Death of Leukemia Cells and Platelets Induced by 3,3′-Dihydroxy-4,5-Dimethoxybibenzyl Is Mediated by p38 Mitogen-Activated Protein Kinase Pathway
Abstract
1. Introduction
2. Results
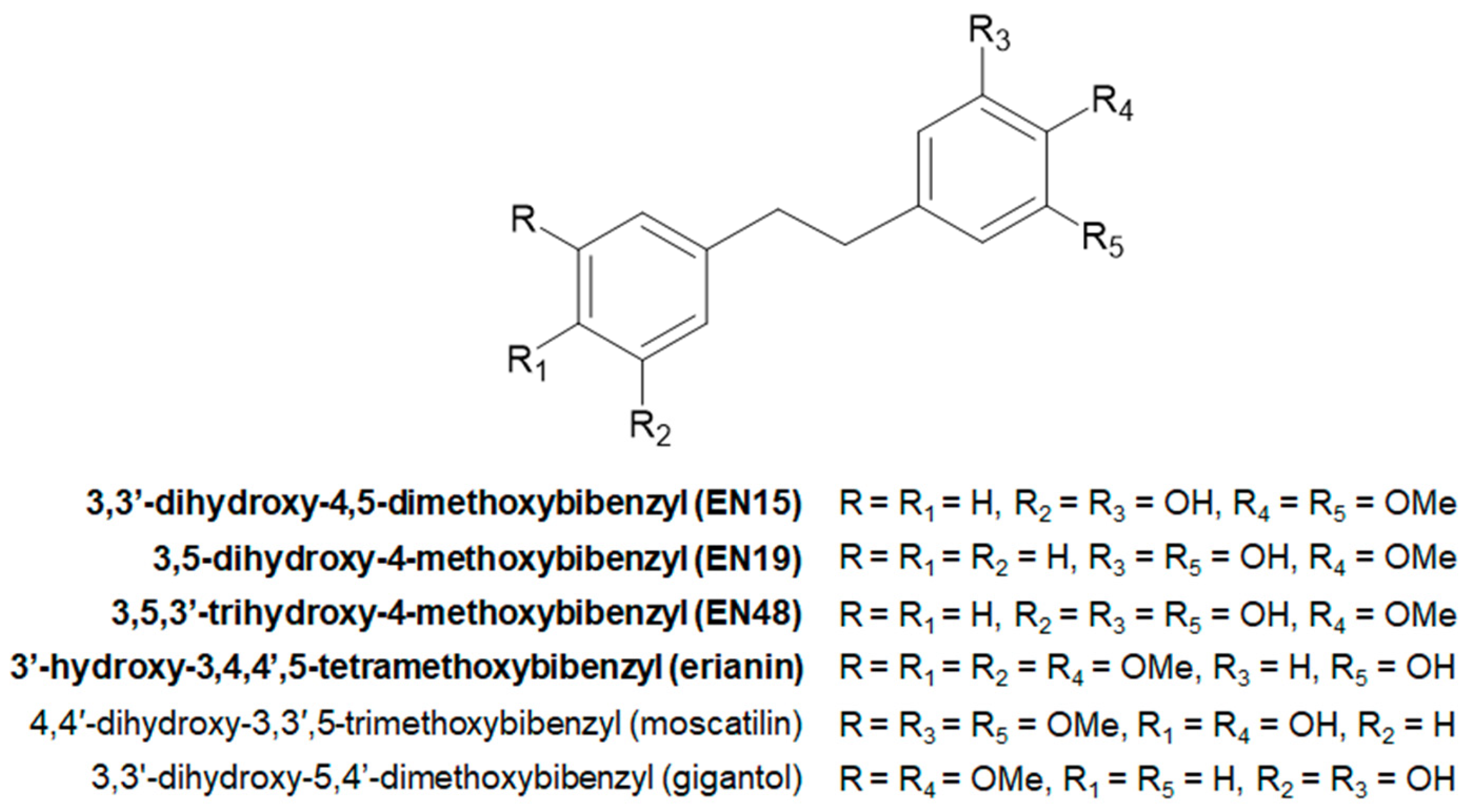
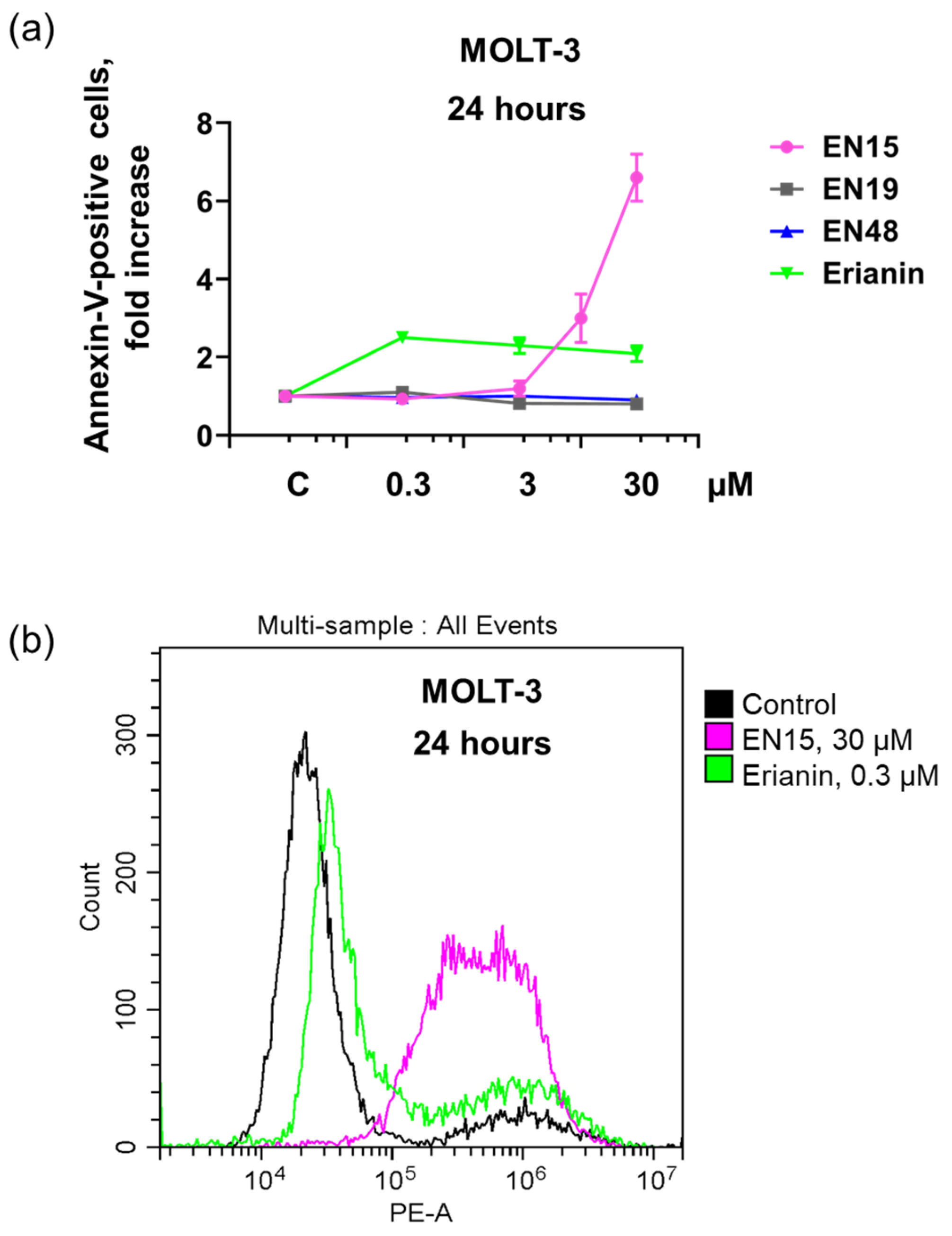
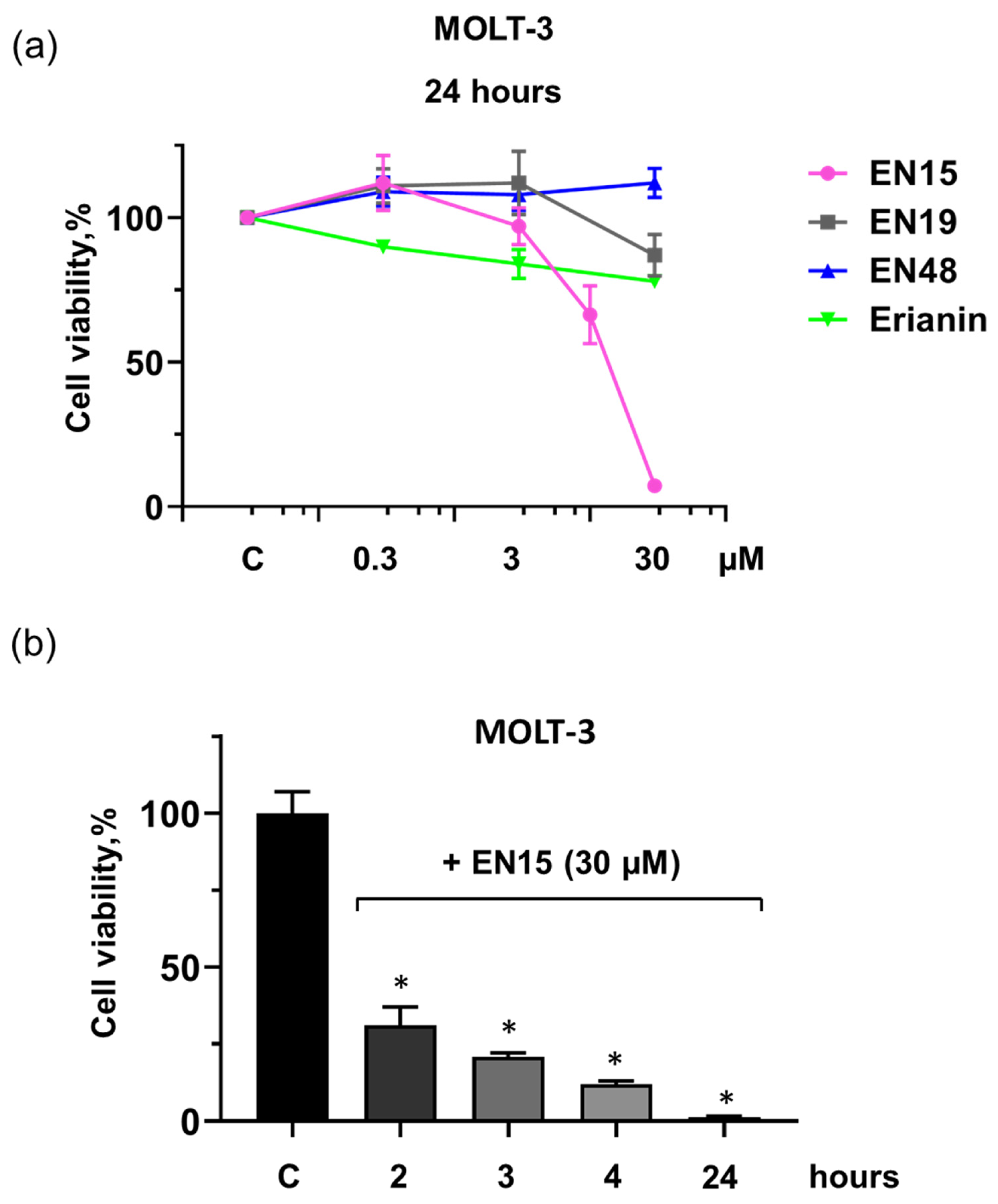
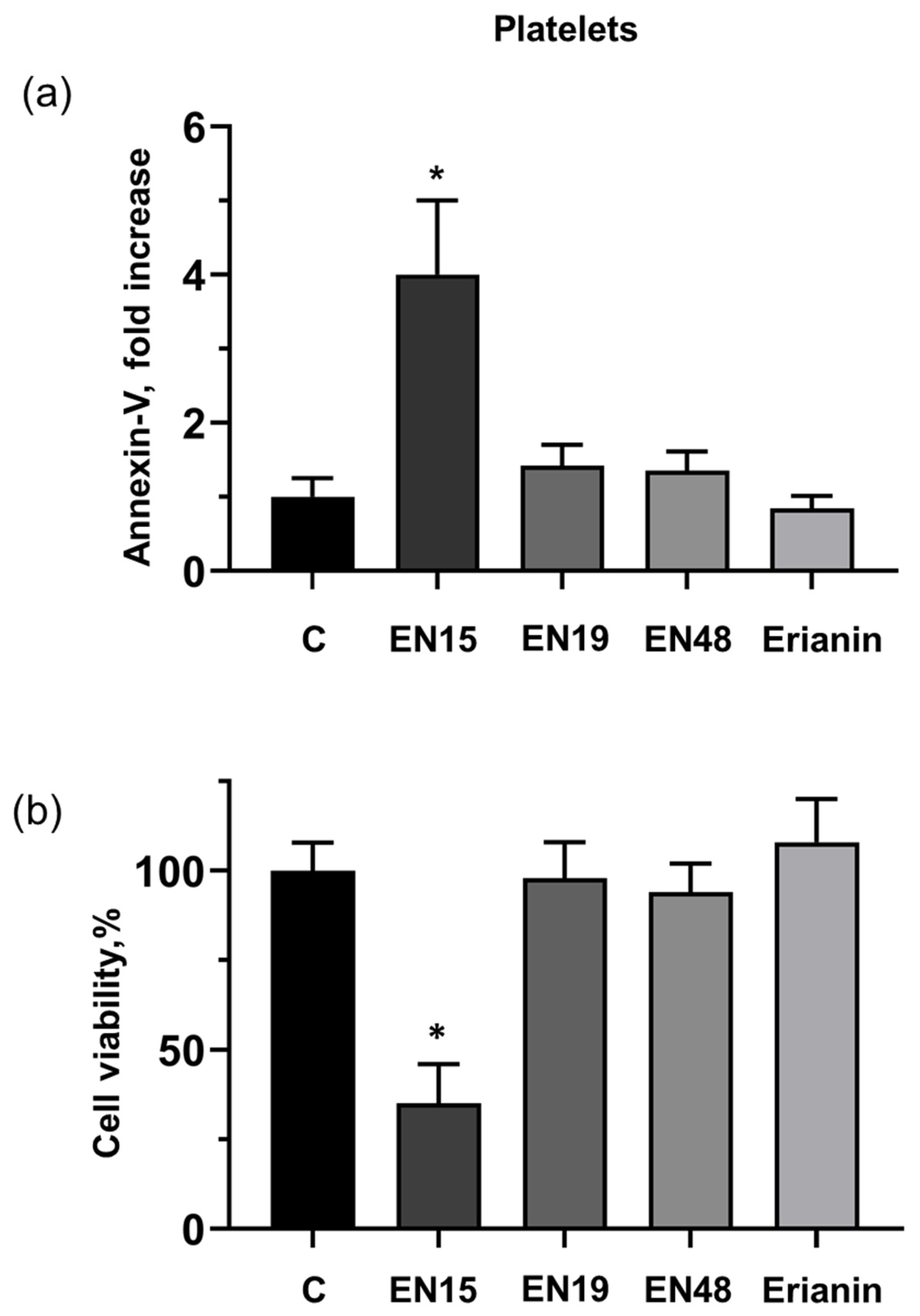
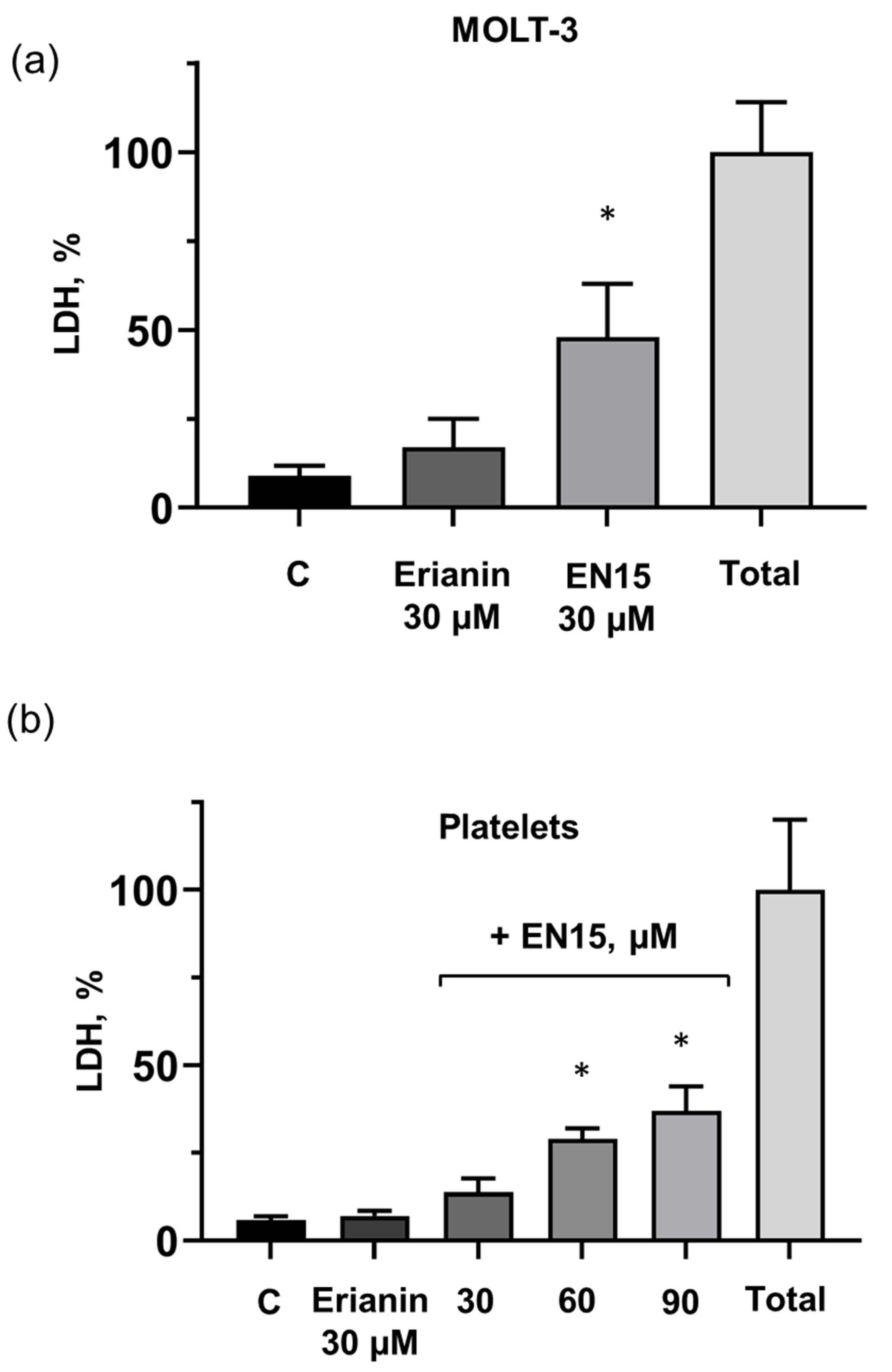
2.1. Effect of Bibenzyls on Phosphatidylserine Surface Exposure and Viability of MOLT-3 Cells and Platelets
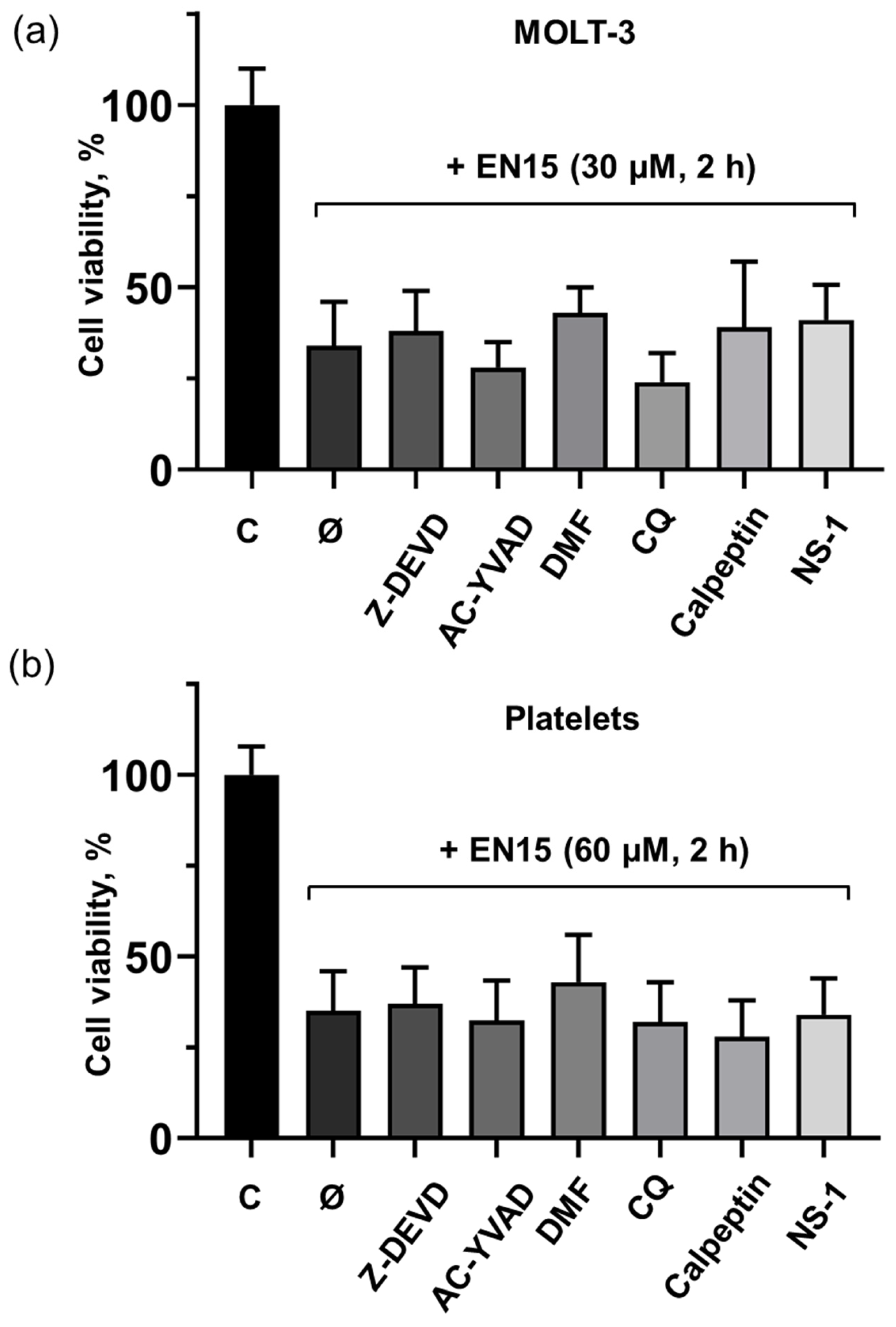
2.2. EN15-Induced Death of MOLT-3 Cells and Platelets Is Not Mediated by Apoptosis, Pyroptosis, Necroptosis, or Autophagy Pathways
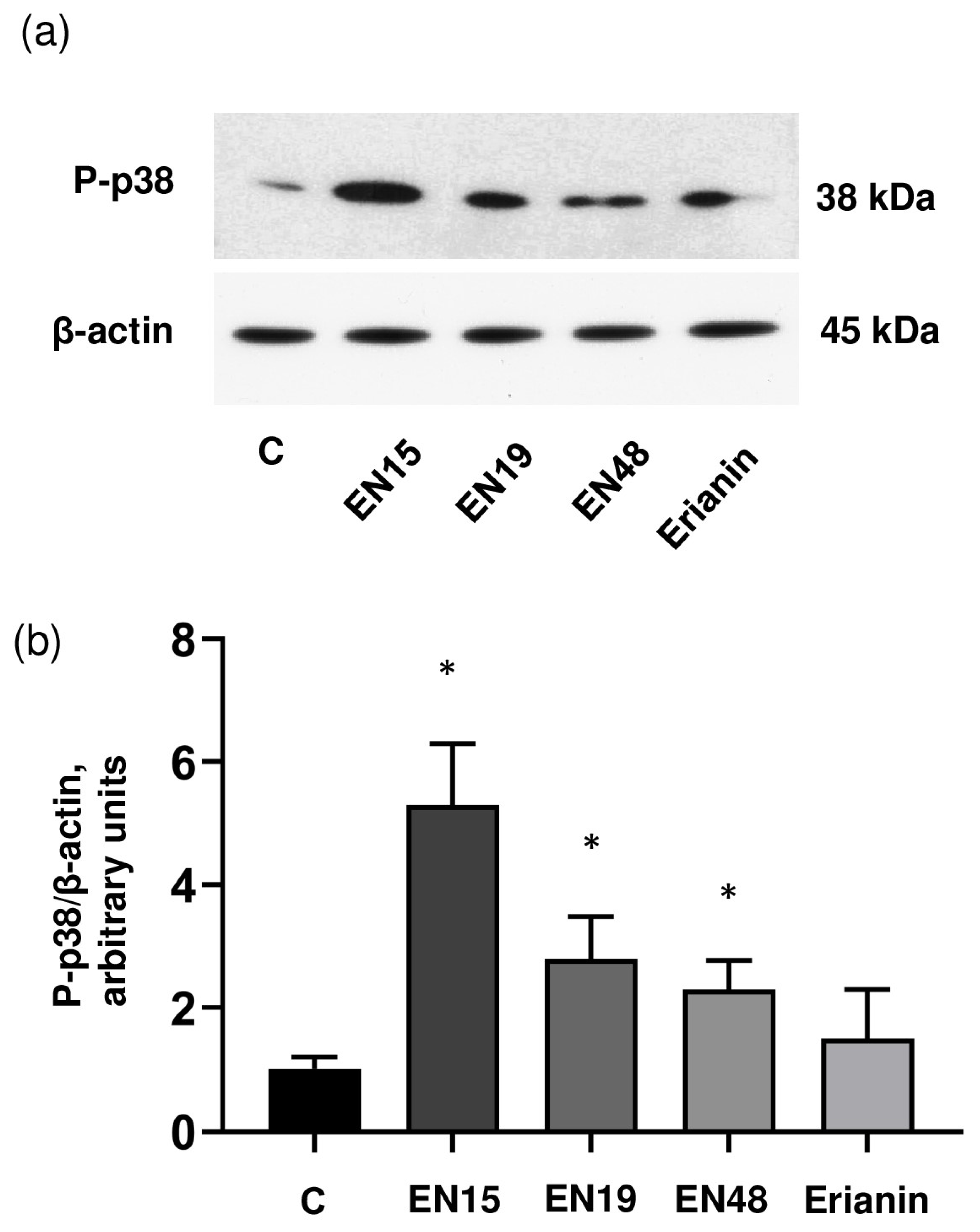
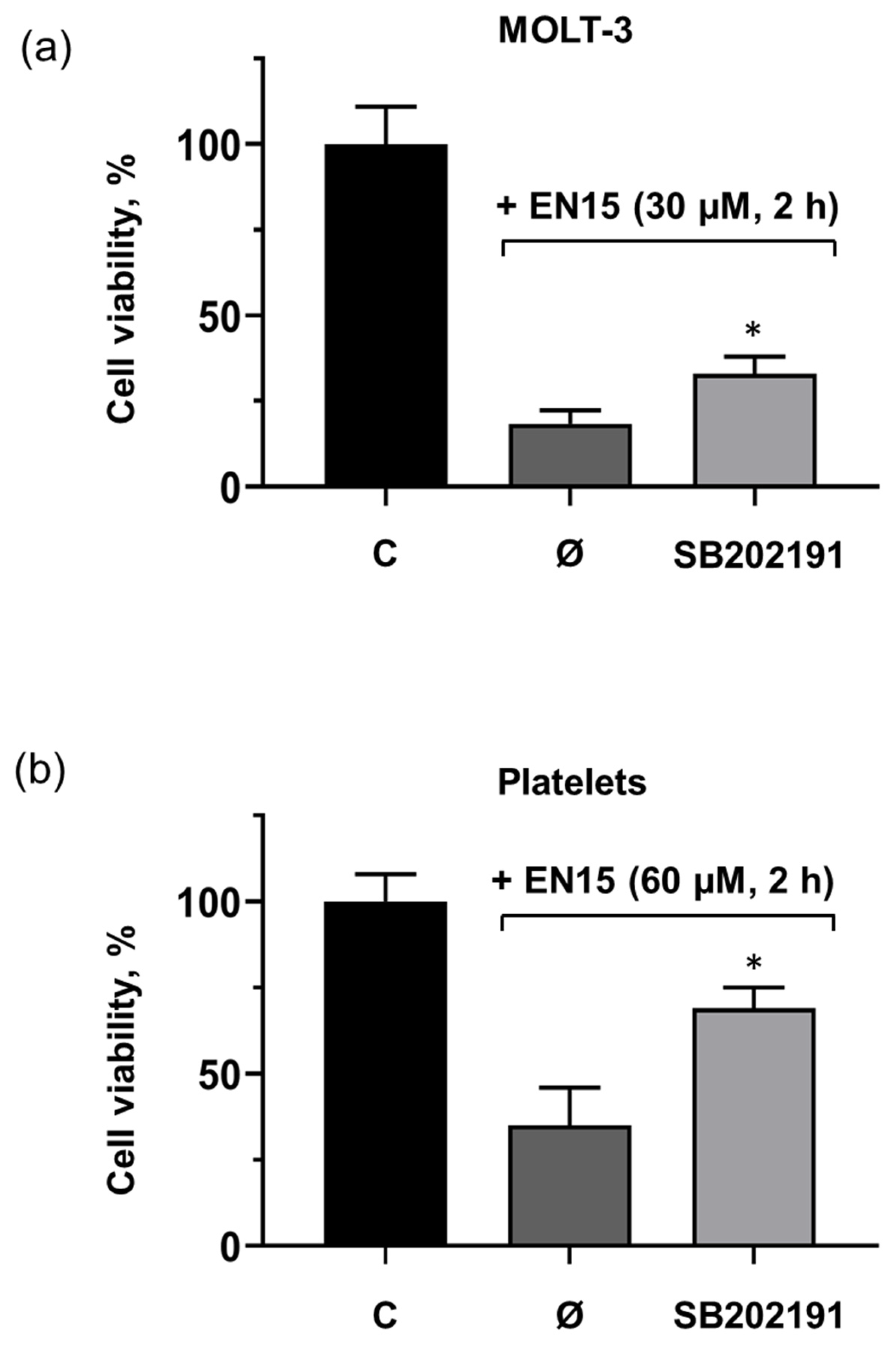
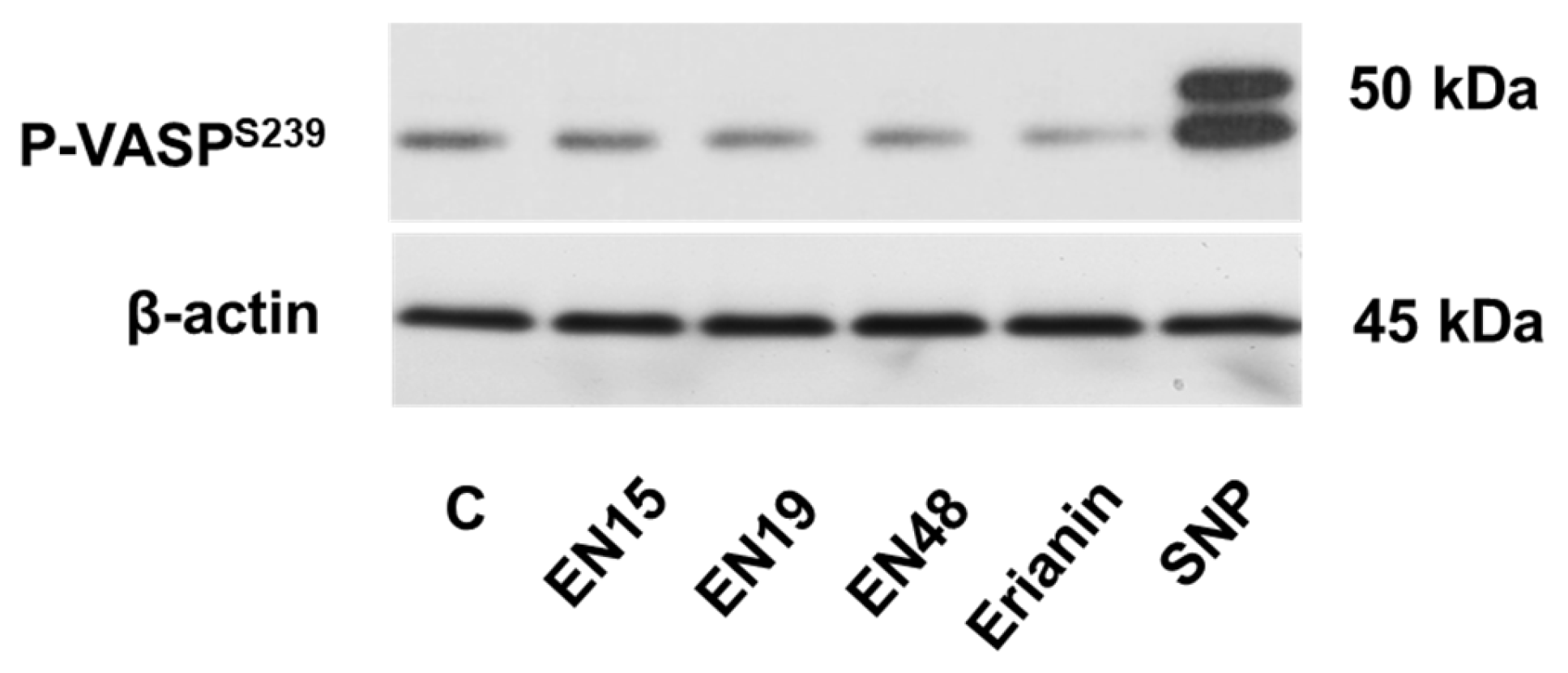
2.3. EN15 Induces Cells Death by Activation of p38 MAP Kinase Pathway
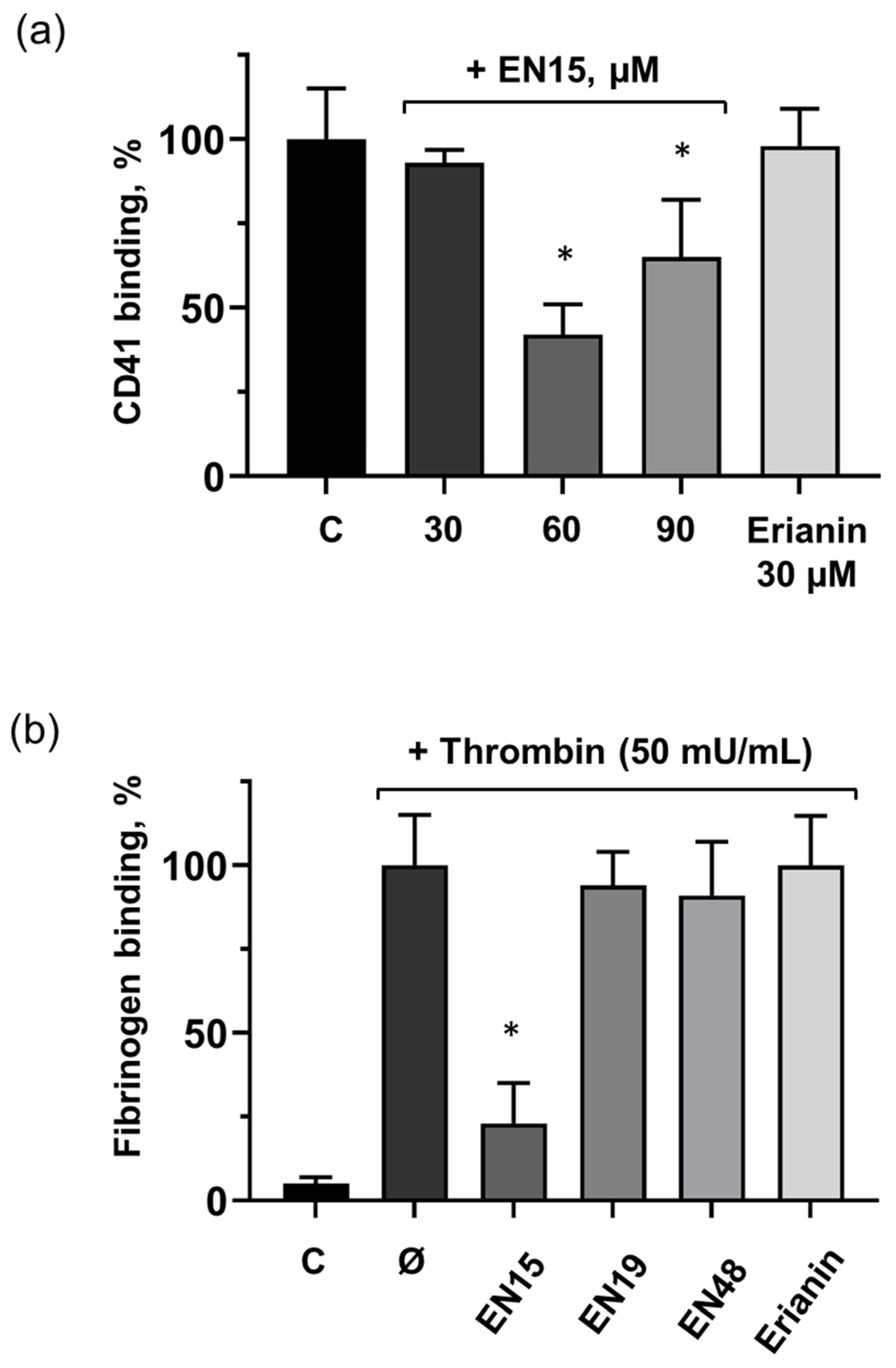
2.4. EN15 Inhibits Thrombin-Induced Platelet Activation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Human Platelet Preparation
4.3. LDH Assay
4.4. MTT Assay
4.5. Flow Cytometry Analysis of Platelets
4.6. Platelet αIIbβ3 Integrin Activation, Phosphatidylserine Surface Exposure, Cell Viability
4.7. MOLT-3 Cell Phosphatidylserine Surface Exposure
4.8. Western Blot Analysis
4.9. Data Analysis
4.10. Bibenzyl Isolation and Structural Elucidation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; McIntyre, E.; Camfield, D.A. Plant-based medicines for anxiety disorders, part 2: A review of clinical studies with supporting preclinical evidence. CNS Drugs 2013, 27, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Lamponi, S. Bioactive Natural Compounds with Antiplatelet and Anticoagulant Activity and Their Potential Role in the Treatment of Thrombotic Disorders. Life 2021, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Stewart, L.E.; Darley, B.A.; Pham, A.M.; Esteban, I.; Panda, S.S. Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates. Molecules 2021, 26, 6197. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; He, Q.; Yang, X. Exploration of the Use of Natural Compounds in Combination with Chemotherapy Drugs for Tumor Treatment. Molecules 2023, 28, 1022. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, D.; Shri, R.; Kumar, S. Mechanistic Insights and Docking Studies of Phytomolecules as Potential Candidates in the Management of Cancer. Curr. Pharm. Des. 2022, 28, 2704–2724. [Google Scholar] [CrossRef]
- He, L.; Su, Q.; Bai, L.; Li, M.; Liu, J.; Liu, X.; Zhang, C.; Jiang, Z.; He, J.; Shi, J.; et al. Recent research progress on natural small molecule bibenzyls and its derivatives in Dendrobium species. Eur. J. Med. Chem. 2020, 204, 112530. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Wei, F.; Liu, N. Progressive study of effects of erianin on anticancer activity. Onco Targets Ther. 2019, 12, 5457–5465. [Google Scholar] [CrossRef]
- Sun, Y.; Li, G.; Zhou, Q.; Shao, D.; Lv, J.; Zhou, J. Dual Targeting of Cell Growth and Phagocytosis by Erianin for Human Colorectal Cancer. Drug Des. Dev. Ther. 2020, 14, 3301–3313. [Google Scholar] [CrossRef]
- Chen, Y.T.; Hsieh, M.J.; Chen, P.N.; Weng, C.J.; Yang, S.F.; Lin, C.W. Erianin Induces Apoptosis and Autophagy in Oral Squamous Cell Carcinoma Cells. Am. J. Chin. Med. 2020, 48, 183–200. [Google Scholar] [CrossRef]
- Wang, T.F.; Carrier, M.; Carney, B.J.; Kimpton, M.; Delluc, A. Anticoagulation management and related outcomes in patients with cancer-associated thrombosis and thrombocytopenia: A systematic review and meta-analysis. Thromb. Res. 2023, 227, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, T. Cancer-associated thrombosis and bleeding. Int. J. Hematol. 2024, 119, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Arellano, M.L.; Borthakur, G.; Berger, M.; Luer, J.; Raza, A. A phase II, multicenter, open-label study of obatoclax mesylate in patients with previously untreated myelodysplastic syndromes with anemia or thrombocytopenia. Clin. Lymphoma Myeloma Leuk. 2014, 14, 534–539. [Google Scholar] [CrossRef]
- Kaefer, A.; Yang, J.; Noertersheuser, P.; Mensing, S.; Humerickhouse, R.; Awni, W.; Xiong, H. Mechanism-based pharmacokinetic/pharmacodynamic meta-analysis of navitoclax (ABT-263) induced thrombocytopenia. Cancer Chemother. Pharmacol. 2014, 74, 593–602. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Agerbaek, M.O.; Gogenur, I. The impact of platelets on the metastatic potential of tumour cells. Heliyon 2024, 10, e34361. [Google Scholar] [CrossRef]
- Chen, C.C.; Wu, L.G.; Ko, F.N.; Teng, C.M. Antiplatelet aggregation principles of Dendrobium loddigesii. J. Nat. Prod. 1994, 57, 1271–1274. [Google Scholar] [CrossRef]
- Fan, C.; Wang, W.; Wang, Y.; Qin, G.; Zhao, W. Chemical constituents from Dendrobium densiflorum. Phytochemistry 2001, 57, 1255–1258. [Google Scholar] [CrossRef]
- Hu, J.M.; Chen, J.J.; Yu, H.; Zhao, Y.X.; Zhou, J. Two novel bibenzyls from Dendrobium trigonopus. J. Asian Nat. Prod. Res. 2008, 10, 653–657. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Zhang, S.; Wong, C.E.; Liang, Q.; Pang, S.; Wu, Y.; Zhao, M.; Yu, H. Pangeneric genome analyses reveal the evolution and diversity of the orchid genus Dendrobium. Nat. Plants 2025, 11, 421–437. [Google Scholar] [CrossRef]
- Bezverkhniaia, E.A.; Ermilova, E.V.; Kadyrova, T.V.; Krasnov, E.A.; Brazovskii, K.S.; Ponkratova, A.O.; Luzhanin, V.G.; Belousov, M.V. Phytochemistry, ethnopharmacology and pharmacology of the genus Empetrum: A review. Adv. Tradit. Med. 2023, 23, 659–672. [Google Scholar] [CrossRef]
- Ponkratova, A.O.; Whaley, A.K.; Balabas, O.A.; Smirnov, S.N.; Proksch, P.; Luzhanin, V.G. A new bibenzyl and 9,10-dihydrophenanthrene derivative from aerial parts of crowberry (Empetrum nigrum L.). Phytochem. Lett. 2021, 42, 15–17. [Google Scholar] [CrossRef]
- Whaley, A.K.; Minakov, D.A.; Orlova, A.A.; Ponkratova, A.O.; Fock, E.; Rukoyatkina, N.; Gambaryan, S.; Luzhanin, V.G. Analysis of Empetrum nigrum L. lipophilic secondary metabolites, their metabolomic profiles and antioxidant activity. J. Essent. Oil Res. 2023, 35, 310–323. [Google Scholar] [CrossRef]
- Fock, E.; Pronin, N.; Rukoyatkina, N.; Whaley, A.; Gambaryan, S.; Whaley, A. Thrombin-induced platelet inhibition by 2′,4′-dihydroxychalcone and 2′,4′-dihydroxydihydrochalcone is mediated by inhibition of ROS production and Thromboxane synthase a activity: Structure-activity relationship of polyphenol secondary metabolites from Empetrum nigrum. Nat. Prod. Res. 2025, 39, 3382–3390. [Google Scholar]
- Aljeldah, M.M. Evaluation of the anticancer and antibacterial activities of moscatilin. Heliyon 2024, 10, e31131. [Google Scholar] [CrossRef]
- Yu, W.; Li, B.; Chen, L.; Chen, Q.; Song, Q.; Jin, X.; Yin, Y.; Tong, H.; Xue, L. Gigantol ameliorates DSS-induced colitis via suppressing beta2 integrin mediated adhesion and chemotaxis of macrophage. J. Ethnopharmacol. 2024, 328, 118123. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Q.; Feng, J.; Yan, L.; Sun, Y.; Liu, S.; Xiang, Y.; Zhang, M.; Pan, T.; Chen, X.; et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Target. Ther. 2020, 5, 51. [Google Scholar] [CrossRef]
- Kudaravalli, S.; den Hollander, P.; Mani, S.A. Role of p38 MAP kinase in cancer stem cells and metastasis. Oncogene 2022, 41, 3177–3185. [Google Scholar] [CrossRef]
- Sarg, N.H.; Zaher, D.M.; Abu Jayab, N.N.; Mostafa, S.H.; Ismail, H.H.; Omar, H.A. The interplay of p38 MAPK signaling and mitochondrial metabolism, a dynamic target in cancer and pathological contexts. Biochem. Pharmacol. 2024, 225, 116307. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Mindukshev, I.; Walter, U.; Gambaryan, S. Dual role of the p38 MAPK/cPLA2 pathway in the regulation of platelet apoptosis induced by ABT-737 and strong platelet agonists. Cell Death Dis. 2013, 4, e931. [Google Scholar] [CrossRef]
- Smolenski, A. Novel roles of cAMP/cGMP-dependent signaling in platelets. J. Thromb. Haemost. 2012, 10, 167–176. [Google Scholar] [CrossRef]
- Laurent, P.A.; Severin, S.; Hechler, B.; Vanhaesebroeck, B.; Payrastre, B.; Gratacap, M.P. Platelet PI3Kbeta and GSK3 regulate thrombus stability at a high shear rate. Blood 2015, 125, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.A.; Hechler, B.; Solinhac, R.; Ragab, A.; Cabou, C.; Anquetil, T.; Severin, S.; Denis, C.V.; Mangin, P.H.; Vanhaesebroeck, B.; et al. Impact of PI3Kalpha (Phosphoinositide 3-Kinase Alpha) Inhibition on Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kang, S.; Deng, X.; Li, H.; Zhao, Y.; Tang, W.; Sheng, M. Erianin inhibits the progression of triple-negative breast cancer by suppressing SRC-mediated cholesterol metabolism. Cancer Cell Int. 2024, 24, 166. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, Q.; Liu, L.; Wang, D.; Liu, J.; Zhu, Q.; Xu, Z.; Chen, Q.; Xu, W. Discovery of the Natural Bibenzyl Compound Erianin in Dendrobium Inhibiting the Growth and EMT of Gastric Cancer through Downregulating the LKB1-SIK2/3-PARD3 Pathway. Int. J. Mol. Sci. 2024, 25, 7973. [Google Scholar] [CrossRef]
- Hong, J.; Xie, Z.; Yang, F.; Jiang, L.; Jian, T.; Wang, S.; Guo, Y.; Huang, X. Erianin suppresses proliferation and migration of cancer cells in a pyruvate carboxylase-dependent manner. Fitoterapia 2022, 157, 105136. [Google Scholar] [CrossRef]
- Yong, H.Y.; Koh, M.S.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef]
- Hanson, S.; Dharan, A.; P V, J.; Pal, S.; Nair, B.G.; Kar, R.; Mishra, N. Paraptosis: A unique cell death mode for targeting cancer. Front. Pharmacol. 2023, 14, 1159409. [Google Scholar] [CrossRef]
- Majumder, P.L.; Guha, S.; Sen, S. Bibenzyl derivatives from the orchid Dendrobium amoenum. Phytochemistry 1999, 52, 1365–1369. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rukoyatkina, N.; Sokolova, T.; Pronin, N.; Whaley, A.; Whaley, A.O.; Gambaryan, S. Death of Leukemia Cells and Platelets Induced by 3,3′-Dihydroxy-4,5-Dimethoxybibenzyl Is Mediated by p38 Mitogen-Activated Protein Kinase Pathway. Molecules 2025, 30, 2965. https://doi.org/10.3390/molecules30142965
Rukoyatkina N, Sokolova T, Pronin N, Whaley A, Whaley AO, Gambaryan S. Death of Leukemia Cells and Platelets Induced by 3,3′-Dihydroxy-4,5-Dimethoxybibenzyl Is Mediated by p38 Mitogen-Activated Protein Kinase Pathway. Molecules. 2025; 30(14):2965. https://doi.org/10.3390/molecules30142965
Chicago/Turabian StyleRukoyatkina, Natalia, Tatyana Sokolova, Nikita Pronin, Andrei Whaley, Anastasiia O. Whaley, and Stepan Gambaryan. 2025. "Death of Leukemia Cells and Platelets Induced by 3,3′-Dihydroxy-4,5-Dimethoxybibenzyl Is Mediated by p38 Mitogen-Activated Protein Kinase Pathway" Molecules 30, no. 14: 2965. https://doi.org/10.3390/molecules30142965
APA StyleRukoyatkina, N., Sokolova, T., Pronin, N., Whaley, A., Whaley, A. O., & Gambaryan, S. (2025). Death of Leukemia Cells and Platelets Induced by 3,3′-Dihydroxy-4,5-Dimethoxybibenzyl Is Mediated by p38 Mitogen-Activated Protein Kinase Pathway. Molecules, 30(14), 2965. https://doi.org/10.3390/molecules30142965






