Abstract
Aloysia triphylla is widely used in traditional medicine from Peru for its sedative, digestive and anti-inflammatory properties. However, comprehensive studies on the biological activities of its essential oil (EO), particularly from Peruvian sources, remain limited. This study aimed to analyze the chemical composition and enantiomeric profile of A. triphylla EO and evaluate its antibacterial, antioxidant, anticholinesterase, and cytotoxic activities. The EO was obtained by steam distillation and analyzed using gas chromatography–mass spectrometry (GC-MS). A total of 62 compounds were identified, with (E)-caryophyllene (16.80%), β-pinene (9.96%), and germacrene D (10.00%) being the major components. Enantiomeric analysis revealed specific chiral signatures, including (−)-α-pinene, (+)-limonene, and (R)-(−)-linalool. The EO exhibited significant antibacterial activity, particularly against Bacillus subtilis (MIC = 5 µg/mL), and weak antioxidant activity (IC50 = 7720 and 4648 µg/mL for DPPH and ABTS, respectively). Additionally, the EO demonstrated moderate acetylcholinesterase inhibition (IC50 = 87.8 µg/mL) and cytotoxicity in the Artemia salina assay (LC50 = 964 µg/mL). These findings suggest that A. triphylla EO possesses promising bioactivities with potential applications in pharmaceutical and cosmetic fields.
1. Introduction
Infectious diseases are the leading cause of death worldwide, and antibiotics prescribed to combat them are acquiring high resistance [1]. However, while antimicrobial resistance occurs naturally, the public health crisis also leads to problems, such as the overuse of antibiotics, their accumulation in the environment, and their application in the animal and food industries [2]. Additionally, the high prevalence of antimicrobial resistance in pathogenic microorganisms is a major challenge to global public health, compromising treatment efficacy, and contributing to the persistence and recurrence of infections [3]. Therefore, there has been growing interest in identifying new antimicrobial agents derived from natural products, including plants, bacteria, fungi, and marine and animal sources. On the other hand, essential oils (EOs) have garnered attention due to their antibacterial activity and ability to target multiple cellular structures and functions in microorganisms [4]. The discovery of novel antibacterial compounds is critical for the development of new therapeutic strategies and preservative agents applicable in the pharmaceutical, cosmetic, and food industries.
Oxidative stress plays a key role in the pathogenesis of several chronic diseases. The accumulation of reactive oxygen species (ROS) in living organisms, including hydroxyl radicals, hydrogen peroxide, superoxide anions, and singlet oxygen, generated during normal cellular respiration particularly during the incomplete reduction of oxygen within the mitochondria, can result in significant cellular damage [5]. Natural antioxidants, especially EOs, have been increasingly investigated for their capacity to mitigate oxidative stress, and some EOs have demonstrated interesting activities, including those rich in oxygenated monoterpenes and sesquiterpenes [6].
Recently, there has been growing interest in EOs for their potential to inhibit acetylcholinesterase (AChE), an enzyme responsible for modulating the neurotransmitter acetylcholine. AChE inhibitors are vital in treating symptoms of neurodegenerative conditions such as Alzheimer’s disease, which are characterized by a significant decline in cholinergic function [7]. Synthetic AChE inhibitors, such as donepezil, rivastigmine, and galantamine, are commonly used to enhance cognitive function and slow disease progression. However, these medications often have side effects, including gastrointestinal problems, liver toxicity, and diminishing effectiveness over time [8]. Consequently, there is increasing interest in identifying natural AChE inhibitors from plants, including essential oils [9], which may provide safer alternatives with a range of biological activities. Therefore, assessing the anticholinesterase potential of A. triphylla EO is part of an ongoing effort to discover new neuroprotective agents from natural sources.
Aloysia triphylla Royle (Synonym: Aloysia citrodora), also known as “cedrón” (in Spanish), is an aromatic plant in the Verbenaceae family. It is native to South America (Peru, Argentina, and Bolivia), is widely distributed in tropical and subtropical regions, and is traditionally used to treat gastrointestinal, neurological, and respiratory diseases through aerial part (leaves and flowers) infusions [10]. In Peru, A. triphylla grows in the Andes at an altitude of approximately 2000 to 3000 m, although it also grows in the coastal region. The chemical composition of EO is abundant in monoterpenes and sesquiterpenes, and is highly influenced by environmental factors such as altitude, climate, and soil conditions, which might affect its biological activities [11]. For instance, in a study in Palestine, geranial, neral, and curcumene were identified as the main volatile components [12]. The EO from other regions was abundant in limonene and citral [13,14]. These differences may affect the antibacterial and antioxidant activities.
Based on previous studies, this study aimed to determine the antibacterial, antioxidant, and anticholinesterase activities of A. triphylla EO obtained from Peru by steam distillation. The chemical composition of the volatile components was assessed using gas chromatography–mass spectrometry (GC-MS). In vitro antioxidant activity was assessed through DPPH (2,2-diphenyl-1-picrylhydrazyl) and 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays. Antibacterial activity was evaluated against both Gram-positive and Gram-negative strains using a resazurin-based colorimetric microdilution assay. Anticholinesterase activity was assessed spectrophotometrically using the Ellman method, and the lethal concentration 50 (LC50) was determined using the toxicity model of Artemia salina.
2. Results
2.1. Chemical Characterization of Essential Oil
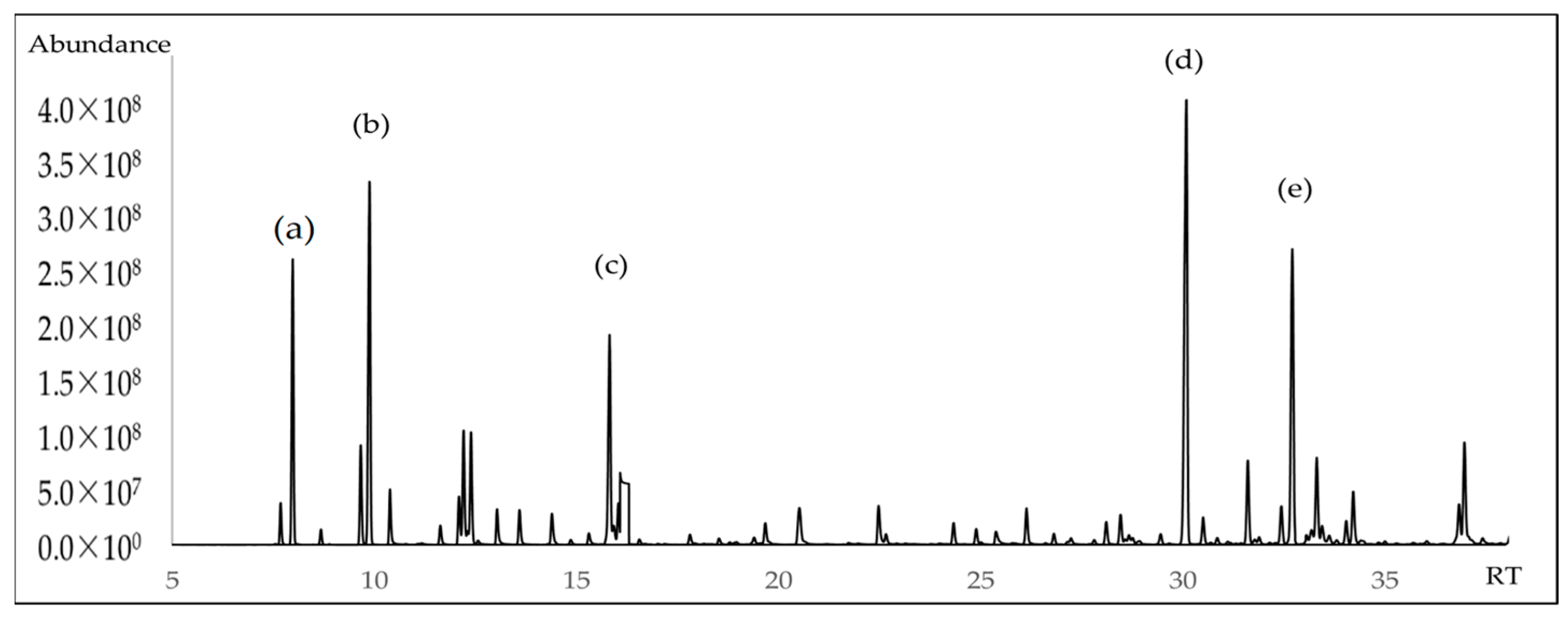
The chemical composition of A. triphylla EO revealed the presence of 62 compounds, representing 99.84% of the total EO content (Table 1). Figure 1 and Figure 2 show the abundant compounds identified as (E)-caryophyllene (16.80 ± 1.00%), β-pinene (9.96 ± 0.95%), α-pinene (6.71 ± 0.85%), linalool (6.30 ± 0.90%), and germacrene D (10.00 ± 0.95%).

Table 1.
Volatile components of A. triphylla EO identified on DB5-ms column.
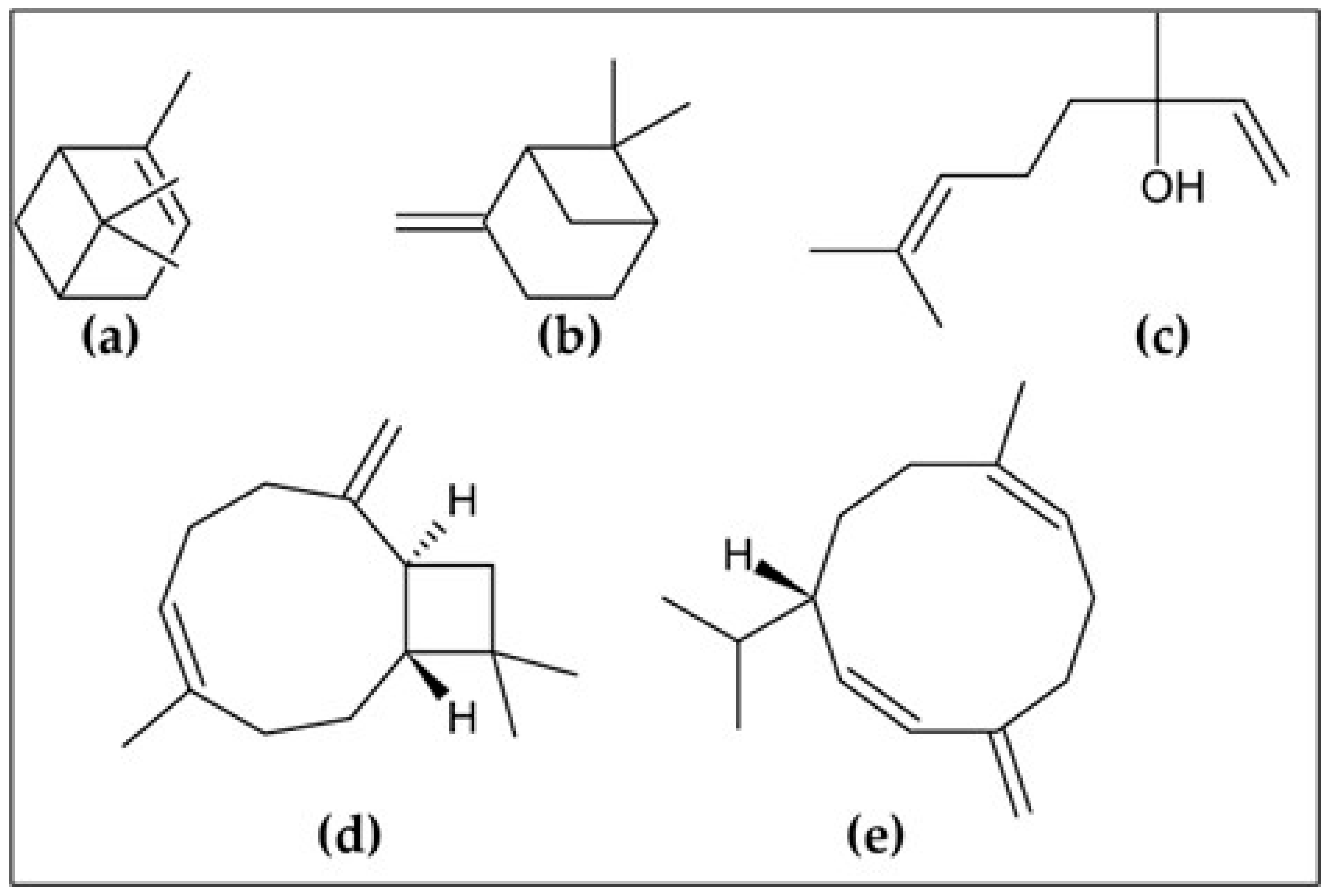
Figure 1.
The most abundant compounds of A. triphylla EO: (a) α-pinene; (b) β-pinene; (c) linalool; (d) (E)-caryophyllene; and (e) germacrene D.
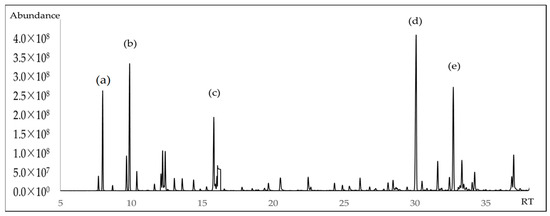
Figure 2.
GC-MS chromatogram of A. triphylla EO on a nonpolar DB5-ms column (a) α-pinene; (b) β-pinene; (c) linalool; (d) (E)-caryophyllene; (e) Germacrene D.
2.2. Enantiomeric Distribution of A. triphylla Essential Oil
The enantiomeric analysis performed in this study by gas chromatography coupled to mass spectrometry (GC-MS) allowed the identification of the stereoisomeric profile of the essential oil of A. triphylla (Table 2 and Figures S1–S7). This analysis revealed the presence of three pairs of enantiomers, namely (1S,5S)-(−)-α-pinene and its enantiomer (1R,5R)-(+)-α-pinene, (1S,5S)-(−)-β-pinene and its enantiomer (1R,5R)-(+)-β-pinene, and (R)-(−)-linalool enantiomer with its enantiomer (S)-(+)-linalool, and three chiral compounds present exclusively in one of their isomers, namely (1S,5S)-(−)-sabinene, (R)-(+)-limonene, and (R)-(+)-germacrene D.

Table 2.
Enantiomeric distribution of A. triphylla EO on a cyclodextrin column. Mean ± SD (n = 3). e.e.= enantiomeric excess.
2.3. Antibacterial Activity of the A. triphylla EO
The antibacterial potential of A. triphylla essential oil was evaluated against four bacterial strains using the colorimetric microdilution method to determine the minimum inhibitory concentration (MIC). The results revealed notable variations in susceptibility among microorganisms, as shown in Table 3. The EO exhibited a moderate inhibitory effect against S. aureus and B. subtilis, with MIC values of 80 ± 2.00 µg/mL and 5 ± 1.00 µg/mL, respectively. However, a significantly higher MIC was observed for E. coli (320 ± 0.58 µg/mL), suggesting the reduced susceptibility of this Gram-negative bacterium. P. aeruginosa displayed the highest resistance, as no inhibition was observed at the tested concentrations (MIC > 500 µg/mL). Ciprofloxacin, which was used as a positive control, demonstrated considerably lower MIC values across all bacterial strains, confirming its superior antibacterial potency. Statistical analysis through two-way ANOVA indicated significant differences (p < 0.0001) in antibacterial activity between treatments. Additionally, Sidak’s multiple comparison test confirmed the statistical significance of these differences across bacterial strains.

Table 3.
Determination of the minimum inhibitory concentrations (MIC) of A. triphylla EO and ciprofloxacin using the colorimetric microdilution method.
2.4. Antioxidant Activity of A. triphylla EO
Table 4 shows the antioxidant capacity of A. triphylla EO, evaluated using DPPH and ABTS radical scavenging assays. The results showed markedly lower antioxidant activity than that of the Trolox standard, as indicated by the significantly higher IC50 values for the essential oil in both assays. Nonetheless, the calculated TEAC values demonstrate that the oil possesses a measurable antioxidant potential. Statistical analysis confirmed that the differences between the EO and Trolox controls were highly significant (p < 0.0001) for both methods.

Table 4.
Antioxidant activity of A. triphylla EO. Mean ± SD (n = 3).
2.5. Acetylcholinesterase Inhibition of A. triphylla EO
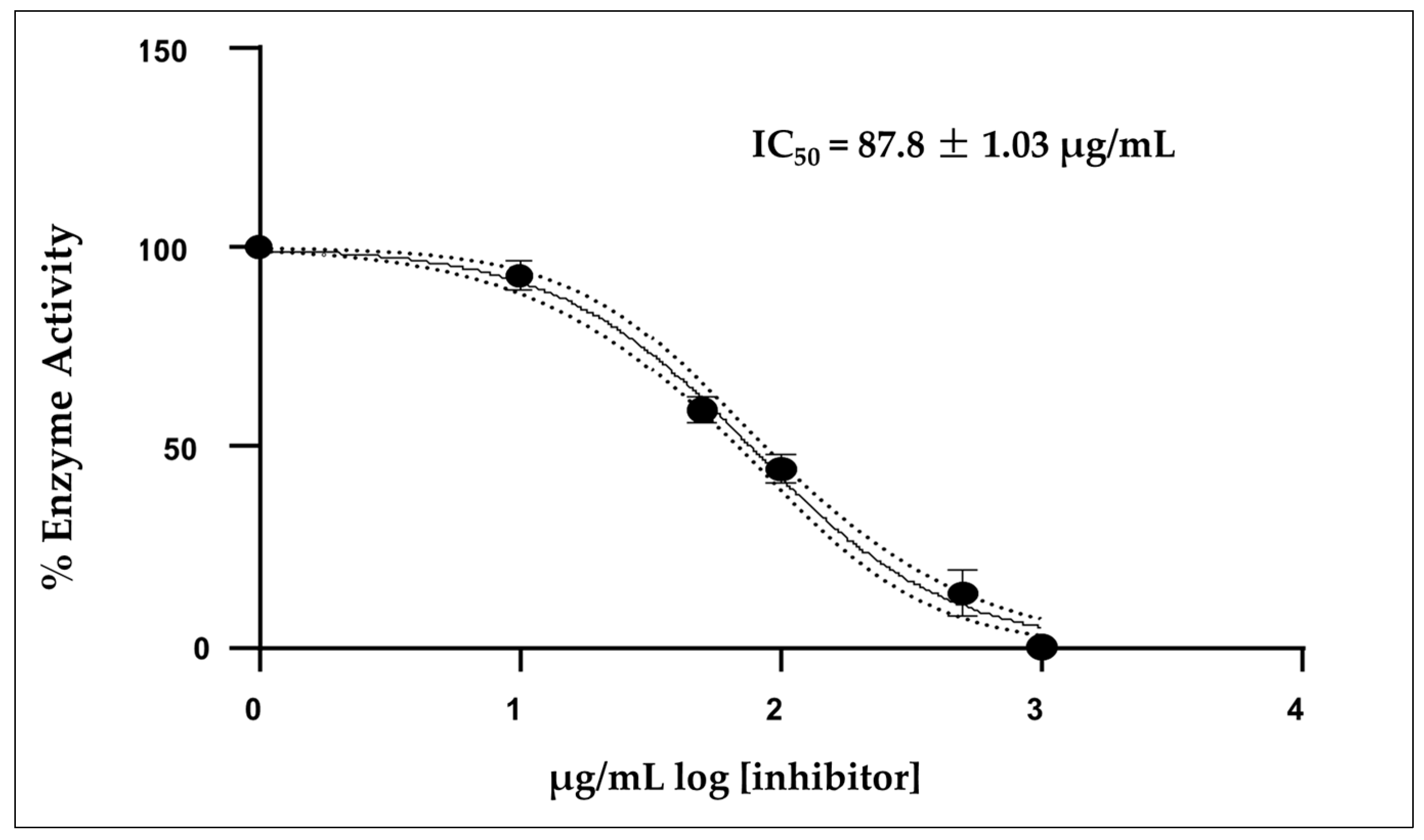
Figure 3 shows that the mean inhibitory concentration (IC50) of the EO was 87.8 ± 1.03 µg/mL, which was calculated from three independent measurements. Donepezil was used as a positive control, showing a IC50 value of 12.40 µg/mL under the same experimental conditions.
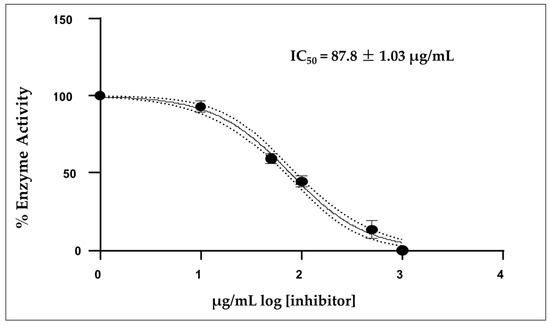
Figure 3.
Acetylcholinesterase activity of IC50 concentration of the essential oil A. triphylla EO from Perú; Mean ± SD (n = 3).
2.6. Cytotoxicity of A. triphylla EO
The brine shrimp lethality assay was used to evaluate the cytotoxic potential of A. triphylla EO. The test revealed moderate toxicity, as indicated by the LC50 value shown in Table 5. In contrast, the solvent control showed no toxic effects, confirming the reliability of the assay. Potassium dichromate, which was used as a positive control, showed high toxicity, as expected, validating the sensitivity of the method.

Table 5.
Cytotoxicity of A. triphylla EO against Artemia salina. Mean ± SD (n = 3).
3. Discussion
Phytochemical analysis revealed that the EO contained three major components, which were identified as (E)-caryophyllene (16.80 ± 1.00%), germacrene D (10.00 ± 0.95%) and β-pinene (9.96 ± 0.95%). However, our findings differ from those obtained in studies on the content of volatile components; the essential oil of A. citrodora from Portugal contained citral isomers geranial (18.7–21.1%) and neral (15.3–16.2%) as the main compounds [14]. In a study on A. citrodora from Algeria, the major compounds were citral (13.80%), D-limonene, (12.16%), and neral (10.67%) [15]. EO obtained by the hydrodistillation of leaves and inflorescences from Brazil contained two compounds, β-pinene (22.1%) and trans-pinocamphone (13.1%) [16]. Another sample from Brazil showed that the concentration of volatile components can vary according to the season, with limonene and citral being the main constituents. However, in some seasons, caryophyllene and/or caryophyllene oxide were detected in significant concentrations [17]. This variation might be explained by several factors (weather, growing conditions, cultivation conditions, and harvest season) that influence the contents of caryophyllene and β-pinene instead of limonene or citral, which are commonly found in A. triphylla species from other regions. A study conducted in Brazil revealed that winter was the best season for detecting α-citral, limonene, and β-citral [18]. In contrast, in some species of aromatic plants such as Rhodomyrtus tomentosa, α-pinene and β-caryophyllene were found in higher concentrations in young leaves than in older leaves [19]. Similar to our report, our samples were abundant in months with rainy weather and temperatures ranging between 15 and 20 °C, and only young leaves were used in this study. For the first time, this study identified the stereoisomeric profile of A. triphylla essential oil using GC-MS with a chiral column, which is relevant for assessing the authenticity and purity of the oil, because the enantiomers of chiral compounds can vary according to botanical origin, environmental conditions, or adulteration [20].
The antioxidant activity of our EO was similar to that obtained using the DPPH and ABTS methods. In other investigations, EOs obtained using the Clevenger apparatus and microwave-assisted hydrodistillation varied in their antioxidant activity against DPPH, with values of 9.583 ± 0.005 mg/mL and 8.631 ± 0.005 mg/mL, respectively [14]. However, these results are similar to our findings. Hashemi et al. investigated the extraction of EO from A. citrodora and reported EC50 values ranging from 5 to 10 mg/mL and 10 to 15 mg/mL for hydrodistilled and ultrasound-assisted extraction, respectively [21]. In another study, EO from Jordan did not show antioxidant activity against DPPH [22]. The limited antioxidant activity of our EO might be explained by the presence of low-oxygenated sesquiterpenes in contrast to oxygenated monoterpenes, which tend to have limited radical scavenging capacity [23]. Furthermore, the presence of a phenolic group containing an electron-repelling group at the ortho position to the phenolic group is required to achieve a strong radical scavenging effect [24,25].
In contrast, antibacterial activity was found to be effective against Gram-positive bacteria, especially B. subtilis. Previous studies have reported the antibacterial activity of EO against important clinical strains, such as S. aureus and E. coli [26]. In other studies, A. triphylla EO from Brazil was shown to be effective against six bacterial strains. It was particularly effective against Enterococcus faecium ATCC 10541 (MIC = 0.05 mg/mL), B. subtilis (MIC = 0.50 mg/mL) and Salmonella cholerasuis (MIC = 0.60 mg/mL), but presented moderate inhibition against S. aureus (MIC = 0.80 mg/mL), with the major components detected being neral and geranial [27]. In another study, A. triphylla EO from Argentina exhibited antibacterial activity against a wide range of microorganisms, including S. aureus, Staphylococcus epidermidis, B. cereus, Micrococcus luteus, E. faecalis, E. coli, Klebsiella sp., and Proteus mirabilis. Notably, it was one of the most effective compounds against B. cereus and S. aureus, with MICs of 56.25 µg/disk for both bacteria. However, it was not effective against P. aeruginosa, which appeared to be the most resistant microorganism in this study [28]. According to our results and those of other investigations, A. triphylla EO exhibits promising antibacterial properties against a wide range of bacteria, particularly against gram-positive strains. These findings suggest that this EO possesses potential for application used in food preservation and as an alternative treatment for infections caused by Gram-positive bacteria.
The lipophilic nature of EOs allows them to penetrate and disrupt the cell membrane structure, thereby affecting their integrity and function [29]. Additionally, the cell wall structure of Gram-positive bacteria facilitates the penetration of hydrophobic molecules, allowing them to act on both the cell wall and the cytoplasm. Phenolic compounds, also found in EOs, typically exhibit antimicrobial activity against Gram-positive bacteria and are likely to be more susceptible to terpene activity [30]. Interestingly, EOs often demonstrate stronger antibacterial activity against Gram-positive bacteria than Gram-negative bacteria. This is likely due to the simpler cell wall structure of Gram-positive bacteria, which lacks the outer membrane present in Gram-negative bacteria, making them more susceptible to EO penetration [31]. Regarding the most abundant components in our EO, compared to (+)-α-pinene, (+)-β-pinene was two to twelve times more efficient against both Gram-positive and Gram-negative bacteria, according to Van Zyl et al. [32], and (E)-caryophyllene showed more antimicrobial activity towards Gram-positive bacteria than Gram-negative bacteria, showing no inhibition of P. aeruginosa [33].
The essential oil of A. triphylla showed moderate inhibitory activity on AChE, with an IC50 of 87.8 µg/mL, indicating that a relatively high amount of oil is needed for 50% enzyme inhibition compared to the drug donepezil with an IC50 of 12.40 µg/mL, designed specifically for this purpose. This finding is relevant in the context of the search for natural compounds with potential applications in the treatment of neurodegenerative diseases, such as Alzheimer’s disease, where the reversible inhibition of AChE may help maintain adequate levels of acetylcholine in the central nervous system [34]. Several studies have shown that some of these compounds can directly interact with AChE. (E)-β-Caryophyllene has previously been reported to inhibit AChE from Electrophorus electricus at a concentration of 0.06 mM of 32% [34]. Linalool, for example, has shown the ability to inhibit AChE in vitro [35]. β-pinene and α-pinene have also been linked to neurological activity [36]. It should be noted that the activity observed in this study is probably the result of a synergistic effect between multiple compounds present in the oil, rather than the individual action of just one of them [37].
Artemia salina has been widely used as a model organism for assessing the eco-toxicity of various substances, including EOs. The brine shrimp Artemia salina offers numerous advantages as a toxicity model, including simplicity, low cost, and reproducibility [38]. EOs from Argentina, such as Aloysia polystachia and A. triphylla, had values ranging between LC50 = 6459 µg/mL and LC50 = 1279 µg/mL, respectively [39]. In our study, the LC50 of the essential oil showed a value of 964.0 ± 20.6 µg/mL, which is below 1000 µg/mL and is considered a moderate cytotoxic [40]. However, it is important to note that these results should be considered preliminary, and may require validation using other toxicity models or in vivo studies.
4. Materials and Methods
4.1. Chemicals
Resazurin sodium salt, potassium dichromate, potassium persulfate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS), dimethylsulfoxide (DMSO), and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol and other solvents were purchased from Merck-Peruana (Lima, Peru).
4.2. Plant Material and Essential Oil Obtention
Young leaves of A. triphylla were collected from the district of Aucará, province of Lucanas, Department of Ayacucho, Peru. The collection area was located at an altitude of approximately 3231 m.a.s.l. The geographical coordinates of the collection site were 14°16′52″ S, 73°58′29″ W, and sample collection was conducted between January and March 2022 during the rainy season. The material plant was identified by the botanist Hamilton Beltran Santiago at the herbarium of the Universidad Nacional Mayor de San Marcos (Id. 051-2022-USM-MHN). Extraction was performed in a Clevenger-type apparatus for 3 h. The EO yield was 0.95%.
4.3. Determination of Essential Oil Composition
The volatile components of the essential oil A. triphylla were identified using a gas chromatography–mass spectrometry (GC) model Trace 1310 supplied by Thermo Fisher Scientific (Waltham, MA, USA) coupled with a single quadrupole mass spectrometer (MS) model ISQ 7000; all EOs were analyzed using a non-polar capillary column based on 5% phenyl-methylpolysiloxane (30 m × 0.25 mm, 0.20 μm film thickness, Agilent Technologies, Santa Clara, CA, USA). The samples were injected in split mode (40:1) by introducing 1 µL into 1000 µL of cyclohexane solution (1:100). The volatile components of the EOs were identified by comparing the relative retention indices (LRI) and NIST 23 Mass Spectra Library [41]. The LRIs were calculated and compared with a homologous series of n-alkanes C9-C25 (Sigma–Aldrich, St. Louis, MO, USA) [42], as shown in Figure S8.
4.4. Enantioselective Profile of the Essential Oil
Enantioselective analysis was performed using gas chromatography–mass spectrometry (GC-MS) with an enantioselective column based on 2,3-diethyl-6-tert-butyldimethylsilyl-β-cyclodextrin (25 m × 0.25 mm, 0.25 µm film thickness, from Mega, Milan, Italy). The injector temperature and carrier gas flow rate were the same conditions as those used in the GC-MS analysis. The injector was operated in the split mode (ratio of 50:1) to enhance the separation and detection of enantiomeric compounds. The oven temperature program started at 60 °C and was held for 2 min, followed by a gradual increase of 2 °C/min to 220 °C. Linear retention indices (LRIs) were calculated by injecting a standard mixture of n-alkanes C9–C25 (Sigma–Aldrich, St. Louis, MO, USA), following the methods of Van den Dool and Kratz. The enantiomers were identified based on their characteristic mass spectra and elution order, which were confirmed using commercially available, enantiomerically pure reference standards [43].
4.5. Evaluation of the Antibacterial Activity
The antibacterial activity of A. triphylla EO was evaluated using the microdilution method and the colorimetric detection of resazurin to determine the Minimum Inhibitory Concentration (MIC). The test was conducted against four reference bacterial strains, Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Bacillus subtilis ATCC 6633, and Pseudomonas aeruginosa ATCC 27853. A stock solution of EO was prepared in Mueller-Hinton broth, with ten two-fold serial dilutions generated. The ciprofloxacin standard served as the positive control, following CLSI guidelines, and was prepared at an initial concentration of 640 µg/mL. Resazurin was used as a redox indicator for colorimetric detection. A volume of 0.03 mL of the resazurin solution was added to 6 mL of the 2× inoculum suspension, ensuring a visible color change in the presence of bacterial growth. Bacterial inoculum was prepared by culturing the strains on Tryptic Soy Agar at 37 °C for 24 h. Colonies were suspended in 0.9% saline, adjusted to a 0.5 McFarland standard (1–2 × 108 CFU/mL), and further diluted with Mueller–Hinton broth to obtain a final concentration of 1–5 × 105 CFU/mL. The assay was carried out in sterile 96-well microplates, in which 100 µL of EO dilution and 100 µL of resazurin-inoculated bacterial suspension were added to each well. The plates were then incubated at 37 °C for 24 h. MIC values were determined as the lowest concentration that prevented a color change from blue to pink or colorless. All assays were performed in triplicate.
4.6. Antioxidant Activity
In the DPPH assay, EO was diluted in methanol and tested at various concentrations (0–2000 µg/mL). The DPPH solution was prepared using methanol at a final concentration of 0.001 mM. Next, 100 µL of EO was reacted with 900 µL of DPPH for 30 min in the dark at room temperature. Finally, absorbance was measured at 517 nm using a UV-VIS Genesys 150 (Thermo Scientific, Waltham, MA, USA) spectrophotometer. Trolox was used as the reference antioxidant standard. The percentage of DPPH radical inhibition was calculated, and the IC50 (the concentration that inhibited 50% of DPPH radicals) was determined using linear regression analysis [44].
For the ABTS assay, the ABTS radical cation was generated by reacting ABTS with potassium persulfate, producing a green–blue solution with absorption peaks at 645, 734, and 815 nm. The EO was diluted in methanol and tested at various concentrations (0–2000 µg/mL). Then, 20 µL of EO was reacted with 980 µL of ABTS for 7 min in the dark at room temperature. Finally, absorbance was measured at 734 nm using a UV-VIS Genesys 150 spectrophotometer. The antioxidant activity was calculated as the percentage of radical inhibition. Trolox® was used as a standard at a final concentration of 250 µg/mL (1 mM). IC50 values were determined from the inhibition curves via linear regression [45].
4.7. Acetylcholinesterase Inhibition of A. triphylla EO In Vitro
AChE activity was determined spectrophotometrically using Ellman’s method. The reaction mixture contained 40 µL of Tris buffer, 20 µL of EO, 20 µL of acetylthiocholine, and 100 µL of DTNB reagent. Donepezil hydrochloride with a calculated IC50 value of 12.40 ± 1.35 nM was used as a positive control. The samples were preincubated at 25 °C for 3 min with continuous shaking. Subsequently, 20 µL of AChE (0.5 U/mL) was added to initiate the reaction. The reaction was monitored using an EPOCH 2 microplate reader (BIOTEK, Winooski, VT, USA) at 405 nm for 60 min at 25 °C. Finally, 10 mg of the A. triphylla EO was dissolved in 1 mL of MeOH. Additional dilutions were added to obtain final concentrations of 1000, 100 and 10 µg/mL [46].
4.8. Brine Shrimp Cytoxicity Test
The lethality of the A. triphylla EOs was assessed using a lethality bioassay of salt shrimp larvae (Artemia salina). Standard and sample solutions of each EO (0.5–10 mg/mL) were prepared in 1% DMSO. In test tubes, 100 µL of each solution was added to 900 µL of synthetic seawater. Then, 20 larvae of Artemia salina were added. The test was repeated thrice for each concentration. After 24 h of incubation, the samples were observed using an Olympic SZX9 (Olympus, Tokyo, Japan) stereomicroscope. LC50 is defined as the lethal concentration that corresponds to 50% dead larvae and was determined for EO; potassium dichromate was used as a positive control.
4.9. Statistical Analysis
All experimental assays were performed in triplicate and the results are presented as mean ± standard deviation (SD). Antibacterial activity data (MIC values) were analyzed using two-way analysis of variance (ANOVA) followed by Sidak’s multiple comparison test to evaluate the differences among treatment groups and bacterial strains. Antioxidant activity (IC50 values for DPPH and ABTS) was compared with that of the Trolox standard using unpaired Student’s t-tests. For the cytotoxicity assay (Artemia salina), LC50 values were calculated using Probit regression analysis in R Studio (version 4.2.1), employing the MASS and drc packages. A p-value less than 0.05 was considered statistically significant in all tests. GraphPad Prism version 9.0 was used for all other statistical analyses.
5. Conclusions
The essential oil of Aloysia triphylla from Peru exhibited a unique chemical and enantiomeric profile that was rich in sesquiterpenes and monoterpenes, such as (E)-caryophyllene, β-pinene, and germacrene D. The EO demonstrated significant antibacterial activity against Gram-positive strains, weak antioxidant activity against DPPH and ABTS radicals, and moderate inhibitory activity against acetylcholinesterase, suggesting its potential neuroprotective effects. Its moderate cytotoxicity, as indicated by the Artemia salina assay, highlights the need for further toxicological assessment before therapeutic application. Overall, this study provides new insights into the bioactive potential of A. triphylla EO and supports its possible use in pharmaceutical, cosmetic, and functional product development.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30132849/s1, Figure S1: Chromatogram profile of Aloysia triphylla Royle essential oil; Figure S2: Chiral separation of (1S,5S)-(-)-α-pinene and (1R,5R)-(+)-α-pinene standards. Figure S3: Chiral separation of (1S,5S)-(-)-β-pinene and (1R,5R)-(+)-β-pinene standards. Figure S4: Chiral separation of (1R,5R)-(+)-sabinene and (1S,5S)-(–)-sabinene standards. Figure S5: Chiral separation of (S)-(-)-limonene and (R)-(+)-limonene standards. Figure S6: Chiral separation of (R)-(–)-linalool and (S)-(+)-linalool standards. Figure S7: Chromatogram of (R)-(+)-germacrene D standard. Figure S8: Chromatogram of η-alkanes (C9-C23) injected in DB5-ms column.
Author Contributions
Conceptualization, C.M.-R., J.R.R.-Q., M.E.S.-S. and O.H.-C.; methodology, J.R.R.-Q., J.C., J.H.C.-E. and O.H.-C.; validation, O.H.-C. and M.E.S.-S.; formal analysis, H.C. and J.C.; investigation, C.M.-R., J.C., and O.H.-C.; resources, E.L.-G.; writing—original draft preparation, J.C., J.S.A.-G. and J.B.P.-O.; writing—review and editing, J.C. and O.H.-C.; supervision, O.H.-C. and J.R.R.-Q. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank the Universidad Nacional Mayor de San Marcos for their support with this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Farha, M.A.; Tu, M.M.; Brown, E.D. Important Challenges to Finding New Leads for New Antibiotics. Curr. Opin. Microbiol. 2025, 83, 102562. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.M.; Quave, C.L. Opportunities for Plant Natural Products in Infection Control. Curr. Opin. Microbiol. 2018, 45, 189. [Google Scholar] [CrossRef] [PubMed]
- Schneider, Y.K.; Simal-Gandara, J.; Barros, L.; Prieto Lage, M.A. Bacterial Natural Product Drug Discovery for New Antibiotics: Strategies for Tackling the Problem of Antibiotic Resistance by Efficient Bioprospecting. Antibiotics 2021, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Chen, X.; Shang, S.; Yan, F.; Jiang, H.; Zhao, G.; Tian, S.; Chen, R.; Chen, D.; Dang, Y. Antioxidant Activities of Essential Oils and Their Major Components in Scavenging Free Radicals, Inhibiting Lipid Oxidation and Reducing Cellular Oxidative Stress. Molecules 2023, 28, 4559. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of Acetylcholinesterase Inhibitors in Alzheimer’s Disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef]
- Chu, M.H.; Liu, T.W.; Chen, P.H.; Chen, Y.H.; Tang, K.L.; Hsu, S.J.; Iskandar, B.; Yin, H.W.; Lin, M.H.; Lee, C.K. Investigation of the Acetylcholinesterase Inhibitors of Mentha Genus Essential Oils with in Vitro and in Silico Approaches. Ind. Crops Prod. 2025, 227, 120783. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia Citrodora Paláu (Lemon Verbena): A Review of Phytochemistry and Pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef]
- Rojas, J.; Palacios, O.; Ronceros, S. Efecto Del Aceite Esencial de Aloysia Triphylla Britton (Cedrón) Sobre El Trypanosoma Cruzi En Ratones. Rev. Peru. Med. Exp. Salud Publica 2012, 29, 61–68. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Salama, Y.; Al-Hajj, N.; Jaradat, N.; Jobran, N.T.; Warad, I.; Hamdan, L.; Alrob, M.A.; Sawafta, A.; Hidmi, A. Chemical Composition, Anticancer, Antimicrobial Activity of Aloysia Citriodora Palau Essential Oils from Four Different Locations in Palestine. BMC Complement. Med. Ther. 2024, 24, 94. [Google Scholar] [CrossRef] [PubMed]
- Özek, T.; Kirimer, N.; Baser, K.H.C.; Tümen, G. Composition of the Essential Oil of Aloysia triphylla (L’herit.) Britton Grown in Turkey. J. Essent. Oil Res. 1996, 8, 581–583. [Google Scholar] [CrossRef]
- Sprea, R.M.; Fernandes, L.H.M.; Pires, T.C.S.P.; Calhelha, R.C.; Rodrigues, P.J.; Amaral, J.S. Volatile Compounds and Biological Activity of the Essential Oil of Aloysia citrodora Paláu: Comparison of Hydrodistillation and Microwave-Assisted Hydrodistillation. Molecules 2023, 28, 4528. [Google Scholar] [CrossRef] [PubMed]
- Boukabache, M.; Chibani, S.; Otmani, A.; Nouichi, A.; Abdelaziz, O.; Karaca, I. Chemical Composition and Insecticidal Activity of Aloysia citrodora Essential Oil against Aphis Fabae (Hemiptera: Aphididae), Rhopalosiphum Maidis (Hemiptera: Aphididae) and Tribolium castaneum (Coleoptera: Tenebrionidae). Int. J. Trop. Insect Sci. 2023, 43, 455–461. [Google Scholar] [CrossRef]
- dos Santos, P.R.; de Andrade Porto, S.M.; Brandão, F.R.; de Melo Souza, D.C.; Rocha, M.J.S.; de Alexandre Sebastião, F.; Oliveira, M.R.; Chaves, F.C.M.; Chagas, E.C. Efficacy of the Essential Oils of Aloysia triphylla, Lippia Gracilis and Piper aduncum in the Control of Piscinoodinium pillulare (Shaperclaus, 1954) in Colossoma macropomum (Cuvier, 1818). Aquaculture 2023, 565, 739127. [Google Scholar] [CrossRef]
- Parodi, T.V.; Gressler, L.T.; Silva, L.d.L.; Becker, A.G.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M.; Baldisserotto, B. Chemical Composition of the Essential Oil of Aloysia triphylla under Seasonal Influence and Its Anaesthetic Activity in Fish. Aquac. Res. 2020, 51, 2515–2524. [Google Scholar] [CrossRef]
- Sgarbossa, J.; Schmidt, D.; Schwerz, F.; Schwerz, L.; Prochnow, D.; Caron, B.O. Effect of Season and Irrigation on the Chemical Composition of Aloysia triphylla Essential Oil. Rev. Ceres 2019, 66, 85–93. [Google Scholar] [CrossRef]
- He, S.M.; Wang, X.; Yang, S.C.; Dong, Y.; Zhao, Q.M.; Yang, J.L.; Cong, K.; Zhang, J.J.; Zhang, G.H.; Wang, Y.; et al. De Novo Transcriptome Characterization of Rhodomyrtus tomentosa Leaves and Identification of Genes Involved in α/β-Pinene and β-Caryophyllene Biosynthesis. Front. Plant Sci. 2018, 9, 355918. [Google Scholar] [CrossRef]
- Capetti, F.; Marengo, A.; Cagliero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Adulteration of Essential Oils: A Multitask Issue for Quality Control. Three Case Studies: Lavandula angustifolia Mill., Citrus Limon (L.) Osbeck and Melaleuca alternifolia (Maiden & Betche) Cheel. Molecules 2021, 26, 5610. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Koubaa, M.; Barba, F.J.; Abedi, E.; Niakousari, M.; Tavakoli, J. Extraction of Essential Oil from Aloysia Citriodora palau Leaves Using Continuous and Pulsed Ultrasound: Kinetics, Antioxidant Activity and Antimicrobial Properties. Process Biochem. 2018, 65, 197–204. [Google Scholar] [CrossRef]
- Rashid, H.M.; Mahmod, A.I.; Afifi, F.U.; Talib, W.H. Antioxidant and Antiproliferation Activities of Lemon Verbena (Aloysia citrodora): An In Vitro and In Vivo Study. Plants 2022, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant Activity of Medicinal and Aromatic Plants. A Review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; El-Ghorab, A.H.; Hussein, A.M.S.; El-Massry, K.F.; Lotfy, S.N.; Sayed Ahmed, M.Y.; Soliman, T.N. Correlation between Chemical Composition and Radical Scavenging Activity of 10 Commercial Essential Oils: Impact of Microencapsulation on Functional Properties of Essential Oils. Arab. J. Chem. 2020, 13, 6815–6827. [Google Scholar] [CrossRef]
- Belahcene, S.; Kebsa, W.; Omoboyowa, D.A.; Alshihri, A.A.; Alelyani, M.; Bakkour, Y.; Leghouchi, E. Unveiling the Chemical Profiling Antioxidant and Anti-Inflammatory Activities of Algerian Myrtus Communis L. Essential Oils, and Exploring Molecular Docking to Predict the Inhibitory Compounds against Cyclooxygenase-2. Pharmaceuticals 2023, 16, 1343. [Google Scholar] [CrossRef]
- Pérez Zamora, C.M.; Torres, C.A.; Nuñez, M.B. Antimicrobial Activity and Chemical Composition of Essential Oils from Verbenaceae Species Growing in South America. Molecules 2018, 23, 544. [Google Scholar] [CrossRef] [PubMed]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and Antimicrobial Activity of Essential Oils from Aromatic Plants Used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Demo, M.; Oliva, M.D.L.M.; López, M.L.; Zunino, M.P.; Zygadlo, J.A. Antimicrobial Activity of Essential Oils Obtained from Aromatic Plants of Argentina. Pharm. Biol. 2005, 43, 129–134. [Google Scholar] [CrossRef]
- Aouadhi, C.; Jouini, A.; Maaroufi, K.; Maaroufi, A. Antibacterial Effect of Eight Essential Oils against Bacteria Implicated in Bovine Mastitis and Characterization of Primary Action Mode of Thymus capitatus Essential Oil. Antibiotics 2024, 13, 237. [Google Scholar] [CrossRef]
- Dias, K.J.S.D.O.; Miranda, G.M.; Bessa, J.R.; De Araújo, A.C.J.; Freitas, P.R.; De Almeida, R.S.; Paulo, C.L.R.; Neto, J.B.D.A.; Coutinho, H.D.M.; Ribeiro-Filho, J. Terpenes as Bacterial Efflux Pump Inhibitors: A Systematic Review. Front. Pharmacol. 2022, 13, 953982. [Google Scholar] [CrossRef]
- Chao, S.C.; Young, D.G.; Oberg, C.J. Screening for Inhibitory Activity of Essential Oils on Selected Bacteria, Fungi and Viruses. J. Essent. Oil Res. 2000, 12, 639–649. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A.M. The Biological Activities of 20 Nature Identical Essential Oil Constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Schmidt, E.; Bail, S.; Friedl, S.M.; Jirovetz, L.; Buchbauer, G.; Wanner, J.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Antimicrobial Activities of Single Aroma Compounds. Nat. Prod. Commun. 2010, 5, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activity of Pinus Species Essential Oils and Their Constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool Bioactive Properties and Potential Applicability in Drug Delivery Systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Petrachaianan, T.; Chaiyasirisuwan, S.; Athikomkulchai, S.; Sareedenchai, V. Screening of acetylcholinesterase inhibitory activity in essential oil from myrtaceae. Thai J. Pharm. Sci. (TJPS) 2019, 43, 63–68. [Google Scholar]
- Albarano, L.; Ruocco, N.; Lofrano, G.; Guida, M.; Libralato, G. Genotoxicity in Artemia spp.: An Old Model with New Sensitive Endpoints. Aquat. Toxicol. 2022, 252, 106320. [Google Scholar] [CrossRef]
- Oliva, M.D.L.M.; Gallucci, N.; Zygadlo, J.A.; Demo, M.S. Cytotoxic Activity of Argentinean Essential Oils on Artemia salina. Pharm. Biol. 2007, 45, 259–262. [Google Scholar] [CrossRef]
- Waghulde, S.; Kale, M.K.; Patil, V.R. Brine Shrimp Lethality Assay of the Aqueous and Ethanolic Extracts of the Selected Species of Medicinal Plants. Proceedings 2019, 41, 47. [Google Scholar] [CrossRef]
- Guía Para El Libro Del Web de Química Del NIST. Available online: https://webbook.nist.gov/chemistry/guide/ (accessed on 16 June 2025).
- Sparkman, O.D. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 2005, 16, 1902–1903. [Google Scholar] [CrossRef]
- Calva, J.; Cartuche, L.; González, S.; Montesinos, J.V.; Morocho, V. Chemical Composition, Enantiomeric Analysis and Anticholinesterase Activity of Lepechinia betonicifolia Essential Oil from Ecuador. Pharm. Biol. 2022, 60, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Calva, J.; Silva, M.; Morocho, V. Composition and Anti-Acetylcholinesterase Properties of the Essential Oil of the Ecuadorian Endemic Species Eugenia valvata McVaugh. Molecules 2023, 28, 8112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).