Chemical, Sensory, and Nutraceutical Profiling, and Shelf-Life Assessment of High-Quality Extra Virgin Olive Oil Produced in a Local Area near Florence (Italy)
Abstract
1. Introduction
2. Results and Discussion
2.1. EVOO Production
2.2. Profiling of Montespertoli EVOO
2.2.1. Chemical, Sensory, and Nutritional Characterization
2.2.2. Commercial Category of the Montespertoli Oils and Their Profile
- Free acidity ≤ 0.30%
- Peroxide value ≤ 10.0 meqO2/kg
- K232 ≤ 1.90
- K270 ≤ 0.19
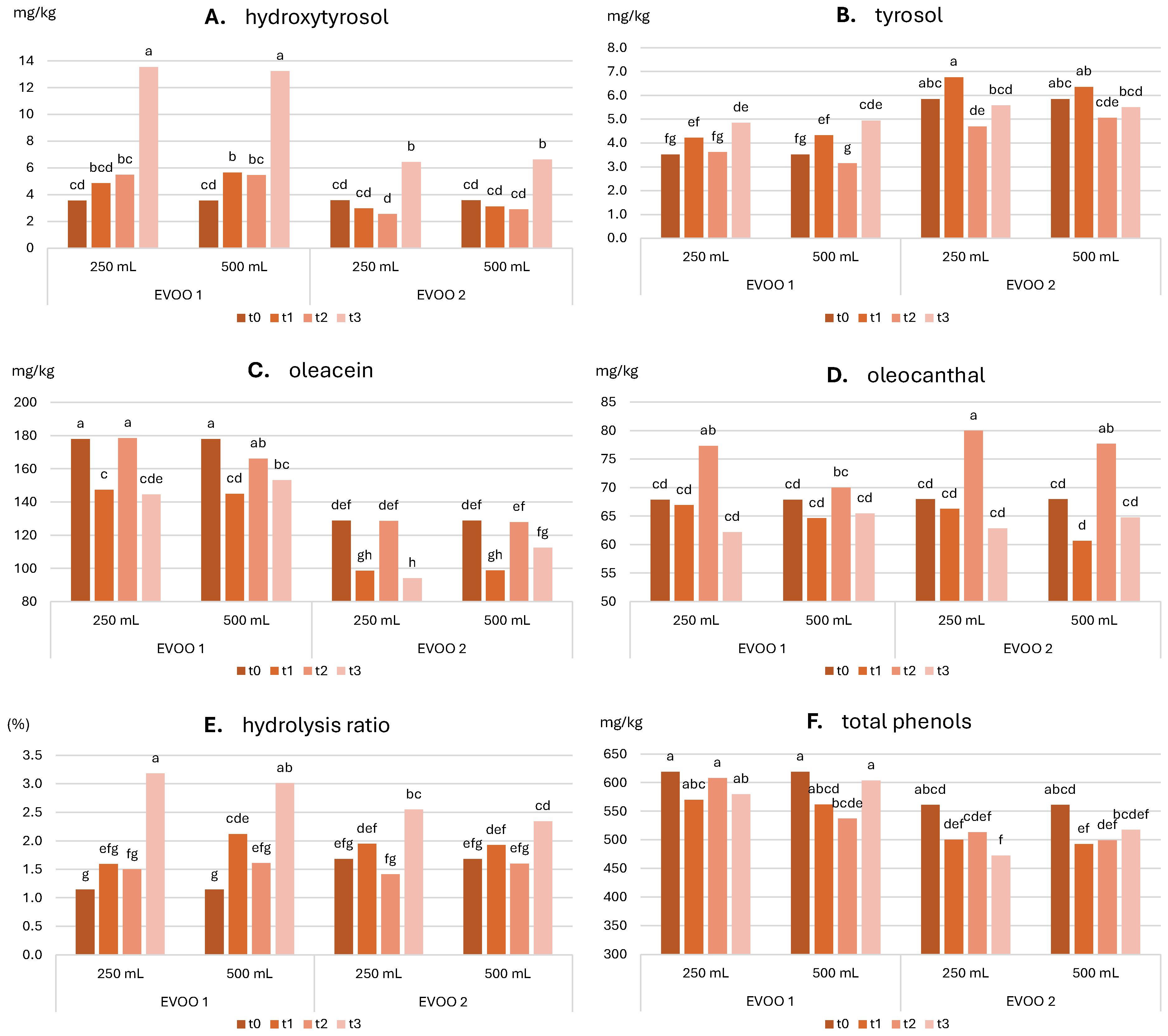
- Total phenolic content ≥ 350.0 mg/kg
- Hydrolysis ratio ≤ 2.0% at production
- Oleacein + oleocanthal content ≥ 50% of the total phenolic content
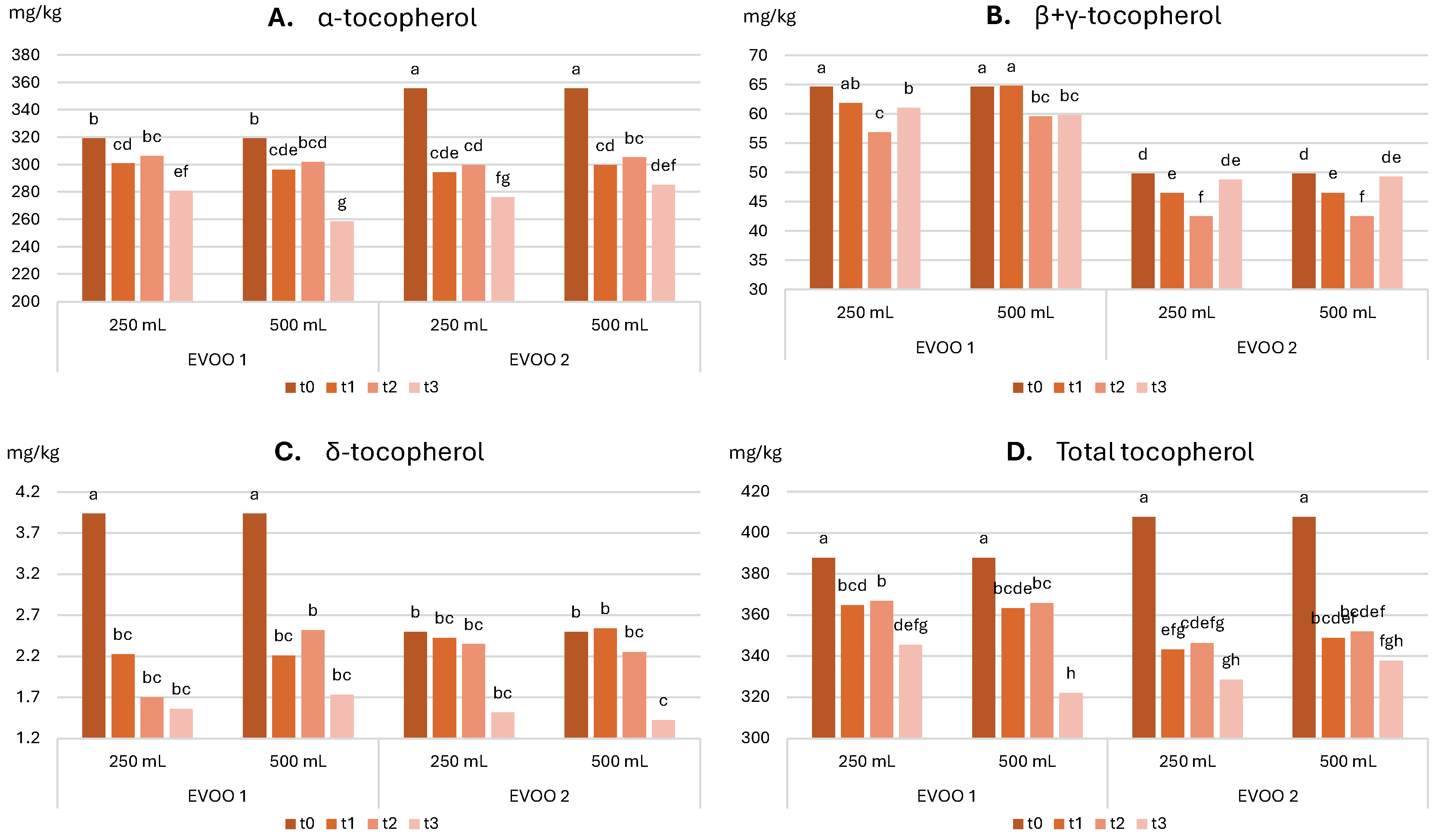
- Total tocopherol content ≥ 250.0 mg/kg
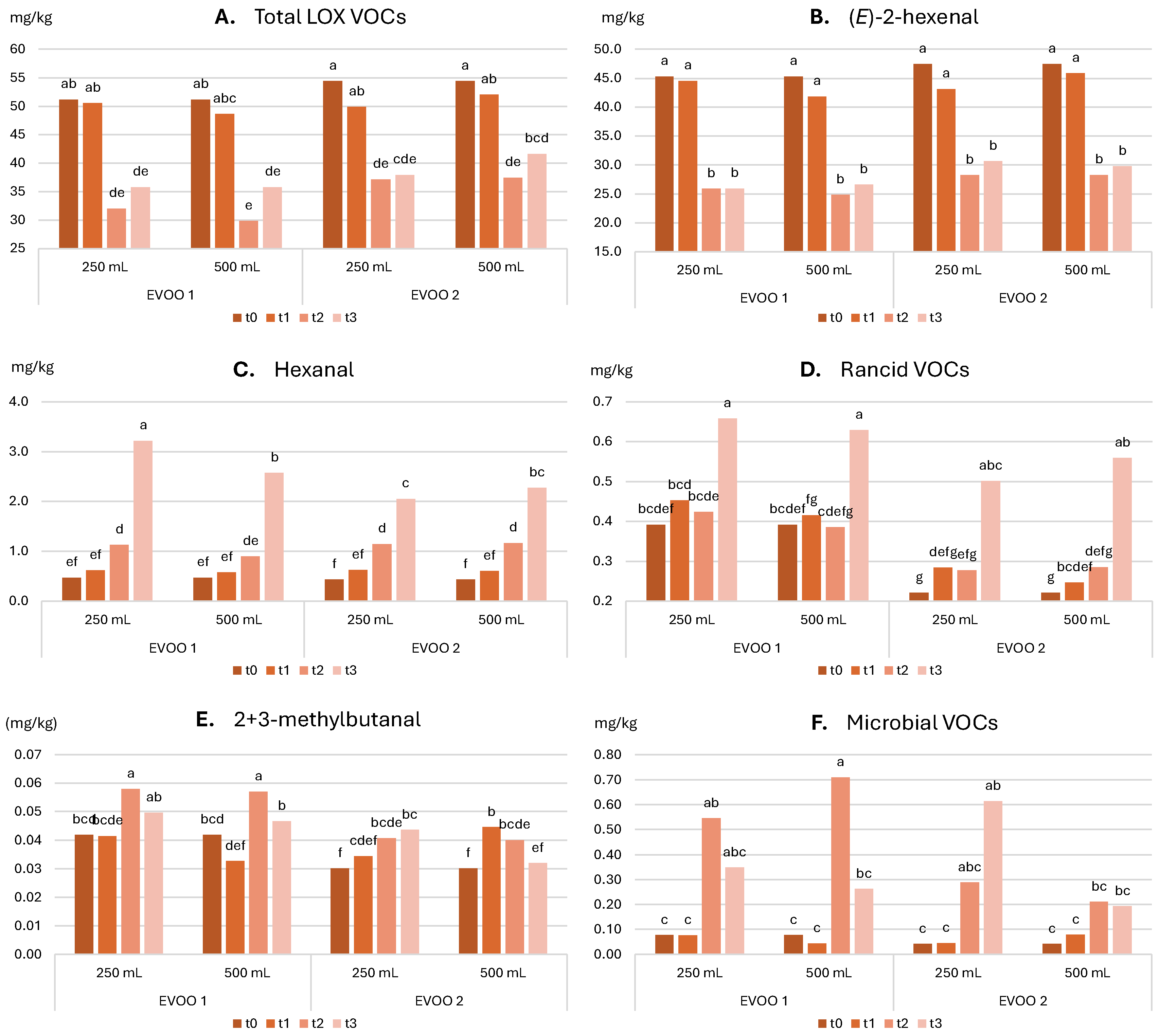
- Total LOX VOC content ≥ 30.0 mg/kg
- (E)-2-hexenal ≥ 80% of total LOX VOCs
- LOX esters ≤ 1% of total LOX VOCs
- 1-penten-3-one, (Z)-3-hexenol, and (E)-2-hexenol: 1–3% of LOX VOCs
- Median of fruitiness ≥ 3
- Median of bitterness ≥ 3
- Median of pungency ≥ 4
- Fruity profile characterized by a prevalence of almond and artichoke.
- NVS ≥ 80.0
2.2.3. The Profile of EVOO and the Consumer Preferences
2.3. Shelf-Life Study
2.3.1. Evolution of Chemical Characteristics over Time
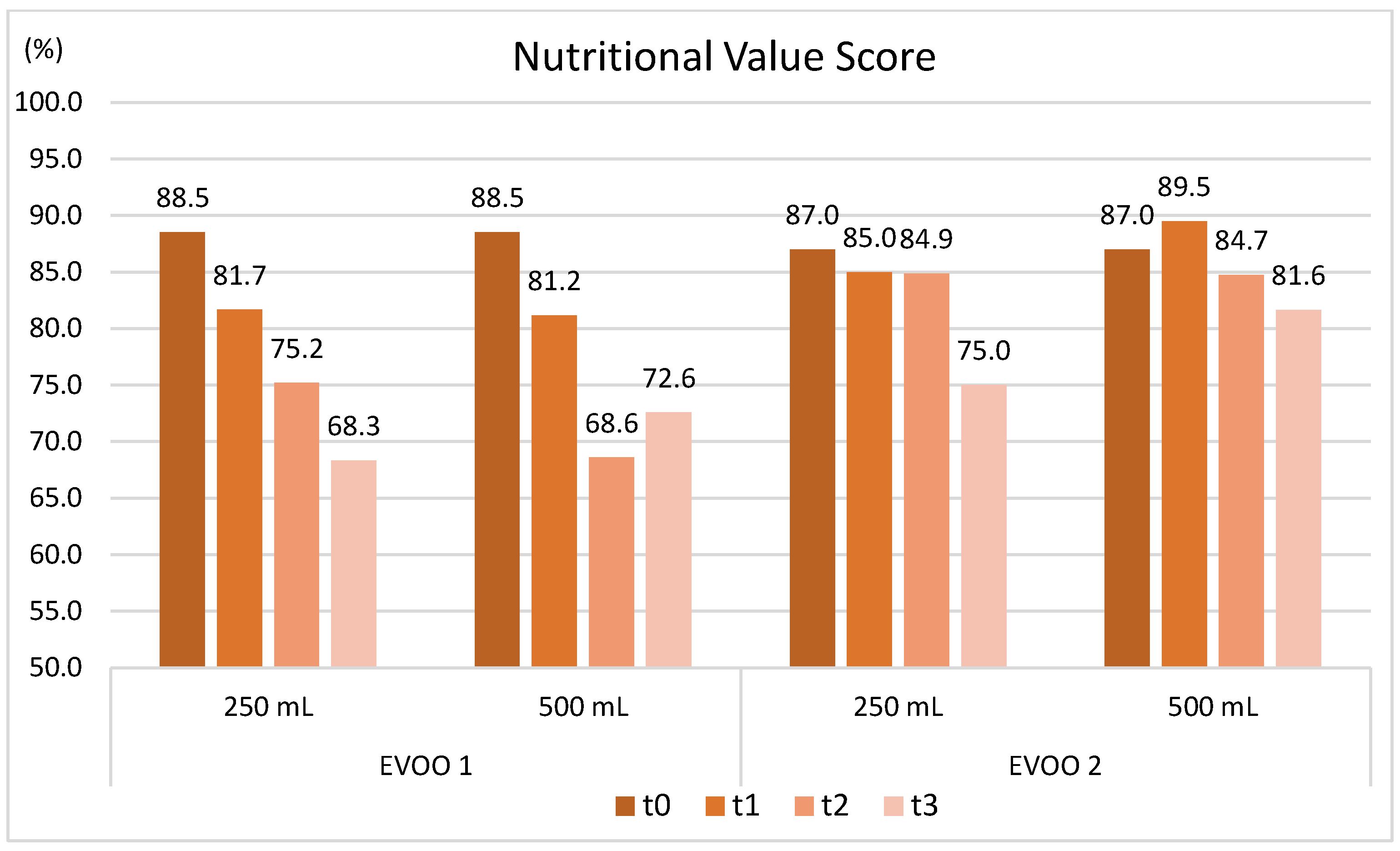
2.3.2. Evolution of Sensory and Nutritional Characteristics over Time
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Virgin Olive Oil Samples Production
3.3. The Shelf-Life Experimental Plan
- EVOO samples: Two samples from 2023 were selected and labelled as EVOO1 and EVOO2.
- Storage time: The oils were analyzed at four different storage times over one year:
- ⮚
- Time 0 (t0): Immediately after production.
- ⮚
- Time 1 (t1): After 2 months of storage.
- ⮚
- Time 2 (t2): After 5 months of storage.
- ⮚
- Time 3 (t3): After 12 months of storage.
- 3.
- Bottle size: Two bottle sizes were selected: 250 mL (B250) and 500 mL (B500), both provided by Fara Srl Vetrerie E Cristallerie (Montespertoli, Italy). This choice was based on the hypothesis that larger bottles better preserve oil quality, similarly to what happens in other beverages like wine [43,44].
3.4. Chemical Analysis
3.4.1. Legal Quality Parameters
3.4.2. Analysis of Phenolic Compounds via HPLC-DAD
3.4.3. Analysis of Tocopherols via HPLC-FLD
3.4.4. Analysis of Volatile Organic Compounds via HS-SPME-GC-MS
3.5. Sensory Analysis
3.6. Nutraceutical Evaluation by Applying the Nutritional Value Score (NVS)
3.7. Consumers’s Preferences by Cognitive Salience Index (CSI)
3.8. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Olive Council. 2025. Available online: https://www.internationaloliveoil.org/olive-world/olive-oil/ (accessed on 28 April 2025).
- European Parliament and the Council (EC). Commission Delegated Regulation (EU) No 2022/2104 of 29 July 2022 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as regards marketing standards for olive oil, and repealing Commission Regulation (EEC) No 2568/1991 and Commission Implementing Regulation (EU) No 29/2012. Off. J. Eur. Union 2022, L284, 1–22. [Google Scholar]
- European Parliament and the Council (EC). Commission Implementing Regulation (EU) 2022/2105 of 29 July 2022 laying down rules on conformity checks of marketing standards for olive oil and methods of analysis of the characteristics of olive oil. Off. J. Eur. Union. 2022, L284, 23–48. [Google Scholar]
- Azcarate, S.M.; Segura-Borrego, M.P.; Ríos-Reina, R.; Callejón, R.M. ¹H-NMR Spectroscopy and Chemometric Fingerprinting for the Authentication of Organic Extra Virgin Olive Oils. Chemosensors 2025, 13, 162. [Google Scholar] [CrossRef]
- Ugolini, T.; Mattagli, F.; Melani, F.; Zanoni, B.; Migliorini, M.; Trapani, S.; Giambanelli, E.; Parenti, A.; Mulinacci, N.; Cecchi, L. HS-SPME-GC-MS and chemometrics for the quality control and clustering of monovarietal extra virgin olive oil: A 3-year study on terpenes and pentene dimers of Italian cultivars. J. Agric. Food Chem. 2024, 72, 11124–11139. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, L.; Casal, S.; Cruz, R.; Peres, A.M.; Baptista, P.; Rodrigues, N. Influence of Interannual Climate Conditions on the Composition of Olive Oil from Centenarian Olive Trees. Agronomy 2023, 13, 2884. [Google Scholar] [CrossRef]
- Ben-Ari, G.; Biton, I.; Many, Y.; Namdar, D.; Samach, A. Elevated temperatures negatively affect olive productive cycle and oil quality. Agronomy 2021, 11, 1492. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Silva-Soto, M.Á.; Carrillo-Fernández, P.; Saez Lancellotti, E.T.; Medina-Jiménez, E.; Mogaburo Alba, J.F.; Catena-Granados, N.; López-Carmona, M.D.; Pérez-Belmonte, L.M.; Prieto Lain, N.; Gómez Hernández, A.I.; et al. Extra Virgin Olive Oil Phenolic Compounds: Modulating Mitochondrial Function and Protecting Against Chronic Diseases—A Narrative Review. Nutrients 2025, 17, 1443. [Google Scholar] [CrossRef]
- Cecchi, L.; Conticelli, F.; Zanoni, B.; Breschi, C.; Bellumori, M.; Mulinacci, N. Chemical Data and Relationships for a Scoring Algorithm of Extra Virgin Olive Oil’s Nutritional Value. Molecules 2024, 29, 525. [Google Scholar] [CrossRef]
- Sanmartin, C.; Venturi, F.; Sgherri, C.; Nari, A.; Macaluso, M.; Flamini, G.; Quartacci, M.F.; Taglieri, I.; Andrich, G.; Zinnai, A. The effects of packaging and storage temperature on the shelf-life of extra virgin olive oil. Heliyon 2018, 4, e00888. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, A.; Quintero-Florez, A.; Ruiz-Mendez, M.V.; Perona, J.S. Virgin olive oil ranks first in a new nutritional quality score due to its compositional profile. Nutrients 2023, 15, 2127. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Muraglia, M.; Crupi, P.; Hachicha Hbaieb, R.; De Santis, S.; Desantis, A.; Corbo, F. The Tower of Babel of pharma-food study on Extra Virgin Olive Oil polyphenols. Foods 2022, 11, 1915. [Google Scholar] [CrossRef]
- Tsimidou, M.Z.; Boskou, D. The health claim on “olive oil polyphenols” and the need for meaningful terminology and effective analytical protocols. Eur. J. Lipid Sci. Technol. 2015, 117, 1091–1094. [Google Scholar] [CrossRef]
- European Commission Regulation EC No. 432/2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, L136, 1–40.
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made onfoods. Off. J. Eur. Union 2006, L404, 9–25.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of health claims related to polyphenols in olive oil and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar]
- Gutierrez-Rosales, F.; Romero, M.P.; Casanovas, M.; Motilva, M.J.; Mínguez-Mosquera, M.I. Metabolites involved in oleuropein accumulation and degradation in fruits of Olea europaea L.: Hojiblanca and Arbequina varieties. J. Agric. Food Chem. 2010, 58, 12924–12933. [Google Scholar] [CrossRef]
- Gutierrez-Rosales, F.; Romero, M.P.; Casanovas, M.; Motilva, M.J.; Mínguez-Mosquera, M.I. β-Glucosidase involvement in the formation and transformation of oleuropein during the growth and development of olive fruits (Olea europaea L. cv. Arbequina) grown under different farming practices. J. Agric. Food Chem. 2012, 60, 4348–4358. [Google Scholar] [CrossRef]
- Cecchi, L.; Innocenti, M.; Melani, F.; Migliorini, M.; Conte, L.; Mulinacci, N. New isobaric lignans from refined olive oils as quality markers for virgin olive oils. Food Chem. 2017, 219, 148–157. [Google Scholar] [CrossRef][Green Version]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Torres-Cobos, B.; Quintanilla-Casas, B.; Romero, A.; Ninot, A.; Alonso-Salces, R.M.; Toschi, T.G.; Bendini, A.; Guardiola, F.; Tres, A.; Vichi, S. Varietal authentication of virgin olive oil: Proving the efficiency of sesquiterpene fingerprinting for Mediterranean Arbequina oils. Food Control 2021, 128, 108200. [Google Scholar] [CrossRef]
- Soldo, B.; Jukić Špika, M.; Pasković, I.; Vuko, E.; Polić Pasković, M.; Ljubenkov, I. The Composition of Volatiles and the Role of Non-Traditional LOX on Target Metabolites in Virgin Olive Oil from Autochthonous Dalmatian Cultivars. Molecules 2024, 29, 1696. [Google Scholar] [CrossRef] [PubMed]
- Brkić Bubola, K.; Lukić, I.; Krapac, M.; Koprivnjak, O. Exploring the Connection between the Occurrence and Intensity of “Grubby” Defect and Volatile Composition of Olive Oil. Foods 2023, 12, 4473. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Aparicio-Ruiz, R.; Morales, M.T.; García-González, D.L. Contribution of specific volatile markers to green and ripe fruity attributes in extra virgin olive oils studied with three analytical methods. Food Chem. 2022, 399, 133942. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Priego-Capote, F. Cultivar influence on the volatile components of olive oil formed in the lipoxygenase pathway. LWT—Food Sci. Technol. 2021, 147, 111485. [Google Scholar] [CrossRef]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Lavelli, V.; Fregapane, G.; Salvador, M.D. Effect of storage on secoiridoid and tocopherol contents and antioxidant activity of monovarietal extra virgin olive oils. J. Agric. Food Chem. 2006, 54, 3002–3007. [Google Scholar] [CrossRef]
- Psomiadou, E.; Tsimidou, M.; Boskou, D. α-Tocopherol content of Greek virgin olive oils. J. Agric. Food Chem. 2000, 48, 1770–1775. [Google Scholar] [CrossRef]
- Bajoub, A.; Bendini, A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Olive oil authentication: A comparative analysis of regulatory frameworks with especial emphasis on quality and authenticity indices, and recent analytical techniques developed for their assessment. A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 832–857. [Google Scholar] [CrossRef]
- Campestre, C.; Angelini, G.; Gasbarri, C.; Angerosa, F. The compounds responsible for the sensory profile in monovarietal virgin olive oils. Molecules 2017, 22, 1833. [Google Scholar] [CrossRef]
- Delgado, C.; Guinard, J.X. How do consumer hedonic ratings for extra virgin olive oil relate to quality ratings by experts and descriptive analysis ratings? Food Qual. Pref. 2011, 22, 213–225. [Google Scholar] [CrossRef]
- Lukić, I.; Lukić, M.; Žanetić, M.; Krapac, M.; Godena, S.; Brkić Bubola, K. Inter-varietal diversity of typical volatile and phenolic profiles of Croatian extra virgin olive oils as revealed by GC-IT-MS and UPLC-DAD analysis. Foods 2019, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Montealegre, C.; Marina Alegre, M.L.; García-Ruiz, C. Traceability markers to the botanical origin in olive oils. J. Agric. Food Chem. 2010, 58, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Stasi, A.; Diotallevi, F.; Marchini, A.; Nardone, G. Italian extra-virgin olive oil: Impact on demand on being market leaders, private labels or small producers. Rev. Financ. Econ 2018, 13, 39–54. [Google Scholar]
- Žanetić, M.; Jukić Špika, M.; Ožić, M.M.; Brkić Bubola, K. Comparative study of volatile compounds and sensory characteristics of dalmatian monovarietal virgin olive oils. Plants 2021, 10, 1995. [Google Scholar] [CrossRef]
- Peres, F.; Martins, L.L.; Ferreira-Dias, S. Influence of enzymes and technology on virgin olive oil composition. Crit. Rev. Food Sci. Nutr. 2017, 57, 3104–3126. [Google Scholar] [CrossRef]
- Peri, C. The extra-virgin olive oil chain. In The Extra-Virgin Olive Oil Handbook; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 3–10. [Google Scholar]
- Mulinacci, N.; Ieri, F.; Ignesti, G.; Romani, A.; Michelozzi, M.; Creti, D.; Innocenti, M.; Calamai, L. The freezing process helps to preserve the quality of extra virgin olive oil over time: A case study up to 18 months. Food Res. Int. 2013, 54, 2008–2015. [Google Scholar] [CrossRef]
- Averbuch, N.C.; Silva, L.T.; Cavalcante, L.S.; Machado, I.C.K.; dos Santos, S.O.; Nogueira, J.L.; Scheid, C.; Merib, J.; Garavaglia, J. Evolution of the quality and sensory characteristics of extra virgin olive oil as affected by phenolic content during long-time storage at room temperature. JSFA Rep. 2023, 3, 320–330. [Google Scholar] [CrossRef]
- International Olive Council. 2018. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-BPS-Doc.-No-1-2018-Eng.pdf (accessed on 3 May 2025).
- Velasco, J.; Dobarganes, C. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 661–676. [Google Scholar] [CrossRef]
- Echave, J.; Barral, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Bottle aging and storage of wines: A review. Molecules 2021, 26, 713. [Google Scholar] [CrossRef]
- Arapitsas, P.; Speri, G.; Angeli, A.; Perenzoni, D.; Mattivi, F. The influence of storage on the “chemical age” of red wines. Metabolomics 2014, 10, 816–832. [Google Scholar] [CrossRef]
- Caipo, L.; Sandoval, A.; Sepúlveda, B.; Fuentes, E.; Valenzuela, R.; Metherel, A.H.; Romero, N. Effect of storage conditions on the quality of arbequina extra virgin olive oil and the impact on the composition of flavor-related compounds (phenols and volatiles). Foods 2021, 10, 2161. [Google Scholar] [CrossRef]
- Esposto, S.; Selvaggini, R.; Taticchi, A.; Veneziani, G.; Sordini, B.; Servili, M. Quality evolution of extra-virgin olive oils according to their chemical composition during 22 months of storage under dark conditions. Food Chem. 2020, 311, 126044. [Google Scholar] [CrossRef] [PubMed]
- Vekiari, S.A.; Papadopoulou, P.; Kiritsakis, A. Effects of processing methods and commercial storage conditions on the extra virgin olive oil quality indexes. Grasas Aceites 2007, 58, 237–242. [Google Scholar] [CrossRef]
- Masella, P.; Parenti, A.; Spugnoli, P. Glass bottles of different sizes for the packaging of virgin olive oil: Influence on shelf life. La Riv. Ital. Sostanze Grasse 2012, 89, 161–165. [Google Scholar]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Gutiérrez-Rosales, F.; Rios, J.J.; Gomez-Rey, M.L. Main polyphenols in the bitter taste of virgin olive oil. Structural confirmation by on-line high-performance liquid chromatography electrospray ionization mass spectrometry. J. Agric. Food Chem. 2003, 51, 6021–6025. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.; de Joode, T.; Groenewegen, A.; Alexandre, H. Sensory properties of virgin olive oil polyphenols: Identification of deacetoxy-ligstroside aglycon as a key contributor to pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef]
- Baldioli, M.; Servili, M.; Perretti, G.; Montedoro, G.F. Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil. J. Am. Oil Chem. Soc. 1996, 73, 1589–1593. [Google Scholar] [CrossRef]
- Curto, S.L.; Dugo, G.; Mondello, L.; Errante, G.; Russo, M.T. Variation in tocopherol content in Italian virgin olive oils. Ital. J. Food Sci. 2001, 13, 221–228. [Google Scholar]
- Angerosa, F.; Lanza, B.; Marsilio, V. Biogenesis of «fusty» defect in virgin olive oils. Grasas Aceites 1996, 47, 142–150. [Google Scholar] [CrossRef]
- Angerosa, F.; Lanza, B.; d’Alessandro, N.; Marsilio, V.; Cumitini, S. Olive oil off-odour compounds produced by Aspergillus and Penicillium. Acta Hortic. 1999, 474, 695–700. [Google Scholar] [CrossRef]
- Fortini, M.; Migliorini, M.; Cherubini, C.; Cecchi, L.; Calamai, L. Multiple internal standard normalization for improving HS-SPME-GC-MS quantitation in virgin olive oil volatile organic compounds (VOO-VOCs) profile. Talanta 2017, 165, 641–652. [Google Scholar] [CrossRef]
- International Olive Council. 2025. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/06/Doc.-No-29-REV-2_ENK.pdf (accessed on 2 May 2025).
- Gliszczyńska-Świgło, A.; Sikorska, E. Simple reversed-phase liquid chromatography method for determination of tocopherols in edible plant oils. J. Chromatog. A 2004, 1048, 195–198. [Google Scholar] [CrossRef]
- Barone, B.; Nogueira, R.M.; Behrens, J.H. Sustainable diet from the urban Brazilian consumer perspective. Food Res. Int. 2019, 124, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Fabbrizzi, S.; Alampi Sottini, V.; Cipollaro, M.; Menghini, S. Sustainability and Natural Wines: An Exploratory Analysis on Consumers. Sustainability 2021, 13, 7645. [Google Scholar] [CrossRef]
- Ginon, E.; Ares, G.; Issanchou, S.; dos Santos Laboissière, L.H.E.; Deliza, R. Identifying motives underlying wine purchase decisions: Results from an exploratory free listing task with Burgundy wine consumers. Food Res. Int. 2014, 62, 860–867. [Google Scholar] [CrossRef]
- Libertino, L.; Ferraris, D.; Osornio, M.L.; Hough, G. Analysis of data from a free-listing study of menus by different income-level populations. Food Qual. Pref. 2012, 24, 269–275. [Google Scholar] [CrossRef]
- Sutrop, U. List task and a cognitive salience index. Field Methods 2001, 13, 263–276. [Google Scholar] [CrossRef]
- Hough, G.; Ferraris, D. Free listing: A method to gain initial insight of a food category. Food Qual. Pref. 2010, 21, 295–301. [Google Scholar] [CrossRef]
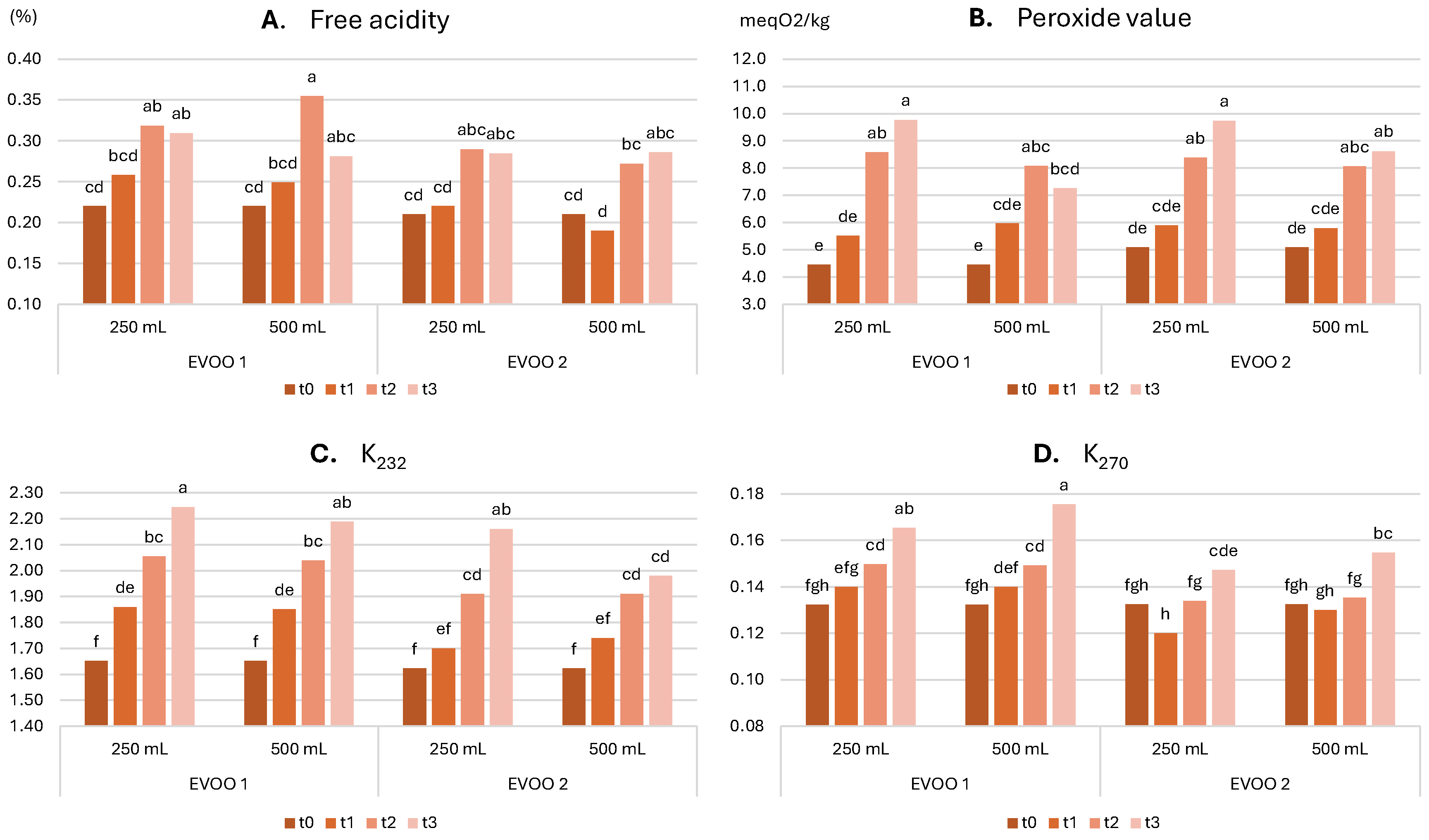
| Sample | FA (%oleic acid) | PV (meqO2/kg) | K232 | K270 | ∆K | TTC | TPC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | |
| EVOO1 | 0.22 | 0.21 | 4.5 | 9.6 | 1.65 | 1.80 | 0.13 | 0.17 | 0.003 | 0.002 | 387.7 | 268.8 | 618.8 | 532.8 |
| EVOO2 | 0.21 | 0.23 | 5.1 | 5.9 | 1.62 | 1.68 | 0.13 | 0.14 | 0.003 | 0.002 | 407.8 | 317.1 | 560.9 | 409.8 |
| EVOO3 | 0.27 | 0.18 | 7.2 | 6.5 | 1.70 | 1.73 | 0.14 | 0.14 | 0.004 | 0.002 | 373.0 | 326.8 | 596.3 | 396.1 |
| EVOO4 | 0.27 | 0.17 | 4.6 | 5.0 | 1.76 | 1.67 | 0.14 | 0.15 | 0.004 | 0.002 | 369.4 | 322.5 | 663.6 | 398.1 |
| EVOO5 | 0.24 | 0.21 | 4.6 | 4.8 | 1.62 | 1.80 | 0.12 | 0.21 | 0.003 | 0.001 | 357.6 | 544.9 | 434.2 | 569.7 |
| EVOO6 | 0.27 | 0.28 | 8.3 | 6.7 | 1.80 | 1.77 | 0.12 | 0.15 | 0.004 | 0.003 | 308.7 | 266.9 | 563.5 | 561.5 |
| EVOO7 | 0.22 | 0.23 | 5.6 | 5.3 | 1.73 | 1.68 | 0.15 | 0.14 | 0.003 | 0.002 | 340.9 | 341.6 | 572.9 | 420.3 |
| EVOO8 | 0.21 | 0.24 | 5.1 | 5.7 | 1.77 | 1.72 | 0.14 | 0.13 | 0.005 | 0.002 | 336.1 | 306.1 | 509.4 | 439.0 |
| EVOO9 | 0.20 | 0.17 | 4.8 | 4.8 | 1.71 | 1.65 | 0.15 | 0.15 | 0.005 | 0.002 | 459.7 | 437.9 | 569.7 | 456.8 |
| EVOO10 | 0.23 | 0.17 | 5.5 | 6.3 | 1.74 | 1.66 | 0.14 | 0.13 | 0.004 | 0.002 | 263.0 | 282.7 | 517.2 | 338.7 |
| EVOO11 | 0.23 | 0.20 | 4.4 | 5.6 | 1.67 | 1.75 | 0.13 | 0.15 | 0.004 | 0.002 | 467.6 | 300.9 | 423.1 | 432.3 |
| EVOO12 | 0.20 | 0.14 | 4.2 | 7.6 | 1.58 | 1.78 | 0.12 | 0.16 | 0.003 | 0.002 | 369.3 | 310.8 | 450.7 | 393.8 |
| Minimum | 0.20 | 0.14 | 4.2 | 4.8 | 1.58 | 1.65 | 0.12 | 0.13 | 0.003 | 0.001 | 263.0 | 266.9 | 423.1 | 338.8 |
| Maximum | 0.27 | 0.28 | 8.3 | 9.6 | 1.80 | 1.80 | 0.15 | 0.21 | 0.005 | 0.003 | 467.6 | 544.9 | 663.6 | 569.7 |
| Mean | 0.23 | 0.20 | 5.5 | 6.3 | 1.70 | 1.72 | 0.13 | 0.15 | 0.004 | 0.002 | 369.3 | 345.6 | 540.5 | 447.0 |
| Sample | Total VOCs (mg/kg) | Total LOX (A) (mg/kg) | (E)-2-Hexenal (B) (mg/kg) | Hexanal (mg/kg) | Total Rancid (mg/kg) | Total Microbial (mg/kg) | 2 + 3-Methylbutanal (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | |
| EVOO1 | 68.5 | 59.0 | 51.4 (75%) | 55.0 (93%) | 45.3 (88%) | 49.8 (91%) | 0.473 | 1.034 | 0.392 | 0.065 | 0.077 | 0.507 | 0.042 | 0.040 |
| EVOO2 | 72.8 | 75.9 | 54.6 (75%) | 67.6 (89%) | 47.4 (87%) | 60.3 (89%) | 0.433 | 1.087 | 0.221 | 0.080 | 0.041 | 0.543 | 0.030 | 0.046 |
| EVOO3 | 54.6 | 71.2 | 38.6 (71%) | 65.1 (91%) | 33.3 (86%) | 57.9 (89%) | 0.623 | 1.237 | 0.601 | 0.073 | 0.075 | 0.500 | 0.057 | 0.050 |
| EVOO4 | 65.3 | 79.2 | 42.2 (65%) | 70.4 (89%) | 36.3 (86%) | 62.5 (89%) | 0.309 | 1.031 | 0.269 | 0.070 | 0.064 | 1.600 | 0.048 | 0.043 |
| EVOO5 | 72.1 | 51.0 | 52.4 (73%) | 43.1 (85%) | 46.0 (88%) | 38.6 (90%) | 0.690 | 0.557 | 0.614 | 0.060 | 0.048 | 0.178 | 0.034 | 0.054 |
| EVOO6 | 51.2 | 73.2 | 31.3 (61%) | 64.0 (87%) | 26.7 (85%) | 56.5 (88%) | 0.339 | 0.706 | 0.601 | 0.074 | 0.066 | 0.275 | 0.041 | 0.064 |
| EVOO7 | 59.2 | 64.1 | 48.8 (82%) | 58.1 (91%) | 43.9 (90%) | 53.1 (91%) | 0.535 | 0.861 | 0.538 | 0.060 | 0.122 | 1.610 | 0.066 | 0.037 |
| EVOO8 | 88.8 | 47.5 | 67.2 (76%) | 43.4 (91%) | 59.6 (89%) | 38.7 (89%) | 0.424 | 0.781 | 0.339 | 0.088 | 0.119 | 0.554 | 0.054 | 0.033 |
| EVOO9 | 48.7 | 54.6 | 28.4 (58%) | 46.5 (85%) | 23.7 (83%) | 41.7 (90%) | 0.467 | 0.560 | 0.506 | 0.043 | 0.078 | 0.234 | 0.055 | 0.035 |
| EVOO10 | na | 72.8 | na | 58.5 (80%) | na | 53.2 (91%) | na | 0.973 | na | 0.127 | na | 0.825 | na | 0.026 |
| EVOO11 | 66.3 | 59.9 | 49.6 (75%) | 53.6 (89%) | 44.1 (89%) | 47.7 (89%) | 0.274 | 0.742 | 0.415 | 0.066 | 0.096 | 0.643 | 0.056 | 0.044 |
| EVOO12 | 72.1 | 68.8 | 48.9 (68%) | 63.7 (93%) | 44.1 (%90) | 57.6 (90%) | 0.307 | 0.983 | 0.521 | 0.072 | 0.090 | 0.134 | 0.042 | 0.040 |
| Minimum | 48.7 | 47.5 | 28.4 (58%) | 43.1 (80%) | 23.7 (83%) | 38.6 (88%) | 0.274 | 0.557 | 0.221 | 0.043 | 0.041 | 0.134 | 0.030 | 0.026 |
| Maximum | 88.8 | 79.2 | 67.2 (82%) | 70.4 (93%) | 59.6 (90%) | 62.5 (91%) | 0.690 | 1.237 | 0.614 | 0.127 | 0.122 | 1.610 | 0.066 | 0.064 |
| Mean | 65.4 | 64.8 | 46.7 (71%) | 57.4 (89%) | 40.9 (87%) | 51.5 (90%) | 0.443 | 0.879 | 0.456 | 0.073 | 0.080 | 0.634 | 0.048 | 0.043 |
| Sample | Defects | Fruitiness | Bitterness | Pungency | ||||
|---|---|---|---|---|---|---|---|---|
| 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | 2023 | 2024 | |
| EVOO1 | - | - | 4.0 | 5.8 | 3.8 | 6.6 | 4.8 | 6.8 |
| EVOO2 | - | - | 4.1 | 4.4 | 2.9 | 5.2 | 4.9 | 5.7 |
| EVOO3 | - | - | 4.4 | 4.7 | 4.2 | 5.4 | 5.3 | 6.2 |
| EVOO4 | - | - | 4.3 | 4.3 | 3.7 | 3.7 | 4.6 | 4.6 |
| EVOO5 | - | - | 4.9 | 5.5 | 3.5 | 5.6 | 4.8 | 6.4 |
| EVOO6 | - | - | 4.0 | 3.7 | 3.4 | 4.1 | 3.7 | 4.6 |
| EVOO7 | - | - | 5.1 | 4.2 | 4.5 | 3.5 | 5.7 | 4.8 |
| EVOO8 | - | - | 5.1 | 4.1 | 4.2 | 4.3 | 5.2 | 5.3 |
| EVOO9 | - | - | 4.3 | 3.8 | 4.0 | 3.0 | 5.0 | 4.1 |
| EVOO10 | - | - | 4.5 | 4.9 | 4.7 | 5.2 | 5.5 | 5.6 |
| EVOO11 | - | - | 4.0 | 3.7 | 2.6 | 4.0 | 3.8 | 3.5 |
| EVOO12 | - | - | 4.1 | 4.2 | 3.3 | 4.4 | 4.0 | 5.0 |
| NVS | EVOO 1 | EVOO 2 | EVOO 3 | EVOO 4 | EVOO 5 | EVOO 6 | EVOO 7 | EVOO 8 | EVOO 9 | EVOO 10 | EVOO 11 | EVOO 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2024 | 88.3 | 89.1 | 88.0 | 87.9 | 75.0 | 86.0 | 90.1 | 90.4 | 93.3 | 86.5 | 89.0 | 85.0 |
| 2023 | 88.5 | 87.0 | 85.0 | 88.6 | 81.9 | 83.1 | 85.0 | 83.1 | 87.8 | 86.9 | 80.4 | 84.5 |
| Semantic Category | CSI | Frequency | Included Terms |
|---|---|---|---|
| Taste and Flavor | 0.5366 | 527 | flavor, taste, delicate, intense, body, good, full-bodied, |
| tasty, sweet, aromatic, spicy, bitter, light, strong, etc. | |||
| Aroma and Scent | 0.1795 | 198 | scent, fragrant, smell, aroma |
| Visual Appearance | 0.1739 | 194 | color, green, yellow, transparency, dark |
| Naturalness and Authenticity | 0.1344 | 131 | natural, organic, genuine, authentic |
| Health and Wellness | 0.1316 | 110 | healthy, wholesome |
| Origin and Italian Identity | 0.0978 | 89 | Italian, local, craft |
| Price and Value | 0.0503 | 73 | price, expensive, good value |
| Factors | Free Acidity | Peroxide Value | K232 | K270 | ΔK | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pr(>F) | Signif. | Pr(>F) | Signif. | Pr(>F) | Signif. | Pr(>F) | Signif. | Pr(>F) | Signif. | |
| EVOO | 0.00023 | *** | 0.1781 | 4.4 × 10−8 | *** | 7.8 × 10−13 | *** | 0.00032 | *** | |
| Time | 1.6 × 10−10 | *** | 1.1 × 10−14 | *** | <2 × 10−16 | *** | <2 × 10−16 | *** | 0.00176 | ** |
| Bottle Size | 0.39749 | 0.0284 | * | 0.0887 | . | 0.0019 | ** | 0.42187 | ||
| EVOO:Time | 0.04919 | * | 0.5517 | 0.0363 | * | 1.1 × 10−6 | *** | 0.07281 | . | |
| EVOO:Bottle Size | 0.42427 | 0.5834 | 0.6073 | 0.7764 | 0.31631 | |||||
| Time:Bottle Size | 0.48375 | 0.0168 | * | 0.018 | * | 0.0166 | * | 0.35210 | ||
| EVOO:Time:Bottle Size | 0.25930 | 0.4774 | 0.2631 | 0.7524 | 0.27538 | |||||
| Factors | Hydroxytyrosol | Tyrosol | Oleacein | Oleocanthal | Total Phenols | Hydr. Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pr(>F) | Signif | Pr(>F) | Signif | Pr(>F) | Signif | Pr(>F) | Signif | Pr(>F) | Signif | Pr(>F) | Signif | |
| EVOO | 4.9 × 10−13 | *** | <2 × 10−16 | *** | <2 × 10−16 | *** | 0.3538 | 1.6 × 10−13 | *** | 0.7122 | ||
| Time | <2 × 10−16 | *** | 2.2 × 10−10 | *** | <2 × 10−16 | *** | 1.1 × 10−12 | *** | 2.2 × 10−7 | *** | <2 × 10−16 | *** |
| Bottle Size | 0.1720 | 0.6270 | 0.3620 | 0.0536 | . | 0.5101 | 0.3228 | |||||
| EVOO:Time | 8.3 × 10−9 | *** | 7.7 × 10−7 | *** | 0.6928 | 0.0131 | * | 0.1329 | 1.2 × 10−7 | *** | ||
| EVOO:Bottle Size | 0.4440 | 0.8550 | 0.0658 | . | 0.9486 | 0.1084 | 0.2427 | |||||
| Time:Bottle Size | 0.1950 | 0.9280 | 0.0008 | *** | 0.0054 | ** | 0.0009 | *** | 0.0384 | * | ||
| EVOO:Time:Bottle Size | 0.1780 | 0.1100 | 0.5195 | 0.2748 | 0.3131 | 0.1700 | ||||||
| Factors | α-Tocopherol | β + γ-Tocopherol | δ-Tocopherol | Total Tocopherol | ||||
|---|---|---|---|---|---|---|---|---|
| Pr(>F) | Signif. | Pr(>F) | Signif. | Pr(>F) | Signif. | Pr(>F) | Signif. | |
| EVOO | 2.47 × 10−7 | *** | <2 × 10−16 | *** | 0.00768 | ** | 0.0491 | * |
| Time | <2 × 10−16 | *** | 9.41 × 10−16 | *** | 1.39 × 10−11 | *** | <2 × 10−16 | *** |
| Bottle Size | 0.40076 | 0.0437 | * | 0.27455 | 0.7204 | |||
| EVOO:Time | 4.72 × 10−9 | *** | 2.05 × 10−6 | *** | 2.74 × 10−6 | *** | 8.16 × 10−8 | *** |
| EVOO:Bottle Size | 0.00065 | *** | 0.1288 | 0.21125 | 0.0052 | ** | ||
| Time:Bottle Size | 0.36106 | 0.0767 | . | 0.5705 | 0.2958 | |||
| EVOO:Time:Bottle Size | 0.0188 | * | 0.0279 | * | 0.29124 | 0.0251 | * | |
| Factors | Hexanal | (E)-2-Hexenal | Total LOX | Rancid VOCs | Microbial VOCs | 2 + 3-Methylbut | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pr(>F) | Signif. | Pr(>F) | Signif. | Pr(>F) | Signif. | Pr(>F) | Signif. | Pr(>F) | Signif. | Pr(>F) | Signif. | |
| EVOO | 0.0015 | ** | 0.0131 | * | 0.0011 | ** | 5.1 × 10−7 | *** | 0.0648 | . | 2.1 × 10−11 | *** |
| Time | <2 × 10−16 | *** | <2 × 10−16 | *** | 9.4 × 10−15 | *** | 1.8 × 10−13 | *** | 3.7 × 10−8 | *** | 8.0 × 10−11 | *** |
| Bottle Size | 0.0600 | . | 0.8801 | 0.8036 | 0.5654 | 0.2098 | 0.0504 | . | ||||
| EVOO:Time | 0.0000 | *** | 0.8045 | 0.4148 | 0.0062 | ** | 0.0016 | ** | 1.4 × 10−7 | *** | ||
| EVOO:Bottle Size | 0.0030 | ** | 0.5303 | 0.2308 | 0.0010 | ** | 0.1292 | 0.1560 | ||||
| Time:Bottle Size | 0.3446 | 0.9972 | 0.8170 | 0.7117 | 0.0602 | . | 0.0152 | * | ||||
| EVOO:Time:Bottle Size | 0.0048 | ** | 0.6320 | 0.9002 | 0.0041 | ** | 0.2711 | 8.4 × 10−5 | *** | |||
| Fusty/Muddy Sediment | Winey/ Winegary | Rancid | Fruityness | Bitterness | Pungency | |
|---|---|---|---|---|---|---|
| B t0 | n.f. | n.f. | n.f. | 4.0 | 3.9 | 4.7 |
| B t1 250 | n.f. | n.f. | n.f. | 4.0 | 5.4 | 6.2 |
| B t2 250 | n.f. | n.f. | n.f. | 3.2 | 6.2 | 4.5 |
| B t3 250 | n.f. | n.f. | n.f. | 4.8 | 6.0 | 5.4 |
| B t0 | n.f. | n.f. | n.f. | 4.0 | 3.9 | 4.7 |
| B t1 500 | n.f. | n.f. | n.f. | 4.5 | 6.2 | 5.9 |
| B t2 500 | n.f. | n.f. | n.f. | 4.3 | 5.8 | 5.4 |
| B t3 500 | n.f. | n.f. | n.f. | 4.5 | 6.3 | 4.6 |
| P t0 | n.f. | n.f. | n.f. | 3.8 | 2.9 | 5.3 |
| P t1 250 | n.f. | n.f. | n.f. | 5.9 | 5.5 | 7.0 |
| P t2 250 | n.f. | n.f. | n.f. | 5.7 | 5.6 | 6.1 |
| P t3 250 | n.f. | n.f. | n.f. | 4.3 | 4.3 | 3.8 |
| P t0 | n.f. | n.f. | n.f. | 3.8 | 2.9 | 5.3 |
| P t1 500 | n.f. | n.f. | n.f. | 6.5 | 7.4 | 7.4 |
| P t2 500 | n.f. | n.f. | n.f. | 5.0 | 6.4 | 5.5 |
| P t3 500 | n.f. | n.f. | n.f. | 4.8 | 6.1 | 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breschi, C.; Cecchi, L.; Mattagli, F.; Zanoni, B.; Ugolini, T.; Ieri, F.; Calamai, L.; Bellumori, M.; Mulinacci, N.; Boncinelli, F.; et al. Chemical, Sensory, and Nutraceutical Profiling, and Shelf-Life Assessment of High-Quality Extra Virgin Olive Oil Produced in a Local Area near Florence (Italy). Molecules 2025, 30, 2811. https://doi.org/10.3390/molecules30132811
Breschi C, Cecchi L, Mattagli F, Zanoni B, Ugolini T, Ieri F, Calamai L, Bellumori M, Mulinacci N, Boncinelli F, et al. Chemical, Sensory, and Nutraceutical Profiling, and Shelf-Life Assessment of High-Quality Extra Virgin Olive Oil Produced in a Local Area near Florence (Italy). Molecules. 2025; 30(13):2811. https://doi.org/10.3390/molecules30132811
Chicago/Turabian StyleBreschi, Carlotta, Lorenzo Cecchi, Federico Mattagli, Bruno Zanoni, Tommaso Ugolini, Francesca Ieri, Luca Calamai, Maria Bellumori, Nadia Mulinacci, Fabio Boncinelli, and et al. 2025. "Chemical, Sensory, and Nutraceutical Profiling, and Shelf-Life Assessment of High-Quality Extra Virgin Olive Oil Produced in a Local Area near Florence (Italy)" Molecules 30, no. 13: 2811. https://doi.org/10.3390/molecules30132811
APA StyleBreschi, C., Cecchi, L., Mattagli, F., Zanoni, B., Ugolini, T., Ieri, F., Calamai, L., Bellumori, M., Mulinacci, N., Boncinelli, F., Canuti, V., & Menghini, S. (2025). Chemical, Sensory, and Nutraceutical Profiling, and Shelf-Life Assessment of High-Quality Extra Virgin Olive Oil Produced in a Local Area near Florence (Italy). Molecules, 30(13), 2811. https://doi.org/10.3390/molecules30132811











