Bioactive Cyclopeptide Alkaloids and Ceanothane Triterpenoids from Ziziphus mauritiana Roots: Antiplasmodial Activity, UHPLC-MS/MS Molecular Networking, ADMET Profiling, and Target Prediction
Abstract
1. Introduction
2. Results
2.1. Antiplasmodial Activity
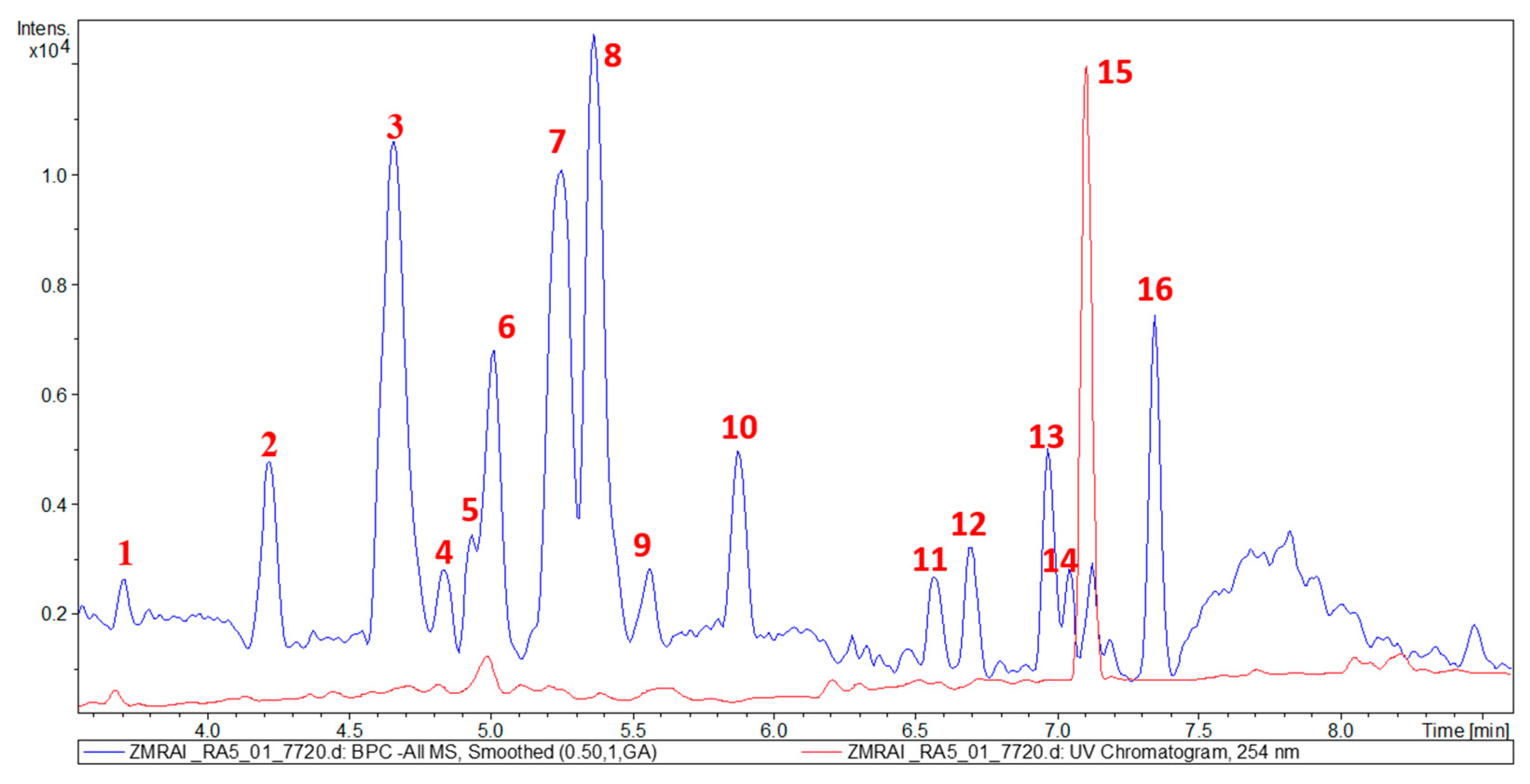
2.2. Metabolic Profiling of Z. mauritiana Roots
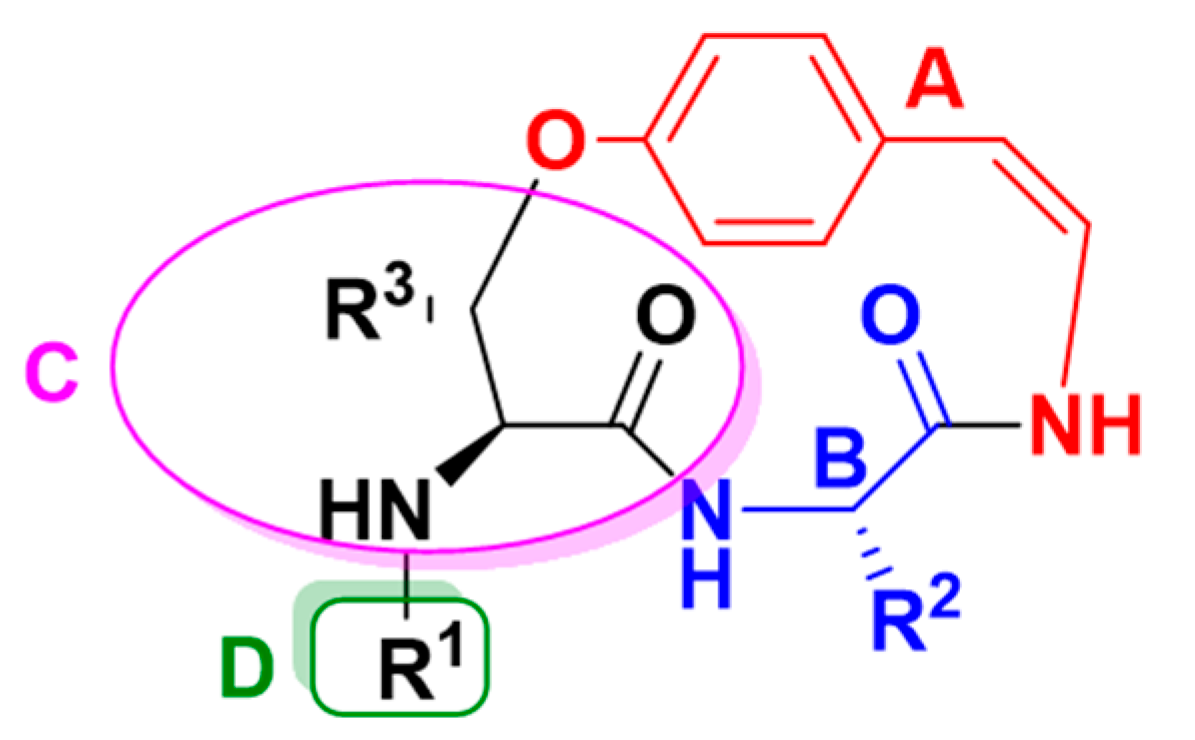
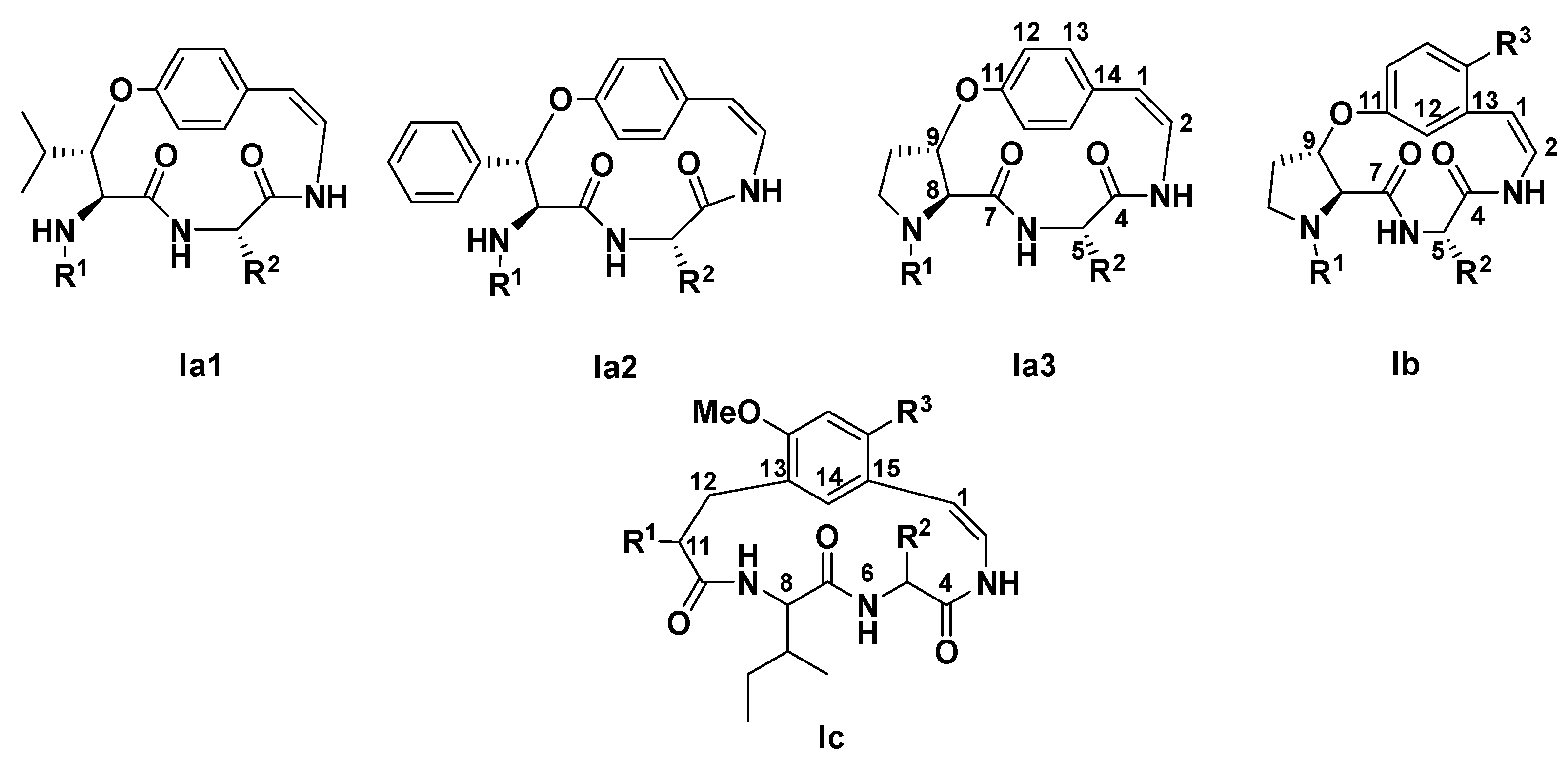
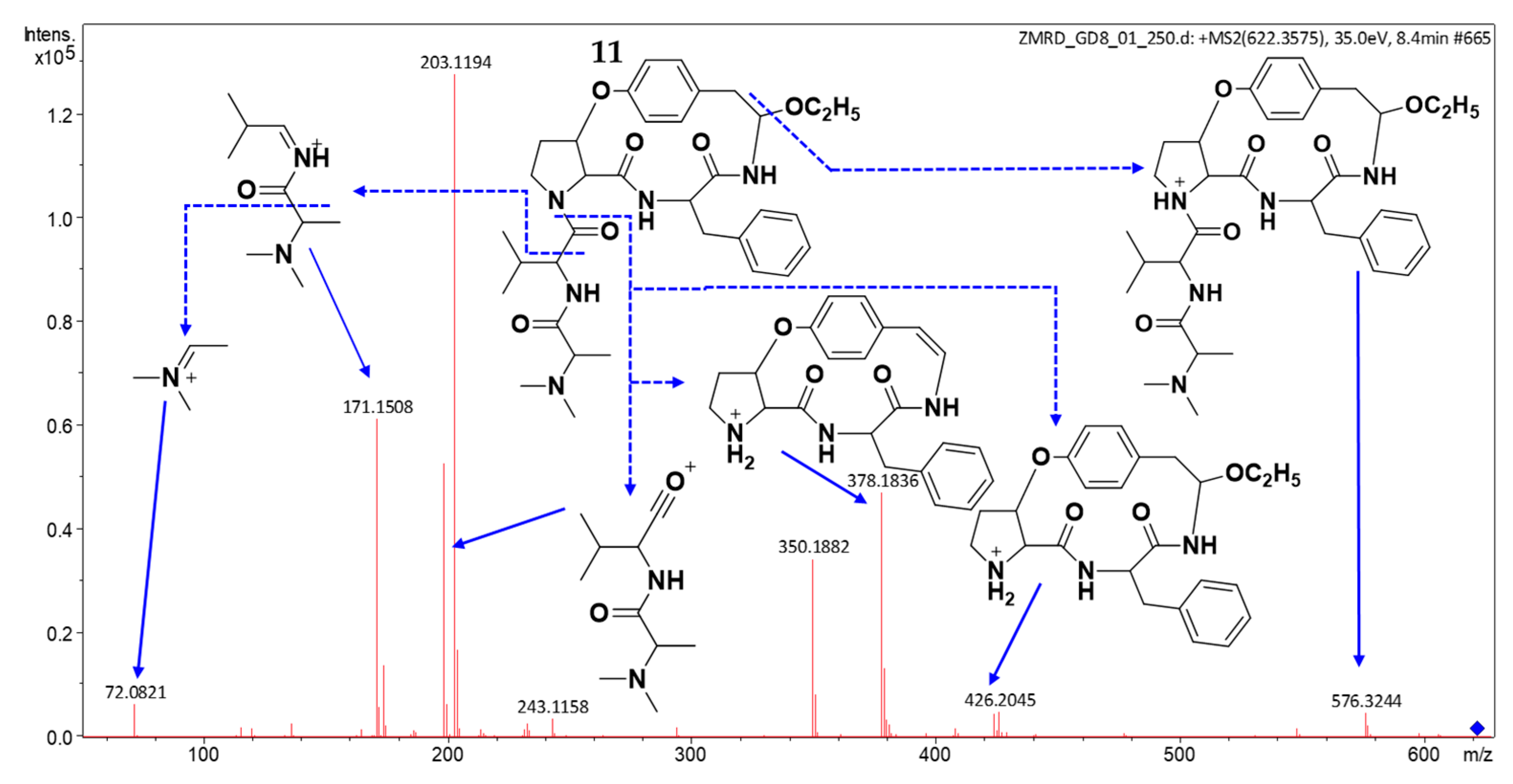
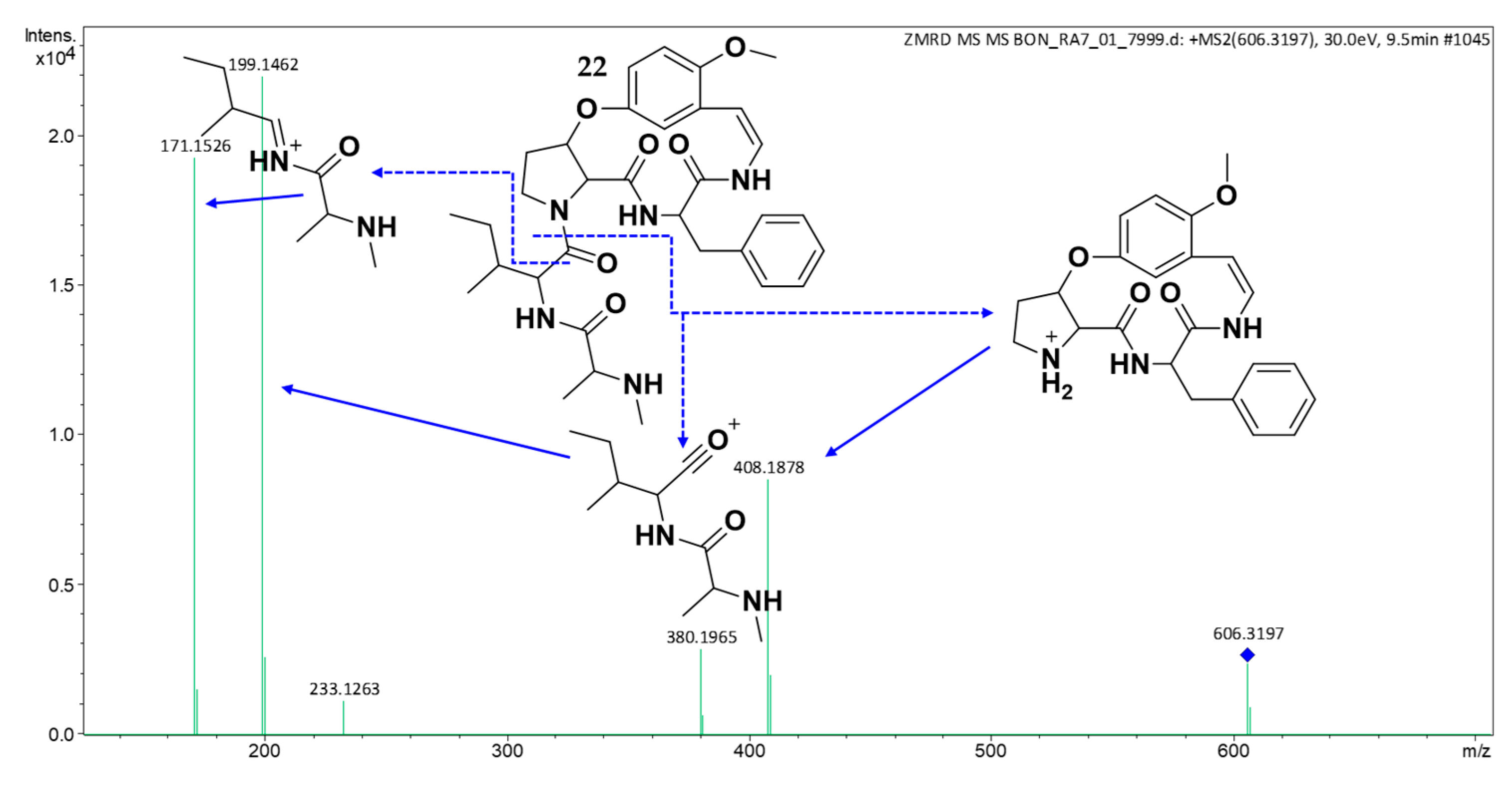
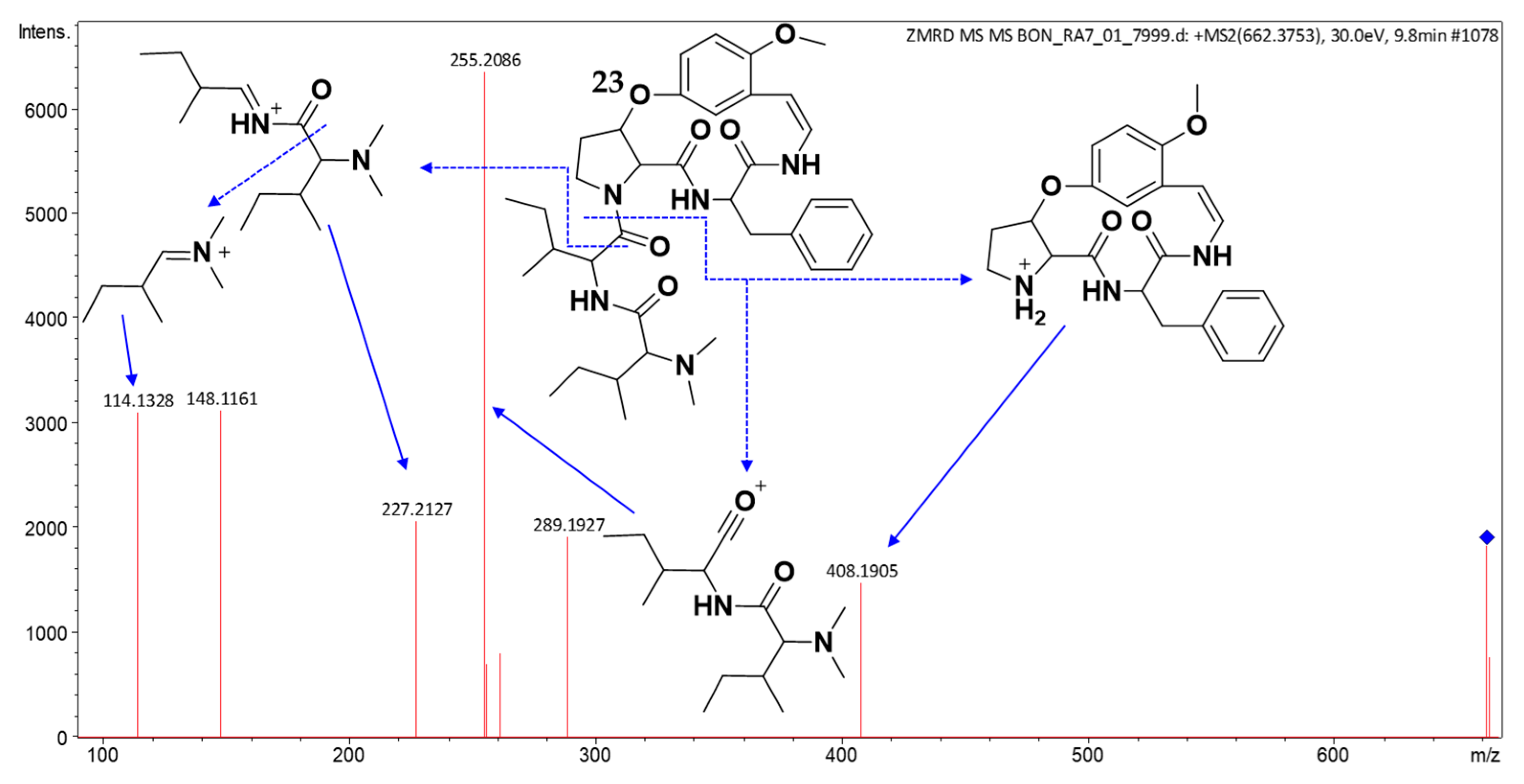
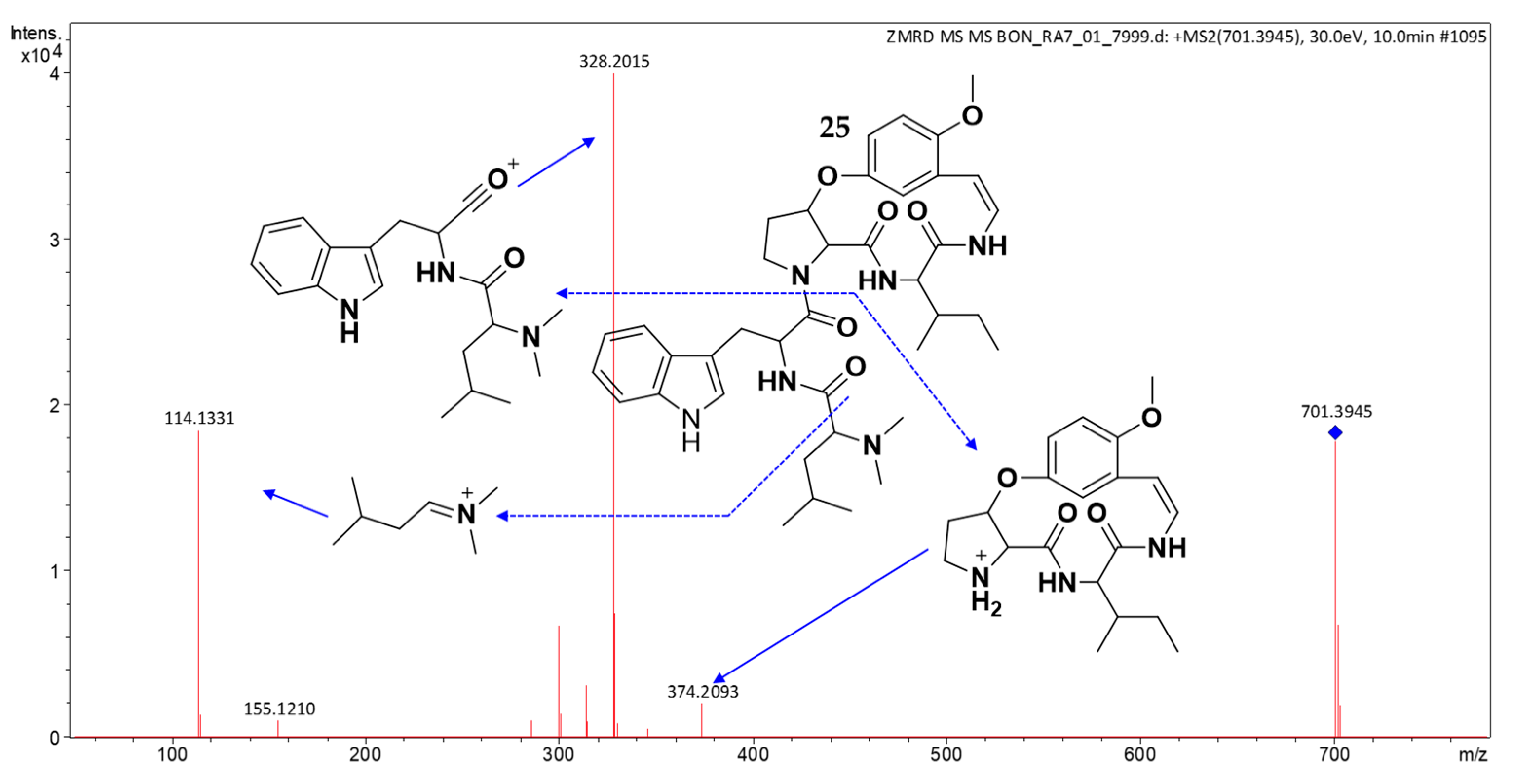
2.3. Structure Elucidation of CPAs Using MS/MS Patterns
2.4. Isolation and Characterization of Compounds
2.5. Chemophenetic Significance
2.6. Antiplasmodial Activities of Compounds
2.7. In Silico Acute Toxicity Prediction of Bioactive Constituents
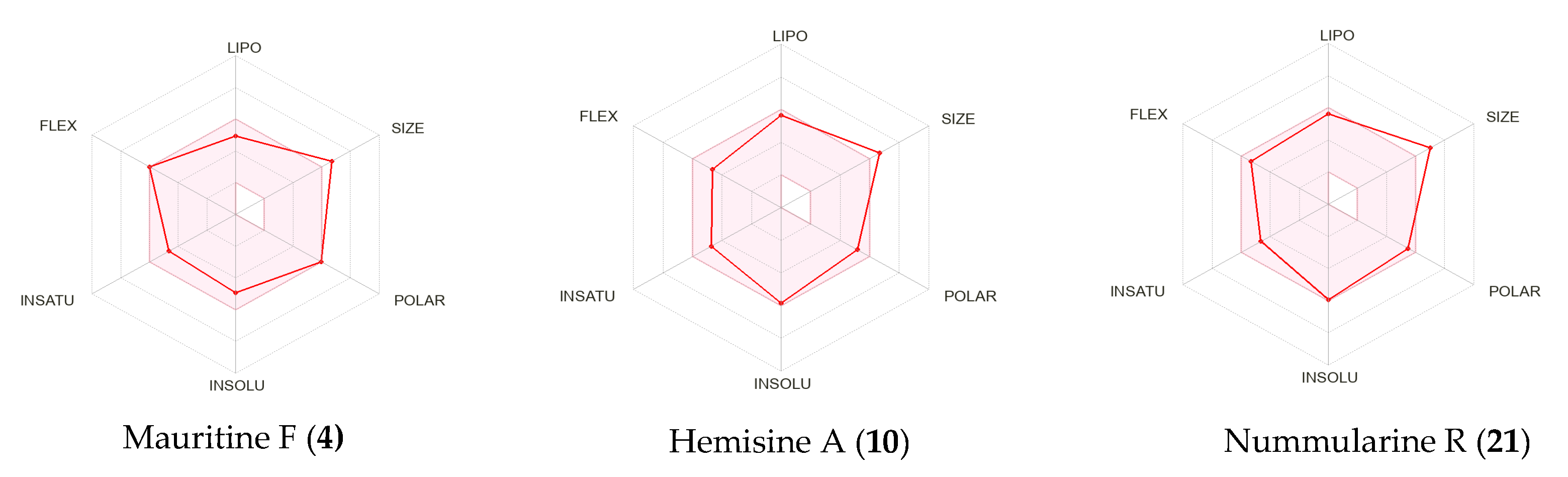
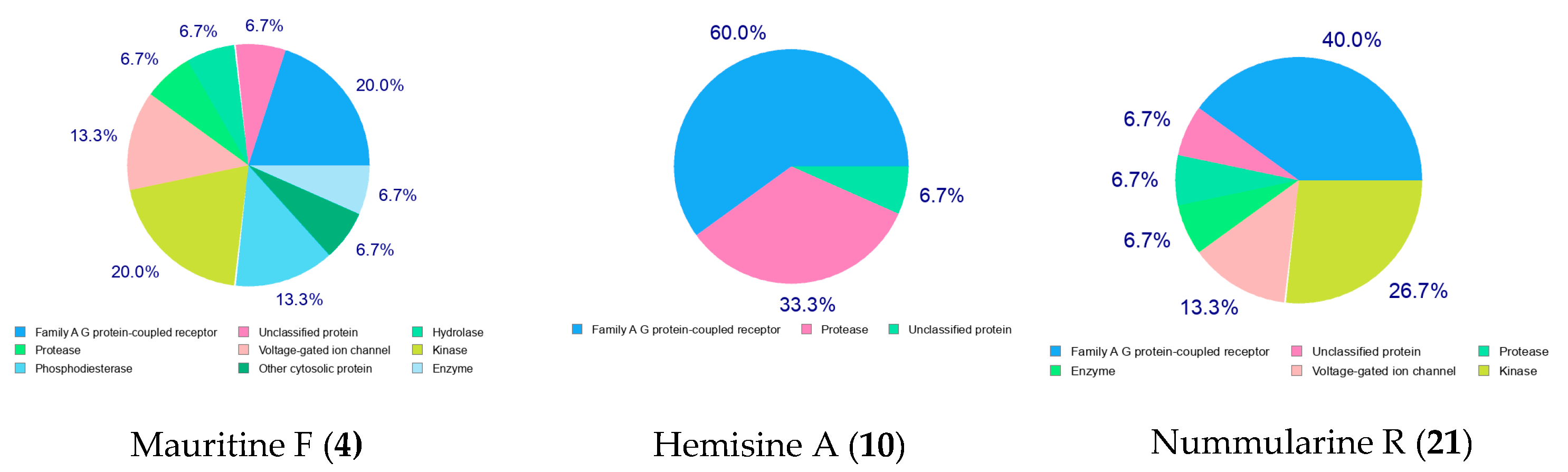
2.8. Pharmacokinetic (ADMET) Profiling, Drug-Likeness, and Molecular Target Prediction of Bioactive Compounds
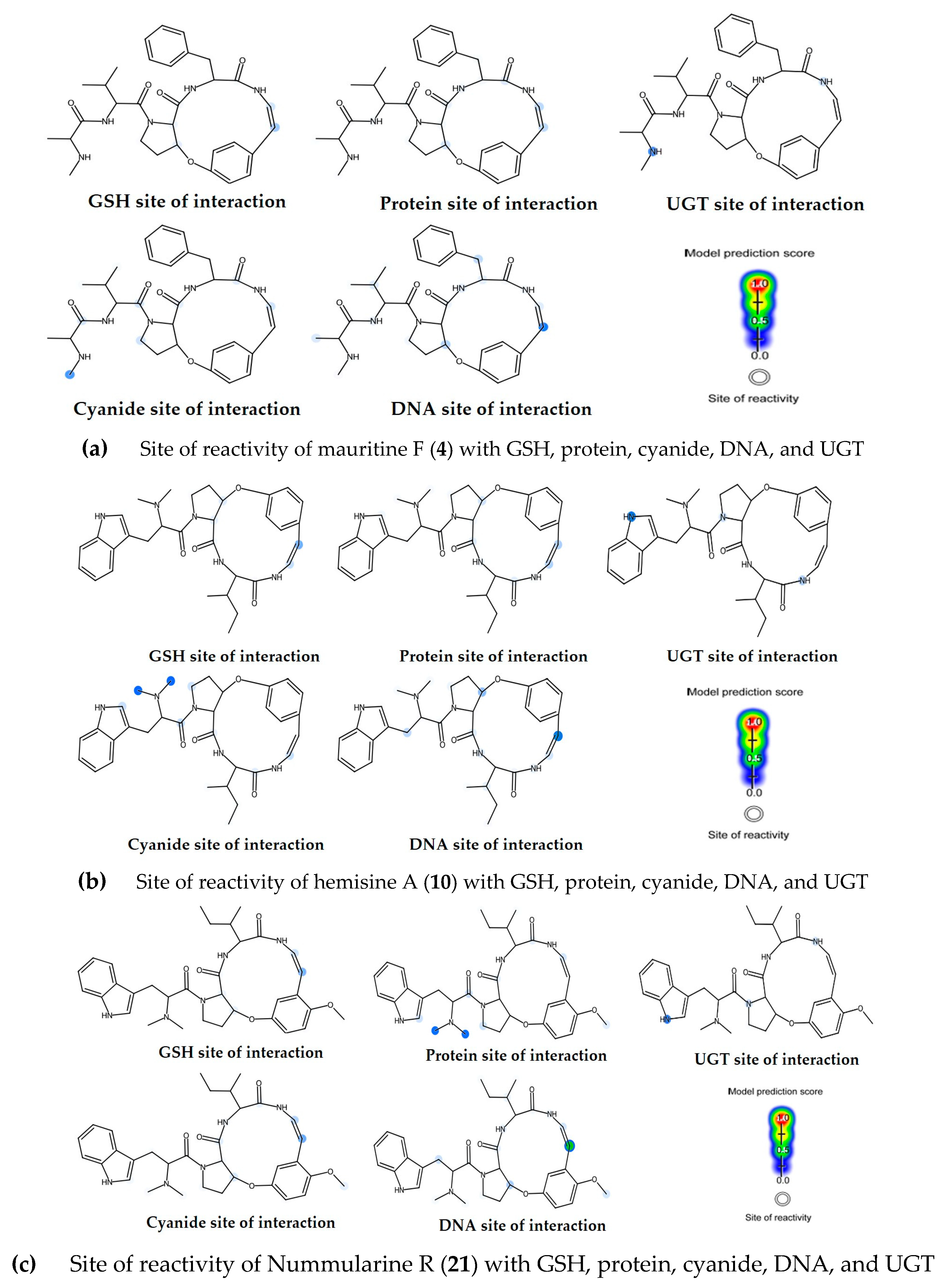
2.9. Toxicity Assessment and Biological Reactivity Profiling of Bioactive Compounds
2.10. Quantification of Bioactive Markers of Z. mauritiana Roots
3. Materials and Methods
3.1. Equipment and General Experimental Procedure
3.2. Plant Material
3.3. Extraction, Fractionation, and Isolation
3.4. UHPLC-DAD-ESI-QTOF MS/MS
3.5. Advanced Mass Spectrometry Data Processing and Annotation
3.6. Antiplasmodial Assay
3.7. In Silico Toxicity, Pharmacokinetic Profiling, Drug-Likeness and Target Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Malaria Report 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 14 February 2025).
- Asua, V.; Conrad, M.D.; Aydemir, O.; Duvalsaint, M.; Legac, J.; Duarte, E.; Tumwebaze, P.; Chin, D.; Cooper, R.; Yeka, A.; et al. Changing Prevalence of Potential Mediators of Aminoquinoline, Antifolate, and Artemisinin Resistance Across Uganda. J. Infect. Dis. 2021, 223, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.R.; Ng, C.L. A proteasome mutation sensitizes P. falciparum Cam3.II K13C580Y Parasites to DHA and OZ439. ACS Infect. Dis. 2021, 7, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Jha, D.; Hangargekar, P.; Akbar, M.; Parihar, A.S.; Kashyap, S.; Joshi, A.; Rahman, M.A. Ziziphus mauritiana: An in-depth Review of its Medicinal Attributes and Pharmacological Activities. Intell. Pharm. 2024, 2, 274–283. [Google Scholar] [CrossRef]
- Mishra, T.; Bhatia, A. Antiplasmodial effects of the aqueous ethanolic seed extract of Ziziphus mauritiana against Plasmodium berghei in Swiss albino mice. Int. J. Pharmacol. Res. 2014, 4, 111–116. [Google Scholar]
- Goyal, M.; Sasmal, D.; Nagori, B. Review on ethnomedicinal uses, Pharmacological activity and phytochemical constituents of Ziziphus mauritiana & jujuba Lam., non Mill). J. Complement. Med. Drug Discov. 2012, 2, 107–116. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestree Database: A Tree Reference and Selection Guide Version 4.0. 2009. Available online: https://apps.worldagroforestry.org/treedb/AFTPDFS/Ziziphus_mauritiana.pdf (accessed on 21 May 2025).
- Prakash, O.; Usmani, S.; Singh, R.; Singh, N.; Gupta, A.; Ved, A. A panoramic view on phytochemical, nutritional, and therapeutic attributes of Ziziphus mauritiana Lam.: A comprehensive review. Phytother. Res. 2021, 35, 63–77. [Google Scholar] [CrossRef]
- Panseeta, P.; Suksamrarn, S. Triterpenes from the root of the Ziziphus mauritiana. JST 2010, 2, 106–118. Available online: https://ph02.tci-thaijo.org/index.php/swujournal/article/view/31901 (accessed on 21 May 2025).
- Nilofar, N.; Sinan, K.I.; Dall’Acqua, S.; Sut, S.; Uba, A.I.; Etienne, O.K.; Ferrante, C.; Ahmad, J.; Zengin, G. Ziziphus mauritiana Lam. Bark and Leaves: Extraction, Phytochemical Composition, In Vitro Bioassays and In Silico Studies. Plants 2024, 13, 2195. [Google Scholar] [CrossRef]
- Ramar, M.K.; Chidambaram, K.; Chandrasekaran, B.; Kandasamy, R. Standardization, in-silico and in-vivo safety assessment of methanol extract of Ziziphus mauritiana Lam leaves. Regul. Toxicol. Pharmacol. 2022, 131, 105144. [Google Scholar] [CrossRef]
- Khanam, A.; Ijaz Hussain, A.; Mohammed, E.H.; Nahar, L.; Rathore, H.A. Phenolic Profile of Seedless Ziziphus mauritiana Fruits and Leaves Extracts with In Vivo Antioxidant and Anti-Inflammatory Activities: Influence on Pro-Inflammatory Mediators. Chem. Biodivers. 2025, 22, e202401728. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.-A.; Li, Y.; Wang, R.; Yan, H.; Qian, D.; Tang, Y.; Su, S. Comparison of the Bioactive Components in Two Seeds of Ziziphus Species by Different Analytical Approaches Combined with Chemometrics. Front. Pharmacol. 2017, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Soraya, S.; Sukara, E.; Sinaga, E. Identification of Chemical Compounds in Ziziphus mauritiana Fruit Juice by GC-MS and LC-MS/MS Analysis. Int. J. Biol. Phys. Chem. Stud. 2022, 4, 11–19. [Google Scholar] [CrossRef]
- Panseeta, P.; Lomchoey, K.; Prabpai, S.; Kongsaeree, P.; Suksamrarn, A.; Ruchirawat, S.; Suksamrarn, S. Antiplasmodial and antimycobacterial cyclopeptide alkaloids from the root of Ziziphus mauritiana. Phytochemistry 2011, 72, 909–915. [Google Scholar] [CrossRef]
- Batool, M.; Afzal, S.; Afzal, K.; Ahmed, B.; Abbas, K.; Muhammad, S.A.; Qadir, M.I. Anticancer activity of Ziziphus mauritiana roots against human breast cancer cell line. Pak. J. Pharm. Sci. 2019, 32, 1715–1716. [Google Scholar]
- Shady, N.H.; Soltane, R.; Maher, S.A.; Saber, E.A.; Elrehany, M.A.; Mostafa, Y.A.; Sayed, A.M.; Abdelmohsen, U.R. Wound Healing and Antioxidant Capabilities of Zizyphus mauritiana Fruits: In-Vitro, In-Vivo, and Molecular Modeling Study. Plants 2022, 11, 1392. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.H.C.; De Carvalho, M.G.; Laub, A.; Franke, K.; Wessjohann, L. UHPLC-ESI-Orbitrap-HR-MS Analysis of Cyclopeptide Alkaloids from Ziziphus joazeiro. Nat. Prod. Commun. 2021, 16, 1934578X2110549. [Google Scholar] [CrossRef]
- Kang, K.B.; Jang, D.S.; Kim, J.; Sung, S.H. UHPLC-ESI-qTOF-MS analysis of cyclopeptide alkaloids in the seeds of Ziziphus jujuba var. spinosa. Mass Spectrom. Lett. 2016, 7, 45–49. [Google Scholar] [CrossRef]
- Collett, L.A.; Davies-Coleman, M.T.; Gournelis, D.C.; Laskaris, G.G.; Rivett, D.E.A.; Verpoorte, R. Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer Chemistry: Vienna, Austria, 1998; pp. 171–179. [Google Scholar] [CrossRef]
- Tschesche, R.; Kaubmann, E.U. The cyclopeptide alkaloids. In The Alkaloids: Chemistry and Physiology; Manske, R.H.F., Ed.; Academic Press: Cambridge, MA, USA, 1975; Volume 15, pp. 165–205. [Google Scholar] [CrossRef]
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software tools and approaches for compound identification of LC-MS/MS data in metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef]
- Tan, N.-H.; Zhou, J. Plant cyclopeptides. Chem. Rev. 2006, 106, 840–895. [Google Scholar] [CrossRef]
- Ji, C.-J.; Zeng, G.-Z.; Han, J.; He, W.-J.; Zhang, Y.-M.; Tan, N.-H. Zizimauritic acids A–C, three novel nortriterpenes from Ziziphus mauritiana. Bioorg. Med. Chem. Lett. 2012, 22, 6377–6380. [Google Scholar] [CrossRef]
- Muñoz-Nuñez, E.; Quiroz-Carreño, S.; Pastene-Navarrete, E.; Seigler, D.S.; Céspedes-Acuña, C.; Martínez Valenzuela, I.; Oppliger Muñoz, M.; Salas-Burgos, A.; Alarcón-Enos, J. Assessments of Ceanothanes Triterpenes as Cholinesterase Inhibitors: An Investigation of Potential Agents with Novel Inspiration for Drug Treatment of Neurodegenerative Diseases. Metabolites 2022, 12, 668. [Google Scholar] [CrossRef]
- Pierre, L.L.; Moses, M.N. Isolation and characterisation of stigmasterol and β-sitosterol from Odontonema Strictum (Acanthaceae). J. Innov. Pharm. Biol. Sci. 2015, 2, 88–96. [Google Scholar]
- Peshin, T.; Kar, H. Isolation and characterization of β-sitosterol-3-O-β-D-glucoside from the extract of the flowers of Viola odorata. Br. J. Pharm. Res. 2017, 16, 1–8. [Google Scholar] [CrossRef]
- Adam, I.A.; Irshad, R.; Wahab, A.T.; Omoboyowa, D.A.; Choudhary, M.I.; Wang, Y. Two new 5 (14)-membered type cyclopeptide alkaloids from root bark of Ziziphus spina-christi (L.) Desf. Nat. Prod. Res. 2022, 37, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ji, C.-J.; He, W.-J.; Shen, Y.; Leng, Y.; Xu, W.-Y.; Fan, J.-T.; Zeng, G.-Z.; Kong, L.-D.; Tan, N.-H. Cyclopeptide alkaloids from Ziziphus apetala. J. Nat. Prod. 2011, 74, 2571–2575. [Google Scholar] [CrossRef]
- Karim, M.A.; Hossain, M.K.; Al-Mansur, M.A.; Shajib, M.S.; Rashid, M.A. Isolation of zizyberenalic acid and biological studies of Ziziphus mauritiana Lam. growing in Bangladesh. Bangladesh J. Bot. 2019, 48, 163–168. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Panseeta, P.; Kunchanawatta, S.; Distaporn, T.; Ruktasing, S. Ceanothane- and lupane-type triterpenes with antiplasmodial and antimycobacterial activities from Ziziphus cambodiana. Chem. Pharm. Bull. 2006, 54, 535–537. [Google Scholar] [CrossRef]
- Tuenter, E.; Segers, K.; Kang, K.; Viaene, J.; Sung, S.; Cos, P.; Maes, L.; Heyden, Y.; Pieters, L. Antiplasmodial activity, cytotoxicity and Structure-Activity Relationship Study of Cyclopeptide Alkaloids. Molecules 2017, 22, 224. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res (Web Server Issue 2024). Available online: https://tox.charite.de/protox3 (accessed on 25 June 2024).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Z.; Surdenikova, L.; Kim, S.; Patel, K.N.; Kim, A.; Ru, F.; Guan, Y.; Weng, H.-J.; Geng, Y.; et al. Sensory Neuron-Specific GPCR Mrgprs Are Itch Receptors Mediating Chloroquine-Induced Pruritus. Cell 2009, 39, 1353–1365. [Google Scholar] [CrossRef]
- Larson, E.T.; Ojo, K.K.; Murphy, R.C.; Johnson, S.M.; Zhang, Z.; Kim, J.E.; Leibly, D.J.; Fox, A.M.W.; Reid, M.C.; Dale, E.J.; et al. Multiple Determinants for Selective Inhibition of Apicomplexan Calcium-Dependent Protein Kinase CDPK1. J. Med. Chem. 2012, 55, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Alberge, B.; Gannoun-Zaki, L.; Bascunana, C.; Tran van Ba, C.; Vial, H.; Cerdan, R. Comparison of the cellular and biochemical properties of Plasmodium falciparum choline and ethanolamine kinases. Biochem. J. 2009, 425, 149–163. [Google Scholar] [CrossRef]
- Zhang, M.V.; Chavchich, M.; Waters, N.C. Targeting Protein Kinases in the Malaria Parasite: Update of an Antimalarial Drug Target. Curr. Top. Med. Chem. 2012, 12, 456–472. [Google Scholar] [CrossRef]
- Doerig, C.; Abdi, A.; Bland, N.; Eschenlauer, S.; Dorin-Semblat, D.; Fennell, C.; Halbert, J.; Holland, Z.; Nivez, M.-P.; Semblat, J.-P.; et al. Malaria: Targeting parasite and host cell kinomes. Biochim. Biophys. Acta 2010, 1804, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Cassiano, G.C.; Tavella, T.A.; Nascimento, M.N.; Rodrigues, D.A.; Cravo, P.V.L.; Andrade, C.H.; Costa, F.T.M. Targeting malaria protein kinases. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 225–274. [Google Scholar] [CrossRef]
- Doerig, C.; Meijer, L. Antimalarial drug discovery: Targeting protein kinases. Expert Opin. Ther. Targets 2007, 11, 279–290. [Google Scholar] [CrossRef]
- Hughes, T.B.; Dang, N.L.; Miller, G.P.; Swamidass, S.J. Modeling Reactivity to Biological Macromolecules with a Deep Multitask Network. ACS Cent. Sci. 2016, 2, 528–537. [Google Scholar] [CrossRef]
- Hughes, T.B.; Miller, G.P.; Swamidass, S.J. Site of reactivity models predict molecular reactivity of diverse chemicals with glutathione. Chem. Res. Toxicol. 2015, 28, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Le Dang, N.; Hughes, T.B.; Krishnamurthy, V.; Swamidass, S.J. A Simple Model Predicts UGT-Mediated Metabolism. Bioinformatics 2016, 32, 3183–3189. [Google Scholar] [CrossRef]
- Schmid, R.; Heuckeroth, S.; Korf, A. Integrative analysis of multimodal mass spectrometry data in MZmine 3. Nat Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef]
- Morehouse, N.J.; Clark, T.N.; McMann, E.; van Santen, J.A.; Haeckl, J.F.P.; Gray, C.A.; Linington, R.G. Annotations of Natural Product Compound Families Using Molecular Networking Topology and Structural Similarity Fingerprinting. Nat. Commun. 2023, 14, 308. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Mohimani, H.; Gurevich, A.; Shlemov, A.; Mikheenko, A.; Korobeynikov, A.; Cao, L.; Shcherbin, E.; Nothias, L.-F.; Dorrestein, P.C.; Pevzner, P.A. Dereplication of microbial metabolites through database search of mass spectra. Nat. Commun. 2018, 9, 4035. [Google Scholar] [CrossRef]
- Cao, L.; Guler, M.; Tagirdzhanov, A.; Lee, Y.-Y.; Gurevich, A.; Mohimani, H. MolDiscovery: Learning mass spectrometry fragmentation of small molecules. Nat. Commun. 2021, 12, 3718. [Google Scholar] [CrossRef]
- Borelli, T.C.; Arini, G.S.; Feitosa, L.G.P.; Dorrestein, P.C.; Lopes, N.P.; Da Silva, R.R. Improving annotation propagation on molecular networks through random walks: Introducing ChemWalker. Bioinformatics 2023, 39, btad078. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. J. Parasitol. 2005, 91, 484–486. [Google Scholar] [CrossRef]
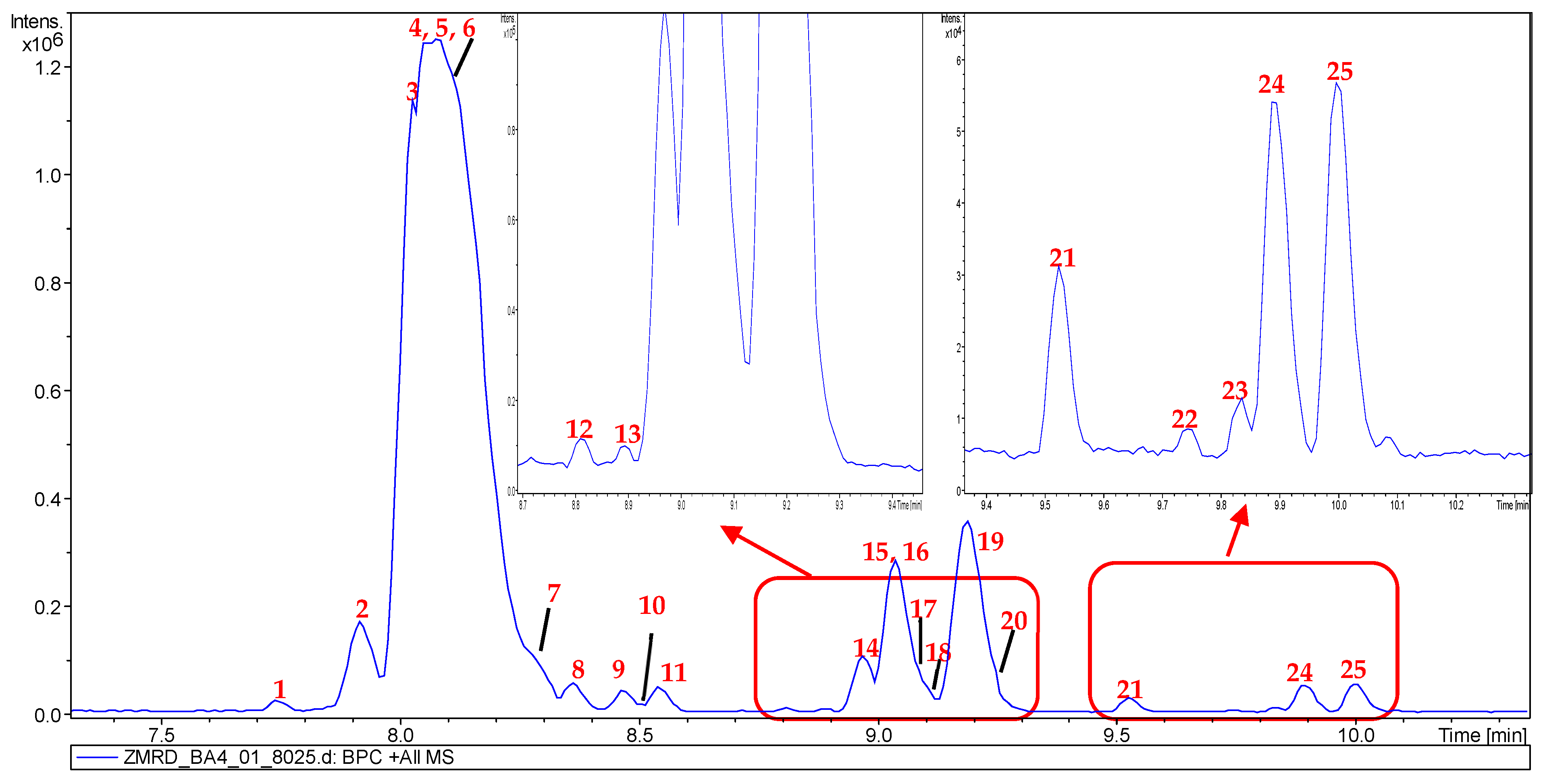
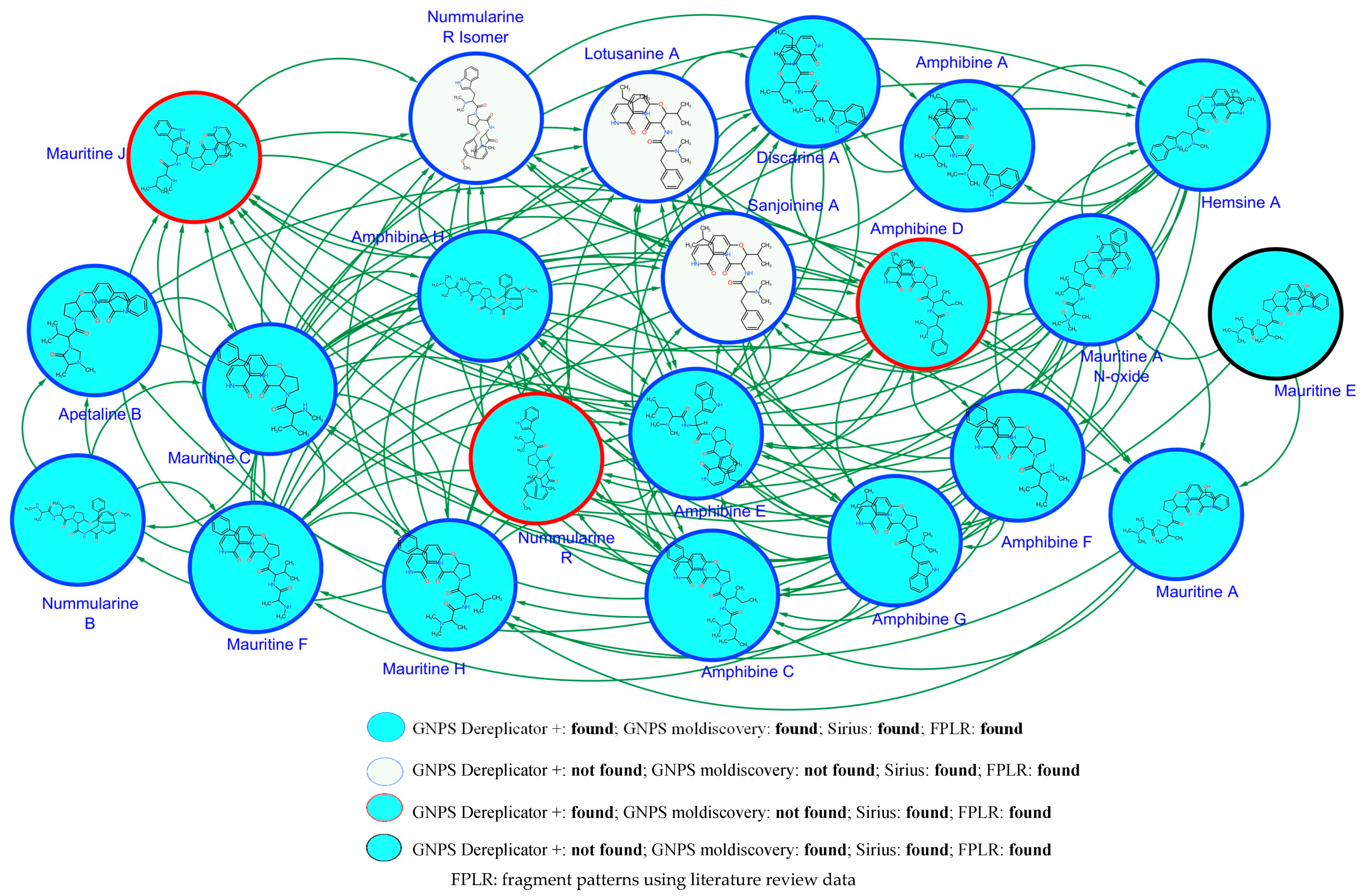


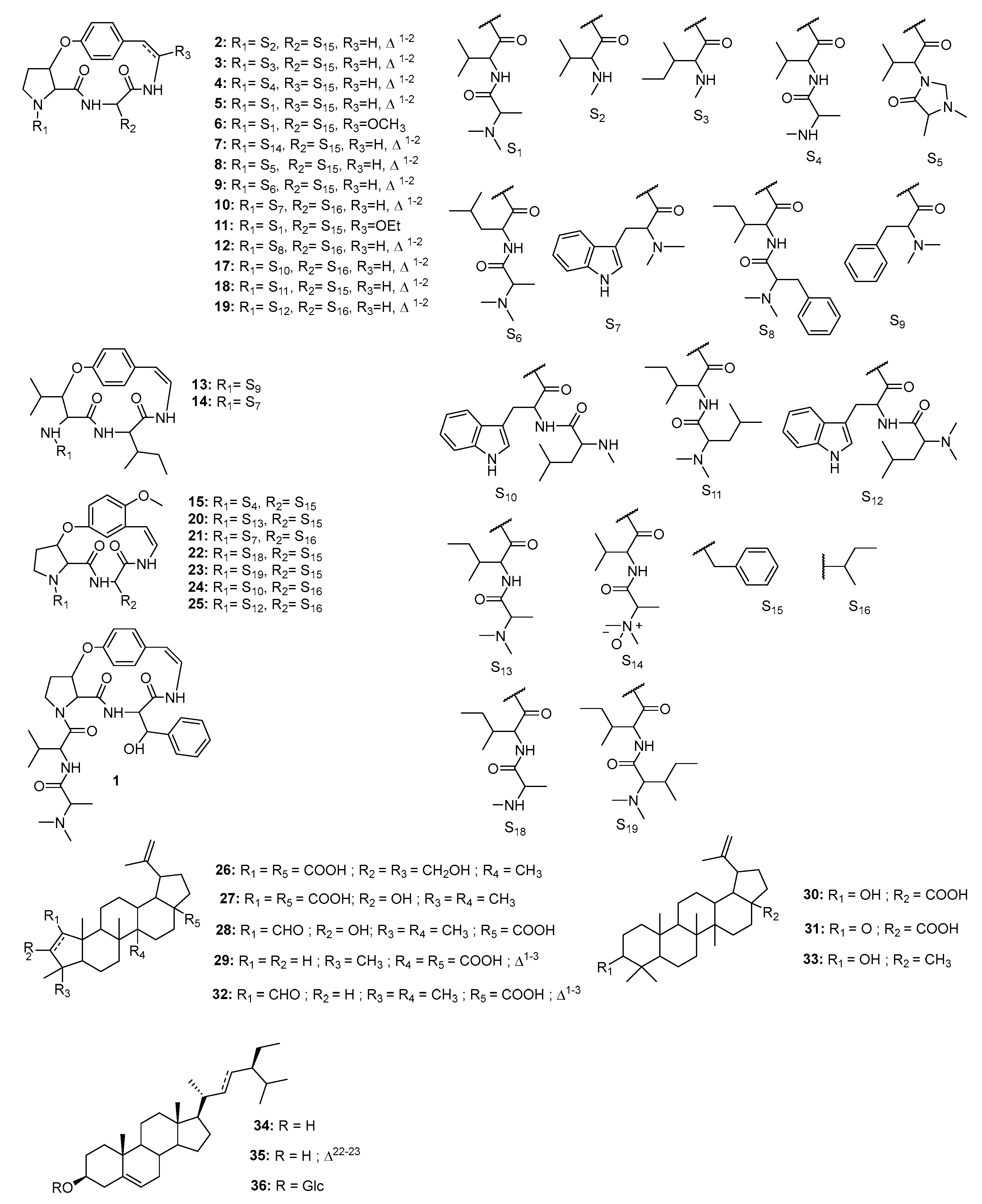
| Peak N° | RT (mins) | m/z (Experimental) | m/z (Theoretical) | Adduct | Molecular Formula | MS/MS Fragments | Potential Compounds | GNPS Dereplicator+ | GNPS Moldiscovery | GNPS2 ChemWalker | Sirius | FBMN (MS/MS Spectral Database) | Literature Review Data |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.74 | 592.3135 | 592.3130 | [M + H]+ | C32H41N5O6 | 394, 366, 199, 171 | Mauritine E (1) | × | ✓ | ✓ | ✓ | × | ✓ |
| 2 | 7.97 | 491.2651 | 491.2653 | [M + H]+ | C28H34N4O4 | 378, 203, 186, 86 | Mauritine C (2) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 3 | 8.03 | 505.2804 | 505.2809 | [M + H]+ | C29H36N4O4 | 378, 203, 100 | Amphibine F (3) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 4 | 8.08 | 562.3020 | 562.3024 | [M + H]+ | C31H39N5O5 | 378, 203, 185, 157 | Mauritine F (4) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 5 | 8.14 | 576.3180 | 576.3180 | [M + H]+ | C32H41N5O5 | 378, 199, 185, 17 1 | Mauritine A (5) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 6 | 8.15 | 608.3448 | 608.3443 | [M + H]+ | C33H46N5O6 | 576, 378, 199, 171 | 1,2-dihydro-2β-methoxymauritine A (6) | ✓ | × | × | × | × | ✓ |
| 7 | 8.33 | 592.3135 | 592.3133 | [M + H]+ | C32H41N5O6 | 531, 378, 215, 116, 72 | Mauritine A N-oxide (7) | ✓ | ✓ | × | ✓ | × | ✓ |
| 8 | 8.45 | 574.3018 | 574.3024 | [M + H]+ | C32H39N5O5 | 378, 197, 169, 141 | Apetaline B (8) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 9 | 8.47 | 590.3326 | 590.3337 | [M + H]+ | C33H43N5O5 | 378, 350, 213, 199, 185, 171 | Mauritine H (9) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 10 | 8.49 | 558.3073 | 558.3075 | [M + H]+ | C32H39N5O4 | 513, 427, 187, 170 | Hemisine A/Amphibine G (10) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 11 | 8.58 | 622.3603 | 622.3599 | [M + H]+ | C33H43N5O7 | 576, 424 378, 199, 171 | (11) | × | × | × | × | × | × |
| 12 | 8.83 | 632.3804 | 632.3806 | [M + H]+ | C36H49N5O5 | 344, 289, 148, 114 | Amphibine D (12) | ✓ | × | ✓ | ✓ | × | ✓ |
| 13 | 8.89 | 535.3277 | 535.3279 | [M + H]+ | C31H42N4O4 | 287, 148 | Lotusanine A (13) | × | × | ✓ | ✓ | × | ✓ |
| 14 | 8.98 | 574.3377 | 574.3388 | [M + H]+ | C33H43N5O4 | 529, 443, 187, 170 | Discarine A/Amphibine A (14) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 15 | 9.01 | 592.3120 | 592.3130 | [M + H]+ | C32H41N5O4 | 408, 185, 157 | Nummularine B (15) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 16 | 9.03 | 606.3277 | 606.3286 | [M + H]+ | C33H43N5O6 | 408, 199, 171, 72 | Amphibine H (16) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 17 | 9.10 | 657.3757 | 657.3759 | [M + H]+ | C37H48N6O5 | 513, 344, 314, 286, 155, 100 | Mauritine J (17) | ✓ | × | ✓ | ✓ | × | ✓ |
| 18 | 9.11 | 632.3802 | 632.3806 | [M + H]+ | C36H49N5O5 | 378, 255, 227, 155 | Amphibine C (18) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 19 | 9.20 | 671.3915 | 671.3915 | [M + H]+ | C38H50N6O5 | 344, 328, 203 114 | Amphibine E (19) | ✓ | ✓ | ✓ | ✓ | × | ✓ |
| 20 | 9.32 | 620.3446 | 620.3443 | [M + H]+ | C34H45N5O6 | 408, 213, 199, 185, 171 | (20) | × | × | × | × | × | × |
| 21 | 9.52 | 588.3189 | 588.3180 | [M + H]+ | C33H41N5O5 | 543, 457, 199, 187, 170 | Nummularine R (21) | ✓ | × | ✓ | ✓ | × | ✓ |
| 22 | 9.74 | 606.3278 | 606.3286 | [M + H]+ | C33H41N5O5 | 408, 380, 199, 171 | (22) | × | × | × | × | × | × |
| 23 | 9.82 | 662.3902 | 662.3912 | [M + H]+ | C37H51N5O6 | 408, 289, 255, 227, 148, 114 | (23) | × | × | × | × | × | × |
| 24 | 9.91 | 687.3859 | 687.3865 | [M + H]+ | C38H50N6O6 | 543, 374, 314, 155, 286, 100 | Mauritine M (24) | ✓ | ✓ | × | ✓ | × | ✓ |
| 25 | 10.00 | 701.4023 | 701.4021 | [M + H]+ | C39H52N6O6 | 374, 328, 300, 155, 114 | (25) | × | × | × | × | × | × |
| Peak N° | RT (mins) | m/z (Experimental) | m/z (Theoretical) | Adduct | Molecular Formula | MS/MS Fragments | Potential Compounds |
|---|---|---|---|---|---|---|---|
| 1 | 3.9 | 329.2186 | / | / | / | / | Not identified |
| 2 | 4.3 | 501.3226 | 501.3222 | [M − H]− | C30H46O6 | 485, 453, 439, 427, 423, 409 | 24-hydroxyceanothic acid (26) |
| 3 | 4.7 | 485.3275 | 485.3272 | [M − H]− | C30H46O5 | 423 | Ceanothic acid (27) |
| 4 | 4.9 | 485.3276 | / | / | / | 423 | Not identified |
| 5 | 5.0 | 471.3387 | / | / | / | / | Not identified |
| 6 | 5.1 | 295.2401 | / | / | / | / | Not identified |
| 7 | 5.3 | 469.3332 | 469.3330 | [M − H]− | C30H46O4 | / | Zizyberanalic acid (28) |
| 8 | 5.4 | 485.3276 | / | / | / | 423 | Not identified |
| 9 | 5.6 | 469.3323 | / | / | / | / | Not identified |
| 10 | 5.9 | 453.3002 | 453.3010 | [M − H]− | C29H42O4 | / | Ceanothenic acid (29) |
| 11 | 6.5 | 455.3531 | 455.3531 | [M − H]− | C30H48O3 | / | Betulinic acid (30) |
| 12 | 6.8 | 439.3877 | / | / | / | / | Not identified |
| 13 | 6.9 | 279.2492 | / | / | / | / | Not identified |
| 14 | 7.0 | 453.3378 | 453.3374 | [M − H]− | C30H46O3 | / | Betulonic acid (31) |
| 15 | 7.1 | 451.3212 | 451.3218 | [M − H]− | C30H44O3 | / | Zizyberenalic acid (32) |
| 16 | 7.3 | 281.2625 | / | / | / | / | Not identified |
| CPAs Type I Core | |||||
|---|---|---|---|---|---|
| Ia | Ib | Ic | |||
| Ia1 | Ia2 | Ia3 | / | / | |
| Number of atoms in the ring | 14 | 14 | 14 | 13 | 15 |
| C unit | leucine | phenylalanine | proline | proline | / |
| Extracts and Fractions | IC50 Pf3D7 (µg/mL) | IC50 PfK1 (µg/mL) | References |
|---|---|---|---|
| ZMRE | 32.70 | NT | / |
| ZMRA | >50 | NT | / |
| ZMRD | 4.75 | NT | / |
| ZMRA II | 11.35 | NT | / |
| Compounds | (µM) | (µM) | / |
| Zizyberenalic acid (32) | 20.45 | 6.62 * | [31] |
| Betulinic acid (30) | 19.0 | / | |
| Mauritine F (4) | NT | 34.2 * | [32] |
| Mauritine M (24) | NT | 3.7 * | [15] |
| Nummularine B (15) | NT | 3.6 * | [32] |
| Nummularine R (21) | NT | 3.2 * | [32] |
| Hemisine A (10) | NT | 7.3 * | [15] |
| Amphibine D (12) | NT | 8.9 * | [32] |
| Reference drugs | (nM) | / | / |
| Chloroquine | 29.9 | / | / |
| Artemisinin | 26.3 | / | / |
| Targets | Hepatotoxicity (SP) | Nephrotoxicity (SP) | Cardiotoxicity (SP) | Respiratory toxicity (SP) | LD50 (mg/kg) | |
|---|---|---|---|---|---|---|
| Compounds | ||||||
| Mauritine F (4) | Inactive (0.65) | Inactive (0.57) | Inactive (0.79) | Active (0.82) | 550 | |
| Hemisine A (10) | Inactive (0.63) | Inactive (0.58) | Inactive (0.83) | Active (0.83) | 550 | |
| Amphibine D (12) | Inactive (0.57) | Inactive (0.60) | Inactive (0.83) | Active (0.77) | 550 | |
| Nummularine B (15) | Inactive (0.64) | Inactive (0.52) | Inactive (0.77) | Active (0.79) | 200 | |
| Nummularine R (21) | Inactive (0.61) | Inactive (0.51) | Inactive (0.81) | Active (0.77) | 386 | |
| Mauritine M (24) | Inactive (0.64) | Active (0.51) | Inactive (80) | Active (0.72) | 386 | |
| Zizyberenalic acid (32) | Inactive (0.59) | Inactive (0.63) | Inactive (0.60) | Active (0.72) | 1000 | |
| Artemisinin | Inactive (0.72) | Inactive (0.56) | Inactive (0.57) | Inactive (0.59) | 4228 | |
| Chloroquine | Inactive (0.90) | Inactive (0.81) | Inactive (0.96) | Inactive (0.91) | 750 | |
| Compounds | Mauritine F (4) | Hemisine A (10) | Amphibine D (12) | Nummularine B (15) | Nummularine R (21) | Mauritine M (24) | Zizyberenalic acid (32) |
|---|---|---|---|---|---|---|---|
| Physicochemical Parameters | |||||||
| Molecular weight | 561.67 | 557.68 | 631.80 | 591.70 | 587.71 | 686.84 | 452.67 |
| Number of rotatable bonds | 9 | 15 | 12 | 10 | 8 | 13 | 3 |
| Number of heavy atoms | 41 | 41 | 46 | 43 | 43 | 50 | 33 |
| Fraction C(sp3) | 0.42 | 0.41 | 0.50 | 0.44 | 0.42 | 0.47 | 0.80 |
| Molar Refractivity | 166.84 | 170.99 | 190.97 | 173.34 | 177.48 | 204.42 | 135.48 |
| TPSA | 128.87 Å2 | 106.77 Å2 | 120.08 Å2 | 138.10 Å2 | 116.00 Å2 | 153.89 Å2 | 54.37 Å2 |
| Lipophilicity | |||||||
| log Po/w (MLOGP) | 0.73 | 1.48 | 1.65 | 0.42 | 1.16 | 0.88 | 5.63 |
| log Po/w (XLOGP3) | 3.15 | 4.36 | 5.30 | 3.12 | 4.33 | 4.93 | 8.04 |
| Water solubility | |||||||
| log S (ESOL) | −4.95 | −5.85 | −6.50 | −5.02 | −5.94 | 6.57 | −7.51 |
| Water solubility class | Moderately soluble | Moderately soluble | Poorly soluble | Moderately soluble | Moderately soluble | Poorly soluble | Poorly soluble |
| Pharmacokinetic | |||||||
| GI absorption | High | High | High | High | High | Low | Low |
| BBB permeant | No | No | No | No | No | No | No |
| P-gp substrate | Yes | Yes | Yes | Yes | Yes | Yes | No |
| CYP1A2 inhibitor | No | No | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No | No | Yes |
| CYP2D6 inhibitor | No | No | No | No | No | No | No |
| CYP3A4 inhibitor | Yes | Yes | Yes | Yes | Yes | Yes | No |
| log Kp (skin permeation) | −7.49 cm/s | −6.61 cm/s | −6.39 cm/s | −7.69 cm/s | −6.81 cm/s | −6.99 cm/s | −3.35 cm/s |
| Drug likeness | |||||||
| Lipinski | Yes; 1 violation | Yes; 1 violation | Yes; 1 violation | No; 2 violations | Yes; 1 violation | No; 2 violations | Yes; 1 violation |
| Ghose | No; 3 violations | No; 3 violations | No; 3 violations | No; 3 violations | No; 3 violations | No; 3 violations | No; 3 violations |
| Veber | Yes | Yes | No; 1 violation | Yes | Yes | No; 2 violations | Yes; 1 violation |
| Egan | Yes | Yes | Yes | No; 1 violation | Yes | No; 1 violation | Yes; 1 violation |
| Bioavailability Score | 0.55 | 0.55 | 0.55 | 0.17 | 0.55 | 0.17 | 0.85 |
| Compounds | Area (In 0.2 mg of ZMRD) | RMCY1 (mg/g of ZMRD) | RMCY2 (mg/g of ZMRD) | Mean ± SD (mg/g) |
|---|---|---|---|---|
| Mauritine F (4) | 3,934,027 | 38.44 | 31.23 | 34.83 ± 3.60 |
| Mauritine A (5) | 51,835,504 | 506.5 | 411.51 | 459.00 ± 47.49 |
| Hemisine A (10) | 765,130 | 7.47 | 6.07 | 6.67 ± 0.70 |
| Amphibine D (12) | 416,025 | 4.06 | 3.30 | 3.68 ± 0.38 |
| Nummularine B (15) | 570,694 | 5.57 | 4.53 | 5.05 ± 0.52 |
| Nummularine R (21) | 87,149 | 0.85 | 0.69 | 0.77 ± 0.08 |
| Mauritine M (24) | 513,715 | 5.01 | 4.07 | 4.54 ± 0.47 |
| Amphibine A (14) | 1,435,994 | 14.03 | 11.4 | 12.71 ± 1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsila, S.S.; Kinyok, M.J.; Tameko, J.E.M.; Mountessou, B.Y.G.; Dongmo, K.J.J.; Garba, J.K.; Efange, N.M.; Ayong, L.; Fongang, Y.S.F.; Bankeu, J.J.K.; et al. Bioactive Cyclopeptide Alkaloids and Ceanothane Triterpenoids from Ziziphus mauritiana Roots: Antiplasmodial Activity, UHPLC-MS/MS Molecular Networking, ADMET Profiling, and Target Prediction. Molecules 2025, 30, 2958. https://doi.org/10.3390/molecules30142958
Tsila SS, Kinyok MJ, Tameko JEM, Mountessou BYG, Dongmo KJJ, Garba JK, Efange NM, Ayong L, Fongang YSF, Bankeu JJK, et al. Bioactive Cyclopeptide Alkaloids and Ceanothane Triterpenoids from Ziziphus mauritiana Roots: Antiplasmodial Activity, UHPLC-MS/MS Molecular Networking, ADMET Profiling, and Target Prediction. Molecules. 2025; 30(14):2958. https://doi.org/10.3390/molecules30142958
Chicago/Turabian StyleTsila, Sylvestre Saidou, Mc Jesus Kinyok, Joseph Eric Mbasso Tameko, Bel Youssouf G. Mountessou, Kevine Johanne Jumeta Dongmo, Jean Koffi Garba, Noella Molisa Efange, Lawrence Ayong, Yannick Stéphane Fotsing Fongang, Jean Jules Kezetas Bankeu, and et al. 2025. "Bioactive Cyclopeptide Alkaloids and Ceanothane Triterpenoids from Ziziphus mauritiana Roots: Antiplasmodial Activity, UHPLC-MS/MS Molecular Networking, ADMET Profiling, and Target Prediction" Molecules 30, no. 14: 2958. https://doi.org/10.3390/molecules30142958
APA StyleTsila, S. S., Kinyok, M. J., Tameko, J. E. M., Mountessou, B. Y. G., Dongmo, K. J. J., Garba, J. K., Efange, N. M., Ayong, L., Fongang, Y. S. F., Bankeu, J. J. K., Sewald, N., & Lenta, B. N. (2025). Bioactive Cyclopeptide Alkaloids and Ceanothane Triterpenoids from Ziziphus mauritiana Roots: Antiplasmodial Activity, UHPLC-MS/MS Molecular Networking, ADMET Profiling, and Target Prediction. Molecules, 30(14), 2958. https://doi.org/10.3390/molecules30142958






