also examined dicoordinate CuCl(H2O)70, starting with C2v symmetry. These are desymmetrized to the unstable C2 and stable Cs structures.
To summarize, for monochloroaquacopper(I), a variety of 2-, 3-, and 4-coordinate structures can be formed, but the lowest-energy form seems to be the hydrated neutral dicoordinate [CuCl(H2O)]0 species. The results using the 6-31G* basis set were not typical of those using the 6-31+G* or 6-311+G* basis sets, with different energy orderings.
2.2. Structures of Dichloroaquacopper(I) Complexes, CuCl2(H2O)n−, n = 1–4, 6
For CuCl2−, a linear D∞h structure is obtained (see Figure 6). We may add a water molecule in one of several ways to give CuCl2(H2O)−. For those with a direct Cu-O interaction, we first consider one of the two possible C2v structures. When both exist, C2v #1 is always lower in energy. When it exists, the C2v #2 has an imaginary A2 mode, whereas C2v #1 does not. This suggests that the C2v #2 would convert to the C2v #1 structure along this mode. The C2v #1 structure usually has imaginary B1 and B2 modes, suggesting desymmetrization to one of two Cs structures. Along the B1 mode, the C2v #1 structure converts to the C2v [2 + 1] structure via a Cs structure by the water wagging motion. Along the B2 mode, it converts to the stable Cs #3 [2 + 1]. There also exists a Cs #4 [2 + 1].
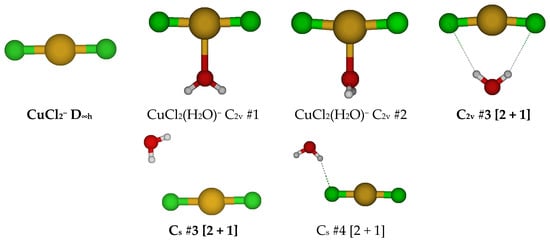
Figure 6.
Dichlorocopper(I) structures, CuCl2(H2O)n−, n = 0, 1.
For CuCl2(H2O)2−, we first examine the two possible D2h square planar structures (Figure 7). For D2h #2, the water molecules dissociated for all levels except B3LYP/6-31G*. The D2h #1 structure has multiple imaginary frequencies of irreducible representation B1g, B3g, B1u, and B3u, whereas the D2h #2 structure additionally has Au and B2g modes, which would convert via a D2 and C2h structure to D2h #1. Desymmetrization of the D2h #1 structure along these modes would lead to C2h #1, C2h #2, C2v #1, and C2v #2 structures. For the C2h #1 structure, the water wags to break coordination with the copper to form potentially a new D2h #3 [2 + 2] structure. Desymmetrization along the ungerade B modes usually results in water detachment, except for B3u at some HF levels, where a chloride detaches instead. For the D2h #3 [2 + 2] structure, desymmetrization along the imaginary B3g, B2u, and B3u modes would give C2h #3, C2v #3, and C2v #4. These in turn desymmetrize to one of a number of Ci, C2, and Cs structures, some of which are local minima at some levels of theory. The Cs structures often ascend in symmetry to C2v #4 or C2h #3. The lowest energy structure on the potential energy surface is the C1 #1 structure, in which a hydrogen bond is formed between the two water molecules. The only stable structures found may be described as hydrated CuCl2− anions.
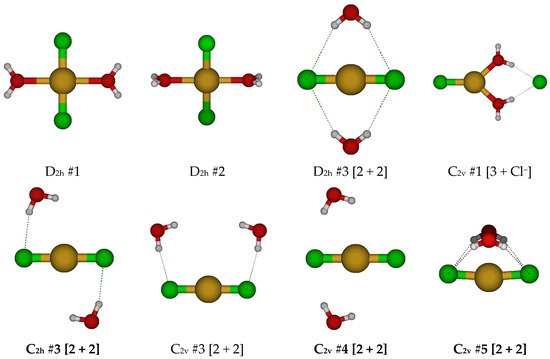
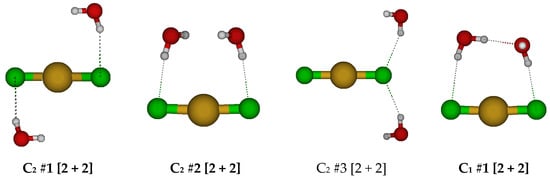
Figure 7.
Dichlorocopper(I) structures, CuCl2(H2O)n−, n = 2.
For CuCl2(H2O)3−, we first examine the two possible D3h trigonal bipyramidal structures (Figure 8). The first has the water molecules bisected by the σh symmetry plane, whereas the second has the water molecules in the σh symmetry plane. In the second case, for diffuse basis sets, the water molecules dissociated, whereas for the 6-31G* basis set, the structures have imaginary E”, A2”, and A1” modes, and at the correlated levels, additional A2’ and E’ modes. In the first case, there are imaginary A2’, E’, E”, and A2” modes at all levels of theory. For D3h #1, desymmetrization along A2’ to give C3h #1 resulted in ascent in symmetry via water wagging to form D3h #3 [2 + 3], which was stable at HF/6-31G*. Desymmetrization along A2” gave the C3v #1 structure in which one of the chlorine atoms dissociated. Desymmetrization along the E’ mode gave one of two possible C2v structures, one (C2v #1, most levels of theory) in which two water molecules wagged to form hydrogen bonds with the chlorine atoms, or one (C2v #2, B3LYP/6-31G* and MP2/6-31G*) in which the two chlorine atoms dissociated. The three C2 structures found either have two dissociated chlorine atoms (C2 #1) or two dissociated water molecules (C2 #2, #3). The two Cs structures, both (Cs #1 and #2), have a dissociated Cl−; in the second one, there is also a dissociated water. The Cs #2 structure was stable at the Hartree–Fock levels. We did not explore the desymmetrization of D3h #2, since it dissociated at all levels with diffuse functions.
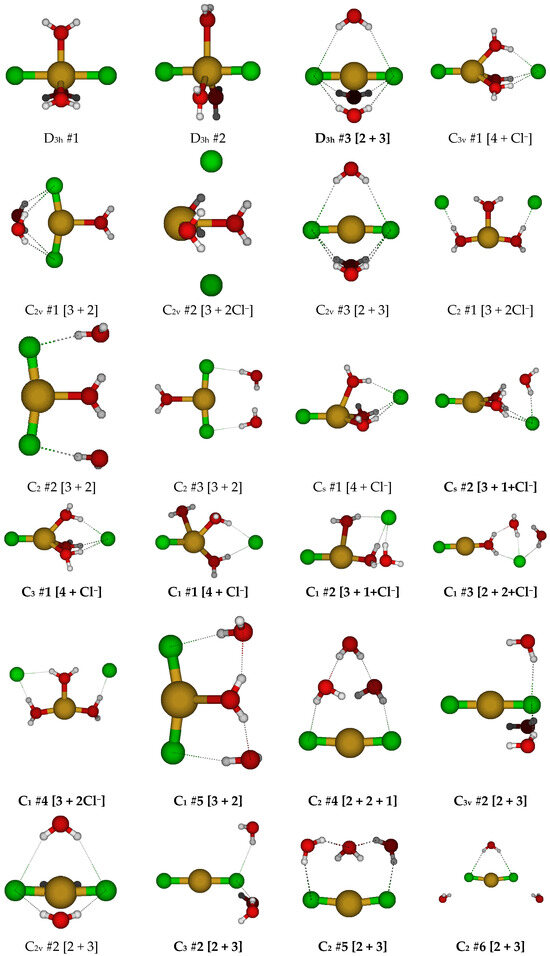
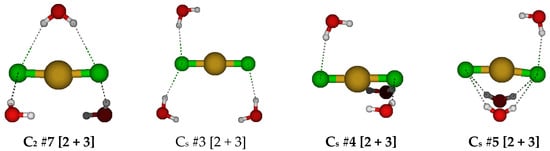
Figure 8.
Dichlorocopper(I) structures, CuCl2(H2O)n−, n = 3.
The C3v #1 structure had imaginary A2 and E modes at all levels of theory. Desymmetrization along the A2 mode would give a C3 structure, which is stable at some levels of theory. Desymmetrizing to give C1 structures often resulted in a wide variety of atomic arrangements. While desymmetrization from a maximally coordinated structure does prove that lower coordination numbers are favored, it is tedious and may not be the best method to find all structures, so we also try desymmetrizing from the high symmetry D3h #3 trihydrated dichlorocuprate(I) ion.
The D3h #3 structure usually contained imaginary A2” and E” modes (and occasionally E’ modes). The resulting C2v #3 structure always has imaginary A2 and B2 (and sometimes B1) modes. The resulting C3v #2 structure was not stable (except at MP2/6-31G*) and contained imaginary A2 and/or E modes. The C2v #3 structure can desymmetrize to a number of C2 and Cs structures, of which C2 #5 is stable at HF/6-31G*, HF/6-311+G* and MP2/6-311+G*; C2 #7 is stable at B3LYP/6-31G*, MP2/6-31G*, and MP2/6-311+G*; Cs #4 is stable at B3LYP/6-31+G* and B3LYP/6-311+G*; and Cs #5 is stable at MP2/6-31+G* and MP2/6-311+G*.
For copper(I) with two chloride ions, it appears that five-coordination was not possible, and the lowest energy structures appeared to be hydrated dicoordinate dichlorocuprate(I), so initial structures with coordination numbers higher than this were not expected to be stable. At this point, we focused on structures having a discrete CuCl2− anion with six water molecules. We started with the D3d #1 structure, which had imaginary A2g, A1u, and usually Eg and Eu modes (see Figure 9). The Hartree–Fock structures had the water molecules farther away from the copper ion by increasing the H…Cl-Cu angle. The resulting S6 #1, D3 #2, and C3h #1 structures were quite close in energy, and at least one of them was a local minimum and the lowest in energy at the HF levels. The S6 #2 structure was the lowest in energy at the MP2 levels, and the D3 #2 structure was the lowest in energy at the B3LYP levels. The D3 #1 structure desymmetrized to give C3 #1, which ascended in symmetry at some HF levels to S6 #2.
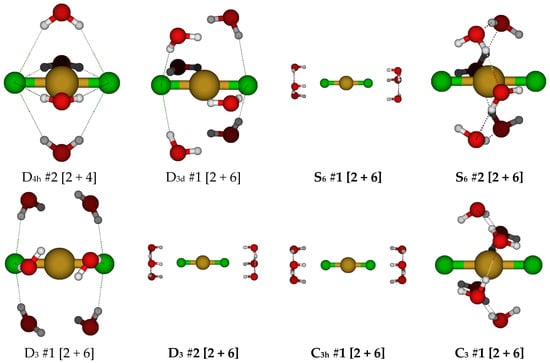
Figure 9.
Dichlorocopper(I) structures, CuCl2(H2O)n−, n = 4, 6.
2.3. Structures of Trichloroaquacopper(I) Complexes, CuCl3(H2O)n2−, n = 1–4, 6
For CuCl32−, a trigonal planar D3h structure is obtained (see Figure 10). We may add a water molecule in one of two ways (Cs #1, Cs #2) to make a tetrahedrally coordinated structure; however, the water molecule always dissociates. For Cs #2, the structure ascends in symmetry to give a C2v #1 [3 + 1] structure. The dissociated structure for Cs #1 is a transition state for water migration between two equivalent C2v #1 structures in which only one hydrogen bond is retained. Other attempts to make tetrahedrally coordinated structures led to structures such as CuCl2− + H2O + Cl−, CuCl2(H2O)− + Cl−, or CuCl(H2O) + 2 Cl−.
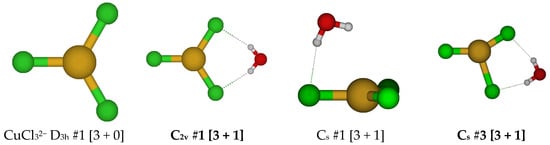
Figure 10.
Trichlorocopper(I) structures, CuCl3(H2O)n2−, n = 0, 1.
For CuCl3(H2O)22−, all attempts to construct a five-coordinate structure led to dissociation of either water or chloride ligand(s). Two stable dihydrated trichlorocuprate(I) structures were found (Figure 11).
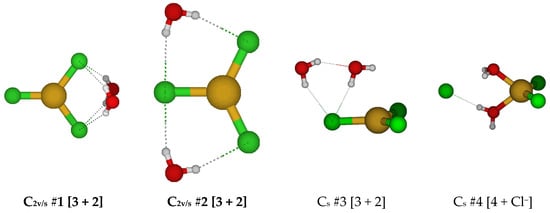
Figure 11.
Trichlorocopper(I) structures, CuCl3(H2O)n2−, n = 2.
For CuCl3(H2O)32−, all attempts to construct a six-coordinate structure failed, resulting in dissociation of chloride and/or water ligands. Two possible trihydrated trichlorocuprate(I) ions were identified, of D3h (C3v at MP2 levels) and Cs symmetry (Figure 12). At the MP2/6-31G* level, the most hydrogen-bonded chloride ion of the Cs #1 structure dissociated.
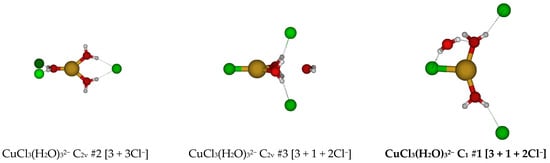
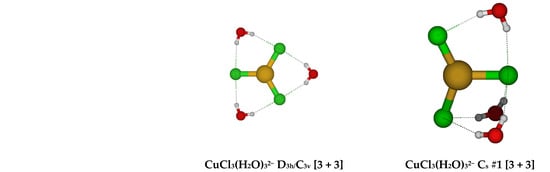
Figure 12.
Trichlorocopper(I) structures, CuCl3(H2O)n2−, n = 3.
At this point, it was decided to focus on hydrated trichlorocuprate(I) ions (Figure 13). With four water molecules, there are two main arrangements that can be considered, with C2v #1 being slightly more stable than C2v #2. With five water molecules, the main arrangement is the C2v structure. For six water molecules, we return to a D3h structure. At some levels of theory, slightly lower symmetry stationary points may be located.
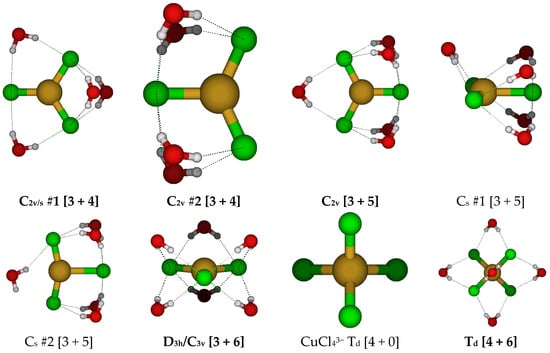
Figure 13.
Trichlorocopper(I), CuCl3(H2O)n2−, n = 4–6, and tetrachlorocopper(I) CuCl4(H2O)n3−, n = 0, 6 structures.
2.4. Structures of Tetrachloroaquacopper(I) Complexes, CuCl4(H2O)n3−, n = 0, 6
For the putative tetrachlorocuprate(I) ion, we only considered two structures, the naked ion and the hexahydrate (Figure 13). The naked ion nearly always has an imaginary T2 mode corresponding to Cu-Cl stretching, suggesting fragmentation of this highly charged ion. However, to our surprise, hydrating this ion with six water molecules results in stabilization of this structure to a local minimum at most levels of theory.
Because of the anomalous structures calculated using the 6-31G* basis set, we do not consider these calculations further.
3. Discussion
In the solid state under ambient conditions, CuCl(s) adopts the sphalerite (zinc-blende) structure (CuCl-II, F-43m), with the tetrahedrally coordinated Cu-Cl distance of 2.382 Å [6], later refined to 2.3407 Å [7] and 2.347 Å [8]. It transforms to the hexagonal wurtzite structure CuCl-I at 681 K before melting at 696 K [9]. In the wurtzite structure, the two Cu-Cl distances are 2.3751 and 2.4717 Å. Above a triple point (1.2 kbar, 724 K), both of the forms transform to the body-centred cubic form CuCl-III (P63mc) upon temperature increase. Increasing the pressure at ambient temperature converts the sphalerite form to CuCl-IV (Pa-3, ~50 kbar), then CuCl-V (~100 kbar) [10]. For CuCl-IV, the Cu-Cl distances are 2.2922, 2.4182 Å (5.52 GPa) and 2.2562, 2.3674 Å (92.4 Gpa), whereas for CuCl-V (halite, Fm-3m), the Cu-Cl distances are 2.4645 Å around these octahedrally coordinated atoms.
In the gas phase, CuCl(g) can be generated by passing HCl(g) over hot Cu(s) metal at 800–1000 °C. Brewer and Lofgren established that the vapor consists of a mixture of CuCl(g) and its trimer Cu3Cl3(g) based on vapor pressure and density measurements [11]. No evidence of the dimer was found. The trimer has a single infrared band at 350 cm−1 [12], whereas the monomer occurs at 416.9 cm−1 [13]. This is consistent with a cyclic structure. The electron diffraction results of Wong and Schomaker fit a planar cyclic model with Cu-Cl = 2.160(15) Å and Cu-Cl-Cu = 87.5° [14]. In the near-ultraviolet, absorbances at 223.5, 218(sh), 238(sh), and 273 nm were observed [15]. Our calculations support a stable D3h trimer structure (Figure 1).
The Cu-Cl distance in select [CuCl(H2O)n]0 structures is given in Table 1. At all levels of theory, the Cu-Cl distance shortens slightly as the first water molecule is added, but then is generally lengthened as more water molecules hydrate the resulting [CuCl(H2O)]0 molecule. The Cu-Cl distance in the unhydrated molecule (2.10 Å, B3LYP/6-311+G*) is much shorter than in the solid state (2.25–2.47 Å, above) but is only slightly shorter than that reported in the gas-phase for the trimer (2.16 Å, above). The predicted trimer Cu-Cl distance is very close (2.1494 Å, MP2/6-311+G*). In the solid state, each copper atom is surrounded by between 4 and 6 chlorine atoms, whereas in the gas-phase monomer, only one atom is nearby, which is why the distance is shorter in the monomer.

Table 1.
Cu-Cl distance (Å) in [CuCl(H2O)n]0. In this and the following tables, n/a = not applicable.
The Cu-Cl vibrational frequencies in select [CuCl(H2O)n]0 structures are given in Table 2. The experimental harmonic value in CuCl(g) is given as 416.9 cm−1, which matches well with the MP2/6-311+G* value of 414 cm−1. For the monohydrate, the Cu-Cl stretching motion couples with the Cu-O stretching motion in a symmetric low-frequency and an asymmetric high-frequency pair. The lower frequency of the hydrated forms of this linear species is only slightly affected by the hydration number, whereas the higher frequency component is significantly affected. A weak band observed at 350 cm−1 in copper(I) chloride solutions, assigned to CuCl0, is in good agreement with the predicted band for hexahydrated [CuCl(H2O)]0 at 365 cm−1 (MP2/6-311+G*).

Table 2.
Cu-Cl(O) stretching vibrational frequency (cm−1) in [CuCl(H2O)n]0.
The discrete dichlorocuprate(I) [CuCl2]− ion is found in the tetraphenylarsonium/phosphonium dichlorocuprate(I) salts, among others [16]. In these the CuCl distances are: [Ph4As]+[CuCl2]−, 2.069(3) and 2.072(3) Å; [Ph4P]+[CuCl2]−, 2.088(2) and 2.090(2) Å. In solution (acetonitrile, dimethyl sulfoxide, pyridine, water), the distances were found to be 2.10(2)–2.12(2) by EXAFS, which demonstrates that the same ion is present [17]. These solid-state and solution distances compare quite favorably to the gas-phase MP2 calculations on the unsolvated dichlorocuprate(I) ion of ~2.10 Å (Table 3). In most of the hydrated structures, the Cu-Cl distance is hardly affected by the hydration, except for the S6 #2 structure, which has a much longer Cu-Cl distance.

Table 3.
Cu-Cl distance (Å) in [CuCl2(H2O)n]−.
The infrared and Raman spectra of solutions and solids containing the dichlorocuprate(I) ion were summarized by Bowmaker et al., who found that in the infrared, a band around 400 cm−1 appears, whereas in the Raman, a band around 300 cm−1 appears [18]. These can be assigned to the antisymmetric and symmetric stretching motions, respectively. In addition, an IR band at 110 cm−1 corresponds to the bending mode. More specifically, in ether extracts of HCl + CuCl, a band occurs at 296 cm−1; in tributylphosphate extracts of HCl + CuCl, at 397 and 301 cm−1; in tributylphosphate solution of CuCl + LiCl, at 405, 109, and 300 cm−1; in polycrystalline tetrabutylammonium dichlorocuprate(I), at 404, 111, and 304 cm−1; and in polycrystalline tetraphenylarsonium dichlorocuprate(I), at 408, 111, and 315 cm−1. The mutual exclusion rule between the IR and Raman spectra for centrosymmetric moieties such as [CuCl2]− can clearly be seen. In aqueous solution, we found a band attributable to [CuCl2]− at 297 cm−1 [4]. Both the MP2 and B3LYP calculations give good agreement with these experiments, with the MP2 calculations being slightly too high and the B3LYP calculations slightly too low (Table 4). The Hartree–Fock calculations severely underestimate the vibrational frequencies, which is atypical because usually Hartree–Fock frequencies are larger than experimental frequencies and are typically scaled by a factor of around 0.9 [19]. The effect of the water molecules upon hydration is to increase the frequency of the stretching modes by 3-14 cm−1. In some cases, coupling between the antisymmetric stretching mode and water librations results in two modes with an appreciable contribution of Cu-Cl stretch (n = 6). The hexahydrate S6 #2 structure has a much longer Cu-Cl distance, resulting in much smaller frequencies.

Table 4.
Cu-Cl stretching vibrational frequency (cm−1) in [CuCl2(H2O)n]−.
The discrete trichlorocuprate ion was found in bis(tetramethylphosphonium) trichlorocuprate(I) salt [20]. The Cu-Cl distances were reported as 2.214(2) and 2.232(3) Å. In aqueous solution, EXAFS gives 2.20(2) or 2.21(3) Å, depending on the analysis [17]. These values are in fair agreement with the MP2 calculations of the hexahydrate shown in Table 5, but the HF and B3LYP calculations are much too long. The general trend is that there is a significant shortening of the Cu-Cl distance on hydration, which suggests a strong stabilization of this small, highly charged ion. For some structures (3, Cs; 4 C2v #2), a pronounced alternation of Cu-Cl bonds occurs as the stabilization by hydrogen bonding results in partial dissociation.

Table 5.
Cu-Cl distance (Å) in [CuCl3(H2O)n]2−.
The increase in the Cu-Cl stretching vibrational frequencies of CuCl32− upon hydration is also pronounced, concomitant with the shortening of the Cu-Cl bonds. In the two cases where the Cu-Cl bond lengthened, one of the vibrational frequencies significantly decreased. Experimentally, we observed a Raman band at 247 cm−1 that we assigned to the Cu-Cl stretching motion of the CuCl32− ion. This is in fair agreement with the totally symmetric band predicted at 235 cm−1 (MP2/6-31+G*, Table 6). We also noticed that at some levels of theory, the predicted symmetry was lower than anticipated, but the calculations of the bond distances and vibrational frequencies at higher symmetry, containing an imaginary mode, were only slightly altered by the lower symmetry.

Table 6.
Cu-Cl stretching vibrational frequency (cm−1) in [CuCl3(H2O)n]2−. The “*” indicates that the structure was not an energy minimum.
We were initially unable to find any experimental literature reports of a discrete CuCl43− anion. Our calculations on the tetrahedrally constrained ion gave long Cu-Cl distances, which shortened considerably upon hydration (Table 7). Although the unhydrated ion was unstable with respect to fragmentation (see the imaginary frequencies in Table 8), the addition of water molecules to form a highly symmetric Td structure stabilized the structure so that it could form a local minimum. In the gas phase, the formation of CuCl32− and CuCl43− by the addition of chloride to the precursor anion would be thermodynamically unfavored. However, the hydration energy would tend to stabilize the highly charged anions in aqueous solutions.

Table 7.
Cu-Cl distance (Å) in [CuCl4(H2O)n]3−.

Table 8.
Cu-Cl stretching vibrational frequency (cm−1) in [CuCl4(H2O)n]3−.
A search of the Cambridge Structural Database revealed 274 structures containing the [CuCl2]- anion, with an average bond distance of 2.096 Å and bond angle of 178° [21]. No hydrated forms of this ion were found, i.e., neither [CuCl2(H2O)]− nor [CuCl2(H2O)2]−, only those of the corresponding copper(II) ion (identifiers ZUKTUX, FUTRUH, KUPHAE, RITYUR). In addition, 24 structures containing the [CuCl3] anion were found, most of which were the [CuCl3]2− anion (Cu-Cl avg 2.243 Å), except for the three instances of the [CuCl3]− anion with copper(II) (identifiers INAYAZ, GEXRIK, MILQIH) and much shorter Cu-Cl distances. Although the average angle is 119.9(6.8)° and the sum 359.6(1.1)°, as expected for a trigonal planar structure, the large standard deviation of 6.8° means that there are some significant in-plane distortions. The 15 [CuCl3(H2O)] and 9 [CuCl3(H2O)2] anions found all had copper in the +2 oxidation state and an overall charge of -1. Of the 744 structures containing [CuCl4], the vast majority contained copper(II) as the [CuCl4]2− ion. These structures were statistically analyzed to find outliers (at least one Cu-Cl distance more than two standard deviations from the average of 2.257(0.031) Å), and these were manually checked. We found 66 such structures, but again the vast majority of these were the [CuCl4]2− ion, and only three instances of the [CuCl4]3− ion were found (identifiers FAMQAL (2.376(0) Å), JETRIN (2.338(0.018) Å), and LADREP (2.202(0.043) Å)), in which all four of the CuCl distances were more than two standard deviations from the average. The last of these is of poor quality due to disorder. Only the tetrachlorocopper(II) structures could also coordinate to one (identifiers COCNEO, COGXOP, HOWCOL, VEWQEX) or two (identifiers DEVVUZ, GADWUD, HERWEK) water molecules. The fact that the di-, tri-, and tetrachlorocopper(I) anions do not directly bind to water molecules supports our computational findings and indicates that the copper(I) ion prefers to bind to the chloride ion rather than the water molecule.
4. Materials and Methods
Preliminary calculations were performed using Gaussian 98 [22]. The MP2 calculations utilize the frozen core approximation. The geometries were optimized using a stepping-stone approach, in which the geometries at the levels HF/STO-3G, HF/3-21G, HF/6-31G*, HF/6-31+G*, MP2/6-31G*, and MP2/6-31+G* were sequentially optimized, with the geometry and molecular orbital reused for the subsequent level. When this approach failed (as one or more ligands dissociated or underwent pseudorotation), the problematic level was skipped. Default optimization specifications were normally used. After each level, where possible, a frequency calculation was performed at the same level, and the resulting Hessian was used in the following optimization. Z-matrix coordinates constrained to the appropriate symmetry were used as required to speed up the optimizations. Because frequency calculations are done at each level, any problems with the Z-matrix coordinates would manifest themselves by giving imaginary frequencies corresponding to modes orthogonal to the spanned Z-matrix space. The Hessian was evaluated at the first geometry (opt = CalcFC) for the first level in a series to aid geometry convergence. For structures that proved not to be local minima at the HF/6-31G* level, the subsequent calculations were not performed.
The calculated structures proved to be very sensitive to the choice of theoretical level, with large changes in optimum geometry sometimes between two sequential calculations. In addition, calculations using the low-level basis sets often converged to incorrect copper(I) ion configurations (s2d8 or s1d9 instead of d10) that were propagated throughout the calculations, as demonstrated by wavefunction stability calculations. It was decided to repeat all calculations starting with the highest possible symmetry and systematically desymmetrizing (Table 9) at all appropriate levels of theory, skipping the STO-3G and 3-21G levels, and using either Gaussian 03 [23] or Gaussian 16 [24]. In addition, the use of the 6-311+G* basis set and the B3LYP functional was explored.

Table 9.
Desymmetrization table (adapted from supplementary material of Ref. [5]). Desymmetrization of a structure with symmetry of a point group along an imaginary mode of irreducible representation given will give a structure with symmetry of a subgroup.
5. Conclusions
The speciation of copper(I) with increasing amounts of chloride is consistent with the existence of [CuCl(H2O)], [CuCl2]−, and [CuCl3]2−. The addition of explicit waters of hydration has been shown to improve the agreement with the experimental solution vibrational frequencies. The MP2 method has been shown to give good results for the geometries and vibrational frequencies of chlorocopper(I) complexes when used with basis sets including diffuse functions. Systematic desymmetrization has proven to be advantageous in finding structures and/or ruling out candidate structures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30153147/s1, Table S1: Total Energies.
Author Contributions
Conceptualization, C.C.P.; methodology, C.C.P.; validation, C.C.P.; formal analysis, C.C.P., C.R.C., D.J.W.M. and D.C.M.W.; investigation, C.C.P., C.R.C., D.J.W.M. and D.C.M.W.; data curation, C.C.P. and D.C.M.W.; writing—original draft preparation, C.C.P.; writing—review and editing, C.C.P. and C.R.C.; visualization, C.C.P.; supervision, C.C.P.; project administration, C.C.P.; funding acquisition, C.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada, Ontario Power Generation, and the Government of Nova Scotia, Canada.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
C.C.P. would like to acknowledge the former support of the Natural Sciences and Engineering Research Council of Canada. C.C.P. acknowledges the support of Peter Tremaine (University of Guelph) and Ontario Power Generation. C.R.C. and D.J.W.M. acknowledge the Nova Scotia Department of Economic Development for co-operative education salary support (C.R.C. Work Term 2, January–April 2001; D.J.W.M., Work Terms 2 and 3, September–December 2002 and January–April 2003). C.C.P., C.R.C., and D.J.W.M. thank the Department of Astronomy and Physics, Saint Mary’s University, for providing access to computing facilities (Cygnus). C.C.P. and D.C.M.W. thank ACENet for access to computing facilities (Placentia). C.C.P. thanks Compute Canada/Digital Research Alliance of Canada for access to computing facilities (Graham). C.C.P. thanks Katherine Robertson for carrying out the CSD search (18 July 2025).
Conflicts of Interest
The authors declare that this study received funding from Ontario Power Generation. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Abbreviations
The following abbreviations are used in this manuscript:
| HF | Hartree–Fock |
| MP2 | Second-order Moller–Plesset Perturbation Theory |
| B3LYP | Becke three-parameter exchange with Lee–Yang–Parr correlation functional |
References
- Klewe, B.; Pedersen, B. The Crystal Structure of Sodium Chloride Dihydrate. Acta Cryst. B 1974, 30, 2363–2371. [Google Scholar] [CrossRef]
- Journaux, B.; Pakhomova, A.; Collings, I.E.; Petitgirard, S.; Ballaran, T.B.; Brown, J.M.; Vance, S.D.; Chariton, S.; Prakapenka, V.B.; Huang, D.; et al. On the identification of hyperhydrated sodium chloride hydrates, stable at icy moon conditions. Proc. Nat. Acad. Sci. USA 2023, 120, e2217125120. [Google Scholar] [CrossRef]
- Pye, C.C.; Gunasekara, C.M. An ab Initio Investigation of the Hydration of Antimony(III). Liquids 2024, 4, 322–331. [Google Scholar] [CrossRef]
- Applegarth, L.M.S.G.A.; Corbeil, C.R.; Mercer, D.J.W.; Pye, C.C.; Tremaine, P.R. Raman and ab Initio Investigation of Aqueous Cu(I) Chloride Complexes from 25 to 80 °C. J. Phys. Chem. B 2014, 118, 204–214. [Google Scholar] [CrossRef]
- Pye, C.C.; Whynot, D.C.M.; Corbeil, C.R.; Mercer, D.J.W. Desymmetrization on geometry optimization: Application to an ab initio study of copper(I) hydration. Pure Appl. Chem. 2020, 92, 1643–1654. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G.; Posnjak, E. The Crystal Structures of the Cuprous Halides. J. Am. Chem. Soc. 1922, 44, 30–36. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystal Structures, 2nd ed.; Interscience: New York, NY, USA, 1963; Volume 1, pp. 85–237. [Google Scholar]
- Hull, S.; Keen, D.A. High-pressure polymorphism of the copper(I) halides: A neutron diffraction study to ~10 GPa. Phys. Rev. B 1994, 50, 5868–5885. [Google Scholar] [CrossRef]
- Rapoport, E.; Pistorius, C.W.F.T. Phase Diagrams of the Cuprous Halides to High Pressures. Phys. Rev. 1968, 172, 838–847. [Google Scholar] [CrossRef]
- Merrill, L. Behavior of the AB-Type Compounds at High Pressures and High Temperatures. J. Phys. Chem. Ref. Data 1977, 6, 1205–1252. [Google Scholar] [CrossRef]
- Brewer, L.; Lofgren, N.L. The Thermodynamics of Gaseous Cuprous Chloride, Monomer and Trimer. J. Am. Chem. Soc. 1950, 72, 3038–3045. [Google Scholar] [CrossRef]
- Klemperer, W.; Rice, S.A.; Berry, R.S. The Infrared Spectrum of Cuprous Chloride Vapor. J. Am. Chem. Soc. 1957, 79, 1810–1811. [Google Scholar] [CrossRef]
- Herzberg, G. Molecular Spectra. Vol 1. The Spectra of Diatomic Molecules, 2nd ed.; D. Van Nostrand: New York, NY, USA, 1950. [Google Scholar]
- Wong, C.-H.; Schomaker, V. An Electron Diffraction Investigation of the Structure of Cuprous Chloride Trimer. J. Phys. Chem. 1957, 61, 358–360. [Google Scholar] [CrossRef]
- Hilden, D.L.; Gregory, N.W. Vapor-Phase Absorbance and Thermodynamic Properties of Cuprous Chloride and Cuprous Bromide. J. Phys. Chem. 1972, 76, 1632–1637. [Google Scholar] [CrossRef]
- Andersson, S.; Jagner, S. Crystal Structures of Tetraphenylarsonium Dichlorocuprate(I), Tetraphenylphosphonium Dichlorocuprate(I) and Tetraphenylphosphonium Dibromocuprate(I). Acta Chem. Scand. A 1985, 39, 297–305. [Google Scholar] [CrossRef]
- Persson, I.; Sandstrom, M.; Steel, A.T.; Zapatero, M.J.; Akesson, R. A Large-Angle X-ray Scattering, XAFS, and Vibrational Spectroscopic Study of Copper(I) Halide Complexes in Dimethyl Sulfoxide, Acetonitrile, Pyridine, and Aqueous Solutions. Inorg. Chem. 1991, 30, 4075–4081. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Brockliss, L.D.; Whiting, R. Bonding in d10 Transition Metal Complexes. I. Infrared, Raman, and N.Q.R. Studies of Some Dihalocuprate(I) Complexes. Aust. J. Chem. 1973, 26, 29–42. [Google Scholar] [CrossRef]
- Pople, J.A.; Scott, A.P.; Wong, M.W.; Radom, L. Scaling Factors for Obtaining Fundamental Vibrational Frequencies and Zero-Point Energies from HF/6-31G* and MP2/6-31G* Harmonic Frequencies. Israel J. Chem. 1993, 33, 345–350. [Google Scholar] [CrossRef]
- Andersson, S.; Jagner, S. Coordination of Copper(I) in Two Novel Chlorocuprate(I) Anions; Structures of Tetramethylphosphonium catena-μ-Chloro-μ3-chloro-[μ-chloro-dicuprate(I)] and Bis(tetramethylphosphonium) Trichlorocuprate(I). Acta Chem. Scand. A 1988, 42, 691–697. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A., Jr.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian 98, Revision A.9 [Computer Program]; Gaussian, Inc.: Pittsburgh, PA, USA, 1998. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision D.02 [Computer Program]; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03 [Computer Program]; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).