Impact of Interactions Between Zn(II) and Selenites in an Aquatic Environment on the Accumulation of Se and Zn in a Fungal Cell
Abstract
1. Introduction
2. Results
2.1. Impact of SeO32−/Zn2+ Molar Ratio in Culture Medium on the Concentration of Se and Zn in the Mushroom Cell Wall
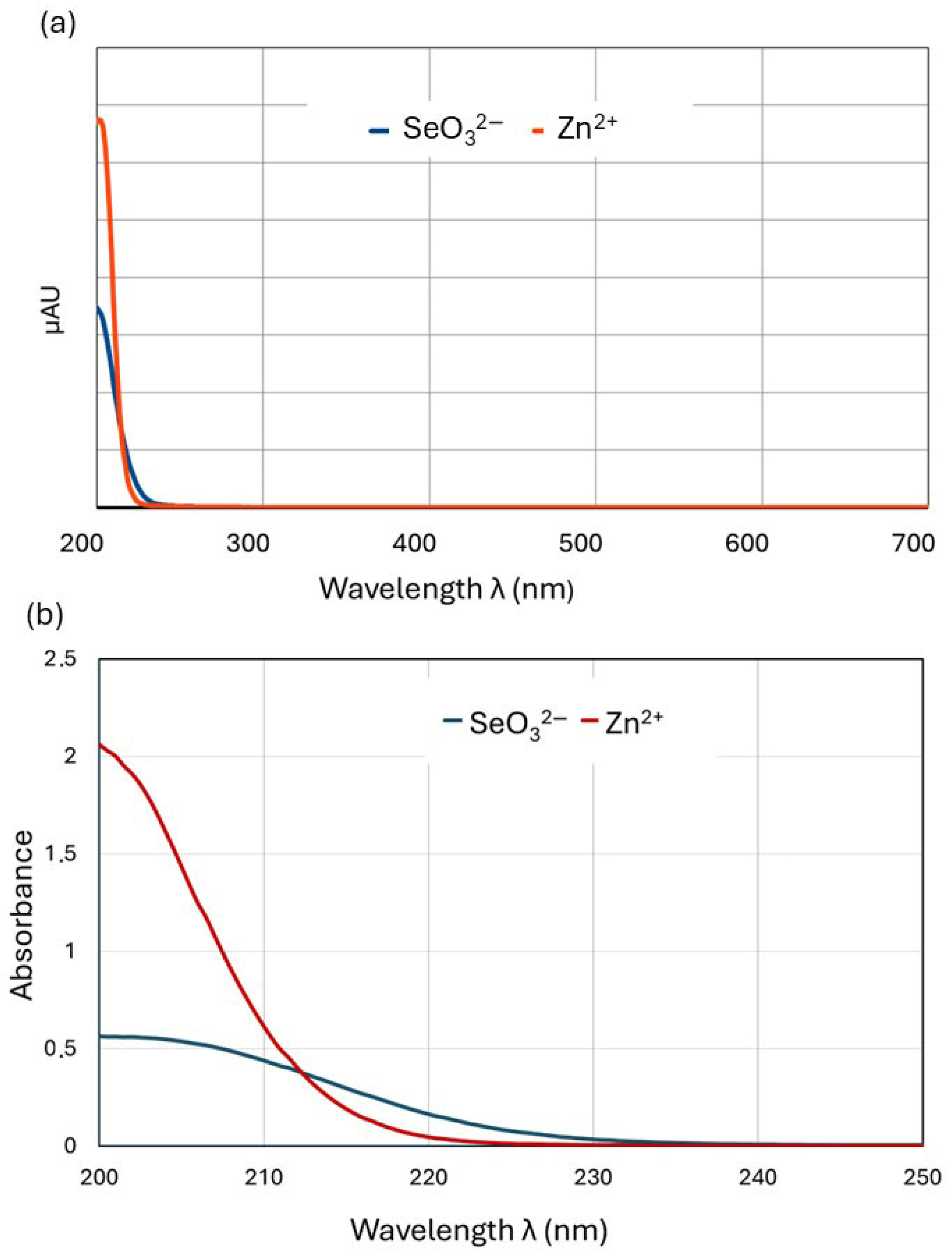
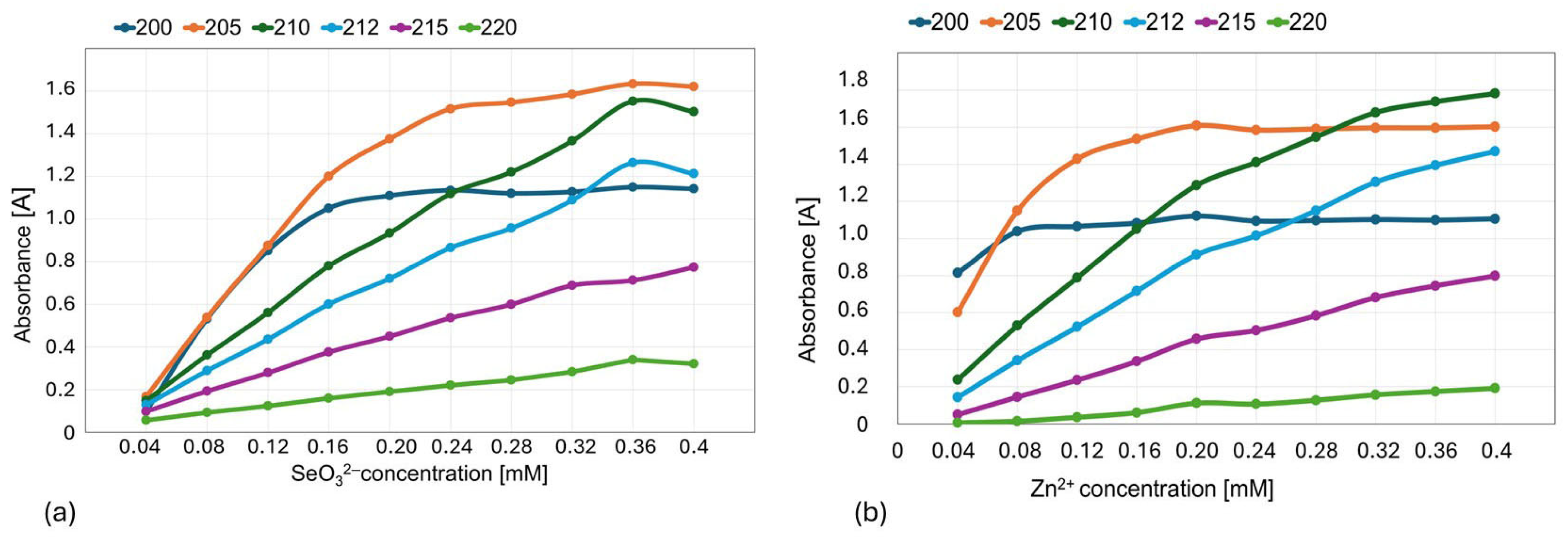
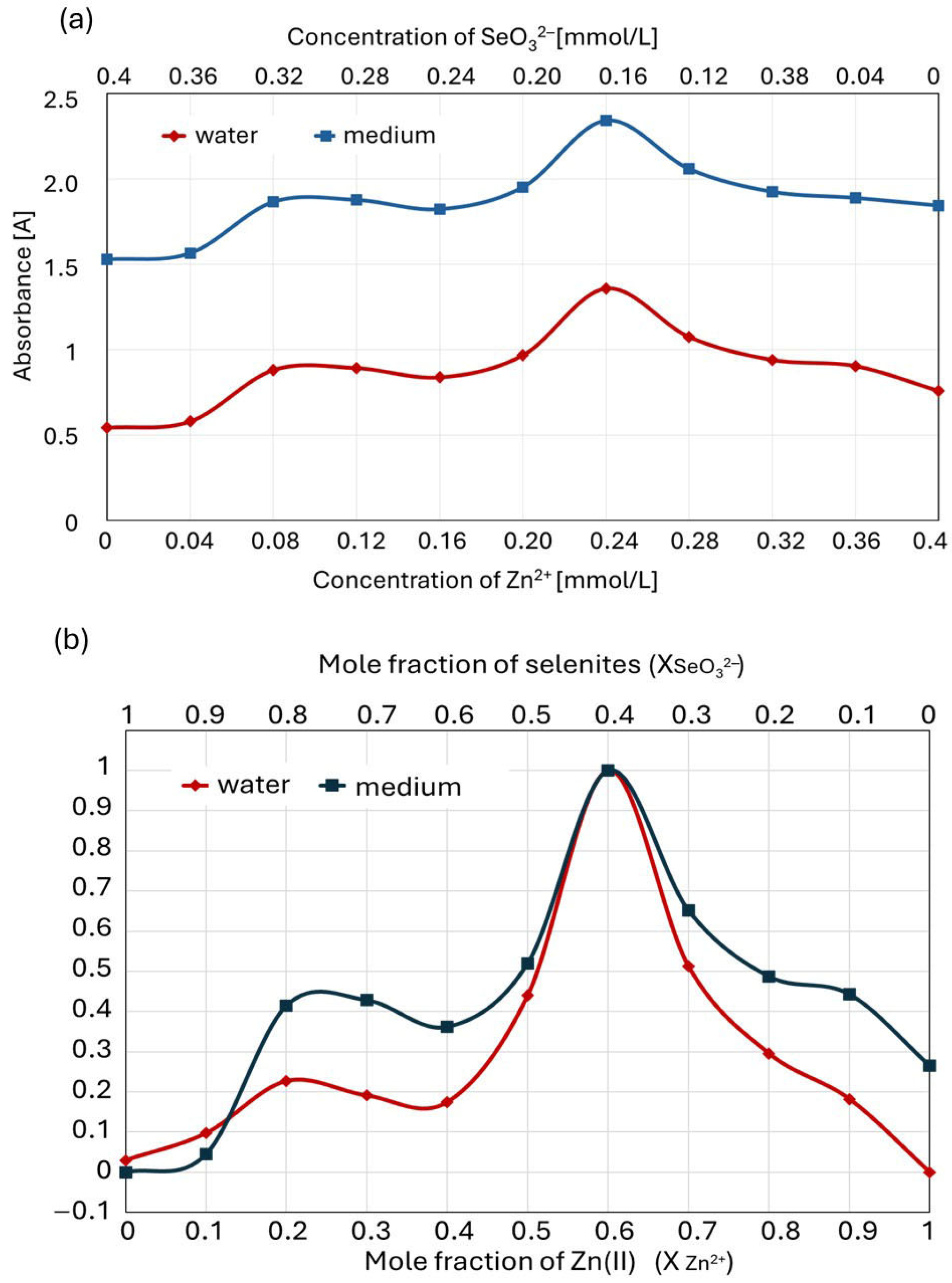
2.2. Determination of the Composition and Stability of Zn(II)-Selenite Complexes in the Liquid Culture Medium Using the Method of Continuous Variations (Job’s Method)
2.3. The Effect of the Zn2+/SeO32− Molar Ratio in the Cultivation Medium on the Concentration of Non-Complexed SeO32− Ions
3. Discussion
4. Conclusions
5. Experimental Procedures
5.1. Microorganism and Cultivation Media
Supplementation of Media and Growth Conditions in Mycelial Shake-Flask Cultures
5.2. Isolation of Mycelial Cell Walls
5.3. Determination of Selenium and Zinc in the Cultivation Medium, Cell Walls, and Mycelial Biomass
5.3.1. Mineralization Procedure
5.3.2. Determination of Zinc Content
5.3.3. Determination of the Total Selenium Content
5.3.4. Determination of Free Selenites in Cultivation Medium
5.4. Determination of the Composition and Stability of Zn(II)-Selenite Complexes in Liquid Culture Medium Using the Method of Continuous Variations (Job’s Method)
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pethkar, A.V.; Paknikar, K.M. Recovery of Gold from Solutions Using Cladosporium cladosporioides Biomass Beads. J. Biotechnol. 1998, 63, 121–136. [Google Scholar] [CrossRef]
- Borovička, J.; Řanda, Z. Distribution of Iron, Cobalt, Zinc and Selenium in Macrofungi. Mycol. Prog. 2007, 6, 249–259. [Google Scholar] [CrossRef]
- Da Silva, M.C.S.; Naozuka, J.; Da Luz, J.M.R.; De Assunão, L.S.; Oliveira, P.V.; Vanetti, M.C.D.; Bazzolli, D.M.S.; Kasuya, M.C.M. Enrichment of Pleurotus ostreatus Mushrooms with Selenium in Coffee Husks. Food Chem. 2012, 131, 558–563. [Google Scholar] [CrossRef]
- Savic, M.; Andjelkovic, I.; Duvnjak, D.; Matijasevic, D.; Avramovic, A.; Pesic-Mikulec, D.; Niksic, M. The Fungistatic Activity of Organic Selenium and Its Application to the Production of Cultivated Mushrooms Agaricus bisporus and Pleurotus spp. Arch. Biol. Sci. 2012, 64, 1455–1463. [Google Scholar] [CrossRef]
- Turło, J.; Gutkowska, B.; Herold, F.; Blażewicz, A.; Kocjan, R. Relationship between Sodium Selenite Concentration in Culture Media and Heavy Metal Uptake by Lentinula edodes (Berk.) in Mycelial Cultures. Fresenius Environ. Bull. 2009, 18, 1035–1038. [Google Scholar]
- Turło, J.; Gutkowska, B.; Herold, F.; Klimaszewska, M.; Suchocki, P. Optimization of Selenium-Enriched Mycelium of Lentinula edodes (Berk.) Pegler as a Food Supplement. Food Biotechnol. 2010, 24, 180–196. [Google Scholar] [CrossRef]
- Malinowska, E.; Klimaszewska, M.; Strączek, T.; Schneider, K.; Kapusta, C.; Podsadni, P.; Łapienis, G.; Dawidowski, M.; Kleps, J.; Górska, S.; et al. Selenized Polysaccharides—Biosynthesis and Structural Analysis. Carbohydr. Polym. 2018, 198, 407–417. [Google Scholar] [CrossRef]
- Kaleta, B.; Górski, A.; Zagożdżon, R.; Cieślak, M.; Kaźmierczak-Barańska, J.; Nawrot, B.; Klimaszewska, M.; Malinowska, E.; Górska, S.; Turło, J. Selenium-Containing Polysaccharides from Lentinula edodes—Biological Activity. Carbohydr. Polym. 2019, 223, 115078. [Google Scholar] [CrossRef]
- Mattila, P.; Suonpää, K.; Piironen, V. Functional Properties of Edible Mushrooms. Nutrition 2000, 16, 694–696. [Google Scholar] [CrossRef]
- Mattila, P.; Könkö, K.; Eurola, M.; Pihlava, J.-M.; Astola, J.; Vahteristo, L.; Hietaniemi, V.; Kumpulainen, J.; Valtonen, M.; Piironen, V. Contents of Vitamins, Mineral Elements, and Some Phenolic Compounds in Cultivated Mushrooms. J. Agric. Food Chem. 2001, 49, 2343–2348. [Google Scholar] [CrossRef]
- Vetter, J. Mineralstoff- Und Aminosäuregehalte Des Eßbaren, Kultivierten Pilzes Shii-Take (Lentinus edodes). Z. Lebensm. Unters. Forsch. 1995, 201, 17–19. [Google Scholar] [CrossRef]
- Wasser, S.P.; Weis, A.L. Medicinal Properties of Substances Occuring in Higher Basidiomycetes Mushrooms. Int. J. Med. Mushrooms 1999, 1, 31–62. [Google Scholar] [CrossRef]
- Chihara, G.; Hamuro, J.; Maeda, Y.Y.; Arai, Y.; Fukuoka, F. Fractionation and Purification of the Polysaccharides with Marked Antitumor Activity, Especially Lentinan, from Lentinus edodes (Berk.) Sing, (an Edible Mushroom). Cancer Res. 1970, 30, 2776–2781. [Google Scholar]
- Akamatsu, S.; Watanabe, A.; Tamesada, M.; Nakamura, R.; Hayashi, S.; Kodama, D.; Kawase, M.; Yagi, K. Hepatoprotective Effect of Extracts from Lentinus edodes Mycelia on Dimethylnitrosamine-Induced Liver Injury. Biol. Pharm. Bull. 2004, 27, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Jie, S.; Hanchuan, D.; Moucheng, W. Characterization and Immunomodulating Activities of Polysaccharide from Lentinus edodes. Int. Immunopharmacol. 2005, 5, 811–820. [Google Scholar] [CrossRef]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.J.; Gordon, S. Dectin-1 Mediates the Biological Effects of β-Glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef]
- Kupfahl, C.; Geginat, G.; Hof, H. Lentinan Has a Stimulatory Effect on Innate and Adaptive Immunity against Murine Listeria monocytogenes Infection. Int. Immunopharmacol. 2006, 6, 686–696. [Google Scholar] [CrossRef]
- Zięba, P.; Kała, K.; Włodarczyk, A.; Szewczyk, A.; Kunicki, E.; Sękara, A.; Muszyńska, B. Selenium and Zinc Biofortification of Pleurotus eryngii Mycelium and Fruiting Bodies as a Tool for Controlling Their Biological Activity. Molecules 2020, 25, 889. [Google Scholar] [CrossRef]
- Ramezannejad, R.; Pourianfar, H.R.; Rezaeian, S. Interactive Effects of Selenium, Zinc, and Iron on the Uptake of Selenium in Mycelia of the Culinary-Medicinal Winter Mushroom Flammulina velutipes (Agaricomycetes). Int. J. Med. Mushrooms 2023, 25, 75–87. [Google Scholar] [CrossRef]
- Avendaño, R.; Chaves, N.; Fuentes, P.; Sánchez, E.; Jiménez, J.I.; Chavarría, M. Production of Selenium Nanoparticles in Pseudomonas putida KT2440. Sci. Rep. 2016, 6, 37155. [Google Scholar] [CrossRef]
- Ning, P.; Fei, P.; Wu, T.; Li, Y.; Qu, C.; Li, Y.; Shi, J.; Tian, X. Combined Foliar Application of Zinc Sulphate and Selenite Affects the Magnitude of Selenium Biofortification in Wheat (Triticum aestivum L.). Food Energy Secur. 2022, 11, e342. [Google Scholar] [CrossRef]
- Sahu, C.; Dwivedi, D.K.; Jena, G.B. Zinc and Selenium Combination Treatment Protected Diabetes-Induced Testicular and Epididymal Damage in Rat. Hum. Exp. Toxicol. 2020, 39, 1235–1256. [Google Scholar] [CrossRef] [PubMed]
- Hasani, M.; Saidpour, A.; Irandoost, P.; Golab, F.; Khazdouz, M.; Qorbani, M.; Agh, F.; Mohammad Sharifi, A.; Vafa, M. Beneficial Effects of Se/Zn Co-Supplementation on Body Weight and Adipose Tissue Inflammation in High-Fat Diet-Induced Obese Rats. Food Sci. Nutr. 2021, 9, 3414–3425. [Google Scholar] [CrossRef] [PubMed]
- Zavros, A.; Andreou, E.; Aphamis, G.; Bogdanis, G.C.; Sakkas, G.K.; Roupa, Z.; Giannaki, C.D. The Effects of Zinc and Selenium Co-Supplementation on Resting Metabolic Rate, Thyroid Function, Physical Fitness, and Functional Capacity in Overweight and Obese People under a Hypocaloric Diet: A Randomized, Double-Blind, and Placebo-Controlled Trial. Nutrients 2023, 15, 3133. [Google Scholar] [CrossRef] [PubMed]
- Turło, J.; Gutkowska, B.; Herold, F.; Gajzlerska, W.; Dawidowski, M.; Dorociak, A.; Zobel, A. Biological Availability and Preliminary Selenium Speciation in Selenium-Enriched Mycelium of Lentinula edodes (Berk.). Food Biotechnol. 2011, 25, 16–29. [Google Scholar] [CrossRef]
- Turło, J.; Gutkowska, B.; Malinowska, E. Relationship between the Selenium, Selenomethionine, and Selenocysteine Content of Submerged Cultivated Mycelium of Lentinula edodes (Berk.). Acta Chromatogr. 2007, 36–48. [Google Scholar]
- Klimaszewska, M.; Górska, S.; Dawidowski, M.; Podsadni, P.; Turło, J. Biosynthesis of Se-Methyl-Seleno-l-Cysteine in Basidiomycetes Fungus Lentinula edodes (Berk.) Pegler. SpringerPlus 2016, 5, 733. [Google Scholar] [CrossRef]
- Turło, J.; Gutkowska, B.; Kałucka, M.; Bujak, M. Accumulation of Zinc by the Lentinus edodes (Berk.) Mycelium Cultivated in Submerged Culture. Acta Pol. Pharm. 2007, 64, 45–51. [Google Scholar]
- Kałucka, M.; Roszczyk, A.; Klimaszewska, M.; Kaleta, B.; Drelich, E.; Błażewicz, A.; Górska-Jakubowska, S.; Malinowska, E.; Król, M.; Prus, A.M.; et al. Optimization of Se- and Zn-Enriched Mycelium of Lentinula edodes (Berk.) Pegler as a Dietary Supplement with Immunostimulatory Activity. Nutrients 2023, 15, 4015. [Google Scholar] [CrossRef]
- Banks, W.H. 210. The Dissociation of the Selenites of Zinc and Cadmium in Water. J. Chem. Soc. 1934, 1010–1012. [Google Scholar] [CrossRef]
- Moriya, H.; Sekine, T. A Solvent-Extraction Study of Zinc(II) Complexes with Several Divalent Anions of Carboxylic and Inorganic Acids. Bull. Chem. Soc. Jpn. 1974, 47, 747–748. [Google Scholar] [CrossRef]
- Somer, G.; Yilmaz, Ü.T. Interference between Selenium and Some Trace Elements during Polarographic Studies and Its Elimination. Talanta 2005, 65, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Feroci, G.; Badiello, R.; Fini, A. Interactions between Different Selenium Compounds and Zinc, Cadmium and Mercury. J. Trace Elem. Med. Biol. 2005, 18, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, A.; Nunes Cechin, C.; Lang, E.S.; Cargnelutti, R.; Ramirez Pineda, N.; Piquini, P.C.; Burrow, R.A.; Santos dos Santos, S.; Bortolotto, T.; Tirloni, B. Organically Templated Zinc Selenite Compounds: Synthesis, Structural Chemistry and DFT Calculations. New J. Chem. 2020, 44, 6699–6703. [Google Scholar] [CrossRef]
- Millange, F.; Serre, C.; Cabourdin, T.; Marrot, J.; Férey, G. Organically Templated Zinc Selenites: MIL-86 or [H2N(CH2)2NH2]2·Zn4(SeO3)4 and MIL-87 or [H3N(CH2)3NH3]4·Zn4(SeO3)8. Solid. State Sci. 2004, 6, 229–233. [Google Scholar] [CrossRef]
- Godjevargova, T.; Mihova, S.; Gabrovska, K. Fixed-Bed Biosorption of Cu2+ by Polyacrylonitrile-Immobilized Dead Cells of Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2004, 20, 273–279. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T. Heavy-Metal Removal from Aqueous Solution by Fungus Mucor rouxii. Water Res. 2003, 37, 4486–4496. [Google Scholar] [CrossRef]
- Renny, J.S.; Tomasevich, L.L.; Tallmadge, E.H.; Collum, D.B. Method of Continuous Variations: Applications of Job Plots to the Study of Molecular Associations in Organometallic Chemistry. Angew. Chem. Int. Ed. 2013, 52, 11998–12013. [Google Scholar] [CrossRef]
- Doiuchi, T.; Minoura, Y. Asymmetric Induction by Copolymerization of Indene with Maleic Anhydride in the Presence of Lecithin. Macromolecules 1978, 11, 270–274. [Google Scholar] [CrossRef]
- Tate, J.F.; Jones, M.M. The Use of Electrical Conductivity Data with Job’s Method. J. Inorg. Nucl. Chem. 1960, 12, 241–251. [Google Scholar] [CrossRef]
- Brown, T.L.; Gerteis, R.L.; Bafus, D.A.; Ladd, J.A. Interaction of Alkyllithium Compounds with Base. Complex Formation between Ethyllithium and Triethylamine in Benzene. J. Am. Chem. Soc. 1964, 86, 2135–2141. [Google Scholar] [CrossRef]
- Que, E.L.; Gianolio, E.; Baker, S.L.; Wong, A.P.; Aime, S.; Chang, C.J. Copper-Responsive Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2009, 131, 8527–8536. [Google Scholar] [CrossRef] [PubMed]
- Loukas, Y.L. Measurement of Molecular Association in Drug: Cyclodextrin Inclusion Complexes with Improved 1H NMR Studies. J. Pharm. Pharmacol. 1997, 49, 944–948. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Khan, V.K.; Taraban, M.B.; Leshina, T.V.; Salakhutdinov, N.F.; Tolstikov, G.A. Complexation of Lappaconitine with Glycyrrhizic Acid: Stability and Reactivity Studies. J. Phys. Chem. B 2005, 109, 24526–24530. [Google Scholar] [CrossRef]
- Addison, C.C.; Sheldon, J.C.; Smith, B.C. Heat of Mixing of Dinitrogen Tetroxide with Organic Liquids. J. Chem. Soc. Dalton Trans. 1974, 999–1002. [Google Scholar] [CrossRef]
- Adachi, K.; Watarai, H. Site-Selective Formation of Optically Active Inclusion Complexes of Alkoxo-Subphthalocyanines with β-Cyclodextrin at the Toluene/Water Interface. Chem. Eur. J. 2006, 12, 4249–4260. [Google Scholar] [CrossRef]
- Goto, H.; Okamoto, Y.; Yashima, E. Metal-Induced Supramolecular Chirality in an Optically Active Polythiophene Aggregate. Chem. Eur. J. 2002, 8, 4027–4036. [Google Scholar] [CrossRef]
- Guan, A.-J.; Shen, M.-J.; Xiang, J.-F.; Zhang, E.-X.; Li, Q.; Sun, H.-X.; Wang, L.-X.; Xu, G.-Z.; Tang, Y.-L.; Xu, L.-J.; et al. G-Quadruplex Induced Chirality of Methylazacalix[6]Pyridine via Unprecedented Binding Stoichiometry: En Route to Multiplex Controlled Molecular Switch. Sci. Rep. 2015, 5, 10479. [Google Scholar] [CrossRef]
- Yan, F.; Copeland, R.; Brittain, H.G. Optical Activity in Tb(III) Mixed-Ligand Complexes Containing Pyridine-2,6-Dicarboxylic Acid and Hydroxyphenyl Derivatives. Inorg. Chim. Acta 1983, 72, 211–216. [Google Scholar] [CrossRef]
- Zha, Y.; Bian, Z.; Liu, Z. The Application of Chiral Groups in Circularly Polarized Luminescent Lanthanide Complexes. Polyhedron 2025, 272, 117452. [Google Scholar] [CrossRef]
- Fenger, J.; Siekierska, K.E.; Skytte Jensen, B. Determination of the Stoichiometry of Precipitates by the Method of Continuous Variations. J. Inorg. Nucl. Chem. 1971, 33, 4366–4368. [Google Scholar] [CrossRef]
- Ouhadi, T.; Hamitou, A.; Jérôme, R.; Teyssié, P. Soluble Bimetallic μ-Oxo-Alkoxides. 8. Structure and Kinetic Behavior of the Catalytic Species in Unsubstituted Lactone Ring-Opening Polymerization. Macromolecules 1976, 9, 927–931. [Google Scholar] [CrossRef]
- Weingarten, H.; Van Wazer, J.R. Exchange of Parts between Molecules at Equilibrium. VI. Scrambling on Titanium of the Alkoxyl, Dimethylamino, and Halogen Substituents. J. Am. Chem. Soc. 1965, 87, 724–730. [Google Scholar] [CrossRef]
- Liou, L.R.; McNeil, A.J.; Toombes, G.E.S.; Collum, D.B. Structures of β-Amino Ester Enolates: New Strategies Using the Method of Continuous Variation. J. Am. Chem. Soc. 2008, 130, 17334–17341. [Google Scholar] [CrossRef][Green Version]
- Novak, D.P.; Brown, T.L. Mixed Complex Formation between Methyllithium and Lithium Bromide or Iodide in Diethyl Ether. J. Am. Chem. Soc. 1972, 94, 3793–3798. [Google Scholar] [CrossRef]
- Desjardins, S.; Flinois, K.; Oulyadi, H.; Davoust, D.; Giessner-Prettre, C.; Parisel, O.; Maddaluno, J. Multinuclear NMR Study of the Aggregates between Methyllithium and Lithium Bromide in Toluene. Organometallics 2003, 22, 4090–4097. [Google Scholar] [CrossRef]
- Kissling, R.M.; Gagné, M.R. Structure and Reactivity of Mixed Alkali Metal Alkoxide/Aryloxide Catalysts. J. Org. Chem. 2001, 66, 9005–9010. [Google Scholar] [CrossRef]
- Ataie, N.J.; Hoang, Q.Q.; Zahniser, M.P.D.; Tu, Y.; Milne, A.; Petsko, G.A.; Ringe, D. Zinc Coordination Geometry and Ligand Binding Affinity: The Structural and Kinetic Analysis of the Second-Shell Serine 228 Residue and the Methionine 180 Residue of the Aminopeptidase from Vibrio proteolyticus. Biochemistry 2008, 47, 7673–7683. [Google Scholar] [CrossRef]
- Clever, H.L.; Derrick, M.E.; Johnson, S.A. The Solubility of Some Sparingly Soluble Salts of Zinc and Cadmium in Water and in Aqueous Electrolyte Solutions. J. Phys. Chem. Ref. Data 1992, 21, 941–1004. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Panunzi, B. Stimuli-Responsive Zinc (II) Coordination Polymers: A Novel Platform for Supramolecular Chromic Smart Tools. Polymers 2021, 13, 3712. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 10-1128. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Lenardon, M.D. Architecture of the dynamic fungal cell wall. Nat. Rev. Microbiol. 2021, 21, 248–259. [Google Scholar] [CrossRef]
- Mahadevan, P.R.; Tatum, E.L. Localization of Structural Polymers in the Cell Wall of Neurospora crassa. J. Cell Biol. 1967, 35, 295–302. [Google Scholar] [CrossRef]
- Schmit, J.C.; Edson, C.M.; Brody, S. Changes in Glucosamine and Galactosamine Levels during Conidial Germination in Neurospora crassa. J. Bacteriol. 1975, 122, 1062–1070. [Google Scholar] [CrossRef]
- Turło, J.; Gutkowska, B.; Herold, F.; Łuczak, I. Investigation of the Kinetics of Selenium Accumulation by Lentinula edodes (Berk.) Mycelial Culture by Use of Reversed-Phase High-Performance Liquid Chromatography with Fluorimetric Detection. Acta Chromatogr. 2009, 21, 1–11. [Google Scholar] [CrossRef]
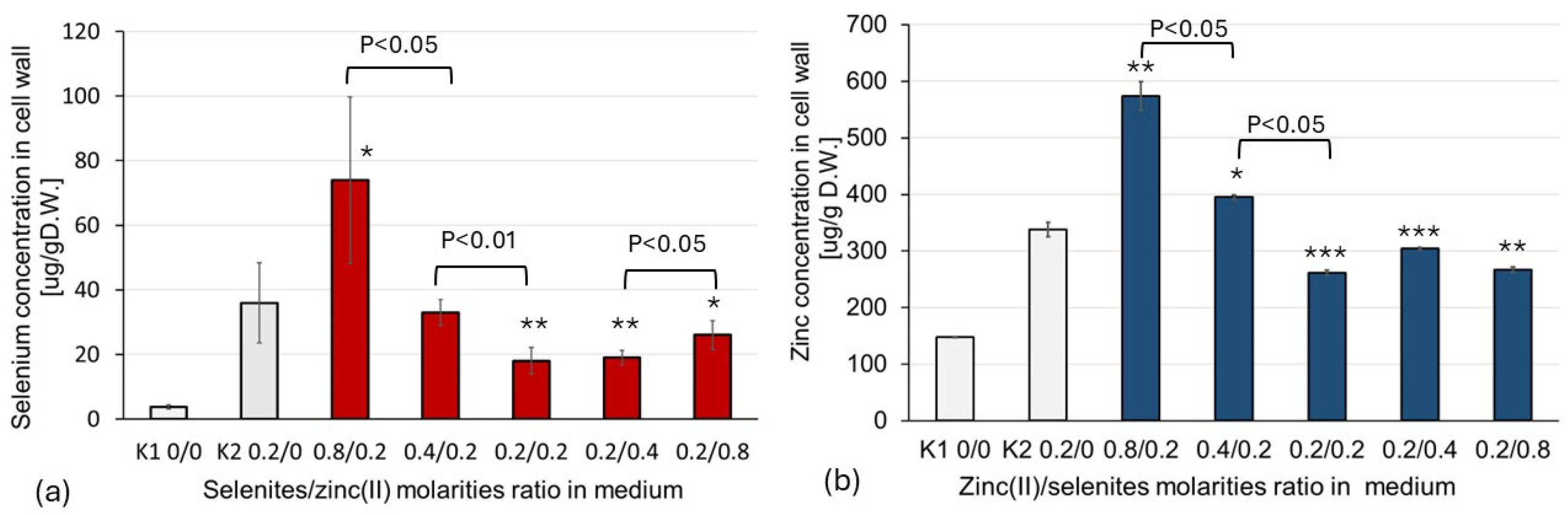
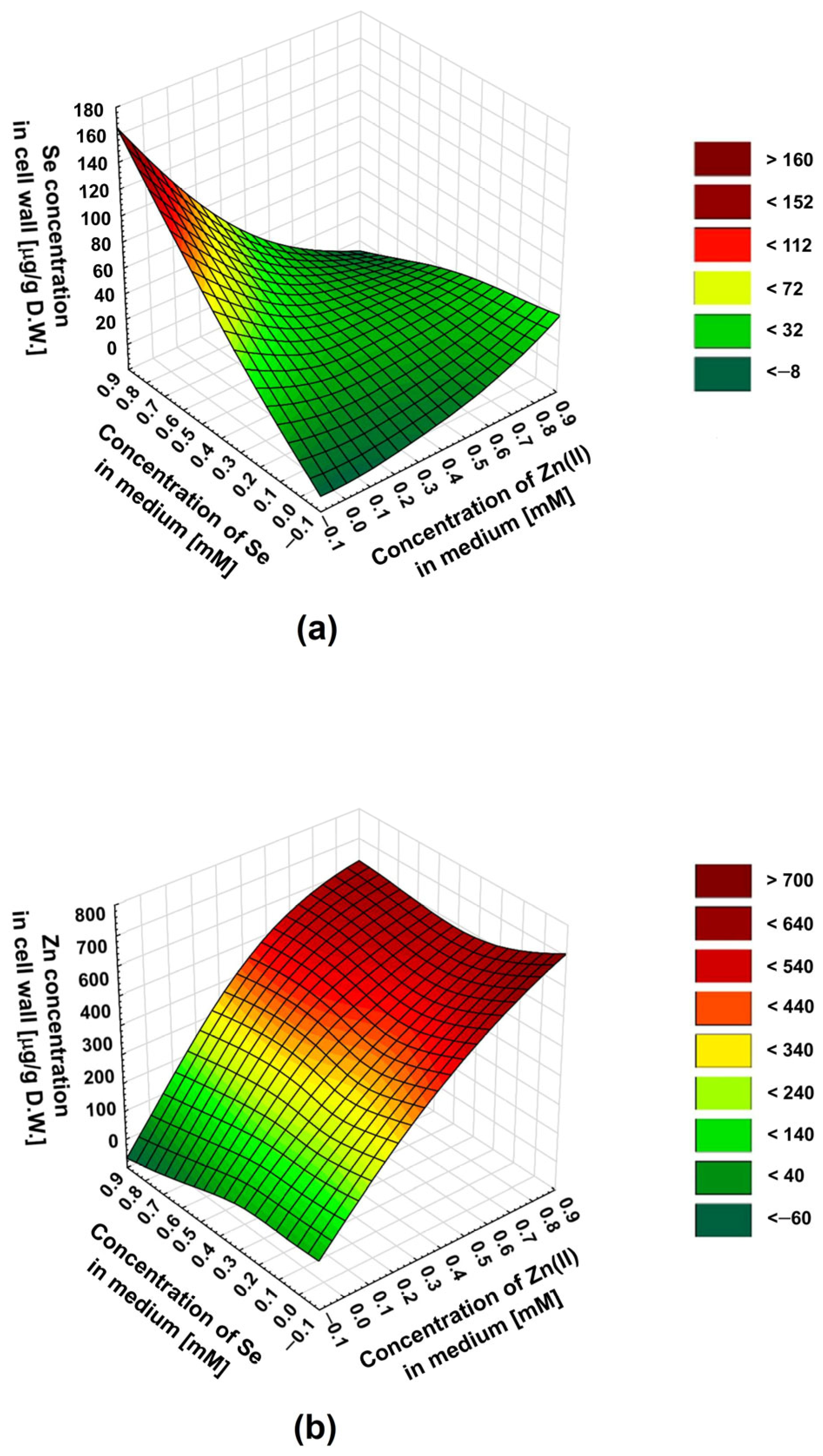
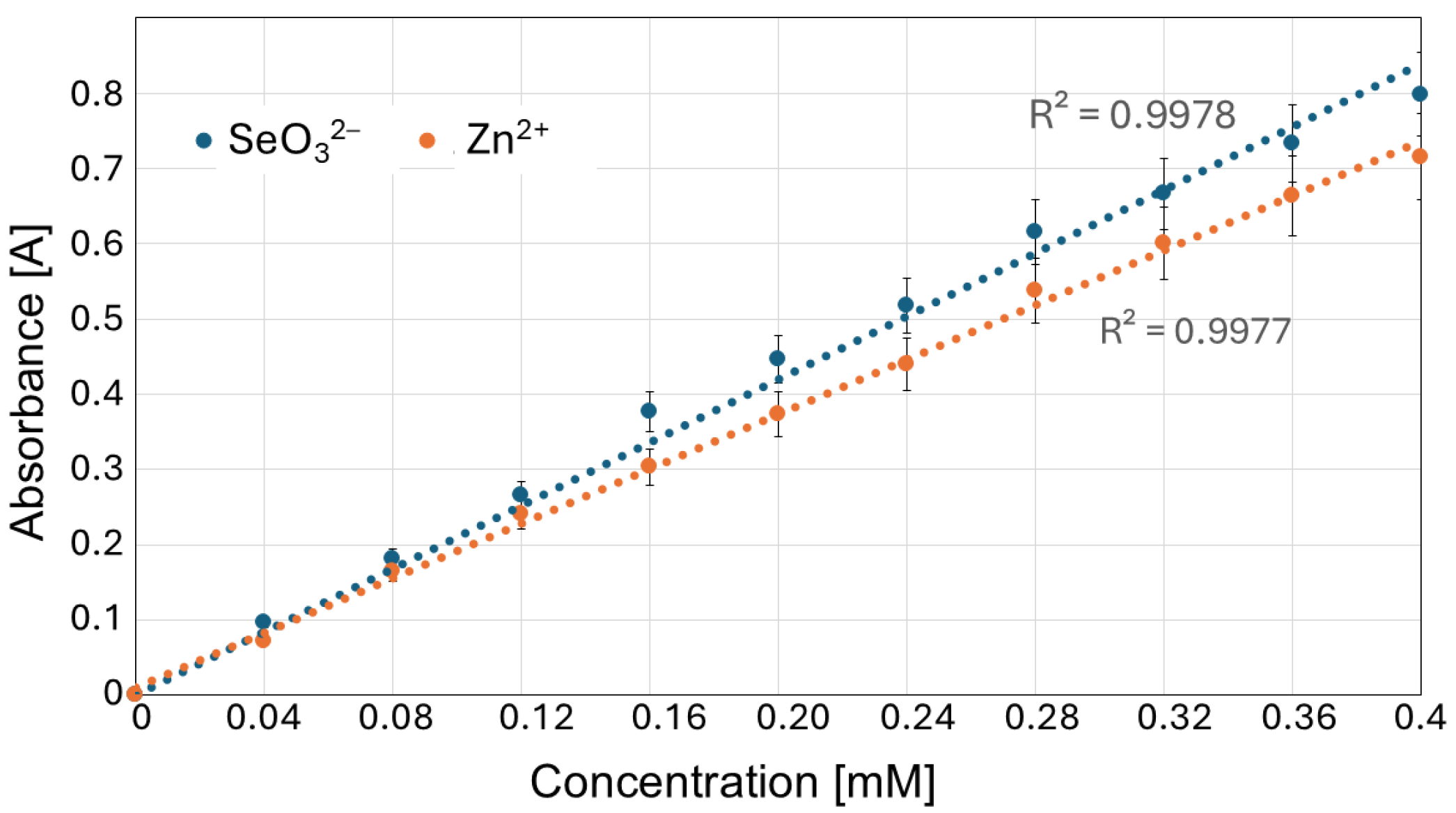
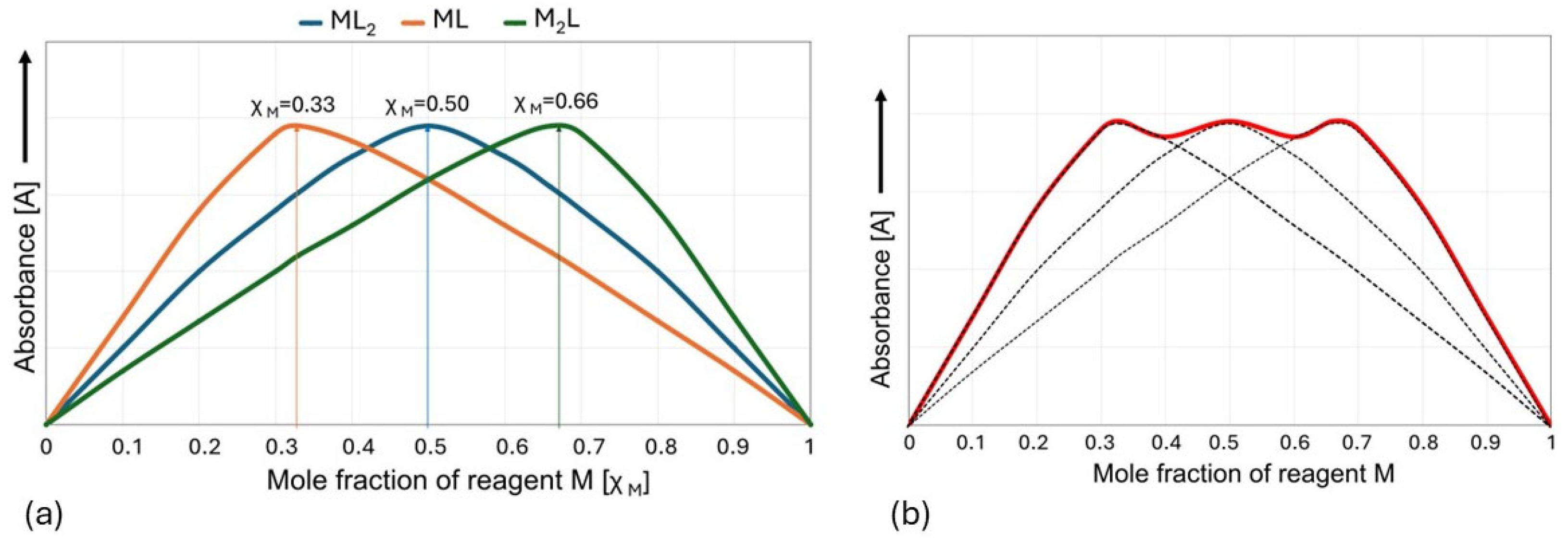
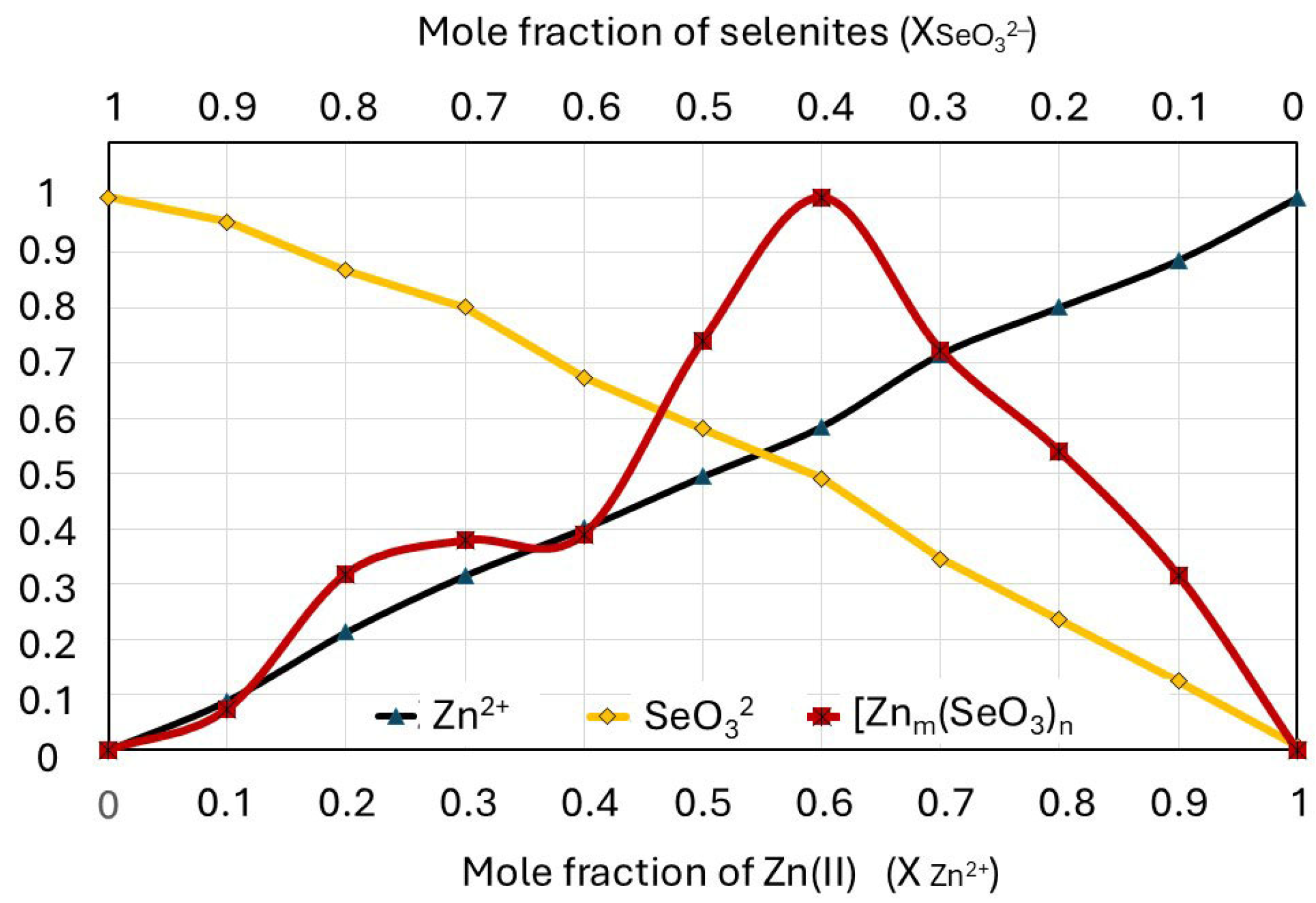
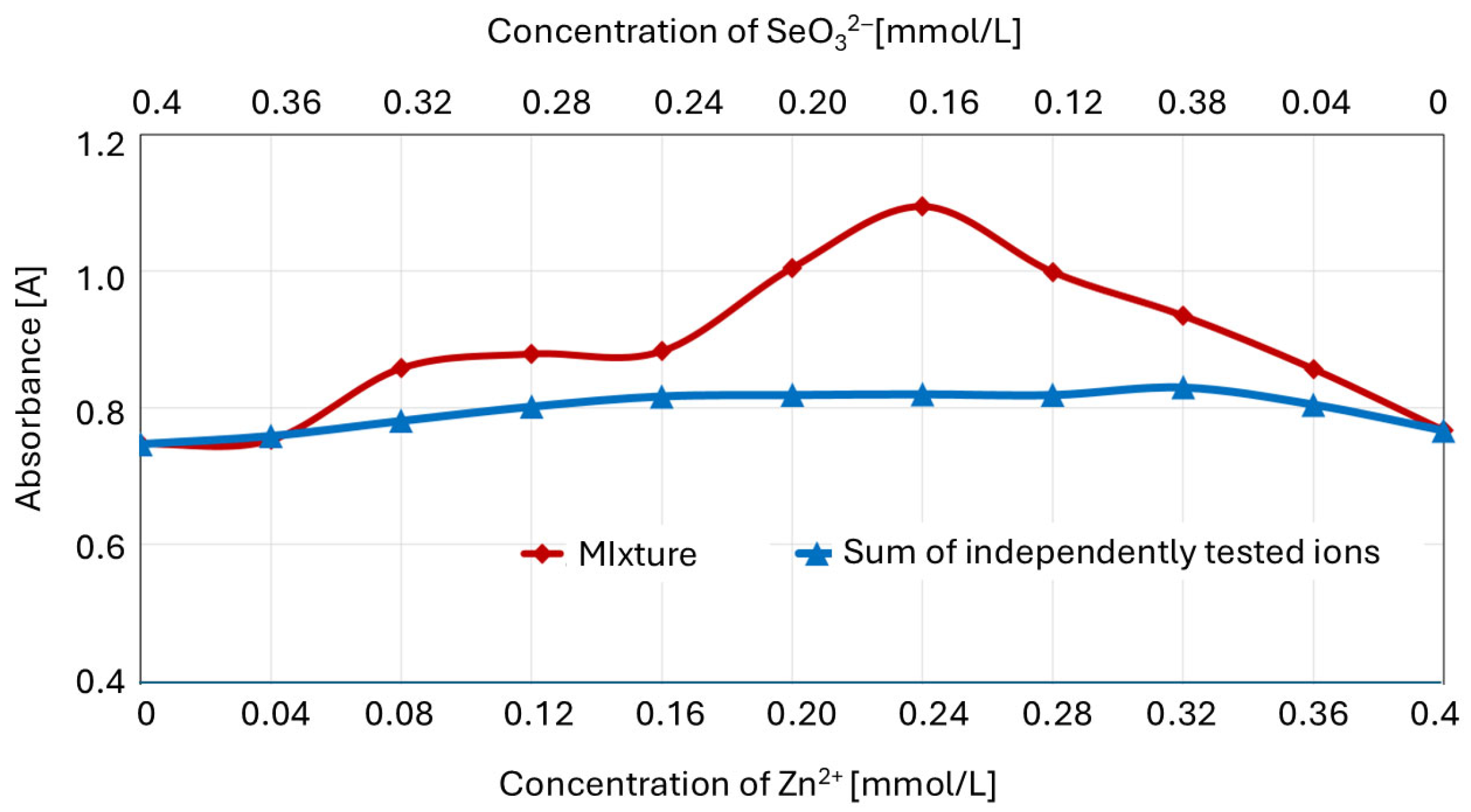

| Concentration of Medium Supplements | Zn2+/SeO32− Molarities Ratio | Cell Wall Composition | Reference Data: Biomass Composition # | |||
|---|---|---|---|---|---|---|
| Zn2+ [mM] | SeO32− [mM] | Zn [μg/g] | Se [μg/g] | Zn [μg/g] | Se [μg/g] | |
| 0 | 0 | N/A | 148.07 (20.27) | 3.77 (0.62) | 298.10 (29.02) | 2.52 (0.17) |
| 0 | 0.2 | N/A | 142.65 (10.28) | 35.97 a (12.43) | 288.96 (50.83) | 310.84 (84.59) |
| 0.2 | 0 | N/A | 337.73 b (59.48) | 2.85 b (0.91) | 920.28 (152.99) | 0.75 (0.28) |
| 0.8 | 0.2 | 4:1 | 574.73 c (44.74) | 26.44 c (4.47) | 874.94 (194.16) | 44.59 (6.76) |
| 0.4 | 0.2 | 2:1 | 395.29 b (72.57) | 19.17 c (2.27) | 278.22 (33.31) | 58.39 (12.65) |
| 0.2 | 0.2 | 1:1 | 261.77 b (18.93) | 18.00 b (4.11) | 639.57 (45.95) | 80.83 (4.16) |
| 0.2 | 0.4 | 1:2 | 303.69 a (72.32) | 33.04 a (3.99) | 603.41 (24.20) | 344.10 (48.65) |
| 0.2 | 0.8 | 1:4 | 266.54 c (8.36) | 74.75 b (25.75) | 591.25 (18.41) | 1395.89 (16.86) |
| Concentration of Medium Supplements | Zn2+/SeO32− Molarity Ratio | SeO32− Concentration [mM] | ||
|---|---|---|---|---|
| Zn2+ [mM] | SeO32− [mM] | Determined | Theoretical | |
| 0 | 0 | N/A | 2 × 10−4 a (10−5) | 0 |
| 0 | 0.2 | N/A | 0.202 (3 × 10−4) | 0.20 |
| 0.2 | 0.2 | 1:1 | 0.073 a (0.002) | 0.2 |
| 0.4 | 0.2 | 2:1 | 0.173 a (0.003) | 0.2 |
| 0.6 | 0.2 | 3:1 | 0.114 a (0.002) | 0.2 |
| 0.8 | 0.2 | 4:1 | 0.078 a (0.005) | 0.2 |
| 0.2 | 0 | N/A | 0.0002 a (3 × 10−5) | 0 |
| 0.2 | 0.2 | 1:1 | 0.073 a (0.002) | 0.2 |
| 0.2 | 0.4 | 1:2 | 0.152 a (0.001) | 0.4 |
| 0.2 | 0.6 | 1:3 | 0.279 a (0.005) | 0.6 |
| 0.2 | 0.8 | 1:4 | 0.390 a (0.007) | 0.8 |
| Precursor | Concentration of Precursor [mM] | |||||||
|---|---|---|---|---|---|---|---|---|
| SeO32− | 0 | 0.2 | 0 | 0.2 | 0.2 | 0.2 | 0.4 | 0.8 |
| Zn2+ | 0 | 0 | 0.2 | 0.8 | 0.4 | 0.2 | 0.2 | 0.2 |
| Ion | 0.4 mM Volume Stock Solution (mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SeO32− | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Zn2+ | 10 | 9 | 8 | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| Ion mole fraction (X) | |||||||||||
| SeO32− | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1 |
| Zn2+ | 1 | 0.9 | 0.8 | 0.7 | 0.6 | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 | 0 |
| Ion concentration (mM/L) | |||||||||||
| SeO32− | 0 | 0.04 | 0.08 | 0.12 | 0.16 | 0.2 | 0.24 | 0.28 | 0.32 | 0.36 | 0.4 |
| Zn2+ | 0.4 | 0.36 | 0.32 | 0.28 | 0.24 | 0.2 | 0.16 | 0.12 | 0.08 | 0.04 | 0 |
| Component | Volume (mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.4 mM SeO32− or Zn2+ | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| H2O | 10 | 9 | 8 | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| Final concentration (mM) | |||||||||||
| SeO32− or Zn2+ | 0 | 0.04 | 0.08 | 0.12 | 0.16 | 0.2 | 0.24 | 0.28 | 0.32 | 0.36 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałucka, M.; Podsadni, P.; Szczepańska, A.; Malinowska, E.; Błażewicz, A.; Turło, J. Impact of Interactions Between Zn(II) and Selenites in an Aquatic Environment on the Accumulation of Se and Zn in a Fungal Cell. Molecules 2025, 30, 3015. https://doi.org/10.3390/molecules30143015
Kałucka M, Podsadni P, Szczepańska A, Malinowska E, Błażewicz A, Turło J. Impact of Interactions Between Zn(II) and Selenites in an Aquatic Environment on the Accumulation of Se and Zn in a Fungal Cell. Molecules. 2025; 30(14):3015. https://doi.org/10.3390/molecules30143015
Chicago/Turabian StyleKałucka, Małgorzata, Piotr Podsadni, Agnieszka Szczepańska, Eliza Malinowska, Anna Błażewicz, and Jadwiga Turło. 2025. "Impact of Interactions Between Zn(II) and Selenites in an Aquatic Environment on the Accumulation of Se and Zn in a Fungal Cell" Molecules 30, no. 14: 3015. https://doi.org/10.3390/molecules30143015
APA StyleKałucka, M., Podsadni, P., Szczepańska, A., Malinowska, E., Błażewicz, A., & Turło, J. (2025). Impact of Interactions Between Zn(II) and Selenites in an Aquatic Environment on the Accumulation of Se and Zn in a Fungal Cell. Molecules, 30(14), 3015. https://doi.org/10.3390/molecules30143015







