Cyclodextrin-Based Quercetin Powders for Potential Nose-to-Brain Transport: Formulation and In Vitro Assessment
Abstract
1. Introduction
2. Results and Discussion
2.1. Yield of Spray-Drying Process
2.2. Que Content in the Powders and Blends
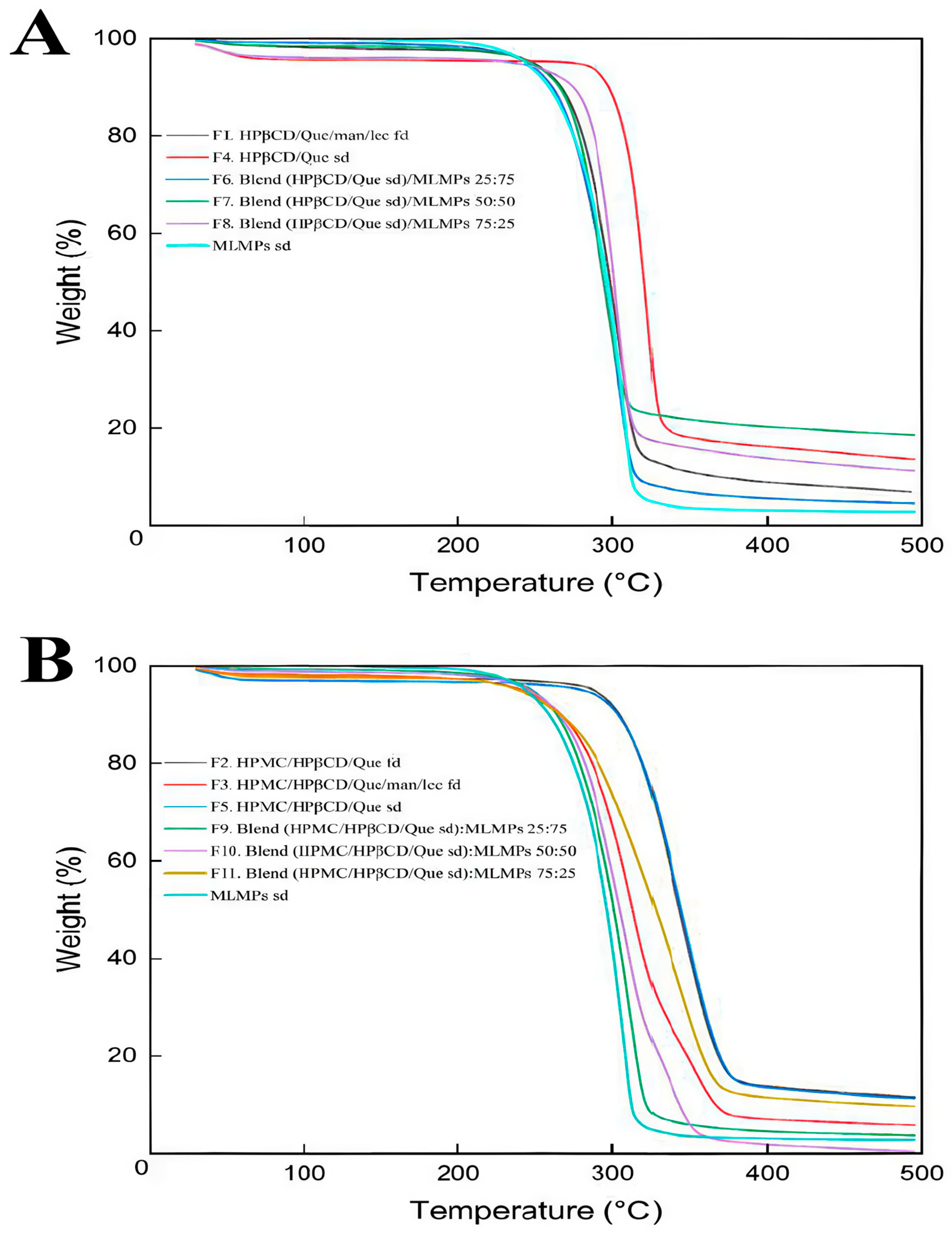
2.3. Decoding System’s Thermal Stability
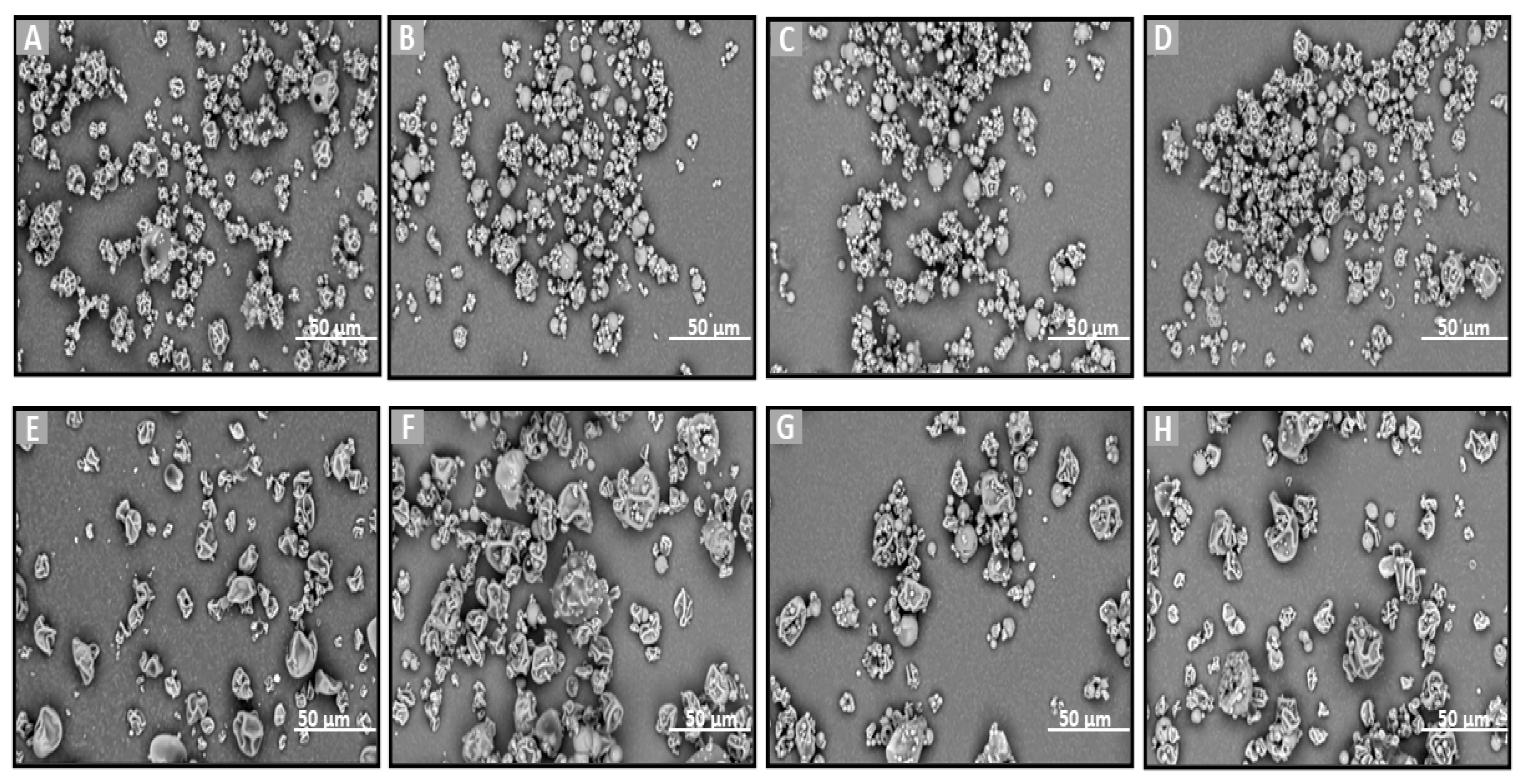
2.4. Morphological Characterization of Que Lyophilized and Spray-Dried Powders and Blends
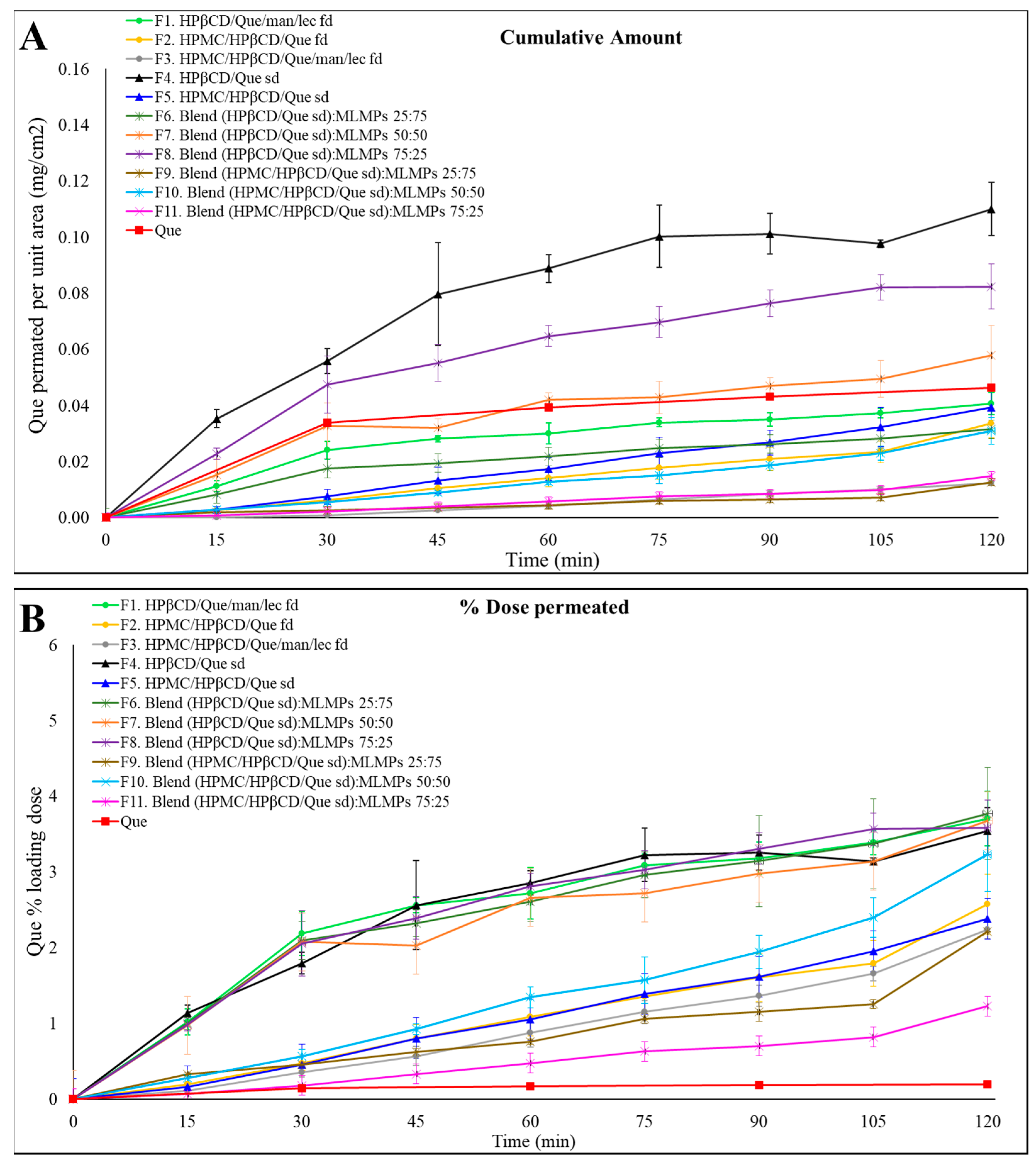
2.5. Que’s Release from the Lyophilized and Spray-Dried Powders and Blends
2.6. In Vitro Device-Based Assessment of Powder Emission
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Methods
3.2.1. Preparation of Freeze-Dried Formulations
3.2.2. Preparation of Spray-Dried Formulations
Preparation of Spray-Dried Que/HPβCD Powder
Preparation of Spray-Dried HPMC/HPβCD/Que Powder
Preparation of Spray-Dried MLMP Powder
3.2.3. Preparation of Blends
3.2.4. Thermogravimetric Analysis
3.2.5. Scanning Electron Microscopy (SEM) Analysis
3.2.6. Quantitative Analysis of Que
3.2.7. In Vitro Diffusion Experiments
3.2.8. In Vitro Device-Based Assessment of Powder Emission
3.2.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Diffusion area |
| API | Active pharmaceutical ingredient |
| BBB | Βlood–brain barrier |
| CI | Confidence interval |
| CNS | Central nervous system |
| HPLC | High-performance liquid chromatography |
| HPMC | Hydroxy-propyl methyl cellulose |
| HPβCD | Hydroxy-propyl-β-CD |
| IQR | Ιnterquartile range |
| J | Flux |
| Que | Quercetin |
| MPs | Microparticles |
| MLMPs | Microparticles of mannitol/lecithin |
| MW | Molecular weight |
| ΜβCD | Methyl-β-CD |
| PDA | Photodiode array |
| PBS | Phosphate buffer solution |
| Que | Quercetin |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
References
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, A.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; Abd El-Hack, Μ.Ε.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Kukolj, M.; Oršolić, N.; Langer Horvat, L.; Nikolić, B.; Ocrt, T.; Branović Čakanić, K.; Gračan, R.; Zrinščak, I.; Jazvinšćak Jembrek, M.; Šimić, G. Quercetin as a Therapeutic Option in a Rat Model of Aluminum Chloride- and D-Galactose-Induced Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 5743. [Google Scholar] [CrossRef]
- Zubčić, K.; Radovanović, V.; Vlainić, J.; Hof, P.R.; Oršolić, N.; Šimić, G.; Jazvinšćak Jembrek, M. PI3K/Akt and ERK1/2 signalling are involved in quercetin-mediated neuroprotection against copper-induced injury. Oxid. Med. Cell. Longev. 2020, 2020, 9834742. [Google Scholar] [CrossRef]
- Das, D.; Banerjee, A.; Mukherjee, S.; Maji, B.K. Quercetin inhibits NF-κB and JAK/STAT signaling via modulating TLR in thymocytes and splenocytes during MSG-induced immunotoxicity: An in vitro approach. Mol. Biol. Rep. 2024, 51, 277. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Muñoz-Manco, J.I.; Ramírez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gómez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, S.; Wang, Y.; Yang, X.; Jiang, J.; Wu, D.; Qu, X.; Fan, H.; Yao, R. Quercetin ameliorates learning and memory via the Nrf2-ARE signaling pathway in d-galactose-induced neurotoxicity in mice. Biochem. Biophys. Res. Commun. 2017, 491, 636–641. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, R.; Huang, H. Anti-Allergic Effects of Quercetin and Quercetin Liposomes in RBL-2H3 Cells. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Papakyriakopoulou, P.; Valsami, G. The nasal route for nose-to-brain drug delivery: Advanced nasal formulations for CNS disorders. Expert Opin. Drug Deliv. 2025, 22, 823–839. [Google Scholar] [CrossRef]
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Zaki, R.M.; Alsaidan, O.A.; Elmowafy, M.; Zafar, A.; Shalaby, K.; Abdelgawad, M.A.; Abo El-Ela, F.I.; Rateb, M.E.; Naguib, I.A.; et al. Intranasal nanotransferosomal gel for quercetin brain targeting: I. Optimization, characterization, brain localization, and cytotoxic studies. Pharmaceutics 2023, 15, 1805. [Google Scholar] [CrossRef]
- Mahmoud, K.Y.; Elhesaisy, N.A.; Rashed, A.R.; Mikhael, E.S.; Fadl, M.I.; Elsadek, M.S.; Mohamed, M.A.; Mostafa, M.A.; Hassan, M.A.; Halema, O.M.; et al. Exploring the potential of intranasally administered naturally occurring quercetin loaded into polymeric nanocapsules as a novel platform for the treatment of anxiety. Sci. Rep. 2023, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- Trenkel, M.; Scherließ, R. Nasal Powder Formulations: In-Vitro Characterisation of the Impact of Powders on Nasal Residence Time and Sensory Effects. Pharmaceutics 2021, 13, 385. [Google Scholar] [CrossRef]
- Buttini, F.; Colombo, P.; Rossi, A.; Sonvico, F.; Colombo, G. Particles and powders: Tools of innovation for non-invasive drug administration. J. Control. Release 2012, 161, 693–702. [Google Scholar] [CrossRef]
- Fasiolo, L.T.; Manniello, M.D.; Tratta, E.; Buttini, F.; Rossi, A.; Sonvico, F.; Bortolotti, F.; Russo, P.; Colombo, G. Opportunity and challenges of nasal powders: Drug formulation and delivery. Eur. J. Pharm. Sci. 2018, 113, 2–17. [Google Scholar] [CrossRef]
- Tanaka, A.; Furubayashi, T.; Tomisaki, M.; Kawakami, M.; Kimura, S.; Inoue, D.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Nasal drug absorption from powder formulations: The effect of three types of hydroxypropyl cellulose (HPC). Eur. J. Pharm. Sci. 2017, 96, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef] [PubMed]
- McAdams, D.; Chen, D.; Kristensen, D. Spray drying and vaccine stabilization. Expert. Rev. Vaccines 2012, 11, 1211–1219. [Google Scholar] [CrossRef]
- Manta, K.; Papakyriakopoulou, P.; Chountoulesi, M.; Diamantis, D.A.; Spaneas, D.; Vakali, V.; Naziris, N.; Chatziathanasiadou, M.V.; Andreadelis, I.; Moschovou, K.; et al. Preparation and Biophysical Characterization of Quercetin Inclusion Complexes with β-Cyclodextrin Derivatives to be Formulated as Possible Nose-to-Brain Quercetin Delivery Systems. Mol. Pharm. 2020, 17, 4241–4255. [Google Scholar] [CrossRef]
- Papakyriakopoulou, P.; Manta, K.; Kostantini, C.; Kikionis, S.; Banella, S.; Ioannou, E.; Christodoulou, E.; Rekkas, D.M.; Dallas, P.; Vertzoni, M.; et al. Nasal powders of quercetin-β-cyclodextrin derivatives complexes with mannitol/lecithin microparticles for Nose-to-Brain delivery: In vitro and ex vivo evaluation. Int. J. Pharm. 2021, 607, 121016. [Google Scholar] [CrossRef] [PubMed]
- Manta, K.; Papakyriakopoulou, P.; Nikolidaki, A.; Balafas, E.; Kostomitsopoulos, N.; Banella, S.; Colombo, G.; Valsami, G. Comparative Serum and Brain Pharmacokinetics of Quercetin after Oral and Nasal Administration to Rats as Lyophilized Complexes with β-Cyclodextrin Derivatives and Their Blends with Mannitol/Lecithin Microparticles. Pharmaceutics 2023, 15, 2036. [Google Scholar] [CrossRef]
- Bentley, K.; Stanton, R.J. Hydroxypropyl Methylcellulose-Based Nasal Sprays Effectively Inhibit In Vitro SARS-CoV-2 Infection and Spread. Viruses 2021, 13, 2345. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.A.; Chauk, D.S.; Mahajan, H.S.; Tekade, A.R.; Gattani, S.G. Formulation and evaluation of nasal mucoadhesive microspheres of sumatriptan succinate. J. Microencapsul. 2009, 26, 711–721. [Google Scholar] [CrossRef]
- Belgamwar, V.S.; Patel, H.S.; Joshi, A.S.; Agrawal, A.; Surana, S.J.; Tekade, A.R. Design and development of nasal mucoadhesive microspheres containing tramadol HCl for CNS targeting. Drug Deliv. 2011, 18, 353–360. [Google Scholar] [CrossRef]
- Sah, M.K.; Gautam, B.; Pokhrel, K.P.; Ghani, L.; Bhattarai, A. Quantification of the Quercetin Nanoemulsion Technique Using Various Parameters. Molecules 2023, 28, 2540. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, Z.; Zhu, R.; Niu, Y. Pyrolysis characteristics and kinetics of β-cyclodextrin and its two derivatives. Pol. J. Chem. Technol. 2015, 17, 1–4. [Google Scholar] [CrossRef]
- Yuan, C.; Liu, B.; Liu, H. Characterization of hydroxypropyl-β-cyclodextrins with different substitution patterns via FTIR, GC–MS, and TG–DTA. Carbohydr. Polym. 2015, 118, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Mazyed, E.A.; Badria, F.A.; ElNaggar, M.H.; El-Masry, S.M.; Helmy, S.A. Development of Cyclodextrin-Functionalized Transethoniosomes of 6-Gingerol: Statistical Optimization, In Vitro Characterization and Assessment of Cytotoxic and Anti-Inflammatory Effects. Pharmaceutics 2022, 14, 1170. [Google Scholar] [CrossRef]
- Zaccaron, C.M.; Oliveira, R.V.B.; Guiotoku, M.; Pires, A.T.N.; Soldi, V. Blends of hydroxypropyl methylcellulose and poly(1-vinylpyrrolidone-co-vinyl acetate): Miscibility and thermal stability. Polym. Degrad. Stab. 2005, 90, 21–27. [Google Scholar] [CrossRef]
- Giang, H.N.; Lê, K.A.T.; Huynh, T.N.A.; Phung, K.; Sakai, W. Effect of additives on fabrication and properties of hydroxypropyl methylcellulose-based hydrogels. Polym. Bull. 2023, 80, 11121–11137. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Gao, W.; Sui, J.; Wang, J.; Cui, B. Effect of Different Proportions of Glycerol and D-Mannitol as Plasticizer on the Properties of Extruded Corn Starch. Front. Nutr. 2024, 11, 1335812. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Li, J.; Mao, J.; Zhou, Q.; Deng, Q.; Chai, Z.; Zheng, L.; Shi, J. The Soybean Lecithin-Cyclodextrin-Vitamin E Complex Nanoparticles Stabilized Pickering Emulsions for the Delivery of β-Carotene: Physicochemical Properties and In Vitro Digestion. Int. J. Biol. Macromol. 2024, 265, 130742. [Google Scholar] [CrossRef]
- de Lima, M.S.A.; Rocha, L.A.; Molina, E.F.; Caetano, B.L.; Marçal, L.; Mello, C.; Ciuffi, K.; Calefi, P.; Nassar, E.J. Thermoanalysis of soybean oil extracted by two methods. Quim. Nova 2008, 31, 527–529. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef]
- Guo, J.; Dong, S.; Ye, M.; Wu, X.; Lv, X.; Xu, H.; Li, Μ. Effects of Hydroxypropyl Methylcellulose on Physicochemical Properties and Microstructure of κ-Carrageenan Film. Foods 2022, 11, 3023. [Google Scholar] [CrossRef]
- Zaharia, M.M.; Bucatariu, F.; Karayianni, M.; Lotos, E.-D.; Mihai, M.; Pispas, S. Synthesis of Thermoresponsive Chitosan-graft-Poly(N-isopropylacrylamide) Hybrid Copolymer and Its Complexation with DNA. Polymers 2024, 16, 1315. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, J.; Tsaknopoulos, K.; Massar, C.; Young, B.; O’Connell, A.; Walde, C.; Birt, A.; Siopis, M.; Cote, D. Comparison of Laser Diffraction and Image Analysis Techniques for Particle Size-Shape Characterization in Additive Manufacturing Applications. Powder Technol. 2021, 391, 20–33. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Wendroth, O.; Liu, B.; Cheng, C.; Huang, T.; Shi, Y. Is the Laser Diffraction Method Reliable for Soil Particle Size Distribution Analysis? Soil Sci. Soc. Am. J. 2019, 83, 276–287. [Google Scholar] [CrossRef]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Jüptner, A.; Scherließ, R. Investigation of powder properties and application aspects impacting nasal deposition of spray-dried powders in a nasal cast. Eur. J. Pharm. Biopharm. 2025, 209, 114666. [Google Scholar] [CrossRef]
- Henriques, P.; Fortuna, A.; Doktorovová, S. Spray dried powders for nasal delivery: Process and formulation considerations. Eur. J. Pharm. Biopharm. 2022, 176, 1–20. [Google Scholar] [CrossRef]
- Cheshmehnoor, P.; Bolourchian, N.; Abdollahizad, Ε.; Derakhshi, A.; Dadashzadeh, S.; Haeri, A. Particle Size Tailoring of Quercetin Nanosuspensions by Wet Media Milling Technique: A Study on Processing and Formulation Parameters. Iran. J. Pharm. Res. 2022, 21, e130626. [Google Scholar] [CrossRef]
- Ibrahim, S.; Bowra, S. Improving Oxidative Stability of Polyphenolic Fraction of Apple Pomace by Encapsulation Using Naturally Occurring Polymers. J. Encapsulation Adsorpt. Sci. 2019, 9, 83–108. [Google Scholar] [CrossRef]
- Giuliani, A.; Balducci, A.G.; Zironi, E.; Colombo, G.; Bortolotti, F.; Lorenzini, L.; Galligioni, V.; Pagliuca, G.; Scagliarini, A.; Calzà, A.; et al. In vivo nose-to-brain delivery of the hydrophilic antiviral ribavirin by microparticle agglomerates. Drug Deliv. 2018, 25, 376–387. [Google Scholar] [CrossRef]
- Otte, A.; Sharifi, F.; Park, K. Interfacial tension effects on the properties of PLGA microparticles. Colloids Surf. B Biointerfaces 2020, 196, 111300. [Google Scholar] [CrossRef]
- Rosita, N.; Ambarwati, N.; Erawati, T.; Hariyadi, D.M. Characterization and in vitro release of inhalation quercetin solid lipid microparticles: Effect of lipid. J. Adv. Pharm. Technol. Res. 2022, 13, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, D.-G.; Williams, G.R.; Wang, Z.-H. Fast-dissolving core-shell composite microparticles of quercetin fabricated using a coaxial electrospray process. PLoS ONE 2014, 9, e92106. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Trotta, V.; Traini, D.; Young, P.M.; Sticozzi, C.; Cervellati, F.; Valacchi, G. Incorporation of quercetin in respirable lipid microparticles: Effect on stability and cellular uptake on A549 pulmonary alveolar epithelial cells. Colloids Surf. B Biointerfaces 2013, 112, 322–329. [Google Scholar] [CrossRef]
- Jaipal, A.; Pandey, M.M.; Charde, S.Y.; Raut, P.P.; Prasanth, K.V.; Prasad, R.G. Effect of HPMC and mannitol on drug release and bioadhesion behavior of buccal discs of buspirone hydrochloride: In-vitro and in-vivo pharmacokinetic studies. Saudi Pharm. J. 2015, 23, 315–326. [Google Scholar] [CrossRef]
- Williams, H.D.; Ward, R.; Hardy, I.J.; Melia, C.D. The extended release properties of HPMC matrices in the presence of dietary sugars. J. Control. Release 2009, 138, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Figueiras, A.; Carvalho, R.A.; Ribeiro, L.; Torres-Labandeira, J.J.; Veiga, F.J.B. Solid-state characterization and dissolution profiles of the inclusion complexes of omeprazole with native and chemically modified beta-cyclodextrin. Eur. J. Pharm. Biopharm. 2007, 67, 531–539. [Google Scholar] [CrossRef]
- Balducci, A.G.; Ferraro, L.; Bortolotti, F.; Nastruzzi, C.; Colombo, P.; Sonvico, F.; Russo, P.; Colombo, G. Antidiuretic effect of desmopressin chimera agglomerates by nasal administration in rats. Int. J. Pharm. 2013, 440, 154–160. [Google Scholar] [CrossRef]
- Pippa, N.; Sentoukas, T.; Pispas, S.; Demetzos, C.; Papalois, A.; Bouropoulos, N. pH-responsive polymeric nanoassemblies encapsulated into alginate beads: Morphological characterization and swelling studies. J. Polym. Res. 2018, 25, 117. [Google Scholar] [CrossRef]
- Diamantis, D.A.; Ramesova, S.; Chatzigiannis, C.M.; Degano, I.; Gerogianni, P.S.; Karadima, K.E.; Perikleous, S.; Rekkas, D.; Gerothanassis, I.P.; Galaris, D.; et al. Exploring the oxidation and iron binding profile of a cyclodextrin encapsulated quercetin complex unveiled a controlled complex dissociation through a chemical stimulus. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1913–1924. [Google Scholar] [CrossRef]
- de Souza Teixeira, L.; Chagas, T.V.; Alonso, A.; Gonzalez-Alvarez, I.; Bermejo, M.; Polli, J.; Rezende, K.R. Biomimetic Artificial Membrane Permeability Assay over Franz Cell Apparatus Using BCS Model Drugs. Pharmaceutics 2020, 12, 988. [Google Scholar] [CrossRef]
- Tai, L.M.; Maldonado Weng, J.; LaDu, M.J.; Brady, S.T. Relevance of transgenic mouse models for Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2021, 177, 1–48. [Google Scholar] [PubMed]

| Formulations | Mass (g) of Produced Powder | Yield (%) |
|---|---|---|
| F4: HPβCD/Que spray-dried | 1.1 | 41.0 |
| F5: HPMC/HPβCD/Que spray-dried | 1.1 | 20.5 |
| MLMPs | 2.6 | 26.0 |
| Formulations | Theoretical Que Content w/w (%) | Experimental Que Content (w/w) ± SD (%) | Recovery ± SD (%) |
|---|---|---|---|
| F1 | 3.26 | 2.79 ± 0.04 | 85.61 ± 1.25 |
| F2 | 4.84 | 3.32 ± 0.08 | 68.61 ± 1.69 |
| F3 | 2.46 | 1.87 ± 0.04 | 76.15 ± 1.66 |
| F4 | 9.39 | 7.92 ± 0.29 | 84.33 ± 3.09 |
| F5 | 4.84 | 4.19 ± 0.01 | 86.63 ± 0.17 |
| F6 | 2.35 | 2.12 ± 0.11 | 90.41 ± 4.54 |
| F7 | 4.71 | 4.01 ± 0.08 | 85.24 ± 1.71 |
| F8 | 7.04 | 5.87 ± 0.14 | 83.37 ± 1.98 |
| F9 | 1.21 | 1.44 ± 0.01 | 119.14 ± 0.48 |
| F10 | 2.42 | 2.19 ± 0.06 | 90.64 ± 2.62 |
| F11 | 3.63 | 3.07 ± 0.04 | 84.72 ± 1.06 |
| Blend/% of Total Population with Specific Size Dimensions | 50–100 μm | 30–50 μm | 10–30 μm | <10 μm |
|---|---|---|---|---|
| F1 | 0 | 9 | 91 | 0 |
| F4 | 1 | 2 | 16 | 81 |
| F5 | 0 | 0 | 17 | 83 |
| F6 | 0 | 1 | 27 | 72 |
| F7 | 0 | 0 | 18 | 82 |
| F8 | 0 | 0 | 10 | 90 |
| F9 | 0 | 0 | 41 | 59 |
| F10 | 0 | 0 | 14 | 86 |
| F11 | 0 | 0 | 7 | 93 |
| MLMPs | 0 | 2 | 49 | 49 |
| Formulation | Que Permeated Per Unit Area (mg/cm2) | Permeated Que (% Loading Dose) | % of Que Dose Retained by Cellulose Membrane |
|---|---|---|---|
| F1 | 0.04 ± 0.00 | 3.70 ± 0.36 | 1.51 ± 0.55 |
| F2 | 0.03 ± 0.01 | 2.57 ± 0.40 | 0.86 ± 0.02 |
| F3 | 0.01 ± 0.00 | 2.24 ± 0.12 | 5.66 ± 0.19 |
| F4 | 0.11 ± 0.01 | 3.54 ± 0.31 | 1.19 ± 0.33 |
| F5 | 0.04 ± 0.01 | 2.38 ± 0.37 | 1.89 ± 0.76 |
| F6 | 0.03 ± 0.01 | 3.77 ± 0.61 | 1.54 ± 0.32 |
| F7 | 0.06 ± 0.01 | 3.68 ± 0.67 | 1.04 ± 0.32 |
| F8 | 0.08 ± 0.01 | 3.58 ± 0.36 | 1.20 ± 0.44 |
| F9 | 0.01 ±0.00 | 2.21 ± 0.00 | 1.51 ± 0.07 |
| F10 | 0.03 ± 0.00 | 3.23 ± 0.49 | 1.94 ± 0.56 |
| F11 | 0.01 ± 0.00 | 1.23 ± 0.07 | 0.91 ± 0.10 |
| Que control | 0.20 ± 0.00 | 1.45 ± 0.59 | 0.05 ± 0.00 |
| Formulation | Loaded Powder (mg) | Emitted Powder (mg) |
|---|---|---|
| F4 | 10.42 ± 0.38 | 7.26 ± 1.57 |
| F8 | 10.43 ± 0.33 | 1.43 ± 0.38 |
| HPβCD/Que freeze-dried | 10.35 ± 0.24 | 0 |
| Blend HPβCD/Que freeze-dried–MLMPs 75:25 | 10.18 ± 0.29 | 2.78 ± 0.37 |
| Formulations | |
|---|---|
| F1 | HPβCD/Que/mannitol/lecithin freeze-dried |
| F2 | HPMC/HPβCD/Que freeze-dried |
| F3 | HPMC/HPβCD/Que/mannitol/lecithin freeze-dried |
| F4 | HPβCD/Que spray-dried |
| F5 | HPMC/HPβCD/Que spray-dried |
| F6 | Blend HPβCD/Que spray-dried–MLMPs 25:75 |
| F7 | Blend HPβCD/Que spray-dried–MLMPs 50:50 |
| F8 | Blend HPβCD/Que spray-dried–MLMPs 75:25 |
| F9 | Blend HPMC/HPβCD/Que spray-dried–MLMPs 25:75 |
| F10 | Blend HPMC/HPβCD/Que spray-dried–MLMPs 50:50 |
| F11 | Blend HPMC/HPβCD/Que spray-dried–MLMPs 75:25 |
| Formulation | Formulation–MLMPs | Experimental Que Content | |
|---|---|---|---|
| % w/w ± SD | Amount (mg) in 25 mg of Formulation ± SD | ||
| F1 | 100:0 | 2.79 ± 0.04 | 0.70 ± 0.01 |
| F2 | 100:0 | 3.32 ± 0.08 | 0.83 ± 0.02 |
| F3 | 100:0 | 1.87 ± 0.04 | 0.47 ± 0.01 |
| F4 | 100:0 | 7.92 ± 0.29 | 1.98 ± 0.07 |
| F5 | 100:0 | 4.19 ± 0.01 | 1.05 ± 0.00 |
| F6 | 25:75 | 2.12 ± 0.11 | 0.53 ± 0.03 |
| F7 | 50:50 | 4.01 ± 0.08 | 1.00 ± 0.02 |
| F8 | 75:25 | 5.87 ± 0.14 | 1.47 ± 0.03 |
| F9 | 25:75 | 1.44 ± 0.01 | 0.36 ± 0.00 |
| F10 | 50:50 | 2.19 ± 0.06 | 0.55 ± 0.02 |
| F11 | 75:25 | 3.07 ± 0.04 | 0.77 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saitani, E.-M.; Papakyriakopoulou, P.; Bogri, T.; Choleva, G.; Kontopoulou, K.; Roboras, S.; Samiou, M.; Vardaxi, A.; Pispas, S.; Valsami, G.; et al. Cyclodextrin-Based Quercetin Powders for Potential Nose-to-Brain Transport: Formulation and In Vitro Assessment. Molecules 2025, 30, 2878. https://doi.org/10.3390/molecules30132878
Saitani E-M, Papakyriakopoulou P, Bogri T, Choleva G, Kontopoulou K, Roboras S, Samiou M, Vardaxi A, Pispas S, Valsami G, et al. Cyclodextrin-Based Quercetin Powders for Potential Nose-to-Brain Transport: Formulation and In Vitro Assessment. Molecules. 2025; 30(13):2878. https://doi.org/10.3390/molecules30132878
Chicago/Turabian StyleSaitani, Elmina-Marina, Paraskevi Papakyriakopoulou, Theodora Bogri, Georgia Choleva, Kyriaki Kontopoulou, Spyridon Roboras, Maria Samiou, Antiopi Vardaxi, Stergios Pispas, Georgia Valsami, and et al. 2025. "Cyclodextrin-Based Quercetin Powders for Potential Nose-to-Brain Transport: Formulation and In Vitro Assessment" Molecules 30, no. 13: 2878. https://doi.org/10.3390/molecules30132878
APA StyleSaitani, E.-M., Papakyriakopoulou, P., Bogri, T., Choleva, G., Kontopoulou, K., Roboras, S., Samiou, M., Vardaxi, A., Pispas, S., Valsami, G., & Pippa, N. (2025). Cyclodextrin-Based Quercetin Powders for Potential Nose-to-Brain Transport: Formulation and In Vitro Assessment. Molecules, 30(13), 2878. https://doi.org/10.3390/molecules30132878









