Chemical Composition and Biological Activities of Chromolaena odorata (L.) R.M.King & H.Rob. Essential Oils from Central Vietnam
Abstract
1. Introduction
2. Results and Discussion
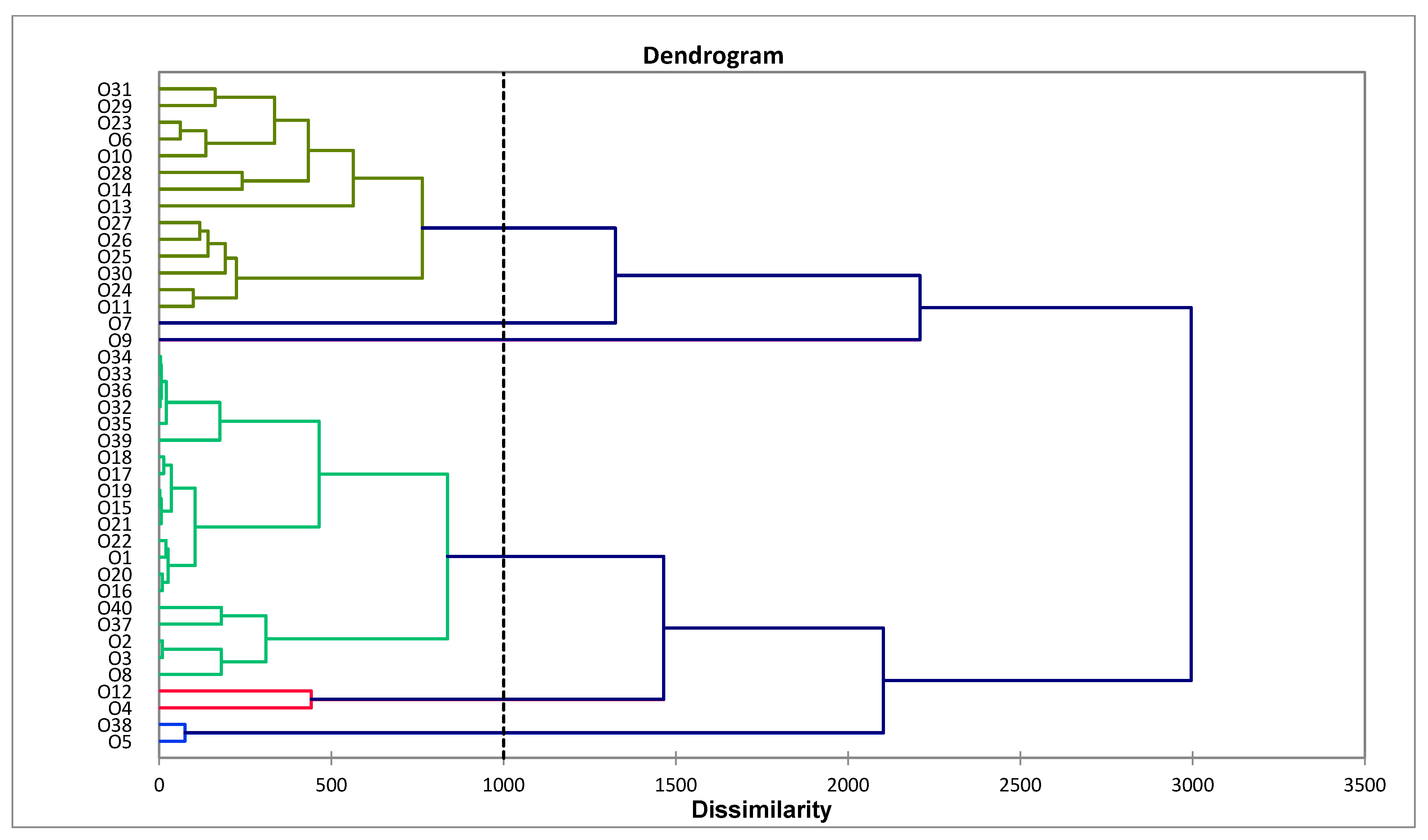
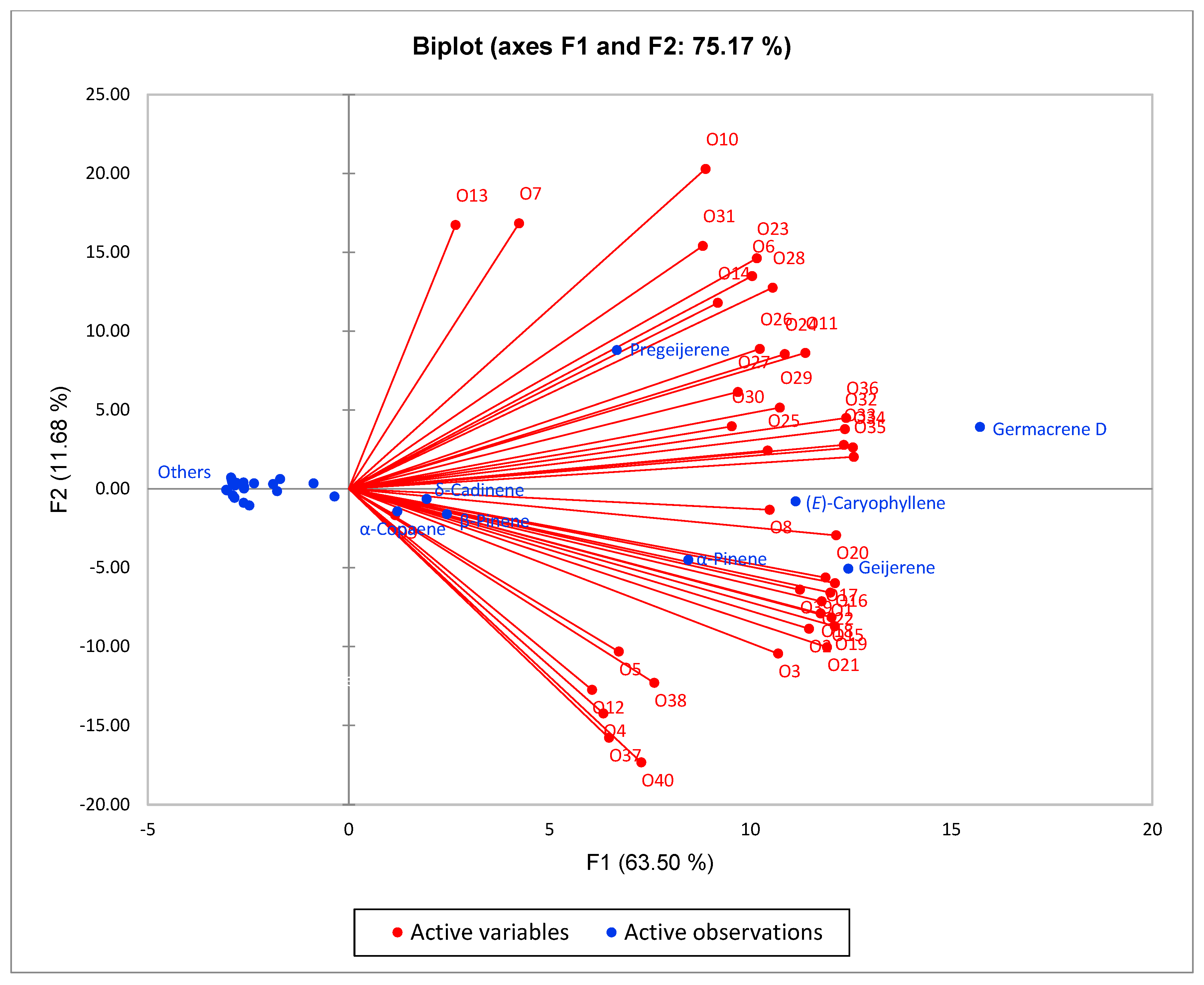
2.1. Chemical Profiles of Essential Oils
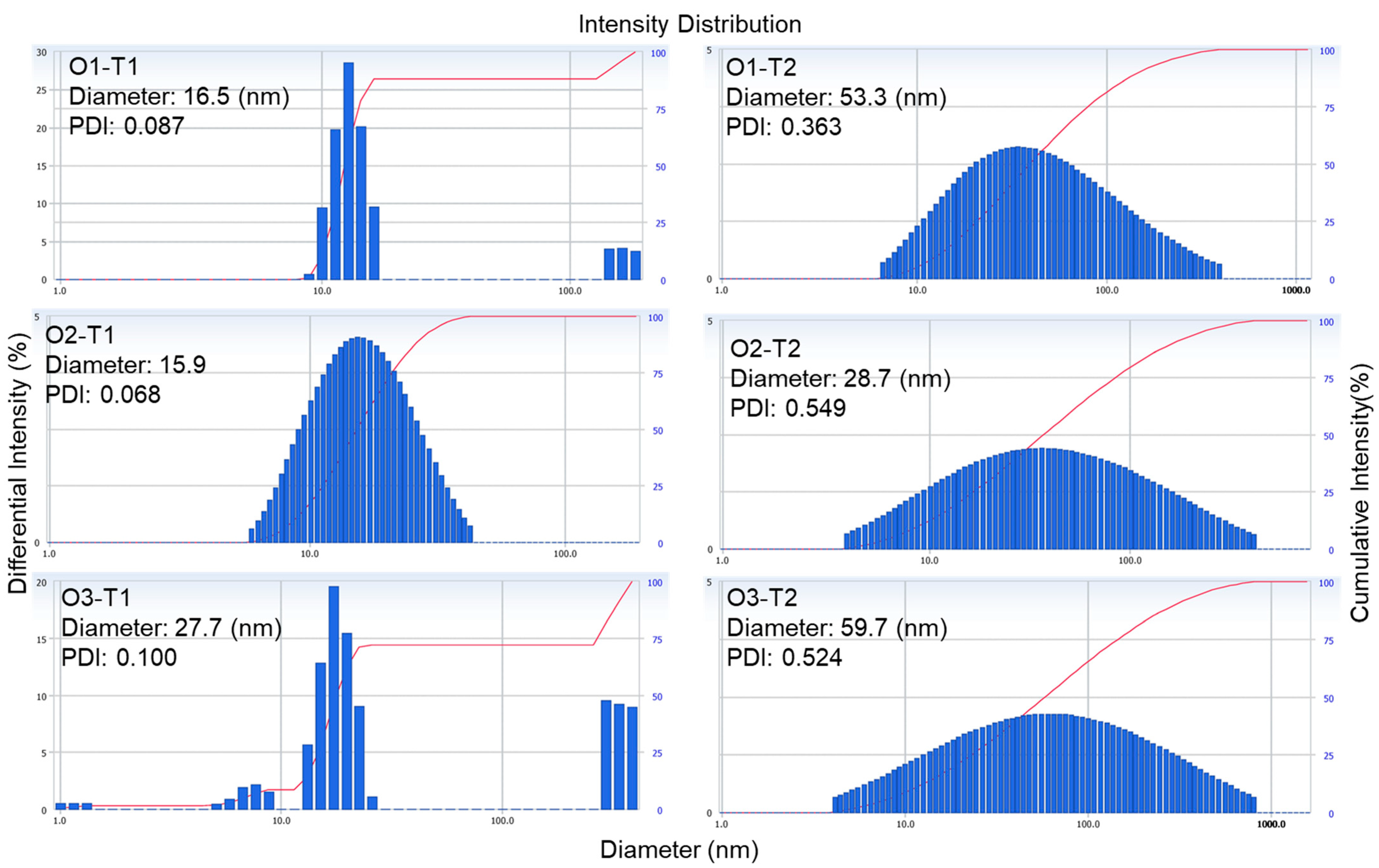
2.2. Characteristics of Microemulsion Formulas
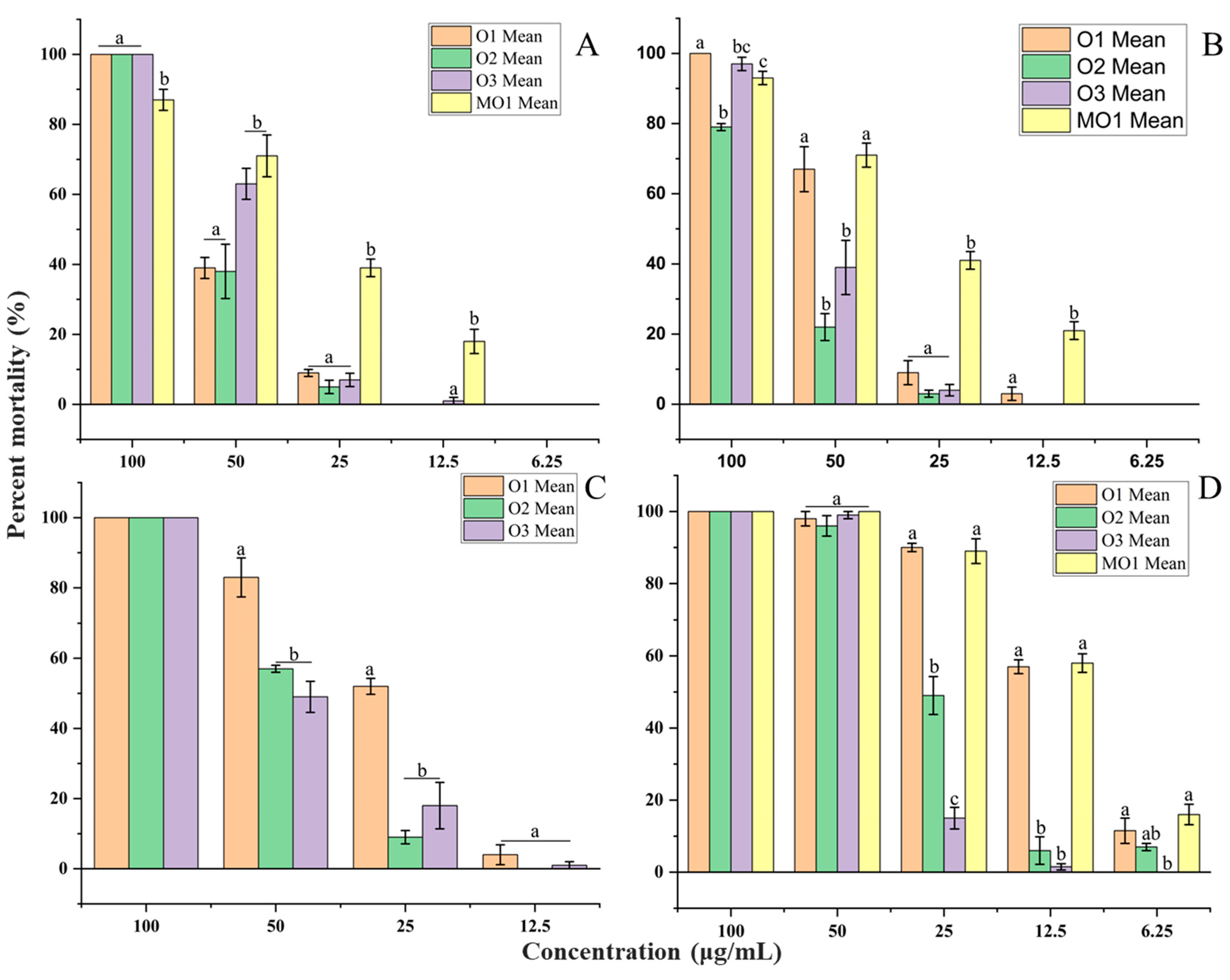
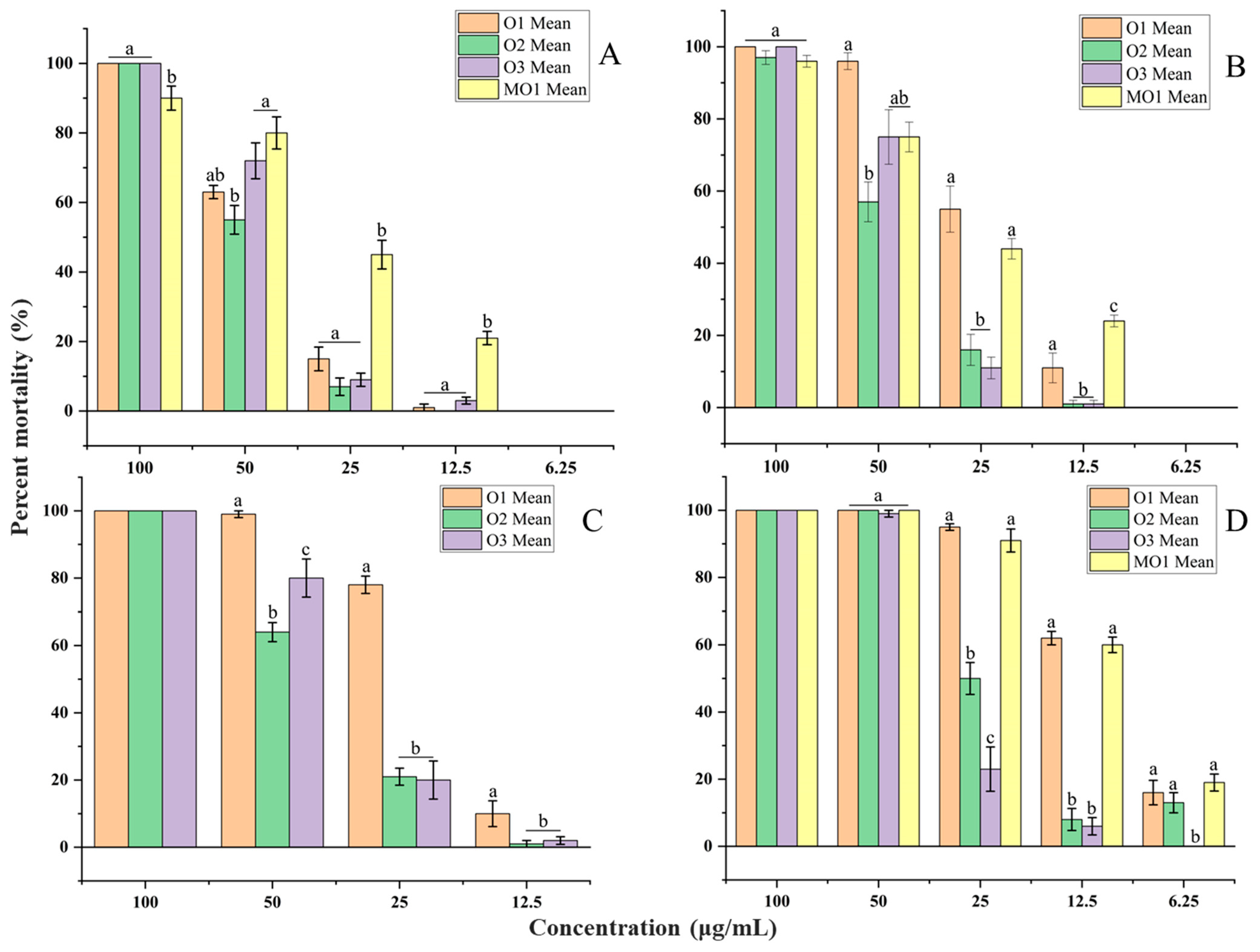
2.3. Larvicidal Activity
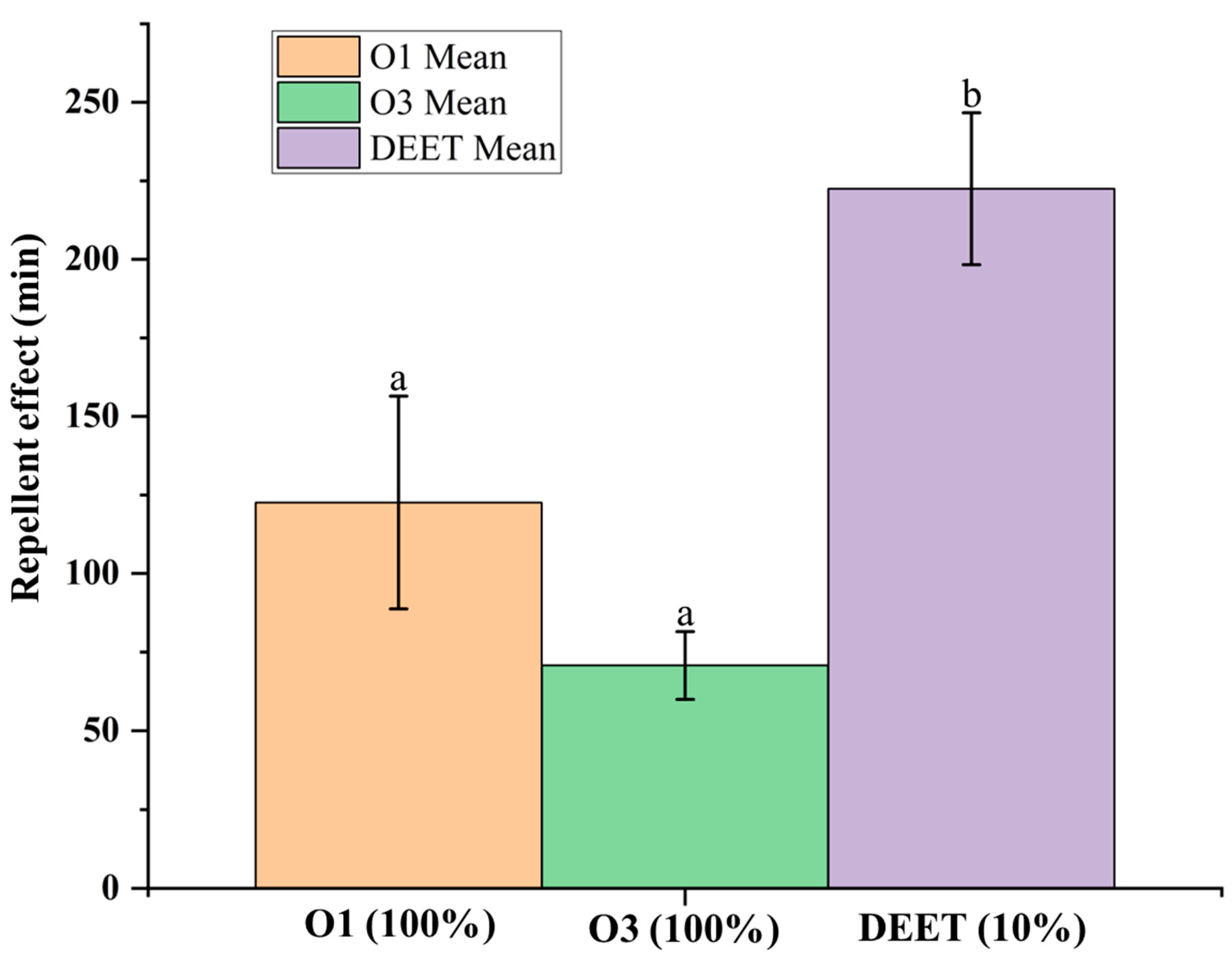
2.4. Repellent Activity
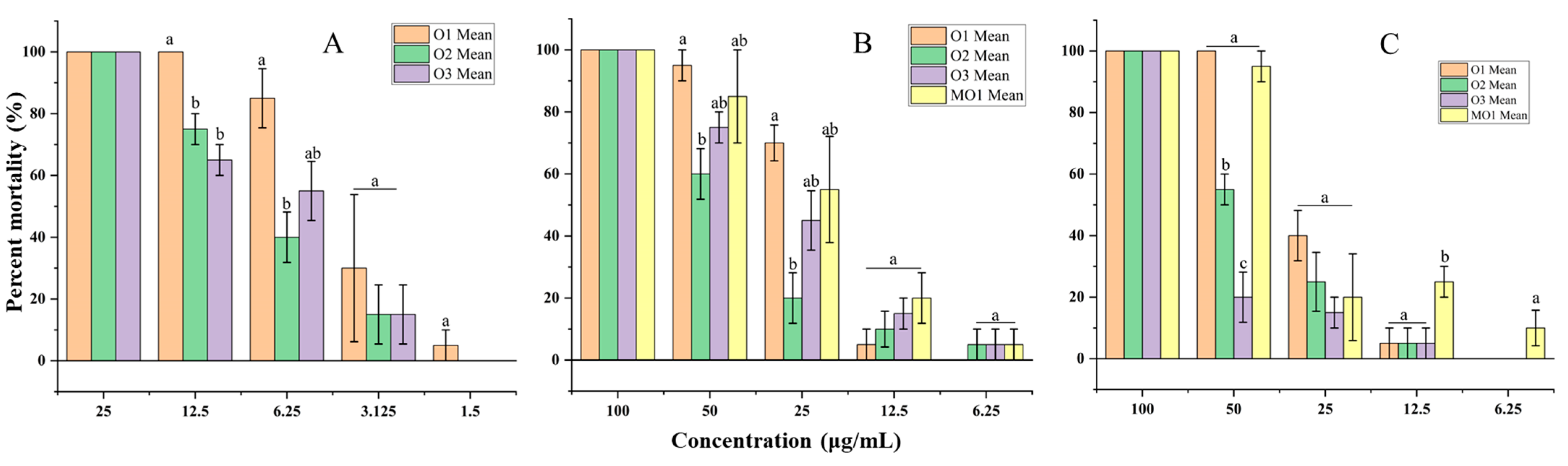
2.5. Fumigation Toxicity
2.6. Molluscicidal Activity
2.7. AChE Inhibitory Activity
2.8. Antimicrobial Activity
3. Materials and Methods
3.1. Plant Material
3.2. Hydrodistillation
3.3. Gas Chromatographic Analysis
3.4. Preparation of Microemulsion Formulas
3.5. Larvicidal Biassays
3.6. Repellency Bioassay
3.7. Fumigant Toxicity
3.8. Molluscicidal Activity
3.9. AChE Inhibitory Activity Assay
3.10. Antimicrobial Activity
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adhikari, P.; Lee, Y.H.; Poudel, A.; Hong, S.H.; Park, Y.S. Global spatial distribution of Chromolaena odorata habitat under climate change: Random forest modeling of one of the 100 worst invasive alien species. Sci. Rep. 2023, 13, 9745. [Google Scholar] [CrossRef]
- Amacher, L.; Silvestri, G.; Walther, G. Status and management of invasive alien species in Switzerland. In Invasive Alien Species; Wiley: Hoboken, NJ, USA, 2021; Volume 2, pp. 253–277. ISBN 9781119607045. [Google Scholar]
- Gogoi, R.; Sarma, N.; Begum, T.; Pandey, S.K.; Lal, M. North-East Indian Chromolaena odorata (L. King Robinson) aerial part essential oil chemical composition, pharmacological activities—Neurodegenerative inhibitory and toxicity study. J. Essent. Oil Bear. Plants 2020, 23, 1173–1191. [Google Scholar] [CrossRef]
- Dougnon, G.; Ito, M. Essential oil from the leaves of Chromolaena odorata, and sesquiterpene caryophyllene oxide induce sedative activity in mice. Pharmaceuticals 2021, 14, 651. [Google Scholar] [CrossRef] [PubMed]
- Carrubba, A. Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-90-481-2715-3. [Google Scholar]
- Johari, N.A.; Voon, K.; Toh, S.Y.; Sulaiman, L.H.; Yap, I.K.S.; Lim, P.K.C. Sylvatic dengue virus type 4 in Aedes aegypti and Aedes albopictus mosquitoes in an urban setting in Peninsular Malaysia. PLoS Negl. Trop. Dis. 2019, 13, e0007889. [Google Scholar] [CrossRef]
- Santos, J.; Meneses, B.M. An integrated approach for the assessment of the Aedes aegypti and Aedes albopictus global spatial distribution, and determination of the zones susceptible to the development of Zika virus. Acta Trop. 2017, 168, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-trotting Aedes aegypti and Aedes albopictus: Risk factors for arbovirus pandemics. Vector-Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.; Shearer, F.M.; Brady, O.J.; Messina, J.P.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci. Data 2015, 2, 150035. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Peyrefitte, C.N.; Mve, M.T.; Jarjaval, F.; Brisse, S.; Iteman, I.; Gravier, P.; Nkoghe, D.; Grandadam, M. Aedes albopictus mosquito: The main vector of the 2007 Chikungunya outbreak in Gabon. PLoS ONE 2009, 4, e4691. [Google Scholar] [CrossRef]
- Paupy, C.; Ollomo, B.; Kamgang, B.; Moutailler, S.; Rousset, D.; Demanou, M.; Hervé, J.P.; Leroy, E.; Simard, F. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in Central Africa. Vector-Borne Zoonotic Dis. 2010, 10, 259–266. [Google Scholar] [CrossRef]
- Simonsen, P.E.; Mwakitalu, M.E. Urban Lymphatic filariasis. Parasitol. Res. 2013, 112, 35–44. [Google Scholar] [CrossRef]
- Samy, A.M.; Elaagip, A.H.; Kenawy, M.A.; Ayres, C.F.J.; Peterson, A.T.; Soliman, D.E. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and Lymphatic filariasis. PLoS ONE 2016, 11, e0163863. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, S.K.; Yadav, R.P.; Srivastava, V.K.; Singh, D.; Tiwari, S. Eco-friendly molluscicides, piscicides and insecticides from common plants. In Trends in Agricultural and Soil Pollution Research; Nova Science: Hauppauge, NY, USA, 2006; pp. 205–230. ISBN 1594543259. [Google Scholar]
- Singh, D.K.; Singh, V.K.; Singh, R.N.; Kumar, P. Fasciolosis: Causes, Challenges and Controls; Springer: Singapore, 2021; ISBN 978-981-16-0258-0. [Google Scholar]
- Min, F.; Wang, J.; Liu, X.; Yuan, Y.; Guo, Y.; Zhu, K.; Chai, Z.; Zhang, Y.; Li, S. Environmental factors affecting freshwater snail intermediate hosts in Shenzhen and Adjacent region, South China. Trop. Med. Infect. Dis. 2022, 7, 426. [Google Scholar] [CrossRef]
- Benelli, G.; Bedini, S.; Flamini, G.; Cosci, F.; Cioni, P.L.; Amira, S.; Benchikh, F.; Laouer, H.; Di Giuseppe, G.; Conti, B. Mediterranean essential oils as effective weapons against the West Nile vector Culex pipiens and the echinostoma intermediate host Physella acuta: What happens around? An acute toxicity survey on non-target mayflies. Parasitol. Res. 2015, 114, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Kvach, Y.; Jurajda, P.; Bryjová, A.; Trichkova, T.; Ribeiro, F.; Přikrylová, I.; Ondračková, M. European distribution for metacercariae of the North American digenean Posthodiplostomum cf. minimum centrarchi (Strigeiformes: Diplostomidae). Parasitol. Int. 2017, 66, 635–642. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, H.C.; Dong, Y.; Zhang, T.; Sun, Y.Y.; Zhong, J.F.; Cao, Y.L.; Shao, S.W.; Pan, Y.L.; Dong, H.Y. New nodule type found in the lungs of Pomacea canaliculata, an intermediate host of Angiostrongylus cantonensis. Iran. J. Parasitol. 2018, 13, 362–368. [Google Scholar] [PubMed]
- Komalamisra, C.; Nuamtanong, S.; Dekumyoy, P. Pila ampullacea and Pomacea canaliculata, as new paratenic hosts of Gnathostoma spinigerum. Southeast Asian J. Trop. Med. Public Health 2009, 40, 243–246. [Google Scholar]
- Naylor, R. Invasions in agriculture: Assessing the cost of the golden apple snail in Asia. Ambio 1996, 25, 443–448. [Google Scholar]
- Muñoz, J.F.; Gade, L.; Chow, N.A.; Loparev, V.N.; Juieng, P.; Berkow, E.L.; Farrer, R.A.; Litvintseva, A.P.; Cuomo, C.A. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat. Commun. 2018, 9, 5346. [Google Scholar] [CrossRef] [PubMed]
- Escolà-Vergé, L.; Los-Arcos, I.; Almirante, B. New antibiotics for the treatment of infections by multidrug-resistant microorganisms. Med. Clin. (Barc) 2020, 154, 351–357. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance. Available online: https://www.who.int/europe/news-room/fact-sheets/item/antimicrobial-resistance (accessed on 11 July 2025).
- Dobetsberger, C.; Buchbauer, G. Actions of essential oils on the central nervous system: An updated review. Flavour Fragr. J. 2011, 26, 300–316. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Khan, M.A.; Ahmad, W.; Shah, M.R.; Imran, M.; Ahmad, S. Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L: A preliminary anti- Alzheimer’s study. Lipids Health Dis. 2015, 14, 141. [Google Scholar] [CrossRef]
- Duñg, N.X.; Bien, L.K.; Leclercq, P.A. The constituents of the leaf oil of Chromolaena odorata (L.) R. M. King and H. Robinson from Vietnam. J. Essent. Oil Res. 1992, 4, 309–310. [Google Scholar] [CrossRef]
- Owolabi, M.S.; Ogundajo, A.; Yusuf, K.O.; Lajide, L.; Villanueva, H.E.; Tuten, J.A.; Setzer, W.N. Chemical composition and bioactivity of the essential oil of Chromolaena odorata from Nigeria. Rec. Nat. Prod. 2010, 4, 72–78. [Google Scholar]
- Pisutthanan, N.; Liawruangrath, B.; Liawruangrath, S.; Baramee, A.; Apisariyakul, A.; Korth, J.; Bremner, J.B. Constituents of the essential oil from aerial parts of Chromolaena odorata from Thailand. Nat. Prod. Res. 2006, 20, 636–640. [Google Scholar] [CrossRef]
- Pitakpawasutthi, Y.; Thitikornpong, W.; Palanuvej, C.; Ruangrungsi, N. Chlorogenic acid content, essential oil compositions, and in vitro antioxidant activities of Chromolaena odorata leaves. J. Adv. Pharm. Technol. Res. 2016, 7, 37. [Google Scholar] [CrossRef]
- Félicien, A.; Guy Alain, A.; Sébastien, D.T.; Fidele, T.; Boniface, Y.; Chantal, M.; Dominique, S. Chemical composition and biological activities of the essential oil extracted from the fresh leaves of Chromolaena odorata (L. Robinson) growing in Benin. ISCA J. Biol. Sci. 2012, 1, 7–13. [Google Scholar]
- Kossouoh, C.; Moudachirou, M.; Adjakidje, V.; Chalchat, J.C.; Figuérédo, G.; Chalard, P. Volatile constituents of Chromolaena odorata (L.) R.M. King & H. Rob. leaves from Benin. J. Essent. Oil Bear. Plants 2011, 14, 224–228. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical composition of the essential oils of aerial parts and flowers of Chromolaena odorata (L.) R. M. King & H. Rob. from western ghats region of North West Karnataka, India. J. Essent. Oil Bear. Plants 2013, 16, 71–75. [Google Scholar] [CrossRef]
- Esse, L.W.; Affia, F.B.; Thierry, A.Y.; Zanahi, F.T. Multifactorial discriminant analysis of leaf oil of C. odorata L. King and Robinson from Cte DIvoire. Afr. J. Pure Appl. Chem. 2015, 9, 18–26. [Google Scholar] [CrossRef]
- Tonzibo, Z.F.; Wognin, E.; Chalchat, J.C.; N’Guessan, Y.T. Chemical investigation of Chromolaena odorata L. King Robinson from ivory coast. J. Essent. Oil Bear. Plants 2007, 10, 94–100. [Google Scholar] [CrossRef]
- Osei-owusu, J.; Acheampong, A.; Afun, J.V.; Acquaah, S.O. Chemical composition of the headspace volatiles from Chromolaena odorata (L.) R.M. King in Ghana. J. Essent. Oil Bear. Plants 2017, 20, 1418–1423. [Google Scholar] [CrossRef]
- Koba, K.; Nénonéné, A.Y.; Catherine, G.; Raynaud, C.; Chaumont, J.-P.; Sanda, K.; Laurence, N. Chemical composition and cytotoxic activity of essential oil of Chromolaena odorata L. growing in Togo. J. Essent. Oil Bear. Plants 2011, 14, 423–429. [Google Scholar] [CrossRef]
- Daouda, T.; Bi, K.F.P.K.; Gustave, B.; Allico, J.; Nathalie, G.; Raphael, O.; Jean, C.C.; Mireille, D.; Felix, T. Effect of geographical location and antibacterial activities of essential oils from ivoirian Chromolaena odorata (L) R. M. King Robinson (Asteraceae). J. Pharmacogn. Phyther. 2014, 6, 70–78. [Google Scholar] [CrossRef]
- Baruah, R.; Leclercq, P. Constituents of the essential oil from the flowers of Chromolaena odorata. Planta Med. 1993, 59, 283. [Google Scholar] [CrossRef]
- Trang, V.M.; Son, N.T.; Luyen, N.D.; Giang, P.M. Essential oils from Chromolaena odorata (L.) R. M. King and H. Robinson stem barks and leaves: Chemical analysis, biological activity and in silico approach. Chem. Biodivers. 2025, 22, e202500091. [Google Scholar] [CrossRef]
- Ganesan, P.; Selvakumaran, J.; Ignacimuthu, S.; Aldahmash, B.; Rady, A.; Alzahrani, F.A.; Almansour, M.; Stalin, A. Activity of essential oils from Pentanema indicum (L.) Y. Ling and Chromolaena odorata (L.) R.M.King & H. Rob against three mosquito species. Entomol. Res. 2025, 55, e70016. [Google Scholar] [CrossRef]
- Swor, K.; Poudel, A.; Satyal, P.; Setzer, W.N. The Essential oil compositions of Ambrosia acanthicarpa Hook., Artemisia ludoviciana Nutt., and Gutierrezia sarothrae (Pursh) Britton & Rusby (Asteraceae) from the Owyhee Mountains of Idaho. Molecules 2024, 29, 1383. [Google Scholar] [CrossRef]
- Poudel, A.; Satyal, P.; Swor, K.; Setzer, W.N. The essential oil characterization of Achillea millefolium var. occidentalis DC. from the Great Basin of North America. J. Essent. Oil Plant Compos. 2024, 2, 130–142. [Google Scholar] [CrossRef]
- Stirling, J.; Platt, B.G.; Satyal, P.; Swor, K.; Setzer, W.N. The essential oils of Rubber Rabbitbrush (Ericameria nauseosa) from North-Central Utah and Southwestern Idaho. Nat. Prod. Commun. 2023, 18, 1934578X231161186. [Google Scholar] [CrossRef]
- Cespi, M.; Quassinti, L.; Perinelli, D.R.; Bramucci, M.; Iannarelli, R.; Papa, F.; Ricciutelli, M.; Bonacucina, G.; Palmieri, G.F.; Maggi, F. Microemulsions enhance the shelf-life and processability of Smyrnium olusatrum L. essential oil. Flavour Fragr. J. 2017, 32, 159–164. [Google Scholar] [CrossRef]
- Date, A.A.; Nagarsenker, M.S. Parenteral microemulsions: An overview. Int. J. Pharm. 2008, 355, 19–30. [Google Scholar] [CrossRef]
- Flanagan, J.; Kortegaard, K.; Neil Pinder, D.; Rades, T.; Singh, H. Solubilisation of soybean oil in microemulsions using various surfactants. Food Hydrocoll. 2006, 20, 253–260. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 175–193. [Google Scholar] [CrossRef]
- Xu, J.; Fan, Q.J.; Yin, Z.Q.; Li, X.T.; Du, Y.H.; Jia, R.Y.; Wang, K.Y.; Lv, C.; Ye, G.; Geng, Y.; et al. The preparation of neem oil microemulsion (Azadirachta indica) and the comparison of acaricidal time between neem oil microemulsion and other formulations in vitro. Vet. Parasitol. 2010, 169, 399–403. [Google Scholar] [CrossRef]
- Wooster, T.J.; Labbett, D.; Sanguansri, P.; Andrews, H. Impact of microemulsion inspired approaches on the formation and destabilisation mechanisms of triglyceride nanoemulsions. Soft Matter 2016, 12, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Awad, T.S.; Asker, D.; Romsted, L.S. Evidence of coexisting microemulsion droplets in oil-in-water emulsions revealed by 2D DOSY 1H NMR. J. Colloid Interface Sci. 2018, 514, 83–92. [Google Scholar] [CrossRef]
- Hung, N.H.; Dai, D.N.; Cong, T.N.; Dung, N.A.; Linh, L.D.; Van Hoa, V.; Hien, T.T.; Chuong, N.T.H.; Hien, V.T.; Nguyen, B.V.; et al. Pesticidal activities of Callicarpa and Premna essential oils from Vietnam. Nat. Prod. Commun. 2022, 17, 1934578X221110660. [Google Scholar] [CrossRef]
- Dias, C.N.; Moraes, D.F.C. Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicides: Review. Parasitol. Res. 2014, 113, 565–592. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Hoi, T.M.; Huong, L.T.; Van Chinh, H.; Hau, D.V.; Satyal, P.; Tai, T.A.; Dai, D.N.; Hung, N.H.; Hien, V.T.; Setzer, W.N. Essential oil compositions of three invasive Conyza species collected in Vietnam and their larvicidal activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Molecules 2020, 25, 4576. [Google Scholar] [CrossRef]
- Huy Hung, N.; Ngoc Dai, D.; Satyal, P.; Thi Huong, L.; Thi Chinh, B.; Quang Hung, D.; Anh Tai, T.; Setzer, W.N. Lantana camara essential oils from Vietnam: Chemical composition, molluscicidal, and mosquito larvicidal activity. Chem. Biodivers. 2021, 18, e2100145. [Google Scholar] [CrossRef]
- Luu, H.V.L.; Nguyen, H.H.; Satyal, P.; Vo, V.H.; Ngo, G.H.; Pham, V.T.; Setzer, W.N. Chemical composition, larvicidal and molluscicidal activity of essential oils of six Guava cultivars grown in Vietnam. Plants 2023, 12, 2888. [Google Scholar] [CrossRef]
- Govindarajan, M. Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (Willd.) Hook. f. Ex Benth (Rutaceae) against three mosquito species. Asian Pac. J. Trop. Med. 2010, 3, 874–877. [Google Scholar] [CrossRef]
- Ravi Kiran, S.; Bhavani, K.; Sita Devi, P.; Rajeswara Rao, B.R.; Janardhan Reddy, K. Composition and larvicidal activity of leaves and stem essential oils of Chloroxylon swietenia DC against Aedes aegypti and Anopheles stephensi. Bioresour. Technol. 2006, 97, 2481–2484. [Google Scholar] [CrossRef] [PubMed]
- Sritabutra, D.; Soonwera, M. Repellent activity of herbal essential oils against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.). Asian Pac. J. Trop. Dis. 2013, 3, 271–276. [Google Scholar] [CrossRef]
- Sutthanont, N.; Sudsawang, M.; Phanpoowong, T.; Sriwichai, P.; Ruangsittichai, J.; Rotejanaprasert, C.; Srisawat, R. Effectiveness of herbal essential oils as single and combined repellents against Aedes aegypti, Anopheles dirus and Culex quinquefasciatus (Diptera: Culicidae). Insects 2022, 13, 658. [Google Scholar] [CrossRef]
- Alı, A.; Tabanca, N.; Kurkcuoglu, M.; Duran, A.; Blythe, E.K.; Khan, I.A.; Can Baser, K.H. Chemical composition, larvicidal, and biting deterrent activity of essential oils of two subspecies of Tanacetum argenteum (Asterales: Asteraceae) and individual constituents against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Tabanca, N.; Demirci, B.; Blythe, E.K.; Ali, Z.; Baser, K.H.C.; Khan, I.A. Chemical composition and biological activity of four Salvia essential oils and individual compounds against two species of mosquitoes. J. Agric. Food Chem. 2015, 63, 447–456. [Google Scholar] [CrossRef]
- Baz, M.M.; Montaser, A.S.; Ali, R.M. Efficacy of mosquito repellent finishes on polyester fabrics using four essential oils against the vector of the West Nile virus, Culex pipiens (Diptera: Culicidae). J. Text. Inst. 2024, 116, 293–308. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Birkett, M.A.; Blande, J.; Hooper, A.M.; Martin, J.L.; Khambay, B.; Prosser, I.; Smart, L.E.; Wadhams, L.J. Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest Manag. Sci. 2005, 61, 1115–1121. [Google Scholar] [CrossRef]
- Ravi Kiran, S.; Pushpalatha, K. Repellency of essential oil and sesquiterpenes from leaves of Chloroxylon swietenia DC. against mosquito bites. Vedic Res. Int. Phytomed. 2013, 1, 103. [Google Scholar] [CrossRef]
- Liakakou, A.; Angelis, A.; Papachristos, D.P.; Fokialakis, N.; Michaelakis, A.; Skaltsounis, L.A. Isolation of volatile compounds with repellent properties against Aedes albopictus (Diptera: Culicidae) using CPC technology. Molecules 2021, 26, 3072. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Ma, Y.P.; Zhang, H.X.; Sun, H.Z.; Su, H.H.; Pei, S.J.; Du, Z.Z. Repellent, larvicidal and adulticidal activities of essential oil from Dai medicinal plant Zingiber cassumunar against Aedes albopictus. Plant Divers. 2021, 43, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Van, N.T.H.; Bach, P.C.; Thuong, V.T.; Tuyen, T.T.; Vien, T.A.; Thuy, D.T.T.; Quynh, D.T.; Nghi, D.H.; Quan, P.M.; Xuan, N.M.; et al. Chemical composition and pesticidal activities against three vector mosquito species of Zanthoxylum armatum DC. essential oils. Chem. Biodivers. 2025, 22, e202500648. [Google Scholar] [CrossRef]
- Lucia, A.; Licastro, S.; Zerba, E.; Audino, P.G.; Masuh, H. Sensitivity of Aedes aegypti adults (Diptera: Culicidae) to the vapors of Eucalyptus essential oils. Bioresour. Technol. 2009, 100, 6083–6087. [Google Scholar] [CrossRef]
- Ma, S.; Jia, R.; Guo, M.; Qin, K.; Zhang, L. Insecticidal activity of essential oil from Cephalotaxus sinensis and its main components against various agricultural pests. Ind. Crops Prod. 2020, 150, 112403. [Google Scholar] [CrossRef]
- Choi, W.S.; Park, B.S.; Lee, Y.H.; Jang, D.Y.; Yoon, H.Y.; Lee, S.E. Fumigant toxicities of essential oils and monoterpenes against Lycoriella mali adults. Crop Prot. 2006, 25, 398–401. [Google Scholar] [CrossRef]
- Kiran, S.R.; Reddy, A.S.; Devi, P.S.; Reddy, K.J. Insecticidal, antifeedant and oviposition deterrent effects of the essential oil and individual compounds from leaves of Chloroxylon swietenia DC. Pest Manag. Sci. 2006, 62, 1116–1121. [Google Scholar] [CrossRef]
- Dai, L.; Wang, W.; Dong, X.; Hu, R.; Nan, X. Molluscicidal activity of cardiac glycosides from Nerium indicum against Pomacea canaliculata and its implications for the mechanisms of toxicity. Environ. Toxicol. Pharmacol. 2011, 27, e00375. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Huang, R.; Zhou, Z.; He, H.; Li, Y. Ambrosia artemisiifolia as a potential resource for management of Golden Apple snails, Pomacea canaliculata (Lamarck). Pest Manag. Sci. 2018, 74, 944–949. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.; Tang, W.; Song, Z. Two new triterpenoids from the bark of Eucalyptus exserta and their molluscicidal and cytotoxic activities. Fitoterapia 2012, 83, 383–387. [Google Scholar] [CrossRef]
- Hung, N.H.; Quan, P.M.; Satyal, P.; Dai, D.N.; Van Hoa, V.; Huy, N.G.; Giang, L.D.; Ha, N.T.; Huong, L.T.; Hien, V.T.; et al. Acetylcholinesterase inhibitory activities of essential oils from Vietnamese traditional medicinal plants. Molecules 2022, 27, 7092. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef]
- Savelev, S.U.; Okello, E.J.; Perry, E.K. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phyther. Res. 2004, 18, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Yilmaz, S.V.; Özdemir, Ö.; Koca, M.; Pınar, N.M.; Demirci, B.; Yıldırım, K.; Sytar, O.; Turkez, H.; Baser, K.H.C. A caryophyllene oxide and other potential anticholinesterase and anticancer agent in Salvia verticillata subsp. amasiaca (Freyn & Bornm.) Bornm. (Lamiaceae). J. Essent. Oil Res. 2020, 32, 512–525. [Google Scholar] [CrossRef]
- Savelev, S.; Okello, E.; Perry, N.S.L.; Wilkins, R.M.; Perry, E.K. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 2003, 75, 661–668. [Google Scholar] [CrossRef]
- Salihu, A.S.; Salleh, W.M.N.H.W.; Ogunwa, T.H. Chemical composition, acetylcholinesterase inhibition and molecular docking studies of essential oil from Knema hookeriana Warb. (Myristicaceae). Nat. Prod. Res. 2024, 38, 2516–2521. [Google Scholar] [CrossRef]
- Luu-dam, N.A.; Le, C.V.C.; Satyal, P.; Le, T.M.H.; Bui, V.H.; Vo, V.H.; Ngo, G.H.; Bui, T.C.; Nguyen, H.H.; Setzer, W.N. Chemistry and bioactivity of croton essential oils: Literature survey and Croton hirtus from Vietnam. Molecules 2023, 28, 2361. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Trinh, N.B.; Satyal, P.; Vo, V.H.; Ngo, G.H.; Le, T.T.T.; Vo, T.T.; Nguyen, V.H.; Nguyen, H.H.; Nguyen, T.T.; et al. Chemical composition, mosquito larvicidal and molluscicidal activities of Magnolia foveolata leaf essential oil. Biochem. Syst. Ecol. 2023, 109, 104666. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Mondello, L. FFNSC 3; Shimadzu Scientific Instruments: Columbia, MD, USA, 2016. [Google Scholar]
- NIST17; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017.
- Giang Le, D.; Satyal, P.; Giang Nguyen, H.; Nhi Nguyen, T.U.; Nhung Nguyen, C.; Hang Le, T.; Huynh Le, V.; Luong Ngo, X.; Hoa Le, T.M.; Hoa Vo, V.; et al. Essential oil and waste hydrosol of Ocimum tenuiflorum L.: A low-cost raw material source of eugenol, botanical pesticides, and therapeutic potentiality. Chem. Biodivers. 2024, 21, e202401161. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
| RIcalc | RIdb | Compound | % | ||
|---|---|---|---|---|---|
| O1 | O2 | O3 | |||
| 927 | 927 | α-Thujene | tr | 0.09 | 0.09 |
| 935 | 933 | α-Pinene | 11.47 | 16.08 | 19.24 |
| 950 | 950 | Camphene | tr | tr | 0.05 |
| 974 | 972 | Sabinene | 0.94 | 1.26 | 1.51 |
| 980 | 978 | β-Pinene | 3.95 | 5.99 | 7.50 |
| 991 | 991 | Myrcene | 0.67 | 0.65 | 0.89 |
| 1026 | 1025 | p-Cymene | tr | 0.08 | 0.15 |
| 1031 | 1030 | Limonene | 0.73 | 0.65 | 0.73 |
| 1037 | 1035 | (Z)-β-Ocimene | 0.13 | 0.13 | 0.13 |
| 1047 | 1046 | (E)-β-Ocimene | 1.00 | 0.94 | 1.08 |
| 1059 | 1058 | γ-Terpinene | tr | tr | 0.09 |
| 1135 | - | iso-Geijerene | 1.40 | 1.21 | 1.07 |
| 1145 | 1143 | Geijerene | 10.55 | 9.71 | 8.96 |
| 1286 | 1286 | Cogeijerene | 0.45 | 0.44 | 0.38 |
| 1295 | 1289 | Pregeijerene | 0.52 | 0.48 | 0.40 |
| 1333 | 1335 | Bicycloelemene | 0.13 | 0.12 | tr |
| 1337 | 1336 | δ-Elemene | 0.32 | 0.35 | 0.30 |
| 1348 | 1348 | α-Cubebene | 0.14 | 0.10 | tr |
| 1378 | 1377 | α-Copaene | 5.26 | 4.45 | 4.02 |
| 1385 | 1382 | β-Bourbonene | 0.17 | 0.30 | 0.19 |
| 1389 | 1387 | β-Cubebene | 0.16 | 0.10 | 0.06 |
| 1391 | 1390 | trans-β-Elemene | 1.74 | 1.39 | 1.30 |
| 1422 | 1418 | (E)-β-Caryophyllene | 11.24 | 10.86 | 9.56 |
| 1431 | 1433 | β-Copaene | 0.53 | 0.47 | 0.48 |
| 1435 | 1431 | Dictamnol | 1.56 | 1.94 | 2.15 |
| 1442 | 1438 | iso-Dictamnol | 0.35 | 0.19 | 0.20 |
| 1450 | 1453 | trans-Muurola-3,5-diene | 0.20 | 0.15 | 0.16 |
| 1457 | 1454 | α-Humulene | 3.22 | 2.97 | 2.97 |
| 1461 | 1458 | allo-Aromadendrene | tr | 0.10 | 0.08 |
| 1473 | 1473 | trans-Cadina-1(6),4-diene | tr | 0.25 | 0.26 |
| 1478 | 1478 | γ-Muurolene | 0.64 | 0.72 | 0.67 |
| 1484 | 1483 | Germacrene D | 15.12 | 13.49 | 11.67 |
| 1493 | 1492 | trans-Muurola-4(14),5-diene | 0.62 | 0.39 | 0.43 |
| 1497 | 1497 | Bicyclogermacrene | 2.42 | 2.10 | 1.72 |
| 1500 | 1500 | α-Muurolene | 0.96 | 0.78 | 0.75 |
| 1507 | 1504 | (E,E)-α-Farnesene | tr | 0.15 | tr |
| 1509 | 1508 | β-Bisabolene | 0.19 | 0.21 | 0.12 |
| 1514 | 1514 | γ-Cadinene | 0.54 | 0.32 | 0.36 |
| 1516 | 1515 | Cubebol | 0.41 | tr | 0.22 |
| 1521 | 1520 | δ-Cadinene | 5.73 | 4.38 | 4.42 |
| 1523 | 1527 | trans-Calamenene | 0.73 | 0.47 | 0.71 |
| 1525 | 1526 | Zonarene | 0.20 | tr | tr |
| 1533 | 1533 | trans-Cadina-1,4-diene | 0.13 | tr | tr |
| 1542 | 1541 | α-Calacorene | 0.62 | 0.56 | 0.60 |
| 1550 | 1549 | α-Elemol | 0.52 | tr | tr |
| 1552 | 1551 | iso-Caryophyllene oxide | 0.41 | 0.45 | 0.43 |
| 1559 | 1560 | Germacrene B | 0.41 | 0.46 | 0.48 |
| 1563 | 1562 | (E)-Nerolidol | 0.34 | 0.25 | 0.32 |
| 1579 | 1578 | Spathulenol | 0.76 | 0.73 | 0.74 |
| 1584 | 1587 | Caryophyllene oxide | 5.02 | 4.40 | 5.33 |
| 1590 | 1592 | Globulol | tr | tr | 0.25 |
| 1595 | 1594 | Viridiflorol | 0.35 | 0.39 | tr |
| 1610 | 1611 | Humulene epoxide II | 1.00 | 0.81 | 0.99 |
| 1617 | 1618 | α-Corocalene | 0.24 | 0.13 | tr |
| 1622 | 1625 | Junenol | 0.22 | 0.25 | 0.12 |
| 1628 | 1628 | 1-epi-Cubenol | 0.10 | 0.48 | 0.35 |
| 1633 | 1629 | iso-Spathulenol | 0.26 | tr | - |
| 1638 | 1644 | allo-Aromadendrene epoxide | - | 0.35 | 0.38 |
| 1643 | 1643 | τ-Cadinol | 0.54 | 0.42 | 0.44 |
| 1645 | 1645 | τ-Muurolol | 0.44 | 0.43 | 0.43 |
| 1647 | 1651 | α-Muurolol (=δ-Cadinol) | 0.22 | tr | tr |
| 1650 | 1647 | cis-Guaia-3,9-dien-11-ol | 0.37 | 0.50 | 0.33 |
| 1656 | 1655 | α-Cadinol | 1.37 | 1.13 | 1.05 |
| 1674 | 1676 | Mustakone | 0.25 | 0.36 | 0.32 |
| 1716 | 1715 | Pentadecanal | 0.18 | 0.27 | 0.33 |
| 2108 | 2109 | Phytol | 1.09 | 1.39 | 1.08 |
| Monoterpene hydrocarbons | 18.89 | 25.87 | 31.46 | ||
| Oxygenated monoterpenoids | 0.00 | 0.00 | 0.00 | ||
| Sesquiterpene hydrocarbons | 51.66 | 45.77 | 41.31 | ||
| Oxygenated sesquiterpenoids | 12.58 | 10.95 | 11.70 | ||
| Diterpenoids | 1.09 | 1.39 | 1.08 | ||
| Other | 15.01 | 14.24 | 13.49 | ||
| Total identified | 99.23 | 98.22 | 99.04 | ||
| Compound | RTdb | RTexp | O1 | O2 | O3 | A.l. | A.a. | A.m.o. | E.n. |
|---|---|---|---|---|---|---|---|---|---|
| (−)-α-Pinene | 15.92 | 15.47 | 54.5 | 58.3 | 54.8 | 50.6–88.0 | 99.3–99.4 | 72.8–87.3 | 59.5–90.6 |
| (+)-α-Pinene | 16.40 | 15.99 | 45.5 | 41.7 | 45.2 | 12.0–49.4 | 0.6–0.7 | 12.7–27.2 | 9.4–41.5 |
| (+)-Sabinene | 19.74 | 19.76 | 63.2 | 64.2 | 60.8 | 13.9–79.4 | 48.2–53.7 | 11.8–56.1 | 0.0–25.6 |
| (−)-Sabinene | 20.60 | 20.79 | 36.8 | 35.8 | 39.2 | 20.6–86.1 | 46.3–51.8 | 43.9–88.2 | 74.4–100.0 |
| (+)-β-Pinene | 20.27 | 20.10 | 95.8 | 96.9 | 96.7 | 2.5–25.8 | 12.1–13.5 | 1.1–18.8 | 0.4–10.4 |
| (−)-β-Pinene | 20.62 | 20.98 | 4.2 | 3.1 | 3.3 | 74.2–97.5 | 86.5–87.9 | 81.2–98.9 | 89.6–99.6 |
| (−)-Limonene | 25.06 | 25.46 | 30.9 | 38.7 | 39.6 | 38.0–100.0 | 50.2–60.4 | 31.8–83.1 | 40.2–95.8 |
| (+)-Limonene | 25.99 | 26.23 | 69.1 | 61.3 | 60.4 | 0.0–62.0 | 39.6–49.8 | 16.9–68.2 | 4.2–59.8 |
| Essential Oil | LC50 (95% Limits) | LC90 (95% Limits) | χ2 | p |
|---|---|---|---|---|
| Aedes aegypti | ||||
| O3 | 43.53 (40.30–46.90) | 68.96 (62.36–79.21) | 4.46 | 0.216 |
| O2 | 53.46 (50.31–57.58 | 71.26 (65.51–80.58) | 2.92 | 0.404 |
| O1 | 52.99 (49.58–57.25) | 73.42 (67.43–82.42) | 7.10 | 0.069 |
| MO1 | 32.43 (28.68–36.79) | 101.93 (83.00–133.89) | 6.62 | 0.085 |
| Aedes albopictus | ||||
| O3 | 53.42 (49.67–57.60) | 83.71 (75.09–97.99) | 2.07 | 0.557 |
| O2 | 69.87 (63.94–76.81) | 127.60 (110.95–155.54) | 0.46 | 0.927 |
| O1 | 44.08 (41.30–47.11) | 61.89 (57.61–67.78) | 1.59 | 0.663 |
| MO1 | 29.81 (26.48–33.62) | 87.62 (72.41–112.62) | 8.11 | 0.044 |
| Culex quinquefasciatus | ||||
| O3 | 44.34 (40.58–48.50) | 83.06 (73.08–98.67) | 14.94 | 0.002 |
| O2 | 44.31 (41.08–47.85) | 72.42 (65.20–83.30) | 3.78 | 0.287 |
| O1 | 27.25 (24.95–29.74) | 52.65 (46.73–61.25) | 7.35 | 0.061 |
| MO1 | Nt | Nt | Nt | Nt |
| Culex fuscocephala | ||||
| O3 | 31.97 (29.72–34.93) | 41.69 (38.13–47.16) | 3.56 | 0.313 |
| O2 | 26.41 (24.31–28.89) | 41.26 (37.54–46.57) | 4.95 | 0.176 |
| O1 | 11.73 (10.60–12.92) | 24.55 (21.40–29.53) | 0.91 | 0.823 |
| MO1 | 11.16 (10.12–12.30) | 23.95 (20.87–28.61) | 2.14 | 0.710 |
| Essential Oil | LC50 (95% Limits) | LC90 (95% Limits) | χ2 | p |
|---|---|---|---|---|
| Aedes aegypti | ||||
| O3 | 45.23 (42.37–48.42) | 63.82 (59.27–70.19) | 1.68 | 0.642 |
| O2 | 46.27 (42.96–49.75) | 72.12 (65.23–83.16) | 4.61 | 0.203 |
| O1 | 41.05 (37.74–44.62) | 71.37 (63.63–83.23) | 5.17 | 0.160 |
| MO1 | 28.54 (25.31–32.22) | 85.72 (70.68–110.50) | 7.55 | 0.056 |
| Aedes albopictus | ||||
| O3 | 38.89 (35.94–42.01) | 61.78 (55.88–70.63) | 1.97 | 0.578 |
| O2 | 43.43 (39.73–47.52) | 81.75 (71.90–97.09) | 3.17 | 0.366 |
| O1 | 23.04 (21.18–25.03) | 40.49 (36.08–47.23) | 2.23 | 0.527 |
| MO1 | 27.08 (24.13–30.40) | 75.52 (63.11–95.53) | 10.59 | 0.014 |
| Culex quinquefasciatus | ||||
| O3 | 34.87 (32.12–37.87) | 58.93 (52.72–68.31) | 1.85 | 0.603 |
| O2 | 39.07 (35.80–42.65) | 71.37 (63.17–83.91) | 7.55 | 0.056 |
| O1 | 19.45 (18.06–20.93) | 30.66 (27.84–34.82) | 1.27 | 0.736 |
| MO1 | Nt | Nt | Nt | Nt |
| Culex fuscocephala | ||||
| O3 | 29.76 (27.70–32.38) | 40.52 (37.09–45.63) | 5.31 | 0.257 |
| O2 | 24.32 (22.34–26.63) | 38.83 (35.26–43.96) | 13.90 | 0.003 |
| O1 | 10.53 (9.57–11.54) | 20.21 (17.79–24.02) | 0.90 | 0.825 |
| MO1 | 9.84 (8.81–10.97) | 25.09 (21.45–30.68) | 13.11 | 0.011 |
| Concentration (%) | KT50 (m) | KT90 (m) | χ2 | p |
|---|---|---|---|---|
| 25 | 31.32 (27.60–35.16) | 63.42 (54.97–76.54) | 14.08 | 0.080 |
| 12.5 | 40.76 (35.51–46.10) | 98.86 (85.27–119.11) | 19.71 | 0.011 |
| 6.25 | 54.87 (48.10–61.73) | 139.45 (120.57–167.47) | 22.99 | 0.003 |
| 3.0 | 212.04 (186.54–243.40) | 824.78 (639.34–1170.59) | 8.79 | 0.360 |
| 1.5 | 550.24 (456.51–697.94) | 2392.87 (1644.53–4098.97) | 4.02 | 0.855 |
| 1.0 | 641.95 (534.11–811.29) | 2306.43 (1634.53–3770.21) | 13.06 | 0.110 |
| 0.5 | 1081.33 (873.80–1422.51) | 3607.33 (2500.65–6138.99) | 7.87 | 0.446 |
| Time (min) | LC50 (%) | LC90 (%) | χ2 | p |
|---|---|---|---|---|
| 15 | 106.03 (47.59–641.77) | 1469.91 (320.34–54115.90) | 3.72 | 0.590 |
| 30 | 40.27 (26.48–81.72) | 314.51 (134.50–1512.64) | 3.73 | 0.590 |
| 60 | 8.64 (7.35–10.28) | 28.87 (22.17–41.64) | 5.94 | 0.312 |
| 90 | 4.68 (4.05–5.43) | 12.95 (10.49–17.16) | 9.91 | 0.078 |
| 120 | 3.59 (3.15–4.09) | 8.08 (6.75–10.31) | 18.23 | 0.003 |
| 180 | 2.69 (2.39–3.05) | 5.72 (4.82–7.25) | 17.77 | 0.003 |
| 240 | 2.36 (2.07–2.69) | 5.57 (4.62–7.19) | 11.80 | 0.038 |
| 300 | 1.95 (1.71–2.23) | 4.88 (4.02–6.38) | 10.50 | 0.062 |
| 360 | 1.85 (1.63–2.12) | 4.63 (3.81–6.06) | 8.71 | 0.121 |
| 1440 | 0.34 (0.19–0.47) | 1.44 (1.13–2.08) | 3.42 | 0.636 |
| Essential Oil | LC50 (95% Limits) | LC90 (95% Limits) | χ2 | p |
|---|---|---|---|---|
| Physa acuta | ||||
| O3 | 6.89 (5.33–8.84) | 17.30 (12.72–29.43) | 5.75 | 0.331 |
| O2 | 7.16 (5.62–9.07) | 16.26 (12.21–26.90) | 2.34 | 0.800 |
| O1 | 3.82 (3.09–4.71) | 6.95 (5.47–11.13) | 0.57 | 0.989 |
| CuSO4 (positive control) | 0.66 (0.55–0.80) | 0.85 (0.72–1.17) | 0.00 | 0.998 |
| Indoplanorbis exustus | ||||
| O3 | 26.97 (20.97–35.00) | 68.70 (49.60–123.03) | 1.77 | 0.779 |
| O2 | 38.57 (29.97–50.74) | 98.63 (69.98–188.00) | 5.73 | 0.220 |
| O1 | 21.88 (18.01–26.24) | 35.22 (28.81–53.59) | 1.12 | 0.878 |
| MO1 | 22.47 (17.41–28.90) | 55.14 (40.29–98.39) | 0.70 | 0.872 |
| CuSO4 (positive control) | 0.28 (0.23–0.33) | 0.43 (0.35–0.64) | 0.3618 | 0.948 |
| Pomacea canaliculata | ||||
| O3 | 54.38 (43.49–69.87) | 112.15 (83.46–207.92) | 11.40 | 0.022 |
| O2 | 39.50 (31.47–50.12) | 83.49 (62.79–143.75) | 3.03 | 0.552 |
| O1 | 25.80 (21.75–30.90) | 38.82 (32.04–64.33) | 1.79 | 0.774 |
| MO1 | 30.06 (24.64–49.72) | 49.72 (41.08–66.80) | 7.15 | 0.128 |
| Positive control (tea saponin) | 24.78 (23.26–26.72) | 32.62 (29.98–37.10) | 0.1301 | 0.988 |
| Concentration (µg/mL) | O1 | Concentration (µg/mL) | Galanthamine | ||
|---|---|---|---|---|---|
| Inhibition (%) | SD | Inhibition (%) | SD | ||
| 500 | 91.72 | 2.77 | 10 | 91.07 | 1.31 |
| 100 | 59.48 | 1.54 | 2 | 56.56 | 1.69 |
| 20 | 25.38 | 1.15 | 0.4 | 21.83 | 0.93 |
| 4 | 8.93 | 0.62 | 0.08 | 9.07 | 0.42 |
| IC50 | 70.85 ± 5.47 | IC50 | 1.70 ± 0.12 | ||
| Microorganism | Essential Oil (µg/mL) | Positive Control (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O1 | O2 | O3 | Streptomycin | Cyclohexamide | ||||||
| MIC | IC50 | MIC | IC50 | MIC | IC50 | MIC | IC50 | MIC | IC50 | |
| Enterococcus faecalis ATCC299212 | 32 | 9.34 ± 1.46 | 2 | 0.67 ± 0.01 | 16 | 5.34 ± 1.32 | 256 | 50.34 ± 2.32 | Nt | Nt |
| Staphylococcus aureus ATCC25923 | 64 | 19.45 ± 2.13 | 2 | 0.54 ± 0.02 | 32 | 12.45 ± 0.05 | 256 | 45.24 ± 1.36 | Nt | Nt |
| Bacillus cereus ATCC14579 | 64 | 21.25 ± 0.23 | 8 | 3.17 ± 0.78 | 32 | 9.45 ± 0.17 | 128 | 20.45 ± 0.39 | Nt | Nt |
| Escherichia coli ATCC25922 | 128 | 42.56 ± 2.56 | 2 | 0.53 ± 0.45 | 32 | 9.76 ± 1.32 | 32 | 9.45 ± 0.35 | Nt | Nt |
| Pseudomonas aeruginosa ATCC27853 | Na | Na | Na | Na | Na | Na | 256 | 64.67 ± 1.89 | Nt | Nt |
| Salmonella enterica ATCC13076 | 64 | 40.34 ± 3.21 | 8 | 3.23 ± 0.06 | 32 | 9.24 ± 0.74 | 128 | 45.67 ± 2.30 | Nt | Nt |
| Candida albicans ATCC10231 | 32 | 20.45 ± 1.04 | 2 | 0.67 ± 0.021 | 32 | 9.27 ± 0.96 | Nt | Nt | 32 | 10.46 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, H.V.; Satyal, P.; Vo, T.T.; Le, T.T.-T.; Nguyen, A.T.-G.; Vu, H.T.; Nguyen, T.T.; Nguyen, H.H.; Setzer, W.N. Chemical Composition and Biological Activities of Chromolaena odorata (L.) R.M.King & H.Rob. Essential Oils from Central Vietnam. Molecules 2025, 30, 3602. https://doi.org/10.3390/molecules30173602
Vo HV, Satyal P, Vo TT, Le TT-T, Nguyen AT-G, Vu HT, Nguyen TT, Nguyen HH, Setzer WN. Chemical Composition and Biological Activities of Chromolaena odorata (L.) R.M.King & H.Rob. Essential Oils from Central Vietnam. Molecules. 2025; 30(17):3602. https://doi.org/10.3390/molecules30173602
Chicago/Turabian StyleVo, Hoa Van, Prabodh Satyal, Thuong Thanh Vo, Truc Thi-Thanh Le, An Thi-Giang Nguyen, Hien Thi Vu, Trung Thanh Nguyen, Hung Huy Nguyen, and William N. Setzer. 2025. "Chemical Composition and Biological Activities of Chromolaena odorata (L.) R.M.King & H.Rob. Essential Oils from Central Vietnam" Molecules 30, no. 17: 3602. https://doi.org/10.3390/molecules30173602
APA StyleVo, H. V., Satyal, P., Vo, T. T., Le, T. T.-T., Nguyen, A. T.-G., Vu, H. T., Nguyen, T. T., Nguyen, H. H., & Setzer, W. N. (2025). Chemical Composition and Biological Activities of Chromolaena odorata (L.) R.M.King & H.Rob. Essential Oils from Central Vietnam. Molecules, 30(17), 3602. https://doi.org/10.3390/molecules30173602









