Formulation and Evaluation of Liposome-Encapsulated Phenolic Compounds from Olive Mill Waste: Insights into Encapsulation Efficiency, Antioxidant, and Cytotoxic Activities
Abstract
1. Introduction
2. Results
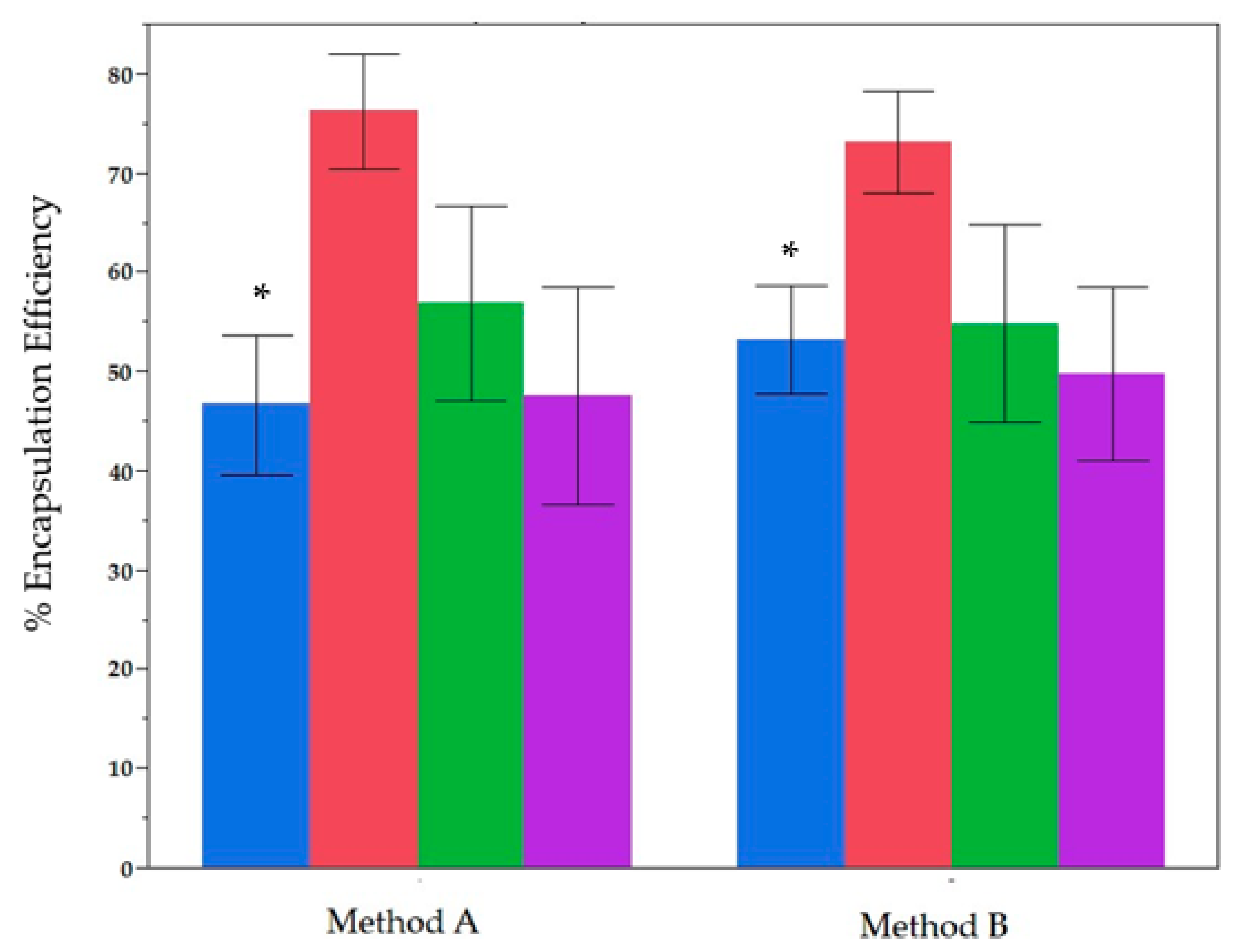
2.1. Comparative Analysis of the Two Methods of Encapsulation
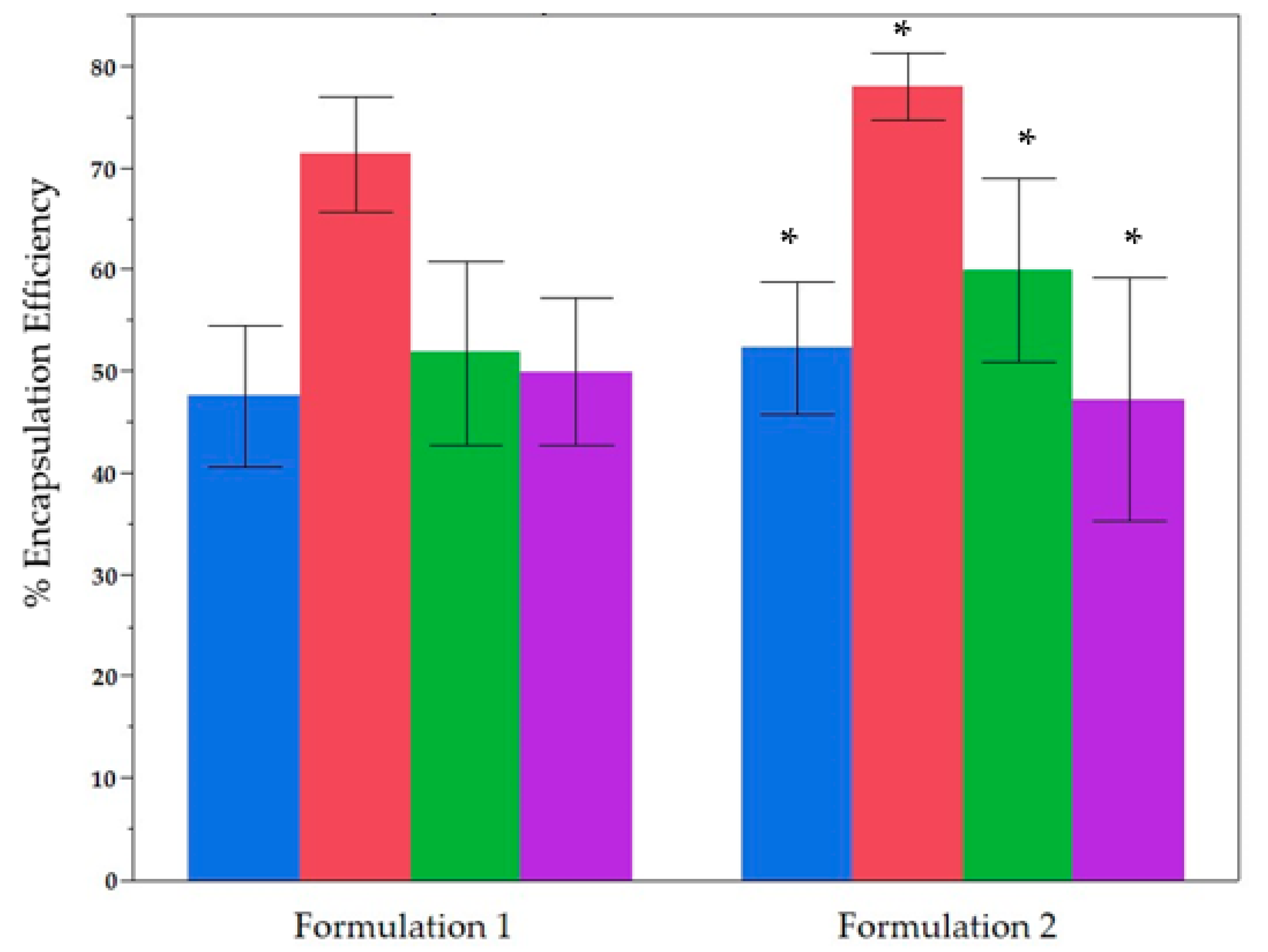
2.2. Comparative Analysis of the Two Formulations Used for Encapsulation
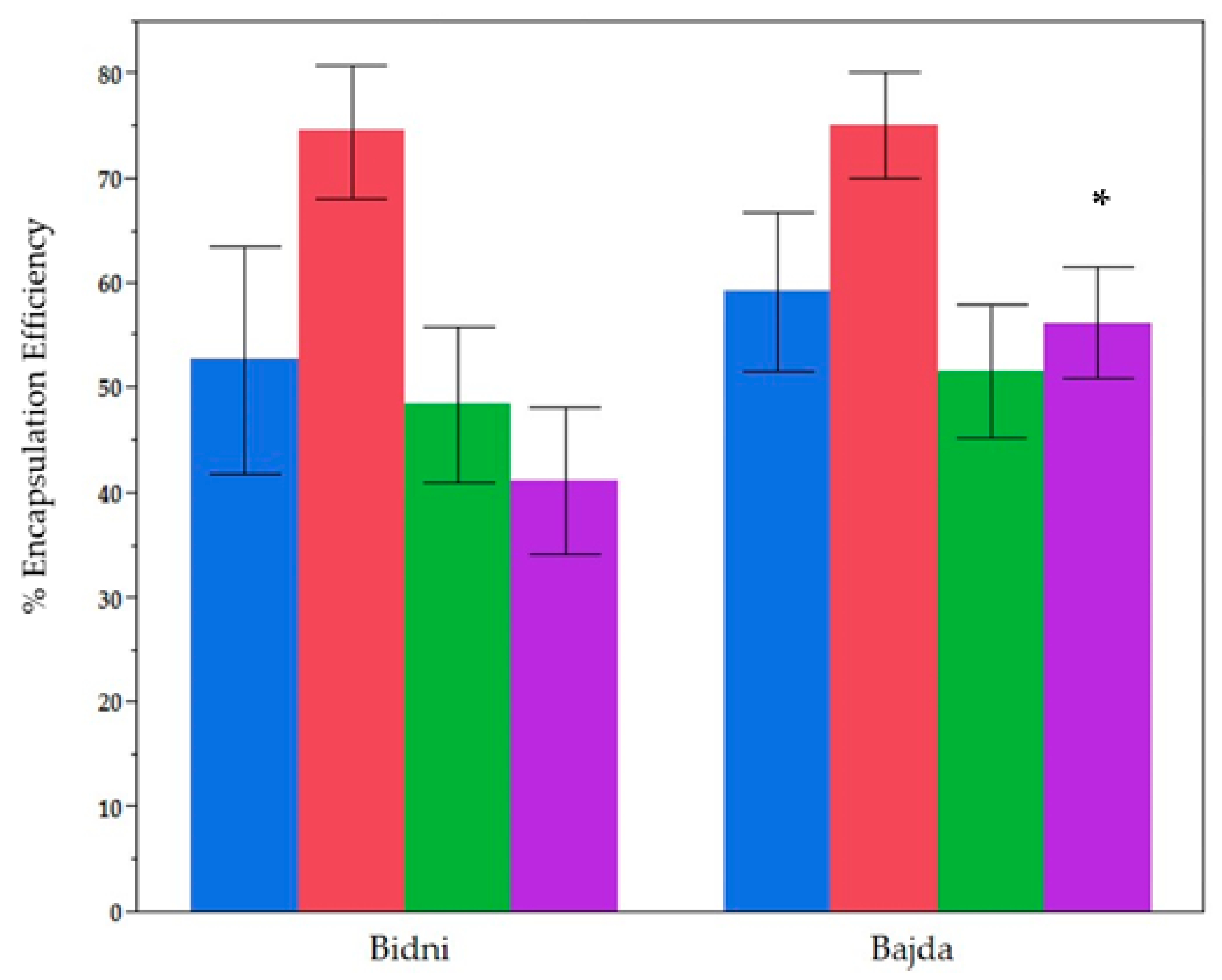
2.3. Comparative Analysis of Encapulsation Effiencies for Two Different Cultivars
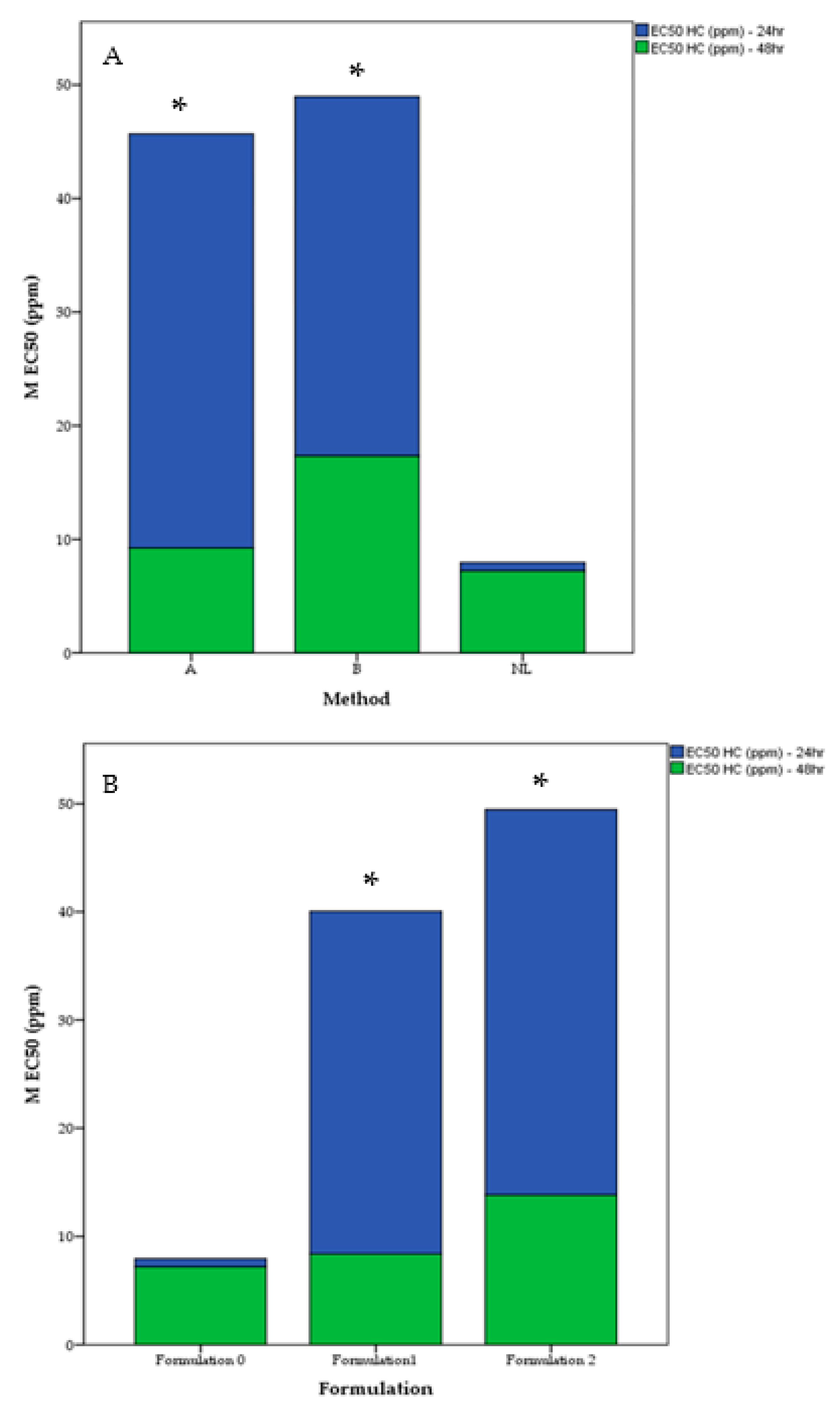
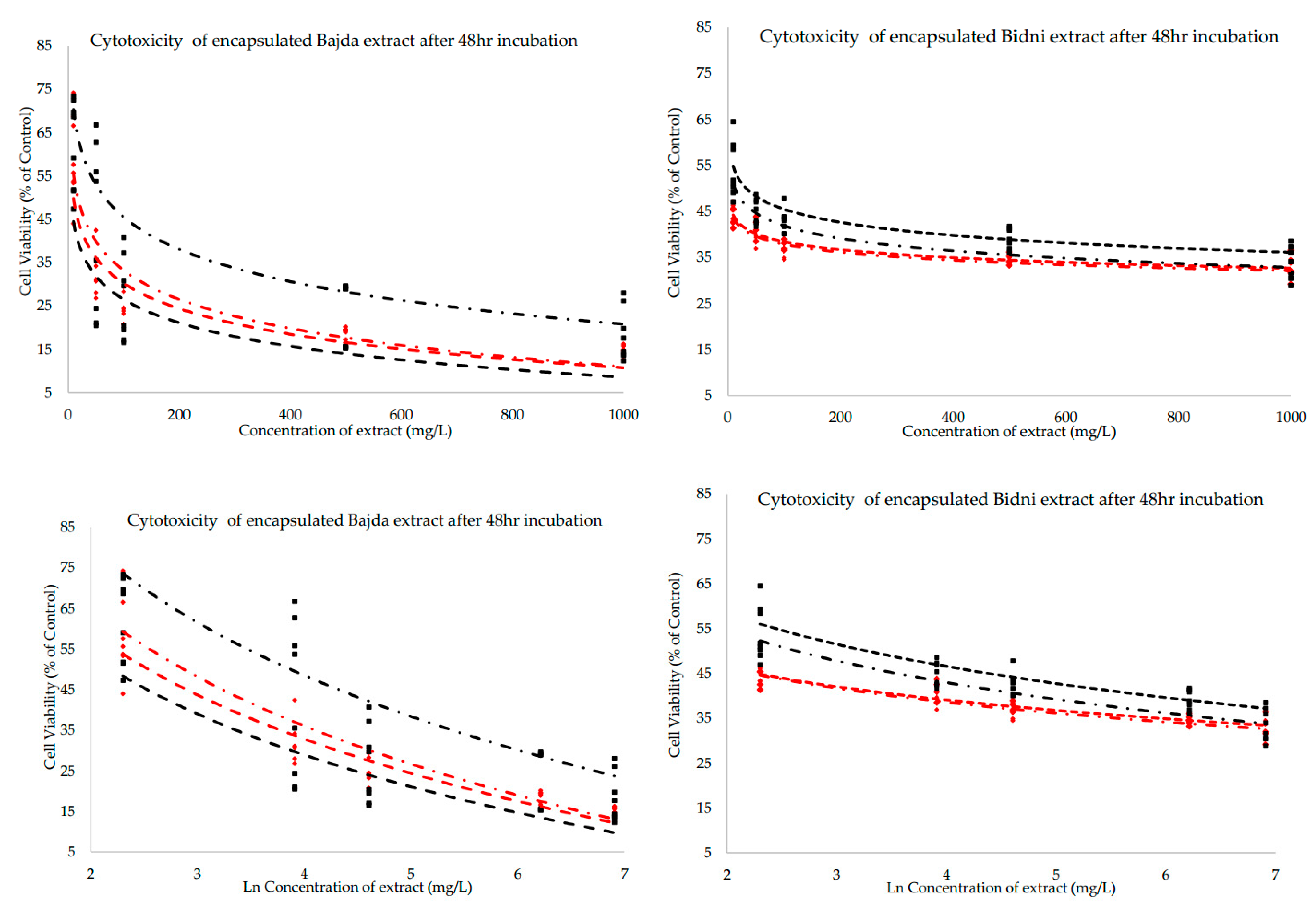
2.4. Cytotoxicity (IC50) After 48 H with Respect to Method, Formulation and Cultivar
3. Discussion
4. Materials and Methods
4.1. Preparation of Olive Mill Waste Extracts
4.2. Liposomal Encapsulation of OMW Extracts
4.3. Determination of Encapsulation Efficiency
4.4. Determination of Total Phenolic Content
4.5. Determination of Total Flavonoid Content
4.6. Determination of Total Ortho-Diphenolic Content
4.7. Determination of CUPRAC
4.8. Cell Culture Seeding
4.9. Cytotoxicity Testing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TPC | Total Phenolic Content |
| TFC | Total Flavonoid Content |
| TdOPC | Total Ortho-Diphenolic Content |
| CUPRAC | Cupric Reducing Antioxidant Capacity |
| PC | Phosphatidylcholine |
| Chol | Cholesterol |
| PBS | Phosphate-Buffered Saline |
| EE% | Encapsulation Efficiency Percentage |
| OMW | Olive Mill Waste |
| OMWW | Olive Mill Waste Water |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (used in cytotoxicity assays) |
| IC50 | Half Maximal Inhibitory Concentration |
| DMSO | Dimethyl Sulfoxide |
| HL-60 | Human Leukemia Cell Line |
| LOQ | Limit of Quantification |
| LOD | Limit of Detection |
| TPSA | Topological Polar Surface Area |
| XLogP | Partition Coefficient (Lipophilicity Measure) |
References
- Passeri, V.; Sammut, C.; Mifsud, D.; Domesi, A.; Stanzione, V.; Bladoni, L.; Mousavi, S.; Mariotti, R.; Pandolfi, S.; Cinosi, N.; et al. The Ancient Olive Trees (Olea europaea L.) of the Maltese Islands: A Rich and Unexplored Patrimony to Enhance Oliviculture. Plants 2023, 12, 1988. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, O.; Calleja, A.; Sadella, S.; Farrugia, C.; Zammit-Mangion, M. Analysis of the molecular diversity of Olea europaea in the Mediterranean Island of Malta Genetic Resources and Crop Evolution. Genet. Resour. Crop Evol. 2015, 62, 1021–1027. [Google Scholar] [CrossRef]
- Lavee, S.O. Handbook of Fruit Set and Development; Monselise, S.P., Ed.; CRC Press: Boca Raton, FL, USA, 1986; pp. 261–274. [Google Scholar]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Lia, F.; Attard, K. Bioactive Potential of Olive Mill Waste Obtained from Cultivars Grown in the Island of Malta. Foods 2024, 13, 1152. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.d.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.-A. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef] [PubMed]
- Hormozi, M.; Salehi Marzijerani, A.; Baharvand, P. Effects of Hydroxytyrosol on Expression of Apoptotic Genes and Activity of Antioxidant Enzymes in LS180 Cells. Cancer Manag. Res. 2020, 12, 7913–7919. [Google Scholar] [CrossRef]
- Bisignano, G.; Tomaino, A.; Lo Cascio, R.; Crisafi, G.; Uccella, N.; Saija, A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999, 51, 971–974. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, M.-Y.; Cho, J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef]
- Pérez de la Lastra, J.M.; Curieses Andrés, C.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Hydroxytyrosol and Arginine as Antioxidant, Anti-Inflammatory and Immunostimulant Dietary Supplements for COVID-19 and Long COVID. Foods 2023, 12, 1937. [Google Scholar] [CrossRef]
- Guzowski, C.; Murawska, J.; Michalska, M.; Winiarek, K.; Czernicka, A.; Kędziora-Kornatowska, K. Tyrosol and Hydroxytyrosol: Their Role in Cardioprotection. J. Educ. Health Sport. 2024, 63, 40–54. [Google Scholar] [CrossRef]
- Sun, W.; Frost, B.; Liu, J. Oleuropein. unexpected benefits! Oncotarget 2017, 8, 17409. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ai, Q.; Wei, Y. Potential role of hydroxytyrosol in neuroprotection. J. Funct. Foods 2021, 82, 104506. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification. preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Malekar, S.A.; Sarode, A.L.; Bach, A.C., II; Worthen, D.R. The Localization of Phenolic Compounds in Liposomal Bilayers and Their Effects on Surface Characteristics and Colloidal Stability. AAPS PharmSciTech 2016, 17, 1468–1476. [Google Scholar] [CrossRef]
- Rafiee, Z.; Barzegar, M.; Sahari, M.A.; Maherani, B. Nanoliposomes Containing Pistachio Green Hull’s Phenolic Compounds as Natural Bio-Preservatives for Mayonnaise. Eur. J. Lipid Sci. Technol. 2018, 120, 1800086. [Google Scholar] [CrossRef]
- Gibis, M.; Vogt, E.; Weiss, J. Encapsulation of polyphenolic grape seed extract in polymer-coated liposomes. Food Funct. 2012, 3, 246–254. [Google Scholar] [CrossRef]
- Santonocito, D.; Sarpietro, M.G.; Carbone, C.; Panico, A.; Campisi, A.; Siciliano, E.A.; Sposito, G.; Castelli, F.; Puglia, C. Curcumin Containing PEGylated Solid Lipid Nanoparticles for Systemic Administration: A Preliminary Study. Molecules 2020, 25, 2991. [Google Scholar] [CrossRef]
- Prevete, G.; Carvalho, L.G.; del Carmen Razola-Díaz, M.; Verardo, V.; Mancini, G.; Fiore, A.; Mazzonna, M. Ultrasound assisted extraction and liposome encapsulation of olive leaves and orange peels: How to transform biomass waste into valuable resources with antimicrobial activity. Ultrason. Sonochem. 2024, 102, 106765. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef]
- Piacentini, E.; Poerio, T.; Bazzarelli, F.; Giorno, L. Microencapsulation by Membrane Emulsification of Biophenols Recovered from Olive Mill Wastewaters. Membranes 2016, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.B.; Muthusamy, N.; Byrd, J.C.; Lee, R.J. Cholesterol as a bilayer anchor for PEGylation and targeting ligand in folate-receptor-targeted liposomes. J. Pharm. Sci. 2007, 96, 2424–2435. [Google Scholar] [CrossRef]
- Efimova, A.A.; Kostenko, S.N.; Orlov, V.N.; Yaroslavov, A.A. Effect of cholesterol on the phase state and permeability of mixed liposomes composed of anionic diphosphatidylglycerol and zwitterionic dipalmitoylphosphatidylcholine. Mendeleev Commun. 2016, 26, 99–100. [Google Scholar] [CrossRef]
- Cagdas, F.M.; Ertugral, N.; Bucak, S.; Atay, N.Z. Effect of preparation method and cholesterol on drug encapsulation studies by phospholipid liposomes. Pharm. Dev. Technol. 2011, 16, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Hudiyanti, D.; Aminah, S.; Hikmahwati, Y.; Siahaan, P. Cholesterol Implications on Coconut Liposomes Encapsulation of Beta-Carotene and Vitamin C; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Paredes, C.; Cegarra, J.; Roig, A.; Sánchez-Monedero, M.A.; Bernal, M.P. Characterization of olive mill wastewater (alpechin) and its sludge for agricultural purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Chen, B.-H.; Chiu, C.P.; Lu, Y.-F. The influence of phytosterols on the encapsulation efficiency of cholesterol liposomes. Int. J. Food Sci. Technol. 2004, 39, 985–995. [Google Scholar] [CrossRef]
- Gulati, M.; Grover, M.; Singh, M.P.; Singh, S. Study of azathioprine encapsulation into liposomes. J. Microencapsul. 1998, 15, 485–494. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.; Santana, M.H. Production of Liposomes in a Multitubular System Useful for Scaling Up of Processes in Progress in Colloid and Polymer Science; Springer: Berlin/Heidelberg, Germany, 2004; pp. 273–277. [Google Scholar] [CrossRef]
- Hızır-Kadı, İ; Gültekin-Özgüven, M.; Altin, G.; Demircan, E.; Özçelik, B. Liposomal nanodelivery systems generated from proliposomes for pollen extract with improved solubility and in vitro bioaccessibility. Heliyon 2020, 6, e05030. [Google Scholar] [CrossRef]
- Bf, H.; Majdizadeh, M.; Taebpour, M. Fabrication and Characterization of Liposomal Nanoparticles Containing Hydroalcoholic Extract of Artemisia Absinthiumand Its Toxicity against MCF-7 Breast Cancer Cell Line. Iran. J. Breast Dis. 2021, 14, 64–77. [Google Scholar] [CrossRef]
- Keshavarz, F.; Dorfaki, M.; Bardania, H.; Khosravani, F.; Nazari, P.; Ghalamfarsa, G. Quercetin-loaded Liposomes Effectively Induced Apoptosis and Decreased the Epidermal Growth Factor Receptor Expression in Colorectal Cancer Cells: An In Vitro Study. Iran. J. Med. Sci. 2023, 48, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Gholamian, R.; Nikoonahad, N.; Haghiralssadat, B. Evaluation of Antioxidant and Cytotoxic Effects of Liposomes Containing Pineapple Fruit Extract on Melanoma Skin Cancer (A375 Cell Line). J. Shahid Sadoughi Univ. Med. Sci. 2020, 28. [Google Scholar] [CrossRef]
- Calvagno, M.G.; Celia, C.; Paolino, D.; Cosco, D.; Iannone, M.; Castelli, F.; Doldo, P.; Frest, M. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes. Curr. Drug Deliv. 2007, 4, 89–101. [Google Scholar] [CrossRef]
- Pereira, S.; Egbu, R.; Jannati, G.; Al-Jamal, W.T. Docetaxel-loaded liposomes: The effect of lipid composition and purification on drug encapsulation and in vitro toxicity. Int. J. Pharm. 2016, 514, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Attard, K.; Oztop, M.H.; Lia, F. The Effect of Hydrolysis on the Antioxidant Activity of Olive Mill Waste. Appl. Sci. 2022, 12, 12187. [Google Scholar] [CrossRef]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1522, pp. 17–22. [Google Scholar] [CrossRef]
- Zhai, L.; Luo, C.; Gao, H.; Du, S.; Shi, J.; Wang, F. A Dual pH-Responsive DOX-Encapsulated Liposome Combined with Glucose Administration Enhanced Therapeutic Efficacy of Chemotherapy for Cancer. Int. J. Nanomed. 2021, 16, 3185–3199. [Google Scholar] [CrossRef]
- Dipali, S.R.; Kulkarni, S.B.; Betageri, G.V. Comparative Study of Separation of Non-encapsulated Drug from Unilamellar Liposomes by Various Methods. J. Pharm. Pharmacol. 1996, 48, 1112–1115. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of Phenols. Flavones, and Lignans in Virgin Olive Oils by Solid-Phase Extraction and High-Performance Liquid Chromatography with Diode Array Ultraviolet Detection Journal of Agricultural and Food Chemistry. Am. Chem. Soc. 2001, 49, 2185–2192. [Google Scholar] [CrossRef]
- Özyürek MGüçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. TrAC Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
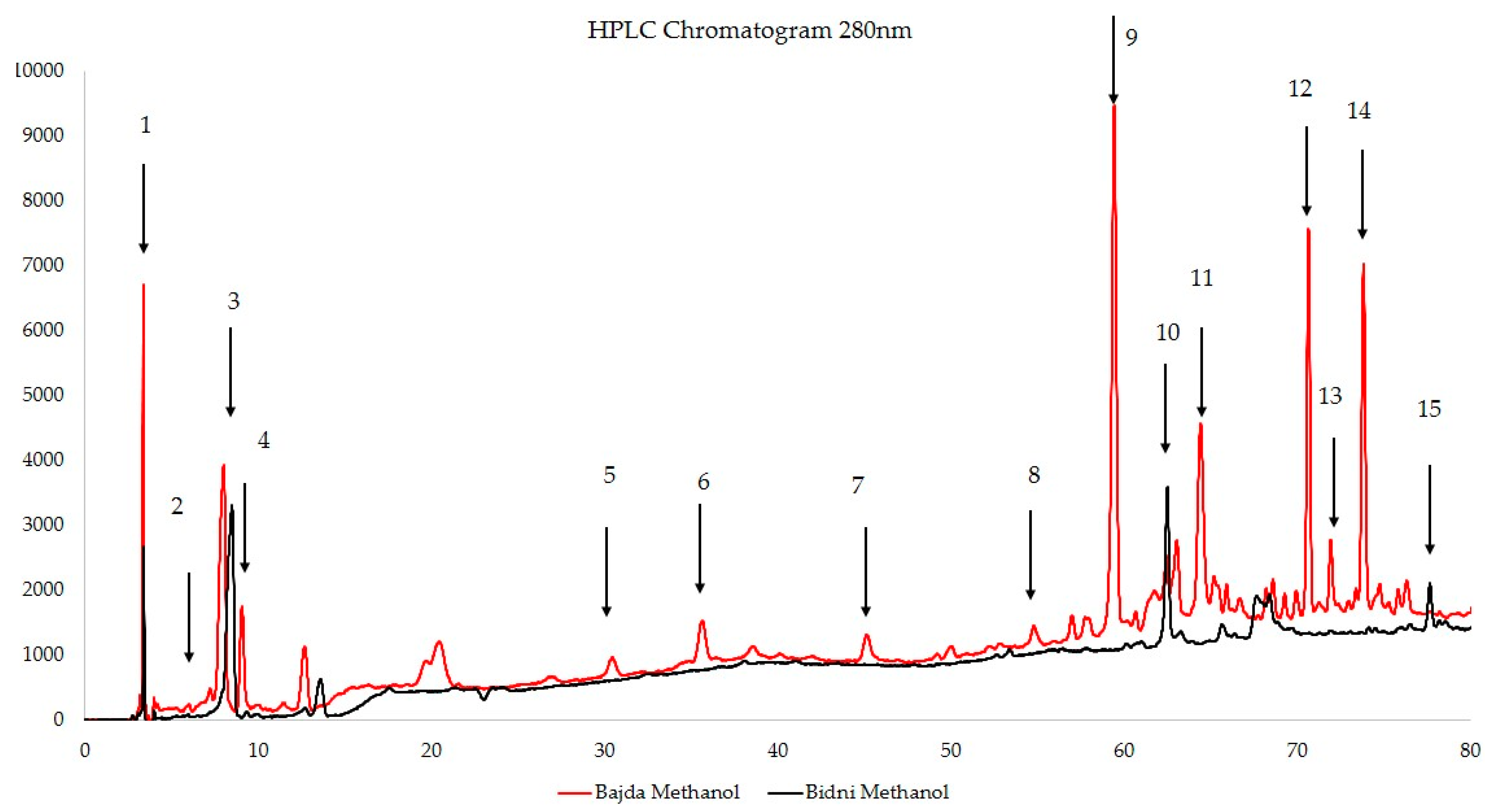
| Compound | Peak number | Bajda Methanol (μg/mL) | Bidni Methanol (μg/mL) |
|---|---|---|---|
| 5,7-dihydroxy flavone | 15 | <LOD | 1.641 ± 0.030 |
| Quercetin | 14 | 2.064 ± 0.001 | <LOD |
| 3′,4,5,7-tetrahydroxy flavone | 13 | 3.551 ± 0.001 | <LOD |
| Ligstroside | 12 | 1.303 ± 0.013 | 0.286 ± 0.006 |
| Oleocanthal | 11 | 1.284 ± 0.069 | <LOD |
| Oleuropein | 10 | 6.265 ± 0.042 | 2.398 ± 0.240 |
| Oleacein | 9 | 11.085 ± 0.021 | 5.651 ± 0.126 |
| Apigenin 7-glucoside | 8 | 8.109 ± 0.178 | 3.343 ± 0.129 |
| Ellagic acid | 7 | 0.218 ± 0.001 | <LOD |
| p-coumaric acid | 6 | 2.348 ± 0.013 | 1.927 ± 0.007 |
| Vanillin | 5 | 4.084 ± 0.001 | <LOD |
| Vanillic acid | 4 | 2.011 ± 0.013 | <LOD |
| 2-(4-hydroxy phenyl) ethanol | 3 | 3.763 ± 0.010 | 3.608 ± 0.019 |
| 3-Hydroxytyrosol | 2 | 6.034 ± 0.044 | 6.801 ± 0.312 |
| Gallic acid | 1 | 0.505 ± 0.006 | <LOD |
| Compound Name | TPSA (Ų) | XLogP | Hydrophilicity/Lipophilicity |
|---|---|---|---|
| Tetrahydroxyflavone | 107 | 2.5 | Moderately lipophilic |
| Quercetin | 127 | 1.5 | Mildly lipophilic |
| p-coumaric acid | 57.5 | 1.5 | Balanced hydrophilic-lipophilic profile |
| Vanillin | 46.5 | 1.2 | Slightly lipophilic |
| Oleacein | 101 | 1.1 | Mildly lipophilic |
| 2-(4-hydroxyphenyl) ethanol | 40.5 | 0.4 | Slightly hydrophilic |
| Apigenin 7-glucoside | 166 | −0.1 | Hydrophilic |
| Oleuropein | 202 | −0.4 | Strongly hydrophilic |
| 3-hydroxytyrosol | 60.7 | −0.7 | Highly hydrophilic |
| ‘Bidni’ Cultivar | ‘Bajda’ Cultivar | |
|---|---|---|
| Formulation 1 (80:20) | Method A | Method A |
| Method B | Method B | |
| Formulation 2 (70:30) | Method A | Method A |
| Method B | Method B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camilleri, D.; Attard, K.; Lia, F. Formulation and Evaluation of Liposome-Encapsulated Phenolic Compounds from Olive Mill Waste: Insights into Encapsulation Efficiency, Antioxidant, and Cytotoxic Activities. Molecules 2025, 30, 2351. https://doi.org/10.3390/molecules30112351
Camilleri D, Attard K, Lia F. Formulation and Evaluation of Liposome-Encapsulated Phenolic Compounds from Olive Mill Waste: Insights into Encapsulation Efficiency, Antioxidant, and Cytotoxic Activities. Molecules. 2025; 30(11):2351. https://doi.org/10.3390/molecules30112351
Chicago/Turabian StyleCamilleri, David, Karen Attard, and Frederick Lia. 2025. "Formulation and Evaluation of Liposome-Encapsulated Phenolic Compounds from Olive Mill Waste: Insights into Encapsulation Efficiency, Antioxidant, and Cytotoxic Activities" Molecules 30, no. 11: 2351. https://doi.org/10.3390/molecules30112351
APA StyleCamilleri, D., Attard, K., & Lia, F. (2025). Formulation and Evaluation of Liposome-Encapsulated Phenolic Compounds from Olive Mill Waste: Insights into Encapsulation Efficiency, Antioxidant, and Cytotoxic Activities. Molecules, 30(11), 2351. https://doi.org/10.3390/molecules30112351









