Brewers’ Spent Grain from Different Types of Malt: A Comprehensive Evaluation of Appearance, Structure, Chemical Composition, Antimicrobial Activity, and Volatile Emissions
Abstract
1. Introduction
2. Results and Discussion
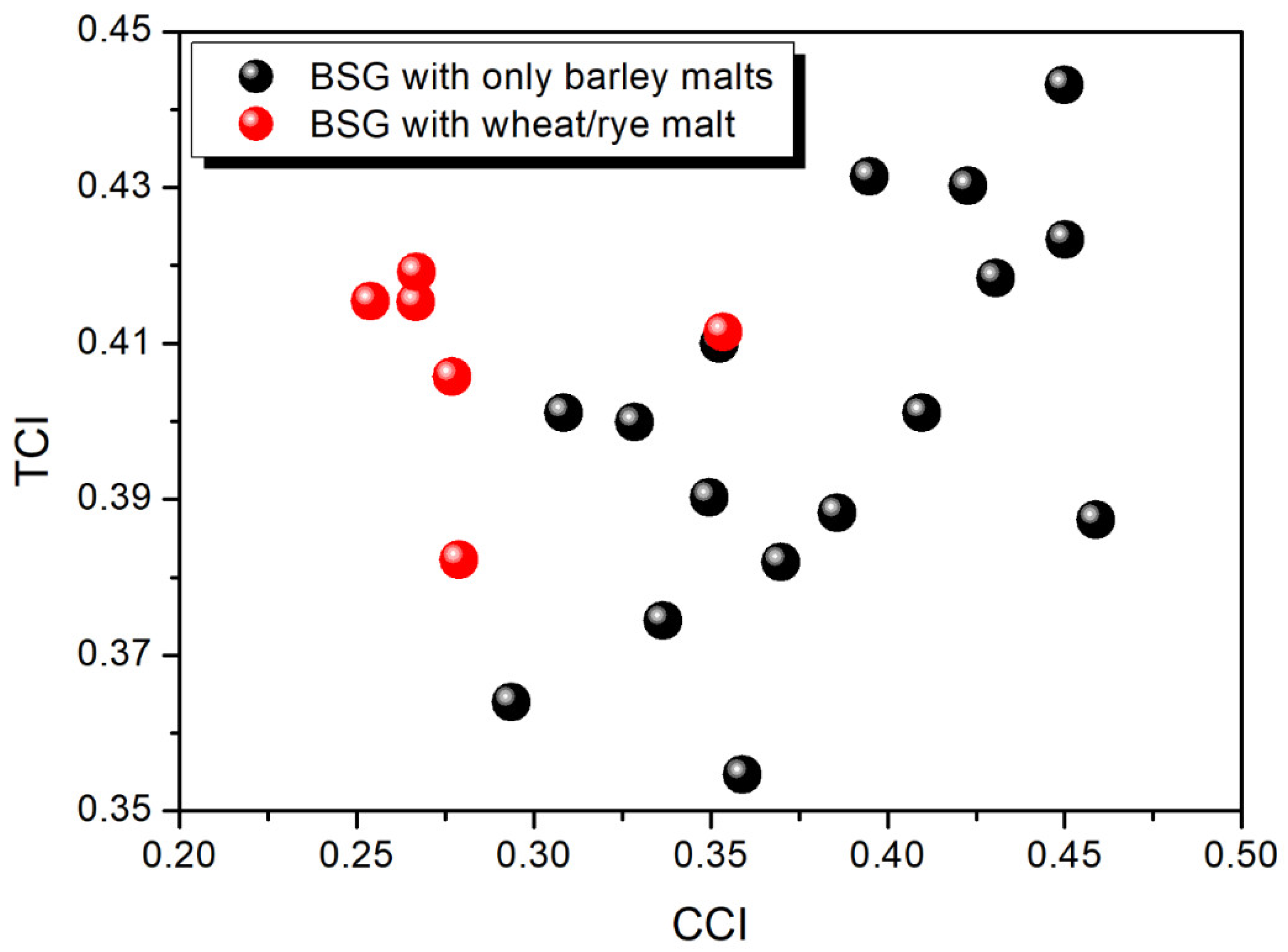
2.1. Visual Assessment of the Obtained BSG
2.2. Composition and Chemical Structure of the Obtained BSG
2.3. Fourier Transform Infrared Spectroscopy and X-Ray Diffraction Analysis of the Obtained BSG
2.4. Assessment of BSG Antimicrobial Activity
2.5. Volatile Organic Compound Emissions from BSG
3. Materials and Methods
3.1. Materials
3.2. Laboratory-Scale Brewing and BSG Generation
3.3. Characterization Techniques
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statista Inc. U.S. Beer Lovers Have the Luxury of Choice. Available online: https://www.statista.com/chart/28572/popularity-of-beer-styles-in-the-us/ (accessed on 30 May 2025).
- Fortune Business Insights Pvt. Ltd. The Global Craft Beer Market Is Projected to Grow from $102.59 Billion in 2021 to $210.78 Billion in 2028 at a CAGR of 10.83% in Forecast Period, 2021–2028; Fortune Business Insights Pvt. Ltd.: Pune, India, 2022. [Google Scholar]
- Jesús Callejo, M.; Tesfaye, W.; Carmen González, M.; Morata, A. Craft Beers: Current Situation and Future Trends. In New Advances on Fermentation Processes; IntechOpen: London, UK, 2020. [Google Scholar][Green Version]
- Durán-Sánchez, A.; de la Cruz del Río-Rama, M.; Álvarez-García, J.; Oliveira, C. Analysis of Worldwide Research on Craft Beer. Sage Open 2022, 12, 215824402211081. [Google Scholar] [CrossRef]
- Baiano, A. Craft Beer: An Overview. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1829–1856. [Google Scholar] [CrossRef]
- Donadini, G.; Porretta, S. Uncovering Patterns of Consumers’ Interest for Beer: A Case Study with Craft Beers. Food Res. Int. 2017, 91, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Statista.com. Beer Production Worldwide from 1998 to 2023 (in Billion Hectoliters). Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 30 May 2025).
- Reid, N. Craft Beer Tourism: The Search for Authenticity, Diversity, and Great Beer. In Regional Science Perspectives on Tourism and Hospitality; Springer: Cham, Switzerland, 2021; pp. 317–337. [Google Scholar]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A. More than Just a Beer—The Potential Applications of by-Products from Beer Manufacturing in Polymer Technology. Emergent Mater. 2021, 5, 765–783. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M. The Variability of Physico-Chemical Properties of Brewery Spent Grain from 8 Different Breweries. Heliyon 2021, 7, e06583. [Google Scholar] [CrossRef]
- Robertson, J.A.; I’Anson, K.J.A.; Treimo, J.; Faulds, C.B.; Brocklehurst, T.F.; Eijsink, V.G.H.; Waldron, K.W. Profiling Brewers’ Spent Grain for Composition and Microbial Ecology at the Site of Production. LWT-Food Sci. Technol. 2010, 43, 890–896. [Google Scholar] [CrossRef]
- Jin, Z.; Lan, Y.; Ohm, J.-B.; Gillespie, J.; Schwarz, P.; Chen, B. Physicochemical Composition, Fermentable Sugars, Free Amino Acids, Phenolics, and Minerals in Brewers’ Spent Grains Obtained from Craft Brewing Operations. J. Cereal Sci. 2022, 104, 103413. [Google Scholar] [CrossRef]
- Pereira de Freitas, F.P.; Steel, C.J.; Lannes, S.C.S. Brewers’ Spent Grain from Three Styles of Craft Beer: Characterization and Their Rheological Effects on Wheat Flour Dough. Cereal Chem. 2023, 100, 752–761. [Google Scholar] [CrossRef]
- Shellhammer, T.H.; Bamforth, C.W. Assessing Color Quality of Beer. ACS Symp. Ser. 2008, 983, 192–202. [Google Scholar]
- Dadali, G.; Demirhan, E.; Özbek, B. Color Change Kinetics of Spinach Undergoing Microwave Drying. Dry. Technol. 2007, 25, 1713–1723. [Google Scholar] [CrossRef]
- Pepa, L.S.; Rodríguez, S.D.; dos Santos Ferreira, C.; Buera, M.d.P. Interpretation of the Color Due to the Ubiquitous Nonenzymatic Browning Phenomena in Foods. Color. Res. Appl. 2021, 46, 446–455. [Google Scholar] [CrossRef]
- Chen, Q.; Koh, H.K.; Park, J.B. Color Evaluation of Red Pepper Powder. Trans. ASAE 1999, 42, 749–752. [Google Scholar] [CrossRef]
- Horváth, Z. Procedure for Setting the Colour Characteristics of Paprika Grist Mixtures. Acta Aliment. 2007, 36, 75–88. [Google Scholar] [CrossRef]
- Jiang, M.; Nakano, S. Application of Image Analysis for Algal Biomass Quantification: A Low-Cost and Non-Destructive Method Based on HSI Color Space. J. Appl. Phycol. 2021, 33, 3709–3717. [Google Scholar] [CrossRef]
- Sass, L.; Majer, P.; Hideg, É. Leaf Hue Measurements: A High-Throughput Screening of Chlorophyll Content. In High-Throughput Phenotyping in Plants; Springer: Cham, Switzerland, 2012; pp. 61–69. [Google Scholar]
- Bassey, F.I.; Chinnan, M.S.; Ebenso, E.E.; Edem, C.A.; Iwegbue, C.M.A. Colour Change: An Indicator of the Extent of Maillard Browning Reaction in Food System. Asian J. Chem. 2013, 25, 9325–9328. [Google Scholar] [CrossRef]
- Bal, L.M.; Kar, A.; Satya, S.; Naik, S.N. Kinetics of Colour Change of Bamboo Shoot Slices during Microwave Drying. Int. J. Food Sci. Technol. 2011, 46, 827–833. [Google Scholar] [CrossRef]
- Bakar, B.F.A.; Tahir, P.M.; Karimi, A.; Bakar, E.S.; Uyup, M.K.A.; Yong Choo, A.C. Evaluations of Some Physical Properties for Oil Palm as Alternative Biomass Resources. Wood Mater. Sci. Eng. 2013, 8, 119–128. [Google Scholar] [CrossRef]
- Dervilly-Pinel, G.; Rimsten, L.; Saulnier, L.; Andersson, R.; Åman, P. Water-Extractable Arabinoxylan from Pearled Flours of Wheat, Barley, Rye and Triticale. Evidence for the Presence of Ferulic Acid Dimers and Their Involvement in Gel Formation. J. Cereal Sci. 2001, 34, 207–214. [Google Scholar] [CrossRef]
- Buffington, J. The Economic Potential of Brewer’s Spent Grain (BSG) as a Biomass Feedstock. Adv. Chem. Eng. Sci. 2014, 04, 308–318. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Rocha, G.J.M.; Roberto, I.C. Hydrogen Peroxide Bleaching of Cellulose Pulps Obtained from Brewer’s Spent Grain. Cellulose 2008, 15, 641–649. [Google Scholar] [CrossRef]
- Kemppainen, K.; Rommi, K.; Holopainen, U.; Kruus, K. Steam Explosion of Brewer’s Spent Grain Improves Enzymatic Digestibility of Carbohydrates and Affects Solubility and Stability of Proteins. Appl. Biochem. Biotechnol. 2016, 180, 94–108. [Google Scholar] [CrossRef]
- Niemi, P.; Martins, D.; Buchert, J.; Faulds, C.B. Pre-Hydrolysis with Carbohydrases Facilitates the Release of Protein from Brewer’s Spent Grain. Bioresour. Technol. 2013, 136, 529–534. [Google Scholar] [CrossRef]
- Yu, D.; Sun, Y.; Wang, W.; O’Keefe, S.F.; Neilson, A.P.; Feng, H.; Wang, Z.; Huang, H. Recovery of Protein Hydrolysates from Brewer’s Spent Grain Using Enzyme and Ultrasonication. Int. J. Food Sci. Technol. 2020, 55, 357–368. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of Extraction Solvents on the Recovery of Antioxidant Phenolic Compounds from Brewer’s Spent Grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Xiros, C.; Topakas, E.; Katapodis, P.; Christakopoulos, P. Hydrolysis and Fermentation of Brewer’s Spent Grain by Neurospora Crassa. Bioresour. Technol. 2008, 99, 5427–5435. [Google Scholar] [CrossRef]
- Carvalheiro, F. Production of Oligosaccharides by Autohydrolysis of Brewery’s Spent Grain. Bioresour. Technol. 2004, 91, 93–100. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. The Effects of Malting and Mashing on Barley Protein Extractability. J. Cereal Sci. 2006, 44, 203–211. [Google Scholar] [CrossRef]
- Kanauchi, O.; Mitsuyama, K.; Araki, Y. Development of a Functional Germinated Barley Foodstuff from Brewer’s Spent Grain for the Treatment of Ulcerative Colitis. J. Am. Soc. Brew. Chem. 2001, 59, 59–62. [Google Scholar] [CrossRef]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, Protein and Mineral Fortification of Wheat Bread through Milled and Fermented Brewer’s Spent Grain Enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; del Nozal, M.J. Variability of Brewer’s Spent Grain within a Brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Lynch, K.M.; Strain, C.R.; Johnson, C.; Patangia, D.; Stanton, C.; Koc, F.; Gil-Martinez, J.; O’Riordan, P.; Sahin, A.W.; Ross, R.P.; et al. Extraction and Characterisation of Arabinoxylan from Brewers Spent Grain and Investigation of Microbiome Modulation Potential. Eur. J. Nutr. 2021, 60, 4393–4411. [Google Scholar] [CrossRef]
- Mathias, T.R.d.S.; Alexandre, V.M.F.; Cammarota, M.C.; de Mello, P.P.M.; Sérvulo, E.F.C. Characterization and Determination of Brewer’s Solid Wastes Composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef]
- Thiex, N. Evaluation of Analytical Methods for the Determination of Moisture, Crude Protein, Crude Fat, and Crude Fiber in Distillers Dried Grains with Solubles. J. AOAC Int. 2009, 92, 61–73. [Google Scholar] [CrossRef]
- Wolters, M.G.E.; Verbeek, C.; Van Westerop, J.J.M.; Hermus, R.J.J.; Voragen, A.G.J. Comparison of Different Methods for Determination of Dietary Fiber. J. AOAC Int. 1992, 75, 626–634. [Google Scholar] [CrossRef]
- Fahey, G.C.; Novotny, L.; Layton, B.; Mertens, D.R. Critical Factors in Determining Fiber Content of Feeds and Foods and Their Ingredients. J. AOAC Int. 2019, 102, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Villacreces, S.; Blanco, C.A.; Caballero, I. Developments and Characteristics of Craft Beer Production Processes. Food Biosci. 2022, 45, 101495. [Google Scholar] [CrossRef]
- Muñoz-Insa, A.; Selciano, H.; Zarnkow, M.; Becker, T.; Gastl, M. Malting Process Optimization of Spelt (Triticum spelta L.) for the Brewing Process. LWT - Food Sci. Technol. 2013, 50, 99–109. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the Extraction Solvent on Phenolic Content, Antioxidant, Antimicrobial and Antimutagenic Activities of Brewers’ Spent Grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- Carciochi, R.; Sologubik, C.; Fernández, M.; Manrique, G.; D’Alessandro, L. Extraction of Antioxidant Phenolic Compounds from Brewer’s Spent Grain: Optimization and Kinetics Modeling. Antioxidants 2018, 7, 45. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Dulf, F.V.; Tofană, M.; Mudura, E.; Diaconeasa, Z. Volatile Profile, Fatty Acids Composition and Total Phenolics Content of Brewers’ Spent Grain by-Product with Potential Use in the Development of New Functional Foods. J. Cereal Sci. 2015, 64, 34–42. [Google Scholar] [CrossRef]
- Szwajgier, D.; Waśko, A.; Targoński, Z.; Niedźwiadek, M.; Bancarzewska, M. The Use of a Novel Ferulic Acid Esterase from Lactobacillus Acidophilus K1 for the Release of Phenolic Acids from Brewer’s Spent Grain. J. Inst. Brew. 2010, 116, 293–303. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Temviriyanukul, P.; Chaikham, P.; Kemsawasd, V. Effects of Freeze Drying and Convective Hot-Air Drying on Predominant Bioactive Compounds, Antioxidant Potential and Safe Consumption of Maoberry Fruits. LWT 2023, 184, 114992. [Google Scholar] [CrossRef]
- Bravi, E.; De Francesco, G.; Sileoni, V.; Perretti, G.; Galgano, F.; Marconi, O. Brewing By-Product Upcycling Potential: Nutritionally Valuable Compounds and Antioxidant Activity Evaluation. Antioxidants 2021, 10, 165. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of Nonextractable Phenolic Compounds in Foods: The Current State of the Art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar] [CrossRef]
- Moreira, M.M.; Morais, S.; Carvalho, D.O.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. Brewer’s Spent Grain from Different Types of Malt: Evaluation of the Antioxidant Activity and Identification of the Major Phenolic Compounds. Food Res. Int. 2013, 54, 382–388. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidant Potential of Barley as Affected by Alkaline Hydrolysis and Release of Insoluble-Bound Phenolics. Food Chem. 2009, 117, 615–620. [Google Scholar] [CrossRef]
- Kitrytė, V.; Šaduikis, A.; Venskutonis, P.R. Assessment of Antioxidant Capacity of Brewer’s Spent Grain and Its Supercritical Carbon Dioxide Extract as Sources of Valuable Dietary Ingredients. J. Food Eng. 2015, 167, 18–24. [Google Scholar] [CrossRef]
- Petrón, M.J.; Andrés, A.I.; Esteban, G.; Timón, M.L. Study of Antioxidant Activity and Phenolic Compounds of Extracts Obtained from Different Craft Beer By-Products. J. Cereal Sci. 2021, 98, 103162. [Google Scholar] [CrossRef]
- Fogarasi, A.-L.; Kun, S.; Tankó, G.; Stefanovits-Bányai, É.; Hegyesné-Vecseri, B. A Comparative Assessment of Antioxidant Properties, Total Phenolic Content of Einkorn, Wheat, Barley and Their Malts. Food Chem. 2015, 167, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Abramovič, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Shopska, V.; Denkova-Kostova, R.; Dzhivoderova-Zarcheva, M.; Teneva, D.; Denev, P.; Kostov, G. Comparative Study on Phenolic Content and Antioxidant Activity of Different Malt Types. Antioxidants 2021, 10, 1124. [Google Scholar] [CrossRef]
- Özcan, M.M.; Aljuhaimi, F.; Uslu, N. Effect of Malt Process Steps on Bioactive Properties and Fatty Acid Composition of Barley, Green Malt and Malt Grains. J. Food Sci. Technol. 2018, 55, 226–232. [Google Scholar] [CrossRef]
- Tomková-Drábková, L.; Psota, V.; Sachambula, L.; Leišová-Svobodová, L.; Mikyška, A.; Kučera, L. Changes in Polyphenol Compounds and Barley Laccase Expression during the Malting Process. J. Sci. Food Agric. 2016, 96, 497–504. [Google Scholar] [CrossRef]
- Oh, S.; Yi, B.; Ka, H.J.; Song, J.; Park, J.; Jung, J.; Kim, M.-J.; Park, K.W.; Lee, J. Evaluation of In Vitro Antioxidant Properties of Roasted Hulled Barley (Hordeum vulgare L.). Food Sci. Biotechnol. 2014, 23, 1073–1079. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Faria, J.d.A.F.; Cruz, A.G. Characterization of Brazilian Lager and Brown Ale Beers Based on Color, Phenolic Compounds, and Antioxidant Activity Using Chemometrics. J. Sci. Food Agric. 2011, 91, 563–571. [Google Scholar] [CrossRef]
- Gąsior, J.; Kawa-Rygielska, J.; Kucharska, A.Z. Carbohydrates Profile, Polyphenols Content and Antioxidative Properties of Beer Worts Produced with Different Dark Malts Varieties or Roasted Barley Grains. Molecules 2020, 25, 3882. [Google Scholar] [CrossRef] [PubMed]
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Antioxidant Activity and the Most Abundant Phenolics in Commercial Dark Beers. Int. J. Food Prop. 2017, 20, S595–S609. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Lu, J.; Zhao, M. Phenolic Profiles and Antioxidant Activities of Commercial Beers. Food Chem. 2010, 119, 1150–1158. [Google Scholar] [CrossRef]
- Liguori, L.; De Francesco, G.; Orilio, P.; Perretti, G.; Albanese, D. Influence of Malt Composition on the Quality of a Top Fermented Beer. J. Food Sci. Technol. 2021, 58, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Orbán, C.; Galló, N.; Kun, S.; Vecseri-Hegyes, B.; Kun-Farkas, G. Folic Acid Content and Antioxidant Activity of Different Types of Beers Available in Hungarian Retail. J. Food Sci. Technol. 2017, 54, 1158–1167. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of Phenolics Content and Antioxidant Activity of Different Beer Types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef]
- Habschied, K.; Lončarić, A.; Mastanjević, K. Screening of Polyphenols and Antioxidative Activity in Industrial Beers. Foods 2020, 9, 238. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Olanrewaju, O.A.; Usman, M.A.; Adeosun, S.O. Lignin from Brewers’ Spent Grain: Structural and Thermal Evaluations. Polymers 2023, 15, 2346. [Google Scholar] [CrossRef]
- Mishra, P.K.; Gregor, T.; Wimmer, R. Utilising Brewer’s Spent Grain as a Source of Cellulose Nanofibres Following Separation of Protein-Based Biomass. Bioresources 2016, 12, 107–116. [Google Scholar] [CrossRef]
- Santos, D.M.D.; de Lacerda Bukzem, A.; Ascheri, D.P.R.; Signini, R.; de Aquino, G.L.B. Microwave-Assisted Carboxymethylation of Cellulose Extracted from Brewer’s Spent Grain. Carbohydr. Polym. 2015, 131, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Hidar, N.; Noufid, A.; Mourjan, A.; El Adnany, E.M.; Mghazli, S.; Mouhib, M.; Jaouad, A.; Mahrouz, M. Effect of Preservation Methods on Physicochemical Quality, Phenolic Content, and Antioxidant Activity of Stevia Leaves. J. Food Qual. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Barczewski, M.; Aniśko, J.; Hejna, A.; Mysiukiewicz, O.; Kosmela, P.; Sałasińska, K.; Boczkowska, A.; Przybylska-Balcerek, A.; Stuper-Szablewska, K. Ground Lemon and Stevia Leaves as Renewable Functional Fillers with Antioxidant Activity for High-Density Polyethylene Composites. Clean. Technol. Environ. Policy 2023, 25, 3345–3361. [Google Scholar] [CrossRef]
- Comino, P.; Shelat, K.; Collins, H.; Lahnstein, J.; Gidley, M.J. Separation and Purification of Soluble Polymers and Cell Wall Fractions from Wheat, Rye and Hull Less Barley Endosperm Flours for Structure-Nutrition Studies. J. Agric. Food Chem. 2013, 61, 12111–12122. [Google Scholar] [CrossRef]
- Skendi, A.; Papageorgiou, M.; Papastergiadis, E. The Effect of Malting on the Crystallites and Microstructure in Greek Barley Cultivar Using X-Ray Diffraction and Microscopic Analysis. Millenium J. Educ. Technol. Health 2018, 2, 67–78. [Google Scholar] [CrossRef]
- Lekjing, S.; Venkatachalam, K. Effects of Germination Time and Kilning Temperature on the Malting Characteristics, Biochemical and Structural Properties of HomChaiya Rice. RSC Adv. 2020, 10, 16254–16265. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Schultz, T.P.; McGinnis, G.D.; Bertran, M.S. Estimation of Cellulose Crystallinity Using Fourier Transform-Infrared Spectroscopy and Dynamic Thermogravimetry. J. Wood Chem. Technol. 1985, 5, 543–557. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Vilas Boas, A.A.; Coscueta, E.R.; Costa, E.M.; Silva, S.; Campos, D.; Teixeira, J.A.; Pintado, M. Bioactive Extracts from Brewer’s Spent Grain. Food Funct. 2020, 11, 8963–8977. [Google Scholar] [CrossRef]
- Marchel, M.; Cieśliński, H.; Boczkaj, G. Thermal Instability of Choline Chloride-Based Deep Eutectic Solvents and Its Influence on Their Toxicity—Important Limitations of DESs as Sustainable Materials. Ind. Eng. Chem. Res. 2022, 61, 11288–11300. [Google Scholar] [CrossRef]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro)Plastic Crisis: Un-Ignorable Contribution to Global Greenhouse Gas Emissions and Climate Change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Arunan, I.; Crawford, R.H. Greenhouse Gas Emissions Associated with Food Packaging for Online Food Delivery Services in Australia. Resour. Conserv. Recycl. 2021, 168, 105299. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Gonçalves, L.M.; Guido, L.F. Overall Antioxidant Properties of Malt and How They Are Influenced by the Individual Constituents of Barley and the Malting Process. Compr. Rev. Food Sci. Food Saf. 2016, 15, 927–943. [Google Scholar] [CrossRef]
- Du, H.; Liu, Q.; Chen, Q.; Xia, X.; Xu, M.; Kong, B. Effect of Woodchip Types on Heterocyclic Aromatic Amine Formation and Quality Characteristics of Smoked Bacon. Food Biosci. 2022, 47, 101709. [Google Scholar] [CrossRef]
- Guillén, M.D.; Manzanos, M.J. Study of the Volatile Composition of an Aqueous Oak Smoke Preparation. Food Chem. 2002, 79, 283–292. [Google Scholar] [CrossRef]
- Hitzel, A.; Pöhlmann, M.; Schwägele, F.; Speer, K.; Jira, W. Polycyclic Aromatic Hydrocarbons (PAH) and Phenolic Substances in Meat Products Smoked with Different Types of Wood and Smoking Spices. Food Chem. 2013, 139, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A.; Marć, M.; Kowalkowska-Zedler, D.; Pladzyk, A.; Barczewski, M. Insights into the Thermo-Mechanical Treatment of Brewers’ Spent Grain as a Potential Filler for Polymer Composites. Polymers 2021, 13, 879. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, Y.; Wang, M. Discovery and Engineering of Cytochrome P450s for Terpenoid Biosynthesis. Trends Biotechnol. 2019, 37, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Wahlen, C.; Frey, H. Anionic Polymerization of Terpene Monomers: New Options for Bio-Based Thermoplastic Elastomers. Macromolecules 2021, 54, 7323–7336. [Google Scholar] [CrossRef]
- Farcas, A.C.; Socaci, S.A.; Chiș, M.S.; Pop, O.L.; Fogarasi, M.; Păucean, A.; Igual, M.; Michiu, D. Reintegration of Brewers Spent Grains in the Food Chain: Nutritional, Functional and Sensorial Aspects. Plants 2021, 10, 2504. [Google Scholar] [CrossRef]
- van Meeningen, Y.; Schurgers, G.; Rinnan, R.; Holst, T. BVOC Emissions from English Oak (Quercus Robur) and European Beech (Fagus Sylvatica) along a Latitudinal Gradient. Biogeosciences 2016, 13, 6067–6080. [Google Scholar] [CrossRef]
- National Fire Protection Association NFPA 704: A Standard System for the Identification of the Hazards of Materials for Emergency Response. Available online: https://www.nfpa.org/codes-and-standards/nfpa-704-standard-development/704 (accessed on 30 May 2025).
- European Parliament and the Council Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No. 1907/2006. 2008. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:353:0001:1355:EN:PDF (accessed on 30 May 2025).
- United States Environmental Protection Agency National Volatile Organic Compound Emission Standards for Consumer Products—Background for Promulgated Standards. 1998. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=2000NYMV.TXT (accessed on 30 May 2025).
- Evans, D.E.; Fox, G.P. Comparison of Diastatic Power Enzyme Release and Persistence during Modified Institute of Brewing 65°C and Congress Programmed Mashes. J. Am. Soc. Brew. Chem. 2017, 75, 302–311. [Google Scholar] [CrossRef]
- International Commission on Illumination CIE Recommendations on Uniform Color Spaces, Color-Difference Equations, and Metric Color Terms. Color. Res. Appl. 1977, 2, 5–6. [CrossRef]
- Hejna, A.; Barczewski, M.; Skórczewska, K.; Szulc, J.; Chmielnicki, B.; Korol, J.; Formela, K. Sustainable Upcycling of Brewers’ Spent Grain by Thermo-Mechanical Treatment in Twin-Screw Extruder. J. Clean. Prod. 2021, 285, 124839. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M.; Tyśkiewicz, K.; Olba-Zięty, E.; Graban, Ł.; Lajszner, W.; Załuski, D.; Wiejak, R.; Kamiński, P.; et al. How Does Extraction of Biologically Active Substances with Supercritical Carbon Dioxide Affect Lignocellulosic Biomass Properties? Wood Sci. Technol. 2020, 54, 519–546. [Google Scholar] [CrossRef]
- Marć, M.; Zabiegała, B. An Investigation of Selected Monoaromatic Hydrocarbons Released from the Surface of Polystyrene Lids Used in Coffee-to-Go Cups. Microchem. J. 2017, 133, 496–505. [Google Scholar] [CrossRef]
- Marć, M.; Namieśnik, J.; Zabiegała, B. The Miniaturised Emission Chamber System and Home-Made Passive Flux Sampler Studies of Monoaromatic Hydrocarbons Emissions from Selected Commercially-Available Floor Coverings. Build. Environ. 2017, 123, 1–13. [Google Scholar] [CrossRef]
- Bociaga, E.; Trzaskalska, M. Influence of Polymer Processing Parameters and Coloring Agents on Gloss and Color of Acrylonitrile-Butadiene-Styrene Terpolymer Moldings. Polimery 2016, 61, 544–550. [Google Scholar] [CrossRef]
- Hunt, R.W.G. The Reproduction of Colour; Wiley: Hoboken, NJ, USA, 2004; ISBN 9780470024256. [Google Scholar]
- López, F.; Valiente, J.M.; Baldrich, R.; Vanrell, M. Fast Surface Grading Using Color Statistics in the CIE Lab Space. In Pattern Recognition and Image Analysis; Springer: Berlin/Heidelberg, Germany, 2005; pp. 666–673. [Google Scholar]
- ADOBE SYSTEMS INCORPORATED Adobe® RGB. Color Image Encoding. 1998. Available online: https://www.adobe.com/digitalimag/adobergb.html (accessed on 30 May 2025).
- Rojas-Lema, S.; Torres-Giner, S.; Quiles-Carrillo, L.; Gomez-Caturla, J.; Garcia-Garcia, D.; Balart, R. On the Use of Phenolic Compounds Present in Citrus Fruits and Grapes as Natural Antioxidants for Thermo-Compressed Bio-Based High-Density Polyethylene Films. Antioxidants 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Massold, E.; Bähr, C.; Salthammer, T.; Brown, S.K. Determination of VOC and TVOC in Air Using Thermal De-sorption GC-MS – Practical Implications for Test Chamber Experiments. Chromatographia 2005, 62, 75–85. [Google Scholar] [CrossRef]
- Formela, K.; Marć, M.; Namieśnik, J.; Zabiegała, B. The Estimation of Total Volatile Organic Compounds Emissions Generated from Peroxide-Cured Natural Rubber/Polycaprolactone Blends. Microchem. J. 2016, 127, 30–35. [Google Scholar] [CrossRef]
- Zabiegała, B.; Sărbu, C.; Urbanowicz, M.; Namieśnik, J. A Comparative Study of the Performance of Passive Samplers. J. Air Waste Manag. Assoc. 2011, 61, 260–268. [Google Scholar] [CrossRef]
- Marć, M.; Zabiegała, B.; Namieśnik, J. Application of Passive Sampling Technique in Monitoring Research on Quality of Atmospheric Air in the Area of Tczew, Poland. Int. J. Environ. Anal. Chem. 2014, 94, 151–167. [Google Scholar] [CrossRef]
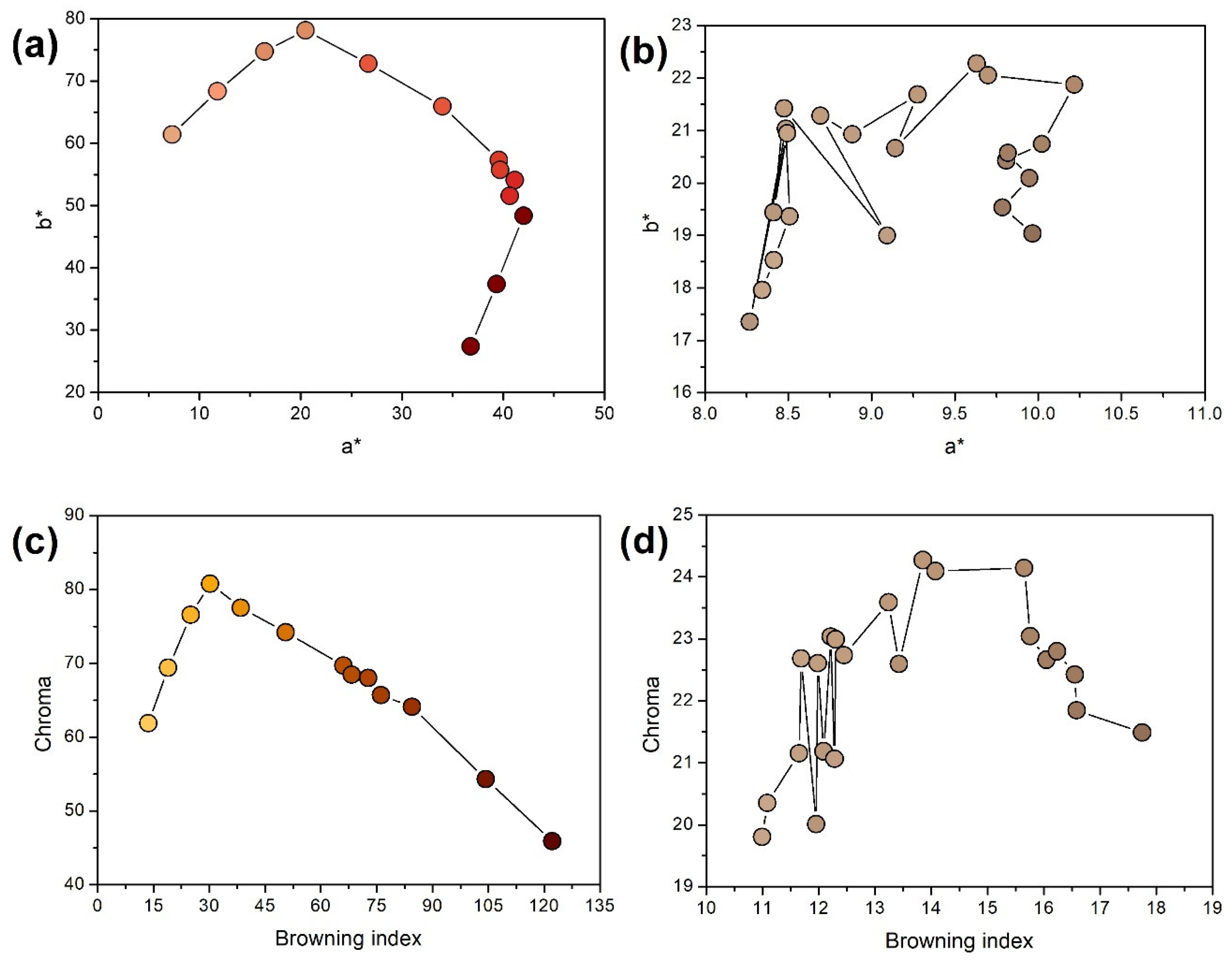
| BSG Sample | Beer | BSG | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Color, EBC | L* | a* | b* | BI | Chroma | Hue | Color | L* | a* | b* | BI | Chroma | Hue | Color | |
| Pilsen | 5.5 | 84.09 | 7.29 | 61.42 | 13.67 | 61.85 | 83.23 | 66.63 | 8.48 | 21.42 | 12.21 | 23.04 | 68.41 | ||
| Wheat 50% | 6.1 | 84.09 | 7.29 | 61.42 | 13.67 | 61.85 | 83.23 | 70.37 | 8.41 | 18.53 | 11.08 | 20.35 | 65.58 | ||
| Wheat 100% | 6.8 | 81.25 | 11.78 | 68.38 | 18.98 | 69.39 | 80.23 | 69.98 | 8.34 | 17.96 | 10.99 | 19.80 | 65.08 | ||
| Rye 50% | 7.2 | 81.25 | 11.78 | 68.38 | 18.98 | 69.39 | 80.23 | 65.27 | 8.41 | 19.44 | 12.09 | 21.18 | 66.60 | ||
| Rye 100% | 8.8 | 77.33 | 16.42 | 74.76 | 25.03 | 76.54 | 77.61 | 64.11 | 8.27 | 17.35 | 11.81 | 19.22 | 64.52 | ||
| Munich I | 14.8 | 67.38 | 26.67 | 72.8 | 38.50 | 77.53 | 69.88 | 65.15 | 9.63 | 22.28 | 13.85 | 24.27 | 66.62 | ||
| Munich II | 20.8 | 58.50 | 33.99 | 65.96 | 50.59 | 74.20 | 62.74 | 64.29 | 9.70 | 22.06 | 14.07 | 24.10 | 66.26 | ||
| Vienna | 7.5 | 81.25 | 11.78 | 68.38 | 18.98 | 69.39 | 80.23 | 67.63 | 8.49 | 20.95 | 11.98 | 22.61 | 67.93 | ||
| Abbey | 12.6 | 74.34 | 20.47 | 78.16 | 30.25 | 80.80 | 75.32 | 63.45 | 9.14 | 20.66 | 13.43 | 22.60 | 66.13 | ||
| Brown | 29.2 | 47.56 | 39.57 | 57.34 | 66.07 | 69.67 | 55.39 | 54.36 | 9.95 | 20.09 | 16.55 | 22.42 | 63.66 | ||
| Coffee light | 35.2 | 40.50 | 40.65 | 51.57 | 76.19 | 65.66 | 51.75 | 59.91 | 10.22 | 21.87 | 15.65 | 24.14 | 64.96 | ||
| Special B | 38.7 | 36.47 | 42.00 | 48.38 | 84.46 | 64.07 | 49.04 | 57.90 | 10.02 | 20.75 | 15.76 | 23.04 | 64.21 | ||
| Coffee 500 | 33.9 | 43.49 | 41.14 | 54.11 | 72.76 | 67.97 | 52.75 | 55.19 | 9.82 | 20.58 | 16.24 | 22.80 | 64.49 | ||
| Chocolate 400 | 30.6 | 45.63 | 39.69 | 55.74 | 68.35 | 68.43 | 54.55 | 55.72 | 9.81 | 20.43 | 16.05 | 22.66 | 64.35 | ||
| Chocolate 900 | 51.9 | 25.62 | 39.33 | 37.41 | 104.34 | 54.28 | 43.57 | 53.21 | 9.79 | 19.53 | 16.58 | 21.85 | 63.39 | ||
| Roasted barley | 62.9 | 18.76 | 36.77 | 27.40 | 122.23 | 45.86 | 36.69 | 50.08 | 9.97 | 19.04 | 17.75 | 21.49 | 62.36 | ||
| Smoked 25% | 6.6 | 84.09 | 7.29 | 61.42 | 13.67 | 61.85 | 83.23 | 67.25 | 8.89 | 20.93 | 12.45 | 22.74 | 67.00 | ||
| Smoked 50% | 7.7 | 81.25 | 11.78 | 68.38 | 18.98 | 69.39 | 80.23 | 65.85 | 9.28 | 21.69 | 13.24 | 23.59 | 66.84 | ||
| Grodziski 25% | 7.1 | 81.25 | 11.78 | 68.38 | 18.98 | 69.39 | 80.23 | 68.25 | 8.51 | 19.37 | 11.65 | 21.15 | 66.28 | ||
| Grodziski 50% | 8.6 | 81.25 | 11.78 | 68.38 | 18.98 | 69.39 | 80.23 | 67.73 | 9.09 | 19.00 | 12.28 | 21.06 | 64.42 | ||
| Peated 25% | 5.5 | 84.09 | 7.29 | 61.42 | 13.67 | 61.85 | 83.23 | 69.38 | 8.49 | 21.03 | 11.69 | 22.68 | 68.03 | ||
| Peated 50% | 5.5 | 84.09 | 7.29 | 61.42 | 13.67 | 61.85 | 83.23 | 67.28 | 8.69 | 21.29 | 12.30 | 22.99 | 67.78 | ||
| BSG Sample | Content, %DW | Elemental Content, %DW | Troloxeq, mg/L | TEAC, mg/gDW | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CWEs | HWEs | NDEs | NDF | ADF | Hemicellulose | Cellulose | Lignin | Ash | Protein * | C | H | N | S | Cl | |||
| Pilsen | 41.04 (1.32) | 48.01 (1.19) | 10.49 (0.65) | 41.50 (0.53) | 12.90 (0.01) | 28.60 (0.53) | 9.67 (0.10) | 3.22 (0.10) | 3.05 (0.01) | 19.75 (0.02) | 51.29 (0.41) | 7.55 (0.12) | 3.16 (0.01) | 0.143 (0.001) | 0.073 (0.003) | 22.36 (8.36) | 0.89 (0.33) |
| Wheat 50% | 51.80 (0.01) | 58.99 (0.19) | 10.96 (0.50) | 30.05 (0.30) | 9.69 (0.44) | 20.36 (0.13) | 7.27 (0.31) | 2.42 (0.13) | 2.65 (0.08) | 23.06 (0.29) | 50.42 (0.67) | 7.75 (0.06) | 3.69 (0.05) | 0.157 (0.001) | 0.027 (0.009) | 18.11 (7.36) | 0.72 (0.29) |
| Wheat 100% | 52.41 (0.28) | 60.69 (0.45) | 9.64 (0.08) | 29.67 (0.37) | 7.52 (0.18) | 22.15 (0.19) | 5.33 (0.16) | 2.20 (0.02) | 2.11 (0.03) | 22.99 (0.20) | 49.24 (0.46) | 7.37 (0.18) | 3.68 (0.03) | 0.169 (0.002) | 0.025 (0.014) | 10.48 (2.37) | 0.42 (0.09) |
| Rye 50% | 52.03 (0.85) | 58.13 (1.83) | 9.56 (0.55) | 32.32 (1.28) | 9.81 (0.67) | 22.50 (0.61) | 6.68 (0.57) | 3.13 (0.10) | 2.75 (0.07) | 21.11 (0.07) | 50.88 (0.66) | 7.61 (0.12) | 3.38 (0.01) | 0.141 (0.003) | 0.030 (0.004) | 21.76 (2.69) | 0.87 (0.11) |
| Rye 100% | 58.85 (0.28) | 63.74 (1.07) | 7.86 (0.29) | 28.40 (0.78) | 8.78 (0.67) | 19.62 (0.11) | 5.59 (0.54) | 3.19 (0.13) | 2.59 (0.03) | 19.66 (0.02) | 50.26 (0.63) | 7.48 (0.11) | 3.14 (0.01) | 0.139 (0.009) | 0.047 (0.004) | 21.42 (5.89) | 0.86 (0.24) |
| Munich I | 54.91 (0.83) | 61.34 (0.51) | 6.03 (0.07) | 32.63 (0.44) | 8.60 (0.18) | 24.03 (0.62) | 6.59 (0.04) | 2.01 (0.10) | 2.37 (0.15) | 17.93 (0.16) | 46.72 (0.46) | 6.47 (0.13) | 2.87 (0.03) | 0.176 (0.004) | 0.014 (0.002) | 16.86 (3.71) | 0.67 (0.15) |
| Munich II | 52.41 (0.04) | 59.82 (0.02) | 6.51 (0.42) | 33.68 (0.40) | 10.38 (0.16) | 23.30 (0.24) | 7.61 (0.09) | 2.77 (0.06) | 2.56 (0.02) | 19.79 (0.32) | 47.11 (0.35) | 6.51 (0.12) | 3.17 (0.05) | 0.200 (0.004) | 0.020 (0.001) | 20.66 (11.19) | 0.83 (0.45) |
| Vienna | 52.00 (0.09) | 58.18 (0.06) | 7.65 (0.16) | 34.16 (0.22) | 9.69 (0.15) | 24.48 (0.06) | 7.29 (0.01) | 2.40 (0.16) | 2.63 (0.05) | 16.97 (0.01) | 46.56 (0.31) | 6.43 (0.09) | 2.72 (0.01) | 0.170 (0.004) | 0.023 (0.001) | 18.69 (3.62) | 0.75 (0.15) |
| Abbey | 47.91 (0.22) | 53.89 (0.36) | 9.35 (0.01) | 36.76 (0.37) | 10.31 (0.42) | 26.45 (0.05) | 7.45 (0.19) | 2.80 (0.23) | 2.63 (0.05) | 18.06 (0.15) | 48.00 (0.10) | 6.67 (0.05) | 2.89 (0.02) | 0.180 (0.002) | 0.015 (0.004) | 13.14 (1.34) | 0.53 (0.05) |
| Brown | 52.14 (0.37) | 56.95 (0.42) | 7.58 (0.05) | 35.47 (0.47) | 13.13 (0.20) | 22.34 (0.27) | 7.77 (0.23) | 5.36 (0.02) | 2.63 (0.01) | 18.47 (0.21) | 48.13 (0.20) | 6.67 (0.20) | 2.96 (0.03) | 0.180 (0.007) | 0.026 (0.003) | 15.70 (2.96) | 0.63 (0.12) |
| Coffee light | 55.46 (0.13) | 61.95 (0.08) | 7.56 (0.35) | 30.49 (0.28) | 9.98 (0.41) | 20.51 (0.13) | 7.17 (0.23) | 2.81 (0.17) | 2.43 (0.07) | 17.65 (0.25) | 48.15 (0.47) | 6.68 (0.14) | 2.82 (0.04) | 0.172 (0.002) | 0.034 (0.004) | 19.79 (2.67) | 0.79 (0.11) |
| Special B | 50.64 (0.50) | 56.49 (0.28) | 7.09 (0.16) | 36.42 (0.13) | 11.40 (0.46) | 25.02 (0.33) | 6.94 (0.18) | 4.46 (0.28) | 2.53 (0.03) | 18.67 (0.11) | 47.97 (0.03) | 6.70 (0.01) | 2.99 (0.02) | 0.172 (0.003) | 0.060 (0.005) | 19.10 (11.87) | 0.76 (0.47) |
| Coffee 500 | 52.29 (0.08) | 57.63 (0.20) | 7.06 (0.32) | 35.31 (0.12) | 12.66 (0.10) | 22.65 (0.02) | 7.57 (0.19) | 5.09 (0.08) | 2.66 (0.09) | 17.35 (0.06) | 47.56 (0.62) | 6.57 (0.13) | 2.78 (0.01) | 0.171 (0.001) | 0.021 (0.003) | 18.22 (4.55) | 0.73 (0.18) |
| Chocolate 400 | 52.88 (0.18) | 57.57 (0.79) | 7.08 (0.58) | 35.35 (0.21) | 12.64 (0.13) | 22.71 (0.34) | 8.32 (0.11) | 4.32 (0.24) | 2.73 (0.04) | 17.49 (0.27) | 48.04 (0.37) | 6.66 (0.11) | 2.80 (0.04) | 0.175 (0.001) | 0.028 (0.002) | 18.94 (9.86) | 0.76 (0.39) |
| Chocolate 900 | 48.64 (0.16) | 53.12 (0.38) | 8.19 (0.07) | 38.69 (0.31) | 12.98 (0.18) | 25.71 (0.13) | 7.76 (0.03) | 5.22 (0.21) | 2.77 (0.01) | 18.40 (0.03) | 49.01 (0.01) | 6.72 (0.05) | 2.94 (0.01) | 0.179 (0.002) | 0.037 (0.002) | 17.56 (6.26) | 0.70 (0.25) |
| Roasted barley | 48.57 (0.34) | 54.28 (0.01) | 8.51 (0.35) | 37.20 (0.34) | 12.72 (0.33) | 24.48 (0.02) | 8.18 (0.25) | 4.54 (0.07) | 2.89 (0.03) | 17.69 (0.09) | 48.08 (0.09) | 6.57 (0.01) | 2.83 (0.01) | 0.172 (0.002) | 0.016 (0.004) | 15.42 (6.83) | 0.62 (0.27) |
| Smoked 25% | 43.26 (0.40) | 50.13 (0.33) | 10.56 (0.21) | 39.31 (0.12) | 10.46 (0.10) | 28.85 (0.22) | 7.26 (0.24) | 3.20 (0.14) | 2.63 (0.01) | 19.10 (0.17) | 48.23 (0.43) | 6.68 (0.02) | 3.06 (0.03) | 0.190 (0.001) | 0.037 (0.002) | 19.06 (1.55) | 0.76 (0.06) |
| Smoked 50% | 43.04 (0.29) | 49.61 (0.28) | 11.72 (0.15) | 38.67 (0.13) | 10.11 (0.09) | 28.56 (0.04) | 6.30 (0.17) | 3.81 (0.08) | 2.77 (0.08) | 20.12 (0.06) | 48.84 (0.18) | 6.81 (0.01) | 3.22 (0.01) | 0.195 (0.001) | 0.010 (0.004) | 17.63 (1.34) | 0.71 (0.05) |
| Grodziski 25% | 47.45 (0.41) | 56.99 (0.19) | 13.30 (0.31) | 29.71 (0.11) | 7.82 (0.43) | 21.89 (0.32) | 5.40 (0.29) | 2.43 (0.14) | 2.16 (0.03) | 17.79 (0.09) | 47.60 (0.08) | 6.72 (0.05) | 2.85 (0.01) | 0.179 (0.001) | 0.018 (0.001) | 16.30 (6.99) | 0.65 (0.28) |
| Grodziski 50% | 49.62 (0.17) | 59.65 (0.60) | 11.01 (0.16) | 29.34 (0.43) | 7.77 (0.05) | 21.57 (0.48) | 5.38 (0.06) | 2.39 (0.01) | 2.12 (0.02) | 18.16 (0.11) | 48.28 (0.04) | 6.77 (0.21) | 2.91 (0.02) | 0.184 (0.003) | 0.030 (0.002) | 11.68 (2.68) | 0.47 (0.11) |
| Peated 25% | 46.16 (0.19) | 52.57 (0.18) | 9.19 (0.06) | 38.24 (0.12) | 10.17 (0.11) | 28.07 (0.23) | 7.54 (0.13) | 2.63 (0.02) | 2.51 (0.05) | 17.92 (0.01) | 47.94 (0.10) | 6.71 (0.06) | 2.87 (0.01) | 0.179 (0.004) | 0.032 (0.002) | 16.50 (0.14) | 0.66 (0.01) |
| Peated 50% | 42.84 (0.23) | 48.64 (0.09) | 7.89 (0.21) | 43.48 (0.12) | 11.48 (0.20) | 32.00 (0.08) | 8.18 (0.09) | 3.30 (0.11) | 2.70 (0.05) | 18.78 (0.19) | 49.18 (0.39) | 6.82 (0.02) | 3.01 (0.03) | 0.198 (0.003) | 0.039 (0.002) | 13.06 (2.02) | 0.52 (0.08) |
| Content, %DW | Reference | |||||
|---|---|---|---|---|---|---|
| NDF | Hemicellulose | Cellulose | Lignin | Ash | Proteins | |
| - | - | - | - | 3.8 | 26.9 | [39] |
| - | - | - | - | 3.4 | 21.6–26.4 | [37] |
| - | - | - | - | 3.3 | 26.7 | [34] |
| - | 25.4 | 21.8 | 11.9 | 2.4 | 24.0 | [35] |
| - | 16.8 | 28.4 | 27.8 | 4.6 | - | [27] |
| 51.0 | - | - | - | 4.1 | 23.4 | [30] |
| 43.5 | - | - | - | 4.1 | 22.6 | [28] |
| 42.2 | - | - | - | 4.7 | 22.8 | [29] |
| 48.2 | - | 22.2 | - | - | 22.1 | [36] |
| - | 21.7 | 19.3 | 19.4 | 4.2 | 24.7 | [31] |
| - | 12.0 | 40.2 | 11.5 | 3.3 | 14.2 | [32] |
| - | 22.2 | 26.8 | 14.1 | - | - | [26] |
| - | 21.9 | 29.6 | 21.7 | 1.2 | 24.6 | [33] |
| 24.0–40.1 | - | - | - | 3.1–3.4 | 15.2–23.9 | [13] |
| 43.0 | - | - | - | 3.6 | 31.0 | [38] |
| - | - | - | - | 3.3–4.3 | 22.2–30.2 | [11] |
| 41.5 | 28.6 | 9.7 | 3.2 | 3.1 | 19.8 | Presented study (Pilsen malt) |
| 28.4–43.5 | 19.6–32.0 | 5.3–9.7 | 2.0–5.2 | 2.1–3.1 | 17.0–23.1 | Presented study (all samples) |
| BSG Sample | Presentation | BGIZ, mm | ZMIBG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli, P. aeruginosa, S. aureus, S. epidermidis, S. pneumoniae | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Control agar disk without BSG |  | − | − | − | − | − | − | − | − | − | − |
| Pilsen |  | − | − | − | − | − | + | − P | + | + | + P |
| Wheat 50% |  | − | − | − | − | − | + | − P | + | + | + P |
| Wheat 100% |  | − | − | − | − | − | + | − P | + | + | + P |
| Rye 50% |  | − | − | − | − | − | + | − | + | + | + P |
| Rye 100% |  | − | − | − | − | − | + | − P | + | + | + P |
| Munich I |  | − | − | − | − | − | + | − P | + | + | + P |
| Munich II |  | − | − | − | − | − | + | − P | + | + | + P |
| Vienna |  | − | − | − | − | − | + | − P | + | + | + P |
| Abbey |  | − | − | − | − | − | + | − P | + | + | + P |
| Brown |  | − | − | − | − | − | + | − P | + | + | + P |
| Coffee light |  | − | − | − | − | − | + | − P | + | + | + P |
| Special B |  | − | − | − | − | − | + | − P | + | + | + P |
| Coffee 500 |  | − | − | − | − | − | + | − | + | + | + P |
| Chocolate 400 |  | − | − | − | − | − | + | − | + | + | + P |
| Chocolate 900 |  | − | − | − | − | − | + | − P | + | + | + P |
| Roasted barley |  | − | − | − | − | − | + | − | + | + | + P |
| Smoked 25% |  | − | − | − | − | − | + | − P | + | + | + P |
| Smoked 50% |  | − | − | − | − | − | + | − P | + | + | + P |
| Grodziski 25% |  | − | − | − | − | − | + | − | + | + | + P |
| Grodziski 50% |  | − | − | − | − | − | + | − | + | + | + P |
| Peated 25% |  | − | − | − | − | − | + | + P | + | + | + P |
| Peated 50% |  | − | − | − | − | − | + | + P | + | + | + P |
| BSG Sample | TVOC | Benzene | Toluene | Ethylbenzene | p,m-Xylene | Styrene | α-Pinene | Camphene | β-Pinene | 3-Carene + α-Terpinene | (R)-(+)-Limonene | γ-Terpinene | L-(-)-Fenchone | Fenchol | (1R)-(+)-Camphor | Isoborneol + DL-Menthol | (+/−)-β-Citronellol | (R)-(+)-Pulegone | Geranyl Acetate | α-Cedrene | α-Humulene | Nerolidol 1 | (+)-Cedrol | α-Bisabolol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ng/g | ||||||||||||||||||||||||
| Pilsen | 1647 | 15.0 | LD | LD | LD | LD | LQ | 5.6 | LD | LD | 3.8 | 6.5 | LD | LD | 8.3 | 16.0 | LD | LD | LQ | LD | LD | LD | LD | LD |
| Wheat 50% | 1626 | 16.6 | LQ | LD | LD | LD | 27.7 | 35.2 | LQ | LD | 6.4 | LD | LD | LD | 4.7 | 20.2 | LD | LD | LQ | LQ | LD | LD | LD | LD |
| Wheat 100% | 1434 | 11.0 | LD | LD | LD | LD | 5.0 | LD | LD | 1.8 | 7.4 | 2.3 | LD | LD | LQ | 9.6 | LD | LQ | LD | LD | LD | LD | LD | LD |
| Rye 50% | 1997 | 12.4 | LD | LD | LQ | LD | LQ | 12.6 | LQ | LD | 6.3 | LQ | LD | 4.1 | LD | 13.2 | LD | LQ | LQ | LD | LD | LD | LD | LD |
| Rye 100% | 1844 | 11.4 | LD | LD | LD | LD | LQ | LD | LD | LD | 6.9 | 2.3 | LD | LD | LD | 11.4 | LD | 14.2 | LD | LD | LD | LD | LD | LD |
| Munich I | 2617 | 11.5 | LD | LD | LD | LD | 2.6 | 16.0 | 4.5 | LD | 7.7 | 2.4 | LD | LD | LQ | 17.3 | LD | 4.4 | LD | LD | LD | LD | LD | LD |
| Munich II | 5889 | 16.0 | LQ | LD | 3.6 | 10.3 | 34.9 | 15.8 | LQ | 2.1 | 8.4 | 3.3 | LD | LD | LD | 4.8 | LD | LQ | 8.4 | LD | LD | LD | LD | LD |
| Vienna | 1240 | 12.2 | LQ | LD | LQ | 4.2 | 4.2 | 18.1 | LD | LD | 5.8 | LQ | LD | LD | 11.7 | 23.8 | LQ | LQ | LQ | LQ | LD | LD | LD | LD |
| Abbey | 2289 | 12.4 | LD | LD | LD | LD | 10.6 | 21.2 | LD | 2.3 | 14.1 | 2.4 | LD | 4.4 | 7.7 | 10.5 | LD | 8.5 | 5.0 | 8.8 | LD | LD | LD | LD |
| Brown | 2195 | 26.7 | LQ | LD | 2.9 | 6.0 | LD | LD | LD | LD | LQ | LD | LD | LD | 5.2 | 28.5 | LD | LD | LQ | 1.9 | LD | LD | LD | LD |
| Coffee light | 2526 | 23.4 | LQ | LD | 3.9 | LD | 17.8 | 9.3 | LQ | LD | 7.4 | LD | LD | LD | LD | 15.2 | LD | LQ | LQ | LQ | LD | LD | LD | LD |
| Special B | 1276 | 13.9 | LD | LD | LD | 5.3 | LQ | 5.1 | LD | 1.1 | 8.5 | 2.4 | LD | 7.0 | 3.2 | 11.0 | LD | 15.3 | LD | LQ | LD | LD | LD | LD |
| Coffee 500 | 1263 | 10.8 | LQ | LD | 2.4 | 3.1 | LD | 26.7 | LD | LD | 8.8 | LD | LD | LD | 5.9 | 12.0 | LD | 4.0 | 9.7 | LQ | LD | LD | LD | LD |
| Chocolate 400 | 1469 | 19.1 | LQ | LD | LD | LD | LD | 8.1 | LQ | LD | 12.2 | LD | LD | LD | LD | 17.0 | LD | LQ | 4.4 | LD | LD | LD | LD | LD |
| Chocolate 900 | 1783 | 15.3 | LD | LD | LD | LD | 2.6 | LD | LD | LD | 11.4 | 28.1 | LD | 4.3 | 3.5 | 13.3 | LD | LD | LQ | LD | LD | LD | LD | LD |
| Roasted barley | 1428 | 13.0 | 14.5 | LD | LD | 8.4 | 8.8 | 12.3 | LD | LD | 4.5 | 7.3 | LD | LD | 14.6 | 18.1 | LD | 4.6 | LD | LD | LD | LD | LD | LD |
| Smoked 25% | 1779 | 14.4 | 16.7 | LD | LD | 5.7 | 5.8 | 24.1 | LD | LD | 7.2 | 25.9 | 5.8 | 5.2 | 2.0 | 24.8 | 10.1 | 3.8 | 5.0 | 28.9 | 11.6 | LD | 9.1 | LD |
| Smoked 50% | 1849 | 15.0 | 17.1 | LD | LD | 5.8 | 6.1 | 25.5 | LD | LD | 7.4 | 26.5 | 6.0 | 5.4 | 2.1 | 25.7 | 10.3 | 3.9 | 5.1 | 29.5 | 12.0 | LD | 9.3 | LD |
| Grodziski 25% | 1284 | 12.6 | LD | LD | LD | LD | 4.7 | 15.8 | LD | LD | 7.9 | 12.8 | LD | 6.6 | 7.0 | 14.6 | LD | 3.9 | LD | LD | LD | LD | LD | LD |
| Grodziski 50% | 892 | 9.9 | LD | LD | LD | LD | LD | 5.7 | LD | LD | 4.0 | 27.8 | LD | LD | LD | 11.8 | LD | LQ | LD | LD | LD | LD | LD | LD |
| Peated 25% | 1637 | 12.4 | 4.3 | LD | LD | LD | 6.7 | 24.3 | LD | LD | 6.9 | 12.9 | LD | LD | 6.3 | 24.8 | LD | 4.6 | 6.5 | LD | 5.4 | LD | LD | LD |
| Peated 50% | 1492 | 13.8 | LD | LD | LD | 5.6 | 7.2 | LD | LQ | LD | 5.4 | 14.6 | 3.7 | 7.2 | 4.1 | 16.7 | LQ | 5.3 | LD | LQ | LD | LD | 10.6 | LD |
| Name | Producer | Extract, % | Color, EBC | Total Protein, % | Max. Kilning Temperature, °C | Additional Information |
|---|---|---|---|---|---|---|
| Pilsen | La Malterie du Château SA (Beloeil, Belgium) | >82.0 | 3.5 | <12.0 | 80–85 | - |
| Wheat | Palatia Malz GmbH (Kreimbach-Kaulbach, Germany) | >82.0 | 3.6–6.0 | <13.5 | 80–85 * | - |
| Rye | Viking Malt Sp. z o.o. (Sierpc, Poland) | >80 | 4.0–10.0 | <11 * | 72–80 | - |
| Munich Light | La Malterie du Château SA (Beloeil, Belgium) | >80 | 13–17 | <12 | 100–105 | - |
| Munich | La Malterie du Château SA (Beloeil, Belgium) | >80 | 21–28 | <12 | 100–105 | - |
| Vienna | La Malterie du Château SA (Beloeil, Belgium) | >80 | 4.0–7.0 | <12 | 85–90 | - |
| Abbey | La Malterie du Château SA (Beloeil, Belgium) | >78 | 41–49 | <11.5 | 110 | - |
| Brown | Thomas Fawcett & Sons Ltd. (Castleford, UK) | >70.0 | 175–200 | <11.6 | 175 * | - |
| Cafe Light | La Malterie du Château SA (Beloeil, Belgium) | >77 | 220–280 | <11.5 * | 200 | - |
| Special B | La Malterie du Château SA (Beloeil, Belgium) | >77 | 260–320 | <12.5 * | 93 * | - |
| Cafe | La Malterie du Château SA (Beloeil, Belgium) | >75.5 | 420–520 | <11.5 * | 220 | - |
| Czekoladowy jasny | Viking Malt Sp. z o.o. (Sierpc, Poland) | >68 | 350–450 | <11.5 * | 220 * | - |
| Czekoladowy ciemny | Viking Malt Sp. z o.o. (Sierpc, Poland) | >67 | 800–1000 | <11.5 * | 220 * | - |
| Roasted barley | La Malterie du Château SA (Beloeil, Belgium) | >65 | 1000–1400 | <11.6 * | 230 | - |
| Smoked | La Malterie du Château SA (Beloeil, Belgium) | >77 | 4.0–12.0 | <11.5 | 85 * | Phenols 1.6–4.0 ppm |
| Grodziski | Viking Malt Sp. z o.o. (Sierpc, Poland) | >81 | 8.0–12.0 | <13.5 | 85 * | Phenols 5–10 ppm |
| Peated | La Malterie du Château SA (Beloeil, Belgium) | >81 | 3.5 | <11.7 | 85 * | Phenols 5–10 ppm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hejna, A.; Aniśko-Michalak, J.; Skórczewska, K.; Barczewski, M.; Sulima, P.; Przyborowski, J.A.; Cieśliński, H.; Marć, M. Brewers’ Spent Grain from Different Types of Malt: A Comprehensive Evaluation of Appearance, Structure, Chemical Composition, Antimicrobial Activity, and Volatile Emissions. Molecules 2025, 30, 2809. https://doi.org/10.3390/molecules30132809
Hejna A, Aniśko-Michalak J, Skórczewska K, Barczewski M, Sulima P, Przyborowski JA, Cieśliński H, Marć M. Brewers’ Spent Grain from Different Types of Malt: A Comprehensive Evaluation of Appearance, Structure, Chemical Composition, Antimicrobial Activity, and Volatile Emissions. Molecules. 2025; 30(13):2809. https://doi.org/10.3390/molecules30132809
Chicago/Turabian StyleHejna, Aleksander, Joanna Aniśko-Michalak, Katarzyna Skórczewska, Mateusz Barczewski, Paweł Sulima, Jerzy Andrzej Przyborowski, Hubert Cieśliński, and Mariusz Marć. 2025. "Brewers’ Spent Grain from Different Types of Malt: A Comprehensive Evaluation of Appearance, Structure, Chemical Composition, Antimicrobial Activity, and Volatile Emissions" Molecules 30, no. 13: 2809. https://doi.org/10.3390/molecules30132809
APA StyleHejna, A., Aniśko-Michalak, J., Skórczewska, K., Barczewski, M., Sulima, P., Przyborowski, J. A., Cieśliński, H., & Marć, M. (2025). Brewers’ Spent Grain from Different Types of Malt: A Comprehensive Evaluation of Appearance, Structure, Chemical Composition, Antimicrobial Activity, and Volatile Emissions. Molecules, 30(13), 2809. https://doi.org/10.3390/molecules30132809










