Unveiling the Chemical Composition, Enantiomeric Profile, Antibacterial, Anticholinesterase and Antioxidant Activity of the Essential Oil of Aloysia triphylla Royle
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization of Essential Oil
2.2. Enantiomeric Distribution of A. triphylla Essential Oil
2.3. Antibacterial Activity of the A. triphylla EO
2.4. Antioxidant Activity of A. triphylla EO
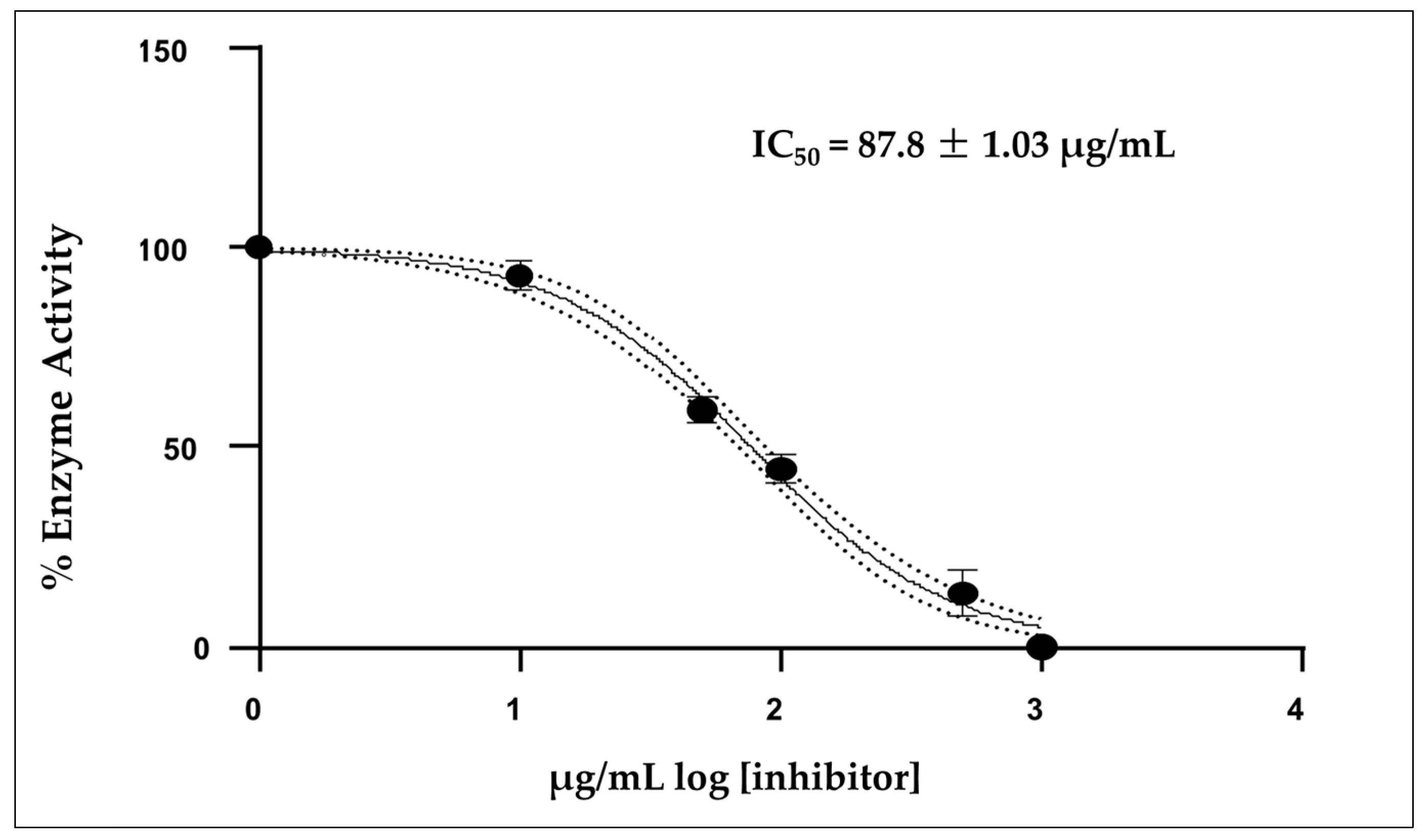
2.5. Acetylcholinesterase Inhibition of A. triphylla EO
2.6. Cytotoxicity of A. triphylla EO
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Essential Oil Obtention
4.3. Determination of Essential Oil Composition
4.4. Enantioselective Profile of the Essential Oil
4.5. Evaluation of the Antibacterial Activity
4.6. Antioxidant Activity
4.7. Acetylcholinesterase Inhibition of A. triphylla EO In Vitro
4.8. Brine Shrimp Cytoxicity Test
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farha, M.A.; Tu, M.M.; Brown, E.D. Important Challenges to Finding New Leads for New Antibiotics. Curr. Opin. Microbiol. 2025, 83, 102562. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.M.; Quave, C.L. Opportunities for Plant Natural Products in Infection Control. Curr. Opin. Microbiol. 2018, 45, 189. [Google Scholar] [CrossRef] [PubMed]
- Schneider, Y.K.; Simal-Gandara, J.; Barros, L.; Prieto Lage, M.A. Bacterial Natural Product Drug Discovery for New Antibiotics: Strategies for Tackling the Problem of Antibiotic Resistance by Efficient Bioprospecting. Antibiotics 2021, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Chen, X.; Shang, S.; Yan, F.; Jiang, H.; Zhao, G.; Tian, S.; Chen, R.; Chen, D.; Dang, Y. Antioxidant Activities of Essential Oils and Their Major Components in Scavenging Free Radicals, Inhibiting Lipid Oxidation and Reducing Cellular Oxidative Stress. Molecules 2023, 28, 4559. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of Acetylcholinesterase Inhibitors in Alzheimer’s Disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Chu, M.H.; Liu, T.W.; Chen, P.H.; Chen, Y.H.; Tang, K.L.; Hsu, S.J.; Iskandar, B.; Yin, H.W.; Lin, M.H.; Lee, C.K. Investigation of the Acetylcholinesterase Inhibitors of Mentha Genus Essential Oils with in Vitro and in Silico Approaches. Ind. Crops Prod. 2025, 227, 120783. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia Citrodora Paláu (Lemon Verbena): A Review of Phytochemistry and Pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef]
- Rojas, J.; Palacios, O.; Ronceros, S. Efecto Del Aceite Esencial de Aloysia Triphylla Britton (Cedrón) Sobre El Trypanosoma Cruzi En Ratones. Rev. Peru. Med. Exp. Salud Publica 2012, 29, 61–68. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Salama, Y.; Al-Hajj, N.; Jaradat, N.; Jobran, N.T.; Warad, I.; Hamdan, L.; Alrob, M.A.; Sawafta, A.; Hidmi, A. Chemical Composition, Anticancer, Antimicrobial Activity of Aloysia Citriodora Palau Essential Oils from Four Different Locations in Palestine. BMC Complement. Med. Ther. 2024, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- Özek, T.; Kirimer, N.; Baser, K.H.C.; Tümen, G. Composition of the Essential Oil of Aloysia triphylla (L’herit.) Britton Grown in Turkey. J. Essent. Oil Res. 1996, 8, 581–583. [Google Scholar] [CrossRef]
- Sprea, R.M.; Fernandes, L.H.M.; Pires, T.C.S.P.; Calhelha, R.C.; Rodrigues, P.J.; Amaral, J.S. Volatile Compounds and Biological Activity of the Essential Oil of Aloysia citrodora Paláu: Comparison of Hydrodistillation and Microwave-Assisted Hydrodistillation. Molecules 2023, 28, 4528. [Google Scholar] [CrossRef] [PubMed]
- Boukabache, M.; Chibani, S.; Otmani, A.; Nouichi, A.; Abdelaziz, O.; Karaca, I. Chemical Composition and Insecticidal Activity of Aloysia citrodora Essential Oil against Aphis Fabae (Hemiptera: Aphididae), Rhopalosiphum Maidis (Hemiptera: Aphididae) and Tribolium castaneum (Coleoptera: Tenebrionidae). Int. J. Trop. Insect Sci. 2023, 43, 455–461. [Google Scholar] [CrossRef]
- dos Santos, P.R.; de Andrade Porto, S.M.; Brandão, F.R.; de Melo Souza, D.C.; Rocha, M.J.S.; de Alexandre Sebastião, F.; Oliveira, M.R.; Chaves, F.C.M.; Chagas, E.C. Efficacy of the Essential Oils of Aloysia triphylla, Lippia Gracilis and Piper aduncum in the Control of Piscinoodinium pillulare (Shaperclaus, 1954) in Colossoma macropomum (Cuvier, 1818). Aquaculture 2023, 565, 739127. [Google Scholar] [CrossRef]
- Parodi, T.V.; Gressler, L.T.; Silva, L.d.L.; Becker, A.G.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M.; Baldisserotto, B. Chemical Composition of the Essential Oil of Aloysia triphylla under Seasonal Influence and Its Anaesthetic Activity in Fish. Aquac. Res. 2020, 51, 2515–2524. [Google Scholar] [CrossRef]
- Sgarbossa, J.; Schmidt, D.; Schwerz, F.; Schwerz, L.; Prochnow, D.; Caron, B.O. Effect of Season and Irrigation on the Chemical Composition of Aloysia triphylla Essential Oil. Rev. Ceres 2019, 66, 85–93. [Google Scholar] [CrossRef]
- He, S.M.; Wang, X.; Yang, S.C.; Dong, Y.; Zhao, Q.M.; Yang, J.L.; Cong, K.; Zhang, J.J.; Zhang, G.H.; Wang, Y.; et al. De Novo Transcriptome Characterization of Rhodomyrtus tomentosa Leaves and Identification of Genes Involved in α/β-Pinene and β-Caryophyllene Biosynthesis. Front. Plant Sci. 2018, 9, 355918. [Google Scholar] [CrossRef]
- Capetti, F.; Marengo, A.; Cagliero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Adulteration of Essential Oils: A Multitask Issue for Quality Control. Three Case Studies: Lavandula angustifolia Mill., Citrus Limon (L.) Osbeck and Melaleuca alternifolia (Maiden & Betche) Cheel. Molecules 2021, 26, 5610. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Koubaa, M.; Barba, F.J.; Abedi, E.; Niakousari, M.; Tavakoli, J. Extraction of Essential Oil from Aloysia Citriodora palau Leaves Using Continuous and Pulsed Ultrasound: Kinetics, Antioxidant Activity and Antimicrobial Properties. Process Biochem. 2018, 65, 197–204. [Google Scholar] [CrossRef]
- Rashid, H.M.; Mahmod, A.I.; Afifi, F.U.; Talib, W.H. Antioxidant and Antiproliferation Activities of Lemon Verbena (Aloysia citrodora): An In Vitro and In Vivo Study. Plants 2022, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant Activity of Medicinal and Aromatic Plants. A Review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; El-Ghorab, A.H.; Hussein, A.M.S.; El-Massry, K.F.; Lotfy, S.N.; Sayed Ahmed, M.Y.; Soliman, T.N. Correlation between Chemical Composition and Radical Scavenging Activity of 10 Commercial Essential Oils: Impact of Microencapsulation on Functional Properties of Essential Oils. Arab. J. Chem. 2020, 13, 6815–6827. [Google Scholar] [CrossRef]
- Belahcene, S.; Kebsa, W.; Omoboyowa, D.A.; Alshihri, A.A.; Alelyani, M.; Bakkour, Y.; Leghouchi, E. Unveiling the Chemical Profiling Antioxidant and Anti-Inflammatory Activities of Algerian Myrtus Communis L. Essential Oils, and Exploring Molecular Docking to Predict the Inhibitory Compounds against Cyclooxygenase-2. Pharmaceuticals 2023, 16, 1343. [Google Scholar] [CrossRef]
- Pérez Zamora, C.M.; Torres, C.A.; Nuñez, M.B. Antimicrobial Activity and Chemical Composition of Essential Oils from Verbenaceae Species Growing in South America. Molecules 2018, 23, 544. [Google Scholar] [CrossRef] [PubMed]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and Antimicrobial Activity of Essential Oils from Aromatic Plants Used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Demo, M.; Oliva, M.D.L.M.; López, M.L.; Zunino, M.P.; Zygadlo, J.A. Antimicrobial Activity of Essential Oils Obtained from Aromatic Plants of Argentina. Pharm. Biol. 2005, 43, 129–134. [Google Scholar] [CrossRef]
- Aouadhi, C.; Jouini, A.; Maaroufi, K.; Maaroufi, A. Antibacterial Effect of Eight Essential Oils against Bacteria Implicated in Bovine Mastitis and Characterization of Primary Action Mode of Thymus capitatus Essential Oil. Antibiotics 2024, 13, 237. [Google Scholar] [CrossRef]
- Dias, K.J.S.D.O.; Miranda, G.M.; Bessa, J.R.; De Araújo, A.C.J.; Freitas, P.R.; De Almeida, R.S.; Paulo, C.L.R.; Neto, J.B.D.A.; Coutinho, H.D.M.; Ribeiro-Filho, J. Terpenes as Bacterial Efflux Pump Inhibitors: A Systematic Review. Front. Pharmacol. 2022, 13, 953982. [Google Scholar] [CrossRef]
- Chao, S.C.; Young, D.G.; Oberg, C.J. Screening for Inhibitory Activity of Essential Oils on Selected Bacteria, Fungi and Viruses. J. Essent. Oil Res. 2000, 12, 639–649. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A.M. The Biological Activities of 20 Nature Identical Essential Oil Constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Schmidt, E.; Bail, S.; Friedl, S.M.; Jirovetz, L.; Buchbauer, G.; Wanner, J.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Antimicrobial Activities of Single Aroma Compounds. Nat. Prod. Commun. 2010, 5, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activity of Pinus Species Essential Oils and Their Constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool Bioactive Properties and Potential Applicability in Drug Delivery Systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Petrachaianan, T.; Chaiyasirisuwan, S.; Athikomkulchai, S.; Sareedenchai, V. Screening of acetylcholinesterase inhibitory activity in essential oil from myrtaceae. Thai J. Pharm. Sci. (TJPS) 2019, 43, 63–68. [Google Scholar]
- Albarano, L.; Ruocco, N.; Lofrano, G.; Guida, M.; Libralato, G. Genotoxicity in Artemia spp.: An Old Model with New Sensitive Endpoints. Aquat. Toxicol. 2022, 252, 106320. [Google Scholar] [CrossRef]
- Oliva, M.D.L.M.; Gallucci, N.; Zygadlo, J.A.; Demo, M.S. Cytotoxic Activity of Argentinean Essential Oils on Artemia salina. Pharm. Biol. 2007, 45, 259–262. [Google Scholar] [CrossRef]
- Waghulde, S.; Kale, M.K.; Patil, V.R. Brine Shrimp Lethality Assay of the Aqueous and Ethanolic Extracts of the Selected Species of Medicinal Plants. Proceedings 2019, 41, 47. [Google Scholar] [CrossRef]
- Guía Para El Libro Del Web de Química Del NIST. Available online: https://webbook.nist.gov/chemistry/guide/ (accessed on 16 June 2025).
- Sparkman, O.D. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 2005, 16, 1902–1903. [Google Scholar] [CrossRef]
- Calva, J.; Cartuche, L.; González, S.; Montesinos, J.V.; Morocho, V. Chemical Composition, Enantiomeric Analysis and Anticholinesterase Activity of Lepechinia betonicifolia Essential Oil from Ecuador. Pharm. Biol. 2022, 60, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Calva, J.; Silva, M.; Morocho, V. Composition and Anti-Acetylcholinesterase Properties of the Essential Oil of the Ecuadorian Endemic Species Eugenia valvata McVaugh. Molecules 2023, 28, 8112. [Google Scholar] [CrossRef]
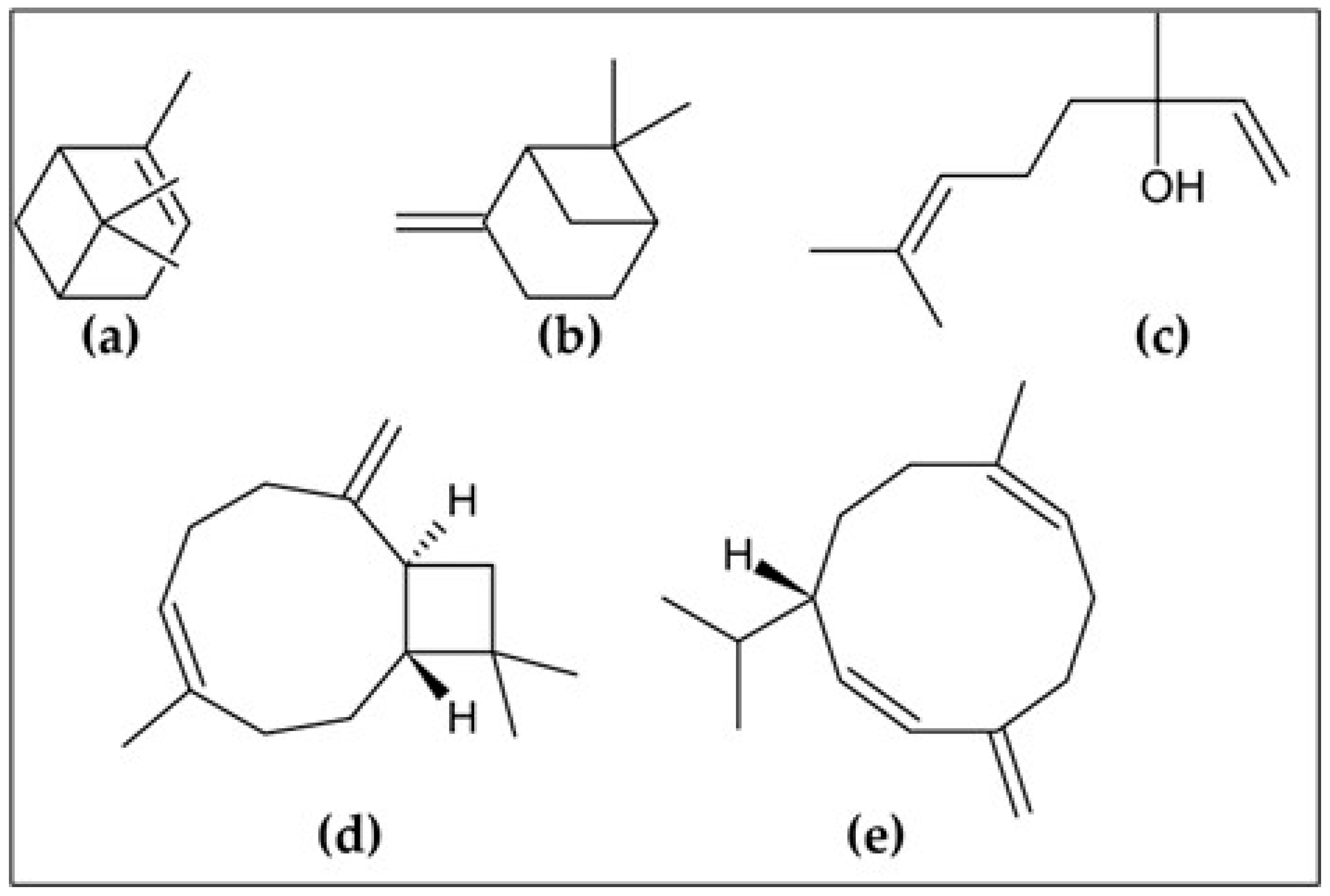
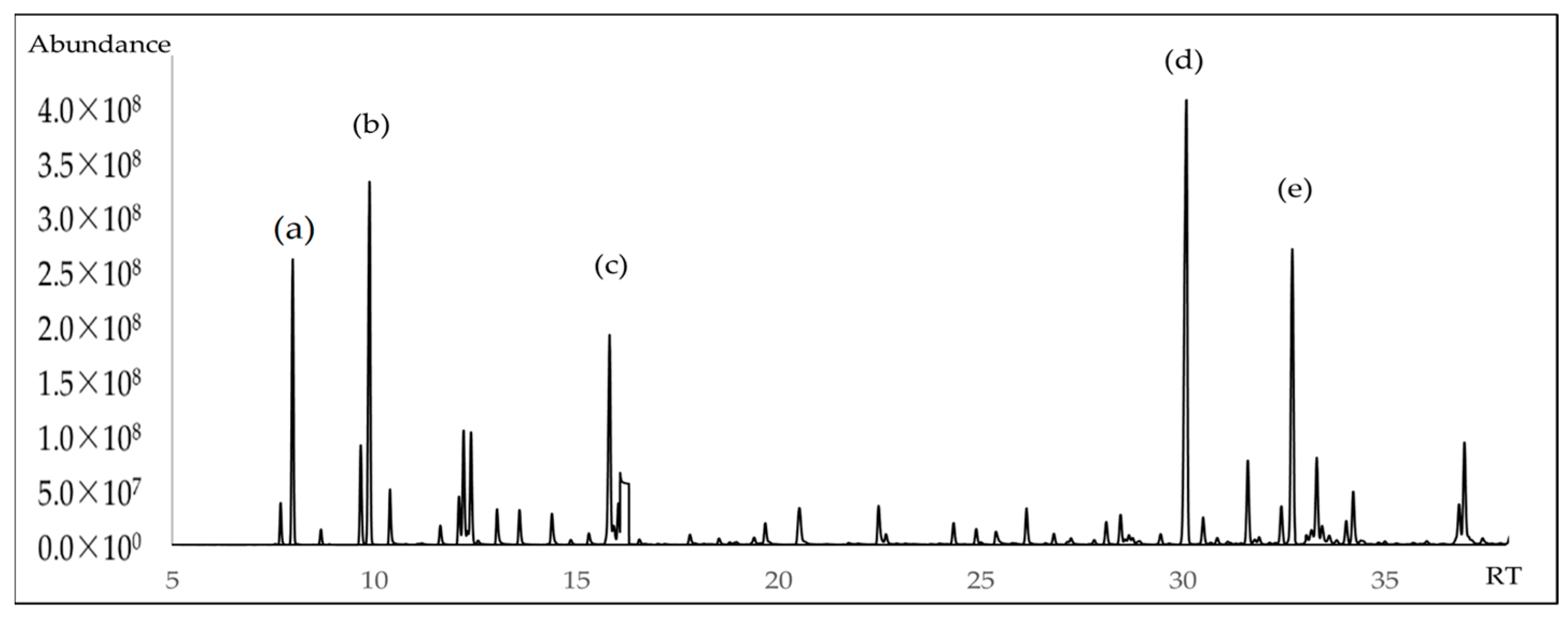
| No. | Compound | Retention Time | LRI a | LRI b | %Δ | % ± SD |
|---|---|---|---|---|---|---|
| 1 | α-Thujene | 7.67 | 927 | 924 | 0.32 | 0.90 ± 0.15 |
| 2 | α-Pinene | 7.97 | 934 | 932 | 0.21 | 6.71 ± 0.85 |
| 3 | α-Fenchene | 8.67 | 951 | 945 | 0.63 | 0.36 ± 0.12 |
| 4 | Sabinene | 9.65 | 975 | 969 | 0.62 | 2.43 ± 0.38 |
| 5 | β-Pinene | 9.87 | 981 | 974 | 0.72 | 9.96 ± 0.95 |
| 6 | myrcene | 10.38 | 993 | 988 | 0.51 | 1.37 ± 0.35 |
| 7 | δ-2-Carene | 11.16 | 1011 | 1001 | 1.00 | 0.10 ± 0.04 |
| 8 | Limonene | 12.09 | 1031 | 1024 | 0.68 | 1.26 ± 0.42 |
| 9 | Sylvestrene | 12.20 | 1033 | 1025 | 0.78 | 2.97 ± 0.43 |
| 10 | β-Phellandrene | 12.30 | 1035 | 1025 | 0.98 | 0.30 ± 0.14 |
| 11 | 1,8-Cineole | 12.41 | 1038 | 1026 | 1.17 | 2.92 ± 0.51 |
| 12 | Not Identified | 12.56 | 1041 | -- | 0.17 ± 0.06 | |
| 13 | (E)-β-Ocimene | 13.03 | 1051 | 1044 | 0.67 | 1.01 ± 0.40 |
| 14 | γ-Terpinene | 13.58 | 1063 | 1054 | 0.85 | 0.99 ± 0.16 |
| 15 | Mentha-2,4(8)-diene | 14.85 | 1090 | 1085 | 0.46 | 0.16 ± 0.03 |
| 16 | Linalol | 15.81 | 1111 | 1095 | 1.46 | 6.30 ± 0.90 |
| 17 | trans-Sabinene hydrate | 15.91 | 1113 | 1098 | 1.37 | 0.60 ± 0.13 |
| 18 | 1-Octen-3-yl acetate | 16.03 | 1115 | 1110 | 0.45 | 1.27 ± 0.37 |
| 19 | 3-Octanol acetate | 16.55 | 1126 | 1120 | 0.54 | 0.14 ± 0.05 |
| 20 | iso-Menthone | 18.52 | 1168 | 1158 | 0.86 | 0.20 ± 0.09 |
| 21 | Rosefuran epoxide | 19.39 | 1186 | 1173 | 1.11 | 0.23 ± 0.11 |
| 22 | Terpinen-4-ol | 19.66 | 1192 | 1174 | 1.53 | 0.67 ± 0.17 |
| 23 | Pulegone | 21.72 | 1237 | 1233 | 0.32 | 0.06 ± 0.02 |
| 24 | Linalool acetate | 22.46 | 1253 | 1254 | 0.08 | 1.27 ± 0.44 |
| 25 | trans-Sabinene hydrate acetate | 22.64 | 1257 | 1253 | 0.32 | 0.35 ± 0.10 |
| 26 | trans-Pinocarvyl acetate | 24.88 | 1305 | 1298 | 0.54 | 0.53 ± 0.16 |
| 27 | trans-Carvyl acetate | 26.60 | 1345 | 1339 | 0.45 | 0.04 ± 0.03 |
| 28 | Silphinene | 26.80 | 1349 | 1345 | 0.30 | 0.33 ± 0.13 |
| 29 | α-Terpinyl acetate | 27.15 | 1357 | 1346 | 0.82 | 0.08 ± 0.04 |
| 30 | α-Ylangene | 27.80 | 1372 | 1373 | 0.07 | 0.17 ± 0.07 |
| 31 | Z-β-Damascone | 28.45 | 1387 | 1386 | 0.07 | 0.92 ± 0.18 |
| 32 | β-Cubebene | 28.66 | 1391 | 1387 | 0.29 | 0.47 ± 0.11 |
| 33 | β-Elemene | 28.75 | 1393 | 1389 | 0.29 | 0.29 ± 0.09 |
| 34 | Longifolene | 29.45 | 1410 | 1407 | 0.21 | 0.37 ± 0.15 |
| 35 | (E)-Caryophyllene | 30.08 | 1425 | 1417 | 0.56 | 16.80 ± 1.00 |
| 36 | β-Copaene | 30.49 | 1435 | 1430 | 0.35 | 0.79 ± 0.14 |
| 37 | α-Guaiene | 30.84 | 1443 | 1437 | 0.42 | 0.21 ± 0.08 |
| 38 | α-Humulene | 31.59 | 1461 | 1452 | 2.54 ± 0.29 | |
| 39 | allo-Aromadendrene | 31.77 | 1466 | 1458 | 0.55 | 0.17 ± 0.05 |
| 40 | cis-Muurola-4(14),5-diene | 31.88 | 1468 | 1465 | 0.20 | 0.24 ± 0.12 |
| 41 | γ-Muurolene | 32.43 | 1481 | 1478 | 0.20 | 1.16 ± 0.39 |
| 42 | Germacrene D | 32.70 | 1488 | 1480 | 0.54 | 10.00 ± 0.95 |
| 43 | γ-Amorphene | 33.17 | 1499 | 1495 | 0.27 | 0.53 ± 0.18 |
| 44 | Bicyclogermacrene | 33.30 | 1502 | 1500 | 0.13 | 2.75 ± 0.45 |
| 45 | trans-β-Guaiene | 33.43 | 1506 | 1502 | 0.27 | 0.66 ± 0.19 |
| 46 | (E, E)-α-Farnesene | 33.62 | 1510 | 1505 | 0.33 | 0.35 ± 0.11 |
| 47 | γ-Cadinene | 34.03 | 1521 | 1513 | 0.53 | 0.73 ± 0.13 |
| 48 | δ-Cadinene | 34.21 | 1525 | 1522 | 0.20 | 1.71 ± 0.22 |
| 49 | trans-Calamenene | 34.39 | 1530 | 1521 | 0.59 | 0.22 ± 0.10 |
| 50 | α-Cadinene | 34.99 | 1545 | 1537 | 0.52 | 0.11 ± 0.04 |
| 51 | (E)-Nerolidol | 36.02 | 1571 | 1561 | 0.64 | 0.10 ± 0.03 |
| 52 | Spathulenol | 36.82 | 1591 | 1577 | 0.89 | 1.52 ± 0.41 |
| 53 | Caryophyllene oxide | 36.95 | 1595 | 1582 | 0.82 | 3.33 ± 0.43 |
| 54 | β-Copaen-4-α-ol | 37.07 | 1598 | 1590 | 0.50 | 0.18 ± 0.06 |
| 55 | Not Identified | 37.13 | 1599 | -- | 0.16 ± 0.07 | |
| 56 | Salvial-4(14)-en-1-one | 37.41 | 1607 | 1594 | 0.82 | 0.25 ± 0.08 |
| 57 | Humulene epoxide II | 38.08 | 1625 | 1608 | 1.06 | 0.33 ± 0.16 |
| 58 | Muurola-4,10(14)-dien-1-β-ol | 38.42 | 1634 | 1630 | 0.25 | 0.07 ± 0.03 |
| 59 | allo-Aromadendrene epoxide | 39.01 | 1649 | 1639 | 0.61 | 0.13 ± 0.05 |
| 60 | Cedr-8(15)-en-9-α-ol | 39.23 | 1655 | 1650 | 0.30 | 0.11 ± 0.06 |
| 61 | Selin-11-en-4-α-ol | 39.33 | 1658 | 1658 | 0.00 | 0.08 ± 0.02 |
| 62 | 7-epi-α-Eudesmol | 39.88 | 1672 | 1662 | 0.60 | 0.35 ± 0.12 |
| 63 | Eudesma-4(15),7-dien-1β-ol | 40.68 | 1694 | 1687 | 0.41 | 0.06 ± 0.04 |
| 64 | Guaiol acetate | 41.35 | 1712 | 1725 | 0.75 | 0.18 ± 0.08 |
| Monoterpene hydrocarbons (%) | 25.10 ± 1.00 | |||||
| Oxygenated monoterpenes (%) | 31.12 ± 0.90 | |||||
| Sesquiterpene hydrocarbons (%) | 37.40 ± 0.45 | |||||
| Oxygenated sesquiterpenes (%) | 6.66 ± 0.41 | |||||
| Other compounds/unidentified | 2.65 ± 0.08 | |||||
| Total identified | 99.84% | |||||
| Enantiomer | LRI | Enantiomeric Distribution (%) | e.e. (%) |
|---|---|---|---|
| (1S,5S)-(−)-α-pinene | 928 | 95.76 | 91.51 |
| (1R,5R)-(+)-α-pinene | 930 | 4.25 | |
| (1S,5S)-(−)-β-pinene | 979 | 55.48 | 11.05 |
| (1R,5R)-(+)-β-pinene | 980 | 44.43 | |
| (1S,5S)-(–)-sabinene | 991 | 100 | 100 |
| (R)-(+)-limonene | 1069 | 100 | 100 |
| (R)-(–)-linalool | 1182 | 57.53 | 15.06 |
| (S)-(+)-linalool | 1192 | 42.46 | |
| (R)-(+)-germacrene D | 1461 | 100 | 100 |
| Microorganism | MIC | Two-Way ANOVA Test | Sidak’s Multiple Comparison Test | |
|---|---|---|---|---|
| Essential Oil | Positive Control | |||
| A. triphylla (µg/mL) | Ciprofloxacin (µg/mL) | |||
| S. aureus | 80.0 ± 2.00 | 15.0 ± 0.05 | p < 0.0001 | p < 0.0001 |
| B. subtilis | 5.0 ± 1.00 | 0.625 ± 0.01 | p < 0.0001 | |
| E. coli | 320.0 ± 0.58 | 0.625 ± 0.01 | p < 0.0001 | |
| P. aeruginosa | >500 | 1.25 ± 0.05 | p < 0.0001 | |
| Antioxidant Assay | Essential Oil | Trolox | p Value (T-Student’s Test) | |
|---|---|---|---|---|
| DPPH | µmol TE/g | 0.459 ± 0.002 | - | p < 0.0001 |
| IC50 (µg/mL) | 7720.0 ± 12.23 | 3.81 ± 0.002 | ||
| ABTS | µmol TE/g | 0.462± 0.001 | - | p < 0.0001 |
| IC50 (µg/mL) | 4648.6 ± 1.25 | 2.31 ± 0.001 | ||
| Substances | LC50 µg/mL ± SD |
|---|---|
| Essential oil | 964.0 ± 20.6 |
| Potassium dichromate | 70.5 ± 2.2 |
| Sea water with DMSO 1% | No toxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejia-Ramos, C.; Ruiz-Quiroz, J.R.; Salazar-Salvatierra, M.E.; Calva, J.; Loyola-Gonzales, E.; Chávez, H.; Chavez-Espinoza, J.H.; Pari-Olarte, J.B.; Almeida-Galindo, J.S.; Herrera-Calderon, O. Unveiling the Chemical Composition, Enantiomeric Profile, Antibacterial, Anticholinesterase and Antioxidant Activity of the Essential Oil of Aloysia triphylla Royle. Molecules 2025, 30, 2849. https://doi.org/10.3390/molecules30132849
Mejia-Ramos C, Ruiz-Quiroz JR, Salazar-Salvatierra ME, Calva J, Loyola-Gonzales E, Chávez H, Chavez-Espinoza JH, Pari-Olarte JB, Almeida-Galindo JS, Herrera-Calderon O. Unveiling the Chemical Composition, Enantiomeric Profile, Antibacterial, Anticholinesterase and Antioxidant Activity of the Essential Oil of Aloysia triphylla Royle. Molecules. 2025; 30(13):2849. https://doi.org/10.3390/molecules30132849
Chicago/Turabian StyleMejia-Ramos, Cinthia, Julio Reynaldo Ruiz-Quiroz, Maria Elena Salazar-Salvatierra, James Calva, Eddie Loyola-Gonzales, Haydee Chávez, Javier Hernán Chavez-Espinoza, Josefa Bertha Pari-Olarte, José Santiago Almeida-Galindo, and Oscar Herrera-Calderon. 2025. "Unveiling the Chemical Composition, Enantiomeric Profile, Antibacterial, Anticholinesterase and Antioxidant Activity of the Essential Oil of Aloysia triphylla Royle" Molecules 30, no. 13: 2849. https://doi.org/10.3390/molecules30132849
APA StyleMejia-Ramos, C., Ruiz-Quiroz, J. R., Salazar-Salvatierra, M. E., Calva, J., Loyola-Gonzales, E., Chávez, H., Chavez-Espinoza, J. H., Pari-Olarte, J. B., Almeida-Galindo, J. S., & Herrera-Calderon, O. (2025). Unveiling the Chemical Composition, Enantiomeric Profile, Antibacterial, Anticholinesterase and Antioxidant Activity of the Essential Oil of Aloysia triphylla Royle. Molecules, 30(13), 2849. https://doi.org/10.3390/molecules30132849










