Comparative Phytochemical Analysis and Antimicrobial Properties of Ethanol and Macerated Extracts from Aerial and Root Parts of Achillea nobilis
Abstract
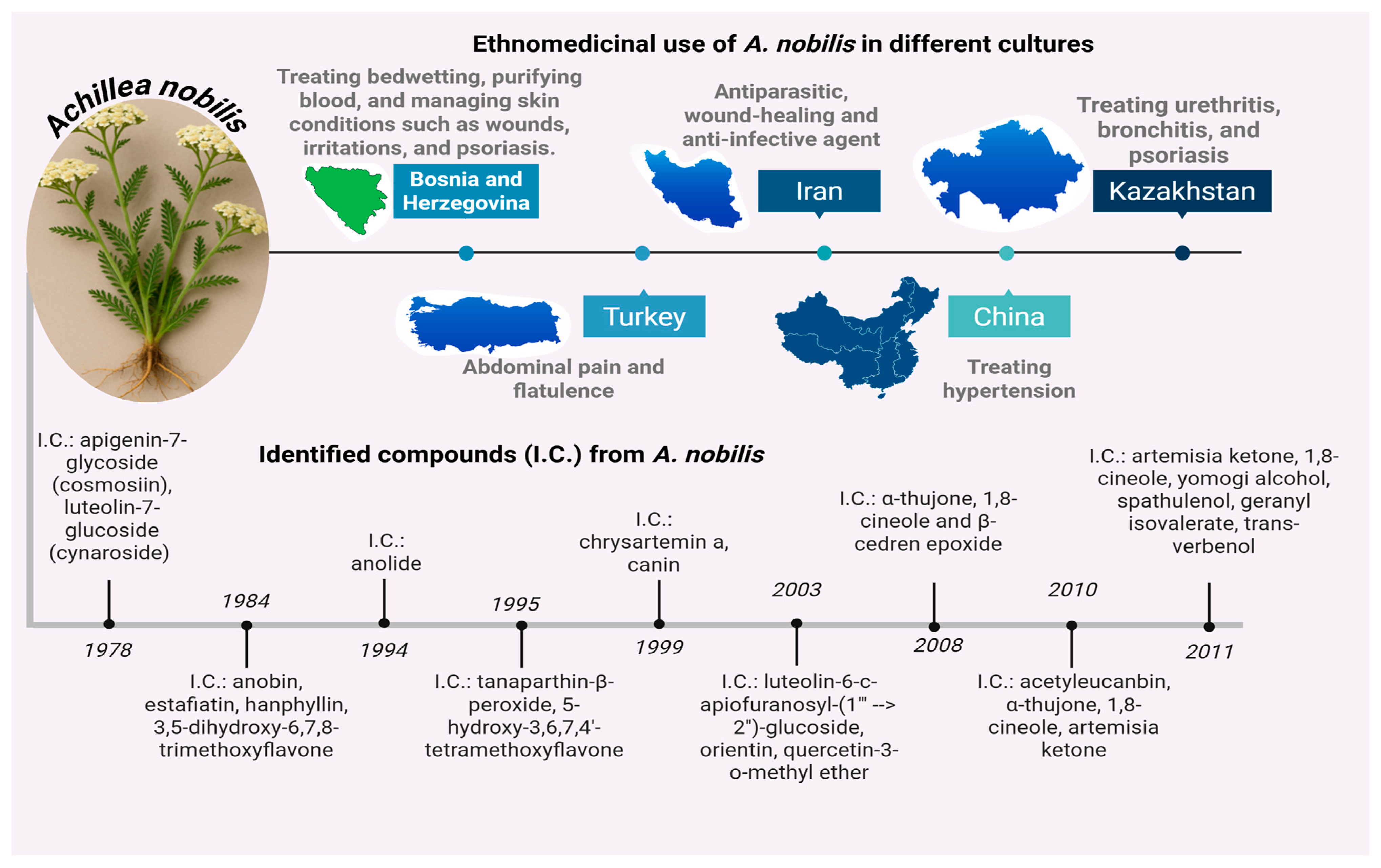
1. Introduction
2. Results
2.1. Screening for Bioactive Phytochemical Classes
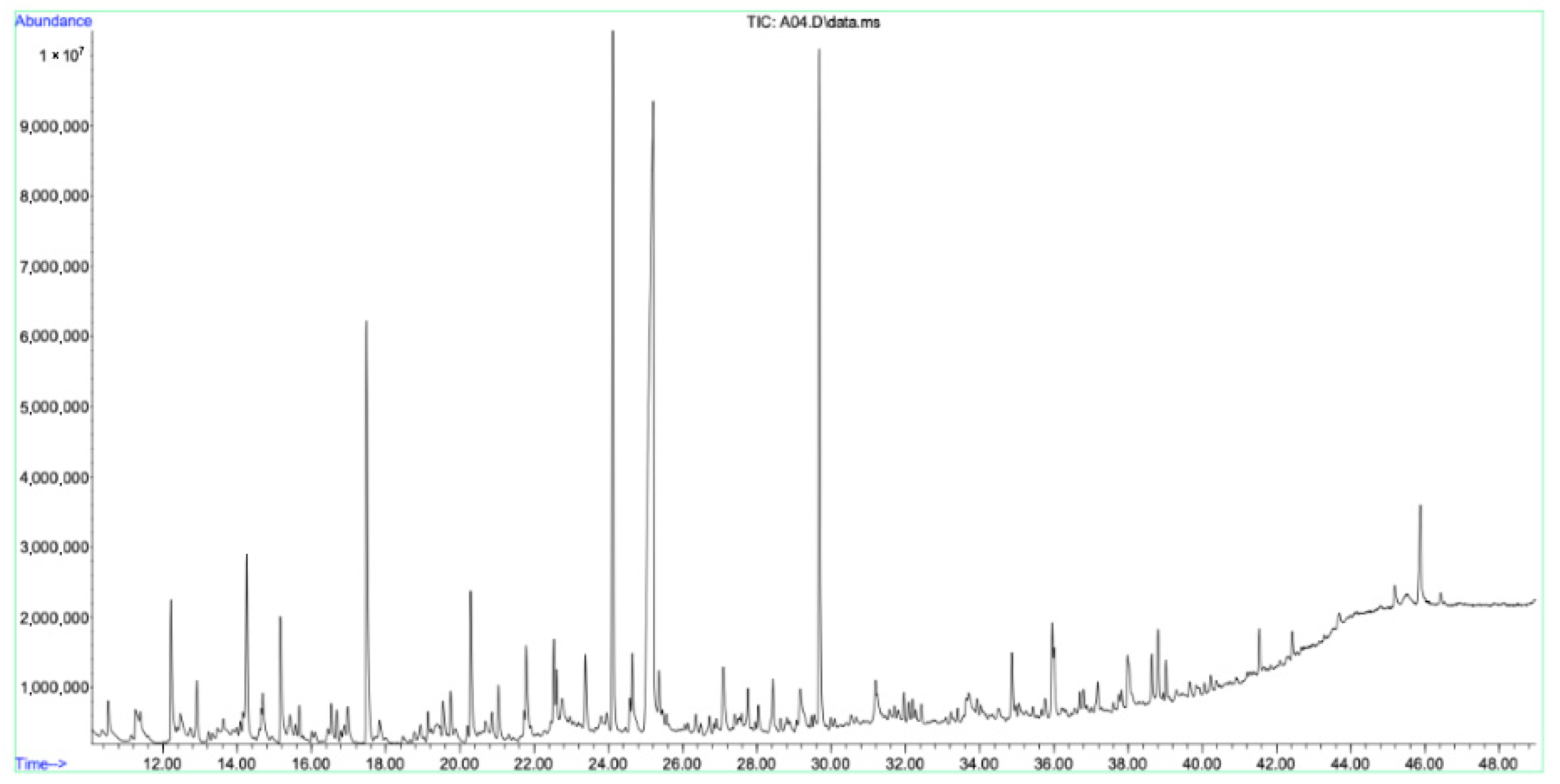
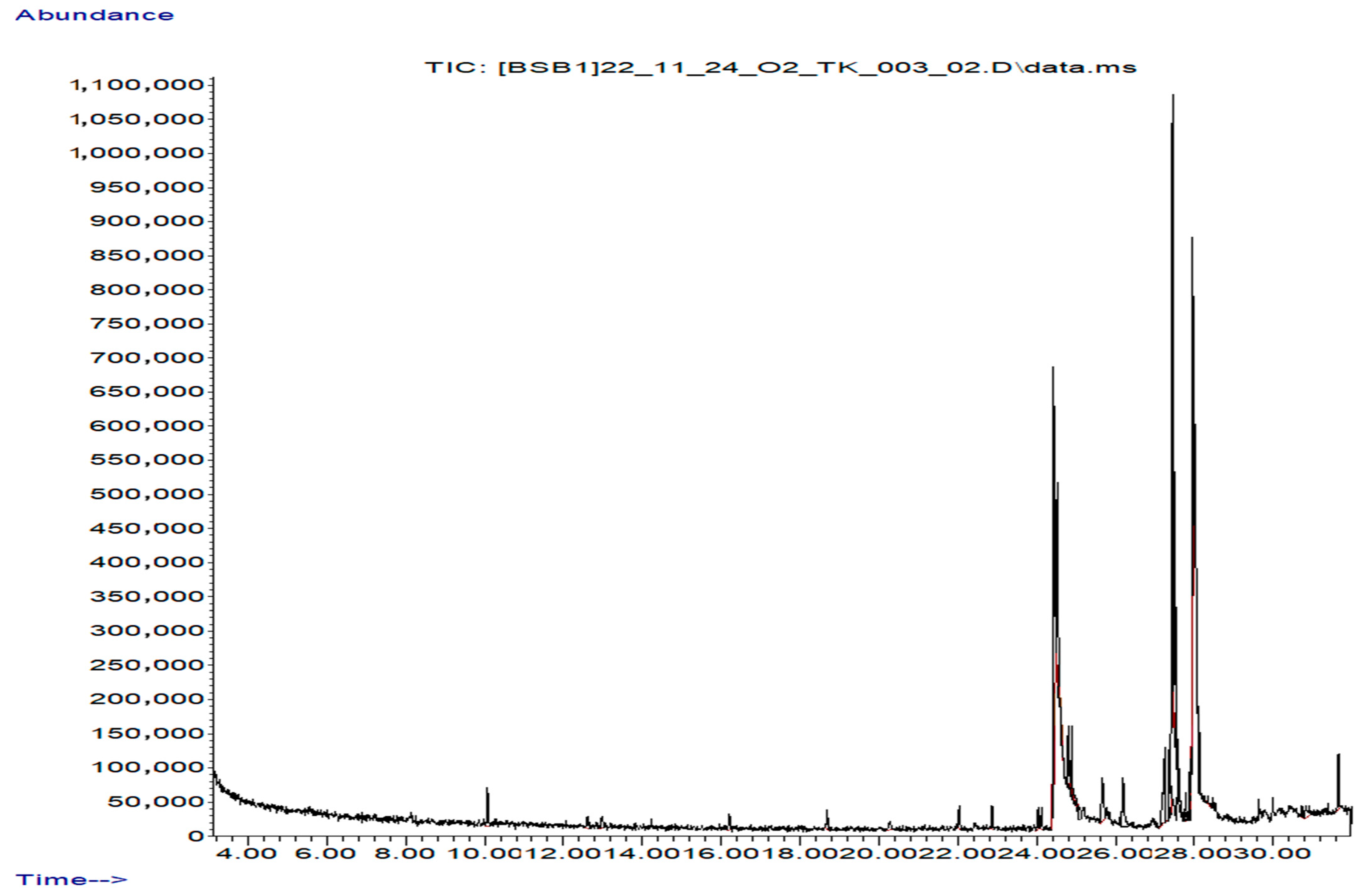
2.2. Secondary Metabolite Analysis (GC–MS)
2.3. Determination of Antimicrobial Potency
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Identification
4.2. Chemicals and Reagents
4.3. Vortex-Assisted Extraction Method (VAM)
4.4. Oil-Based Maceration Extraction
4.5. Preliminary Qualitative Analysis
4.6. GC–MS Profiling
4.7. Antimicrobial Activity
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shevchenko, A.; Akhelova, A.; Nokerbek, S.; Kaldybayeva, A.; Sagyndykova, L.; Raganina, K.; Dossymbekova, R.; Meldebekova, A.; Amirkhanova, A.; Ikhsanov, Y.; et al. Phytochemistry, Pharmacological Potential, and Ethnomedicinal Relevance of Achillea nobilis and Its Subspecies: A Comprehensive Review. Molecules 2025, 30, 2460. [Google Scholar] [CrossRef] [PubMed]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Kukula-Koch, W. Achillea species as sources of active phytochemicals for dermatological and cosmetic applications. Oxid. Med. Cell. Longev. 2021, 2021, 6643827. [Google Scholar] [CrossRef]
- USDA; NRCS. The PLANTS Database; National Plant Data Team: Greensboro, NC, USA, 2025. Available online: http://plants.usda.gov (accessed on 17 April 2025).
- Barda, C.; Grafakou, M.-E.; Tomou, E.-M.; Skaltsa, H. Phytochemistry and Evidence-Based Traditional Uses of the Genus Achillea L.: An Update (2011–2021). Sci. Pharm. 2021, 89, 50. [Google Scholar] [CrossRef]
- Solomko, O.V.; Dzhumyrko, S.F.; Kompantsev, V.A. Flavonoids of Achillea nobilis. Chem. Nat. Compd. 1978, 14, 224. [Google Scholar] [CrossRef]
- Adekenov, S.M.; Mukhametzhanov, M.N.; Kagarlitskii, A.D.; Turmukhambetov, A.Z. A chemical investigation of Achillea nobilis. Chem. Nat. Compd. 1984, 20, 568–571. [Google Scholar] [CrossRef]
- Soliman, G.A.; Abood, S.H.; Hassen, H.F.; El-Kased, R.F. The Potential Anticonvulsant Activity of the Ethanolic Extracts of Achillea nobilis and Momordica charantia in Rats. J. Pharm. Pharmacogn. Res. 2016, 4, 107–114. [Google Scholar] [CrossRef]
- Karabay-Yavaşoğlu, N.Ü.; Karamenderes, C.; Baykan, S.; Apaydın, S. Antinociceptive and Anti-Inflammatory Activities and Acute Toxicity of Achillea nobilis subsp. neilreichii Extract in Mice and Rats. Pharm. Biol. 2007, 45, 162–168. [Google Scholar] [CrossRef]
- Karamenderes, C.; Apaydın, S. Antispasmodic Effect of Achillea nobilis L. subsp. sipylea (O. Schwarz) Bässler on the Rat Isolated Duodenum. J. Ethnopharmacol. 2003, 84, 175–179. [Google Scholar] [CrossRef]
- Konyalıoğlu, S.; Karamenderes, C. The Protective Effects of Achillea L. Species Native in Turkey against H2O2-Induced Oxidative Damage in Human Erythrocytes and Leucocytes. J. Ethnopharmacol. 2005, 102, 221–227. [Google Scholar] [CrossRef]
- Trudybekov, K.M.; Turmukhambetov, A.Z.; Adekenov, S.M.; Struchkov, Y.T. Anolide—A new guaianolide from Achillea nobilis. Chem. Nat. Compd. 1994, 30, 460–463. [Google Scholar] [CrossRef]
- Kastner, U.; Breuer, J.; Glasl, S.; Baumann, A.; Robien, W.; Jurenitsch, J.; Kubelka, W. Guaianolide-Endoperoxide and Monoterpene-Hydroperoxides from Achillea nobilis. Planta Medica 1995, 61, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Turmukhambetov, A.Z.; Buketova, G.K.; Gafurov, N.M.; Adekenov, S.M. Chrysartemin A and canin from Achillea nobilis. Chem. Nat. Compd. 1999, 35, 102. [Google Scholar] [CrossRef]
- Krenn, L.; Miron, A.; Pemp, E.; Petr, U.; Kopp, B. Flavonoids from Achillea nobilis L. Z. Naturforsch. C J. Biosci. 2003, 58, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Palić, R.; Stojanović, G.; Nasković, T.; Ranelović, N. Composition and Antibacterial Activity of Achillea crithmifolia and Achillea nobilis Essential Oils. J. Essent. Oil Res. 2003, 15, 434–437. [Google Scholar] [CrossRef]
- Serkerov, S.V.; Mustafaeva, S.J. Detection of acetyleucanbin in Achillea nobilis. Chem. Nat. Compd. 2010, 46, 666. [Google Scholar] [CrossRef]
- Azizi, M.; Chizzola, R.; Ghani, A.; Oroojalian, F. Composition at different development stages of the essential oil of four Achillea species grown in Iran. Nat. Prod. Commun. 2010, 5, 283–290. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Masoudi, S.; Ezatpour, L.; Danaii, E.; Taherkhani, M.; Aghajani, Z. Composition of the essential oils of Anthemis hyalina DC., Achillea nobilis L., and Cichorium intybus L.—Three Asteraceae herbs growing wild in Iran. J. Essent. Oil Bear. Plants 2011, 14, 472–480. [Google Scholar] [CrossRef]
- Karaalp, C.; Yurtman, A.N.; Karabay Yavasoglu, N.U. Evaluation of antimicrobial properties of Achillea L. flower head extracts. Pharm. Biol. 2009, 47, 86–91. [Google Scholar] [CrossRef]
- Taşkın, D.; Taşkın, T.; Rayaman, E. Phenolic composition and biological properties of Achillea nobilis L. subsp. neilreichii (Kerner) Formanek. Ind. Crops Prod. 2018, 111, 555–562. [Google Scholar] [CrossRef]
- Ozdemir, F.A. Potential effects of essential oil compositions on antibacterial activities of Achillea nobilis L. subsp. neilreichii. J. Essent. Oil Bear. Plants 2019, 22, 574–580. [Google Scholar] [CrossRef]
- Moradi, M.T.; Rafieian-Koupaei, M.; Imani-Rastabi, R.; Nasiri, J.; Shahrani, M.; Rabiei, Z.; Alibabaei, Z. Antispasmodic effects of yarrow (Achillea millefolium L.) extract in the isolated ileum of rat. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Luo, Q.; Cui, H.; Deng, H.; Kuang, P.; Liu, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Sodium fluoride causes oxidative stress and apoptosis in the mouse liver. Aging 2017, 9, 1623–1639. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial production of 2,3-butanediol for industrial applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z. Recent Advances on Production of 2,3-Butanediol Using Engineered Microbes. Biotechnol. Adv. 2019, 37, 569–578. [Google Scholar] [CrossRef]
- Akdemir, H.; Liu, Y.; Zhuang, L.; Zhang, H.; Koffas, M.A. Utilization of Microbial Cocultures for Converting Mixed Substrates to Valuable Bioproducts. Curr. Opin. Microbiol. 2022, 68, 102157. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Glycerin as Used in Cosmetics. Int. J. Toxicol. 2019, 38 (Suppl. S3), 6S–22S. [Google Scholar] [CrossRef]
- Goldman, E.; Choueiri, T.K.; Mainelis, G.; Ramachandran, G.; Schaffner, D.W. Triethylene Glycol Can Be Predeployed as a Safe Virus-Killing Indoor Air Treatment. J. Infect. Dis. 2022, 226, 2040–2041. [Google Scholar] [CrossRef]
- Lee, S.J.; Moon, T.W.; Lee, J. Increases of 2-Furanmethanol and Maltol in Korean Red Ginseng during Explosive Puffing Process. J. Food Sci. 2010, 75, C147–C151. [Google Scholar] [CrossRef]
- Dhall, H.; Sikka, P.; Kumar, A.; Mishra, A.K. Recent Advancements and Biological Activities of Aryl Propionic Acid Derivatives: (A Review). Orient. J. Chem. 2016, 32, 1831–1838. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Liang, W.; Wong, C.C.; Qin, H.; Gao, Y.; Liang, M.; Song, L.; Zhang, Y.; Fan, M.; et al. Fusobacterium nucleatum Facilitates Anti-PD-1 Therapy in Microsatellite Stable Colorectal Cancer. Cancer Cell 2024, 42, 1729–1746.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Xi, T.F.; Zheng, Y.F. Surface Modification by Natural Biopolymer Coatings on Magnesium Alloys for Biomedical Applications. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 301–333. [Google Scholar]
- Martiryan, A.I.; Galstyan, A.S.; Tadevosyan, L.G.; Petrosyan, I.A. Synthesis of γ-Hydroxy Acid Hydrazides of a New Structure and Study of Their Antioxidant Properties. Proc. YSU B Chem. Biol. Sci. 2020, 54, 188–195. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.; Zhang, Q.; Qin, Y.; Li, K.; Xie, Y.; Zhang, T.; Wang, X.; Yang, X.; Zhang, L.; et al. Linoleic Acid Promotes Mitochondrial Biogenesis and Alleviates Acute Lung Injury. Clin. Respir. J. 2024, 18, e70004. [Google Scholar] [CrossRef] [PubMed]
- Nava Lauson, C.B.; Tiberti, S.; Corsetto, P.A.; Conte, F.; Tyagi, P.; Machwirth, M.; Ebert, S.; Loffreda, A.; Scheller, L.; Sheta, D.; et al. Linoleic Acid Potentiates CD8+ T Cell Metabolic Fitness and Antitumor Immunity. Cell Metab. 2023, 35, 633–650.e9. [Google Scholar] [CrossRef]
- Okumu, A.; Lu, Y.; Dellos-Nolan, S.; Papa, J.L.; Koci, B.; Cockroft, N.T.; Gallucci, J.; Wozniak, D.J.; Yalowich, J.C.; Mitton-Fry, M.J. Novel Bacterial Topoisomerase Inhibitors Derived from Isomannide. Eur. J. Med. Chem. 2020, 199, 112324. [Google Scholar] [CrossRef]
- Pineda Molina, C.; Hussey, G.S.; Eriksson, J.; Shulock, M.A.; Cárdenas Bonilla, L.L.; Giglio, R.M.; Gandhi, R.M.; Sicari, B.M.; Wang, D.; Londono, R.; et al. 4-Hydroxybutyrate Promotes Endogenous Antimicrobial Peptide Expression in Macrophages. Tissue Eng. Part A 2019, 25, 693–706. [Google Scholar] [CrossRef]
- Molina, C.P.; Hussey, G.S.; Liu, A.; Eriksson, J.; D’Angelo, W.A.; Badylak, S.F. Role of 4-Hydroxybutyrate in Increased Resistance to Surgical Site Infections Associated with Surgical Meshes. Biomaterials 2021, 267, 120493. [Google Scholar] [CrossRef]
- Jarzyński, S.; Rapacz, A.; Dziubina, A.; Pękala, E.; Popiół, J.; Piska, K.; Wojtulewski, S.; Rudolf, B. Mechanochemical Synthesis and Anticonvulsant Activity of 3-Aminopyrrolidine-2,5-dione Derivatives. Biomed. Pharmacother. 2023, 168, 115749. [Google Scholar] [CrossRef]
- Mokhtari, M.; Jackson, M.D.; Brown, A.S.; Ackerley, D.F.; Ritson, N.J.; Keyzers, R.A.; Munkacsi, A.B. Bioactivity-Guided Metabolite Profiling of Feijoa (Acca sellowiana) Cultivars Identifies 4-Cyclopentene-1,3-dione as a Potent Antifungal Inhibitor of Chitin Synthesis. J. Agric. Food Chem. 2018, 66, 5531–5539. [Google Scholar] [CrossRef]
- Poma, P.; Rigogliuso, S.; Labbozzetta, M.; Carfì Pavia, F.; Carbone, C.; Ma, J.; Cusimano, A.; Notarbartolo, M. Antimigratory Effects of a New NF-κB Inhibitor, (S)-β-Salicyloylamino-α-exo-methylene-γ-butyrolactone, in 2D and 3D Breast Cancer Models. Biomed. Pharmacother. 2024, 180, 117552. [Google Scholar] [CrossRef]
- Premkumar, J.; Sampath, P.; Sanjay, R.; Chandrakala, A.; Rajagopal, D. Synthetic Guaiacol Derivatives as Promising Myeloperoxidase Inhibitors Targeting Atherosclerotic Cardiovascular Disease. ChemMedChem 2020, 15, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhang, Y.; Shi, J.; Mohamed, S.R.; Xu, J.; Liu, X. The Antioxidant Guaiacol Exerts Fungicidal Activity against Fungal Growth and Deoxynivalenol Production in Fusarium graminearum. Front. Microbiol. 2021, 12, 762844. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Ozasa, N.; Ueda, Y.; Takagi, N. Pharmacological Studies on the Anti-Inflammatory Action of Phenolic Compounds. J. Dent. Res. 1986, 65, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Morshidi, N.A.A.B.; Lee, J.; Sim, W.; Kim, J.H. 2-Methoxy-4-vinylphenol Mitigates Malignancy of Cholangiocarcinoma Cells through the Blockade of Sonic Hedgehog Signalling. Biochem. Biophys. Res. Commun. 2025, 754, 151515. [Google Scholar] [CrossRef]
- Sathiyamoorthi, E.; Faleye, O.S.; Lee, J.H.; Lee, J. Hydroquinone Derivatives Attenuate Biofilm Formation and Virulence Factor Production in Vibrio spp. Int. J. Food Microbiol. 2023, 384, 109954. [Google Scholar] [CrossRef]
- Ma, C.; He, N.; Zhao, Y.; Xia, D.; Wei, J.; Kang, W. Antimicrobial Mechanism of Hydroquinone. Appl. Biochem. Biotechnol. 2019, 189, 1291–1303. [Google Scholar] [CrossRef]
- Pałasz, A.; Cież, D. In Search of Uracil Derivatives as Bioactive Agents. Uracils and Fused Uracils: Synthesis, Biological Activity and Applications. Eur. J. Med. Chem. 2015, 97, 582–611. [Google Scholar] [CrossRef]
- Benedek, B.; Kopp, B. Achillea millefolium L. s.l.–Is the Anti-inflammatory Activity Mediated by Protease Inhibition? J. Ethnopharmacol. 2007, 113, 312–317. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Czop, M.; Sakipova, Z.; Głowniak, K.; Kukula-Koch, W. Achillea millefolium L. and Achillea biebersteinii Afan. Hydroglycolic Extracts–Bioactive Ingredients for Cosmetic Use. Molecules 2020, 25, 3368. [Google Scholar] [CrossRef]
- Widyawati, T.; Syahputra, R.A.; Syarifah, S.; Sumantri, I.B. Analysis of Antidiabetic Activity of Squalene via In Silico and In Vivo Assay. Molecules 2023, 28, 3783. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Duan, X.; Si, H.; Luo, H.; Chen, S.; Liu, L.; He, H.; Wang, Z.; Liao, S. Antifungal Activity and Mechanism of Camphor Derivatives against Rhizoctonia solani: A Promising Alternative Antifungal Agent for Rice Sheath Blight. J. Agric. Food Chem. 2024, 72, 11415–11428. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Kovaleva, K.S.; Yarovaya, O.I.; Bormotov, N.I.; Shishkina, L.N.; Serova, O.A.; Sergeev, A.A.; Agafonov, A.P.; Maksuytov, R.A.; Salakhutdinov, N.F. (+)-Camphor and (−)-Borneol Derivatives as Potential Anti-Orthopoxvirus Agents. Arch. Pharm. 2021, 354, e2100038. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, R.; Mazumder, A.; Salahuddin; Yadav, R.K.; Chauhan, B.; Abdulah, M.M. Camphor and Menthol as Anticancer Agents: Synthesis, Structure-Activity Relationship and Interaction with Cancer Cell Lines. Anticancer Agents Med. Chem. 2023, 23, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Mozirandi, W.; Tagwireyi, D.; Mukanganyama, S. Evaluation of Antimicrobial Activity of Chondrillasterol Isolated from Vernonia adoensis (Asteraceae). BMC Complement. Altern. Med. 2019, 19, 249. [Google Scholar] [CrossRef]
- Chen, M.; Ghelfi, M.; Poon, J.F.; Jeon, N.; Boccalon, N.; Rubsamen, M.; Valentino, S.; Mehta, V.; Stamper, M.; Tariq, H.; et al. Antioxidant-Independent Activities of Alpha-Tocopherol. J. Biol. Chem. 2025, 301, 108327. [Google Scholar] [CrossRef]
- La Torre, M.E.; Cianciulli, A.; Monda, V.; Monda, M.; Filannino, F.M.; Antonucci, L.; Valenzano, A.; Cibelli, G.; Porro, C.; Messina, G.; et al. α-Tocopherol Protects Lipopolysaccharide-Activated BV2 Microglia. Molecules 2023, 28, 3340. [Google Scholar] [CrossRef]
- Uchihara, Y.; Ueda, F.; Tago, K.; Nakazawa, Y.; Ohe, T.; Mashino, T.; Yokota, S.; Kasahara, T.; Tamura, H.; Funakoshi-Tago, M. Alpha-Tocopherol Attenuates the Anti-Tumor Activity of Crizotinib against Cells Transformed by NPM-ALK. PLoS ONE 2017, 12, e0183003. [Google Scholar] [CrossRef]
- Kopańska, M.; Batoryna, M.; Banaś-Ząbczyk, A.; Błajda, J.; Lis, M.W. The Effect of α-Tocopherol on the Reduction of Inflammatory Processes and the Negative Effect of Acrylamide. Molecules 2022, 27, 965. [Google Scholar] [CrossRef]
- Frański, R.; Beszterda-Buszczak, M. Comment on Villalva et al. Antioxidant, Anti-Inflammatory, and Antibacterial Properties of an Achillea millefolium L. Extract and Its Fractions Obtained by Supercritical Anti-Solvent Fractionation against Helicobacter pylori. Antioxidants 2022, 11, 1849. Antioxidants 2023, 12, 1226. [Google Scholar] [CrossRef]
- El-Kalamouni, C.; Venskutonis, P.R.; Zebib, B.; Merah, O.; Raynaud, C.; Talou, T. Antioxidant and Antimicrobial Activities of the Essential Oil of Achillea millefolium L. Grown in France. Medicines 2017, 4, 30. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory Effects of Rosemary Extracts, Carnosic Acid and Rosmarinic Acid on the Growth of Various Human Cancer Cell Lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru 2011, 19, 173–186. [Google Scholar] [PubMed] [PubMed Central]
- Sökmen, A.; Sökmen, M.; Daferera, D.; Polissiou, M.; Candan, F.; Unlü, M.; Akpulat, H.A. The in vitro antioxidant and antimicrobial activities of the essential oil and methanol extracts of Achillea biebersteinii Afan. (Asteraceae). Phytother. Res. 2004, 18, 451–456. [Google Scholar] [CrossRef]
- Plaskova, A.; Mlcek, J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front. Nutr. 2023, 10, 1118761. [Google Scholar] [CrossRef] [PubMed]
- Demirci, F.; Guven, K.; Demirci, B.; Dadalioglu, I.; Baser, K.H.C. Characterization and Biological Activity of Achillea teretifolia Willd. and A. nobilis L. subsp. neilreichii (Kerner) Formanek Essential Oils. Turk. J. Biol. 2009, 33, 129–136. [Google Scholar] [CrossRef]
- Stojanović, G.; Radulović, N.; Hashimoto, T.; Palić, R. In Vitro Antimicrobial Activity of Extracts of Four Achillea Species: The Composition of Achillea clavennae L. (Asteraceae) Extract. J. Ethnopharmacol. 2005, 101, 185–190. [Google Scholar] [CrossRef]
- Apel, L.; Lorenz, P.; Urban, S.; Sauer, S.; Spring, O.; Stintzing, F.C.; Kammerer, D.R. Phytochemical Characterization of Different Yarrow Species (Achillea sp.) and Investigations into Their Antimicrobial Activity. Z. Naturforsch. C J. Biosci. 2020, 76, 55–65. [Google Scholar] [CrossRef]
- Ghavam, M.; Castangia, I.; Manconi, M.; Bacchetta, G.; Manca, M.L. Chemical Composition and Antimicrobial Activity of a Newly Identified Chemotype of Achillea wilhelmsii K. Koch from Kashan, Iran. Sci. Rep. 2024, 14, 22655. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Gazizova, A.; Datkhayev, U.; Amirkhanova, A.; Ustenova, G.; Kozhanova, K.; Ikhsanov, Y.; Kapsalyamova, E.; Kadyrbayeva, G.; Allambergenova, Z.; Kantureyeva, A.; et al. Phytochemical Profiling of Mentha asiatica Boriss. Leaf Extracts: Antioxidant and Antibacterial Activities. ES Food Agrofor. 2025, 19, 1355. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Used in Medicinal Plants, Principle, Strength, and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Barros, L.; Oliveira, S.; Carvalho, A.M.; Ferreira, I.C. In vitro antioxidant properties and characterisation in nutrients and phytochemicals of six medicinal plants from the Portuguese folk medicine. Ind. Crops Prod. 2010, 32, 572–579. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for In Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- CLSI M27-A3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard-Third Edition; CLSI: Wayne, PA, USA, 2008.




| Phytochemical Groups | Reagent | Observation | Aerial Ethanol Extract | Aerial Oil Extract | Root Ethanol Extract |
|---|---|---|---|---|---|
| Alcohols | Ferric chloride | Intense blue or green coloration | + | + | + |
| Aldehydes | Schiff’s reagent | Pink to magenta color formation | + | + | + |
| Amines | Ninhydrin reagent | Purple coloration | + | − | + |
| Amides | Sodium hydroxide + heat | Ammonia-like smell or evolution of gas | + | − | + |
| Flavonoids | Ferric chloride | Yellowish appearance clears after acid (HCl) addition | + | − | + |
| Tannins | Gelatin | Dirty (brownish) green precipitates | − | − | + |
| Alkaloids | Dragendorff’s | Reddish-orange precipitate | + | − | + |
| Triterpenoids | Liebermann–Burchard | Brown ring | + | + | + |
| Glycosides | Keller–Killiani | Reddish-brown layer | + | − | + |
| No. | Name | Molecular Formula | Molecular Mass, g/mol | Retention Indices (RI) | Retention Time (min) | PubChem Compound CID | Similarities | Area, % |
|---|---|---|---|---|---|---|---|---|
| 1 | Propanoic acid | C3H6O2 | 74.08 | 878 | 10.58 | 1032 | 93 | 0.92 |
| 2 | 2,3-Butanediol | C4H10O2 | 90.12 | 1055 | 11.40 | 262 | 96 | 6.08 |
| 3 | R-(–)-1,2-propanediol | C3H8O2 | 76.09 | 946 | 11.62 | 259994 | 91 | 0.89 |
| 4 | Gamma-Butyrolactone | C4H6O2 | 86.09 | 960 | 12.32 | 7302 | 96 | 0.95 |
| 5 | Butanoic acid | C4H8O2 | 88.11 | 950 | 12.40 | 264 | 95 | 1.89 |
| 6 | 4-Hydroxybutanoic acid | C4H8O3 | 104.1 | 1026 | 12.99 | 10413 | 96 | 7.76 |
| 7 | (L)-α-Terpineol | C10H18O | 154.25 | 1195 | 14.22 | 443162 | 92 | 1.53 |
| 8 | N-Nitrosohexamethyleneimine | C6H12N2O | 128.17 | 1120 | 14.35 | 13613 | 90 | 0.19 |
| 9 | 1,2-Cyclopentanedione | C5H6O2 | 98.1 | 985 | 15.28 | 566657 | 89 | 0.54 |
| 10 | Methyl N-hydroxybenzenecarboximidate | C8H9NO2 | 151.16 | 1210 | 15.58 | 9602988 | 98 | 2.69 |
| 11 | Guaiacol | C7H8O2 | 124.14 | 1105 | 17.01 | 460 | 85 | 2.40 |
| 12 | Ethanol, 2,2’-oxybis- | C4H10O3 | 106.12 | 1035 | 19.14 | 161927 | 87 | 1.82 |
| 13 | Phenol | C6H6O | 94.11 | 1080 | 19.77 | 996 | 90 | 2.01 |
| 14 | Phenol, 4-ethyl-2-methoxy- | C9H12O2 | 152.19 | 1255 | 20.35 | 62465 | 87 | 1.08 |
| 15 | 2-Pyrrolidinone | C4H7NO | 85.1 | 1010 | 20.65 | 12025 | 97 | 3.22 |
| 16 | 1,3-Propanediol | C3H8O2 | 76.09 | 920 | 22.57 | 10442 | 95 | 2.20 |
| 17 | Eugenol | C10H12O2 | 164.2 | 1356 | 22.75 | 3314 | 87 | 1.49 |
| 18 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.17 | 1320 | 23.43 | 332 | 85 | 3.26 |
| 19 | Dianhydromannitol | C6H10O4 | 146.14 | 1350 | 23.66 | 23619611 | 95 | 1.07 |
| 20 | Phenol, 2,6-dimethoxy- | C8H10O3 | 154.16 | 1280 | 24.66 | 7041 | 85 | 2.85 |
| 21 | Glycerin | C3H8O3 | 92.09 | 1150 | 25.04 | 753 | 86 | 15.97 |
| 22 | Triethylene glycol | C6H14O4 | 150.17 | 1175 | 25.33 | 8172 | 91 | 2.80 |
| 23 | 1,4:3,6-Dianhydro- α-d-glucopyranose | C6H8O4 | 144.13 | 1385 | 26.35 | 22213879 | 87 | 1.76 |
| 24 | Benzofuran, 2,3-dihydro- | C8H8O | 120.15 | 1150 | 26.75 | 10329 | 88 | 0.60 |
| 25 | 5-tert-Butylpyrogallol | C10H14O3 | 182.22 | 1400 | 27.08 | 597592 | 83 | 0.60 |
| 26 | Succinimide | C4H5NO2 | 99.09 | 990 | 27.62 | 11439 | 94 | 0.48 |
| 27 | Isosorbide | C6H10O4 | 146.14 | 1310 | 29.53 | 12597 | 90 | 7.91 |
| 28 | 3’,5’-Dimethoxyacetophenone | C10H12O3 | 180.2 | 1450 | 29.73 | 95997 | 86 | 2.54 |
| 29 | Tetraethylene glycol | C8H18O5 | 194.23 | 1225 | 31.20 | 8200 | 96 | 3.22 |
| 30 | 3-Methyl-4-phenyl-1H-pyrrole | C11H11N | 157.21 | 1420 | 31.98 | 15164561 | 85 | 0.61 |
| 31 | Hydroquinone | C6H6O2 | 110.11 | 1155 | 35.97 | 785 | 89 | 1.74 |
| 32 | 3-Isobutylhexahydropyrrolo [1,2-a]pyrazine-1,4-dione | C11H18N2O2 | 210.27 | 1325 | 36.69 | 102892 | 87 | 1.27 |
| 33 | Hexaethylene glycol | C12H26O7 | 282.33 | 1275 | 37.18 | 17472 | 88 | 3.56 |
| 34 | Octadecanoic acid | C18H36O2 | 284.48 | 1980 | 37.75 | 5281 | 93 | 1.42 |
| 35 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | C10H16N2O2 | 196.25 | 1350 | 38.65 | 98951 | 84 | 2.19 |
| 36 | β-D-Glucopyranose, 1,6-anhydro- | C6H10O5 | 162.14 | 1400 | 39.66 | 11947765 | 83 | 1.39 |
| 37 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- | C7H10N2O2 | 154.17 | 1295 | 41.55 | 193540 | 84 | 1.07 |
| 38 | 4-Methyleneproline | C6H9NO2 | 127.14 | 1230 | 45.85 | 558375 | 86 | 5.63 |
| No. | Name | Molecular Formula | Molecular Mass, g/mol | Retention Indices (RI) | Retention Time (min) | PubChem Compound CID | Similarities | Area, % |
|---|---|---|---|---|---|---|---|---|
| 1 | Propanoic acid | C3H6O2 | 74.08 | 880 | 10.52 | 1032 | 85 | 0.96 |
| 2 | 4-Cyclopentene-1,3-dione | C5H4O2 | 96.08 | 900 | 11.27 | 70258 | 80 | 0.81 |
| 3 | 4-Hydroxybutanoic acid | C4H8O3 | 104.10 | 920 | 12.22 | 10413 | 82 | 2.34 |
| 4 | 2-Propenoic acid | C3H4O2 | 72.06 | 940 | 12.48 | 6581 | 85 | 1.16 |
| 5 | 2-Furanmethanol | C5H6O2 | 98.10 | 960 | 12.92 | 7361 | 86 | 1.14 |
| 6 | 2(5H)-Furanone, 3-methyl- | C5H6O2 | 98.10 | 980 | 14.16 | 30945 | 82 | 0.39 |
| 7 | 2,4-Dimethyl-2-oxazoline-4-methanol | C6H11NO2 | 129.16 | 1000 | 14.26 | 98073 | 80 | 3.01 |
| 8 | 2(5H)-Furanone | C4H4O2 | 84.07 | 1020 | 14.69 | 10341 | 82 | 0.97 |
| 9 | 1,2-Cyclopentanedione | C5H6O2 | 98.1 | 1040 | 15.16 | 566657 | 85 | 2.55 |
| 10 | 1,2-Cyclopentanedione, 3-methyl- | C6H8O2 | 112.13 | 1060 | 16.54 | 61209 | 94 | 0.69 |
| 11 | Guaiacol | C7H8O2 | 124.14 | 1080 | 16.98 | 460 | 88 | 0.72 |
| 12 | Benzaldehyde, 3-hydroxy-, oxime | C7H7NO2 | 137.14 | 1100 | 17.49 | 9603073 | 86 | 6.65 |
| 13 | 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- | C7H10O2 | 126.15 | 1120 | 17.84 | 62752 | 82 | 0.54 |
| 14 | Ethanone, 1-(1H-pyrrol-2-yl)- | C6H7NO | 109.13 | 1140 | 19.14 | 14079 | 80 | 0.45 |
| 15 | Phenol | C6H6O | 94.11 | 1160 | 19.75 | 996 | 82 | 0.61 |
| 16 | 2-Pyrrolidinone | C4H7NO | 85.10 | 1180 | 20.68 | 12025 | 85 | 0.65 |
| 17 | 2(3H)-Furanone, 5-heptyldihydro- | C11H18O2 | 182.26 | 1200 | 20.86 | 7714 | 94 | 0.70 |
| 18 | Cyclopropyl carbinol | C4H8O | 72.11 | 1220 | 21.04 | 75644 | 88 | 1.06 |
| 19 | 1,3-Dioxol-2-one,4,5-dimethyl- | C5H6O3 | 114.10 | 1240 | 21.79 | 142210 | 90 | 1.39 |
| 20 | α-Hydroxy-gamma-butyrolactone | C4H6O3 | 102.09 | 1260 | 22.53 | 19444 | 85 | 1.26 |
| 21 | Allyl acetate | C5H8O2 | 100.12 | 1280 | 22.61 | 11584 | 80 | 0.85 |
| 22 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.17 | 1300 | 23.38 | 332 | 82 | 1.10 |
| 23 | 2,3-Dimethylhydroquinone | C8H10O2 | 138.17 | 1320 | 23.95 | 69100 | 85 | 0.41 |
| 24 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H6O4 | 142.11 | 1340 | 24.12 | 119838 | 94 | 9.39 |
| 25 | 1,2,3-Propanetriol, 1-acetate | C5H10O4 | 134.13 | 1360 | 24.58 | 33510 | 88 | 0.42 |
| 26 | Phenol, 2,6-dimethoxy- | C8H10O3 | 154.16 | 1380 | 24.65 | 7041 | 80 | 1.57 |
| 27 | Glycerin | C3H8O3 | 92.09 | 1400 | 25.21 | 753 | 82 | 26.73 |
| 28 | Triethylene glycol | C6H14O4 | 150.17 | 1420 | 25.36 | 8172 | 85 | 1.05 |
| 29 | 1,4:3,6-Dianhydro-α-d-glucopyranose | C6H8O4 | 144.13 | 1440 | 26.35 | 39923607 | 94 | 0.40 |
| 30 | Benzofuran, 2,3-dihydro- | C8H8O | 120.15 | 1460 | 26.72 | 10329 | 85 | 0.41 |
| 31 | Acetaminophen | C8H9NO2 | 151.16 | 1480 | 26.91 | 1983 | 88 | 0.09 |
| 32 | Benzoic acid, 3-pyridyl ester | C12H9NO2 | 199.20 | 1500 | 27.10 | 569697 | 87 | 1.88 |
| 33 | Succinimide | C4H5NO2 | 99.09 | 1520 | 27.59 | 11439 | 76 | 0.34 |
| 34 | (S)-(+)-2’,3’-Dideoxyribonolactone | C4H6O3 | 102.09 | 1540 | 27.76 | 32780 | 89 | 0.96 |
| 35 | 2-Aminopyrimidine-1-oxide | C4H5N3O | 111.10 | 1560 | 28.04 | 139694 | 80 | 0.53 |
| 36 | 2,5-Dimethyl-4-phenylpyridine | C13H13N | 183.25 | 1580 | 28.64 | 603086 | 82 | 0.24 |
| 37 | 3-Pyridinol, 6-methyl- | C6H7NO | 109.13 | 1600 | 28.81 | 14275 | 85 | 0.32 |
| 38 | Butyl 9-decenoate | C14H26O2 | 226.36 | 1620 | 29.17 | 17825102 | 94 | 1.58 |
| 39 | Isosorbide | C6H10O4 | 146.14 | 1640 | 29.54 | 12597 | 88 | 0.31 |
| 40 | 3’,5’-Dimethoxyacetophenone | C10H12O3 | 180.20 | 1660 | 29.68 | 95997 | 80 | 9.97 |
| 41 | DL-Proline, 5-oxo-, methyl ester | C6H9NO3 | 143.14 | 1680 | 29.99 | 500249 | 82 | 0.12 |
| 42 | Tetraethylene glycol | C8H18O5 | 194.23 | 1700 | 31.20 | 8200 | 85 | 1.95 |
| 43 | 3-Methyl-4-phenyl-1H-pyrrole | C11H11N | 157.21 | 1720 | 31.97 | 15164561 | 86 | 0.48 |
| 44 | 3-(1H-Pyrrol-3-yl)propionic acid, methyl ester | C8H11NO2 | 153.18 | 1740 | 32.09 | 556813 | 92 | 0.46 |
| 45 | 2,6-Dimethylphenyl isocyanate | C9H9NO | 147.17 | 1760 | 32.19 | 98787 | 86 | 0.46 |
| 46 | Ethyl N-(o-anisyl)formimidate | C10H13NO2 | 179.22 | 1780 | 32.43 | 601627 | 90 | 0.38 |
| 47 | 2-Naphthalenamine | C10H9N | 143.18 | 1800 | 33.40 | 7057 | 83 | 0.19 |
| 48 | Benzaldehyde, 4-hydroxy-3,5-dimethoxy- | C9H10O4 | 182.17 | 1820 | 35.06 | 8655 | 98 | 0.38 |
| 49 | Hydroquinone | C6H6O2 | 110.11 | 1840 | 35.95 | 785 | 93 | 1.35 |
| 50 | Pentaethylene glycol | C10H22O6 | 238.28 | 1860 | 37.18 | 62551 | 89 | 0.50 |
| 51 | Uric acid | C5H4N4O3 | 168.11 | 1880 | 37.83 | 1175 | 93 | 0.70 |
| 52 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | C11H18N2O2 | 210.27 | 1900 | 37.99 | 98951 | 86 | 1.55 |
| 53 | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | C10H12O3 | 180.20 | 1920 | 39.02 | 1549095 | 88 | 0.59 |
| 54 | β-D-Glucopyranose, 1,6-anhydro- | C6H10O5 | 162.14 | 1940 | 39.66 | 11947765 | 89 | 0.33 |
| 55 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- | C7H10N2O2 | 154.17 | 1960 | 41.53 | 193540 | 93 | 0.78 |
| 56 | Hexaethylene glycol | C12H26O7 | 282.33 | 1980 | 42.43 | 17472 | 86 | 0.45 |
| 57 | Uracil | C4H4N2O2 | 112.09 | 2000 | 45.19 | 1174 | 88 | 0.56 |
| 58 | 4-Methyleneproline | C6H9NO2 | 127.14 | 2020 | 45.88 | 558375 | 98 | 2.25 |
| No. | Name | Molecular Formula | Molecular Mass, g/mol | Retention Indices (RI) | Retention Time (min) | PubChem Compound CID | Similarities | Area, % |
|---|---|---|---|---|---|---|---|---|
| 1 | Camphor | C10H16O | 152.23 | 1173 | 10.07 | 2537 | 93 | 1.41 |
| 2 | 2,4-Decadienal | C10H16O | 152.23 | 1280 | 12.61 | 5283349 | 96 | 0.46 |
| 3 | 2-Furanacetaldehyde, α-propyl- | C10H12O2 | 152.19 | 1076 | 12.99 | 557292 | 91 | 0.69 |
| 4 | 3-(Hydroxymethylene)indolin-2-one | C9H7NO2 | 161.16 | 1490 | 16.21 | 595118 | 96 | 0.66 |
| 5 | Hedycaryol | C15H26O | 222.37 | 1534 | 18.67 | 6432240 | 95 | 0.70 |
| 6 | 6-Methyl-2,4(1H,3H)-pteridinedione | C7H6N4O2 | 178.15 | 1590 | 20.27 | 601068 | 96 | 0.57 |
| 7 | Tridecanoic acid, methyl ester | C4H28O2 | 228.37 | 1608 | 22.03 | 15608 | 92 | 0.92 |
| 8 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284.50 | 1994 | 22.87 | 12366 | 86 | 1.15 |
| 9 | 11,14-Octadecadienoic acid, methyl ester | C19H34O2 | 294.50 | 2089 | 24.03 | 5365677 | 90 | 0.68 |
| 10 | 4-Methyl-3-pentenal | C6H10O | 98.14 | 942 | 24.13 | 21457 | 92 | 0.60 |
| 11 | 6-Tetradecyne | C14H26 | 194.36 | 1395 | 24.43 | 138027 | 87 | 14.99 |
| 12 | Linoleic acid | C18H32O2 | 280.40 | 2095 | 24.52 | 5280450 | 85 | 8.08 |
| 13 | Linoleic acid ethyl ester | C20H36O2 | 308.50 | 2145 | 24.80 | 5282184 | 93 | 1.75 |
| 14 | Oleic acid ethyl ester | C20H38O2 | 310.50 | 2175 | 24.89 | 5363269 | 88 | 2.28 |
| 15 | 2,2,2-Trifluoro-N-(hydroxymethyl)acetamide | C3H4F3NO2 | 143.06 | 2197 | 25.66 | 3084931 | 95 | 3.35 |
| 16 | Palmitoyl chloride | C16H34ClO | 274.90 | 2256 | 26.19 | 8206 | 91 | 2.50 |
| 17 | α -Tocopherol | C29H50O2 | 430.70 | 3100 | 27.26 | 14985 | 86 | 8.36 |
| 18 | E,Z-1,3,12-Nonadecatriene | C19H34 | 262.50 | 1916 | 27.44 | 5365680 | 88 | 22.25 |
| 19 | Oleic acid, 3-hydroxypropyl ester | C21H40O3 | 340.50 | 2076 | 27.51 | 5352775 | 90 | 6.92 |
| 20 | 2-Dodecylcyclobutanone | C16H30O | 238.41 | 1600 | 27.77 | 161875 | 90 | 1.24 |
| 21 | Isopropyl linoleate | C21H38O2 | 322.50 | 2150 | 27.96 | 5352860 | 81 | 14.64 |
| 22 | cis-13,16-Docasadienoic acid | C22H40O2 | 336.60 | 2566 | 28.28 | 5312554 | 91 | 1.42 |
| 23 | 1-(Trimethylsilyl)-1-propyne | C6H12Si | 112.240 | 2509 | 30.71 | 80363 | 85 | 0.49 |
| 24 | Chondrillasterol | C29H48O | 412.70 | 3380 | 30.86 | 5283663 | 89 | 1.54 |
| 25 | Squalene | C30H50 | 410.70 | 2814 | 31.65 | 638072 | 90 | 2.35 |
| No. | Chemical Class | Subclass | Name | Known Pharmacological Activities |
|---|---|---|---|---|
| 1. | Alcohol | Diol | 2,3-Butanediol a | Antitumor activity, immunomodulatory effects, cryoprotective agent, solvent and drug carrier, anti-inflammatory properties, neuroprotective effects, probiotic metabolite potential, inhibitory effect on certain pathogens [26,27] |
| 2. | Diol | R-(–)-1,2-propanediol a | – | |
| 3. | Diol | 1,3-Propanediol a | Solvent and drug delivery agent, moisturizing and humectant properties, stabilizing agent [28] | |
| 4. | Monoterpene alcohol | (L)-α-Terpineol a | – | |
| 5. | Triol | Glycerin a,r | Humectant and lubricant [29] | |
| 6. | Polyether diol | Triethylene glycol a,r | Antimicrobial activity, antiviral activity, disinfectant properties, low toxicity profile, plasticizer and solvent in pharmaceuticals, air sanitizing agent, humectant in topical formulations [30] | |
| 7. | Polyether diol | Tetraethylene glycol a,r | – | |
| 8. | Polyether diol | Hexaethylene glycol a,r | – | |
| 9. | Furan derivative | 2-Furanmethanol r | Antimicrobial, antifungal, and anticancer [31] | |
| 10. | Cyclopropyl alcohol | Cyclopropyl carbinol r | – | |
| 11. | Polyether | Pentaethylene glycol r | – | |
| 12. | Aldehyde | Oxime | Benzaldehyde, 3-hydroxy-, oxime r | – |
| 13. | α,β-Unsaturated aliphatic aldehyde | 2,4-Decadienal b | – | |
| 14. | α,β-Unsaturated aldehyde | 4-Methyl-3-pentenal b | – | |
| 15. | Phenolic aldehyde | Benzaldehyde, 4-hydroxy-3,5-dimethoxy- r | – | |
| 16. | Furan derivative | 2-Furanacetaldehyde, α-propyl- b | – | |
| 17. | Aromatic compound | Benzofuran derivative | Benzofuran, 2,3-dihydro- a,r | – |
| 18. | Amino acid | Non-proteinogenic amino acid | 4-Methyleneproline b | – |
| 19. | Amino acid derivative | Lactam ester | DL-Proline, 5-oxo-, methyl ester r | – |
| 20. | Amine | Aromatic amine | 2-Naphthalenamine r | – |
| 21. | Amide | Aniline derivative | Acetaminophen r | – |
| 22. | Trifluoroacetamide derivative | 2,2,2-Trifluoro-N-(hydroxymethyl)acetamide b | – | |
| 23. | Carboxylic acid | Short-chain fatty acid | Propanoic acid a,r | Antimicrobial activity, anti-inflammatory properties, anticancer potential, lipid metabolism regulation, gut microbiota modulation, histone deacetylase (HDAC) inhibition, immune response modulation [32] |
| 24. | Short-chain fatty acid | Butanoic acid a | HDAC inhibition [33] | |
| 25. | Alpha, beta-unsaturated acid | 2-Propenoic acid r | – | |
| 26. | Saturated fatty acid | Octadecanoic acid a | Antimicrobial activity, anti-inflammatory properties, antioxidant activity, anticancer potential, emollient and skin-conditioning agent, cholesterol-lowering effects, immune-modulating activity [34] | |
| 27. | Polyunsaturated fatty acid | Linoleic acid b | Anti-proliferative, anti-invasive, pro-apoptotic, cell cycle arrest (G1 phase), ROS-inducing, mitochondrial membrane potential disruption, anti-inflammatory, antioxidant, epithelial-mesenchymal transition (EMT) inhibition, angiogenesis inhibition, immune modulation, mitochondrial biogenesis stimulation, PGC-1α/NRF1/TFAM pathway activation [34,35] | |
| 28. | Polyunsaturated fatty acid | cis-13,16-Docasadienoic acid b | – | |
| 29. | γ-hydroxy acid | 4-hydroxybutanoic acid a,r | CNS depressant activity, sedative effects, anesthetic properties, muscle relaxant, euphoric effects [36], treatment of narcolepsy, treatment of alcohol dependence, potential neuroprotective effects [37], upregulation of expression of the Cramp gene (encoding cathelicidin LL-37) in murine bone marrow-derived macrophages [38], promotion of endogenous antimicrobial peptide expression in macrophages [39] | |
| 30. | Carboxylic acid ester | Pyrrole derivative | 3-(1H-Pyrrol-3-yl)propionic acid, methyl ester r | – |
| 31. | Saturated methyl ester | Tridecanoic acid, methyl ester b | – | |
| 32. | Saturated ethyl ester | Hexadecanoic acid, ethyl ester b | – | |
| 33. | Polyunsaturated methyl ester | 11,14-Octadecadienoic acid, methyl ester b | – | |
| 34. | Polyunsaturated ethyl ester | Linoleic acid ethyl ester b | – | |
| 35. | Monounsaturated ethyl ester | Oleic acid ethyl ester b | – | |
| 36. | Polyunsaturated ester | Isopropyl linoleate b | – | |
| 37. | Monounsaturated ester | Oleic acid, 3-hydroxypropyl ester b | – | |
| 38. | Carbohydrate | Dianhydrosugar alcohol | Dianhydromannitol a | Diuretic activity, osmotic laxative effect, low toxicity, potential use as a pharmaceutical excipient, stabilizing agent, osmoprotective properties [40] |
| 39. | Sugar derivative | 1,4:3,6-Dianhydro-α-d-glucopyranose a,r | – | |
| 40. | Sugar alcohol derivative | Isosorbide a,r | – | |
| 41. | Monosaccharide derivative | β-D-Glucopyranose, 1,6-anhydro- a,r | – | |
| 42. | Carbonate | Dioxolone | 1,3-Dioxol-2-one,4,5-dimethyl- r | – |
| 43. | Ether | Diol ether | Ethanol, 2,2’-oxybis- a | – |
| 44. | Allyl ester | Allyl acetate r | – | |
| 45. | Acylglycerol | 1,2,3-Propanetriol, 1-acetate r | – | |
| 46. | Pyridine ester | Benzoic acid, 3-pyridyl ester r | – | |
| 47. | Fatty acid ester | Butyl 9-decenoate r | – | |
| 48. | Heterocycle | Lactam | 2-Pyrrolidinone a,r | – |
| 49. | Pyrrole derivative | 3-Methyl-4-phenyl-1H-pyrrole a,r | – | |
| 50. | Lactam | 3- Isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione a | – | |
| 51. | Lactam | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- a,r | – | |
| 52. | Lactam | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- a,r | – | |
| 53. | Oxazoline derivative | 2,4-Dimethyl-2-oxazoline-4-methanol r | – | |
| 54. | Pyrimidine derivative | 2-Aminopyrimidine-1-oxide r | – | |
| 55. | Pyridine derivative | 2,5-Dimethyl-4-phenylpyridine r | – | |
| 56. | Indole derivative | 3-(Hydroxymethylene)indolin-2-one b | – | |
| 57. | Pteridine derivative | 6-Methyl-2,4(1H,3H)-pteridinedione b | – | |
| 58. | Imidate | Aromatic imidate | Methyl N-hydroxybenzenecarboximidate a | – |
| 59. | Aromatic imidate | Ethyl N-(o-anisyl)formimidate r | – | |
| 60. | Imide | Cyclic imide | Succinimide a,r | Anticonvulsant activity, antiepileptic effects, central nervous system depressant, muscle relaxant properties, sedative effects, enzyme inhibitor potential [41] |
| 61. | Isocyanate | Aromatic isocyanate | 2,6-Dimethylphenyl isocyanate r | – |
| 62. | Ketone | Diketone | 1,2-Cyclopentanedione a | – |
| 63. | Acetophenone derivative | 3’,5’-Dimethoxyacetophenone a,r | – | |
| 64. | Cyclic diketone | 4-Cyclopentene-1,3-dione r | Antifungal [42] | |
| 65. | Cyclic diketone | 1,2-Cyclopentanedione r | – | |
| 66. | Cyclic diketone | 1,2-Cyclopentanedione, 3-methyl- r | – | |
| 67. | Hydroxycyclopentenone | 2-Cyclopenten-1-one, 3-ethyl-2-hydroxy- r | – | |
| 68. | Aryl ketone | Ethanone, 1-(1H-pyrrol-2-yl)- r | – | |
| 69. | Cyclobutanone derivative | 2-Dodecylcyclobutanone b | – | |
| 70. | Lactone | Cyclic ester | γ-Butyrolactone a | CNS depressant activity, sedative and hypnotic effects, anxiolytic properties, anesthetic effects, muscle relaxant, prodrug of gamma-hydroxybutyric acid (GHB), treatment of narcolepsy (via GHB), potential abuse and dependence liability [43] |
| 71. | Furanone | 2(5H)-Furanone, 3-methyl- r | – | |
| 72. | Furanone derivative | 2(3H)-Furanone, 5-heptyldihydro- r | – | |
| 73. | Furanone | 2(5H)-Furanone r | – | |
| 74. | Hydroxybutyrolactone | Aα-Hydroxy-γ-butyrolactone r | – | |
| 75. | Sugar lactone | (S)-(+)-2’,3’-Dideoxyribonolactone r | – | |
| 76. | Nitrosoamine | Cyclic nitrosamine | N-Nitrosohexamethyleneimine a | – |
| 77. | Phenol | Methoxyphenol | Guaiacol a,r | Expectorant activity, antiseptic, analgesic, anti-inflammatory, antioxidant, local anesthetic, antimicrobial [44] |
| 78. | Allyl-substituted methoxyphenol | Eugenol a | Antibacterial, antiviral, antifungal, anticancer, anti-inflammatory and antioxidant [45] | |
| 79. | Monohydroxybenzene | Phenol a,r | Antiseptic, anesthetic, antibacterial, antifungal, antiparasitic, disinfectant, cauterizing agent, local analgesic [46] | |
| 80. | Alkylated methoxyphenol | Phenol, 4-ethyl-2-methoxy- a | – | |
| 81. | Vinyl-substituted methoxyphenol | 2-Methoxy-4-vinylphenol a,r | Antioxidant, anti-inflammatory, antimicrobial, anticancer, antiplatelet, hepatoprotective, cytoprotective [47] | |
| 82. | Dimethoxyphenol | Phenol, 2,6-dimethoxy- a,r | – | |
| 83. | Trihydroxybenzene derivative | 5-tert-Butylpyrogallol a | – | |
| 84. | Dihydroxybenzene | Hydroquinone a,r | Skin depigmenting agent, antioxidant activity, anticancer potential, antibacterial activity, antifungal activity, melanin synthesis inhibition, anti-inflammatory [47,48] | |
| 85. | Hydroquinone derivative | 2,3-Dimethylhydroquinone r | Antioxidant activity, antimicrobial activity, cytotoxic effects, potential anticancer activity, redox-modulating [49] | |
| 86. | Pyridinol | 3-Pyridinol, 6-methyl- r | – | |
| 87. | Lignan derivative | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol r | – | |
| 88. | Pyranone | Hydroxypyranone | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- r | – |
| 89. | Purine derivative | Heterocyclic compound | Uric acid r | Potential biomarker for cardiovascular and metabolic disorders, pro-inflammatory effects in hyperuricemia, crystal-induced inflammation (e.g., gout) [50] |
| 90. | Pyrimidine base | Nucleobase | Uracil r | Antiviral, anticancer, enzyme inhibition, involvement in DNA/RNA synthesis, radiosensitizing, antimicrobial, immunomodulatory [51] |
| 91. | Hydrocarbon | Alkyne | 6-Tetradecyne b | – |
| 92. | Polyunsaturated alkene | E,Z-1,3,12-Nonadecatriene b | – | |
| 93. | Terpene | Triterpene | Squalene b | Anticancer, antiinflammatory, antioxidant, and antidiabetic [52] |
| 94. | Terpenoid | Camphor b | Antifungal, antiviral, and anticancer pharmacological activities, including strong inhibitory effects against Rhizoctonia solani and other fungal pathogens [53], antiviral activity against orthopoxviruses [54], and cytotoxic effects on various cancer cell lines via modulation of cellular pathways and structure–activity relationships [55] | |
| 95. | Terpenoid | Hedycaryol b | – | |
| 96. | Phytosterol | Sterol | Chondrillasterol b | Antibacterial activity against Staphylococcus aureus (25% inhibition), Klebsiella pneumoniae (38% inhibition), and Pseudomonas aeruginosa (65% inhibition); complete biofilm disruption of P. aeruginosa at 1.6 μg/mL; complete inhibition of biofilm formation at 100 μg/mL [56] |
| 97. | Vitamin | Tocopherol antioxidant | α-Tocopherol b | Antioxidant activity, anti-inflammatory effects, gene-regulatory activity, neuroprotective activity, cytoprotective effects, mitochondrial protection, anti-apoptotic effects, modulation of signal transduction pathways, suppression of endoplasmic reticulum stress, selective protection of non-cancerous cells during chemotherapy, attenuation of drug-induced cytotoxicity, modulation of lipid metabolism, reduction of hepatic steatosis, prevention of nonalcoholic steatohepatitis progression, interference with anti-cancer drug efficacy [57,58,59,60] |
| 98. | Organosilicon | Silylated alkyne | 1-(Trimethylsilyl)-1-propyne b | – |
| 99. | Acyl chloride | Fatty acid derivative | Palmitoyl chloride b | – |
| Comp. | Name | Aerial Ethanol Extract | Root Ethanol Extract | Comp. | Name | Aerial Oil Extract |
|---|---|---|---|---|---|---|
| Area, % | Area, % | Area, % | ||||
| 1 | Propanoic acid | 0.92 | 0.96 | 23 | Camphor | 1.41 |
| 2 | 4-hydroxybutanoic acid | 7.76 | 2.34 | 24 | Linoleic acid | 8.08 |
| 3 | Guaiacol | 2.40 | 0.72 | 25 | 2,2,2-Trifluoro-N-(hydroxymethyl)acetamide | 3.35 |
| 4 | Phenol | 2.01 | 0.61 | 26 | Hexadecanoic acid, ethyl ester | 1.15 |
| 5 | 2-Pyrrolidinone | 3.22 | 0.65 | 27 | 6-Tetradecyne | 14.99 |
| 6 | 2-Methoxy-4-vinylphenol | 3.26 | 1.10 | 28 | Palmitoyl chloride | 2.50 |
| 7 | Phenol, 2,6-dimethoxy- | 2.85 | 1.57 | 29 | Linoleic acid ethyl ester | 1.75 |
| 8 | Glycerin | 15.97 | 26.73 | 30 | Oleic acid ethyl ester | 2.28 |
| 9 | Triethylene glycol | 2.80 | 1.05 | 31 | cis-13,16-Docasadienoic acid | 1.42 |
| 10 | 1,4:3,6-Dianhydro-α-d-glucopyranose | 1.76 | 0.40 | 32 | α -Tocopherol | 8.36 |
| 11 | Benzofuran, 2,3-dihydro- | 0.60 | 0.41 | 33 | E,Z-1,3,12-Nonadecatriene | 22.25 |
| 12 | Succinimide | 0.48 | 0.34 | 34 | Isopropyl linoleate | 14.64 |
| 13 | Isosorbide | 7.91 | 0.31 | 35 | Oleic acid, 3-hydroxypropyl ester | 6.92 |
| 14 | 3’,5’-Dimethoxyacetophenone | 2.54 | 9.97 | 36 | 2-Dodecylcyclobutanone | 1.24 |
| 15 | Tetraethylene glycol | 3.22 | 1.95 | 37 | Chondrillasterol | 1.54 |
| 16 | 3-Methyl-4-phenyl-1H-pyrrole | 0.61 | 0.48 | 38 | Squalene | 2.35 |
| 17 | Hydroquinone | 1.74 | 1.35 | |||
| 18 | Hexaethylene glycol | 3.56 | 0.45 | |||
| 19 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- | 2.19 | 1.55 | |||
| 20 | β-D-Glucopyranose, 1,6-anhydro- | 1.39 | 0.33 | |||
| 21 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- | 1.07 | 0.78 | |||
| 22 | 4-Methyleneproline | 5.63 | 2.25 |
| Microorganisms | Aerial Ethanol Extract, mg/mL | Aerial Oil Extract, mg/mL | Root Ethanol Extract, mg/mL |
|---|---|---|---|
| C. albicans | 0.75 | 1.20 | 1.25 |
| A. fumigatus | 1.50 | 2.50 | 2.00 |
| C. neoformans | 0.85 | 1.40 | 1.50 |
| MRSA | 0.50 | 0.90 | 1.00 |
| E. coli | 1.00 | 1.50 | 1.80 |
| P. aeruginosa | 2.00 | 3.20 | 3.00 |
| K. pneumoniae | 1.20 | 2.10 | 2.00 |
| VRE | 1.00 | 1.60 | 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berdgaleeva, A.; Zhalimova, Z.; Saginbazarova, A.; Tulegenova, G.; Zharylkassynova, D.; Bazargaliyeva, A.; Kuanbay, Z.; Sakhanova, S.; Ramazanova, A.; Bilkenova, A.; et al. Comparative Phytochemical Analysis and Antimicrobial Properties of Ethanol and Macerated Extracts from Aerial and Root Parts of Achillea nobilis. Molecules 2025, 30, 2957. https://doi.org/10.3390/molecules30142957
Berdgaleeva A, Zhalimova Z, Saginbazarova A, Tulegenova G, Zharylkassynova D, Bazargaliyeva A, Kuanbay Z, Sakhanova S, Ramazanova A, Bilkenova A, et al. Comparative Phytochemical Analysis and Antimicrobial Properties of Ethanol and Macerated Extracts from Aerial and Root Parts of Achillea nobilis. Molecules. 2025; 30(14):2957. https://doi.org/10.3390/molecules30142957
Chicago/Turabian StyleBerdgaleeva, Aiman, Zere Zhalimova, Akzharkyn Saginbazarova, Gulbanu Tulegenova, Dana Zharylkassynova, Aliya Bazargaliyeva, Zhaidargul Kuanbay, Svetlana Sakhanova, Akmaral Ramazanova, Akzhamal Bilkenova, and et al. 2025. "Comparative Phytochemical Analysis and Antimicrobial Properties of Ethanol and Macerated Extracts from Aerial and Root Parts of Achillea nobilis" Molecules 30, no. 14: 2957. https://doi.org/10.3390/molecules30142957
APA StyleBerdgaleeva, A., Zhalimova, Z., Saginbazarova, A., Tulegenova, G., Zharylkassynova, D., Bazargaliyeva, A., Kuanbay, Z., Sakhanova, S., Ramazanova, A., Bilkenova, A., & Sartayeva, A. (2025). Comparative Phytochemical Analysis and Antimicrobial Properties of Ethanol and Macerated Extracts from Aerial and Root Parts of Achillea nobilis. Molecules, 30(14), 2957. https://doi.org/10.3390/molecules30142957






