Potential Use of Cefiderocol and Nanosilver in Wound Dressings to Control Multidrug-Resistant Gram-Negative Bacteria
Abstract
1. Introduction
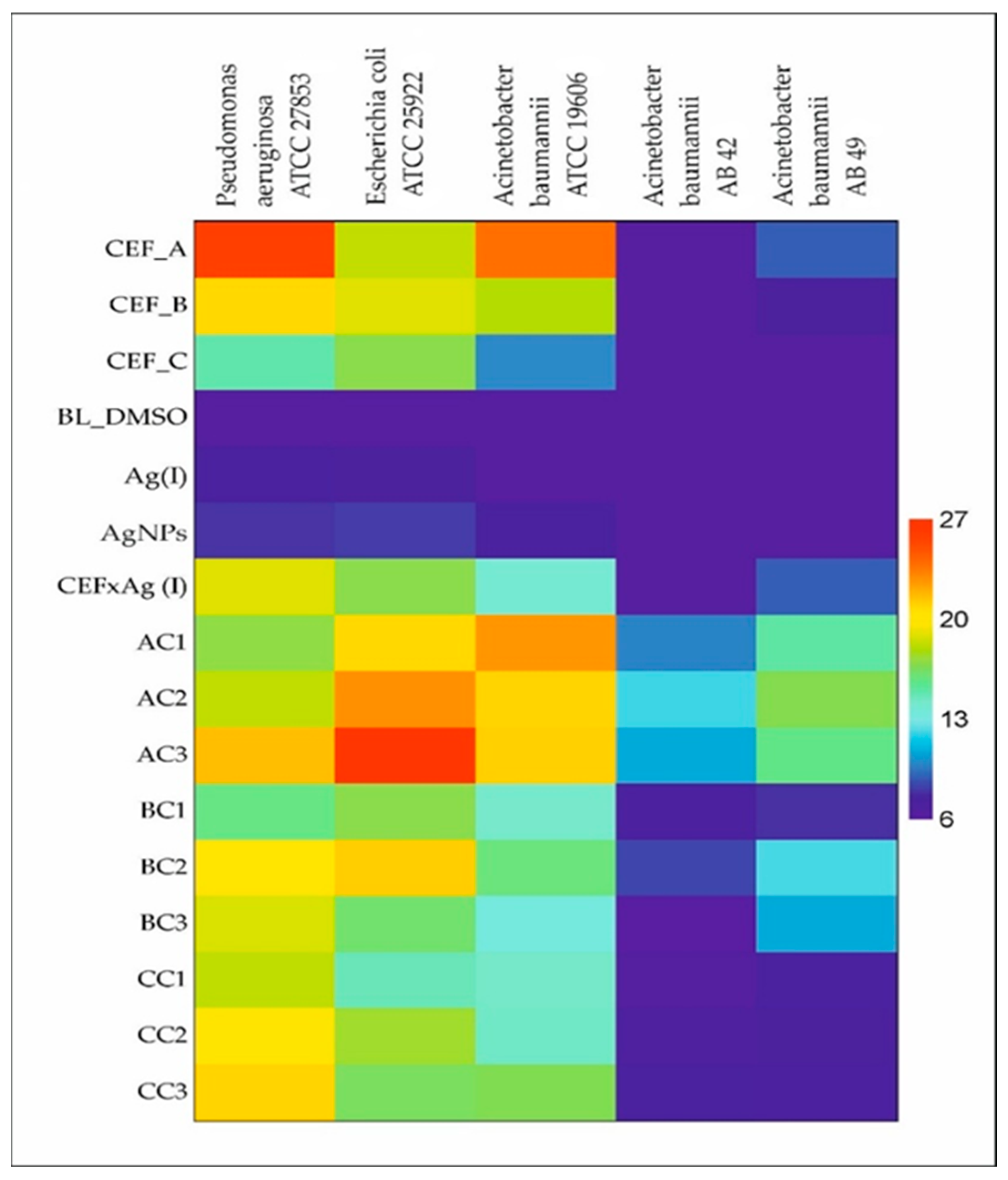
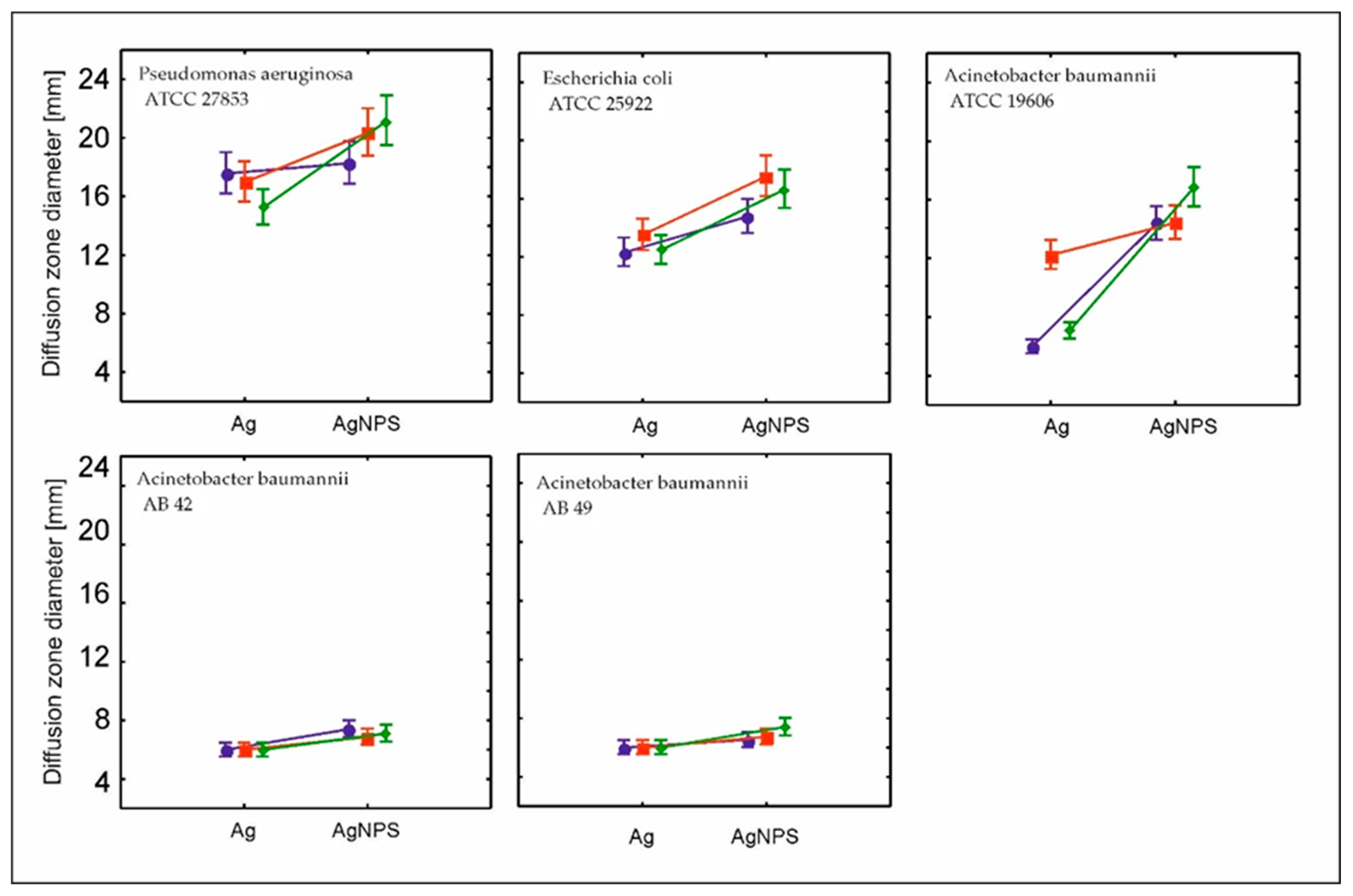
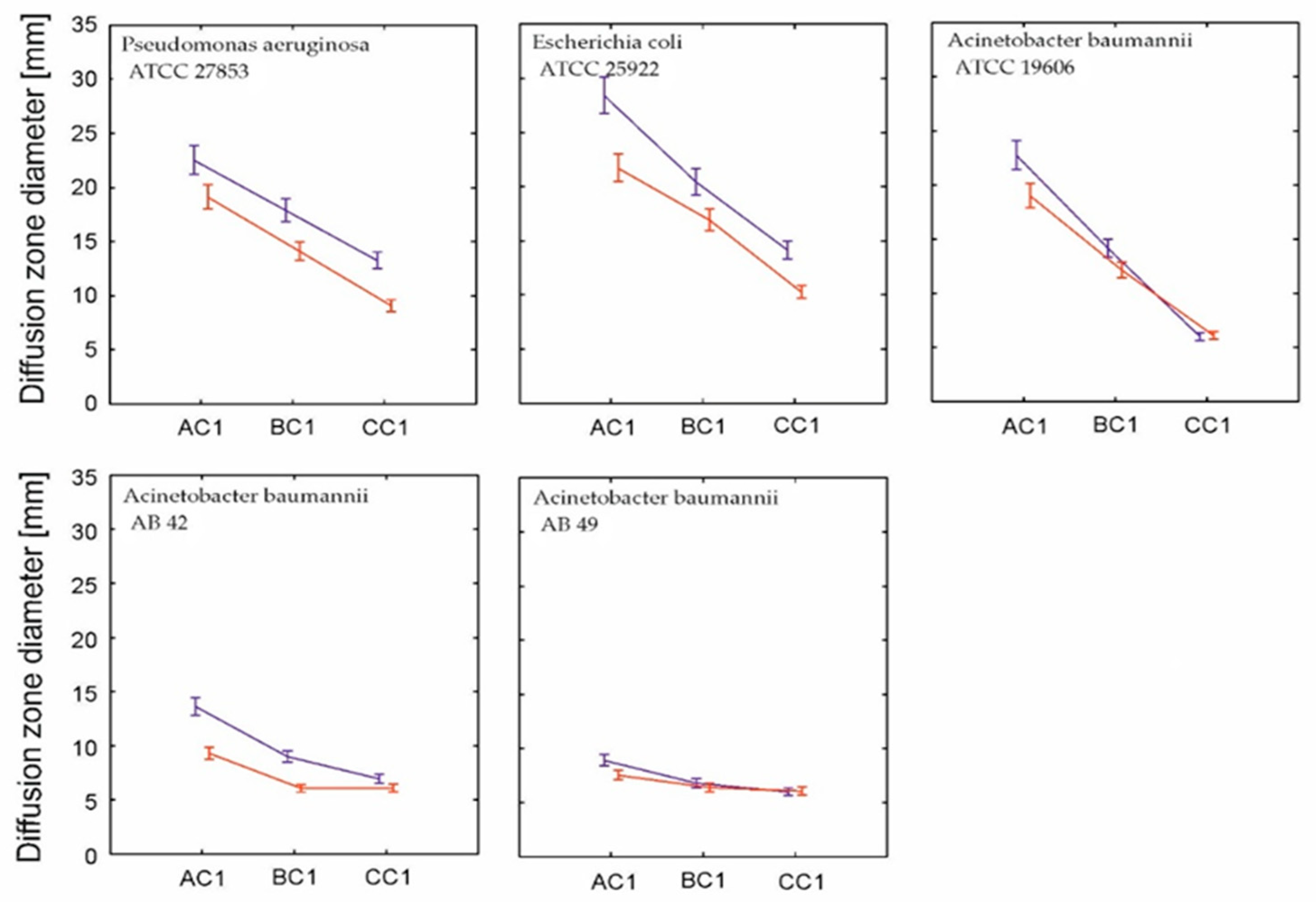
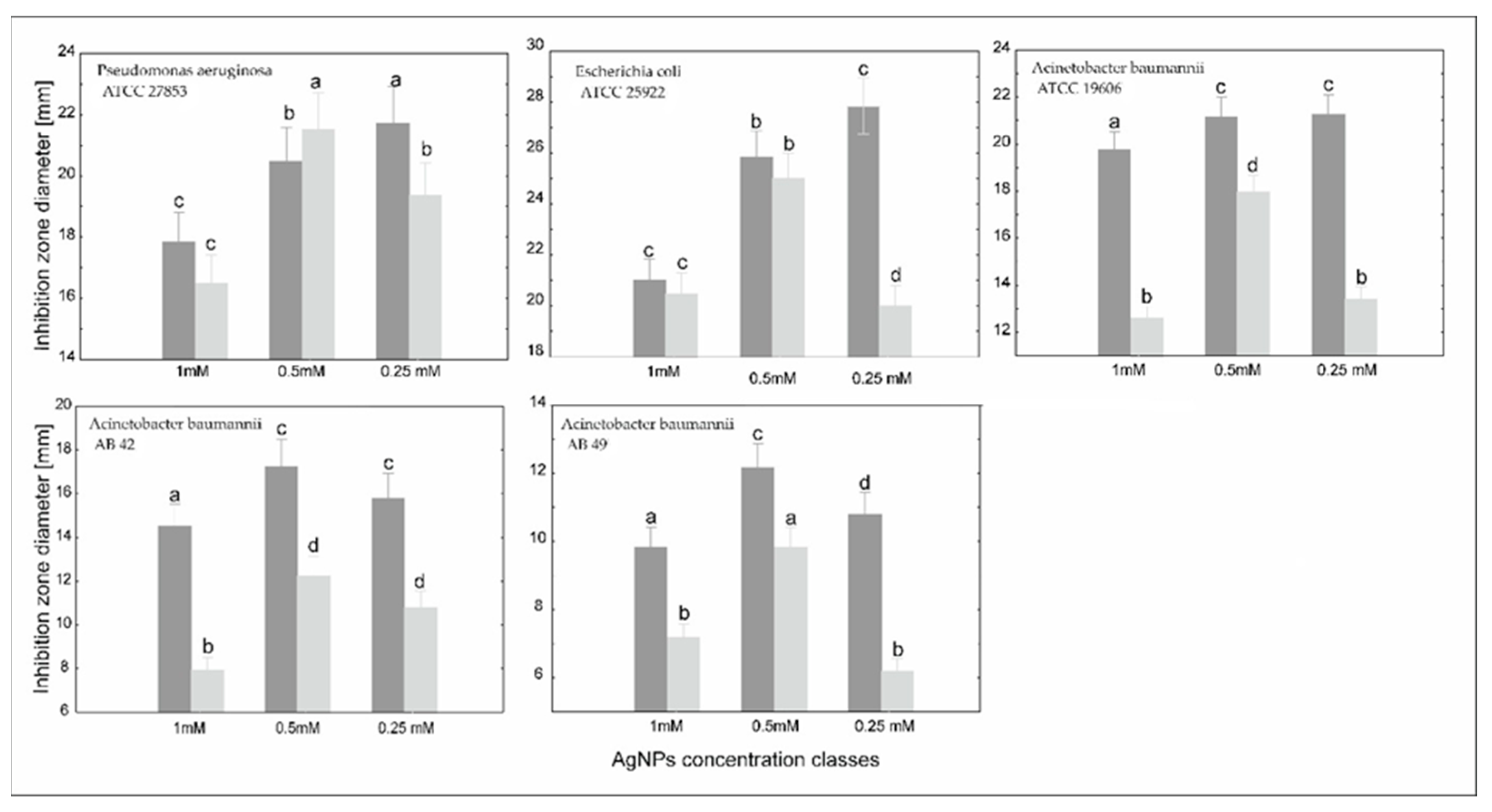
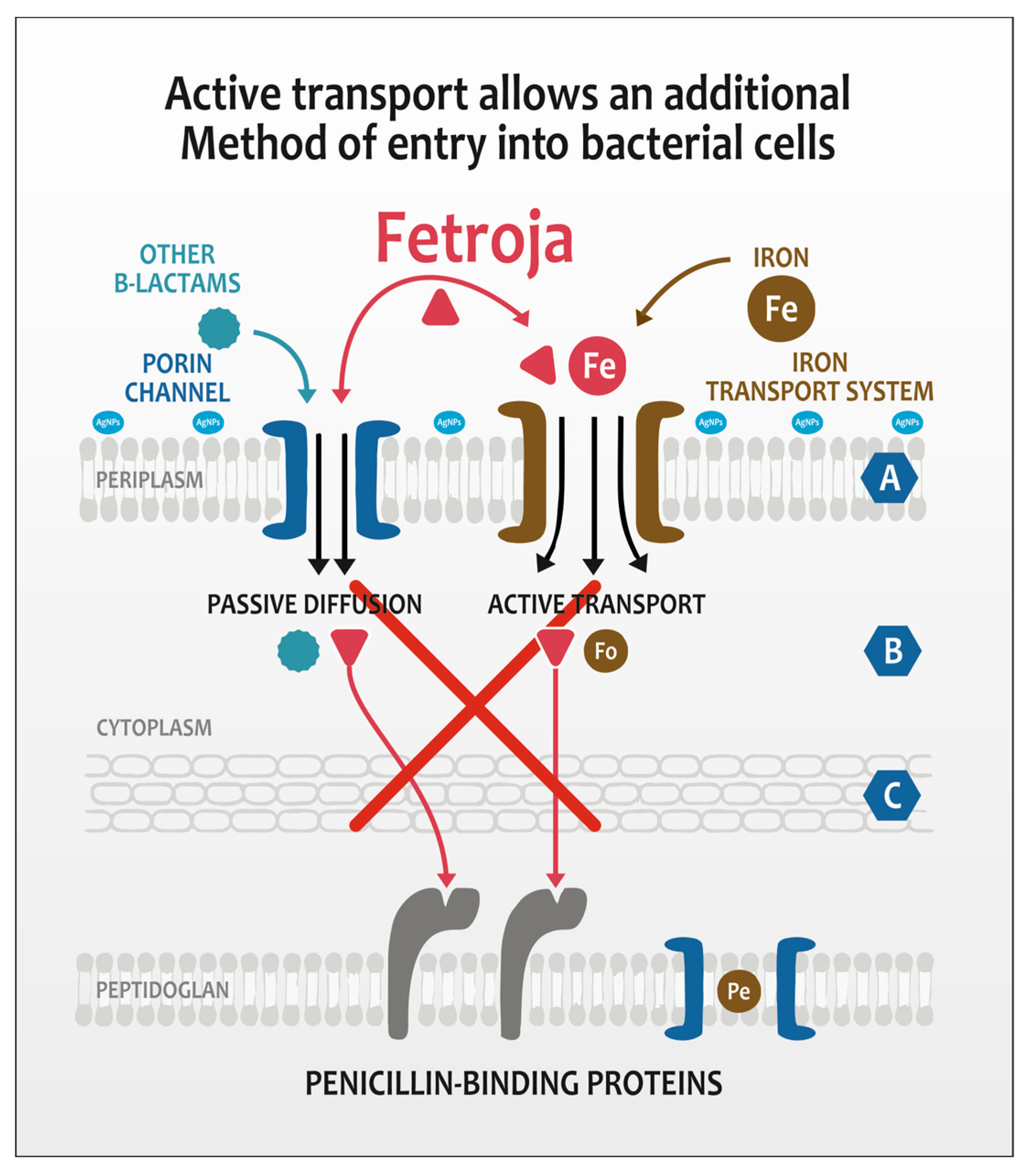
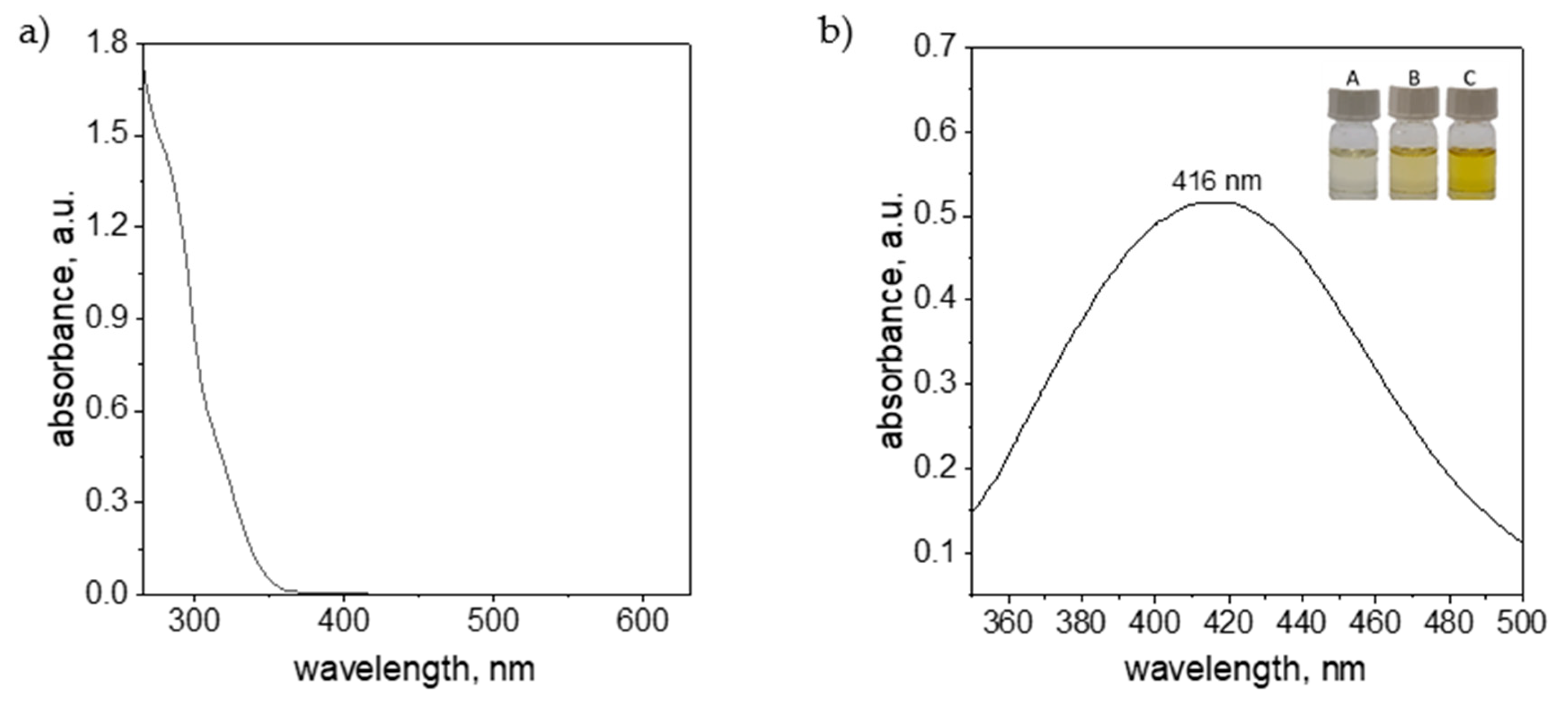
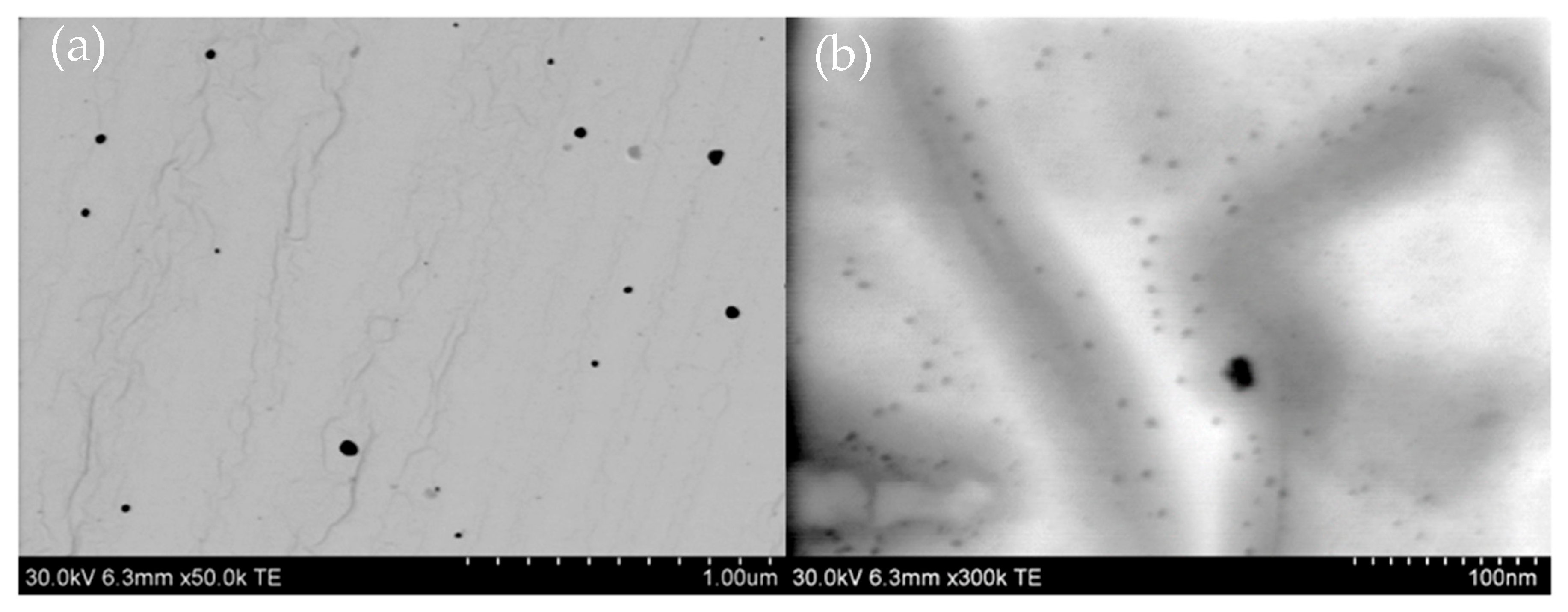
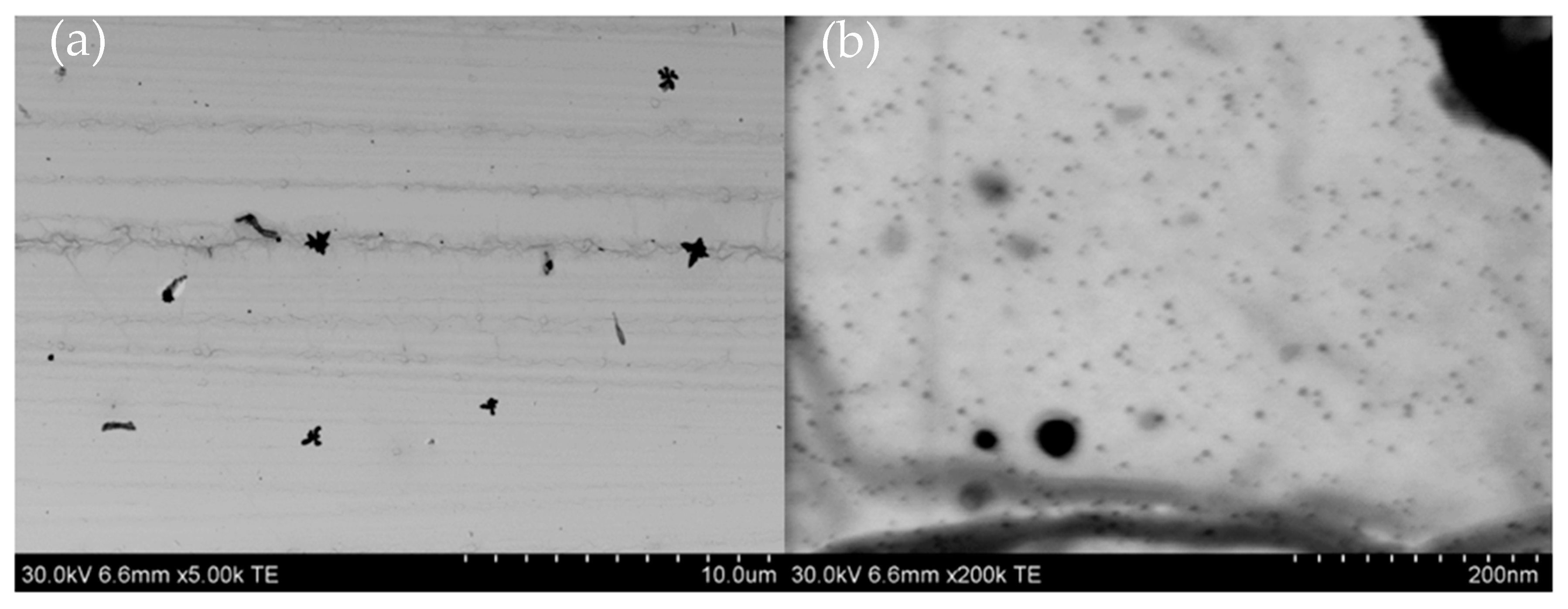
2. Results and Discussion
3. Experimental Section
3.1. Materials Preparation
3.1.1. Preparation of Unmodified Silver(I) Solutions
3.1.2. Synthesis of AgNPs
3.1.3. Preparation of Cefiderocol (Fetroja®) Solutions
3.2. Method Analysis
3.2.1. Microbiological Analysis
3.2.2. UV-Vis Analysis
3.2.3. DLS Analysis
3.2.4. Testing Microstructure
3.3. Reagents
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blanchette, V.; Kuhnke, J.L. The Current Situation for Non-healing Wounds. Wound Care Can. 2021, 19, 60–69. [Google Scholar]
- Sharma, A.; Shankar, R.; Yadav, A.K.; Pratap, A.; Ansari, M.A.; Srivastava, V. Burden of chronic nonhealing wounds: An overview of the worldwide humanistic and economic burden to the healthcare system. Int. J. Low. Extrem. Wounds 2024, 15347346241246339. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.O.; Tettelbach, W.H.; Ciprandi, G.; Downie, F.; Hampton, J.; Hodgson, H.; Percival, S.L. Best practice for wound debridement. J. Wound Care 2024, 33 (Suppl. 6b), S1–S32. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhang, Y.; Li, X.; Li, Q.; Xiu, W.; He, A.; Mou, Y. Simultaneous biofilm disruption, bacterial killing, and inflammation elimination for wound treatment using silver embellished polydopamine nanoplatform. Small 2024, 20, 2400927. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.; Bjarnsholt, T.; James, G.A.; Leaper, D.J.; McBain, A.J.; Malone, M. Global Wound Biofilm Expert Panel. (2017). Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017, 25, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D.G.; Bowler, P.G. Biofilm delays wound healing: A review of the evidence. Burn. Trauma 2013, 1, 2321–3868. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.D. Bacteria and antibiotics in wound healing. Surg. Clin. 2020, 100, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G. Antibiotic resistance and biofilm tolerance: A combined threat in the treatment of chronic infections. J. Wound Care 2018, 27, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, X.; Zheng, H.; Tang, Y.; Zeng, K.; Shao, L.; Li, L. Nanomaterials applied in wound healing: Mechanisms, limitations and perspectives. J. Control Release 2021, 337, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, V.; Brooks, M.; Mostow, E. Advanced therapies for chronic wounds: NPWT, engineered skin, growth factors, extracellular matrices. Dermatol. Ther. 2013, 26, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Obaid, A.; Abdulmalik, A.; Muhammad, S.N.; Sami, I.A.; Waleed, H.A.; Aqsa, T.; Bibi, M. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.K.; Qureshi, A.T.; Moll, A.N.; Hayes, D.J.; Monroe, W.T. Silver Nanoscale Antisense Drug Delivery System for Photoactivated Gene Silencing. ACS Nano 2013, 7, 2948–2959. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Koh, Y.S. A novel antibiotic agent, cefiderocol, for multidrug-resistant Gram-negative bacteria. J. Bacteriol. Virol. 2020, 50, 218–226. [Google Scholar] [CrossRef]
- McCreary, E.K.; Heil, E.L.; Tamma, P.D. New perspectives on antimicrobial agents: Cefiderocol. Antimicrob. Agents Chemother. 2021, 65, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S. Synthesis of silver nanoparticles using fresh bark of Pongamia pinnata and characterization of its antibacterial activity against gram positive and gram negative pathogens. Resour.-Effic. Technol. 2016, 2, 30–35. [Google Scholar]
- Beg, M. Green synthesis of silver nanoparticles using Pongamia pinnata seed: Characterization, antibacterial property, and spectroscopic investigation of interaction with human serum albumin. J. Mol. Recognit. 2017, 30, 2565. [Google Scholar] [CrossRef] [PubMed]
- Portsmouth, S.; van Veenhuyzen, D.; Echols, R.; Machida, M.; Ferreira, J.C.A.; Ariyasu, M.; Tenke, P.; Nagata, T.D. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: A phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 2018, 18, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Parsels, K.A.; Mastro, K.A.; Steele, J.M.; Thomas, S.J.; Kufel, W.D. Cefiderocol: A novel siderophore cephalosporin for multidrug-resistant Gram-negative bacterial infections. J. Antimicrob. Chemother. 2021, 76, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Sadouki, Z.; Vickers, A. In vitro activity of cefiderocol, a siderophore-cephalosporin, against multidrug-resistant Gram-negative bacteria. Antimicrob. Agents Chemother. 2020, 64, e01582-20. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Serapide, F. From Clinical Trials to Real-World Experiences: Evidence About Cefiderocol Use and Potential Role in Empirical Therapy. Infect. Dis. Ther. 2025, 14, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Chhangte, V.; Vanlalhruaii, R.; Hlawncheu, Z.; Jasha, M.A.; Lallianrawnae, S.; Lalthazuala, S. A review of microbes mediated biosynthesis of silver nanoparticles and their enhanced antimicrobial activities. Heliyon 2024, 10, e32333. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, D.; Wang, Y.; Ni, W. Cefiderocol for the treatment of multidrug-resistant Gram-negative bacteria: A systematic review of currently available evidence. Front. Pharmacol. 2022, 13, 896971. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zhang, R.; Zheng, Y.; Wang, J.; Khatib, M.; Jiang, X.; Zhou, C.; Omar, R.; Saliba, W.; Wu, W.; et al. Highly efficient self-healing multifunctional dressing with antibacterial activity for sutureless wound closure and infected wound monitoring. Adv. Mater. 2022, 34, 2106842. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M. The growing burden of antimicrobial resistance. J. Antimicrob. Chemother. 2008, 62, i1–i9. [Google Scholar] [CrossRef] [PubMed]
- Page, M.G. The role of iron and siderophores in infection, and the development of siderophore antibiotics. Clin. Infect. Dis. 2019, 69, S529–S537. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, G.; Seshagiri Rao, J.V.L.N.; Dhanaraju, M.D. Elucidating pharmacodynamic interaction of silver nanoparticle-topical deliverable antibiotics. Sci. Rep. 2016, 6, 29982. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wei, X.; Gao, P.; Wang, C.; de Jong, A.; Hon Kwan Chen, J.; Rodríguez-Sánchez, M.J.; Rodríguez-Nogales, A.; Diez-Echave, P.; Gálvez, J.; et al. Bismuth-based drugs sensitize Pseudomonas aeruginosa to multiple antibiotics by disrupting iron homeostasis. Nat. Microbiol. 2024, 9, 2600–2613. [Google Scholar] [CrossRef] [PubMed]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Mutakabbir, J.; Alosaimy, S.; Morrisette, T.; Kebriaei, R.; Rybak, M.J. Cefiderocol: A Novel Siderophore Cephalosporin against Multidrug-Resistant Gram-Negative Pathogens. Pharmacotherapy 2020, 40, 1228–1247. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Nishikawa, T.; Matsumoto, S.; Yoshizawa, H.; Sato, T.; Nakamura, R.; Tsuji, M.; Yamano, Y. Siderophore Cephalosporin Cefiderocol Utilizes Ferric Iron Transporter Systems for Antibacterial Activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 7396–7401. [Google Scholar] [CrossRef] [PubMed]
- Mollmann, U.; Heinisch, L.; Bauernfeind, A.; Kohler, T.; Ankel-Fuchs, D. Siderophores as drug delivery agents: Application of the “Trojan Horse” strategy. Biometals 2009, 22, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Page, M.G.; Dantier, C.; Desarbre, E. In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant Gram-negative bacilli. Antimicrob. Agents Chemother. 2010, 54, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Kriz, R.; Spettel, K.; Pichler, A.; Schefberger, K.; Sanz-Codina, M.; Lötsch, F.; Harrison, N.; Willinger, B.; Zeitlinger, M.; Burgmann, H.; et al. In vitro resistance development gives insights into molecular resistance mechanisms against cefiderocol. J. Antibiot. 2024, 77, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef] [PubMed]
- Palau, M.; Muñoz, E.; Gusta, M.; Larrosa, N.; Gomis, X.; Gilabert, J.; Almirante, B.; Puntes, V.; Texidó, R.; Gavaldà, J. In Vitro Antibacterial Activity of Silver Nanoparticles Conjugated with Amikacin and Combined with Hyperthermia against Drug-Resistant and Biofilm-Producing Strains UVVis. Microbiol. Spectr. 2023, 15, e0028023. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Mezhuev, Y.; Chistyakov, E. Nanoparticle-Containing Wound Dressing: Antimicrobial and Healing Effects. Gels 2022, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.G.; Peng, Q.L.; Gurunathan, S. Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver nanoparticles in therapeutics: Development of an antimicrobial gep formulation for topical use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Kędziora, A.; Wieczorek, R.; Speruda, M.; Matolínová, I.; Goszczyński, M.T.; Litwin, I.; Matolín, V.; Bugla-Płoskońska, G. Comparison of Antibacterial Mode of Action of Silver Ions and Silver Nanoformulations With Different Physico-Chemical Properties: Experimental and computational studies. Front. Microbiol. 2021, 1, 659614. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69, S538–S543. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Sato, T.; Ota, M.; Takemura, M.; Nishikawa, T.; Toba, S.; Kohira, N.; Miyagawa, S.; Ishibashi, N.; Matsumoto, S.; et al. In Vitro Antibacterial Properties of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2017, 62, e01454-17. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Mensa, J. Mechanism of action of cefiderocol. Rev. Esp. Quimioter. 2022, 35, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Boattini, M.; Cricca, M.; Diella, L.; Gatti, M.; Rossi, L.; Bartoletti, M.; Sambri, V.; Signoretto, C.; Fonnesu, R.; et al. Updates on Activity, Efficacy and Emerging Mechanisms of Resistance to Cefiderocol. Curr. Issues Mol. Biol. 2024, 46, 14132–14153. [Google Scholar] [CrossRef] [PubMed]
- El-Lababidi, R.M.; Rizk, J.G. Cefiderocol: A Siderophore Cephalosporin. Ann. Pharmacother. 2020, 54, 1215–1231. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, S.; Kohta, M.; Ohyabu, y.; Iwasaki, T. In vitro parallel evaluation of antibacterial activity and cytotoxicity of commercially available silver containing wound dressings. Chronic Wound Care Manag. Res. 2015, 2, 1–9. [Google Scholar]
- Syed, Y.Y. Cefiderocol: A review in serious Gram-negative bacterial infections. Drugs 2021, 81, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Braun, V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol. Rev. 1995, 16, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver enhances antibiotic activity against gram-negative bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef] [PubMed]
- Auda, S.H.; Mrestani, Y.; Ahmed, M.S.; Neubert, H.H.R. Characterization of the interaction of cefadroxil with different metal ions using. Electrophoresis 2009, 30, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Auda, S.H.; Knütter, I.; Bretschneider, B.; Brandsch, M.; Mrestani, Y.; Große, C.; Neubert, R. Effect of Different Metal Ions on the Biological Properties of Cefadroxil. Pharmaceuticals 2009, 2, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, N.; Yan, X.; Zou, H. DFT-based Analysis of Siderophore-Metal Ion Interaction for E cient Heavy Metal Remediation. Environ. Sci. Pollut. Res. 2023, 30, 91780–91793. [Google Scholar] [CrossRef] [PubMed]
- Kircheva, N.; Dobrev, S.; Petkova, V.; Yocheva, L.; Angelova, S.; Dudev, T. In Silico Analysis of the Ga3+/Fe3+ Competition for Binding the Iron-Scavenging Siderophores of P. aeruginosa—Implementation of Three Gallium-Based Complexes in the “Trojan Horse” Antibacterial Strategy. Biomolecules 2024, 14, 487. [Google Scholar] [CrossRef] [PubMed]
- Adamowska, M.; Pałuba, B.; Hyk, W. Electrochemical Determination of Nanoparticle Size: Combined Theoretical and Experimental Study for Matrixless Silver Nanoparticles. Molecules 2022, 27, 2592. [Google Scholar] [CrossRef] [PubMed]
- Rodriques, A.S.; Batista, J.G.S.; Rodriques, M.A.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugao, B.A. Advances in silver nanoparticles: A comprehensive revew on their popential as antimicrobial agents and their mechanisms of acion elucidated by proteomics. Front. Microbiol. 2024, 31, 1440065. [Google Scholar] [CrossRef] [PubMed]
- Ekatarina, O.; Mikhailova, O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Noinaj, N.; Guillier, M.; Barnard, J.T.; Buchanan, K.S. TonB-dependent transporters: Regulation, structure, and function. Ann. Rev. Microbiol. 2010, 64, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Kędziora, A.; Speruda, M.; Wernecki, M.; Dudek, B.; Kapczyńska, K.; Krzyżwska, E.; Rybka, J.; Bugla-Płoskońska, G. How Bacteria Change after Exposure to Silver Nanoformulations: Analysis of the Genome and Outer Membrane Proteome. Pathogens 2021, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; American Society for Microbiology: Washington, DC, USA, 2009. [Google Scholar]
- Bozdogan, H. Model selection and Akaike’s Information Criterion (AIC): The general theory and its analytical extensions. Psychometrika 1987, 52, 345–370. [Google Scholar] [CrossRef]
| Effect | df | Wald’s Stat. | p |
|---|---|---|---|
| Intercept | 1 | 99,378.27 | 0.00 |
| Strain | 4 | 3144.05 | 0.00 |
| Silver | 1 | 340.34 | 0.00 |
| Ag conc | 2 | 30.87 | 0.00 |
| Strain × Silver | 4 | 153.61 | 0.00 |
| Strain × Ag conc | 8 | 62.12 | 0.00 |
| Silver × Ag conc | 2 | 30.49 | 0.00 |
| Strain × Silver × Ag conc | 8 | 87.51 | 0.00 |
| Effect | df | Wald’s Stat. | p |
|---|---|---|---|
| Intercept | 1 | 193,111.7 | 0 |
| CEF | 4 | 4285.5 | 0 |
| Position | 2 | 2617.3 | 0 |
| Strain | 1 | 328.4 | 0 |
| CEF × position | 8 | 597 | 0 |
| CEF × strain | 4 | 70.5 | 0 |
| Position × strain | 2 | 7.5 | 0.02 |
| CEF × position × strain | 8 | 56.4 | 0 |
| Variables | df | Wald’s Stat | p |
|---|---|---|---|
| Pseudomonas aeruginosa ATCC 27853 | |||
| Intercept | 1 | 70,448.01 | 0.0000 |
| Cef | 1 | 4.49 | 0.0341 |
| AgNPs | 2 | 65.14 | 0.0000 |
| Cef × AgNPs | 2 | 10.1 | 0.0064 |
| Escherichia coli ATCC 25922 | |||
| Intercept | 1 | 148,964.9 | 0.0000 |
| Cef | 1 | 62.9 | 0.0000 |
| AgNPs | 2 | 105.8 | 0.0000 |
| Cef × AgNPs | 2 | 76.5 | 0.0000 |
| Acinetobacter baumannii ATCC 19606 | |||
| Intercept | 1 | 127,778.3 | 0.0000 |
| Cef | 1 | 507.1 | 0.0000 |
| AgNPs | 2 | 120.4 | 0.0000 |
| Cef × AgNPs | 2 | 73.7 | 0.0000 |
| Acinetobacter baumannii AB 42 | |||
| Intercept | 1 | 31,053.35 | 0.0000 |
| Cef | 1 | 234.25 | 0.0000 |
| AgNPs | 2 | 76.45 | 0.0000 |
| Cef × AgNPs | 2 | 16.1 | 0.0003 |
| Acinetobacter baumannii AB 49 | |||
| Intercept | 1 | 33,892.2 | 0.0000 |
| Cef | 1 | 227.16 | 0.0000 |
| AgNPs | 2 | 119.72 | 0.0000 |
| Cef × AgNPs | 2 | 35.68 | 0.0000 |
| Sample | Preparation | Ingredients in Sample (After Mixing) |
|---|---|---|
| BL_DMSO | DMSO | DMSO |
| BL_A | 100 µL Ag(I) (2 mM) + 100 µL DMSO | AgNO3 concentration 1 mM |
| BL_B | No dilution | AgNPs concentration 1 mM |
| BL_C | 100 µL CEF (1.5 mg/mL) + 100 µL DMSO | CEF concentration 0.75 mg/mL |
| Sample | Composition After Mixing: (100 µL A/B/C + 100 µL C1/C2/C3) |
|---|---|
| A_C1 | CEF concentration 0.75 mg/mL + AgNPs (1 mM) |
| A_C2 | CEF concentration 0.75 mg/mL + AgNPs (0.5 mM) |
| A_C3 | CEF concentration 0.75 mg/mL + AgNPs (0.25 mM) |
| B_C1 | CEF concentration 0.075 mg/mL + AgNPs (1 mM) |
| B_C2 | CEF concentration 0.075 mg/mL + AgNPs (0.5 mM) |
| B_C3 | CEF concentration 0.075 mg/mL + AgNPs (0.25 mM) |
| C_C1 | CEF concentration 0.0075 mg/mL + AgNPs (1 mM) |
| C_C2 | CEF concentration 0.0075 mg/mL + AgNPs (0.5 mM) |
| C_C3 | CEF concentration 0.0075 mg/mL + AgNPs (0.25 mM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binert-Kusztal, Ż.; Krakowska, A.; Skiba-Kurek, I.; Luty-Błocho, M.; Kula, A.; Olechowska-Jarząb, A.; Dorożyński, P.; Skalski, T. Potential Use of Cefiderocol and Nanosilver in Wound Dressings to Control Multidrug-Resistant Gram-Negative Bacteria. Molecules 2025, 30, 3072. https://doi.org/10.3390/molecules30153072
Binert-Kusztal Ż, Krakowska A, Skiba-Kurek I, Luty-Błocho M, Kula A, Olechowska-Jarząb A, Dorożyński P, Skalski T. Potential Use of Cefiderocol and Nanosilver in Wound Dressings to Control Multidrug-Resistant Gram-Negative Bacteria. Molecules. 2025; 30(15):3072. https://doi.org/10.3390/molecules30153072
Chicago/Turabian StyleBinert-Kusztal, Żaneta, Agata Krakowska, Iwona Skiba-Kurek, Magdalena Luty-Błocho, Anna Kula, Aldona Olechowska-Jarząb, Przemysław Dorożyński, and Tomasz Skalski. 2025. "Potential Use of Cefiderocol and Nanosilver in Wound Dressings to Control Multidrug-Resistant Gram-Negative Bacteria" Molecules 30, no. 15: 3072. https://doi.org/10.3390/molecules30153072
APA StyleBinert-Kusztal, Ż., Krakowska, A., Skiba-Kurek, I., Luty-Błocho, M., Kula, A., Olechowska-Jarząb, A., Dorożyński, P., & Skalski, T. (2025). Potential Use of Cefiderocol and Nanosilver in Wound Dressings to Control Multidrug-Resistant Gram-Negative Bacteria. Molecules, 30(15), 3072. https://doi.org/10.3390/molecules30153072










