Abstract
In this study, a novel adsorbent, UiO-66-H3IMDC, was successfully prepared by functionalizing UiO-66 with imidazole-4,5-dicarboxylic acid (H3IMDC). The effective functionalization of H3IMDC on UiO-66 was confirmed by powder X-ray diffraction (PXRD) and Fourier transform infrared spectroscopy (FT-IR). The relationships between the adsorption of U(VI) on UiO-66-H3IMDC and the contact time, the pH of the solution, as well as the initial concentration of U(VI) were investigated. Additionally, the selective adsorption of U(VI) by UiO-66-H3IMDC and its cyclic regeneration performance were also studied. The results demonstrate that the UiO-66-H3IMDC adsorbent exhibits excellent adsorption performance for uranium in aqueous solutions.
1. Introduction
During uranium mining, nuclear fuel processing, nuclear power generation, and spent fuel reprocessing, substantial amounts of uranium-containing radioactive waste are generated [1]. Upon entering the human body, uranium primarily accumulates in the liver, kidneys, and skeletal system, potentially causing acute/chronic poisoning, multi-organ diseases, hepatic damage, and carcinogenic effects. Effective management of such radioactive waste is imperative, with uranium-contaminated wastewater treatment representing a critical component of radwaste disposal [2]. Currently, the treatment methods are divided into three major categories: chemical methods [3], physical-chemical methods [4], and biological methods [5]. Specific treatment technologies include traditional methods such as evaporation concentration [6], chemical precipitation [7], ion exchange [8], electrochemical methods [9], and microbial treatment [10]. With the development of materials science, new types of adsorbent materials keep emerging, and the treatment of radioactive wastewater by the adsorption method has received special attention in recent years [11].
Metal-organic frameworks (MOFs) are a class of porous crystalline materials composed of organic ligands connected via coordination bonds [12]. These extensively studied materials demonstrate exceptional potential for radioactive wastewater remediation due to their unique combination of properties, including tunable pore architectures, structural customizability, and high crystallinity. While pristine MOFs typically exhibit limited intrinsic adsorption capacities for radionuclides, targeted modifications have been shown to significantly enhance their adsorption performance. This optimization enables more efficient removal of radioactive species such as uranyl ions (UO22+) from contaminated water systems [13,14,15,16,17].
Understandably, MOFs serving as adsorbents require stability in aqueous or acidic environments. A breakthrough came with Lillerud and colleagues’ synthesis of a zirconium(IV) dicarboxylate porous material, designated as UiO-66 [18]. This framework demonstrated exceptional surface area and unprecedented chemical stability, establishing a new benchmark in the field. Subsequent research efforts have extensively investigated the uranyl ion (UO22+) adsorption capabilities of both pristine UiO-66 and its structural analogs, driving innovations in nuclear waste treatment technologies. Luo et al. [19] studied the ability of UIO-66-NH2 to capture U(VI) from an aqueous solution. Under the condition of pH 5.5, the adsorption of U(VI) reached equilibrium in approximately 4 h, and the maximum adsorption capacity was 114.9 mg g−1. RAJAEI et al. [20] studied the modification of UIO-66 and vacant UIO-66 (UiO-66-vac) by immobilizing tributyl phosphate (TBP), and these modified materials were used for the removal of uranyl ions from an aqueous solution. The research results showed that the maximum adsorption capacities of UiO-66-TBP and UiO-66-vac-TBP for uranium were 201.9 and 203.5 mg g−1, respectively. That is, the immobilization of TBP significantly enhanced the adsorption capacity of MOFs for uranyl ions. It can be seen that although researchers have carried out various modifications on UiO-66 to improve its uranium adsorption capacity, the results are not entirely satisfactory. Therefore, on the basis of taking into account the high chemical stability of UiO-66, it is of great significance to explore how to develop a functionalized UiO-66 adsorbent with a high adsorption capacity.
Imidazole-4,5-dicarboxylic acid (H3IMDC) is a versatile ligand containing imidazole nitrogen and two carboxyl groups [21]. Its molecular structure features two carboxylic acid groups and an imidazole ring nitrogen atom, which together provide multiple coordination sites to form stable chelates with metal ions [22]. Metal complexes derived from this ligand exhibit strong stability under high-temperature and chemically harsh environments, making them advantageous for the regeneration and reuse of adsorbent materials [23]. Furthermore, the amphoteric nature of the imidazole ring—capable of protonation/deprotonation—enables it to maintain adsorption activity across a broad pH range, allowing adaptability to diverse wastewater treatment conditions [24]. This unique combination of structural and chemical properties positions imidazole-4,5-dicarboxylic acid as a promising candidate for designing robust and reusable materials for environmental remediation applications [25,26,27].
Therefore, in this study, UiO-66(Zr) was functionalized with H3IMDC to enhance its uranium adsorption performance. Compared to pristine UiO-66 and H3IMDC individually, the UiO-66-H3IMDC composite exhibited a significant improvement in U(VI) ion adsorption capacity. Key factors influencing adsorption behavior—including pH, initial U(VI) concentration, contact time, ionic competition, and regeneration cycles—were systematically investigated. Furthermore, Fourier transform infrared (FT-IR) spectroscopy was employed to elucidate the adsorption mechanism of UiO-66-H3IMDC.
2. Results and Discussion
2.1. Characterization of MOFs
Figure 1a displays the PXRD patterns of UiO-66, UiO-66-H3IMDC, and pristine H3IMDC. The characteristic diffraction peaks of both UiO-66 and H3IMDC are distinctly present in the UiO-66-H3IMDC composite. This dual observation not only confirms the successful synthesis of crystalline UiO-66-H3IMDC but also demonstrates the effective functionalization of UiO-66 through H3IMDC incorporation. Additionally, Figure 1b presents the Fourier transform infrared (FT-IR) spectra of pristine UiO-66, H3IMDC, and UiO-66-H3IMDC. The absorption peak at 3174 cm−1 corresponds to N-H stretching vibrations inherent in the pure H3IMDC ligand [25]. Following H3IMDC modification, significant spectral shifts emerge in two critical regions: both the C-N (1469–1457 cm−1) and O-C=O (1585–1578 cm−1, 1389–1397 cm−1) characteristic peaks exhibit notable displacement compared to their original positions in pure H3IMDC. And the O-C=O vibrational signature in UiO-66-H3IMDC (1397 cm−1, 1578 cm−1) displays marked deviation from its counterpart in pristine UiO-66 (1395 cm−1, 1583 cm−1) [28]. These spectral discrepancies collectively confirm the successful covalent grafting of H3IMDC onto the UiO-66 framework. The N2 adsorption-desorption isotherms of UiO-66 and UiO-66-H3IMDC at 77 K exhibit classical Type I behavior (Figure 1c), confirming their microporous architectures. Post functionalization, UiO-66-H3IMDC demonstrates a substantial reduction in BET surface area (from 1297.5 m2 g−1 to 604.2 m2 g−1), primarily due to partial pore blockage caused by H3IMDC grafting. This steric hindrance effect arises from the chelation of H3IMDC carboxylate groups to Zr6O4(OH)4 clusters, which modifies the pore accessibility while preserving the overall framework crystallinity.
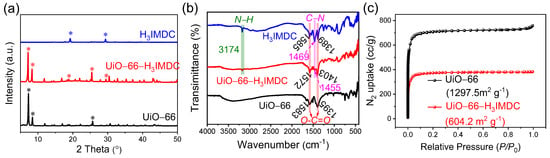
Figure 1.
(a) PXRD pattern and (b) infrared spectra of UiO-66, UiO-66-H3IMDC, and pristine H3IMDC and (c) N2 adsorption-desorption isotherm at 77 K of UiO-66 and UiO-66-H3IMDC. (* * *: Labeling of characteristic peak positions in PXRD).
Furthermore, Figure 2 displays the morphological characteristics of UiO-66 and UiO-66-H3IMDC, respectively. As can be observed, UiO-66 exhibits a smooth surface morphology. Upon grafting H3IMDC onto UiO-66, the crystal structure of the parent material remains intact and undamaged. Notably, irregular particles become distinctly visible and dispersed across the surface of UiO-66-H3IMDC, as highlighted by the circular markers. Further SEM mapping investigated the distribution of carbon (C), oxygen (O), nitrogen (N), and zirconium (Zr) within the sample (Figure S1a–e). Analysis revealed that these elements constituted approximately 58.19%, 10.50%, 29.94%, and 1.38% of the sample composition, respectively (Table S1).
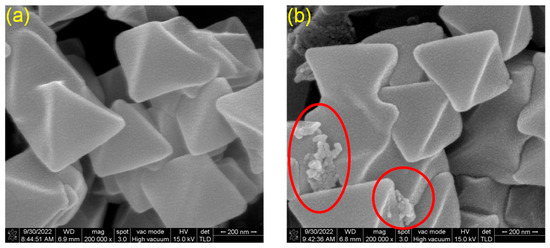
Figure 2.
SEM images of (a) UiO-66 and (b) UiO-66-H3IMDC.
2.2. Effect of Initial pH
Given that solution pH significantly influences the protonation and deprotonation of functional groups on the adsorbent surface, as well as the speciation of metal ions in solution [29], we prioritized exploring the impact of pH on our adsorption experiments. Figure 3a illustrates the adsorption outcomes of UiO-66-H3IMDC for U(VI) at various pH levels. Notably, the adsorption capacity for U(VI) is relatively low at low pH values. This can be attributed to the high concentration of H+ ions in the solution at low pH, which compete with U(VI) ions for adsorption sites on the UiO-66-H3IMDC surface. On the other hand, pH-induced U(VI) speciation may also account for the pH-dependent adsorption. It is well-known that as the pH increases, the U(VI) species gradually transform from free UO22+ to polynuclear hydroxide complexes (Figure 3b). These hydroxide complexes are likely to be more favorably adsorbed by the adsorbent. In the subsequent experiments, a pH of 6.0 was selected as an appropriate condition for further investigation.
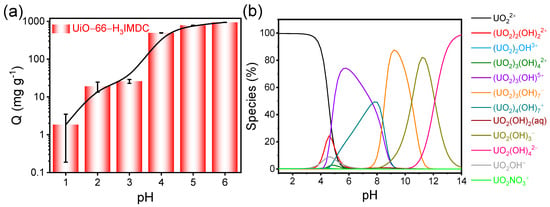
Figure 3.
(a) Effect of pH on UiO-66-H3IMDC on U (VI) adsorption (adsorbent dosage = 3 mg; C0 = 300 mg L−1; t = 8 h; T = 298.15 K). (b) Variation in Th (IV) species with pH of aqueous solution (Visual Minteq-3.1 program).
2.3. Adsorption Isotherm
The uranium sorption performance of UiO-66, H3IMDC, and UiO-66-H3IMDC was systematically evaluated. As shown in Figure 4a, the adsorption isotherms of these three materials exhibit a significant enhancement in uranium uptake capacity after functionalization. The maximum sorption capacities of UiO-66, H3IMDC, and UiO-66-H3IMDC within the tested concentration range were determined as 235.5, 605.3, and 942.8 mg g−1, respectively. UiO-66-H3IMDC demonstrates exceptional uranyl ion (UO22+) adsorption capacity that significantly surpasses most MOF-based adsorbents reported in the current literature (Table S2). Despite the substantially reduced BET surface area of the functionalized UiO-66 (attributed to pore-blocking effects from H3IMDC coordination), the integration of H3IMDC introduced high-efficiency binding sites (e.g., carboxylate and imidazole groups), enabling exceptional uranium affinity that compensates for porosity loss.
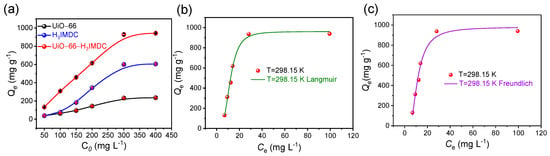
Figure 4.
(a) Adsorption isotherms of UiO-66, H3IMDC, and UiO-66-H3IMDC (T = 298.15 K, t = 8 h; pH = 6.0). (b) Langmuir model and (c) Freundlich model of UiO-66-H3IMDC.
To elucidate the sorption mechanism, the adsorption data were fitted with two classical isotherm models, the Langmuir and Freundlich models [30,31].
Langmuir isotherm equation:
Freundlich isotherm equation:
where Ce (mg L−1) denotes the equilibrium U(VI) concentration in the aqueous phase, Qe (mg g−1) represents the equilibrium adsorption capacity, and Qm (mg g−1) corresponds to the theoretical maximum adsorption capacity derived from the Langmuir isotherm model. The parameter KL is the Langmuir affinity constant, while KF and n are the Freundlich constants characterizing adsorption capacity and heterogeneity, respectively.
The experimental versus modeled adsorption profiles for the Langmuir and Freundlich isotherms are comparatively visualized in Figure 4b,c, with detailed fitting parameters and correlation coefficients (R2) tabulated in Table S3. Notably, the Langmuir model demonstrates superior agreement (R2 = 0.992) over the Freundlich model (R2 = 0.984). This pronounced preference for the Langmuir isotherm strongly supports a monolayer adsorption mechanism governed by homogeneous active sites across the adsorbent surface [32,33,34,35,36,37], where U(VI) species undergo chemo selective coordination with the imidazole-dicarboxylate functionalities of UiO-66-H3IMDC.
2.4. Adsorption Kinetics
The adsorption kinetics were investigated through time-dependent batch experiments, with the temporal evolution of adsorption capacity quantified in Figure 5a. Kinetic analysis revealed a triphasic adsorption mechanism: (1) an initial rapid adsorption stage (0–100 min) dominated by surface complexation, (2) a transitional diffusion-controlled phase (100–300 min) showing progressive site saturation, and (3) an equilibrium plateau (>300 min) achieving maximum adsorption capacity. Notably, >60% of total uranium uptake occurred within the first kinetic phase, followed by gradual pore-filling processes until complete monolayer formation.
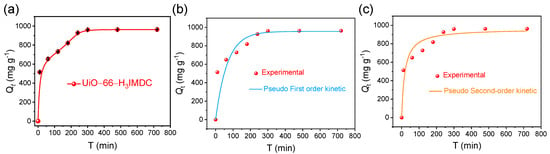
Figure 5.
(a) Effect of uranium adsorption time on UiO-66-H3IMDC (C0 = 300 mg L−1; T = 298.15 K; pH = 6.0); (b) pseudo-first-order model and (c) pseudo-second-order model fitting curves.
The interfacial mass transfer mechanisms governing U(VI) adsorption on UiO-66-H3IMDC were elucidated through kinetic modeling using two classical formulations [38,39]: the pseudo-first-order-model (PFO) Equation (3) for physisorption-dominated processes and the pseudo-second-order model (PSO) Equation (4) describing chemisorption-controlled systems. The linearized rate equations are mathematically expressed as
where k1 (min−1) and k2 (g mg−1 min) are the adsorption constants. Qe and Qt denote the amount of adsorption (mg g−1) at the equilibrium moment and t (min) moment, respectively.
ln(Qe − Qt) = lnQe − k1t
Figure 5b,c present the nonlinear regression analysis of kinetic models, with corresponding goodness-of-fit statistics detailed in Table S4. The pseudo-second-order (PSO) model demonstrates superior predictive capability, evidenced by its coefficient of determination (R2 = 0.927) significantly exceeding that of the pseudo-first-order (PFO) model (R2 = 0.796). This high degree of correlation establishes through rigorous statistical validation that the U(VI) adsorption process on UiO-66-H3IMDC follows PSO kinetics, indicative of rate-limiting chemisorption mechanisms potentially involving surface complexation or ion-exchange reactions [40,41,42].
2.5. Effect of Co-Existing Ions
The remediation of U(VI) from radioactive wastewater necessitates adsorbents with exceptional ion-discrimination capability, given the inherent challenges posed by heterogeneous ionic matrices containing competing cations. In this study, we ascertained the selective adsorption capabilities of U(VI) in multi-ionic solutions, and the obtained results are presented in Figure 6. After the adsorption process, the concentration of U(VI) ions in the coexisting solution is markedly lower than that of other coexisting ions. Notably, the presence of interfering ions does not impede the adsorption of U(VI) by UiO-66-H3IMDC. Thus, this material may be used in the treatment of real radioactive wastewater.
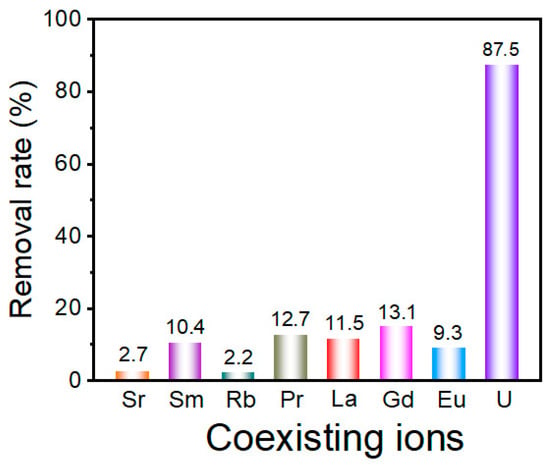
Figure 6.
The effect of interfering ions on adsorption capacity for Th (IV) ion on UiO-66-H3IMDC. (CM+ = 300 mg L−1; t = 8 h; pH = 6.0; T = 298.15K).
2.6. Regeneration and Stability Investigation
In practical scenarios, guaranteeing the reusability and stability of adsorbents is essential. As a result, both the adsorption efficiency and the adsorption capacity throughout the adsorption/desorption cycle have emerged as crucial benchmarks for assessing the performance of adsorbents. As illustrated in Figure 7a, following three regeneration cycles, the adsorption quantity witnesses a 16% reduction. Significantly, the adsorption rates continue to stay elevated throughout this process. Significantly, UiO-66-H3IMDC maintained structural integrity with indistinguishable PXRD patterns from the pristine material after 7-day immersion in aqueous media (pH = 6) (Figure 7b), providing robust evidence of its exceptional hydrolytic stability under environmentally relevant conditions.
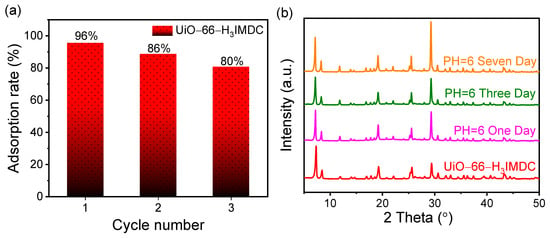
Figure 7.
(a) Cyclic regeneration and (b) water stability of UiO-66-H3IMDC.
2.7. Removal Mechanism
Following the adsorption of U(VI), the PXRD patterns of the UiO-66-H3IMDC show diffraction peaks that precisely match those of the original materials (Figure 8a). This result clearly demonstrates that the crystal structure remains intact during the uranium adsorption process. To further investigate the mechanism of strong chemical interaction between U(VI) ions and UiO-66-H3IMDC, we compared the infrared (IR) spectral shifts of UiO-66 and UiO-66-H3IMDC before and after uranium adsorption (Figure 8b,c). Post adsorption, new O=U=O vibrational bands appeared at 922 cm−1 and 911 cm−1 [43], directly confirming successful uranium coordination on the material’s surface. Notably, the O=U=O peak associated with H3IMDC exhibited a lower vibrational frequency compared to UiO-66, suggesting a stronger adsorption of U(VI) by H3IMDC. Additionally, the -COO-Zr doublet peaks in UiO-66-H3IMDC (1582–1577 cm−1, 1405–1392 cm−1) underwent significantly larger shifts than those in UiO-66 (1583–1580 cm−1, 1395–1398 cm−1), further evidencing enhanced chemical interactions between UiO-66-H3IMDC and U(VI), consistent with its superior adsorption capacity. Furthermore, significant shifts in the N-H (3173–3718 cm−1) and C-N (1457–1469 cm−1) regions indicate that the nitrogen adsorption sites on UiO-66-H3IMDC also interact with U(VI) ions. The mechanism may be as follows: Firstly, the large specific surface area and abundant porous structure of the UiO-66-H3IMDC material enable U(VI) species to efficiently diffuse and come into full contact with the composite material. Subsequently, uranyl ions coordinate with the nitrogen- and oxygen-containing active adsorption sites. Therefore, uranium(VI) can not only interact with the surface of the adsorbent but also undergo complexation with more reactive sites. Additionally, we selected the two ions exhibiting the highest (gadolinium) and lowest (rubidium) removal rates among the competing ions besides uranium to explore whether strong chemical interactions exist between these competing ions and UiO-66-H3IMDC (Figure S2). Results revealed no shifts in the characteristic FTIR peaks corresponding to the adsorption sites (N-H, COO-Zr, C-N) for both rubidium (Rb+) and gadolinium (Gd3+) ions before and after adsorption. This observation further confirms the high adsorption selectivity of UiO-66-H3IMDC for uranium.
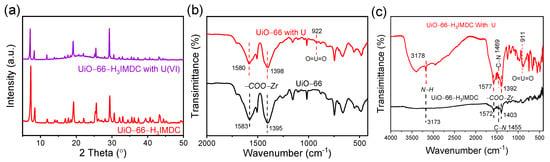
Figure 8.
(a) PXRD of UiO-66-H3IMDC before and after uranium adsorption. FT-IR spectra of (b) UiO-66 and (c) UiO-66-H3IMDC before and after uranium adsorption.
3. Materials and Methods
3.1. Materials
Zirconyl chloride octahydrate (ZrOCl2·8H2O, AR), terephthalic acid (H2BDC, >99.0%), 4,5-Imidazoledicarboxylic acid (C5H4N2O4, AR), Acetate (C2H4O2, AR), N, N-Dimethylformamide (DMF, >99.5%), and methanol (CH3OH, AR) were purchased from MACKLIN reagent (Shanghai, China). Strontium nitrate (SrN2O6, AR), cesium nitrate (CsNO3, AR), samarium nitrate hexahydrate (SmN3O9·6H2O, AR), rubidium nitrate (RbNO3, AR), praseodymium nitrate hexahydrate (PrN3O9·6H2O, AR), lanthanum nitrate hexahydrate (LaN3O9·6H2O, AR), gadolinium nitrate hexahydrate (GdN3O9·6H2O, AR), and europium nitrate hexahydrate (EuN3O9·6H2O, AR) were purchased from Aladdin (Shanghai, China). Uranyl nitrate hexahydrate (UO2(NO3)2·6H2O, AR) was obtained from the China Institute of Atomic Energy. Ultrapure water was prepared from the Millipore system (18.25 MΩ cm) (Direct 8, Millipore, Burlington, MA, USA). All the above reagents were used directly without further purification.
3.2. Preparation of UiO-66(Zr)
UiO-66(Zr) was synthesized by a solvothermal synthesis technique reported in the literature [18]. H2BDC (1.6 g) and ZrOCl2·8H2O (3.2 g) were dissolved in DMF (50 mL)/acetate (50 mL) and mixed completely, then transferred to a 250 mL round-bottomed flask and stirred at 378.15 K for 24 h under reflux in an oil bath. After that, white powder was obtained through centrifugation. Finally, the sample was washed three times with DMF and three times with methanol and then dried in a vacuum drying oven to obtain the sample.
3.3. Preparation of UiO-66-H3IMDC
UiO-66 (Zr) (0.5 g) and H3IMDC (1.0 g) were dissolved in a solvent mixture of 100 mL ethanol and 50 mL DMF. The mixture was sonicated for 20 min to ensure uniform dispersion. After sonication, the solution was subjected to reflux heating at 353.15 K for 24 h. Upon cooling to room temperature, the product was separated via centrifugation, followed by washing the solid product three times with deionized water and ethanol. Finally, the material was vacuum-dried overnight to yield the functionalized UiO-66-H3IMDC composite.
3.4. Characterization Techniques
Powder X-ray diffraction (PXRD) analysis was conducted on a Bruker D8 QUEST diffractometer (Bremen, Germany) to evaluate the crystallinity and phase purity of the MOF materials. Measurements utilized Cu Kα radiation (λ = 1.542 Å) operated at 40 kV and 40 mA, with a scan range of 4–50.0° (2θ) at a rate of 10° min−1. For porosity characterization, nitrogen adsorption-desorption isotherms were recorded at 77 K using a Micromeritics ASAP 2460 analyzer (Norcross, GA, USA). Prior to analysis, MOF samples (~100 mg) were degassed under vacuum at 80 °C for 12 h to remove physisorbed species. Specific surface areas were calculated via the Brunauer-Emmett-Teller (BET) method. Chemical bonding analysis was performed by Fourier transform infrared spectroscopy (FT-IR) on a Bruker TENSOR27 spectrometer (Germany). Spectra were acquired in the 400–4000 cm−1 range to monitor functional group transformations in MOFs. The morphology of the materials was observed using a scanning electron microscope (SEM) (JEM 2100, JEOL, Akishima, Japan) operating at 30.0 kV under high vacuum conditions. The sample was vacuum-dried and affixed to the test bench with a conductive adhesive before gold sputtering for 80 s to facilitate microscopic observation.
3.5. Adsorption Experiments
Batch adsorption experiments were performed by adding 3 mg of adsorbent to 10 mL of U(VI) solution in 15 mL polypropylene centrifuge tubes. The suspensions were agitated at 220 rpm in a temperature-controlled orbital shaker for predetermined time intervals. Subsequently, the mixtures were filtered through 0.22 μm pore-size nylon membranes using a syringe filtration assembly (5 mL capacity). The collected filtrates were acidified with 2% (v/v) HNO3 and analyzed for residual U(VI) concentrations via inductively coupled plasma optical emission spectrometry (ICP-OES, JY2000-2, HORIBA, Bhamboli, Maharashtra). Specific experimental parameters (pH, initial U(VI) concentration, and contact time, etc.) are provided in the captions of relevant figures.
The adsorption capacity (Q) of U(VI) was defined by the following equation:
where C0 (mg L−1) and Ce (mg L−1) are the initial concentration and equilibrium concentration of U(VI) ions, respectively. V (L) is the volume of solution, and m (g) is the usage amount of adsorbent.
4. Conclusions
In conclusion, a novel MOF (UiO-66-H3IMDC) was successfully synthesized through grafting H3IMDC onto UiO-66, demonstrating exceptional U(VI) adsorption capabilities. The modified material exhibited a maximum U(VI) adsorption capacity of 942.8 mg g−1 under optimized conditions (pH = 6), reaching adsorption equilibrium within 300 min. Additionally, the uranium adsorption process of UiO-66-H3IMDC fits better with the Langmuir model and the pseudo-second-order model, indicating that the adsorption process is predominantly a monolayer chemisorption process. Finally, the excellent selectivity and cyclic adsorption ability demonstrated by UiO-66-H3IMDC suggest that UiO-66-H3IMDC has the potential to adsorb U(VI) from actual wastewater.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30142966/s1, Figure S1: (a) SEM image of UiO-66-H3IMDC; (b–e) SEM-EDS mapping of elements C, O, N, and Zr in UiO-66-H3IMDC. Figure S2: FT-IR spectra of UiO-66-H3IMDC before and after rubidium and gadolinium adsorption. Table S1: Elemental distribution of UiO-66-H3IMDC. Table S2: Uranium adsorption capacity of MOFs and their materials. Table S3: Langmuir and Freundlich model fitting parameters for U (VI) adsorption. Table S4: Kinetic fitting parameters of U (VI) adsorption in UiO-66-H3IMDC. Refs. [13,14,15,16,17,19,20,43,44,45,46,47,48,49] are cited in Supplementary Materials.
Author Contributions
Conceptualization, X.D.; Methodology, T.L. and X.L.; Validation, H.C.; Formal analysis, T.L., H.C. and X.D.; Investigation, T.L. and X.L.; Data curation, H.C.; Writing—original draft, T.L.; Writing—review & editing, X.D., J.Z. and S.X.; Supervision, X.D.; Project administration, S.X.; Funding acquisition, J.Z. and S.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Qisun Ye Fund (China) (No. U2441290) and China Institute of Atomic Energy Dean’s Fund (No. YZ222505001103).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rodríguez-Penalonga, L.; Soria, B.Y.M. A Review of the Nuclear Fuel Cycle Strategies and the Spent Nuclear Fuel Management Technologies. Energies 2017, 10, 1235. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, L.; Yu, F.; Xiao, S.; Wang, C.; Yuan, D.; Liu, Y. Sulfonated heteroatom co-doped carbon materials with a porous structure boosting electrosorption capacity for uranium (VI) removal. J. Solid State Chem. 2023, 327, 124262. [Google Scholar] [CrossRef]
- Kadadou, D.; Said, E.A.; Ajaj, R.; Hasan, S.W. Research advances in nuclear wastewater treatment using conventional and hybrid technologies: Towards sustainable wastewater reuse and recovery. J. Water Process Eng. 2023, 52, 103604. [Google Scholar] [CrossRef]
- Srivastava, A.; Parida, V.K.; Majumder, A.; Gupta, B.; Gupta, A.K. Treatment of saline wastewater using physicochemical, biological, and hybrid processes: Insights into inhibition mechanisms, treatment efficiencies and performance enhancement. J. Environ. Chem. Eng. 2021, 9, 105775. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, W.; He, Y. Prediction of Uranium Adsorption Capacity in Radioactive Wastewater Treatment with Biochar. Toxics 2024, 12, 118. [Google Scholar] [CrossRef]
- Wang, Y.; Zhan, L.; Chen, H.; Mao, J.; Chen, H.; Ma, X.; Yang, L. Study on the evaporation performance of concentrated desulfurization wastewater and its products analysis. J. Water Process Eng. 2024, 58, 104862. [Google Scholar] [CrossRef]
- Kang, J.; Sun, W.; Hu, Y.; Gao, Z.; Liu, R.; Zhang, Q.; Liu, H.; Meng, X. The utilization of waste by-products for removing silicate from mineral processing wastewater via chemical precipitation. Water Res. 2017, 125, 318–324. [Google Scholar] [CrossRef]
- José, L.B.; Silva, G.C.; Ladeira, A.C.Q. Pre-concentration and partial fractionation of rare earth elements by ion exchange. Miner. Eng. 2024, 205, 108477. [Google Scholar] [CrossRef]
- Lin, T.; Chen, T.; Jiao, C.; Zhang, H.; Hou, K.; Jin, H.; Liu, Y.; Zhu, W.; He, R. Ion pair sites for efficient electrochemical extraction of uranium in real nuclear wastewater. Nat. Commun. 2024, 15, 4149. [Google Scholar] [CrossRef]
- Moghaddam, A.; Khayatan, D.; Barzegar, P.E.F.; Ranjbar, R.; Yazdanian, M.; Tahmasebi, E.; Alam, M.; Abbasi, K.; Ghaleh, H.E.G.; Tebyaniyan, H. Biodegradation of pharmaceutical compounds in industrial wastewater using biological treatment: A comprehensive overview. Int. J. Environ. Sci. Technol. 2023, 20, 5659–5696. [Google Scholar] [CrossRef]
- Mei, D.; Liu, L.; Yan, B. Adsorption of uranium (VI) by metal-organic frameworks and covalent-organic frameworks from water. Coord. Chem. Rev. 2023, 475, 214917. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- De Decker, J.; Folens, K.; De Clercq, J.; Meledina, M.; Van Tendeloo, G.; Laing, G.D.; Van Der Voort, P. Ship-in-a-bottle CMPO in MIL-101(Cr) for selective uranium recovery from aqueous streams through adsorption. J. Hazard. Mater. 2017, 335, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, Y.; Liu, L.; Ma, F.; Zhang, C.; Dong, H. MOF modified with copolymers containing carboxyl and amidoxime groups and high efficiency U (VI) extraction from seawater. Sep. Purif. Technol. 2022, 291, 120946. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Liu, Q.; Song, D.; Li, R.; Liu, P.; Wang, J. Diaminomaleonitrile functionalized double-shelled hollow MIL-101 (Cr) for selective removal of uranium from simulated seawater. Chem. Eng. J. 2019, 368, 951–958. [Google Scholar] [CrossRef]
- Liu, L.; Fang, Y.; Meng, Y.; Wang, X.; Ma, F.; Zhang, C.; Dong, H. Efficient adsorbent for recovering uranium from seawater prepared by grafting amidoxime groups on chloromethylated MIL-101(Cr) via diaminomaleonitrile intermediate. Desalination 2020, 478, 114300. [Google Scholar] [CrossRef]
- Bi, C.; Zhang, C.; Xu, W.; Ma, F.; Zhu, L.; Zhu, R.; Qi, Q.; Liu, L.; Bai, J.; Dong, H. Highly efficient antibacterial adsorbent for recovering uranium from seawater based on molecular structure design of PCN-222 post-engineering. Desalination 2023, 545, 116169. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- Luo, B.-C.; Yuan, L.-Y.; Chai, Z.-F.; Shi, W.-Q.; Tang, Q. U(VI) capture from aqueous solution by highly porous and stable MOFs: UiO-66 and its amine derivative. J. Radioanal. Nucl. Chem. 2016, 307, 269–276. [Google Scholar] [CrossRef]
- Rajaei, A.; Ghani, K.; Jafari, M. Modification of UiO-66 for removal of uranyl ion from aqueous solution by immobilization of tributyl phosphate. J. Chem. Sci. 2021, 133, 14. [Google Scholar] [CrossRef]
- Zhai, Q.-G.; Zeng, R.-R.; Li, S.-N.; Jiang, Y.-C.; Hu, M.-C. Alkyl substituents introduced into novel d10-metalimidazole-4,5-dicarboxylate frameworks: Synthesis, structure diversities and photoluminescence properties. CrystEngComm 2013, 15, 965–976. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, X.; Yang, Z.; Li, G. Metal-organic frameworks constructed from imidazole dicarboxylates bearing aromatic substituents at the 2-position. CrystEngComm 2012, 14, 7382–7397. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B. Construction of cobalt-imidazole-based dicarboxylate complexes with topological diversity: From metal-organic square to one-dimensional coordination polymer. Inorg. Chem. Commun. 2012, 22, 170–173. [Google Scholar] [CrossRef]
- Shi, L.; Bao, K.; Cao, J.; Qian, Y. Sunlight-assisted fabrication of a hierarchical ZnO nanorod array structure. CrystEngComm 2009, 11, 2009–2014. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, Q.; Zhang, H.; Liu, J.; Yu, J.; Wang, J. High efficiency biosorption of Uranium (VI) ions from solution by using hemp fibers functionalized with imidazole-4,5-dicarboxylic. J. Mol. Liq. 2020, 297, 111739. [Google Scholar] [CrossRef]
- Li, J.; Dai, C.; Cao, Y.; Sun, X.; Li, G.; Huo, Q.; Liu, Y. Lewis basic site (LBS)-functionalized zeolite-like supramolecular assemblies (ZSAs) with high CO2 uptake performance and highly selective CO2/CH4 separation. J. Mater. Chem. 2017, 5, 21429–21434. [Google Scholar] [CrossRef]
- Banerjee, D.; Mondal, B.C.; Das, D.; Das, A.K. Use of Imidazole 4,5-Dicarboxylic Acid Resin in Vanadium Speciation. Microchim. Acta 2003, 141, 107–113. [Google Scholar] [CrossRef]
- Ding, X.; Xiao, S.; Wang, T.; Zeng, Z.; Zhao, X.; Yang, Q. Stability of metal-organic frameworks towards β-ray irradiation: Role of organic groups. Microporous Mesoporous Mater. 2023, 354, 112533. [Google Scholar] [CrossRef]
- Lei, H.; Pan, N.; Wang, X.; Zou, H. Facile Synthesis of Phytic Acid Impregnated Polyaniline for Enhanced U(VI) Adsorption. J. Chem. Eng. Data 2018, 63, 3989–3997. [Google Scholar] [CrossRef]
- Sayari, A.; Hamoudi, S.; Yang, Y. Applications of Pore-Expanded Mesoporous Silica. 1. Removal of Heavy Metal Cations and Organic Pollutants from Wastewater. Chem. Mater. 2005, 17, 212–216. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, Z.; Li, X.; Ma, K.; Jin, T.; Feng, Z.; Lan, T.; Zhao, J.; Xiao, S. Highly radiation-resistant Al-MOF selected based on the radiation stability rules of metal-organic frameworks with ultra-high thorium ion adsorption capacity. Environ. Sci. Nano 2024, 11, 2103–2111. [Google Scholar] [CrossRef]
- Khan, P.N.; Pahan, S.; Sengupta, A.; Dasgupta, K.; Bhattacharyya, K.; Tyagi, D.; Vincent, T. Diglycolic Acid Monoamide-Functionalized UiO-66-Based Metal Organic Framework (MOFDGAMA) for Selective Removal of UO22+ and Th4+. Ind. Eng. Chem. Res. 2024, 63, 10492–10497. [Google Scholar] [CrossRef]
- Xiong, Y.; Gao, Y.; Guo, X.; Wang, Y.; Su, X.; Sun, X. Water-Stable Metal-organic Framework Material with Uncoordinated Terpyridine Site for Selective Th(IV)/Ln(III) Separation. ACS Sustain. Chem. Eng. 2019, 7, 3120–3126. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Xin, Q.; Lei, Z.; Hu, E.; Li, L.; Liang, F.; Wang, H. Enhancement mechanism of chitosan/tannic acid curing and functional group modification on uranium adsorption in five types of wastewater by Cu-MOF. J. Hazard. Mater. 2025, 492, 138185. [Google Scholar] [CrossRef]
- Xin, Q.; Wang, H.; Hu, E.; Luo, K.; Lei, Z.; Hu, F.; Liu, X.; Hu, J.; Wang, Q. Investigation into highly selective uranium adsorption using a water-stable chitosan/ellagic acid/Cu-metal-organic framework material. Desalination 2025, 606, 118768. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Wu, F.; Xiao, W.; Hua, W.; Tang, Z.; Liu, W.; Chen, S.; Wang, Y.; Wu, W.; et al. Photoisomerization-mediated tunable pore size in metal organic frameworks for U(VI)/V(V) selective separation. Nat. Commun. 2025, 16, 2361. [Google Scholar] [CrossRef]
- Wang, W.; Ni, S.; Liu, Y.; Zhao, Y.; Meng, Y.; Yang, L. Structural and coordination microenvironment regulated MOF with phosphorylurea group to boost uranium adsorption. Sep. Purif. Technol. 2024, 346, 127409. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Xiao, S.; Li, X.; Zhao, S.; Zhao, Y.; Yu, C.; Feng, Z.; Ma, K.; Liu, X.; et al. Efficient capture of thorium ions by the hydroxyl-functionalized sp2c-COF through nitrogen-oxygen cooperative mechanism. Green Chem. Eng. 2024, in press. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Zhao, Y.; Chen, L.; Yu, C.; Liu, X.; Zhao, S.; Feng, Z.; Ma, K.; Ding, X.; et al. Efficient and rapid adsorption of thorium by sp2c-COF with one-dimensional regular micropores channels. J. Environ. Chem. Eng. 2024, 12, 114066. [Google Scholar] [CrossRef]
- Gumber, N.; Pai, R.V.; Bahadur, J.; Sengupta, S.; Das, D.; Goutam, U.K. γ-Resistant Microporous CAU-1 MOF for Selective Remediation of Thorium. ACS Omega 2023, 8, 12268–12282. [Google Scholar] [CrossRef]
- Song, A.-M.; Yang, M.-J.; Wu, Z.; Yang, Q.; Lin, B.; Liang, R.-P.; Qiu, J.-D. Rational Designed Metal-organic Framework with Nanocavity Traps for Selectively Recognizing and Separating of Radioactive Thorium in Rare Earth Wastewater. Adv. Funct. Mater. 2024, 34, 2406932. [Google Scholar] [CrossRef]
- Khan, P.N.; Pahan, S.; Sengupta, A.; Dasgupta, K.; Vincent, T. Post-Synthetically Modified Metal Organic Framework Functionalized with a 1,2-Dihydroxybenzene Chelating Unit for Efficient Removal of Thorium and Uranyl Ions from Radioactive Waste. ACS Sustain. Resour. Manag. 2024, 1, 2530–2538. [Google Scholar] [CrossRef]
- Su, S.; Che, R.; Liu, Q.; Liu, J.; Zhang, H.; Li, R.; Jing, X.; Wang, J. Zeolitic Imidazolate Framework-67: A promising candidate for recovery of uranium (VI) from seawater. Colloids Surf. A 2018, 547, 73–80. [Google Scholar] [CrossRef]
- Das, A.; Roy, D.; Erukula, K.; De, S. Synthesis of pH responsive malononitrile functionalized metal organic framework MIL-100(Fe) for efficient adsorption of uranium U(VI) from real-life alkaline leach liquor. Chemosphere 2024, 348, 140780. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Y.; Zhao, X.; Chen, L.; Peng, S.; Ma, C.; Duan, G.; Liu, Z.; Wang, H.; Yuan, Y.; et al. A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater. e-Polymers 2022, 22, 399–410. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Q.; Liu, J.; Zhang, H.; Li, Z.; Li, R.; Liu, L.; Wang, J. Interfacial growth of a metal-organic framework (UiO-66) on functionalized graphene oxide (GO) as a suitable seawater adsorbent for extraction of uranium(vi). J. Mater. Chem. A 2017, 5, 17933–17942. [Google Scholar] [CrossRef]
- Zhao, B.; Yuan, L.; Wang, Y.; Duan, T.; Shi, W. Carboxylated UiO-66 Tailored for U(VI) and Eu(III) Trapping: From Batch Adsorption to Dynamic Column Separation. ACS Appl. Mater. Interfaces 2021, 13, 16300–16308. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, H.; Shan, T.; Yang, P.; Li, S.; Liu, Z.; Liu, C.; Shen, C. MOF-implanted poly (acrylamide-co-acrylic acid)/chitosan organic hydrogel for uranium extraction from seawater. Carbohydr. Polym. 2023, 302, 120377. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Zhang, H.; Liu, Q.; Liu, J.; Zhu, J.; Yu, J.; Chen, R. MOF-derived Co-Ni layered double hydroxides/polyethyleneimine modified chitosan micro-nanoreactor for high-efficiency capture of uranium from seawater. Carbohydr. Polym. 2024, 323, 121426. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).