Nutraceutical Potential of Sideroxylon cinereum, an Endemic Mauritian Fruit of the Sapotaceae Family, Through the Elucidation of Its Phytochemical Composition and Antioxidant Activity
Abstract
1. Introduction
2. Results
2.1. Preliminary Qualitative Phytochemical Screening
2.2. Quantitative Phytochemical Analysis
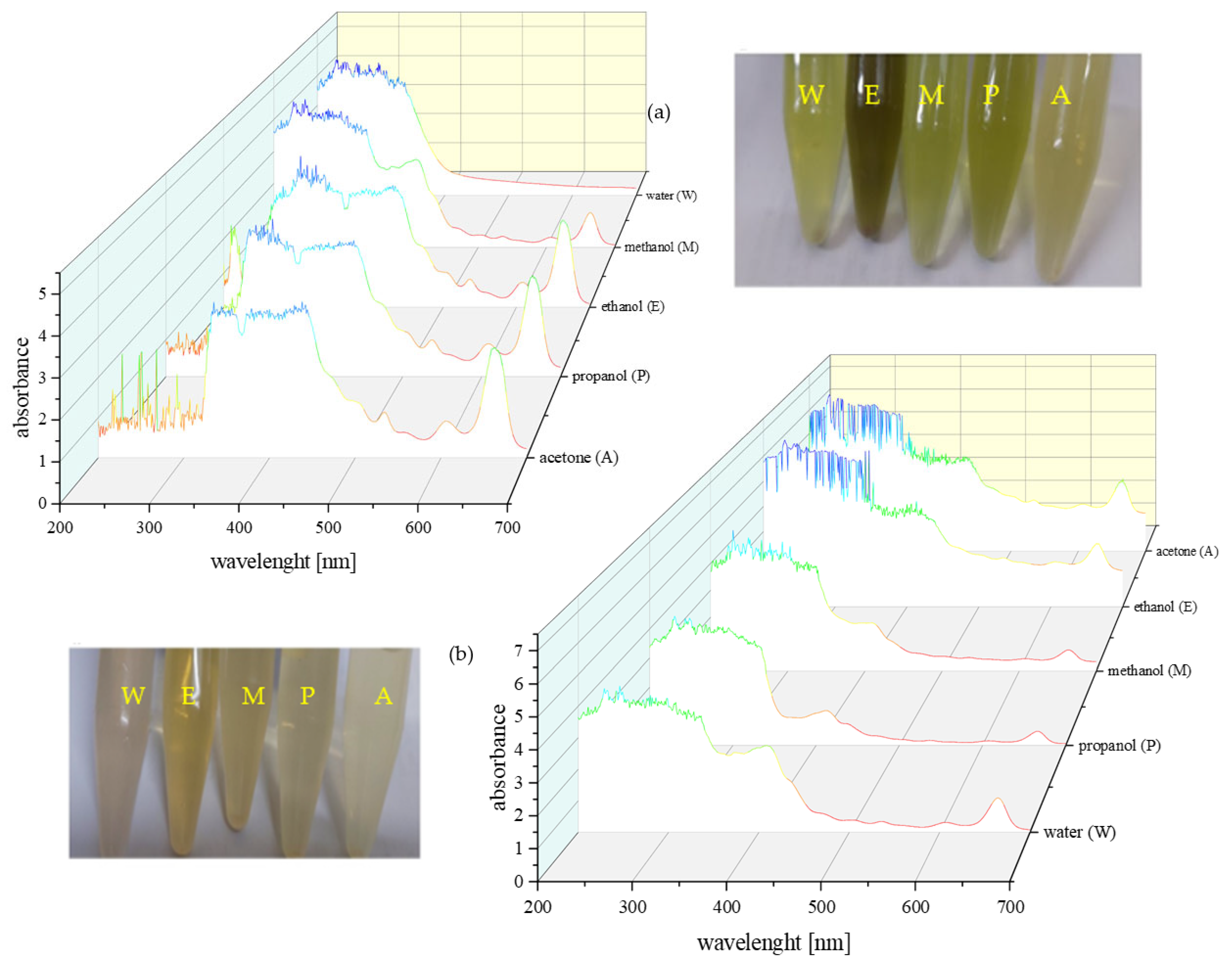
2.3. UV–Vis Absorbance Characteristics
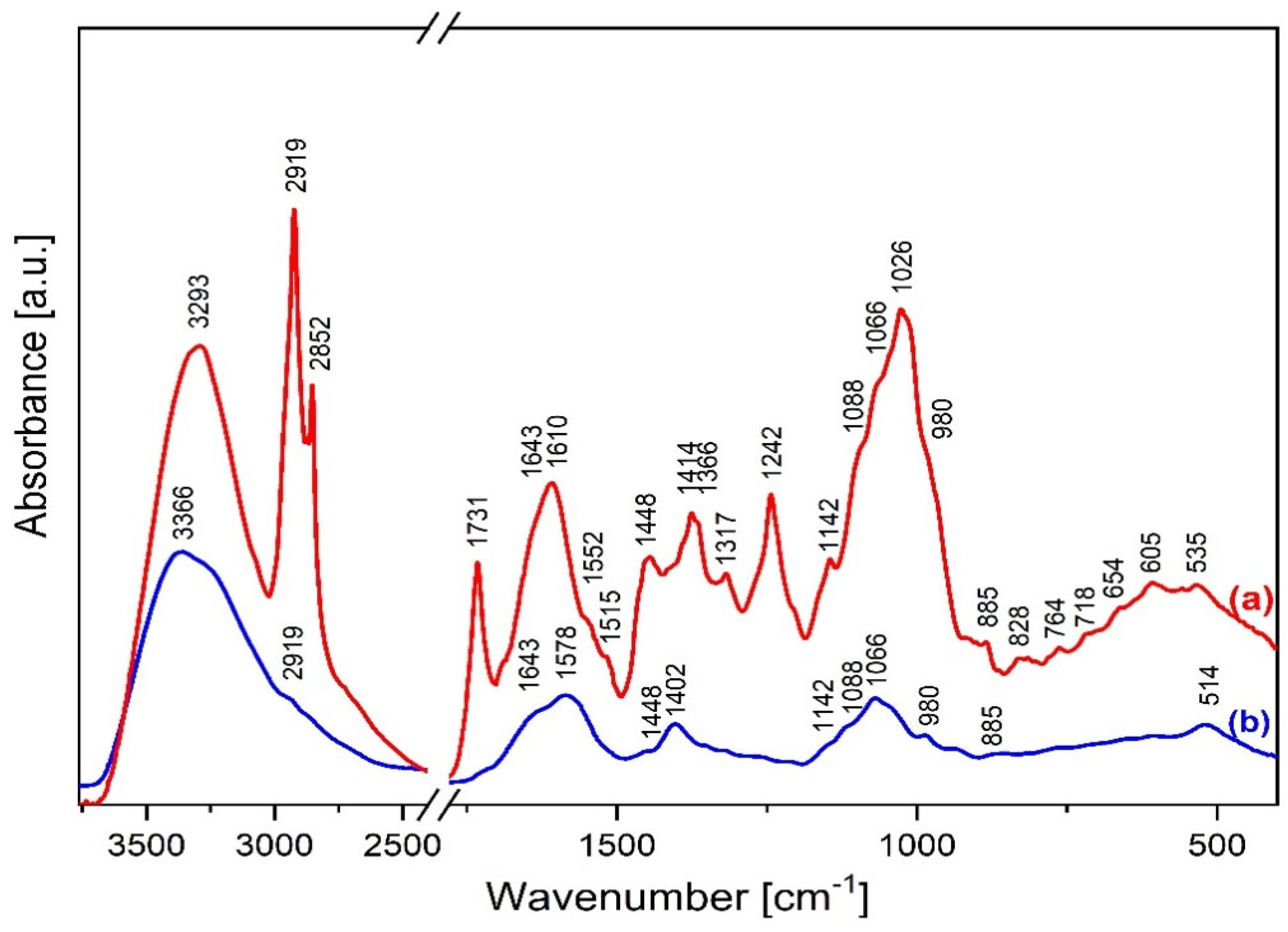
2.4. FTIR Characterization
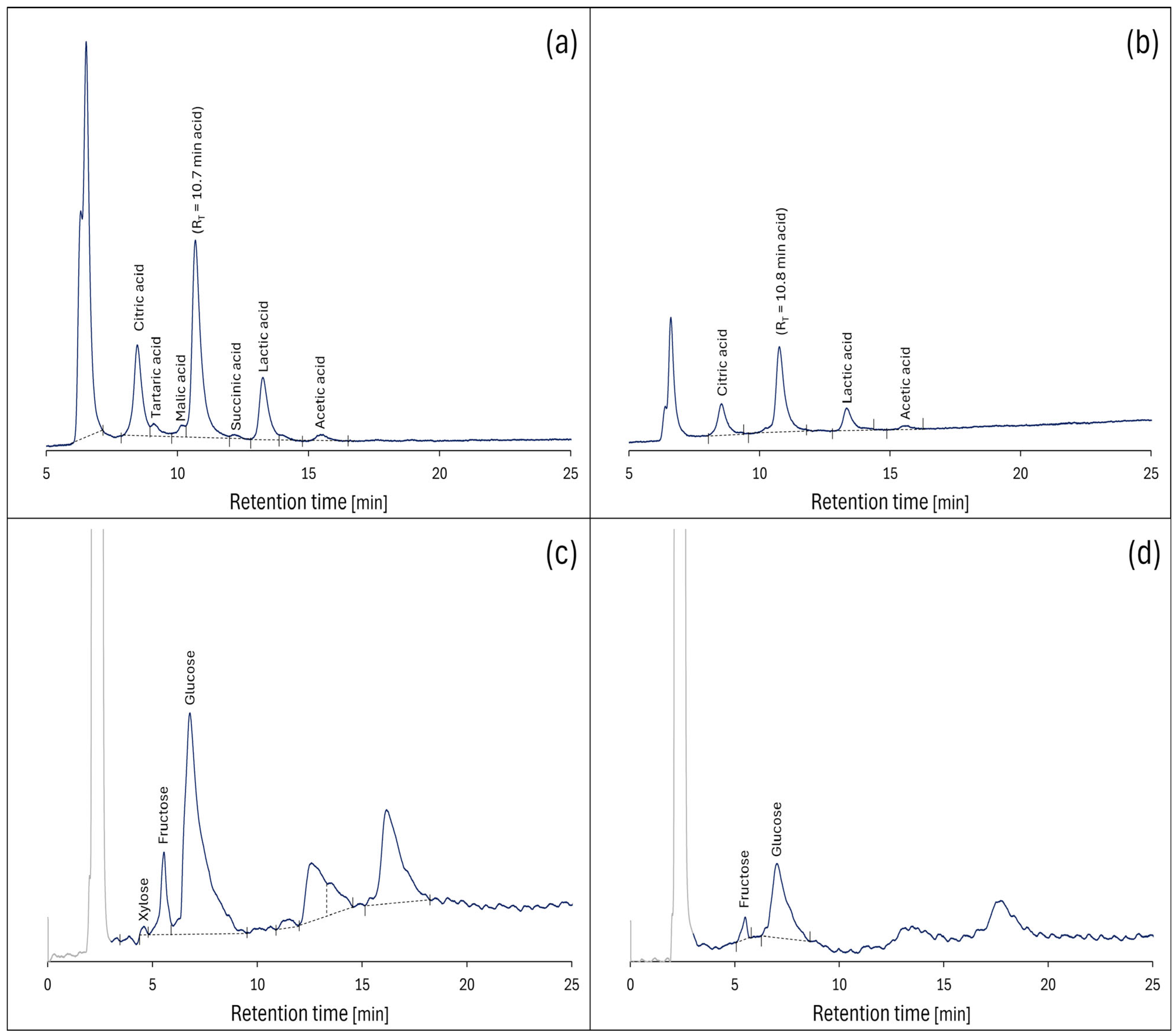
2.5. Saccharides and Organic Acids Characteristics by HPLC
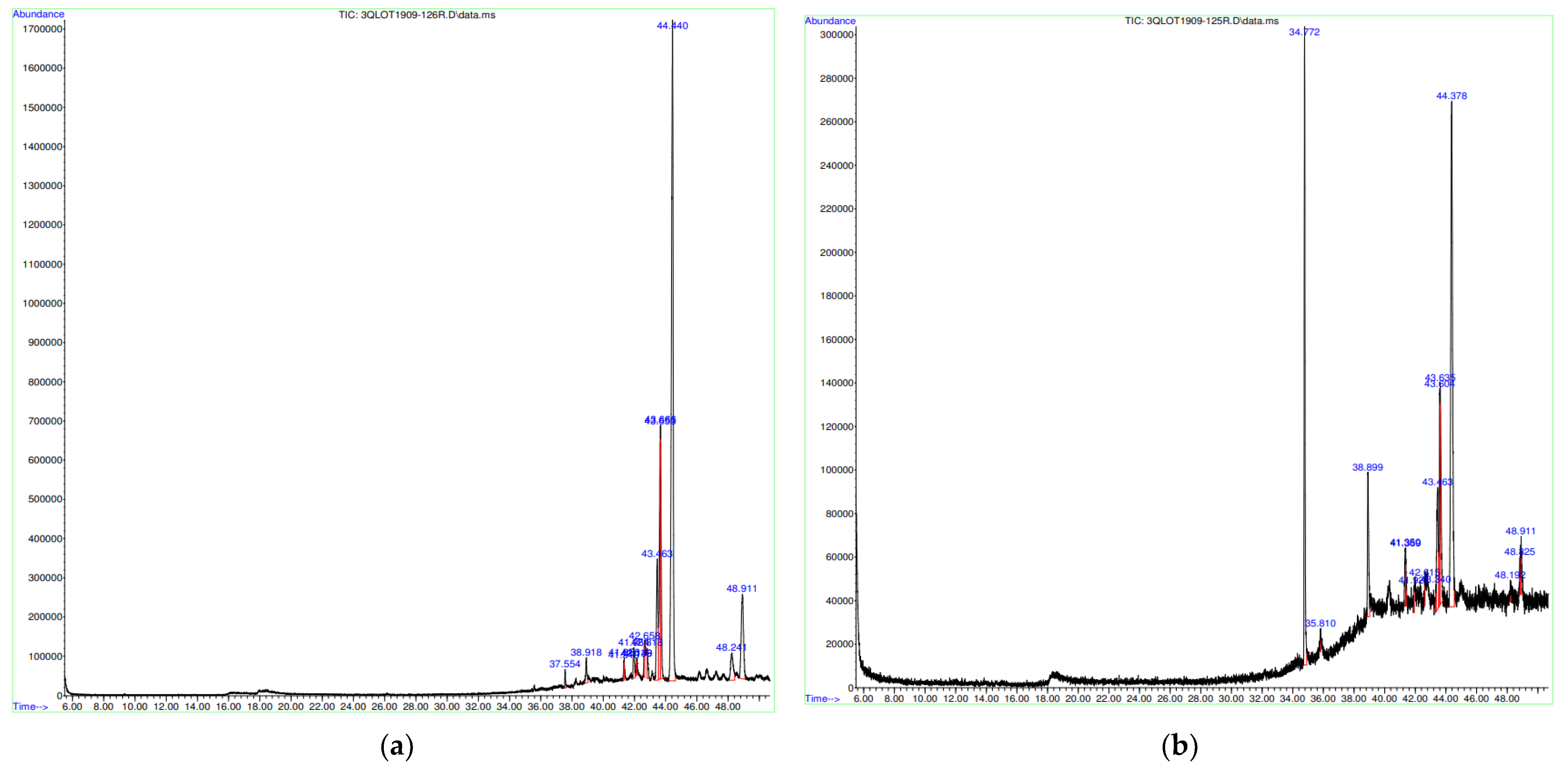
2.6. Compound Identification by GC–MS
2.7. Antioxidant and Oxidoreductive Activity
2.8. Correlation Analysis Between Phytochemical Content and Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Fruit Sampling
4.2. Reagents and Chemicals
4.3. Phytochemical Extraction
4.4. Qualitative Phytochemical Screening
4.5. Quantitative Phytochemical Analysis
4.5.1. Total Phenolic Content (TPC) Determination
4.5.2. Total Flavonoid Content (TFC) Determination
4.5.3. Total Terpenoid Content (TTC) Determination
4.5.4. Reducing Sugar (RS) and Protein Content Determination
4.6. Ultraviolet–Visible (UV–Vis) Spectroscopy
4.7. Mid-Infrared (MIR) Spectroscopy
4.8. Gas Chromatography–Mass Spectrometry (GC–MS)
4.9. Sugar and Organic Acid Determination by HPLC
4.10. Antioxidant and Oxidoreductive Activity Determination
4.10.1. Ferric Reducing Antioxidant Power (FRAP) Assay
4.10.2. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Assay
4.10.3. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
4.11. Statistical Analysis and Correlation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baky, M.H.; Elsaid, M.B.; Farag, M.A. Phytochemical and biological diversity of triterpenoid saponins from family Sapotaceae: A comprehensive review. Phytochemistry 2022, 202, 113345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.P.N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.K.S.; Oz, F. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef] [PubMed]
- Khallouki, F.; Eddouks, M.; Mourad, A.; Breuer, A.; Owen, R.W. Ethnobotanic, Ethnopharmacologic Aspects and New Phytochemical Insights into Moroccan Argan Fruits. Int. J. Mol. Sci. 2017, 18, 2277. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, L.; Sarangi, P.K.; Singh, A.K.; Srivastava, R.K.; Chandel, A.K. Pre-, pro-, and postbiotics development from vegetable, fruit, and lignocellulosic biomass: A perspective. Food Biosci. 2024, 61, 2212–4292. [Google Scholar] [CrossRef]
- Khunnonkwao, P.; Thitiprasert, S.; Jaiaue, P.; Khumrangsee, K.; Cheirsilp, B.; Thongchul, N. The outlooks and key challenges in renewable biomass feedstock utilization for value-added platform chemical via bioprocesses. Heliyon 2024, 10, 2405–8440. [Google Scholar] [CrossRef] [PubMed]
- Coyago-Cruz, E.; Valenzuela, D.; Guachamin, A.; Méndez, G.; Heredia-Moya, J.; Vera, E. Bioactive Compound Profiling and Antioxidant Activity of Phytelephas tenuicaulis and Other Amazonian Fruits. Foods 2024, 13, 2151. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.; Medina, P.J. Identificación Cualitativa De Metabolitos Secundarios Y Determinación De La Citotoxicidad De Extractos De Tempisque (Sideroxylum capiri PITTIER). Biotecnia. 2016, XVIII, 3–8. [Google Scholar] [CrossRef]
- Martínez-Silvestre, K.E.; Santiz-Gómez, J.A.; Luján-Hidalgo, M.C.; Ruiz-Lau, N.; Sánchez-Roque, Y.; Gutiérrez-Miceli, F.A. Effect of UV-B Radiation on Flavonoids and Phenols Accumulation in Tempisque (Sideroxylon capiri Pittier) Callus. Plants 2022, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Kauroo, S.; Govinden-Soulange, J.; Ranghoo-Sanmukhiya, V.M. Extracts of select endemic plants from the Republic of Mauritius exhibiting anti-cancer and immunomodulatory properties. Sci. Rep. 2021, 11, 4272. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 4. [Google Scholar] [CrossRef]
- De Medeiros, M.F.A.; Silva, S.G.B.; Teixeira, C.D.; Lima, S.C.V.C.; Marchioni, D.M.; Jacob, M.C.M. Assessment of Biodiversity in Food Consumption Studies: A Systematic Review. Front. Nutr. 2022, 9, 832288. [Google Scholar] [CrossRef] [PubMed]
- Allaqaband, S.; Dar, A.H.; Patel, U.; Kumar, N.; Nayik, G.A.; Khan, S.A.; Ansari, M.J.; Alabdallah, N.M.; Kumar, P.; Pandey, V.K.; et al. Utilization of Fruit Seed-Based Bioactive Compounds for Formulating the Nutraceuticals and Functional Food: A Review. Front. Nutr. 2022, 9, 902554. [Google Scholar] [CrossRef] [PubMed]
- Mabasa, X.E.; Mathomu, L.M.; Madala, N.E.; Musie, E.M.; Sigidi, M.T. Molecular Spectroscopic (FTIR and UV-Vis) and Hyphenated Chromatographic (UHPLC-qTOF-MS) Analysis and In Vitro Bioactivities of the Momordica balsamina Leaf Extract. Biochem. Res. Int. 2021, 2854217, 12. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley and Sons Ltd.: London, UK, 2004. [Google Scholar]
- Zając, A.; Hanuza, J.; Dymińska, L. Raman spectroscopy in determination of horse meat content in the mixture with other meats. Food Chem. 2014, 156, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Mossoba, M.M.; Milosevic, V.; Milosevic, M.; Kramer, J.K.G.; Azizian, H. Determination of total trans fats and oils by infrared spectroscopy for regulatory compliance. Anal. Bioanal. Chem. 2007, 389, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Żuk, M.; Dymińska, L.; Kulma, A.; Boba, A.; Prescha, A.; Szopa, J.; Mączka, M.; Zając, A.; Szołtysek, K.; Hanuza, J. IR and Raman studies of oil and seedcake extracts from natural and genetically modified flax seeds. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2011, 78, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Turan, M.; Ekinci, M.; Ercisli, S.; Ozturk, H.I.; Aydin, M.; Ilhan, E.; Vicas, S.I.; Iancu, C.V.; Gitea, D.; et al. Composition of Anthocyanins, Specific Sugars, and Organic Acids in Wild Edible Aromatic and Medicinal Vegetables. Horticulturae 2025, 11, 145. [Google Scholar] [CrossRef]
- Thamer, F.A.; Thamer, N. Gas chromatography—Mass spectrometry (GC-MS) profiling reveals newly described bioactive compounds in Citrullus colocynthis (L.) seeds oil extracts. Heliyon 2023, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, N.A.; Almulaiky, Y.Q.; Afifi, M.; Al-Farga, A.; Ali, H.A.; Baeshen, N.N.; Abomughaid, M.M.; Abdelazim, A.M.; Baeshen, M.N. GC-MS Analysis of Bioactive Compounds Extracted from Plant Rhazya stricta Using Various Solvents. Plants 2023, 12, 960. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.; Sinniah, U.R.; Akhtar, M.S. In Vitro Pharmacological Activities and GC-MS Analysis of Different Solvent Extracts of Lantana camara Leaves Collected from Tropical Region of Malaysia. Evid. Based Complement. Altern. Med. 2015, 2015, 506413. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Saini, M. Gas Chromatography Mass Spectrometry Profiling in Methanolic and Ethyl-acetate Root and Stem Extract of Corbichonia decumbens (Forssk.) Exell from Thar Desert of Rajasthan, India. Pharmacogn. Res. 2017, 9, S48–S52. [Google Scholar] [CrossRef] [PubMed]
- Olivia, N.U.; Goodness, U.C.; Obinna, O.M. Phytochemical profiling GC-MSanalysis of aqueous methanol fraction of Hibiscus asper leaves Futur. J. Pharm. Sci. 2021, 7, 59. [Google Scholar] [CrossRef]
- Jabbar, A.A.; Abdullah, F.O.; Abdulrahman, K.K.; Galali, Y.S.; Sardar, A.S.; Hano, C. GC-MS Analysis of Bioactive Compounds in Methanolic Extracts of Papaver decaisnei and Determination of Its Antioxidants and Anticancer Activities. J. Food Qual. 2022, 2022, 12. [Google Scholar] [CrossRef]
- Joel, B.; Johnson, E.J.B.; Hoyos, J.S.; Reynolds, M.R.; Altvater, J.; Naiker, M. Identification of phenolics responsible for the high antioxidant activity in Burdekin plum (Pleiogynium timoriense) fruit. Food Chem. Adv. 2022, 1, 100081. [Google Scholar] [CrossRef]
- Mungwari, P.C.; King’ondu, C.K.; Sigauke, P.; Obadele, B.A. Conventional and modern techniques for bioactive compounds recovery from plants: Review. Sci. Afr. 2025, 27, e02509. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of Techniques and Solvents on the Antimicrobial and Antioxidant Potential of Extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Kefayati, Z.; Motamed, S.M.; Shojaii, A.; Noori, M.; Ghods, R. Antioxidant Activity and Phenolic and Flavonoid Contents of the Extract and Subfractions of Euphorbia splendida Mobayen. Pharmacogn. Res. 2017, 9, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Ranghoo-Sanmukhiya, V.M.; Chellan, Y.; Soulange, J.G. Biochemical and phylogenetic analysis of Eugenia and Syzygium species from Mauritius. J. Appl. Res. Med. Aromat. Plants 2017, 12, 21–29. [Google Scholar] [CrossRef]
- De Andrés, E.G.; Serra-Maluquer, X.; Gazol, A.; Olano, J.M.; García-Plazaola, J.I.; Fernández-Marín, B.; Imbert, J.B.; Coll, L.; Ameztegui, A.; Espelta, J.M.; et al. Constrained trait variation by water availability modulates radial growth in evergreen and deciduous Mediterranean oaks. Agric. For. Meteorol. 2024, 346, 109884. [Google Scholar] [CrossRef]
- Alshammari, G.M.; Ahmed, M.A.; Alsulami, T.; Hakeem, M.J.; Ibraheem, M.A.; Al-Nouri, D.M. Phenolic Compounds, Antioxidant Activity, Ascorbic Acid, and Sugars in Honey from Ingenious Hail Province of Saudi Arabia. Appl. Sci. 2022, 12, 8334. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Hadinoto, K. Deep Eutectic Solvent as Green Solvent in Extraction of Biological Macromolecules: A Review. Int. J. Mol. Sci. 2022, 23, 3381. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, I.; Chin, N.L. Antioxidant Properties of Dried Ginger (Zingiber officinale Roscoe) var. Bentong. Foods 2023, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Genkawa, T.; Ahamed, T.; Noguchi, R.; Takigawa, T.; Ozaki, Y. Simple and rapid determination of free fatty acids in brown rice by FTIR spectroscopy in conjunction with a second-derivative treatment. Food Chem. 2016, 191, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef] [PubMed]
- Miękus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Świergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2021, 25, 3804. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Cabo, N. Relationships between the Composition of Edible Oils and Lard and the Ratio of the Absorbance of Specific Bands of Their Fourier Transform Infrared Spectra. Role of Some Bands of the Fingerprint Region. J. Agric. Food Chem. 1998, 46, 1788–1793. [Google Scholar] [CrossRef]
- Mahmood, T.; Anwar, F.; Abbas, M.; Boyce, M.C.; Saari, N. Compositional variation in sugars and organic acids at different maturity stages in selected small fruits from pakistan. Int. J. Mol. Sci. 2012, 13, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Carbohydrate Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040568. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hwang, S.W.; Kim, S.; Lee, Y.S.; Kim, T.Y.; Lee, S.H.; Kim, S.J.; Yoo, H.J.; Kim, E.N.; Kweon, M.N. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes 2020, 11, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Giuntini, E.B.; Sardá, F.A.H.; De Menezes, E.W. The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives. Foods 2022, 11, 3934. [Google Scholar] [CrossRef] [PubMed]
- Baccichet, I.; Tagliabue, G.A.; Linge, C.; Tura, D.; Chiozzotto, R.; Bassi, D.; Cirilli, M. Sensory perception of citrate and malate and their impact on the overall taste in apricot (Prunus armeniaca L.) fruits. Sci. Hortic. 2023, 321, 112266. [Google Scholar] [CrossRef]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Son, H.; Baek, J.H. Tricarboxylic Acid (TCA) Cycle Intermediates: Regulators of Immune Responses. Life 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, M.; Tang, J.; Wang, N.; Feng, Y.; Ma, H. Research Progress on the Therapeutic Effect of Polysaccharides on Non-Alcoholic Fatty Liver Disease through the Regulation of the Gut–Liver Axis. Int. J. Mol. Sc. 2022, 23, 11710. [Google Scholar] [CrossRef] [PubMed]
- Plehn, S.; Wagle, S.; Rupasinghe, H.P.V. Chaga mushroom triterpenoids as adjuncts to minimally invasive cancer therapies: A review. Curr. Res. Toxicol. 2023, 5, 100137. [Google Scholar] [CrossRef] [PubMed]
- Abu-Lafi, S.; Rayan, M.; Masalha, M.; Abu-Farich, B.; Al-Jaas, H.; Abu-Lafi, M.; Rayan, A. Phytochemical Composition and Biological Activities of Wild Scolymus maculatus L. Medicines 2019, 6, 53. Medicines 2019, 6, 53. [Google Scholar] [CrossRef]
- Radi, M.H.; El-Shiekh, R.A.; El-Halawany, A.M. Friedelin and 3β-Friedelinol: Pharmacol. Activities. Rev. Bras. Farm. 2023, 33, 886–900. [Google Scholar] [CrossRef]
- Kamboj, S.; Singh, R. Chromanone—A Prerogative Therapeutic Scaffold: An Overview. Arab. J. Sci. Eng. 2022, 47, 75–111. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The Effectiveness of Vitamin E Treatment in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef] [PubMed]
- Muehlebach, M.E.; Holstein, S.A. Geranylgeranyl diphosphate synthase: Role in human health, disease and potential therapeutic target. Clin. Transl. Med. 2023, 13, e1167. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, E.S.; Tajbakhsh, A.; Iranshahy, M.; Asili, J.; Kretschmer, N.; Shakeri, A.; Sahebkar, A. Naphthoquinone Derivatives Isolated from Plants: Recent Advances in Biological Activity. Mini Rev. Med. Chem. 2020, 20, 2019–2035. [Google Scholar] [CrossRef] [PubMed]
- Nisa, S.; Bibi, Y.; Masood, S.; Ali, A.; Alam, S.; Sabir, M.; Qayyum, A.; Ahmed, W.; Alharthi, S.; Santali, E.Y.; et al. Isolation, Characterization and Anticancer Activity of Two Bioactive Compounds from Arisaema flavum (Forssk.) Schott. Molecules 2022, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Monroy-García, I.N.; Carranza-Torres, I.E.; Carranza-Rosales, P.; Oyón-Ardoiz, M.; García-Estévez, I.; Ayala-Zavala, J.F.; Morán-Martínez, J.; Viveros-Valdez, E. Phenolic Profiles and Biological Activities of Extracts from Edible Wild Fruits Ehretia tinifolia and Sideroxylon lanuginosum. Foods 2021, 10, 2710. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, D.; Tan, L.H.; Yu, B.; Zhao, S.P.; Cao, W.G. Comparison of the antioxidant properties of various solvent extracts from Dipsacus asperoides and identification of phenolic compounds by LC-ESI-QTOF-MS–MS. S. Afr. J. Bot. 2017, 109, 1–8. [Google Scholar] [CrossRef]
- Yulianti, I.; Noviani, D.; Ismandari, T.T.; Murtilaksono, A.; Ariyanti, R. Phytochemicals and Antioxidant Properties of Ethanol Extract of Terap Fruit Seeds (Artocarpus odoratissimus). Trop. J. Nat. Prod. Res. 2025, 9, 2. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; González-Aguilar, G.A.; Moo-Huchin, M.; Ortiz-Vázquez, E.; Cuevas-Glory, L.; Sauri-Duch, E.; Betancur-Ancona, D. Carotenoid Composition and Antioxidant Activity of Extracts From Tropical Fruits. Chiang Mai J. Sci. 2017, 44, 605–616. [Google Scholar]
- Terpinc, P.; Polak, T.; Segatin, N.; Hanzlowsky, A.; Ulrih, N.; Abramovi, H. Antioxidant properties of 4-vinyl derivatives of hydroxycinnamic acid. Food Chem. 2011, 128, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Xianyan, L.; Huimin, X.; Peng, F.; Yaxi, W.; Junyi, H. Evaluation of environment on polyphenols and flavonoids in Oxalis corymbosa extracts as a potential source of antioxidants. IOP Conf. Ser. Earth Environ. Sci. 2018, 170, 052034. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281365, Geranylgeraniol. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Geranylgeraniol (accessed on 26 June 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 73170, Alpha-Amyrin. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/alpha-Amyrin (accessed on 25 June 2025).
- Viet, T.D.; Xuan, T.D.; Anh, H. α-Amyrin and β-Amyrin Isolated from Celastrus hindsii Leaves and Their Antioxidant, Anti-Xanthine Oxidase, and Anti-Tyrosinase Potentials. Molecules 2021, 26, 23. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5362471, Morphinan-6-ol, 7,8-didehydro-4,5-epoxy-3-methoxy-17-methyl-, monohydrate, (5alpha,6alpha)-. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/codeine-monohydrate (accessed on 25 June 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 53987218, Morphinan-6-ol. 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Morphinan-6-ol (accessed on 25 June 2025).
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Parbuntari, H.; Prestica, Y.; Gunawan, R.; Nurman, M.; Adella, F. Preliminary phytochemical screening (qualitative analysis) of cacao leaves (Theobroma cacao L.). EKSAKTA Berk. Ilm. Bid. MIPA 2018, 19, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Olędzki, R.; Lutosławski, K.; Nowicka, P.; Wojdyło, A.; Harasym, J. Non-commercial grapevines hybrids fruits as a novel food of high antioxidant activity. Foods 2022, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Olędzki, R.; Harasym, J. Boiling vs. microwave heating—The impact on physicochemical characteristics of bell pepper (Capsicum annuum L.) at different ripening stages. Appl. Sci. 2023, 13, 8175. [Google Scholar] [CrossRef]
- Asif, A.; Ahmad, A.; Usman, R.; Shaikh, T.; Husain, M.; Shaikh, Z. Antioxidant activity of leaves solvent extract of Mimusops elengi Linn. Int. J. Pharm. Sci. Res. 2023, 12, 2238–2246. [Google Scholar]
- Truong, D.; Ta, N.T.A.; Pham, T.V.; Huynh, T.D.; Do, Q.T.G.; Dinh, N.C.G.; Dang, C.D.; Nguyen, T.K.C.; Bui, A.V. Effects of solvent—Solvent fractionation on the total terpenoid content and in vitro anti-inflammatory activity of Serevenia buxifolia bark extract. Food Sci. Nutr. 2021, 9, 1720–1735. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Daniel Hare, J.; Compton, S.J. Measuring plant protein with the Bradford assay: Evaluation standard method. J. Chem. Ecol. 1989, 15, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Thrane, J.E.; Kyle, M.; Striebel, M. Spectrophotometric analysis of pigments: A critical assessment of a high-throughput method for analysis of algal pigment mixtures by spectral deconvolution. PLoS ONE 2015, 10, e0137645. [Google Scholar] [CrossRef] [PubMed]
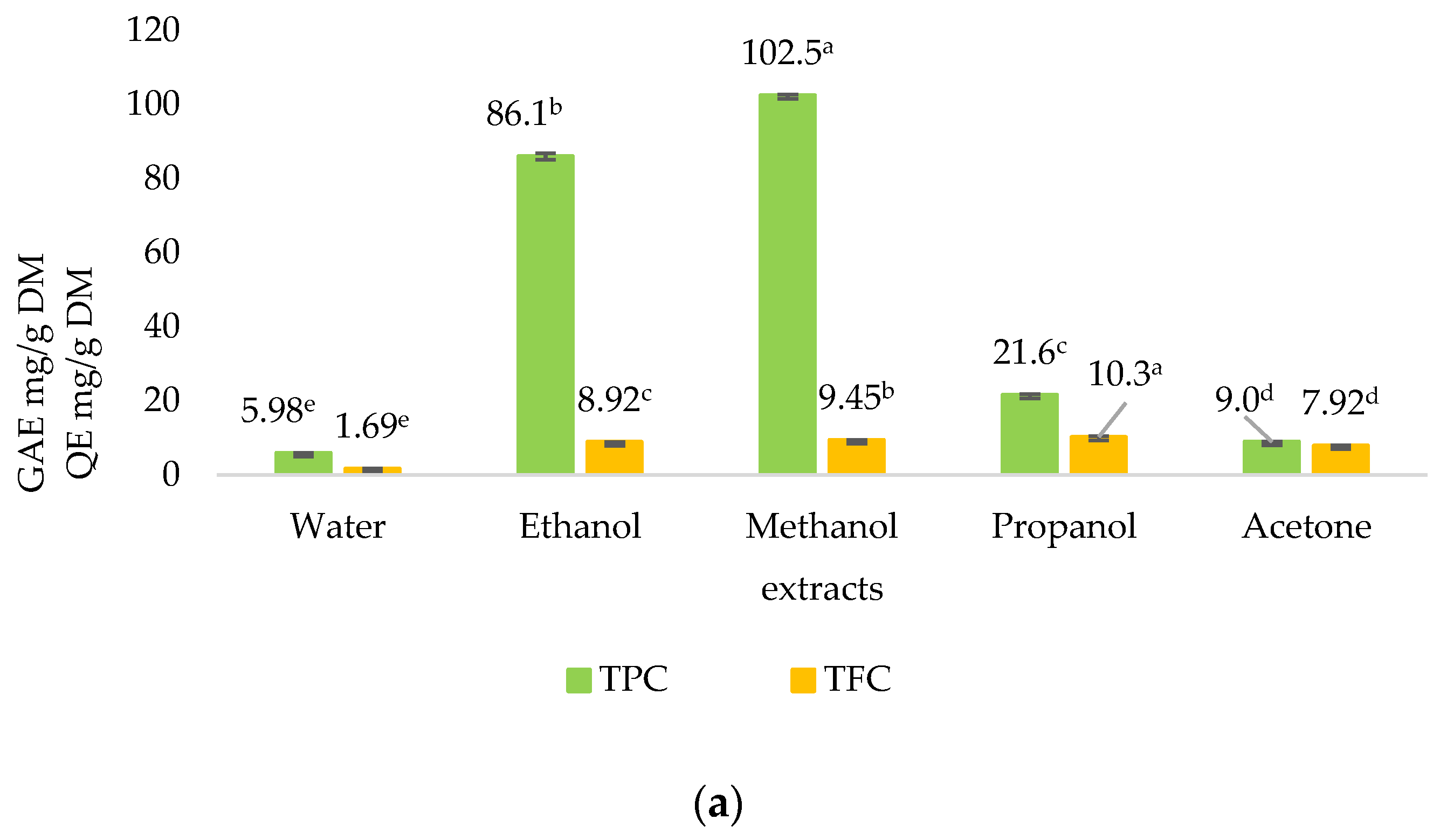
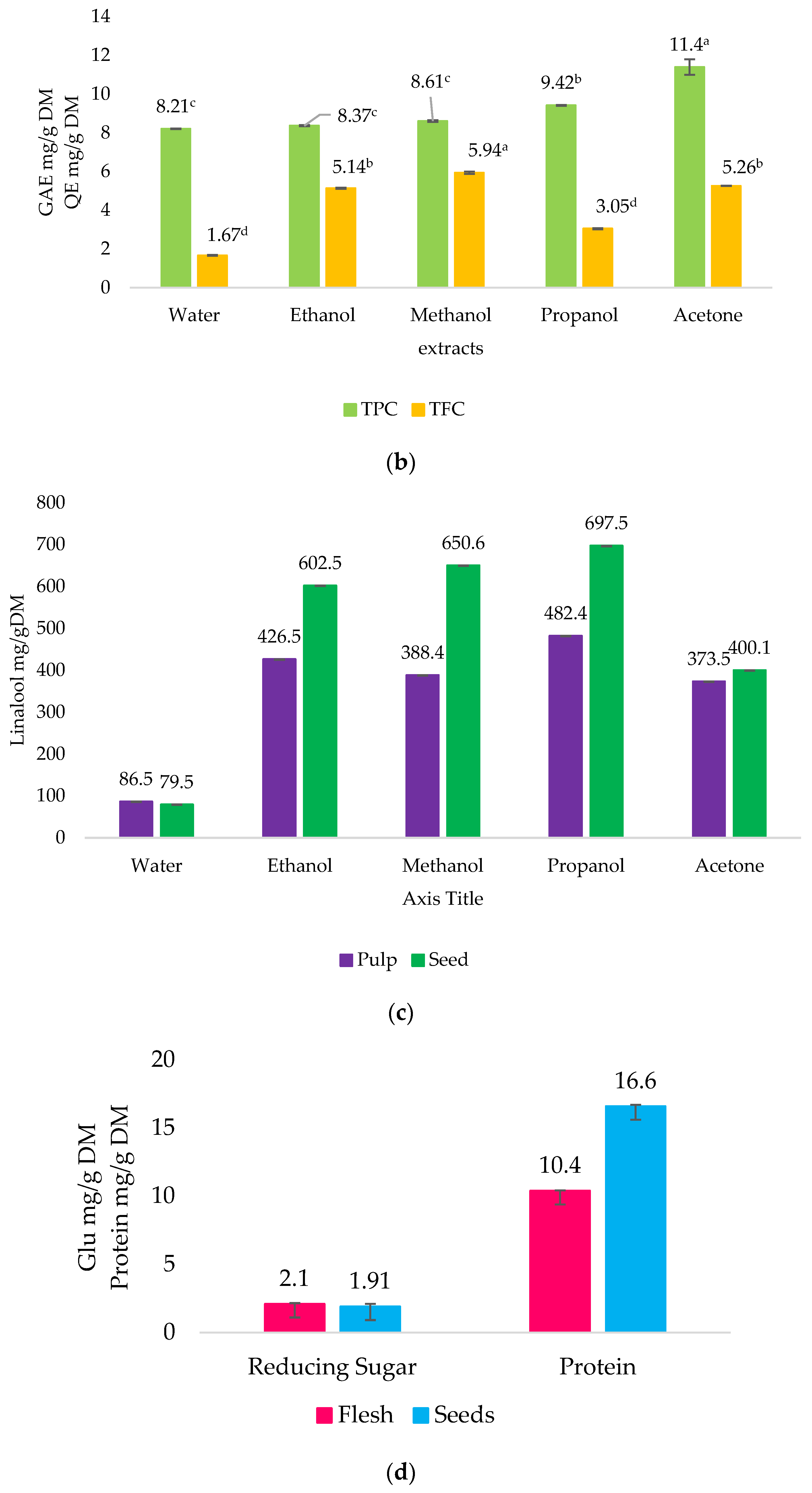


| Phytochemicals | Solvents | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Water | Methanol | Ethanol | Propanol | Acetone | ||||||
| Pulp | Seeds | Pulp | Seeds | Pulp | Seeds | Pulp | Seeds | Pulp | Seeds | |
| Phenols | + | − | ++ | + | ++ | + | + | + | + | + |
| Flavonoids | − | − | − | + | − | + | − | + | − | + |
| Terpenes | − | − | ++ | − | ++ | − | ++ | − | ++ | − |
| Steroids | − | + | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| Saponins | + | + | ++ | ++ | ++ | ++ | + | + | + | + |
| Coumarins | − | − | + | ++ | + | ++ | + | + | + | + |
| Alkaloids | + | − | ++ | ++ | ++ | ++ | + | + | + | + |
| Tannins | − | − | + | ++ | + | ++ | + | + | + | + |
| Reducing sugars | + | + | − | − | − | − | − | − | − | − |
| Proteins | + | + | − | − | + | + | − | − | + | + |
| Retention Time [min] | Acids Saccharides | Content | |
|---|---|---|---|
| In Extract, mg/mL | In Fruit, mg/g (Dry Mass) | ||
| S. cinereum—pulp | |||
| 8.47 | Citric | 1.56 ± 0.08 F,b | 15.63 ± 0.78 E,b |
| 9.08 | Tartaric | 0.26 ± 0.01 BC | 2.65 ± 0.13 B |
| 10.15 | Malic | 0.18 ± 0.01 B | 1.84 ± 0.09 B |
| 12.16 | Succinic | 0.23 ± 0.01 B | 2.33 ± 0.11 B |
| 13.26 | Lactic | 1.41 ± 0.07 F,b | 14.12 ± 0.71 E,b |
| 15.55 | Acetic Xylose | 0.36 ± 0.02 D,b 0.03 ± 0.00 A | 3.64 ± 0.18 C,b 0.35 ± 0.02 A |
| 4.59 | |||
| 5.56 | Fructose | 0.54 ± 0.03 E,b | 5.35 ± 0.27 D,b |
| 6.79 | Glucose | 3.91 ± 0.20 G,b | 39.09 ± 1.95 F,b |
| S. cinereum—seeds | |||
| 8.54 | Citric | 0.73 ± 0.04 D,a | 7.29 ± 0.36 D,a |
| 13.34 | Lactic | 0.57 ± 0.03 C,a | 5.68 ± 0.26 C,a |
| 15.69 | Acetic | 0.25 ± 0.01 B,a | 2.46 ± 0.12 B,a |
| 5.50 | Fructose | 0.11 ± 0.01 A,a | 1.10 ± 0.01 A,a |
| 7.02 | Glucose | 1.16 ± 0.01 E,a | 11.60 ± 0.58 E,a |
| Peak Number | Retention Time (min) | Peak Area (%) | Corresponding Compound | Common Name |
|---|---|---|---|---|
| S. cinereum pulp | ||||
| 1 | 37.554 | 0.64 | 3,7,11,15-tetramethyl-2E,6E,10E,14-hexadecatetraen-1-ol | Geranyl alcohol |
| 2 | 38.918 | 1.28 | (3β)-cholest-5-en-3-ol | Cholesterol |
| 3 | 41.319 | 0.44 | Stigmasta-7,25-dien-3-ol, (3.beta,5.alpha.)- | delta-7,25-stigmastadienol |
| 4 | 41.350 | 0.57 | 5-Chloro-2-iodobenzoic acid | 5-Chloro-2-iodobenzoic acid |
| 5 | 41.964 | 1.84 | Ethanone, 1,1′-(6-hydroxy-2,5-benzofurandiyl)bis- | Euparone |
| 6 | 42.149 | 0.47 | Lanosta-9(11)-en-12-one | Parkeol |
| 7 | 42.658 | 1.32 | 9,19-Cyclolanost-24-en-3-ol, (3. beta.)- | Cycloartenol acetate |
| 8 | 42.818 | 1.14 | Trimethylsilyl ether | Nalmefene |
| 9 | 43.463 | 8.14 | 12-Oleanen-3-yl acetate, (3.α)- | α-amyrin |
| 10 | 43.653 | 10.97 | Lanosta-9(11),24-dien-3-ol, acetate, (3.beta.)- | Perkeol acetate |
| 11 | 43.666 | 8.83 | Octamethylicosahydropicen-3(2H)-one | Friedeline |
| 12 | 44.440 | 52.58 | Lanosta-8,24-dien-3-ol, acetate, (3.beta.)- | Lanosterol acetate |
| 13 | 48.241 | 3.07 | Dammara-20,24-dien-3-ol, 3-acetate, (3.beta) | Dammaradienyl acetate |
| 14 | 48.911 | 8.71 | 2,2,5,7,8-Pentamethyl-chromane-6-sulfonyl chloride | Chromane |
| S. cinereum seeds | ||||
| 1 | 34.772 | 22.41 | 1,2-Benzenedicarboxylic acid | Phthalic acid |
| 2 | 35.810 | 0.50 | 4,5-epoxy-3-methoxy-17-methyl- | Morphinan-6-ol |
| 3 | 38.899 | 7.31 | 26-Nor-5-cholesten-3.beta.-ol-25-one | Norcholesterol |
| 4 | 41.350 | 2.00 | 33-Norgorgosta-5,24(28)-dien-3-ol, (3.beta.)- | 33-Norgorgosta-5,24(28)-dien-3-ol, (3.beta.)- |
| 5 | 41.369 | 1.01 | 2-Acetylamino-5-iodo-4-p-tolyl-thiophene-3-carboxylic acid ethyl ester | |
| 6 | 41.921 | 0.54 | 4-Nitrophenyl-N-(2-chloroethyl)-N-nitrosocarbamate | Carbamic acid |
| 7 | 42.615 | 0.48 | Acetyl Codeine | Acetyl codeine |
| 8 | 43.340 | 0.49 | 2,3,5,6-Tetrachloroiodobenzene | |
| 9 | 43.463 | 7.34 | 4-Allyl-5-pyridin-3-yl-2,4-dihydro-[1,2,4]triazole-3-thione | |
| 10 | 43.635 | 9.42 | Lanosta-7,9(11)-dien-3-ol, acetate, (3.beta.)- | |
| 11 | 44.378 | 45.67 | Lanosta-8,24-dien-3-ol, acetate, (3.beta.)- | Lanosterol acetate |
| 12 | 48.192 | 0.47 | 1,3-Benzenedicarboxylic acid, 5-nitro-, dimethyl ester | Dimethyl 5-nitroisophthalate; |
| 13 | 48.825 | 0.53 | 2-[4-Acetamidophenylsulfonyl]-1,4 naphthoquinone | para-naphthoquinone |
| 14 | 48.911 | 1.83 | Benzo[1,2,5]oxadiazole, 5-(1H-benzoimidazol-2ylsulfanylmethyl)- | Benzo[1,2,5]oxadiazole, 5-(1H-benzoimidazol-2ylsulfanylmethyl)- |
| S. cinereum | Solvent | DPPH (mg TE/g DM) | ABTS (mg TE/g DM) | FRAP (uM Fe2+/g DM) |
|---|---|---|---|---|
| Pulp | Water | 3.11 ± 0.07 b | 2.03 ± 0.26 a | 0.55 ± 0.39 a |
| Methanol | 3.39 ± 0.21 b | 4.63 ± 0.28 b | 1.45 ± 0.39 b | |
| Ethanol | 3.17 ± 0.08 b | 4.52 ± 0.27 b | 2.57 ± 0.47 c | |
| Propanol | 4.29 ± 0.21 c | 5.41 ± 0.70 c | 3.27 ± 0.51 c | |
| Acetone | 2.40 ± 0.27 a | 4.44 ± 0.45 b | 4.69 ± 0.18 d | |
| Seed | Water | 4.22 ± 0.53 bc | 2.71 ± 0.21 a | 6.20 ± 0.10 c |
| Methanol | 3.95 ± 0.15 bc | 4.13 ± 0.31 b | 1.27 ± 0.24 a | |
| Ethanol | 3.77 ± 0.32 b | 4.36 ± 0.47 b | 1.63 ± 0.54 ab | |
| Propanol | 4.49 ± 0.22 c | 3.13 ± 0.05 a | 2.15 ± 0.02 b | |
| Acetone | 3.07 ± 0.07 a | 5.21 ± 0.18 c | 2.12 ± 0.28 b |
| TPC | TFC | TTC | DPPH | ABTS | FRAP | |
|---|---|---|---|---|---|---|
| TPC | 1 | |||||
| TFC | 0.613863 | 1 | ||||
| TTC | 0.597528 | 0.87499 | 1 | |||
| DPPH | 0.245845 | 0.152466 | 0.336171 | 1 | ||
| ABTS | 0.081978 | 0.300145 | 0.480643 | −0.15989 | 1 | |
| FRAP | −0.36201 | −0.3952 | −0.36548 | 0.064946 | −0.0111 | 1 |
| Phytochemicals | Reagents and Method | Positive Result |
|---|---|---|
| Phenols | Few drops of Pb(C2H3O2)2 were added to a few drops of extract. | Appearance of a white precipitate. |
| Flavonoids | Few drops of 1% NH3 were added to a few drops of extract. | Appearance of a yellow coloration. |
| Steroids | Few drops of extract followed by few drops of CHCl3 and H2SO4 were added to the side of a test tube. | Formation of a reddish-brown ring at the interface. |
| Terpenes | Formation of yellow precipitate. | |
| Saponins | Distilled water was added to a few drops of extract, and the mixture was shaken vigorously. | Formation of a froth, which remains for 15–30 min. |
| Alkaloids | To a few drops of extract, few drops of Wagner’s reagent were added. | Appearance of a reddish-brown precipitate. |
| Tannins | Few drops of 5% FeCl3 were added to a few drops of extract. | Appearance of a blue-black or brownish-green precipitate. Appearance of a blue-black or brownish-green precipitate. |
| Coumarins | To a few drops of extract, a few drops of NaOH were added. | Formation of a yellow colour. |
| Reducing Sugars | To 1 mL of extract, 2 mL of Benedict’s reagent was added and heated in a bath of boiling water for 3 to 5 min. | Development of a brick-red coloured precipitate. |
| Proteins | To a few drops of extract, 2 mL of NaOH and 5 to 6 drops of CuSO4 was added and the test tube gently shaken and allowed to stand for 4–5 min. | Appearance of a bluish-violet colour. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhajan, C.; Soulange, J.G.; Ranghoo-Sanmukhiya, V.M.; Olędzki, R.; Ociński, D.; Jacukowicz-Sobala, I.; Zając, A.; Howes, M.-J.R.; Harasym, J. Nutraceutical Potential of Sideroxylon cinereum, an Endemic Mauritian Fruit of the Sapotaceae Family, Through the Elucidation of Its Phytochemical Composition and Antioxidant Activity. Molecules 2025, 30, 3041. https://doi.org/10.3390/molecules30143041
Bhajan C, Soulange JG, Ranghoo-Sanmukhiya VM, Olędzki R, Ociński D, Jacukowicz-Sobala I, Zając A, Howes M-JR, Harasym J. Nutraceutical Potential of Sideroxylon cinereum, an Endemic Mauritian Fruit of the Sapotaceae Family, Through the Elucidation of Its Phytochemical Composition and Antioxidant Activity. Molecules. 2025; 30(14):3041. https://doi.org/10.3390/molecules30143041
Chicago/Turabian StyleBhajan, Cheetra, Joyce Govinden Soulange, Vijayanti Mala Ranghoo-Sanmukhiya, Remigiusz Olędzki, Daniel Ociński, Irena Jacukowicz-Sobala, Adam Zając, Melanie-Jayne R. Howes, and Joanna Harasym. 2025. "Nutraceutical Potential of Sideroxylon cinereum, an Endemic Mauritian Fruit of the Sapotaceae Family, Through the Elucidation of Its Phytochemical Composition and Antioxidant Activity" Molecules 30, no. 14: 3041. https://doi.org/10.3390/molecules30143041
APA StyleBhajan, C., Soulange, J. G., Ranghoo-Sanmukhiya, V. M., Olędzki, R., Ociński, D., Jacukowicz-Sobala, I., Zając, A., Howes, M.-J. R., & Harasym, J. (2025). Nutraceutical Potential of Sideroxylon cinereum, an Endemic Mauritian Fruit of the Sapotaceae Family, Through the Elucidation of Its Phytochemical Composition and Antioxidant Activity. Molecules, 30(14), 3041. https://doi.org/10.3390/molecules30143041









