Engineered PAM-SPION Nanoclusters for Enhanced Cancer Therapy: Integrating Magnetic Targeting with pH-Responsive Drug Release
Abstract
1. Introduction
2. Results
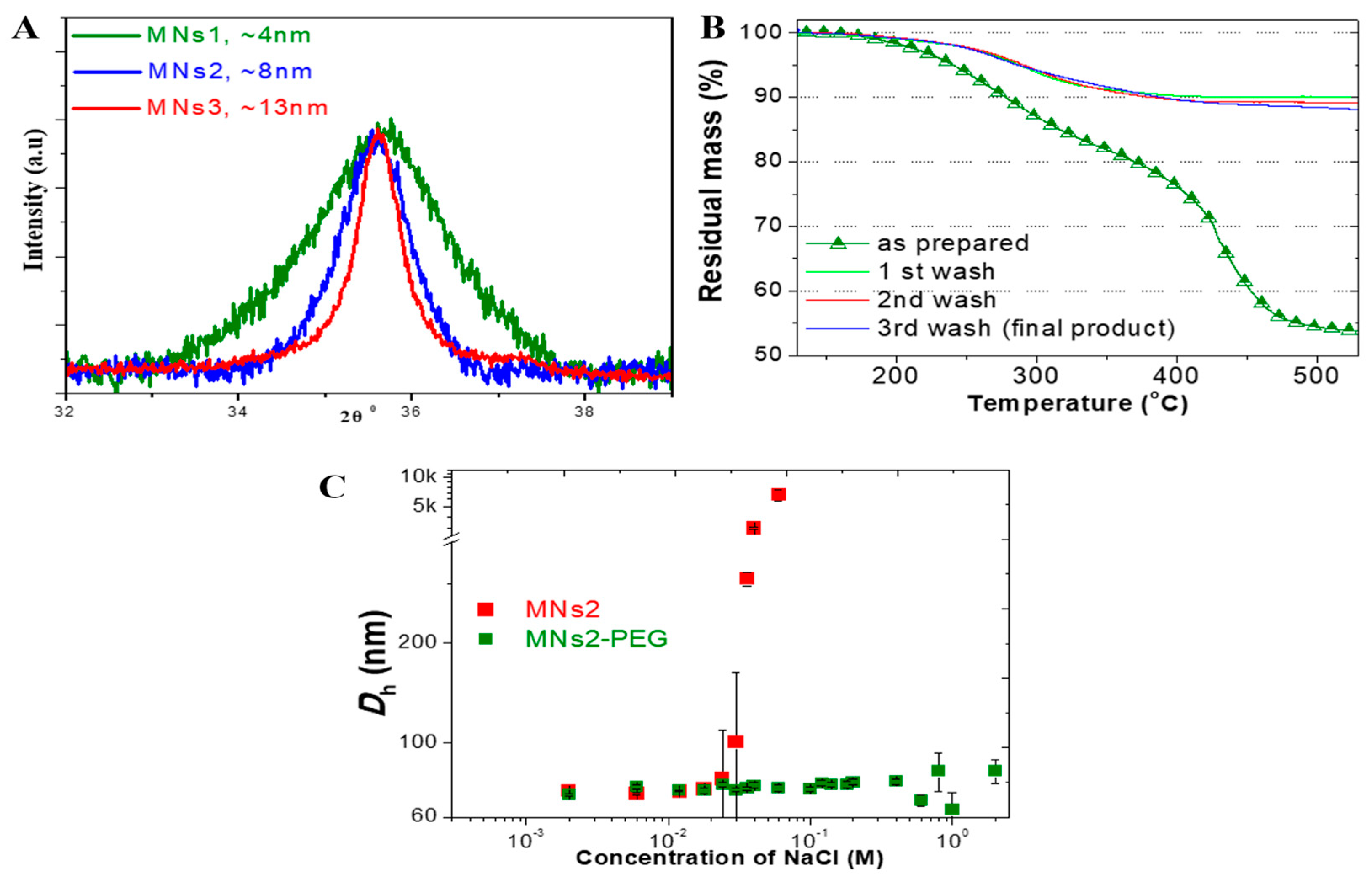
2.1. Development and Characterization of SPION Clusters
2.1.1. Controlled Synthesis and Size Distribution
2.1.2. Surface Chemistry and Modification
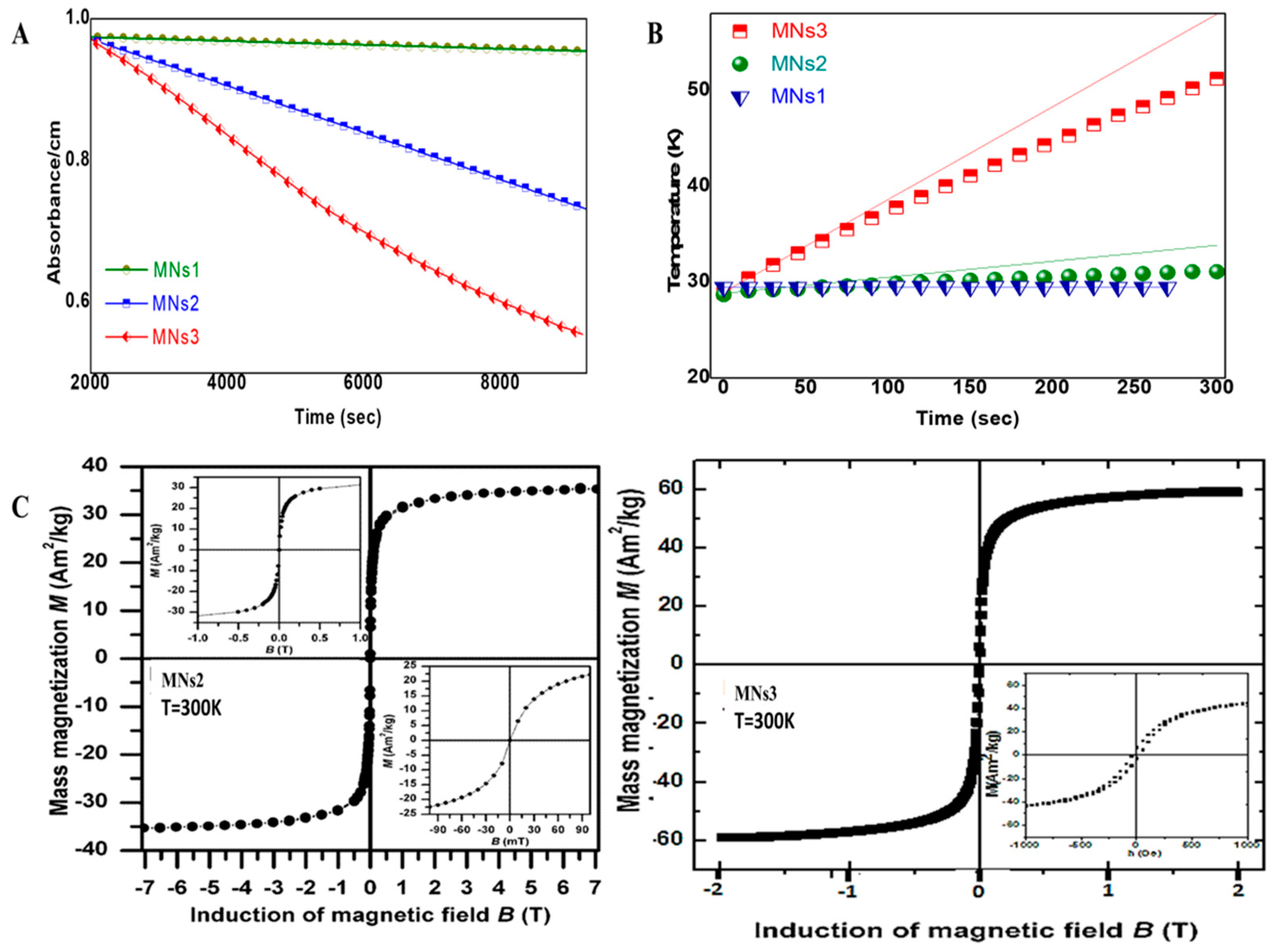
2.1.3. Magnetic Properties and Their Application-Specific Relevance
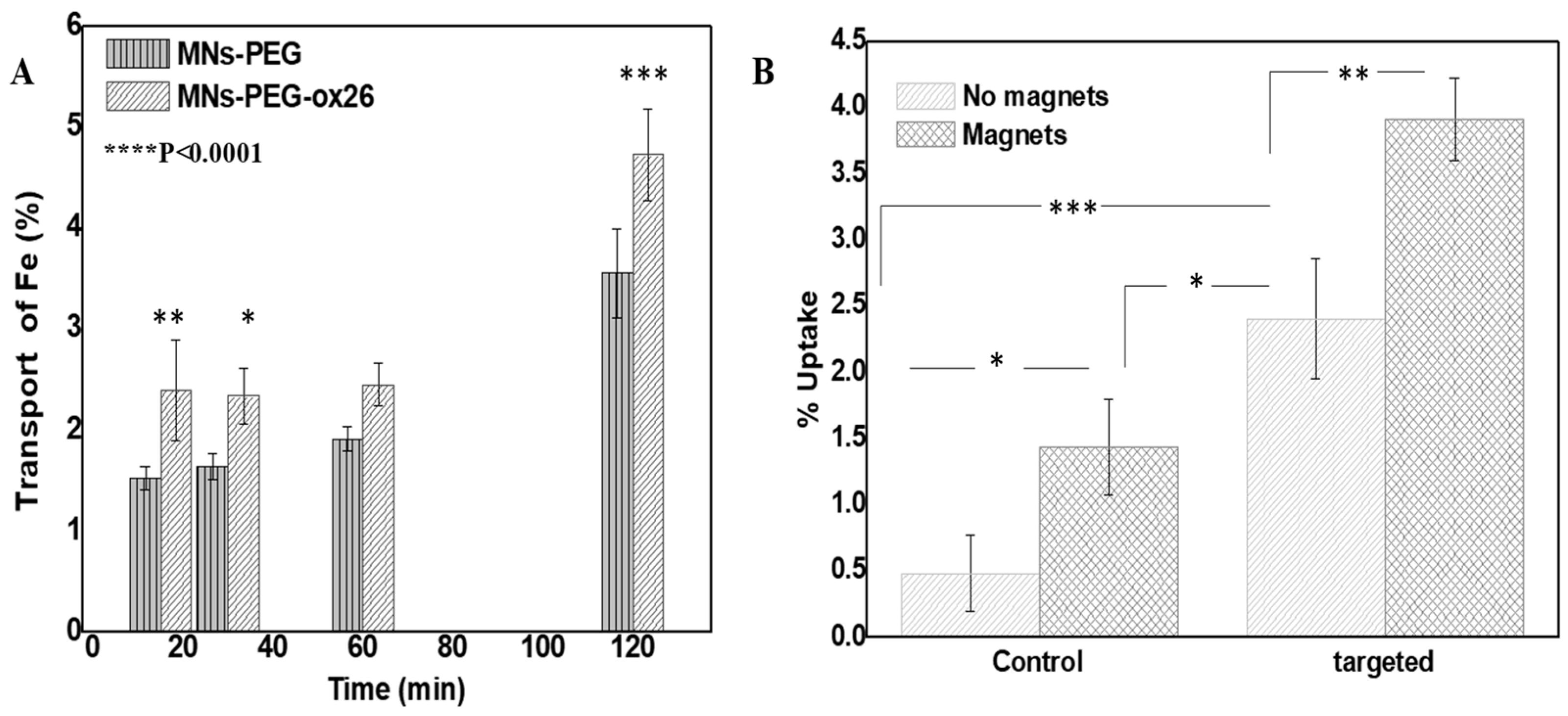
2.2. Functional Capabilities: Targeting Efficiency and hCMEC/D3 Monolayer Transport
2.2.1. Cellular Uptake Studies
2.2.2. hCMEC/D3 Monolayer Transport Studies
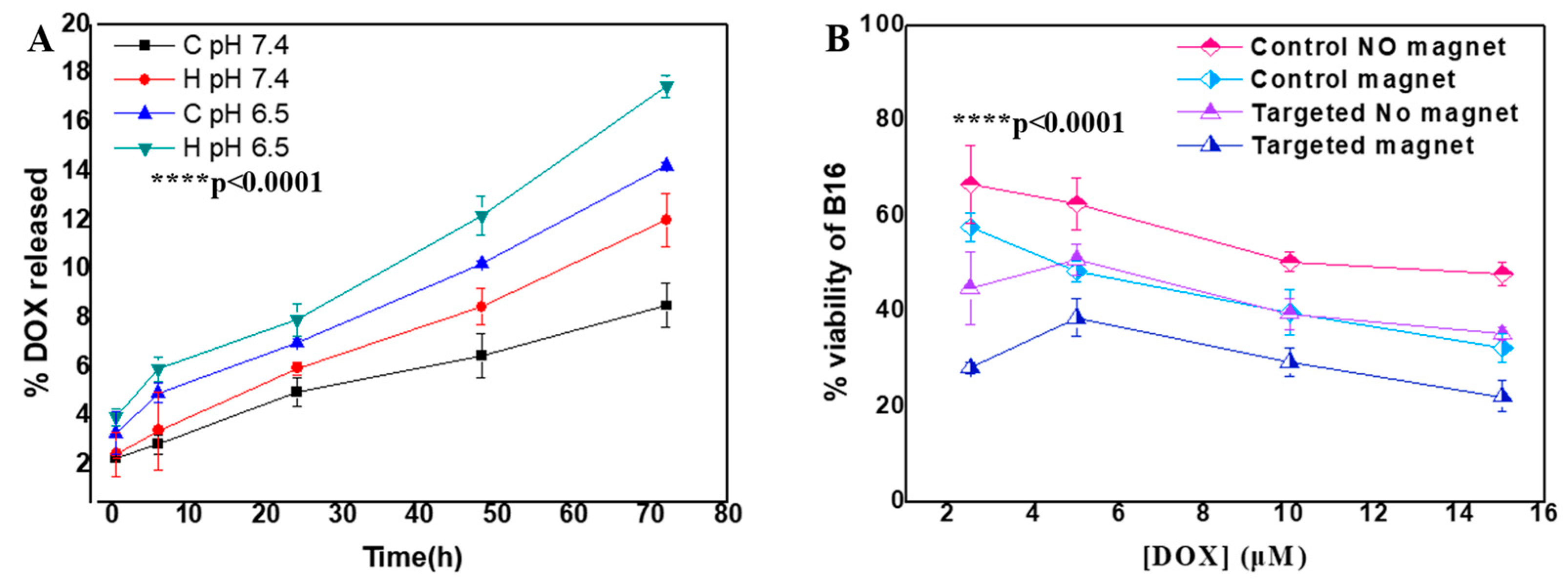
2.3. Therapeutic Performance
2.3.1. Drug Loading and Release Properties
2.3.2. Anti-Proliferative Effects
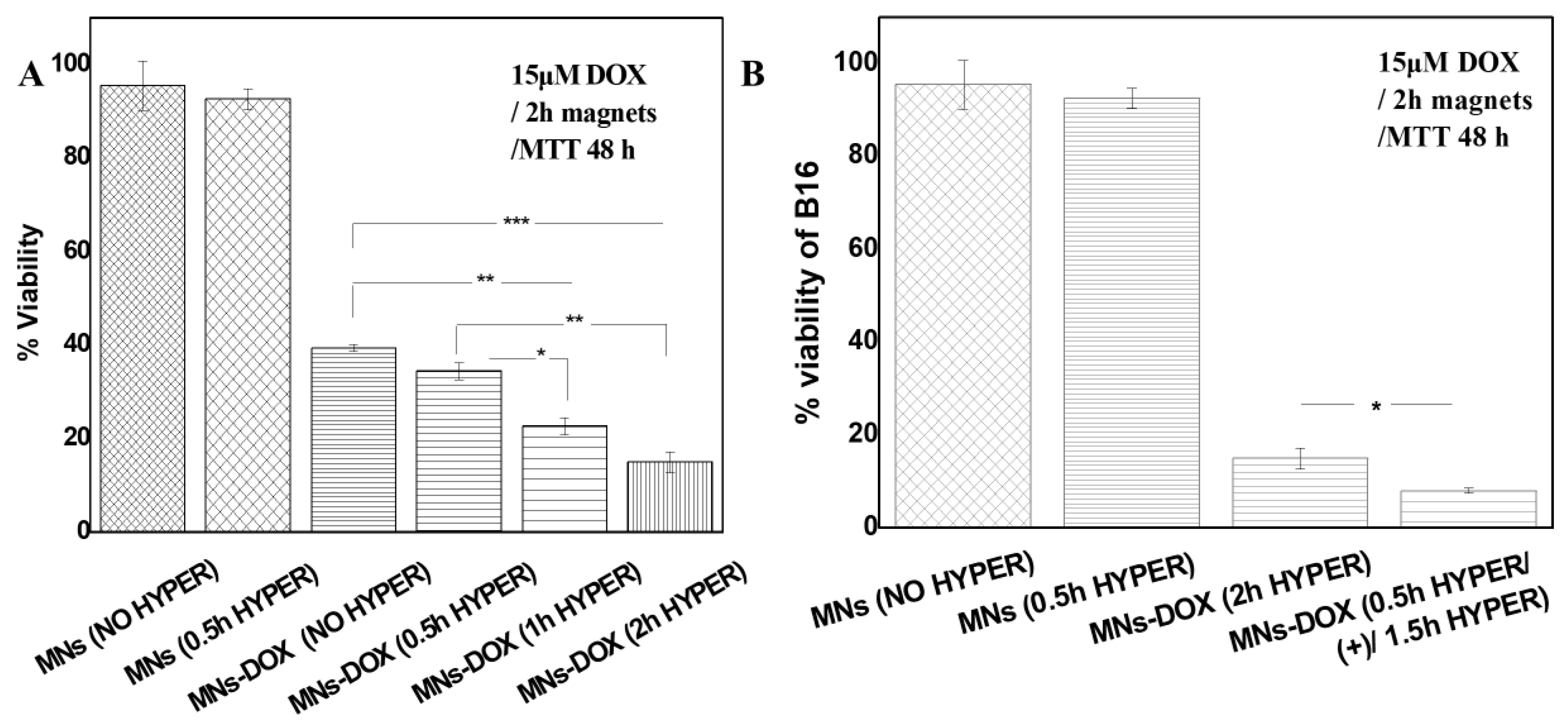
2.3.3. AMF-Enhanced Therapeutic Effects
2.3.4. Superior Efficacy of AMF Pre-Treatment Protocol
3. Discussion
3.1. Enhanced Magnetic Properties Through Controlled Clustering
3.2. Advantages of PAM Coating for Multi-Functional Applications
3.3. Synergistic Benefits of Dual Targeting Approach
3.4. Novel Insights into AMF Pre-Treatment Effects
3.5. Clinical Translation Potential
3.6. Study Limitations and Future Directions
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization
4.2.1. Synthesis of PAM-SPION Nanoclusters
General Synthesis Procedure
Specific Formulations
Purification and Final Processing
4.2.2. PEGylation and Antibody Conjugation
4.2.3. Physicochemical Characterization
4.2.4. Magnetic Characterization
4.3. Functional Studies
4.3.1. Doxorubicin Loading and Release
4.3.2. Cell Culture Studies
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMF | Alternating magnetic field |
| BBB | Blood–brain barrier |
| DOX | Doxorubicin |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide |
| MNs | PAM-stabilized SPIONs |
| MNs-PEG: | PEGylated formulations |
| MNs-PEG-OX26: | Antibody-conjugated final formulations |
| PAM | Poly(acrylic acid-co-maleic acid) |
| PDI | Polydispersity index |
| PEG | Poly(oxyethylene) |
| ROS | Reactive oxygen species |
| SAR | Specific absorption rate |
| S-NHS | N-Hydroxysulfosuccinimide sodium salt |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
Appendix A
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xiao, Y.; Sun, X.; Lin, X.; Koo, S.; Yaremenko, A.V.; Qin, D.; Kong, N.; Farokhzad, O.C.; Tao, W. Cancer nanomedicine toward clinical translation: Obstacles, opportunities, and future prospects. Med 2023, 4, 147–167. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Albino, A.C.; Nozka, A.; Melnyk, A.; Good, H.J.; Rinaldi-Ramos, C.M. Post-synthesis Oxidation of Superparamagnetic Iron Oxide Nanoparticles to Enhance Magnetic Particle Imaging Performance. ACS Appl. Nano Mater. 2024, 7, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef]
- Pucci, C.; Degl’Innocenti, A.; Belenli Gümüş, M.; Ciofani, G. Superparamagnetic iron oxide nanoparticles for magnetic hyperthermia: Recent advancements, molecular effects, and future directions in the omics era. Biomater. Sci. 2022, 10, 2103–2121. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.Z.; Roy, V.A. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 2016, 8, 19421–19474. [Google Scholar] [CrossRef]
- Mehak Thummer, R.P.; Pandey, L.M. Surface modified iron-oxide based engineered nanomaterials for hyperthermia therapy of cancer cells. Biotechnol. Genet. Eng. Rev. 2023, 39, 1187–1233. [Google Scholar] [CrossRef]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wiśniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernandino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Shiji, R.; Joseph, M.M.; Sen, A.; Unnikrishnan, B.S.; Sreelekha, T.T. Galactomannan armed superparamagnetic iron oxide nanoparticles as a folate receptor targeted multi-functional theranostic agent in the management of cancer. Int. J. Biol. Macromol. 2022, 219, 740–753. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244 (Pt A), 108–121. [Google Scholar] [CrossRef]
- Cheng, Y.H.; He, C.; Riviere, J.E.; Monteiro-Riviere, N.A.; Lin, Z. Meta-Analysis of Nanoparticle Delivery to Tumors Using a Physiologically Based Pharmacokinetic Modeling and Simulation Approach. ACS Nano 2020, 14, 3075–3095. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070–8101. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Lartigue, L.; Javed, Y.; Luciani, N.; Pellegrino, T.; Wilhelm, C.; Alloyeau, D.; Gazeau, F. Biotransformations of magnetic nanoparticles in the body. Nano Today 2017, 11, 280–284. [Google Scholar] [CrossRef]
- Lapusan, R.; Borlan, R.; Focsan, M. Advancing MRI with magnetic nanoparticles: A comprehensive review of translational research and clinical trials. Nanoscale Adv. 2024, 6, 2234–2259. [Google Scholar] [CrossRef]
- Usov, N.A.; Rytov, R.A.; Bautin, V.A. Properties of assembly of superparamagnetic nanoparticles in viscous liquid. Sci. Rep. 2021, 11, 6999. [Google Scholar] [CrossRef]
- Jang, Y.J.; Liu, S.; Yue, H.; Park, J.A.; Cha, H.; Ho, S.L.; Marasini, S.; Ghazanfari, A.; Ahmad, M.Y.; Miao, X.; et al. Hydrophilic Biocompatible Poly(Acrylic Acid-co-Maleic Acid) Polymer as a Surface-Coating Ligand of Ultrasmall Gd2O3 Nanoparticles to Obtain a High r1 Value and T1 MR Images. Diagnostics 2020, 11, 2. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Zaloga, J.; Schreiber, E.; Tóth, I.Y.; Tombácz, E.; Lyer, S.; Alexiou, C. Tissue Plasminogen Activator Binding to Superparamagnetic Iron Oxide Nanoparticle-Covalent Versus Adsorptive Approach. Nanoscale Res. Lett. 2016, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Du, J.Z.; Du, X.J.; Mao, C.Q.; Wang, J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef]
- Nowak-Jary, J.; Machnicka, B. In vivo Biodistribution and Clearance of Magnetic Iron Oxide Nanoparticles for Medical Applications. Int. J. Nanomed. 2023, 18, 4067–4100. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ortega, F.; Delgado, Á.V.; Iglesias, G.R. Modulation of the Magnetic Hyperthermia Response Using Different Superparamagnetic Iron Oxide Nanoparticle Morphologies. Nanomaterials 2021, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Laha, S.S.; Sahu, N.K.; Thorat, N.D.; Shankar, B. Recent advancements and clinical aspects of engineered iron oxide nanoplatforms for magnetic hyperthermia-induced cancer therapy. Mater. Today Bio 2024, 29, 101348. [Google Scholar] [CrossRef]
- Couto, D.; Freitas, M.; Vilas-Boas, V.; Dias, I.; Porto, G.; Lopez-Quintela, M.A.; Rivas, J.; Freitas, P.; Carvalho, F.; Fernandes, E. Interaction of polyacrylic acid coated and non-coated iron oxide nanoparticles with human neutrophils. Toxicol. Lett. 2014, 225, 57–65. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH: Hoboken, NJ, USA, 2003. [Google Scholar]
- Lu, Y.; Huang, J.; Neverova, N.V.; Nguyen, K.L. USPIOs as targeted contrast agents in cardiovascular magnetic resonance imaging. Curr. Cardiovasc. Imaging Rep. 2021, 14, 2. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Mehdaoui, B.; Meffre, A.; Carrey, J.; Lachaize, S.; Lacroix, L.-M.; Gougeon, M.; Chaudret, B.; Respaud, M. Optimal Size of Nanoparticles for Magnetic Hyperthermia: A Combined Theoretical and Experimental Study. Adv. Funct. Mater. 2011, 21, 4573–4581. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Johannsen, M.; Thiesen, B.; Wust, P.; Jordan, A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010, 26, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Fiandra, L.; Alessio, G.; Mazzucchelli, S.; Nebuloni, M.; De Palma, C.; Kantner, K.; Pelaz, B.; Rotem, R.; Corsi, F.; et al. Tumour homing and therapeutic effect of colloidal nanoparticles depend on the number of attached antibodies. Nat. Commun. 2016, 7, 13818. [Google Scholar] [CrossRef]
- Markoutsa, E.; Papadia, K.; Giannou, A.D.; Spella, M.; Cagnotto, A.; Salmona, M.; Stathopoulos, G.T.; Antimisiaris, S.G. Mono and dually decorated nanoliposomes for brain targeting, in vitro and in vivo studies. Pharm. Res. 2014, 31, 1275–1289. [Google Scholar] [CrossRef]
- Skouras, A.; Papadia, K.; Mourtas, S.; Klepetsanis, P.; Antimisiaris, S.G. Multifunctional doxorubicin-loaded magnetoliposomes with active and magnetic targeting properties. Eur. J. Pharm. Sci. 2018, 123, 162–172. [Google Scholar] [CrossRef]
- Ramazi, S.; Salimian, M.; Allahverdi, A.; Kianamiri, S.; Abdolmaleki, P. Synergistic cytotoxic effects of an extremely low-frequency electromagnetic field with doxorubicin on MCF-7 cell line. Sci. Rep. 2023, 13, 8844. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Q.; Lv, X.; Lin, T. Progressive Study on the Non-thermal Effects of Magnetic Field Therapy in Oncology. Front. Oncol. 2021, 11, 638146. [Google Scholar] [CrossRef]
- Ashdown, C.P.; Johns, S.C.; Aminov, E.; Unanian, M.; Connacher, W.; Friend, J.; Fuster, M.M. Pulsed Low-Frequency Magnetic Fields Induce Tumor Membrane Disruption and Altered Cell Viability. Biophys. J. 2020, 118, 1552–1563. [Google Scholar] [CrossRef]
- Omote, Y.; Hosokawa, M.; Komatsumoto, M.; Namieno, T.; Nakajima, S.; Kubo, Y.; Kobayashi, H. Treatment of experimental tumors with a combination of a pulsing magnetic field and an antitumor drug. Jpn. J. Cancer Res. Gann 1990, 81, 956–961. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Markoutsa, E.; Pampalakis, G.; Niarakis, A.; Romero, I.A.; Weksler, B.; Couraud, P.O.; Antimisiaris, S.G. Uptake and permeability studies of BBB-targeting immunoliposomes using the hCMEC/D3 cell line. Eur. J. Pharm. Biopharm. 2011, 77, 265–274. [Google Scholar] [CrossRef] [PubMed]
| Sample | Dh (nm) | ζp (mV) |
|---|---|---|
| MNs1 | 61.3 ± 8.1 | −49.3 ± 7.6 |
| MNs2 | 80 ± 9.7 | −48.1 ± 8.5 |
| MNs3 | 100.2 ± 6.4 | −50.2 ± 5.3 |
| MNs2-PEG | 97.3 ± 7.2 | −11.2 ± 4.8 |
| MNs3-PEG | 112.4 ± 10.8 | −10.9 ± 3.3 |
| MNs2-PEG-OX26 | 129.2 ± 4.8 | 1.1 ± 0.8 |
| MNs3-PEG-OX26 | 140.2 ± 8.4 | 1.96 ± 0.92 |
| Model | Parameter | Control pH 7.4 | Hyperthermia pH 7.4 | Control pH 6.5 | Hyperthermia pH 6.5 |
|---|---|---|---|---|---|
| Zero Order | Slope | 0.086 ± 0.005 | 0.130 ± 0.005 | 0.146 ± 0.007 | 0.180 ± 0.011 |
| Intercept | 2.47 ± 0.22 | 2.57 ± 0.19 | 3.60 ± 0.28 | 4.11 ± 0.45 | |
| R2 | 0.988 | 0.996 | 0.993 | 0.989 | |
| First Order | Slope | −0.00091 ± 0.00005 | −0.00140 ± 0.00005 | −0.00160 ± 0.00008 | −0.00202 ± 0.00014 |
| Intercept | 4.58 ± 0.002 | 4.58 ± 0.002 | 4.57 ± 0.003 | 4.56 ± 0.006 | |
| R2 | 0.990 | 0.996 | 0.992 | 0.986 | |
| Higuchi | Slope | 0.80 ± 0.08 | 1.20 ± 0.15 | 1.34 ± 0.18 | 1.64 ± 0.27 |
| Intercept | 1.30 ± 0.42 | 0.87 ± 0.83 | 1.70 ± 0.99 | 1.83 ± 1.47 | |
| R2 | 0.973 | 0.954 | 0.948 | 0.926 | |
| Korsmeyer–Peppas | Slope (n) | 0.51 ± 0.06 | 0.61 ± 0.06 | 0.54 ± 0.06 | 0.54 ± 0.06 |
| Intercept (log k) | 0.38 ± 0.08 | 0.42 ± 0.08 | 0.55 ± 0.07 | 0.63 ± 0.08 | |
| R2 | 0.889 | 0.899 | 0.907 | 0.884 |
| Protocol | AMF Timing | Cell Viability (%) | Cell Death (%) | Relative Efficacy |
|---|---|---|---|---|
| Conventional | 2 h post-treatment | 14.92 ± 1.2 | 85.08 | 1.00 (baseline) |
| Optimized | 0.5 h pre + 1.5 h post | 7.95 ± 0.8 | 92.05 | 1.87 (87% improvement) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzavara, D.; Papadia, K.; Kolokithas-Ntoukas, A.; Antimisiaris, S.G.; Skouras, A. Engineered PAM-SPION Nanoclusters for Enhanced Cancer Therapy: Integrating Magnetic Targeting with pH-Responsive Drug Release. Molecules 2025, 30, 2785. https://doi.org/10.3390/molecules30132785
Tzavara D, Papadia K, Kolokithas-Ntoukas A, Antimisiaris SG, Skouras A. Engineered PAM-SPION Nanoclusters for Enhanced Cancer Therapy: Integrating Magnetic Targeting with pH-Responsive Drug Release. Molecules. 2025; 30(13):2785. https://doi.org/10.3390/molecules30132785
Chicago/Turabian StyleTzavara, Dimitra, Konstantina Papadia, Argiris Kolokithas-Ntoukas, Sophia G. Antimisiaris, and Athanasios Skouras. 2025. "Engineered PAM-SPION Nanoclusters for Enhanced Cancer Therapy: Integrating Magnetic Targeting with pH-Responsive Drug Release" Molecules 30, no. 13: 2785. https://doi.org/10.3390/molecules30132785
APA StyleTzavara, D., Papadia, K., Kolokithas-Ntoukas, A., Antimisiaris, S. G., & Skouras, A. (2025). Engineered PAM-SPION Nanoclusters for Enhanced Cancer Therapy: Integrating Magnetic Targeting with pH-Responsive Drug Release. Molecules, 30(13), 2785. https://doi.org/10.3390/molecules30132785








