Chlorin Activity Enhancers for Photodynamic Therapy
Abstract
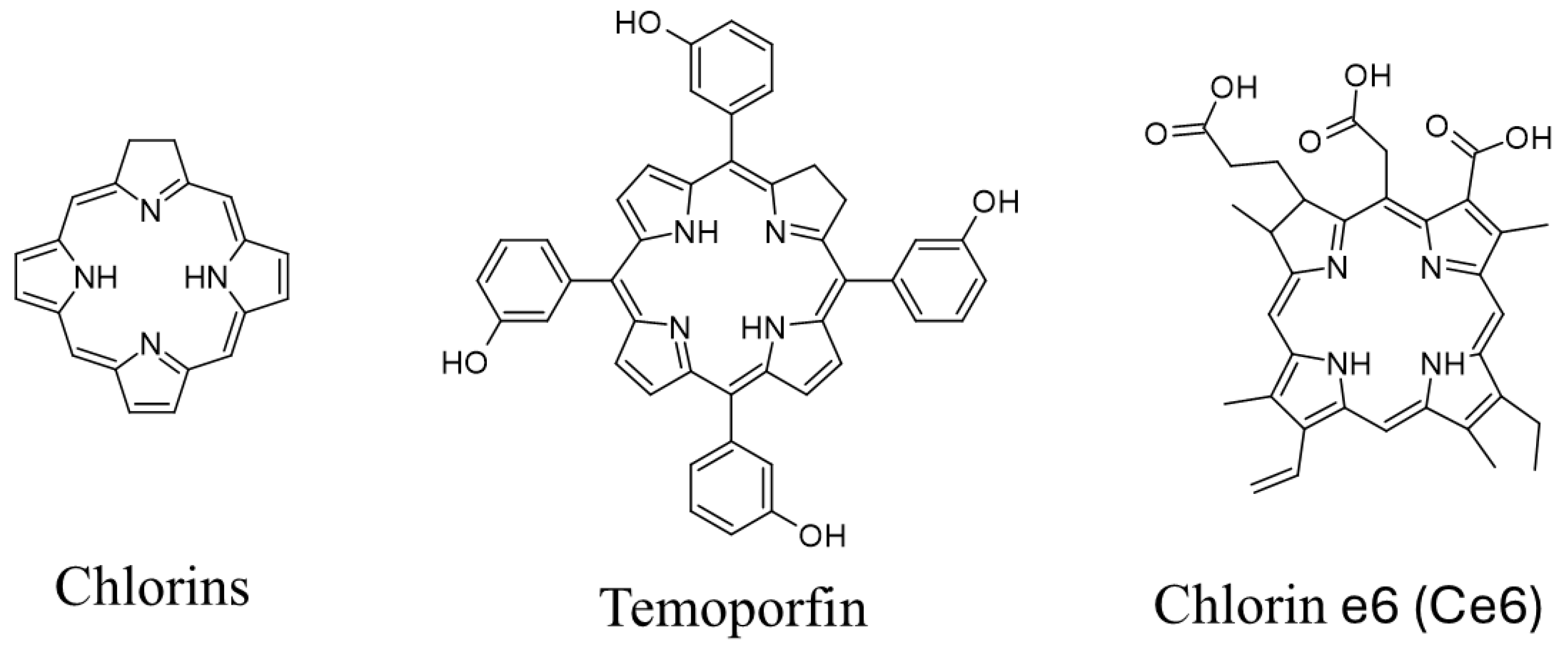
1. Introduction
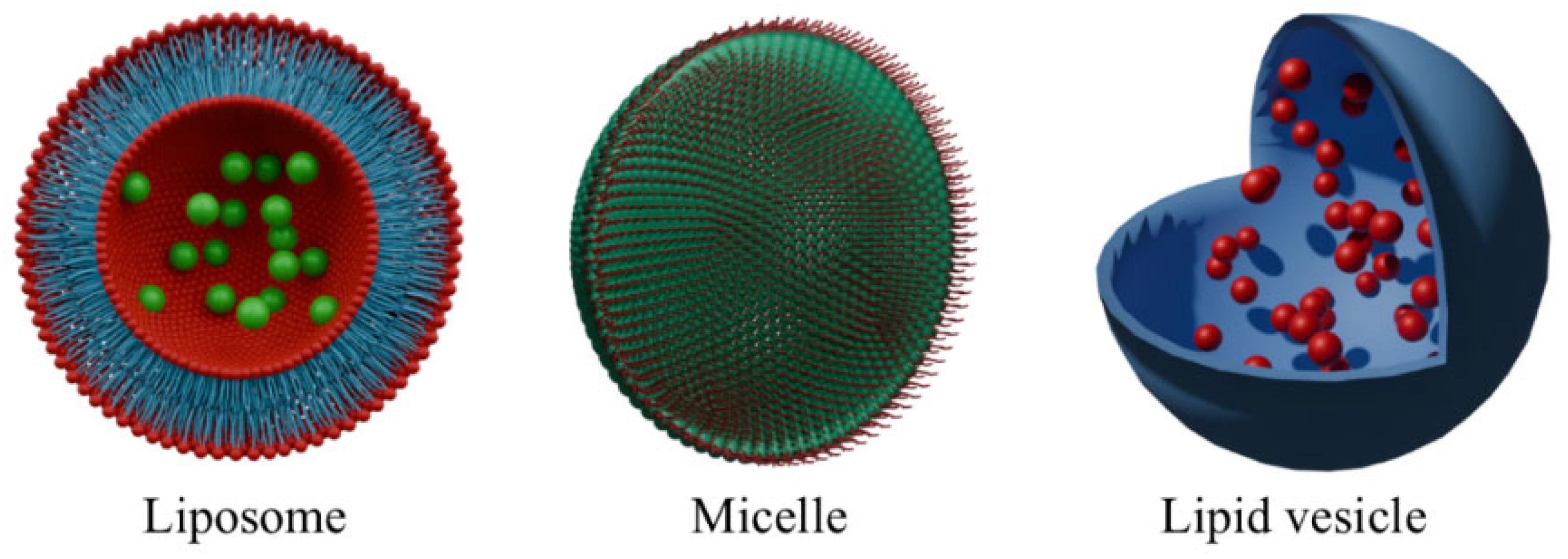
2. Lipid-Based Nanocarriers
2.1. Lipidic Vehicles
2.2. Conjugates with Micelles
| Type of Nanoparticle | Advantages | Disadvantages |
|---|---|---|
| Liposomes | Effective encapsulation and improved pharmacokinetics of photosensitizers [45,46]. Ability to combine PSs with synergistic therapeutic agents (chemotherapeutics, oxygen generators) [49,51,52]. | Limited physicochemical stability under physiological conditions [45,46]. Risk of non-specific biodistribution and premature drug leakage [49,51,52]. |
| Micelles | Precise, stimuli-responsive drug release (ROS, pH) [53]. Synergistic combination of PS with chemotherapeutics or oxygen-generating agents [54,55,56]. | Dependence on sufficient intratumoral ROS or external activation [58]. Stability and predictability challenges in complex biological environments [54,55,56]. |
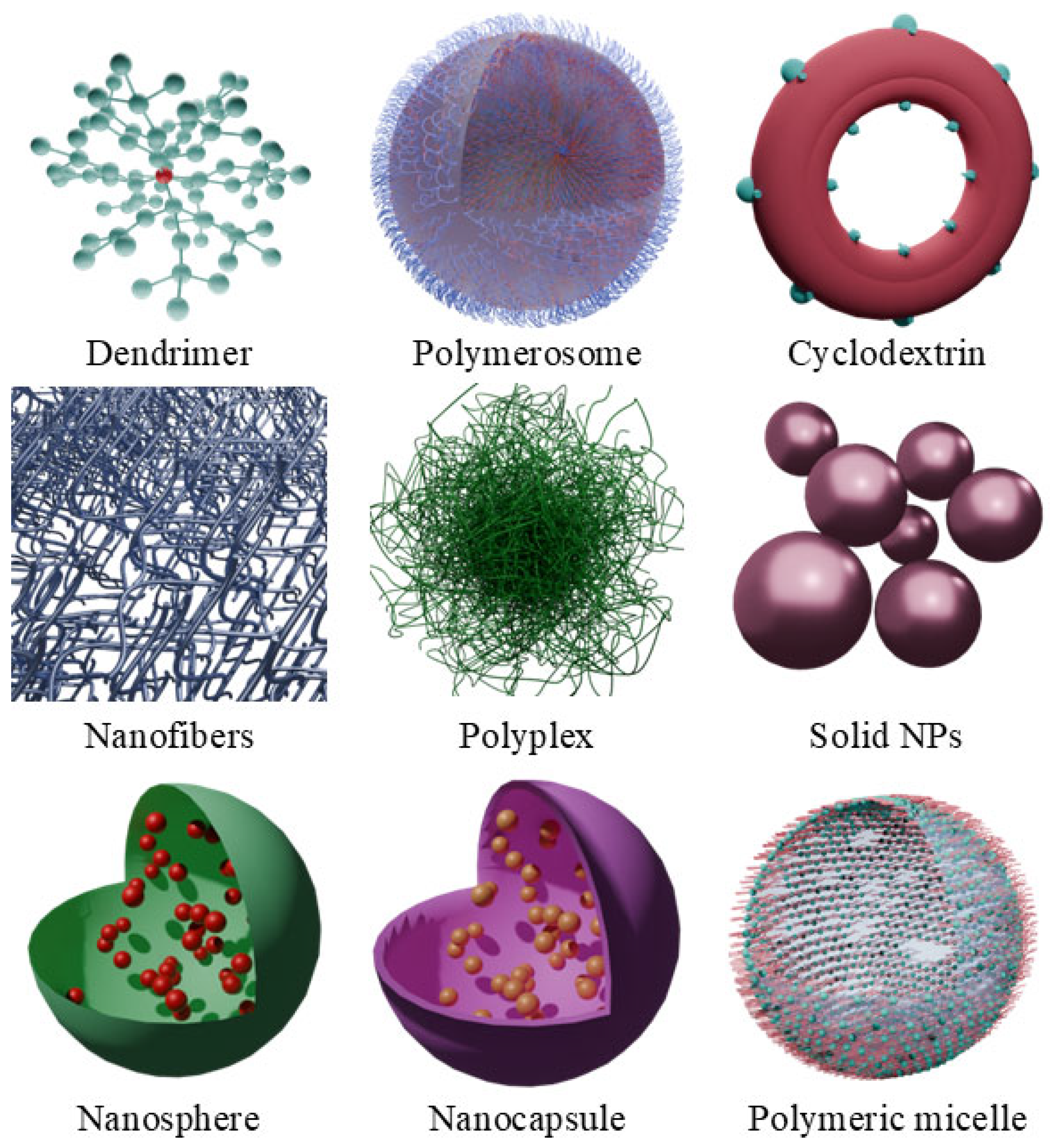
3. Polymer-Based Nanocarriers
3.1. Chlorins and Cellulose
3.2. Chlorins and Chitosan
3.3. Chlorins and Fibroin
3.4. Chlorins and Polyethyleneimine
3.5. Chlorins and PLGA
| Type of Nanoparticle | Advantages | Disadvantages |
|---|---|---|
| Cellulose-based | Excellent biocompatibility and biodegradability suitable for biomedical applications [61]. | Moderate drug release efficiency compared to free PSs [62]. |
| Chitosan-based | High functional versatility and responsiveness to tumor microenvironment stimuli [65,66,67]. | Reduced effectiveness against certain Gram-negative bacteria or structurally resistant cells [64]. |
| Fibroin-based | Exceptional biodegradability and low immunogenicity [69]. | Structural sensitivity to environmental conditions affecting therapeutic consistency [68]. |
| Polyethyleneimine-based (PEI) | Strong functional interactions facilitating effective drug loading [69]. | Potential cytotoxicity requiring additional modifications to improve biocompatibility [71]. |
| PLGA-based | High versatility in encapsulation of diverse therapeutic molecules [73,74]. | Potential interference in combined PS-drug systems (e.g., energy transfer issues) [74]. |
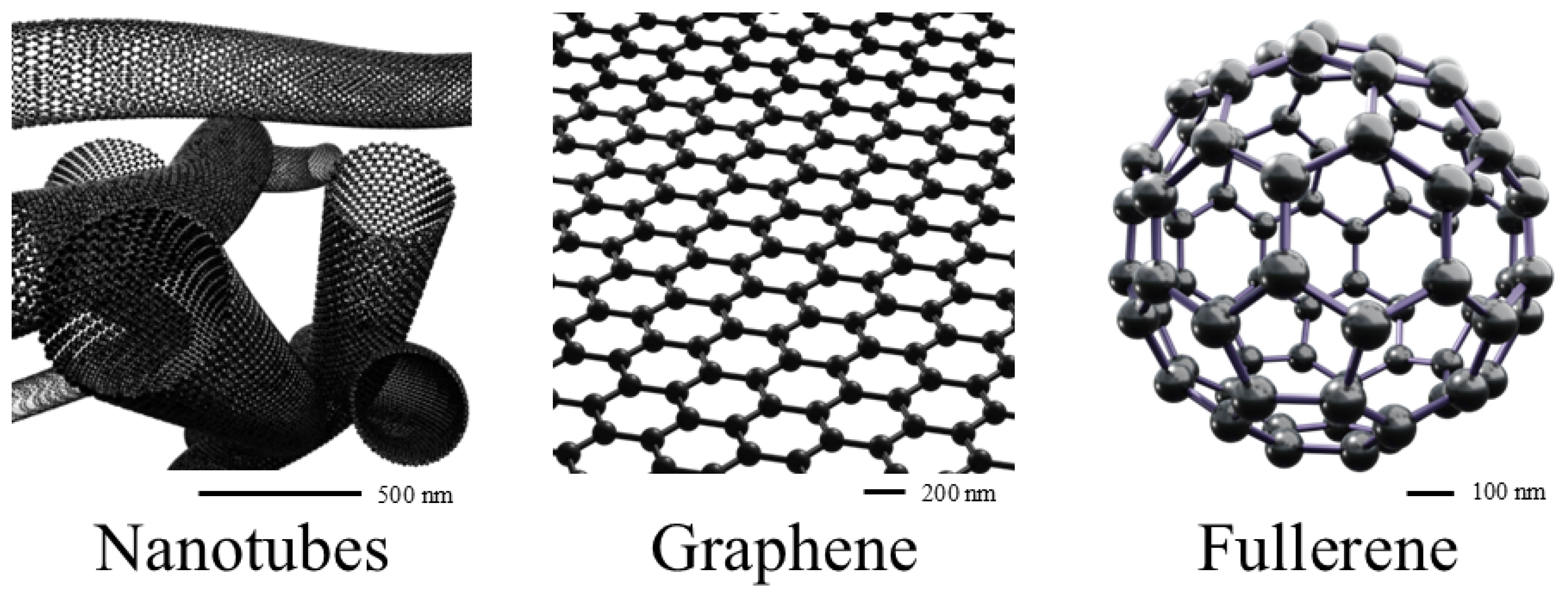
4. Carbon-Based Nanocarriers
4.1. Chlorins and Graphene
4.2. Chlorins and Carbon Quantum Dots
| Type of Nanoparticle | Advantages | Disadvantages |
|---|---|---|
| Graphene Oxide (GO) | Excellent biocompatibility and functionalization potential (e.g., folic acid targeting) [81]. | Stability issues due to possible aggregation or non-specific interactions in biological media [83]. |
| Graphene Quantum Dots (GQDs) | Improved solubility and efficient loading of poorly soluble natural PS (e.g., curcumin) [79]. | Complex preparation methods and potential variability in physicochemical properties [79]. |
| Metal–Organic Frameworks (MOFs) | Enhanced catalytic ability to produce ROS (e.g., iron-based Fenton-like reactions) [80]. | Possible heavy metal-associated cytotoxicity, necessitating careful biocompatibility evaluation [80]. |
| Carbon Quantum Dots (CQDs) | Ability to incorporate multiple functions, including targeted drug delivery and ROS generation [84]. | Potentially inconsistent photodynamic efficacy depending on preparation methods and precursor selection [84]. |
| Black Phosphorus Quantum Dots (BPQDs) | High photothermal conversion efficiency for combined PTT/PDT applications [85]. | Instability and rapid degradation under physiological conditions [85]. |
| Metal-containing Quantum Dots (CdSe/ZnS) | Efficient energy transfer (FRET) for enhanced PS activation [87]. | Concerns regarding heavy-metal toxicity and long-term biocompatibility [87]. |
5. Nanospheres
6. In Vivo Research Progress
6.1. Temoporfin
6.2. Chlorin e6
7. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PDT | photodynamic therapy |
| PS | photosensitizer |
| PLGA | Poly Lactic-co-Glycolic Acid |
| ROS | reactive oxygen species |
| Ce6 | chlorin e6 |
| mTHPC | temoporfin |
| NP | nanoparticles |
| aPDT | Antimicrobial Photodynamic Therapy |
| TGF-β1 | Transforming Growth Factor beta 1 |
| PACT | Photodynamic Antimicrobial Chemotherapy |
| MB | methylene blue |
| PTX | paclitaxel |
| CAT | catalase |
| 3BP | 3-bromopyruvate |
| TME | tumor microenvironment |
| AQ4N | banoxantrone dihydrochloride |
| MIC | Minimal Inhibitory Concentration |
| MBC | Minimal Bactericidal Concentration |
| NIR | near infrared light |
| MRSA | methicillin-resistant Staphylococcus aureus |
| UCON | ultra-small copper oxide |
| PEG | polyethylene glycol |
| SRF | sorafenib |
| CPT | camptothecin |
| PFCEs | perfluorinated crown ethers |
| GSH | glutathione |
| PBC | polymer-based complexes |
| PPDO | Poly(p-dioxanone) |
| PHB | Poly(hydroxybutyrate) |
| PHBV | Poly(hydroxybutyrate-co-hydroxyvalerate) |
| PBS | Poly(butylene succinate) |
| TNBC | triple-negative breast cancer |
| CMC | carboxymethyl cellulose |
| PC | pectin |
| SA | sodium alginate |
| Cp6 | chlorin p6 |
| HPCS | hydroxypropyl chitosan |
| SF | silk fibroin |
| DA | dopamine |
| CDI | CMC-polydopamine-NaI |
| PTT | photothermal therapy |
| PEI | polyethylenimine |
| GQDs | graphene quantum dots |
| HM | hybrid membranes |
| ICG | indocyanine green |
| FRET | fluorescence resonance energy transfer |
| CBNC | carbon-based nanocarriers |
| CD | carbon-dot-based |
| MOFs | metal–organic frameworks |
| GO | graphene oxide |
| CB[7] | cucurbit[7]uril |
| OX | oxaliplatin |
| HA | hyaluronic acid |
| QDs | quantum dots |
| BP | black phosphorus |
| EV | extracellular vesicles |
| TPL | triptolide |
| MSN | mesoporous silica nanospheres |
| PCL-NPs | biocompatible dextran based—multi-component nanomedicine |
| BALB/c | albino, laboratory-bred strain of the house mouse |
| DNA | deoxyribonucleic acid |
| 4T1 | human breast cancer cell line |
| WHO | World Health Organization |
| FDA | Food and Drug Administration |
| BCC | basal cell carcinoma |
| RDT | radiodynamic therapy |
| PLS | Pliss lymphosarcoma |
References
- Raab, O. Ueber Die Wirkung Fluorescirender Stoffe Auf Infusorien; R. Oldenbourg: München, Germany, 1900. [Google Scholar]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past Is Prologue. Photochem Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Active, Recruiting and Not yet Recruiting Clinical Trials for Photodynamic Therapy. 2025. Available online: http://clinicaltrials.gov (accessed on 20 May 2025).
- Ji, B.; Wei, M.; Yang, B. Recent Advances in Nanomedicines for Photodynamic Therapy (PDT)-Driven Cancer Immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Abrahamse, H. Oxygen-Independent Antimicrobial Photoinactivation: Type III Photochemical Mechanism? Antibiotics 2020, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Plekhova, N.; Shevchenko, O.; Korshunova, O.; Stepanyugina, A.; Tananaev, I.; Apanasevich, V. Development of Novel Tetrapyrrole Structure Photosensitizers for Cancer Photodynamic Therapy. Bioengineering 2022, 9, 82. [Google Scholar] [CrossRef]
- Silva, E.F.F.; Serpa, C.; Dąbrowski, J.M.; Monteiro, C.J.P.; Formosinho, S.J.; Stochel, G.; Urbanska, K.; Simões, S.; Pereira, M.M.; Arnaut, L.G. Mechanisms of Singlet-Oxygen and Superoxide-Ion Generation by Porphyrins and Bacteriochlorins and Their Implications in Photodynamic Therapy. Chem. Eur. J. 2010, 16, 9273–9286. [Google Scholar] [CrossRef]
- Boris, M. Magnetic Properties of Reactive Oxygen Species and Their Possible Role in Cancer Therapy. Arch. Cancer Sci. Ther. 2024, 8, 048–053. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Y.; He, S.; Wu, S.; Yang, C. Singlet Oxygen: Properties, Generation, Detection, and Environmental Applications. J. Hazard. Mater. 2024, 461, 132538. [Google Scholar] [CrossRef]
- Minaev, B.F. Electronic Mechanisms of Activation of Molecular Oxygen. Russ. Chem. Rev. 2007, 76, 1059–1083. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, J.; Long, S.; Zhao, X.; Li, H.; Du, J.; Shao, K.; Peng, X. The Concept and Examples of Type-III Photosensitizers for Cancer Photodynamic Therapy. Chem 2022, 8, 197–209. [Google Scholar] [CrossRef]
- Luo, T.; Ni, K.; Culbert, A.; Lan, G.; Li, Z.; Jiang, X.; Kaufmann, M.; Lin, W. Nanoscale Metal–Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 7334–7339. [Google Scholar] [CrossRef]
- Beltukova, D.M.; Belik, V.P.; Chudakov, K.A.; Smirnov, O.V.; Semenova, I.V.; Vasyutinskii, O.S. Time-Resolved Phosphorescence Analysis of Singlet Oxygen Generation and Radachlorin/Ce6 Triplet State Quenching in Solutions with Albumin. Chem. Phys. Lett. 2025, 861, 141826. [Google Scholar] [CrossRef]
- Dias, L.D.; Mfouo-Tynga, I.S. Learning from Nature: Bioinspired Chlorin-Based Photosensitizers Immobilized on Carbon Materials for Combined Photodynamic and Photothermal Therapy. Biomimetics 2020, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Geng, X.; Wang, Y.; Su, X.; Han, R.; Wang, J.; Li, X.; Wang, P.; Zhang, K.; Wang, X. Highly Efficient Water-Soluble Photosensitizer Based on Chlorin: Synthesis, Characterization, and Evaluation for Photodynamic Therapy. ACS Pharmacol. Transl. Sci. 2021, 4, 802–812. [Google Scholar] [CrossRef]
- Warszyńska, M.; Repetowski, P.; Dąbrowski, J.M. Photodynamic Therapy Combined with Immunotherapy: Recent Advances and Future Research Directions. Coord. Chem. Rev. 2023, 495, 215350. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Arnaut, L.G.; Pereira, M.M.; Monteiro, C.J.P.; Urbańska, K.; Simões, S.; Stochel, G. New Halogenated Water-Soluble Chlorin and Bacteriochlorin as Photostable PDT Sensitizers: Synthesis, Spectroscopy, Photophysics, and in Vitro Photosensitizing Efficacy. ChemMedChem 2010, 5, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.I.; Pereira, N.A.M.; Cardoso, A.L.; Nascimento, B.F.O.; Pineiro, M.; Schaberle, F.A.; Gomes-da-Silva, L.C.; Brito, R.M.M.; Pinho E Melo, T.M.V.D. Novel Foscan®-Derived Ring-Fused Chlorins for Photodynamic Therapy of Cancer. Bioorganic Med. Chem. 2023, 93, 117443. [Google Scholar] [CrossRef]
- Ambrósio, J.A.R.; Pinto, B.C.D.S.; Da Silva, B.G.M.; Passos, J.C.D.S.; Beltrame Junior, M.; Costa, M.S.; Simioni, A.R. BSA Nanoparticles Loaded-Methylene Blue for Photodynamic Antimicrobial Chemotherapy (PACT): Effect on Both Growth and Biofilm Formation by Candida albicans. J. Biomater. Sci. Polym. Ed. 2020, 31, 2182–2198. [Google Scholar] [CrossRef]
- He, H.; Du, L.; Xue, H.; Wu, J.; Shuai, X. Programmable Therapeutic Nanoscale Covalent Organic Framework for Photodynamic Therapy and Hypoxia-Activated Cascade Chemotherapy. Acta Biomater. 2022, 149, 297–306. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, H.; Guo, Y.; Tang, J.; Liu, C.; Li, L.; Yao, C.; Yang, D. A Programmable Hybrid DNA Nanogel for Enhanced Photodynamic Therapy of Hypoxic Glioma. Trans. Tianjin Univ. 2020, 26, 450–457. [Google Scholar] [CrossRef]
- Han, L.; Huang, X.; Zhu, H.; Wang, R.; Zhao, B.; Liu, S.; Li, Q.; Qian, W.; Feng, F.; Liu, F.; et al. Programmed Cyclodextrin-Based Core–Shell Nanoparticles for Cooperative TGF-β Blockade to Reverse Immunosuppression Post Photodynamic Therapy. Chem. Eng. J. 2023, 455, 140830. [Google Scholar] [CrossRef]
- Uthaman, S.; Kim, Y.; Lee, J.Y.; Pillarisetti, S.; Huh, K.M.; Park, I.-K. Self-Quenched Polysaccharide Nanoparticles with a Reactive Oxygen Species-Sensitive Cascade for Enhanced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 28004–28013. [Google Scholar] [CrossRef] [PubMed]
- Dlugaszewska, J.; Szczolko, W.; Koczorowski, T.; Skupin-Mrugalska, P.; Teubert, A.; Konopka, K.; Kucinska, M.; Murias, M.; Düzgüneş, N.; Mielcarek, J.; et al. Antimicrobial and Anticancer Photodynamic Activity of a Phthalocyanine Photosensitizer with N -Methyl Morpholiniumethoxy Substituents in Non-Peripheral Positions. J. Inorg. Biochem. 2017, 172, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Parihar, A.S.; Modi, S.; Kalra, A. Photodynamic Therapy for ESKAPE Pathogens: An Emerging Approach to Combat Antimicrobial Resistance (AMR). Microb. Pathog. 2023, 183, 106307. [Google Scholar] [CrossRef] [PubMed]
- Glowacka-Sobotta, A.; Ziental, D.; Czarczynska-Goslinska, B.; Michalak, M.; Wysocki, M.; Güzel, E.; Sobotta, L. Nanotechnology for Dentistry: Prospects and Applications. Nanomaterials 2023, 13, 2130. [Google Scholar] [CrossRef]
- Shabangu, S.M.; Babu, B.; Soy, R.C.; Oyim, J.; Amuhaya, E.; Nyokong, T. Susceptibility of Staphylococcus Aureus to Porphyrin-Silver Nanoparticle Mediated Photodynamic Antimicrobial Chemotherapy. J. Lumin. 2020, 222, 117158. [Google Scholar] [CrossRef]
- Linares, I.A.P.; Uría, M.S.; Graminha, M.A.S.; Iglesias, B.A.; Velásquez, A.M.A. Antileishmanial Activity of Tetra-Cationic Porphyrins with Peripheral Pt(II) and Pd(II) Complexes Mediated by Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2023, 42, 103641. [Google Scholar] [CrossRef]
- Khorsandi, K.; Fekrazad, S.; Vahdatinia, F.; Farmany, A.; Fekrazad, R. Nano Antiviral Photodynamic Therapy: A Probable Biophysicochemical Management Modality in SARS-CoV-2. Expert Opin. Drug Deliv. 2021, 18, 265–272. [Google Scholar] [CrossRef]
- Prandini, J.A.; Castro, K.A.D.F.; Biazzotto, J.C.; Brancini, G.T.P.; Tomé, J.P.C.; Lourenço, L.M.O.; Braga, G.Ú.L.; Da Silva, R.S. Thiopyridinium Phthalocyanine for Improved Photodynamic Efficiency against Pathogenic Fungi. J. Photochem. Photobiol. B Biol. 2022, 231, 112459. [Google Scholar] [CrossRef]
- Ziental, D.; Wysocki, M.; Michalak, M.; Dlugaszewska, J.; Güzel, E.; Sobotta, L. The Dual Synergy of Photodynamic and Sonodynamic Therapy in the Eradication of Methicillin-Resistant Staphylococcus Aureus. Appl. Sci. 2023, 13, 3810. [Google Scholar] [CrossRef]
- Sabino, C.P.; Ribeiro, M.S.; Wainwright, M.; Dos Anjos, C.; Sellera, F.P.; Dropa, M.; Nunes, N.B.; Brancini, G.T.P.; Braga, G.U.L.; Arana-Chavez, V.E.; et al. The Biochemical Mechanisms of Antimicrobial Photodynamic Therapy †. Photochem. Photobiol. 2023, 99, 742–750. [Google Scholar] [CrossRef]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Nakonieczna, J.; Grinholc, M. Development of Antimicrobial Phototreatment Tolerance: Why the Methodology Matters. Int. J. Mol. Sci. 2021, 22, 2224. [Google Scholar] [CrossRef] [PubMed]
- Funari, R.; Shen, A.Q. Detection and Characterization of Bacterial Biofilms and Biofilm-Based Sensors. ACS Sens. 2022, 7, 347–357. [Google Scholar] [CrossRef]
- Garcia De Carvalho, G.; Pacheco Mateo, R.; Costa E Silva, R.; Maquera Huacho, P.M.; De Souza Rastelli, A.N.; De Oliveira, K.T.; Chierici Marcantonio, R.A.; Zandim-Barcelos, D.L.; Palomari Spolidorio, D.M. Chlorin-Based Photosensitizer under Blue or Red-Light Irradiation against Multi-Species Biofilms Related to Periodontitis. Photodiagnosis Photodyn. Ther. 2023, 41, 103219. [Google Scholar] [CrossRef]
- Glowacka-Sobotta, A.; Wrotynski, M.; Kryjewski, M.; Sobotta, L.; Mielcarek, J. Porphyrinoids in Photodynamic Diagnosis and Therapy of Oral Diseases. J. Porphyr. Phthalocyanines 2019, 23, 1–10. [Google Scholar] [CrossRef]
- Araújo, J.L.; Da Silva, P.B.; Fonseca-Santos, B.; Báo, S.N.; Chorilli, M.; De Souza, P.E.N.; Muehlmann, L.A.; Azevedo, R.B. Photodynamic Therapy Directed to Melanoma Skin Cancer by Thermosensitive Hydrogel Containing Chlorophyll A. Pharmaceuticals 2023, 16, 1659. [Google Scholar] [CrossRef] [PubMed]
- Diamond, I.; Granelli, S.G.; McDonagh, A.F.; Nielsen, S.; Wilson, C.B.; Jaenicke, R. Photodynamic Therapy of Malignant Tumours. Lancet 1972, 2, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation Therapy for the Treatment of Malignant Tumors. Cancer Res 1978, 38, 2628–2635. [Google Scholar]
- Wang, Y.; Xie, Y.; Li, J.; Peng, Z.-H.; Sheinin, Y.; Zhou, J.; Oupický, D. Tumor-Penetrating Nanoparticles for Enhanced Anticancer Activity of Combined Photodynamic and Hypoxia-Activated Therapy. ACS Nano 2017, 11, 2227–2238. [Google Scholar] [CrossRef]
- Brown, J.; Wilson, B. Brown JM, Wilson WR.. Exploiting Tumor Hypoxia in Cancer Treatment. Nat. reviews. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Iqbal, Z.; Chen, J.; Chen, Z.; Huang, M. Phthalocyanine-Biomolecule Conjugated Photosensitizers for Targeted Photodynamic Therapy and Imaging. Curr. Drug Metab. 2015, 16, 816–832. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Azevedo, R.B.; Feron, O.; Amorim, C.A. Photodynamic Cancer Therapy Using Liposomes as an Advanced Vesicular Photosensitizer Delivery System. J. Control. Release 2021, 339, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Enzian, P.; Schell, C.; Link, A.; Malich, C.; Pries, R.; Wollenberg, B.; Rahmanzadeh, R. Optically Controlled Drug Release from Light-Sensitive Liposomes with the New Photosensitizer 5,10-DiOH. Mol. Pharm. 2020, 17, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Tararina, L.A.; Ageev, V.P.; Brodovskaya, E.P.; Balashov, V.P.; Maev, I.V.; Zaborovskii, A.V.; Yunina, D.V.; Pyataev, N.A. Comparative Study of the Photocytotoxicity of Two Liposomal Forms of Chlorin E6. Pharm. Chem. J. 2023, 57, 1356–1361. [Google Scholar] [CrossRef]
- Krivosheeva, O.P.; Doctor, M.A.; Larkina, E.A.; Vedenkin, A.S.; Nikolskaya, T.A. Effect of Substituents in Chlorin e Derivatives on the Loading Efficiency of the Photosensitizer into the Liposome Membrane and Their Biological Activity. Photodiagnosis Photodyn. Ther. 2023, 42, 103328. [Google Scholar] [CrossRef]
- Alimu, G.; Yan, T.; Zhu, L.; Du, Z.; Ma, R.; Fan, H.; Chen, S.; Alifu, N.; Zhang, X. Liposomes Loaded with Dual Clinical Photosensitizers for Enhanced Photodynamic Therapy of Cervical Cancer. RSC Adv. 2023, 13, 3459–3467. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, C.; Wang, C.; Jiang, J.; Tang, F.; Li, C.; Yuan, H.; Yang, X.; Tong, Z.; Huang, Y. Lutein-Based pH and Photo Dual-Responsive Novel Liposomes Coated with Ce6 and PTX for Tumor Therapy. ACS Omega 2023, 8, 31436–31449. [Google Scholar] [CrossRef]
- Cong, C.; He, Y.; Zhao, S.; Zhang, X.; Li, L.; Wang, D.; Liu, L.; Gao, D. Diagnostic and Therapeutic Nanoenzymes for Enhanced Chemotherapy and Photodynamic Therapy. J. Mater. Chem. B 2021, 9, 3925–3934. [Google Scholar] [CrossRef]
- Li, X.; Man, J.; Hu, H.; Ye, J.; Jin, Q. Oxygen-Economizing Liposomes for Synergistic Photodynamic and Starvation Therapy. Colloid Interface Sci. Commun. 2022, 47, 100598. [Google Scholar] [CrossRef]
- Wang, C.; Liang, C.; Hao, Y.; Dong, Z.; Zhu, Y.; Li, Q.; Liu, Z.; Feng, L.; Chen, M. Photodynamic Creation of Artificial Tumor Microenvironments to Collectively Facilitate Hypoxia-Activated Chemotherapy Delivered by Coagulation-Targeting Liposomes. Chem. Eng. J. 2021, 414, 128731. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Li, X.; Li, K.; Zhang, G.; Hong, W. Photodynamic Cationic Ultrasmall Copper Oxide Nanoparticles-Loaded Liposomes for Alleviation of MRSA Biofilms. Int. J. Nanomed. 2023, 18, 5441–5455. [Google Scholar] [CrossRef]
- Guo, Z.; Wong, K.H.; Li, E.; Zhou, X.; Jiang, D.; Gao, J.; Chen, M. Co-Delivery of Dimeric Camptothecin and Chlorin E6 via Polypeptide-Based Micelles for Chemo-Photodynamic Synergistic Therapy. Chin Med 2023, 18, 133. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, P.; Wang, L.; Chen, X.; Zhou, Y.; Huang, W.; Yan, D. ROS-responsive Thioether-containing Hyperbranched Polymer Micelles for Light-triggered Drug Release. SmartMat 2022, 3, 522–531. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Qiu, H.; Huang, K.; Xu, Q.; Zhou, B.; Zhang, L.; Zhou, M.; Yi, X. A Co-Delivery System Based on Chlorin E6-Loaded ROS-Sensitive Polymeric Prodrug with Self-Amplified Drug Release to Enhance the Efficacy of Combination Therapy for Breast Tumor Cells. Front. Bioeng. Biotechnol. 2023, 11, 1168192. [Google Scholar] [CrossRef]
- Shu, M.; Tang, J.; Chen, L.; Zeng, Q.; Li, C.; Xiao, S.; Jiang, Z.; Liu, J. Tumor Microenvironment Triple-Responsive Nanoparticles Enable Enhanced Tumor Penetration and Synergetic Chemo-Photodynamic Therapy. Biomaterials 2021, 268, 120574. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, X.; Luo, W.; Mo, Y.; Dai, C.; Zhu, L. PEGA-BA@Ce6@PFCE Micelles as Oxygen Nanoshuttles for Tumor Hypoxia Relief and Enhanced Photodynamic Therapy. Molecules 2023, 28, 6697. [Google Scholar] [CrossRef]
- Paul, M.; Itoo, A.M.; Ghosh, B.; Biswas, S. Hypoxia Alleviating Platinum(IV)/Chlorin E6-Based Combination Chemotherapeutic-Photodynamic Nanomedicine for Oropharyngeal Carcinoma. J. Photochem. Photobiol. B Biol. 2023, 238, 112627. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, T.; Mu, N.; Lam, H.W.; Sun, C.; Yue, L.; Cheng, Q.; Gao, C.; Yuan, Z.; Wang, R. Supramolecular Micelles as Multifunctional Theranostic Agents for Synergistic Photodynamic Therapy and Hypoxia-Activated Chemotherapy. Acta Biomater. 2021, 131, 483–492. [Google Scholar] [CrossRef]
- Adhikari, C. Polymer Nanoparticles-Preparations, Applications and Future Insights: A Concise Review. Polym.-Plast. Technol. Mater. 2021, 60, 1–29. [Google Scholar] [CrossRef]
- Liu, E.-J.; Huang, J.-X.; Hu, R.-Z.; Yao, X.-H.; Zhao, W.-G.; Zhang, D.-Y.; Chen, T. A Hierarchical Porous Cellulose Sponge Modified with Chlorogenic Acid as a Antibacterial Material for Water Disinfection. Sustainability 2022, 15, 773. [Google Scholar] [CrossRef]
- Sharma, M.; Dube, A.; Majumder, S.K. Antibacterial Photodynamic Activity of Photosensitizer-Embedded Alginate-Pectin-Carboxymethyl Cellulose Composite Biopolymer Films. Lasers Med. Sci. 2020, 36, 763–772. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Zhang, Z.; Zhao, J.; Yuan, Y.; Wang, S. Oxidation Triggered Formation of Polydopamine-Modified Carboxymethyl Cellulose Hydrogel for Anti-Recurrence of Tumor. Colloids Surf. B Biointerfaces 2021, 207, 112025. [Google Scholar] [CrossRef]
- Yue, L.; Zheng, M.; Wang, M.; Khan, I.M.; Ding, X.; Zhang, Y.; Wang, Z. Water-Soluble Chlorin E6-Hydroxypropyl Chitosan as a High-Efficiency Photoantimicrobial Agent against Staphylococcus Aureus. Int. J. Biol. Macromol. 2022, 208, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.; Kim, T.G.; Kim, T.; Kim, D.; Kim, D.-H.; Jo, J.; Lee, Y.-J.; Jeong, Y.-I. Phenethyl Isothiocyanate-Conjugated Chitosan Oligosaccharide Nanophotosensitizers for Photodynamic Treatment of Human Cancer Cells. Int. J. Mol. Sci. 2022, 23, 13802. [Google Scholar] [CrossRef]
- Zhu, T.; Shi, L.; Ma, C.; Xu, L.; Yang, J.; Zhou, G.; Zhu, X.; Shen, L. Fluorinated Chitosan-Mediated Intracellular Catalase Delivery for Enhanced Photodynamic Therapy of Oral Cancer. Biomater. Sci. 2021, 9, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-O.; Kook, M.-S.; Jeong, Y.-I.; Park, M.-J.; Yang, S.-W.; Kim, B.-H. Nanophotosensitizers Composed of Phenyl Boronic Acid Pinacol Ester-Conjugated Chitosan Oligosaccharide via Thioketal Linker for Reactive Oxygen Species-Sensitive Delivery of Chlorin E6 against Oral Cancer Cells. Materials 2022, 15, 7057. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.U.D.; Zargar, M.I.; Masoodi, M.H.; Alshehri, S.; Alam, P.; Ghoneim, M.M.; Alshlowi, A.; Shivakumar, H.G.; Ali, M.; Shakeel, F. Silk Fibroin as an Efficient Biomaterial for Drug Delivery, Gene Therapy, and Wound Healing. Int. J. Mol. Sci. 2022, 23, 14421. [Google Scholar] [CrossRef]
- Lin, X.; Cai, L.; Cao, X.; Zhao, Y. Stimuli-responsive Silk Fibroin for On-demand Drug Delivery. Smart Med. 2023, 2, e20220019. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; He, C.; Ling, J. Electrospun Fibers Based Anisotropic Silk Fibroin Film with Photodynamic Antibacterial Therapy for S. Aureus Infected Wound Healing. Int. J. Biol. Macromol. 2024, 254, 127685. [Google Scholar] [CrossRef]
- Mohammadi, N.; Fayazi Hosseini, N.; Nemati, H.; Moradi-Sardareh, H.; Nabi-Afjadi, M.; Kardar, G.A. Revisiting of Properties and Modified Polyethylenimine-Based Cancer Gene Delivery Systems. Biochem. Genet. 2024, 62, 18–39. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Xu, Y.; Zhao, L.; Wang, L.; Yin, Z.; Li, H.; Tan, H.; Liu, K. A Dual Enhanced Anti-Bacterial Strategy Based on High Chlorin E6-Loaded Polyethyleneimine Functionalized Graphene. RSC Adv. 2021, 11, 739–744. [Google Scholar] [CrossRef]
- Fan, J.; Qin, Y.; Xiao, C.; Yuan, L.; Long, Y.; Zhao, Y.; Nguyen, W.; Chen, S.; Chen, W.; Liu, X.; et al. Biomimetic PLGA-Based Nanocomplexes for Improved Tumor Penetration to Enhance Chemo-Photodynamic Therapy against Metastasis of TNBC. Mater. Today Adv. 2022, 16, 100289. [Google Scholar] [CrossRef]
- Shen, J.; Chen, D.; Liu, Y.; Gao, G.; Liu, Z.; Wang, G.; Wu, C.; Fang, X. A Biodegradable Nano-Photosensitizer with Photoactivatable Singlet Oxygen Generation for Synergistic Phototherapy. J. Mater. Chem. B 2021, 9, 4826–4831. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Nie, X.; Zeng, W.; Zhang, Y.; Deng, Y.; Chen, H.; Zeng, X.; Ma, H.; Zheng, Y.; et al. pH-Sensitive and Charge-Reversal Polymeric Nanoplatform Enhanced Photothermal/Photodynamic Synergistic Therapy for Breast Cancer. Front. Bioeng. Biotechnol. 2022, 10, 836468. [Google Scholar] [CrossRef]
- Huang, T.; Xu, X.; Cheng, C.; Wang, J.; Yang, L. Cooperative Phototherapy Based on Bimodal Imaging Guidance for the Treatment of Uveal Melanoma. J. Nanobiotechnol. 2023, 21, 146. [Google Scholar] [CrossRef]
- Lagos, K.J.; Buzzá, H.H.; Bagnato, V.S.; Romero, M.P. Carbon-Based Materials in Photodynamic and Photothermal Therapies Applied to Tumor Destruction. Int. J. Mol. Sci. 2021, 23, 22. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Q.; Nan, F.; Wang, J.; Liang, K.; Li, J.; Xue, X.; Ren, H.; Liu, W.; Ge, J.; et al. Carbon Dots Nanophotosensitizers with Tunable Reactive Oxygen Species Generation for Mitochondrion-Targeted Type I/II Photodynamic Therapy. Biomaterials 2023, 293, 121953. [Google Scholar] [CrossRef]
- Mushtaq, S.; Yasin, T.; Saleem, M.; Dai, T.; Yameen, M.A. Potentiation of Antimicrobial Photodynamic Therapy by Curcumin-Loaded Graphene Quantum Dots. Photochem. Photobiol. 2022, 98, 202–210. [Google Scholar] [CrossRef]
- Sui, C.; Tan, R.; Chen, Y.; Yin, G.; Wang, Z.; Xu, W.; Li, X. MOFs-Derived Fe–N Codoped Carbon Nanoparticles as O2-Evolving Reactor and ROS Generator for CDT/PDT/PTT Synergistic Treatment of Tumors. Bioconjugate Chem. 2021, 32, 318–327. [Google Scholar] [CrossRef]
- Guo, S.; Song, Z.; Ji, D.-K.; Reina, G.; Fauny, J.-D.; Nishina, Y.; Ménard-Moyon, C.; Bianco, A. Combined Photothermal and Photodynamic Therapy for Cancer Treatment Using a Multifunctional Graphene Oxide. Pharmaceutics 2022, 14, 1365. [Google Scholar] [CrossRef] [PubMed]
- Işıklan, N.; Hussien, N.A.; Türk, M. Multifunctional Aptamer-Conjugated Magnetite Graphene Oxide/Chlorin E6 Nanocomposite for Combined Chemo-Phototherapy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128841. [Google Scholar] [CrossRef]
- Ding, Y.-F.; Kwong, C.H.T.; Li, S.; Pan, Y.-T.; Wei, J.; Wang, L.-H.; Mok, G.S.P.; Wang, R. Supramolecular Nanomedicine Derived from Cucurbit [7]Uril-Conjugated Nano-Graphene Oxide for Multi-Modality Cancer Therapy. Biomater. Sci. 2021, 9, 3804–3813. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shen, J.; Ma, Y.; Qin, X.; Qin, X.; Yang, F.; Ostrikov, K.; Zhang, Q.; He, J.; Zhong, X. Cancer-targeting Carbon Quantum Dots Synthesized by Plasma Electrochemical Method for Red-light-activated Photodynamic Therapy. Plasma Process. Polym. 2023, 21, e2300174. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Li, X.; Ge, M.; Liu, S.; Li, S.; Li, J.; Ding, J.; Ragauskas, A.J.; Sun, W.; et al. A Photosensitive Sustainable Lignin Nanoplatform for Multimodal Image-Guided Mitochondria-Targeted Photodynamic and Photothermal Therapy. Mater. Today Chem. 2022, 26, 101000. [Google Scholar] [CrossRef]
- He, M.; Cheng, Z.; Wang, Z.; Li, M.; Liang, H.; Liu, H.; Yu, L.; Zhao, L.; Yu, F. Controllable Regulation of Ag2S Quantum-Dot-Mediated Protein Nanoassemblies for Imaging-Guided Synergistic PDT/PTT/Chemotherapy against Hypoxic Tumor. Adv Healthc. Mater. 2023, 12, 2300752. [Google Scholar] [CrossRef] [PubMed]
- Sangtani, A.; Nag, O.K.; Oh, E.; Stewart, M.H.; Delehanty, J.B. Quantum Dot-Enabled Membrane-Tethering and Enhanced Photoactivation of Chlorin-E6. J. Nanopart. Res. 2021, 23, 159. [Google Scholar] [CrossRef]
- Sheng, Y.; Ren, Q.; Tao, C.; Wen, M.; Qu, P.; Yu, N.; Li, M.; Chen, Z.; Xie, X. Construction of PEGylated Chlorin e6@CuS-Pt Theranostic Nanoplatforms for Nanozymes-Enhanced Photodynamic-Photothermal Therapy. J. Colloid Interface Sci. 2023, 645, 122–132. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, K.; Yang, X.; Liu, M.; Cui, Y.; Li, Y.; Li, C.; Liu, S.; Lu, Y.; Zhang, Z.; et al. Embedding Atomically Dispersed Manganese/Gadolinium Dual Sites in Oxygen Vacancy-Enriched Biodegradable Bimetallic Silicate Nanoplatform for Potentiating Catalytic Therapy. Adv. Sci. 2024, 11, 2307424. [Google Scholar] [CrossRef]
- Palanikumar, L.; Kalmouni, M.; Houhou, T.; Abdullah, O.; Ali, L.; Pasricha, R.; Straubinger, R.; Thomas, S.; Afzal, A.J.; Barrera, F.N.; et al. pH-Responsive Upconversion Mesoporous Silica Nanospheres for Combined Multimodal Diagnostic Imaging and Targeted Photodynamic and Photothermal Cancer Therapy. ACS Nano 2023, 17, 18979–18999. [Google Scholar] [CrossRef]
- Buchholz, J.; Wergin, M.; Walt, H.; Gräfe, S.; Bley, C.R.; Kaser-Hotz, B. Photodynamic Therapy of Feline Cutaneous Squamous Cell Carcinoma Using a Newly Developed Liposomal Photosensitizer: Preliminary Results Concerning Drug Safety and Efficacy. Vet. Intern. Med. 2007, 21, 770–775. [Google Scholar] [CrossRef]
- Millard, M.; Posty, S.; Piffoux, M.; Jasniewski, J.; Lassalle, H.-P.; Yakavets, I.; Gazeau, F.; Wilhelm, C.; Silva, A.K.A.; Bezdetnaya, L. mTHPC-Loaded Extracellular Vesicles Significantly Improve mTHPC Diffusion and Photodynamic Activity in Preclinical Models. Pharmaceutics 2020, 12, 676. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, S.; Li, J.; He, C.; Cheng, Y.; Li, N.; Wang, Y.; Wu, Y.; Zhang, J. Self-Amplified pH/ROS Dual-Responsive Co-Delivery Nano-System with Chemo-Photodynamic Combination Therapy in Hepatic Carcinoma Treatment. Int. J. Nanomed. 2024, 19, 3737–3751. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Shi, Z.; Ou, M.; Li, Y.; Huang, L.; Wang, W.; Huang, Q.; Li, M.; Chen, C.; Zeng, X.; et al. Dextran-Based Micelles for Combinational Chemo-Photodynamic Therapy of Tumors via in Vivo Chemiluminescence. Carbohydr. Polym. 2023, 319, 121192. [Google Scholar] [CrossRef] [PubMed]
- Artsemyeva, T.P.; Tzerkovsky, D.A. Efficacy of Photodynamic Therapy with Chlorine-Based Photosensitizer in the Treatment of Basal Cell Carcinomas. J. Anal. Oncol. 2023, 12, 15–23. [Google Scholar] [CrossRef]
- Cui, J.; Makita, Y.; Okamura, T.; Ikeda, C.; Fujiwara, S.; Tominaga, K. Near-Infrared Light Photodynamic Therapy with PEI-Capped Up-Conversion Nanoparticles and Chlorin E6 Induces Apoptosis of Oral Cancer Cells. J. Funct. Biomater. 2024, 15, 333. [Google Scholar] [CrossRef]
- Tzerkovsky, D.A.; Mazurenko, A.N.; Kozlovsky, D.I.; Borychevsky, F.F. Radiodynamic Therapy with Chlorine-Based Photosensitizer on Pliss Lymphosarcoma Solid Tumor: In Vivo Experiment. J. Anal. Oncol. 2022, 11, 33–38. [Google Scholar] [CrossRef]

| Type of Nanoparticle | Advantages | Disadvantages |
|---|---|---|
| Copper(II) sulfide-based nanospheres (CuS-Pt) | Synergistic photothermal and photodynamic action enhanced by CAT-like platinum activity [88]. | Potential stability issues due to complex surface modifications [88]. |
| Mesoporous silica-based nanospheres (Mn-Gd MSNs) | Multifunctional catalytic activity (CAT-, oxidase-, peroxidase-like) enhancing ROS generation and PDT efficiency [89]. | Complex preparation procedure and potential concerns regarding biocompatibility of multiple metals [89]. |
| Lanthanide-doped MSN-based nanospheres (NaYF4:Er,Yb,Gd@Bi2Se3) | Efficient NIR-triggered simultaneous PDT/PTT with controlled Ce6 release [90]. | Risk of toxicity and stability issues due to lanthanide and heavy metal core components [90]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, M.; Szymczyk, J.; Pawska, A.; Wysocki, M.; Janiak, D.; Ziental, D.; Ptaszek, M.; Güzel, E.; Sobotta, L. Chlorin Activity Enhancers for Photodynamic Therapy. Molecules 2025, 30, 2810. https://doi.org/10.3390/molecules30132810
Michalak M, Szymczyk J, Pawska A, Wysocki M, Janiak D, Ziental D, Ptaszek M, Güzel E, Sobotta L. Chlorin Activity Enhancers for Photodynamic Therapy. Molecules. 2025; 30(13):2810. https://doi.org/10.3390/molecules30132810
Chicago/Turabian StyleMichalak, Maciej, Jakub Szymczyk, Aleksandra Pawska, Marcin Wysocki, Dominika Janiak, Daniel Ziental, Marcin Ptaszek, Emre Güzel, and Lukasz Sobotta. 2025. "Chlorin Activity Enhancers for Photodynamic Therapy" Molecules 30, no. 13: 2810. https://doi.org/10.3390/molecules30132810
APA StyleMichalak, M., Szymczyk, J., Pawska, A., Wysocki, M., Janiak, D., Ziental, D., Ptaszek, M., Güzel, E., & Sobotta, L. (2025). Chlorin Activity Enhancers for Photodynamic Therapy. Molecules, 30(13), 2810. https://doi.org/10.3390/molecules30132810








