Exploring Aroma and Flavor Diversity in Cannabis sativa L.—A Review of Scientific Developments and Applications
Abstract
1. Introduction
2. Biochemistry and Genetic Regulation of Aroma and Flavor Compounds in C. sativa L.

2.1. Terpenes
2.2. Flavonoids and Other Aroma and Flavor Compounds
2.3. Trait Associations and Exclusivity
2.4. Practical Implications for Breeding
3. Strategies to Influence C. sativa L. Aroma and Flavor Profiles
4. Stability of C. sativa L. Aroma and Flavor Compounds
4.1. Factors Affecting the Stability of Aroma and Flavor Compounds
4.2. Preservation Strategies
5. Cannabis Flavor Profiling Framework
5.1. Extraction and Quantification Methods for Aroma and Flavor Compounds
5.2. Aroma and Flavor Wheel Development
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, A.N.; DiVerdi, J.A. Consumer Perceptions of Strain Differences in Cannabis Aroma. PLoS ONE 2018, 13, e0192247. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, A.L.; Naibauer, S.K.; McGlaughlin, M.E.; Gilbert, A.N. Human Olfactory Discrimination of Genetic Variation within Cannabis Strains. Front. Psychol. 2022, 13, 942694. [Google Scholar] [CrossRef]
- Kwásnica, A.; Pachura, N.; Masztalerz, K.; Figiel, A.; Zimmer, A.; Nski, R.K.; Wujcikowska, K.; Carbonell-Barrachina, A.A.; Szumny, A.; Nski, H.R. Volatile Composition and Sensory Properties as Quality Attributes of Fresh and Dried Hemp Flowers (Cannabis sativa L.). Foods 2020, 9, 1118. [Google Scholar] [CrossRef]
- Plumb, J.; Demirel, S.; Sackett, J.L.; Russo, E.B.; Wilson-Poe, A.R. The Nose Knows: Aroma, but Not THC Mediates the Subjective Effects of Smoked and Vaporized Cannabis Flower. Psychoactives 2022, 1, 70–86. [Google Scholar] [CrossRef]
- Booth, J.K.; Yuen, M.M.S.; Jancsik, S.; Madilao, L.L.; Page, A.J.E. Terpene Synthases and Terpene Variation in Cannabis sativa. Plant Physiol. 2020, 184, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.; McElroy, M.; Migicovsky, Z.; Maassen, H.; van Velzen, R.; Myles, S. Cannabis Labelling Is Associated with Genetic Variation in Terpene Synthase Genes. Nat. Plants 2021, 7, 1330–1334. [Google Scholar] [CrossRef]
- Ahsan, S.; Hoque, M.; Kumar, A.; Shaffique, S.; Rahman, M.; Choi, H. Plant Improvement and Metabolite Production in Cannabis sativa: Recent Biotechnological Advances. Plant Trends 2024, 2, 74–91. [Google Scholar] [CrossRef]
- Fiorentino, N.; Formisano, C.; Delfine, S.; Chianese, G. Editorial: Environmental and Agronomic Factors Affecting the Chemical Composition and Biological Activity of Cannabis Extracts. Front. Plant Sci. 2024, 15, 1407262. [Google Scholar] [CrossRef]
- Addo, P.W.; Desaulniers Brousseau, V.; Morello, V.; MacPherson, S.; Paris, M.; Lefsrud, M. Cannabis Chemistry, Post-Harvest Processing Methods and Secondary Metabolite Profiling: A Review. Ind. Crops Prod. 2021, 170, 113743. [Google Scholar] [CrossRef]
- Kaminski, K.P.; Hoeng, J.; Goffman, F.; Schlage, W.K.; Latino, D. Opportunities, Challenges, and Scientific Progress in Hemp Crops. Molecules 2024, 29, 2397. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; Elsohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Kneubühl, M.; André, A.; Chetschik, I. Characterisation of the Key-Aroma Compounds Among the Volatile Constituents in Different Hemp Strains (Cannabis sativa L.). In Proceedings of the 16th Weurman Flavour Research Symposium, Online, 4–6 May 2021. [Google Scholar] [CrossRef]
- Prescott, J. Multisensory Processes in Flavour Perception and Their Influence on Food Choice. Curr. Opin. Food Sci. 2015, 3, 47–52. [Google Scholar] [CrossRef]
- ISO 5492:2008; Sensory Analysis—Vocabulary. ISO: Geneva, Switzerland, 2008.
- Auvray, M.; Spence, C. The Multisensory Perception of Flavor. Conscious. Cogn. 2008, 17, 1016–1031. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Prescott, J. Odor/Taste Integration and the Perception of Flavor. Exp. Brain Res. 2005, 166, 345–357. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain”, No Gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef]
- Meehan-Atrash, J.; Strongin, R.M. Thermal Degradation of Cannabinoids and Cannabis Terpenes. In Recent Advances in the Science of Cannabis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 215–234. [Google Scholar]
- Moir, D.; Rickert, W.S.; Levasseur, G.; Larose, Y.; Maertens, R.; White, P.; Desjardins, S. A Comparison of Mainstream and Sidestream Marijuana and Tobacco Cigarette Smoke Produced under Two Machine Smoking Conditions. Chem. Res. Toxicol. 2008, 21, 494–502. [Google Scholar] [CrossRef]
- García-Valverde, M.T.; Sánchez-Carnerero Callado, C.; Díaz-Liñán, M.C.; Sánchez de Medina, V.; Hidalgo-García, J.; Nadal, X.; Hanuš, L.; Ferreiro-Vera, C. Effect of Temperature in the Degradation of Cannabinoids: From a Brief Residence in the Gas Chromatography Inlet Port to a Longer Period in Thermal Treatments. Front. Chem. 2022, 10, 1038729. [Google Scholar] [CrossRef]
- Meehan-Atrash, J.; Luo, W.; McWhirter, K.J.; Dennis, D.G.; Sarlah, D.; Jensen, R.P.; Afreh, I.; Jiang, J.; Barsanti, K.C.; Ortiz, A.; et al. The Influence of Terpenes on the Release of Volatile Organic Compounds and Active Ingredients to Cannabis Vaping Aerosols. RSC Adv. 2021, 11, 11714–11723. [Google Scholar] [CrossRef]
- Gieringer, D.; Laurent, J.S.; Goodrich, S. Cannabis Vaporizer Combines Efficient Delivery of THC with Effective Suppression of Pyrolytic Compounds. J. Cannabis Ther. 2004, 4, 7–27. [Google Scholar] [CrossRef]
- Oswald, I.W.H.; Ojeda, M.A.; Pobanz, R.J.; Koby, K.A.; Buchanan, A.J.; Del Rosso, J.; Guzman, M.A.; Martin, T.J. Identification of a New Family of Prenylated Volatile Sulfur Compounds in Cannabis Revealed by Comprehensive Two-Dimensional Gas Chromatography. ACS Omega 2021, 6, 31667–31676. [Google Scholar] [CrossRef] [PubMed]
- Oswald, I.W.H.; Paryani, T.R.; Sosa, M.E.; Ojeda, M.A.; Altenbernd, M.R.; Grandy, J.J.; Shafer, N.S.; Ngo, K.; Peat, J.R.; Melshenker, B.G.; et al. Minor, Nonterpenoid Volatile Compounds Drive the Aroma Differences of Exotic Cannabis. ACS Omega 2023, 8, 39203–39216. [Google Scholar] [CrossRef] [PubMed]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef]
- Sainz Martinez, A.; Lanaridi, O.; Stagel, K.; Halbwirth, H.; Schnürch, M.; Bica-Schröder, K. Extraction Techniques for Bioactive Compounds of Cannabis. Nat. Prod. Rep. 2023, 40, 676–717. [Google Scholar] [CrossRef]
- Rettberg, N.; Biendl, M.; Garbe, L.A. Hop Aroma and Hoppy Beer Flavor: Chemical Backgrounds and Analytical Tools—A Review. J. Am. Soc. Brew. Chem. 2018, 76, 1–20. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research Progress of Wine Aroma Components: A Critical Review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef]
- Angeloni, S.; Mustafa, A.M.; Abouelenein, D.; Alessandroni, L.; Acquaticci, L.; Nzekoue, F.K.; Petrelli, R.; Sagratini, G.; Vittori, S.; Torregiani, E.; et al. Characterization of the Aroma Profile and Main Key Odorants of Espresso Coffee. Molecules 2021, 26, 3856. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.T.; Zheng, X.; Li, S. Tea Aroma Formation. Food Sci. Hum. Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Leffingwell, J.C. Chemical Constituents of Tobacco Leaf and Differences among Tobacco Types. Leffingwell Rep. 2001, 1, 1–56. [Google Scholar]
- Schmelzle, A. The Beer Aroma Wheel: Updating Beer Flavour Terminology According to Sensory Standards. BrewingScience 2009, 62, 26–32. [Google Scholar]
- Lawless, L.J.R.; Hottenstein, A.; Ellingsworth, J. The McCormick Spice Wheel: A Systematic and Visual Approach to Sensory Lexicon Development. J. Sens. Stud. 2012, 27, 37–47. [Google Scholar] [CrossRef]
- De Pelsmaeker, S.; De Clercq, G.; Gellynck, X.; Schouteten, J.J. Development of a Sensory Wheel and Lexicon for Chocolate. Food Res. Int. 2019, 116, 1183–1191. [Google Scholar] [CrossRef]
- Koch, I.S.; Muller, M.; Joubert, E.; van der Rijst, M.; Næs, T. Sensory Characterization of Rooibos Tea and the Development of a Rooibos Sensory Wheel and Lexicon. Food Res. Int. 2012, 46, 217–228. [Google Scholar] [CrossRef]
- Spencer, M.; Sage, E.; Velez, M.; Guinard, J.X. Using Single Free Sorting and Multivariate Exploratory Methods to Design a New Coffee Taster’s Flavor Wheel. J. Food Sci. 2016, 81, S2997–S3005. [Google Scholar] [CrossRef]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef]
- Tanney, C.A.S.; Backer, R.; Geitmann, A.; Smith, D.L. Cannabis Glandular Trichomes: A Cellular Metabolite Factory. Front. Plant Sci. 2021, 12, 721986. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- van Bakel, H.; Stout, J.M.; Cote, A.G.; Tallon, C.M.; Sharpe, A.G.; Hughes, T.R.; Page, J.E. The Draft Genome and Transcriptome of Cannabis sativa. Genome Biol. 2011, 12, R102. [Google Scholar] [CrossRef]
- Braich, S.; Baillie, R.C.; Spangenberg, G.C.; Cogan, N.O.I. A New and Improved Genome Sequence of Cannabis sativa. GigaByte 2020, 2020, gigabyte10. [Google Scholar] [CrossRef]
- Gao, S.; Wang, B.; Xie, S.; Xu, X.; Zhang, J.; Pei, L.; Yu, Y.; Yang, W.; Zhang, Y. A High-Quality Reference Genome of Wild Cannabis sativa. Hortic. Res. 2020, 7, 73. [Google Scholar] [CrossRef]
- Ryu, B.-R.; Gim, G.-J.; Shin, Y.-R.; Kang, M.-J.; Kim, M.-J.; Kwon, T.-H.; Lim, Y.-S.; Park, S.-H.; Lim, J.-D. Chromosome-Level Haploid Assembly of Cannabis sativa L. Cv. Pink Pepper. Sci. Data 2024, 11, 1442. [Google Scholar] [CrossRef] [PubMed]
- Zager, J.J.; Lange, I.; Srividya, N.; Smith, A.; Markus Lange, B. Gene Networks Underlying Cannabinoid and Terpenoid Accumulation in Cannabis. Plant Physiol. 2019, 180, 1877–1897. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; McKernan, K.; Pauli, C.; Roe, J.; Torres, A.; Gaudino, R. Genomic Characterization of the Complete Terpene Synthase Gene Family from Cannabis sativa. PLoS ONE 2019, 14, e0222363. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary Metabolism in Cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Kaur, J.; Sun, N.; Hill, J.E. Comprehensive Profiling of Terpenes and Terpenoids in Different Cannabis Strains Using GC × GC-TOFMS. Separations 2023, 10, 500. [Google Scholar] [CrossRef]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From Plant Genome to Humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis Glandular Trichomes Alter Morphology and Metabolite Content during Flower Maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef]
- McDougall, J.J.; McKenna, M.K. Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis. Int. J. Mol. Sci. 2022, 23, 7891. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—What Are the Potential Health Benefits of This Flavouring and Aroma Agent? Front. Nutr. 2021, 8, 699666. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-Limonene: A Multifunctional Compound with Potent Therapeutic Effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A Review of the Potential Use of Pinene and Linalool as Terpene-Based Medicines for Brain Health: Discovering Novel Therapeutics in the Flavours and Fragrances of Cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, M.; Steuer, C. α-Pinene: A Never-Ending Story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Hǎncianu, M.; Costache, I.I.; Miron, A. Linalool: A Review on a Key Odorant Molecule with Valuable Biological Properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Machado, K.d.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A Systematic Review on the Neuroprotective Perspectives of Beta-Caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef] [PubMed]
- Blake, K. Beta-Caryophyllene: A Review of Current Research. Altern. Complement. Ther. 2021, 27, 222–226. [Google Scholar] [CrossRef]
- Mendes de Lacerda Leite, G.; de Oliveira Barbosa, M.; Pereira Lopes, M.J.; de Araújo Delmondes, G.; Bezerra, D.S.; Araújo, I.M.; Carvalho de Alencar, C.D.; Melo Coutinho, H.D.; Peixoto, L.R.; Barbosa-Filho, J.M.; et al. Pharmacological and Toxicological Activities of α-Humulene and Its Isomers: A Systematic Review. Trends Food Sci. Technol. 2021, 115, 255–274. [Google Scholar] [CrossRef]
- Novak, J.; Zitterl-Eglseer, K.; Deans, S.G.; Franz, C.M. Essential Oils of Different Cultivars of Cannabis sativa L. And Their Antimicrobial Activity. Flavour Fragr. J. 2001, 16, 259–262. [Google Scholar] [CrossRef]
- de Sousa, J.M.S.; Nunes, T.A.d.L.; Rodrigues, R.R.L.; de Sousa, J.P.A.; Val, M.d.C.A.; Coelho, F.A.d.R.; dos Santos, A.L.S.; Maciel, N.B.; de Souza, V.M.R.; Machado, Y.A.A.; et al. Cytotoxic and Antileishmanial Effects of the Monoterpene β-Ocimene. Pharmaceuticals 2023, 16, 183. [Google Scholar] [CrossRef]
- Javed, H.; Azimullah, S.; Abul Khair, S.B.; Ojha, S.; Haque, M.E. Neuroprotective Effect of Nerolidol Against Neuroinflammation and Oxidative Stress Induced by Rotenone. BMC Neurosci. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.Y.; Marin Rodriguez, A.A.; Menchaca Vega, D.S.; Sussmann, R.A.C.; Kimura, E.A.; Katzin, A.M. Antimalarial Activity of the Terpene Nerolidol. Int. J. Antimicrob. Agents 2016, 48, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Ito, K.; Ito, M. The Sedative Effect of Inhaled Terpinolene in Mice and Its Structure-Activity Relationships. J. Nat. Med. 2013, 67, 833–837. [Google Scholar] [CrossRef]
- Menezes, I.O.; Scherf, J.R.; Martins, A.O.B.P.B.; Ramos, A.G.B.; Quintans, J.d.S.S.; Coutinho, H.D.M.; Ribeiro-Filho, J.; de Menezes, I.R.A. Biological Properties of Terpinolene Evidenced by in Silico, in Vitro and in Vivo Studies: A Systematic Review. Phytomedicine 2021, 93, 153768. [Google Scholar] [CrossRef]
- Laws, J.S.; Shrestha, S.; Smid, S.D. Cannabis Terpenes Display Variable Protective and Anti-Aggregatory Actions against Neurotoxic β Amyloid in Vitro: Highlighting the Protective Bioactivity of α-Bisabolol in Motorneuronal-like NSC-34 Cells. Neurotoxicology 2022, 90, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.B.; Jha, N.K.; Goyal, S.N.; Agrawal, Y.O.; Subramanya, S.B.; Bastaki, S.M.A.; Ojha, S. Health Benefits, Pharmacological Effects, Molecular Mechanisms, and Therapeutic Potential of α-Bisabolol. Nutrients 2022, 14, 1470. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.G.; Dufresne, J.; Sabatinos, S.A. Cannabinoid Inheritance Relies on Complex Genetic Architecture. Cannabis Cannabinoid Res. 2020, 5, 105–116. [Google Scholar] [CrossRef]
- Barcaccia, G.; Palumbo, F.; Scariolo, F.; Vannozzi, A.; Borin, M.; Bona, S. Potentials and Challenges of Genomics for Breeding Cannabis Cultivars. Front. Plant Sci. 2020, 11, 573299. [Google Scholar] [CrossRef]
- de Ronne, M.; Lapierre, É.; Torkamaneh, D. Genetic Insights into Agronomic and Morphological Traits of Drug-Type Cannabis Revealed by Genome-Wide Association Studies. Sci. Rep. 2024, 14, 9162. [Google Scholar] [CrossRef]
- Borin, M.; Palumbo, F.; Vannozzi, A.; Scariolo, F.; Sacilotto, G.B.; Gazzola, M.; Barcaccia, G. Developing and Testing Molecular Markers in Cannabis sativa (Hemp) for Their Use in Variety and Dioecy Assessments. Plants 2021, 10, 2174. [Google Scholar] [CrossRef] [PubMed]
- Shiels, D.; Prestwich, B.D.; Koo, O.; Kanchiswamy, C.N.; O’Halloran, R.; Badmi, R. Hemp Genome Editing—Challenges and Opportunities. Front. Genome Ed. 2022, 4, 823486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, G.; Cheng, C.; Lei, L.; Sun, J.; Xu, Y.; Deng, C.; Dai, Z.; Yang, Z.; Chen, X.; et al. Establishment of an Agrobacterium-Mediated Genetic Transformation and CRISPR/Cas9-Mediated Targeted Mutagenesis in Hemp (Cannabis sativa L.). Plant Biotechnol. J. 2021, 19, 1979–1987. [Google Scholar] [CrossRef]
- Desaulniers Brousseau, V.; Wu, B.S.; MacPherson, S.; Morello, V.; Lefsrud, M. Cannabinoids and Terpenes: How Production of Photo-Protectants Can Be Manipulated to Enhance Cannabis sativa L. Phytochemistry. Front. Plant Sci. 2021, 12, 620021. [Google Scholar] [CrossRef] [PubMed]
- Payment, J.; Cvetkovska, M. The Responses of Cannabis sativa to Environmental Stress: A Balancing Act. Botany 2023, 101, 318–332. [Google Scholar] [CrossRef]
- Ingvardsen, C.R.; Brinch-Pedersen, H. Challenges and Potentials of New Breeding Techniques in Cannabis sativa. Front. Plant Sci. 2023, 14, 1154332. [Google Scholar] [CrossRef]
- Landi, S.; Berni, R.; Capasso, G.; Hausman, J.F.; Guerriero, G.; Esposito, S. Impact of Nitrogen Nutrition on Cannabis sativa: An Update on the Current Knowledge and Future Prospects. Int. J. Mol. Sci. 2019, 20, 5803. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen Source Matters: High NH4/NO3 Ratio Reduces Cannabinoids, Terpenoids, and Yield in Medical Cannabis. Front. Plant Sci. 2022, 13, 830224. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Effect of Potassium (K) Supply on Cannabinoids, Terpenoids and Plant Function in Medical Cannabis. Agronomy 2022, 12, 1242. [Google Scholar] [CrossRef]
- Seaman, C. Cultivation Stress Techniques and the Production of Secondary Metabolites in Cannabis sativa. In Recent Advances in the Science of Cannabis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–30. [Google Scholar]
- Garrido, J.; Rico, S.; Corral, C.; Sánchez, C.; Vidal, N.; Martínez-Quesada, J.J.; Ferreiro-Vera, C. Exogenous Application of Stress-Related Signaling Molecules Affect Growth and Cannabinoid Accumulation in Medical Cannabis (Cannabis sativa L.). Front. Plant Sci. 2022, 13, 1082554. [Google Scholar] [CrossRef] [PubMed]
- Sands, L.B.; Haiden, S.R.; Ma, Y.; Berkowitz, G.A. Hormonal Control of Promoter Activities of Cannabis sativa Prenyltransferase 1 and 4 and Salicylic Acid Mediated Regulation of Cannabinoid Biosynthesis. Sci. Rep. 2023, 13, 8620. [Google Scholar] [CrossRef] [PubMed]
- Soltan, K.; Dadkhah, B. Studies of the Major Gene Expression and Related Metabolites in Cannabinoids Biosynthesis Pathway Influenced by Ascorbic Acid. Planta Medica Int. Open 2022, 9, e116–e122. [Google Scholar] [CrossRef]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl Jasmonate and Salicylic Acid as Powerful Elicitors for Enhancing the Production of Secondary Metabolites in Medicinal Plants: An Updated Review. Plant Cell Tissue Organ Cult. 2023, 153, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, C.; Simon, J.E. Terpenoid Essential Oil Metabolism in Basil (Ocimum basilicum L.) Following Elicitation. J. Essent. Oil Res. 2006, 18, 618–621. [Google Scholar] [CrossRef]
- Melo, E.R.; Fabri, E.G.; Magalhães, H.M.; Gorni, P.H.; Pacheco, A.C. In Vivo Elicitation Is Efficient in Increasing Essential Oil Yield with High Anti-Inflammatory Sesquiterpene Content in Varronia curassavica Jacq. Chil. J. Agric. Res. 2023, 83, 369–379. [Google Scholar] [CrossRef]
- Afkar, S. Assessment of Chemical Compositions and Antibacterial Activity of the Essential Oil of Mentha piperita in Response to Salicylic Acid. Nat. Prod. Res. 2024, 38, 3562–3573. [Google Scholar] [CrossRef]
- El-Ziat, R.A.; Soliman, D.M.; El-Sayed, I.M. Exogenous Utilization of Jasmonic Acid and Methyl Jasmonate Stimulates Growth and Biochemical Composition of Lavender (Lavandula angustifolia) Plant. Egypt. Pharm. J. 2023, 22, 372–379. [Google Scholar] [CrossRef]
- Choi, M.S.; Park, D.J.; Song, H.J.; Min, J.Y.; Kang, S.M.; Lee, C.K.; Cho, K.M.; Karigar, C.; Kim, H.K.; Kang, Y.M. Enhancing Production of Terpenoids in Metabolically Engineered Transgenic Spearmint (Mentha spicata L.) by Salt and Fungal Elicitors. J. For. Environ. Sci. 2014, 30, 243–252. [Google Scholar] [CrossRef]
- Huang, M.; Abel, C.; Sohrabi, R.; Petri, J.; Haupt, I.; Cosimano, J.; Gershenzon, J.; Tholl, D. Variation of Herbivore-Induced Volatile Terpenes among Arabidopsis Ecotypes Depends on Allelic Differences and Subcellular Targeting of Two Terpene Synthases, TPS02 and TPS03. Plant Physiol. 2010, 153, 1293–1310. [Google Scholar] [CrossRef]
- Trancoso, I.; de Souza, G.A.R.; dos Santos, P.R.; dos Santos, K.D.; de Miranda, R.M.d.S.N.; da Silva, A.L.P.M.; Santos, D.Z.; García-Tejero, I.F.; Campostrini, E. Cannabis sativa L.: Crop Management and Abiotic Factors That Affect Phytocannabinoid Production. Agronomy 2022, 12, 1492. [Google Scholar] [CrossRef]
- Rutnik, K.; Ocvirk, M.; Košir, I.J. Changes in Hop (Humulus lupulus L.) Oil Content and Composition during Long-Term Storage under Different Conditions. Foods 2022, 11, 3089. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wongso, I.; Putnam, D.; Khir, R.; Pan, Z. Effect of Hot Air and Infrared Drying on the Retention of Cannabidiol and Terpenes in Industrial Hemp (Cannabis sativa L.). Ind. Crops Prod. 2021, 172, 114051. [Google Scholar] [CrossRef]
- Pavlovic, R.; Nenna, G.; Calvi, L.; Panseri, S.; Borgonovo, G.; Giupponi, L.; Cannazza, G.; Giorgi, A. Quality Traits of “Cannabidiol Oils”: Cannabinoids Content, Terpene Fingerprint and Oxidation Stability of European Commercially Available Preparations. Molecules 2018, 23, 1230. [Google Scholar] [CrossRef]
- Baek, Y.; Grab, H.; Chen, C. Postharvest Drying and Curing Affect Cannabinoid Contents and Microbial Levels in Industrial Hemp (Cannabis sativa L.). Plants 2025, 14, 414. [Google Scholar] [CrossRef]
- Das, P.C.; Vista, A.R.; Tabil, L.G.; Baik, O.-D. Postharvest Operations of Cannabis and Their Effect on Cannabinoid Content: A Review. Bioengineering 2022, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.; Leuer, E.; Kearney, M.; Green, E.H.; Greenbaum, E.A. The Preservation and Augmentation of Volatile Terpenes in Cannabis Inflorescence. J. Cannabis Res. 2020, 2, 27. [Google Scholar] [CrossRef]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and extraction methods of medicinal cannabis: A narrative review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, O.; Li, D.P.; Rodriguez-Juarez, R.; Golubov, A.; Hudson, D.; Kovalchuk, I. The Effect of Cannabis Dry Flower Irradiation on the Level of Cannabinoids, Terpenes and Anti-Cancer Properties of the Extracts. Biocatal. Agric. Biotechnol. 2020, 29, 101736. [Google Scholar] [CrossRef]
- Addo, P.W.; Poudineh, Z.; Shearer, M.; Taylor, N.; MacPherson, S.; Raghavan, V.; Orsat, V.; Lefsrud, M. Relationship between Total Antioxidant Capacity, Cannabinoids and Terpenoids in Hops and Cannabis. Plants 2023, 12, 1225. [Google Scholar] [CrossRef] [PubMed]
- Addo, P.W.; Chauvin-Bossé, T.; Taylor, N.; MacPherson, S.; Paris, M.; Lefsrud, M. Freeze-Drying Cannabis sativa L. Using Real-Time Relative Humidity Monitoring and Mathematical Modeling for the Cannabis Industry. Ind. Crops Prod. 2023, 199, 116754. [Google Scholar] [CrossRef]
- Spadafora, N.D.; Felletti, S.; Chenet, T.; Sirangelo, T.M.; Cescon, M.; Catani, M.; De Luca, C.; Stevanin, C.; Cavazzini, A.; Pasti, L. The Influence of Drying and Storage Conditions on the Volatilome and Cannabinoid Content of Cannabis sativa L. Inflorescences. Anal. Bioanal. Chem. 2024, 416, 3797–3809. [Google Scholar] [CrossRef]
- Kanabus, J.; Bryła, M.; Roszko, M. Effect of Selected Drying Methods on the Cannabinoid Profile of Cannabis sativa L. Var. Sativa Inflorescences Leaves. Pol. J. Food Nutr. Sci. 2024, 74, 408–418. [Google Scholar] [CrossRef]
- Kłosowska, A.; Wawrzyńczak, A.; Feliczak-Guzik, A. Microencapsulation as a Route for Obtaining Encapsulated Flavors and Fragrances. Cosmetics 2023, 10, 26. [Google Scholar] [CrossRef]
- Singh, A.P.; Fathordoobady, F.; Guo, Y.; Singh, A.; Kitts, D.D. Antioxidants help favorably regulate the kinetics of lipid peroxidation, polyunsaturated fatty acids degradation and acidic cannabinoids decarboxylation in hempseed oil. Sci. Rep. 2020, 10, 10567. [Google Scholar] [CrossRef] [PubMed]
- López-Olmos, C.; García-Valverde, M.T.; Hidalgo, J.; Ferrerio-Vera, C.; Sánchez de Medina, V. Comprehensive Comparison of Industrial Cannabinoid Extraction Techniques: Evaluation of the Most Relevant Patents and Studies at Pilot Scale. Front. Nat. Prod. 2022, 1, 1043147. [Google Scholar] [CrossRef]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (Hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef]
- Aiello, A.; Pizzolongo, F.; Scognamiglio, G.; Romano, A.; Masi, P.; Romano, R. Effects of Supercritical and Liquid Carbon Dioxide Extraction on Hemp (Cannabis sativa L.) Seed Oil. Int. J. Food Sci. Technol. 2020, 55, 2472–2480. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Separation of Aroma Compounds from Industrial Hemp Inflorescences (Cannabis sativa L.) by Supercritical CO2 Extraction and on-Line Fractionation. Ind. Crops Prod. 2014, 58, 99–103. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Natolino, A. Ultrasound-Assisted Extraction of Volatile Compounds from Industrial Cannabis sativa L. Inflorescences. Int. J. Appl. Res. Nat. Prod. 2014, 7, 1–14. [Google Scholar]
- Kobus, Z.; Pecyna, A.; Buczaj, A.; Krzywicka, M.; Przywara, A.; Nadulski, R. Optimization of the Ultrasound-Assisted Extraction of Bioactive Compounds from Cannabis sativa L. Leaves and Inflorescences Using Response Surface Methodology. Appl. Sci. 2022, 12, 6747. [Google Scholar] [CrossRef]
- Micalizzi, G.; Vento, F.; Alibrando, F.; Donnarumma, D.; Dugo, P.; Mondello, L. Cannabis sativa L.: A Comprehensive Review on the Analytical Methodologies for Cannabinoids and Terpenes Characterization. J. Chromatogr. A 2021, 1637, 461864. [Google Scholar] [CrossRef] [PubMed]
- Lipan, L.; Issa-Issa, H.; Sendra, E.; Noguera-Artiaga, L.; Carbonell-Pedro, A.Á.; Carbonell-Barrachina, Á.A. An Overview on Sensory Evaluation, Volatile Compounds, and Legal Regulations of Cannabis Sativa. In Current Applications, Approaches and Potential Perspectives for Hemp: Crop Management, Industrial Usages, and Functional Purposes; Academic Press: Cambridge, MA, USA, 2023; pp. 447–491. ISBN 9780323898676. [Google Scholar] [CrossRef]
- Noble, A.C.; Arnold, R.A.; Buechsenstein, J.; Leach, E.J.; Schmidt, J.O.; Stern, P.M. Modification of a Standardized System of Wine Aroma Terminology. Am. J. Enol. Vitic. 1987, 38, 143–146. [Google Scholar] [CrossRef]
- ISO 8586:2023; Selection and Training of Sensory Assessors. ISO: Geneva, Switzerland, 2023.
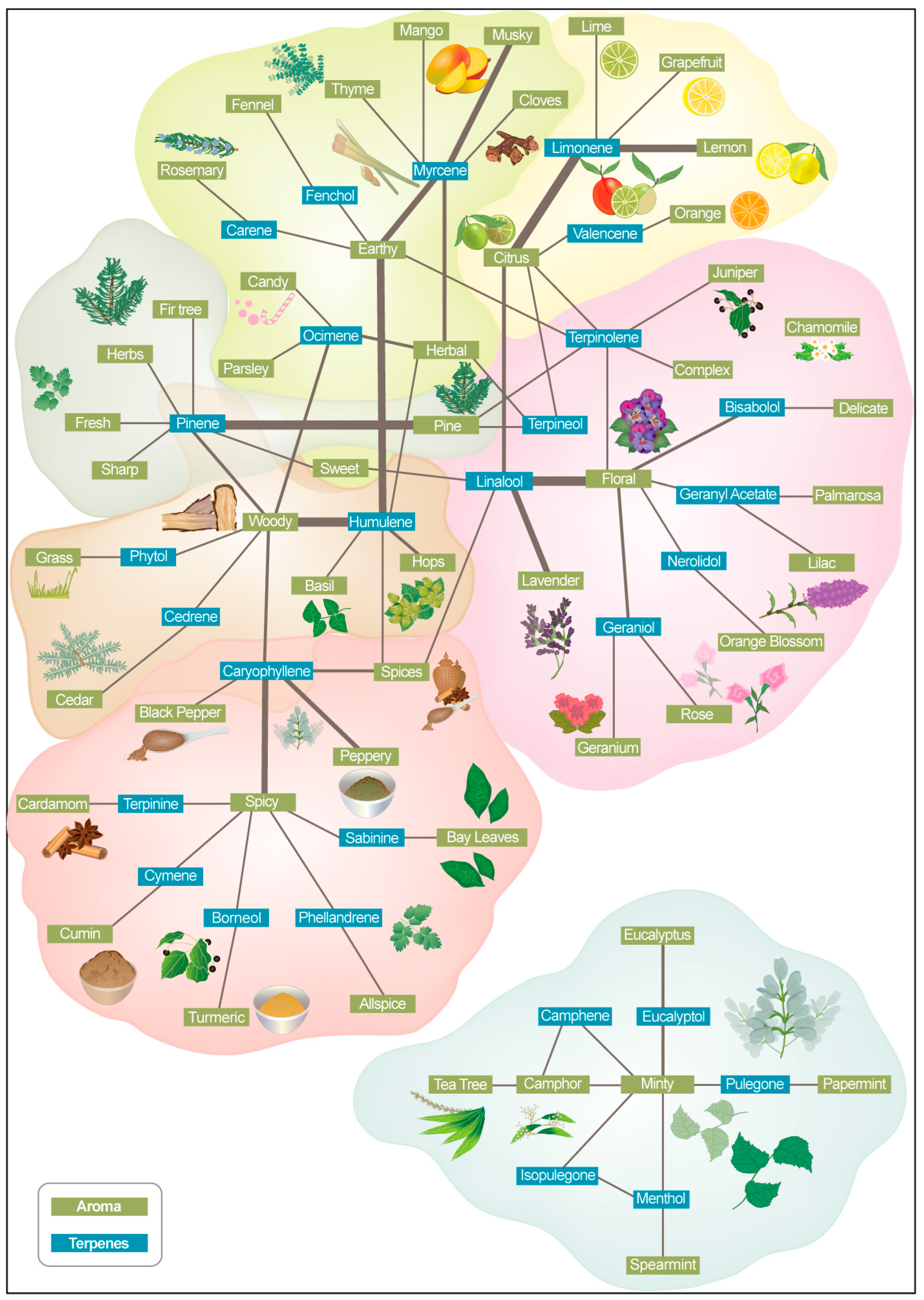
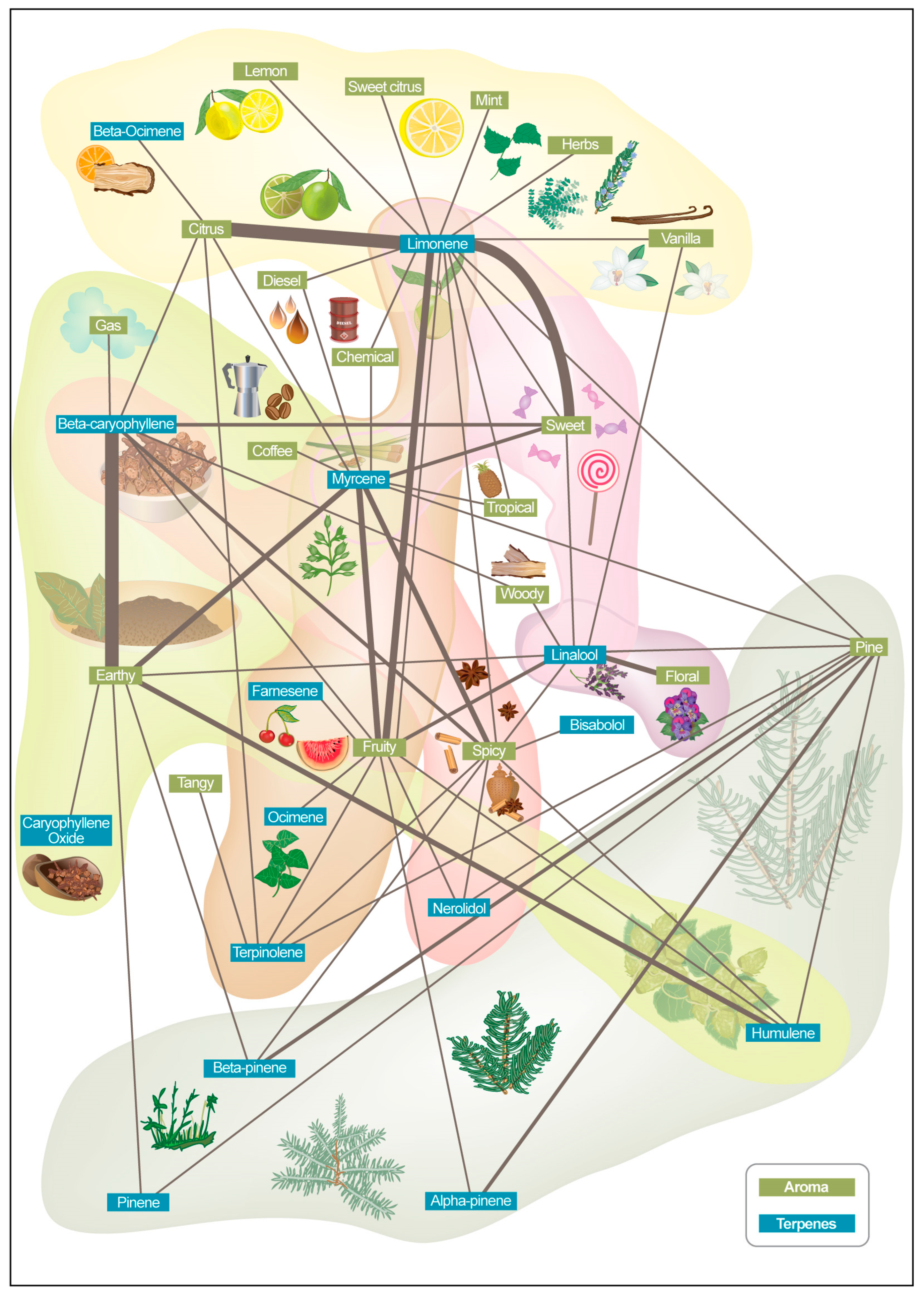
| Terpene | Class | Aroma Description 1 | Flavor Impression 1 |
|---|---|---|---|
| Myrcene | Monoterpene | Musky, earthy, fruity | Mildly sweet, herbal |
| α-Pinene | Monoterpene | Piney, resinous | Sharp, woody |
| β-Pinene | Monoterpene | Woody, turpentine-like | Resinous, slightly bitter |
| Limonene | Monoterpene | Citrus (lemon, orange) | Sweet, citrusy |
| Linalool | Monoterpene | Floral, lavender | Lightly spicy, floral |
| Terpinolene | Monoterpene | Floral, herbal, citrus | Mild, slightly bitter |
| Ocimene | Monoterpene | Sweet, herbal, woody | Subtle, slightly fruity |
| α-Terpinene | Monoterpene | Herbal, citrus | Mild, spicy |
| γ-Terpinene | Monoterpene | Citrus, lemon-like | Clean, tangy |
| α-Phellandrene | Monoterpene | Peppery, citrus | Pungent, slightly bitter |
| Sabinene | Monoterpene | Spicy, peppery | Warm, bitter |
| 1,8-Cineole | Monoterpene | Eucalyptus, minty | Cooling, spicy |
| Camphene | Monoterpene | Earthy, woody | Pungent |
| δ-3-Carene | Monoterpene | Sweet, cedar-like | Earthy |
| β-Caryophyllene | Sesquiterpene | Spicy, woody, peppery | Warm, peppery |
| α-Humulene | Sesquiterpene | Earthy, woody | Bitter, hoppy |
| Farnesene | Sesquiterpene | Green apple, woody | Mild, sweet |
| Nerolidol | Sesquiterpene | Woody, citrusy | Subtle, bitter |
| Bisabolol | Sesquiterpene | Floral, sweet | Mildly bitter, soft |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaminski, K.P.; Hoeng, J.; Lach-Falcone, K.; Goffman, F.; Schlage, W.K.; Latino, D. Exploring Aroma and Flavor Diversity in Cannabis sativa L.—A Review of Scientific Developments and Applications. Molecules 2025, 30, 2784. https://doi.org/10.3390/molecules30132784
Kaminski KP, Hoeng J, Lach-Falcone K, Goffman F, Schlage WK, Latino D. Exploring Aroma and Flavor Diversity in Cannabis sativa L.—A Review of Scientific Developments and Applications. Molecules. 2025; 30(13):2784. https://doi.org/10.3390/molecules30132784
Chicago/Turabian StyleKaminski, Kacper Piotr, Julia Hoeng, Kasia Lach-Falcone, Fernando Goffman, Walter K. Schlage, and Diogo Latino. 2025. "Exploring Aroma and Flavor Diversity in Cannabis sativa L.—A Review of Scientific Developments and Applications" Molecules 30, no. 13: 2784. https://doi.org/10.3390/molecules30132784
APA StyleKaminski, K. P., Hoeng, J., Lach-Falcone, K., Goffman, F., Schlage, W. K., & Latino, D. (2025). Exploring Aroma and Flavor Diversity in Cannabis sativa L.—A Review of Scientific Developments and Applications. Molecules, 30(13), 2784. https://doi.org/10.3390/molecules30132784








