Natural-Origin Edible Gels as Delivery Systems for Green Tea Extract: Formulation, Physicochemical, and Biopharmaceutic Profile Assessment
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Dry Green Tea Extract
4.2.2. Development of Experimental Edible Gels
4.2.3. High-Performance Liquid Chromatography Assay for the Determination of Individual Chemical Compounds
4.2.4. Determination of Total Phenolic Compounds by Spectrophotometric Method
4.2.5. Determination of pH Value
4.2.6. Determination of the Flow Curve of Gels
4.2.7. Dissolution Test
4.2.8. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascuta, M.S.; Varvara, R.A.; Teleky, B.E.; Szabo, K.; Plamada, D.; Nemeş, S.A.; Mitrea, L.; Martău, G.A.; Ciont, C.; Călinoiu, L.F.; et al. Polysaccharide-Based Edible Gels as Functional Ingredients: Characterization, Applicability, and Human Health Benefits. Gels 2022, 8, 524. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Shavandi, A.; Jodjaja, T.; Birch, J.; The, S.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Saeedi, P.; Bekhit, A.A. Flaxseed: Composition, detoxification, utilization, and opportunities. Biocatal. Agric. Biotechnol. 2018, 13, 129–152. [Google Scholar] [CrossRef]
- Cao, T.; Wei, Z.; Xue, C. Recent advances in nutraceutical delivery systems constructed by protein–polysaccharide complexes: A systematic review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70115. [Google Scholar] [CrossRef]
- Korčok, M.; Calle, J.; Veverka, M.; Vietoris, V. Understanding the health benefits and technological properties of β-glucan for the development of ea. Crit. Rev. Food Sci. Nutr. 2023, 63, 11504–11521. [Google Scholar] [CrossRef]
- Miao, W.; Jiang, H.; Li, X.; Sang, S.; Jiang, L.; Lin, Q.; Zhang, Z.; Chen, L.; Long, J.; Jiao, A.; et al. Recent advances in natural gums as additives to help the construction and application of edible biopolymer gels: The example of hydrogels and oleogels. Crit. Rev. Food Sci. Nutr. 2024, 64, 12702–12719. [Google Scholar] [CrossRef]
- Maruyama, S.; Streleckaya, N.A.; Lim, J. Clean label: Why this ingredient but not that one? Food Qual. Prefer. 2021, 87, 104062. [Google Scholar] [CrossRef]
- Haseeb, M.T.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A.; Sheikh, F.A. Flaxseed (Linum usitatissimum) mucilage: A versatile stimuli responsive functional biomaterial for pharmaceuticals and healthcare. Int. J. Biol. Macromol. 2024, 278, 134817. [Google Scholar] [CrossRef]
- Noreen, S.; Arshad, M.; Ghumman, S.A.; Noureen, S.; Malik, M.Z.; Bukhari, S.N.A. Smart gel system of Linum usitatissimum mucilage as a vehicle of an ophthalmic drug. Bioinspired Biomim. Nanobiomater. 2018, 7, 90–99. [Google Scholar] [CrossRef]
- Ray, A.; Sharma, A.; Singhal, R.S. Valorization of arabinoxylans from Linum usitatissimum (flaxseed) and galactomannans from Leucaena leucocephala (subabul) to develop hybrid hydrogels: Rheological, morphological and thermal characterization. Ind. Crops Prod. 2022, 178, 114575. [Google Scholar] [CrossRef]
- Kumar, L.; Sehrawat, R. Oat proteins: A perspective on functional properties. LWT—Food Sci. Technol. 2021, 152, 112307. [Google Scholar] [CrossRef]
- Scientific Opinion on the Substantiation of Health Claims Related to Beta-Glucans from Oats and Barley and Maintenance of Normal Blood LDL-Cholesterol Concentrations (ID 1236, 1299), Increase in Satiety Leading to a Reduction in Energy Intake (ID 851, 852), Reduction of Post-Prandial Glycaemic Responses (ID 821, 824), and “Digestive Function” (ID 850) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. Available online: https://data.europa.eu/doi/10.2903/j.efsa.2011.2207 (accessed on 30 April 2025).
- Skendi, A.; Biliaderis, C.G.; Lazaridou, A.; Izydorczyk, M.S. Structure and rheological properties of water soluble b-glucans from oat cultivars of Avena sativa and Avena bysantina. J. Cereal Sci. 2003, 38, 15–31. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2024; Monograph 04/2024:2668, Green Tea; Available online: https://pheur.edqm.eu/subhome/11-8 (accessed on 30 April 2025).
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef] [PubMed]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Dietz, C.; Dekker, M. Effect of Green Tea Phytochemicals on Mood and Cognition. Curr. Pharm. Des. 2017, 23, 2876–2905. [Google Scholar] [CrossRef]
- Benlloch-Tinoco, M.; Gentile, P.; Taylor, L.; Giron-Hernandez, J. Alginate edible films as delivery systems for green tea polyphenols. Food Hydrocoll. 2025, 158, 110518. [Google Scholar] [CrossRef]
- Ulfa, L.R.; Ningrum, A.; Supriyadi. Effect of edible coating of gelatin-sodium alginate with the addition of green tea (Camellia sinensis) extract on the characteristics of star fruit (Averrhoa carambola L.) during storage. J. Food Sci. 2024, 89, 6217–6231. [Google Scholar] [CrossRef]
- Radi, M.; Firouzi, E.; Akhavan, H.; Amiri, S. Effect of Gelatin-Based Edible Coatings Incorporated with Aloe vera and Black and Green Tea Extracts on the Shelf Life of Fresh-Cut Oranges. J. Food Qual. 2017, 2017, 9764650. [Google Scholar] [CrossRef]
- Hamann, D.; Puton, B.M.S.; Comin, T.; Colet, R.; Valduga, E.; Zeni, J.; Steffens, J.; Junges, A.; Backes, G.T.; Cansian, R.L.; et al. Active edible films based on green tea extract and gelatin for coating of fresh sausage. Meat Sci. 2022, 194, 108966. [Google Scholar] [CrossRef]
- Giménez, B.; Lopez de Lacey, A.; Perez-Santin, E.; Lopez-Caballero, M.E.; Montero, P. Release of active compounds from agar and agar-gelatin films with green tea extract. Food Hydrocoll. 2013, 30, 264–271. [Google Scholar] [CrossRef]
- Kawee-ai, A. Advancing Gel Systems with Natural Extracts: Antioxidant, Antimicrobial Applications, and Sustainable Innovations. Gels 2025, 11, 125. [Google Scholar] [CrossRef]
- Puligundla, P.; Lim, S. A Review of Extraction Techniques and Food Applications of Flaxseed Mucilage. Foods 2022, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Xia, Q.; Liu, L.; Wu, Z.; Pan, D. Recent advances of cereal β-glucan on immunity with gut microbiota regulation functions and its intelligent gelling application. Crit. Rev. Food Sci. Nutr. 2023, 63, 3895–3911. [Google Scholar] [CrossRef]
- Fernandez, P.; Pablos, F.; Martin, M.J.; Gonzalez, G. Study of Catechin and Xanthine Tea Profiles as Geographical Tracers. Study Catechin Xanthine Tea Profiles Geogr. Tracers 2002, 50, 1833–1839. [Google Scholar] [CrossRef]
- Bansal, S.; Syan, N.; Mathur, P.; Choudhary, S. Pharmacological profile of green tea and its polyphenols: A review. Med. Chem. Res. 2012, 21, 3347–3360. [Google Scholar] [CrossRef]
- Green, R.J.; Murphy, A.S.; Schulz, B.; Watkins, B.A.; Ferruzzi, M.G. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol. Nutr. Food Res. 2007, 51, 1152–1162. [Google Scholar] [CrossRef]
- Ye, Y.; Yan, J.; Cui, J.; Mao, S.; Li, M.; Liao, X.; Tong, H. Dynamic changes in amino acids, catechins, caffeine and gallic acid in green tea during withering. J. Food Compos. Anal. 2018, 66, 98–108. [Google Scholar] [CrossRef]
- Wong, M.; Sirisena, S.; Ng, K. Phytochemical profile of differently processed tea: A review. J. Food Sci. Technol. 2022, 87, 1925–1942. [Google Scholar] [CrossRef]
- Bruckner-Guhmann, M.; Kratzsh, A.; Sozer, N.; Drusch, S. Oat protein as plant-derived gelling agent: Properties and potential of modification. Future Foods 2021, 4, 100053. [Google Scholar] [CrossRef]
- Singh, R.; De, S.; Belkheir, A. Avena sativa (Oat), A Potential Neutraceutical and Therapeutic Agent: An Overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F. Oat proteins as emerging ingredients for food formulation: Where we stand? Eur. Food Res. Technol. 2021, 247, 535–544. [Google Scholar] [CrossRef]
- El Hosary, R.; El-Mancy, S.M.S.; El Deeb, K.S.; Eid, H.H.; El Tantawy, M.E.; Shams, M.M.; Samir, R.; Assar, N.H.; Sleem, A.A. Efficient wound healing composite hydrogel using Egyptian Avena sativa L. polysaccharide containing β-glucan. Int. J. Biol. Macromol. 2020, 149, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Duan, R.; Yu, H.; Liu, S.; Bao, Y. Research Progress in the Extraction, Structural Characteristics, Bioactivity, and Commercial Applications of Oat β-Glucan: A Review. Foods 2024, 13, 4160. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Kabir, Y.; Huffman, F.G. Functional Foods from Cereal Grains. Int. J. Food Prop. 2007, 10, 231–244. [Google Scholar] [CrossRef]
- Li, D.; Chen, M.; Meng, X.; Sun, Y.; Liu, R.; Sun, T. Extraction, purification, structural characteristics, bioactivity and potential applications of polysaccharides from Avena sativa L.: A review. Int. J. Biol. Macromol. 2024, 265, 130891. [Google Scholar] [CrossRef]
- Johnsson, P.; Peerlkamp, N.; Kamal-Eldin, A.; Andresson, R.E.; Andersson, R.; Lunfgren, L.N.; Åman, P. Polymeric fractions containing phenol glucosides in flaxseed. Food Chem. 2002, 76, 207–212. [Google Scholar] [CrossRef]
- Herchi, W.; Arraez-Roman, D.; Trabelsi, H.; Bouali, I.; Boukhchina, S.; Kallel, H.; Segura-Carretero, A.; Fernández-Gutierrez, A. Phenolic Compounds in Flaxseed: A Review of Their Properties and Analytical Methods. An Overview of the Last Decade. J. Oleo Sci. 2014, 63, 7–14. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 45–55. [Google Scholar] [CrossRef]
- Kaunaite, V.; Vilkickyte, G.; Raudone, L. Phytochemical Diversity and Antioxidant Potential of Wild Heather (Calluna vulgaris L.) Aboveground Parts. Plants 2022, 11, 2207. [Google Scholar] [CrossRef]
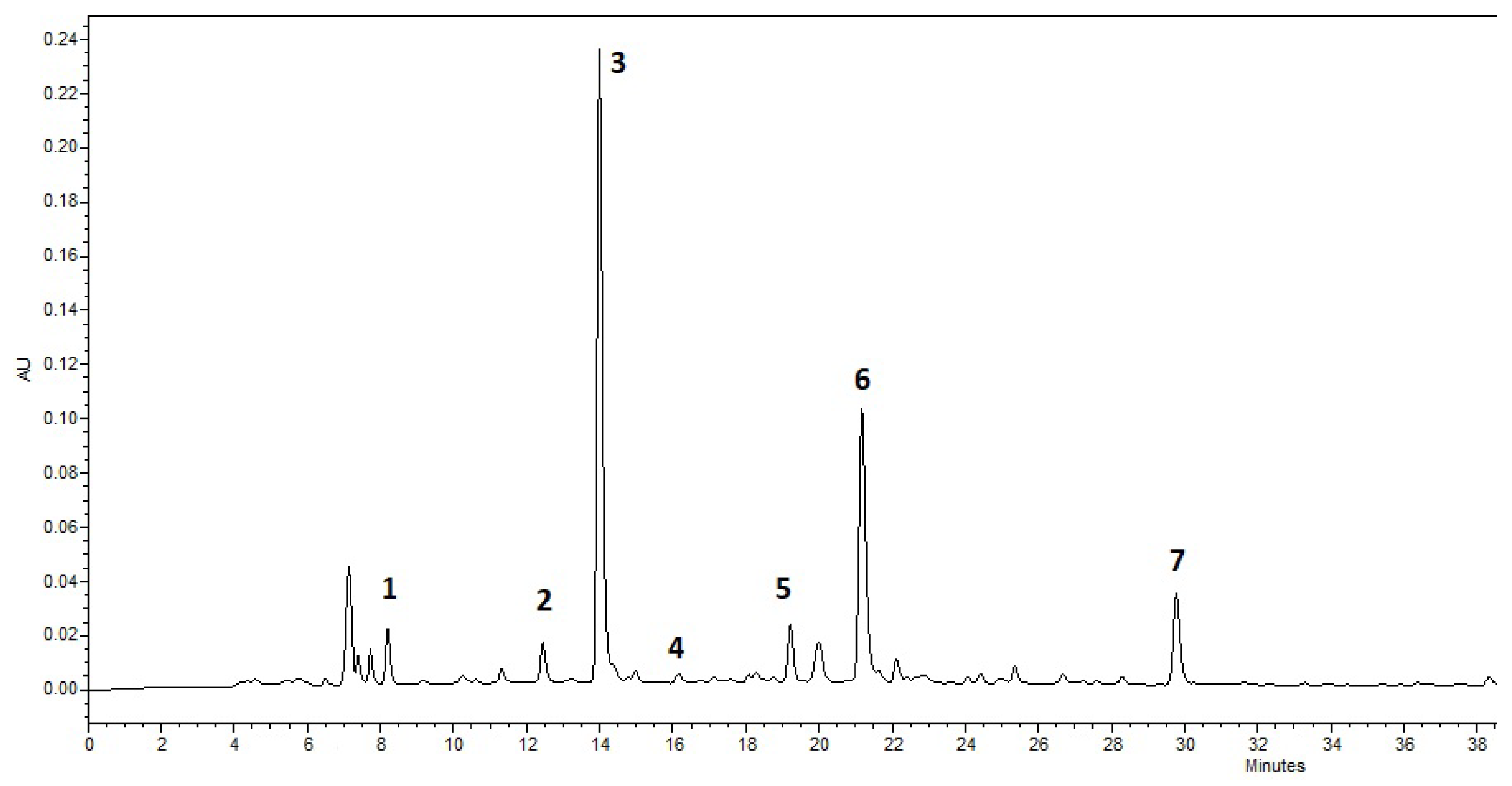









| Compound | Retention Time | Amount (mg/g (SD)) |
|---|---|---|
| Gallic acid | 8.199 | 2.81 (0.31) |
| Epigallocatechin | 12.447 | 29.42 (1.5) |
| Caffeine | 13.997 | 57.05 (1.03) |
| Catechin | 16.164 | 3.55 (0.25) |
| Epicatechin | 19.215 | 18.37 (0.67) |
| Epigallocatechin–3–gallate | 21.174 | 38.39 (1.72) |
| Epicatechin–gallate | 29.774 | 11.72 (0.69) |
| Formulation | Consistency Coefficient, Pa·s | Flow Index |
|---|---|---|
| NL-1 | 2.11 (0.37) a | 0.47 (0.02) a |
| ML-1 | 8.61 (0.54) b | 0.35 (0.01) b |
| VA-1 | 24.33 (2.60) c | 0.31 (0.03) b |
| SA-1 | 9.62 (2.21) b | 0.44 (0.04) a |
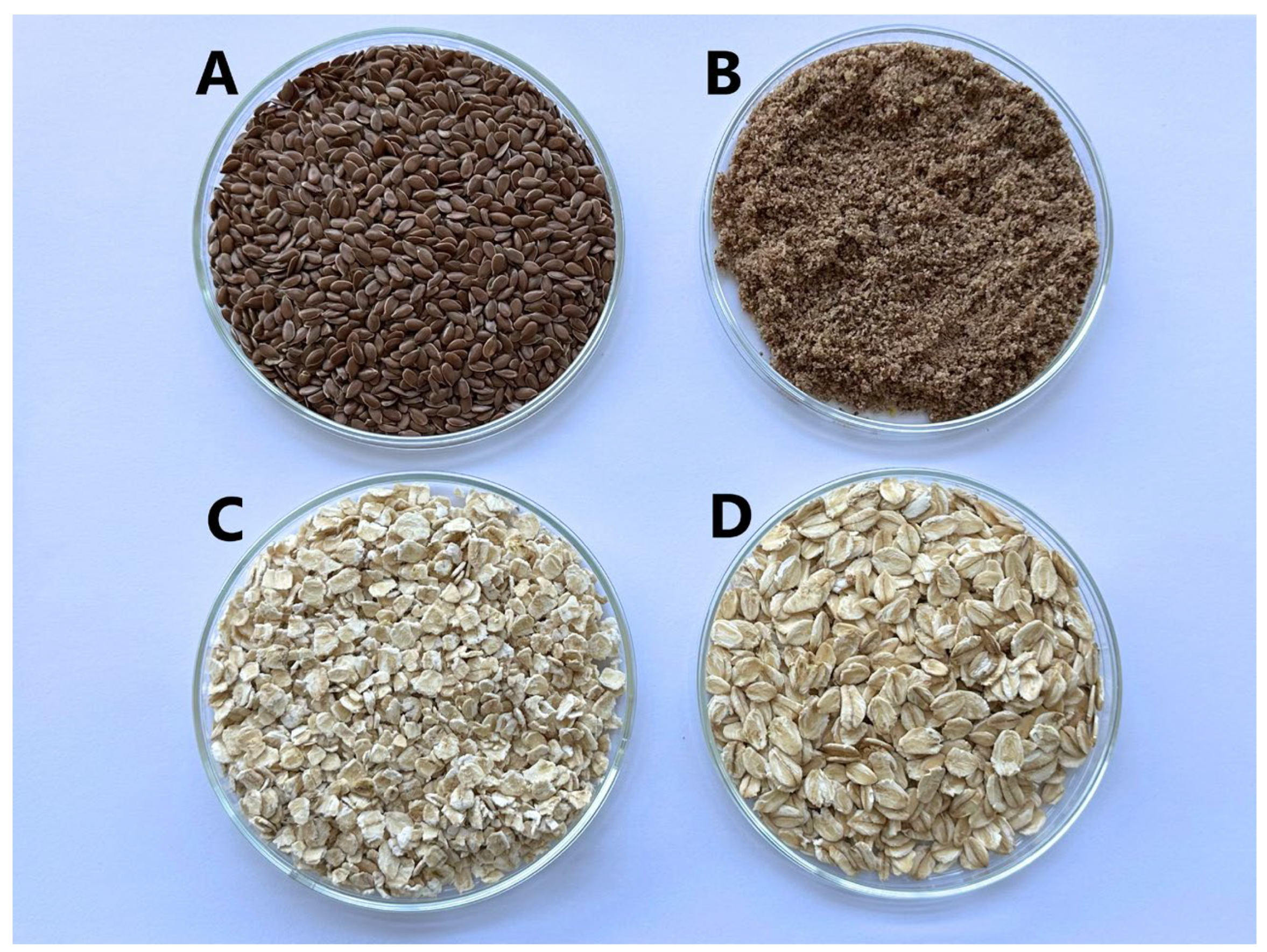
| Formulation | Gelling Agent | Amount of Gelling Agent | Amount of Purified Water (mL) | Amount of Green Tea Dry Extract (%) |
|---|---|---|---|---|
| NL-1 | Whole flaxseeds | 7.0 | 50.0 | 4.64 |
| ML-1 | Ground flaxseeds | 7.0 | 50.0 | 4.64 |
| VA-1 | Medium-size oatmeal | 7.0 | 50.0 | 4.64 |
| SA-1 | Coarse oatmeal | 7.0 | 50.0 | 4.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poceviciute, A.; Mazurkeviciute, A.; Raudone, L. Natural-Origin Edible Gels as Delivery Systems for Green Tea Extract: Formulation, Physicochemical, and Biopharmaceutic Profile Assessment. Molecules 2025, 30, 2789. https://doi.org/10.3390/molecules30132789
Poceviciute A, Mazurkeviciute A, Raudone L. Natural-Origin Edible Gels as Delivery Systems for Green Tea Extract: Formulation, Physicochemical, and Biopharmaceutic Profile Assessment. Molecules. 2025; 30(13):2789. https://doi.org/10.3390/molecules30132789
Chicago/Turabian StylePoceviciute, Andreja, Agne Mazurkeviciute, and Lina Raudone. 2025. "Natural-Origin Edible Gels as Delivery Systems for Green Tea Extract: Formulation, Physicochemical, and Biopharmaceutic Profile Assessment" Molecules 30, no. 13: 2789. https://doi.org/10.3390/molecules30132789
APA StylePoceviciute, A., Mazurkeviciute, A., & Raudone, L. (2025). Natural-Origin Edible Gels as Delivery Systems for Green Tea Extract: Formulation, Physicochemical, and Biopharmaceutic Profile Assessment. Molecules, 30(13), 2789. https://doi.org/10.3390/molecules30132789









