Sucrose-Based Macrocycles: An Update
Abstract
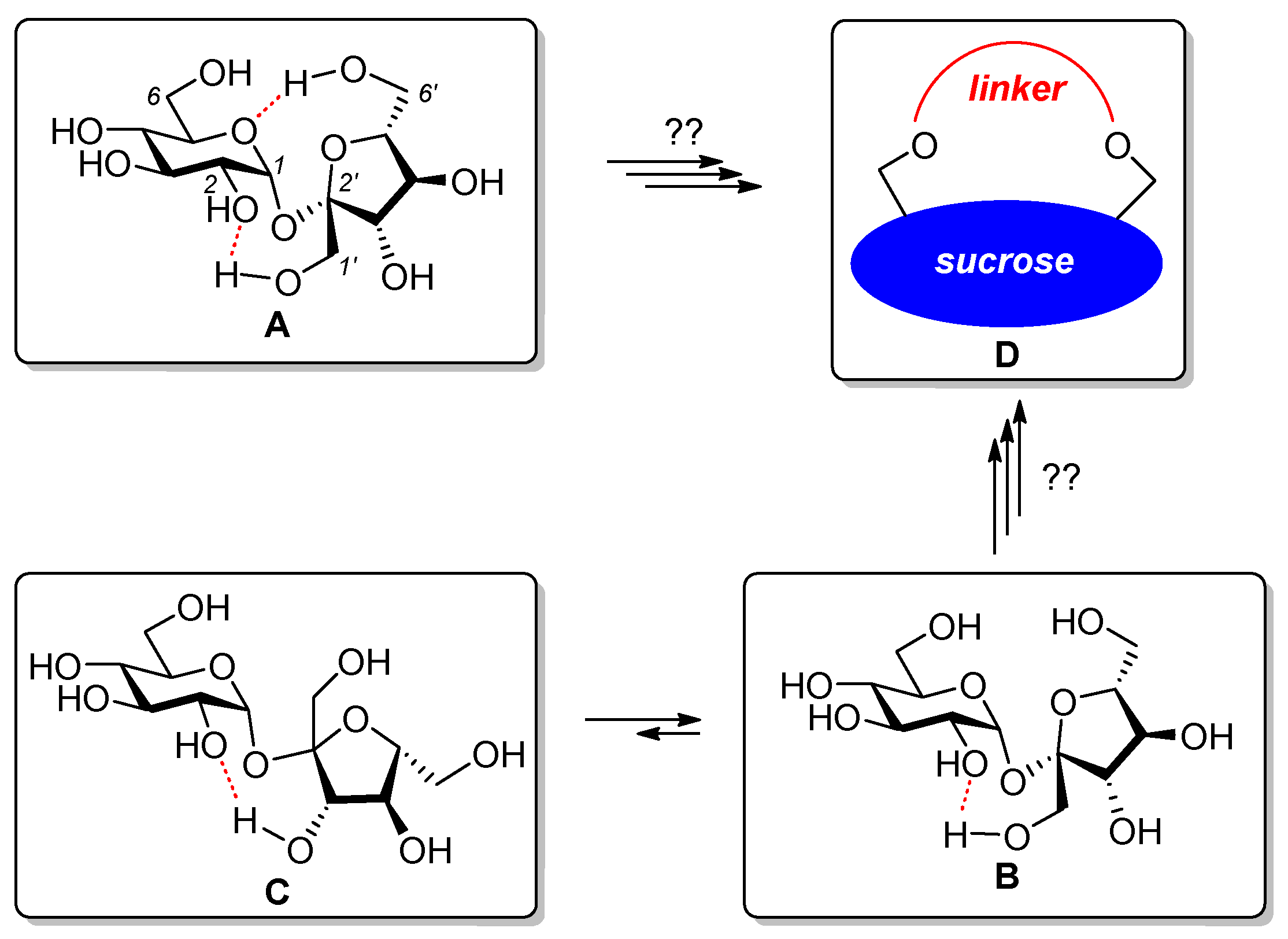
1. Introduction
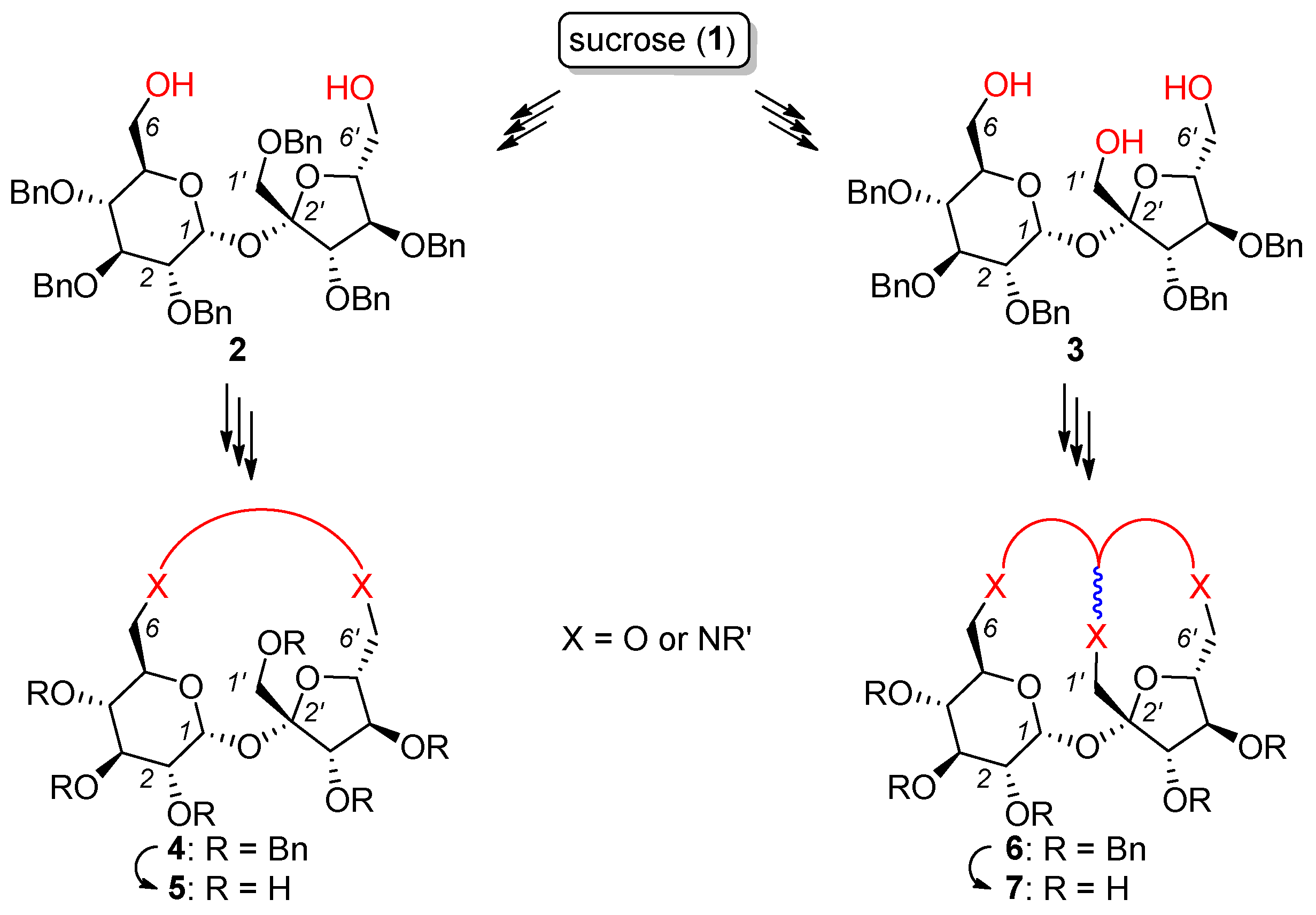
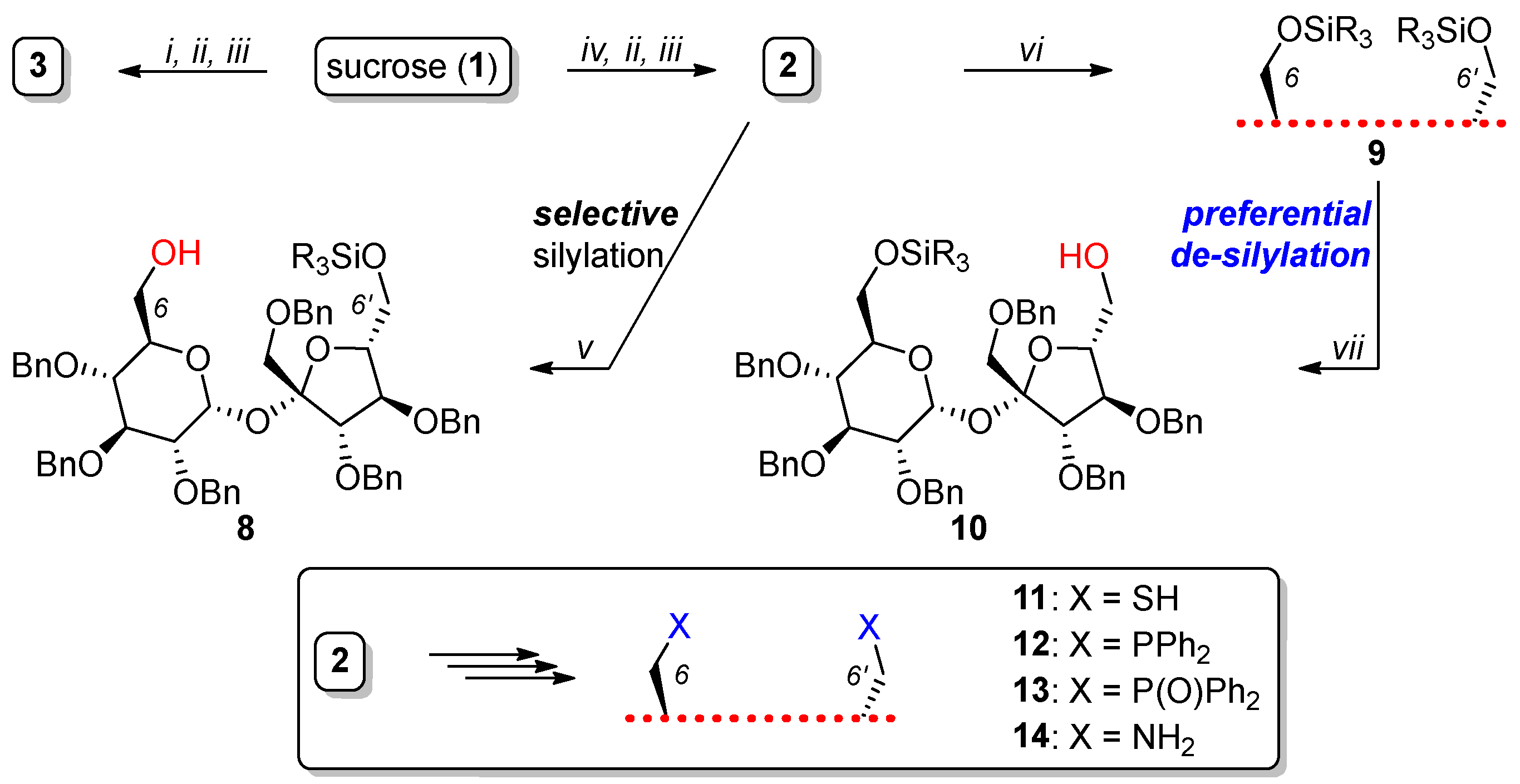
- The protection of all secondary hydroxyl groups with temporary blocks (preferably benzyl) that could be easily removed after the desired transformations without the destruction of a highly sensitive glycosidic bond (Figure 3; compounds 2 and 3). This strategy requires temporary protection of the primary hydroxyl groups (C6, C6′ and eventually C1′), benzylation of all remaining OH groups, and the removal of temporary blocks from C6, C6′ (and eventually C1′) positions. The selective protection of the primary OH groups is well known and can be achieved using very expensive bulky silyl chlorides or much cheaper trityl chlorides. However, the removal of the trityl blocks from per-benzylated intermediates was not trivial since this process can only be realized by careful acidic hydrolysis.
- The connection of the terminal positions (C6, C6′ and eventually C1′) should be connected via a proper link (4 and 6).
- Total deprotection, i.e., the removal of all temporary blocks, which should provide macrocyclic derivatives (potential receptors) soluble in water (5 and 7).
2. Sugar Derived Macrocyclic Derivatives with Sucrose Scaffold
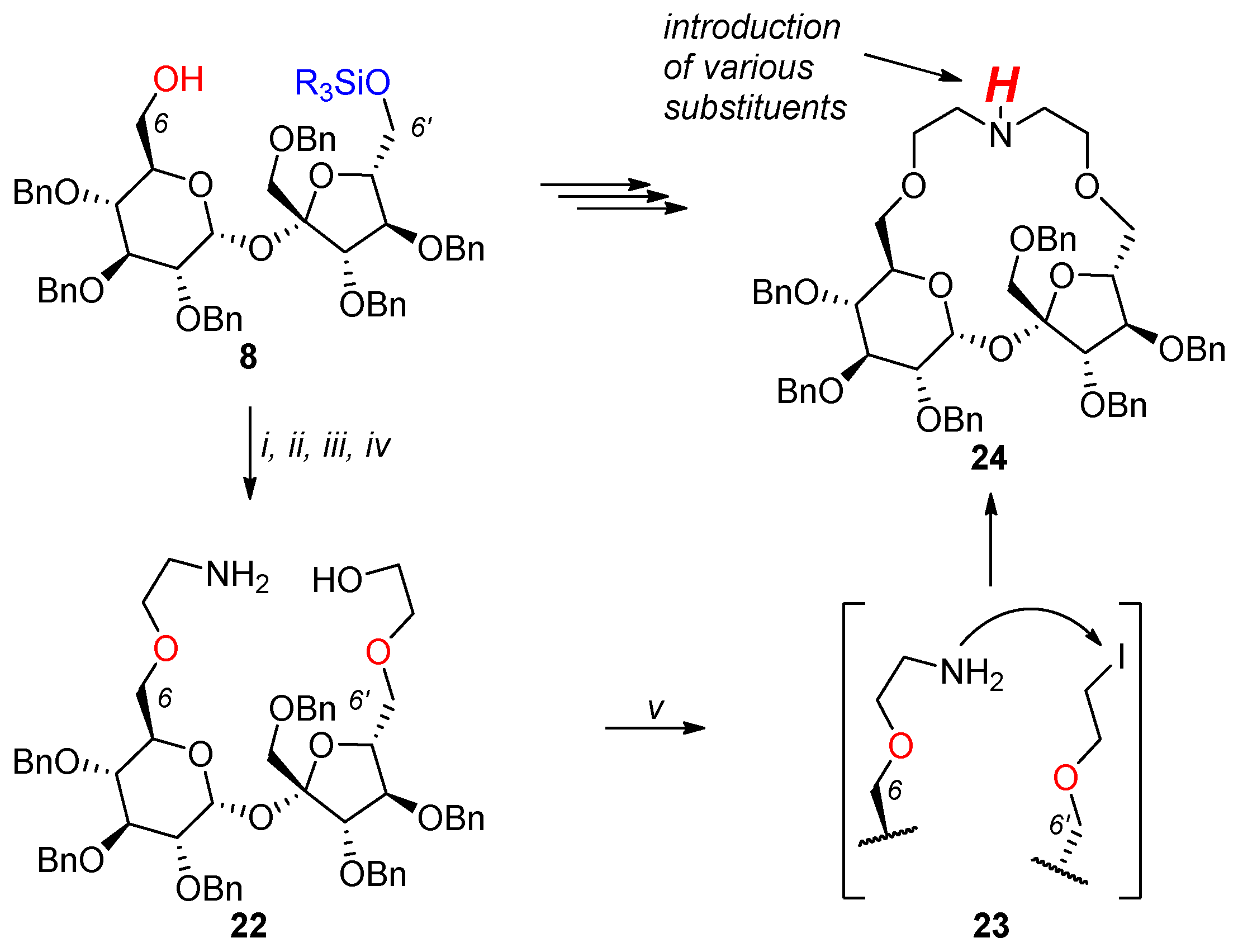
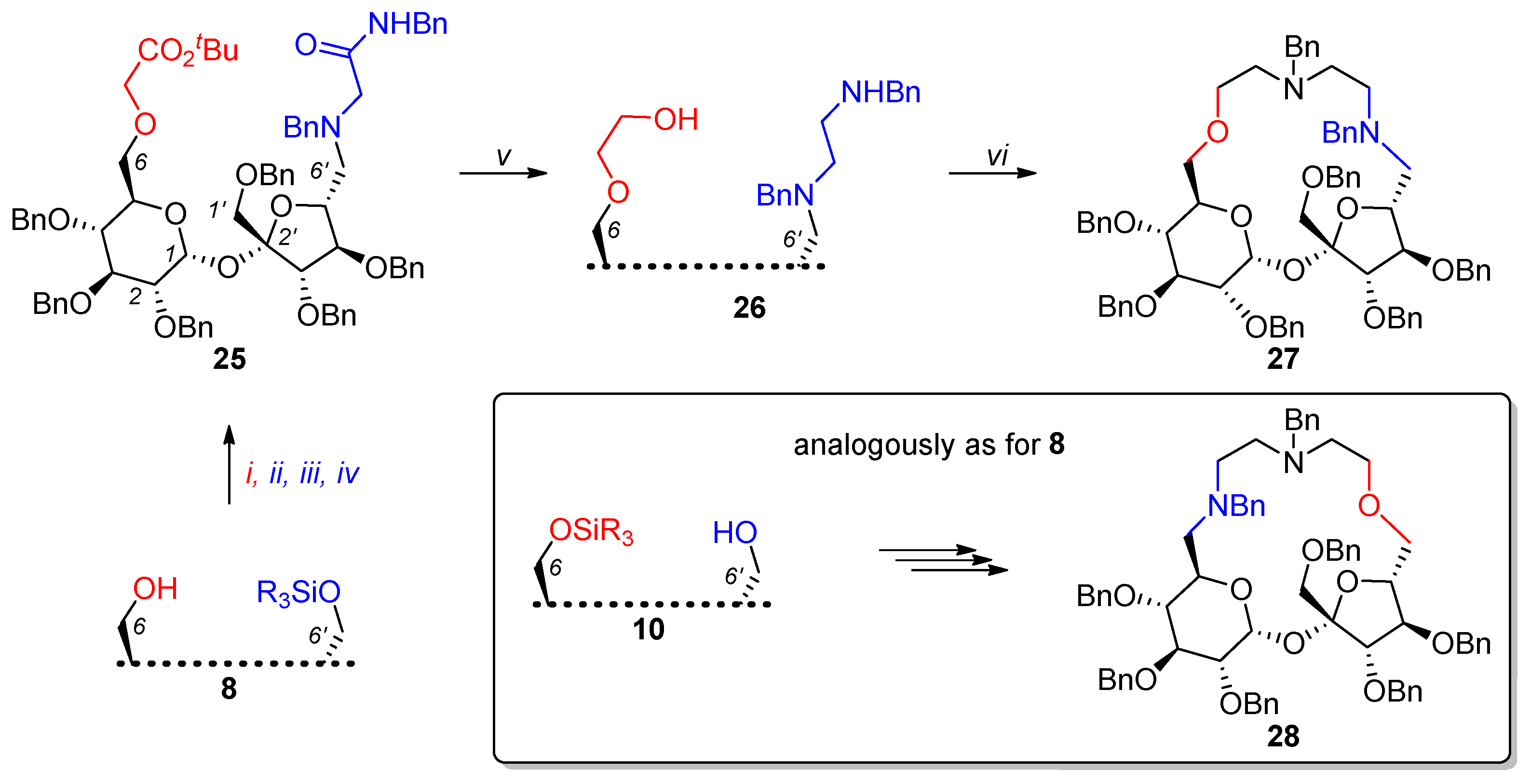
2.1. Synthesis of Macrocyclic Derivatives from “Sucrose Diol” 2
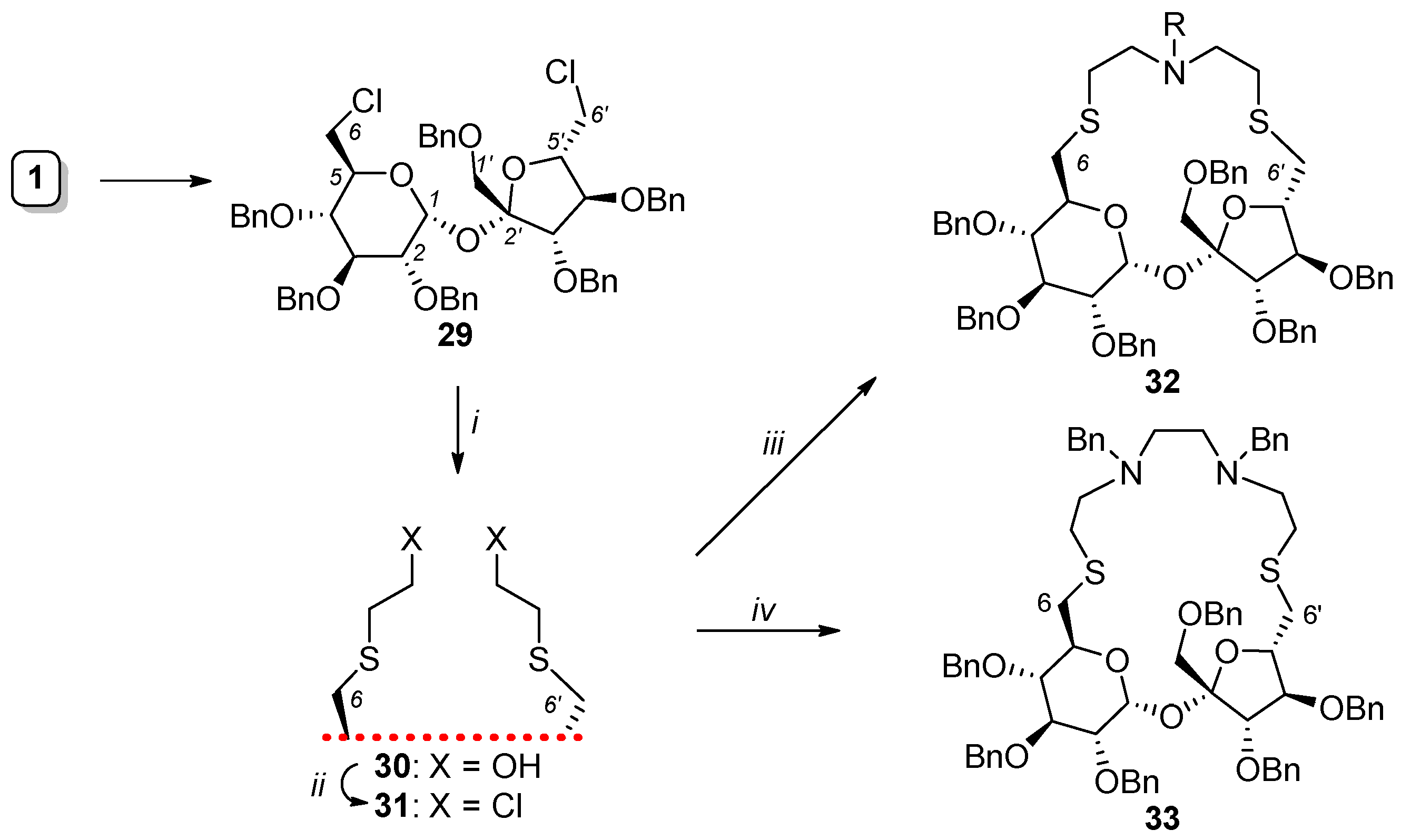
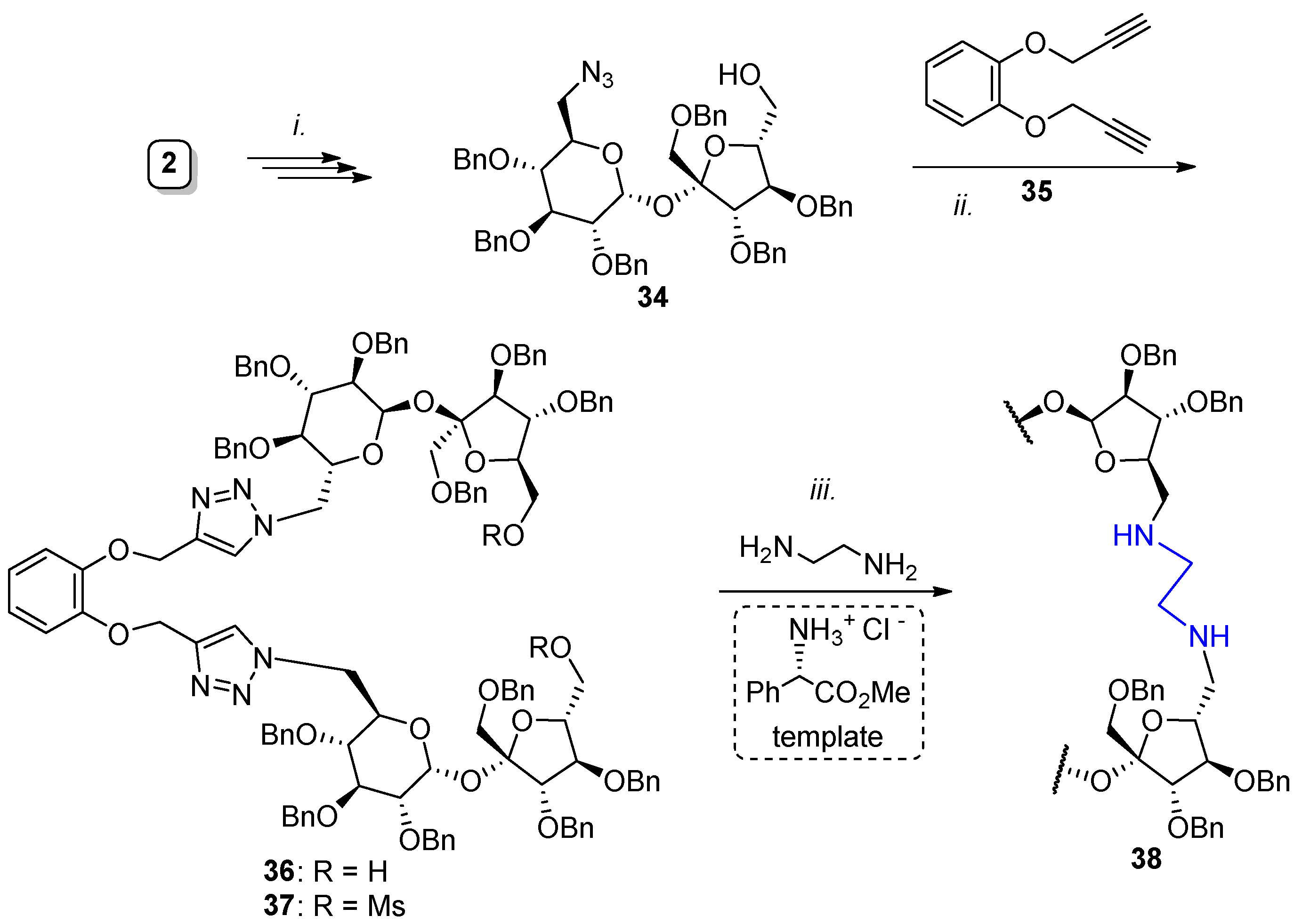
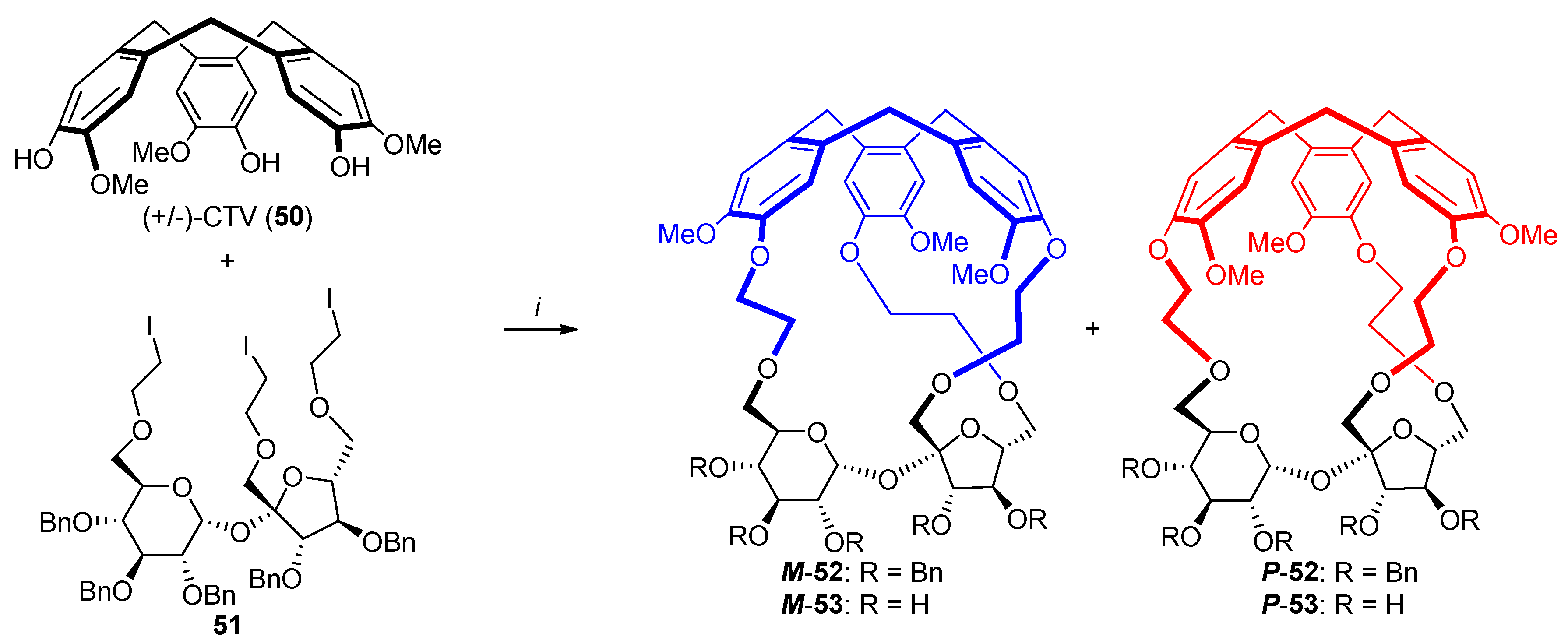
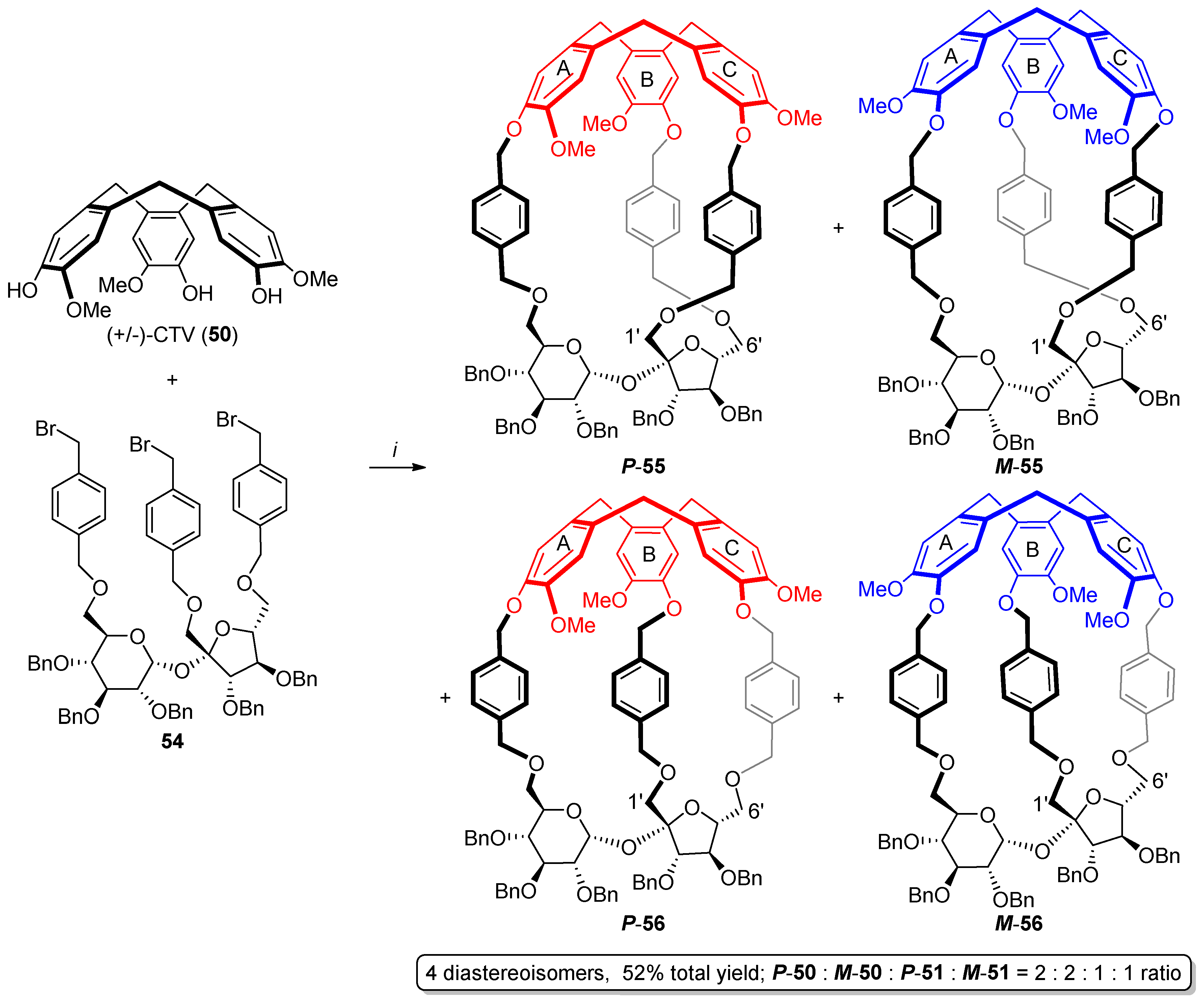
2.2. Synthesis of Other Complex Macrocyclic Sucrose-Based Derivatives
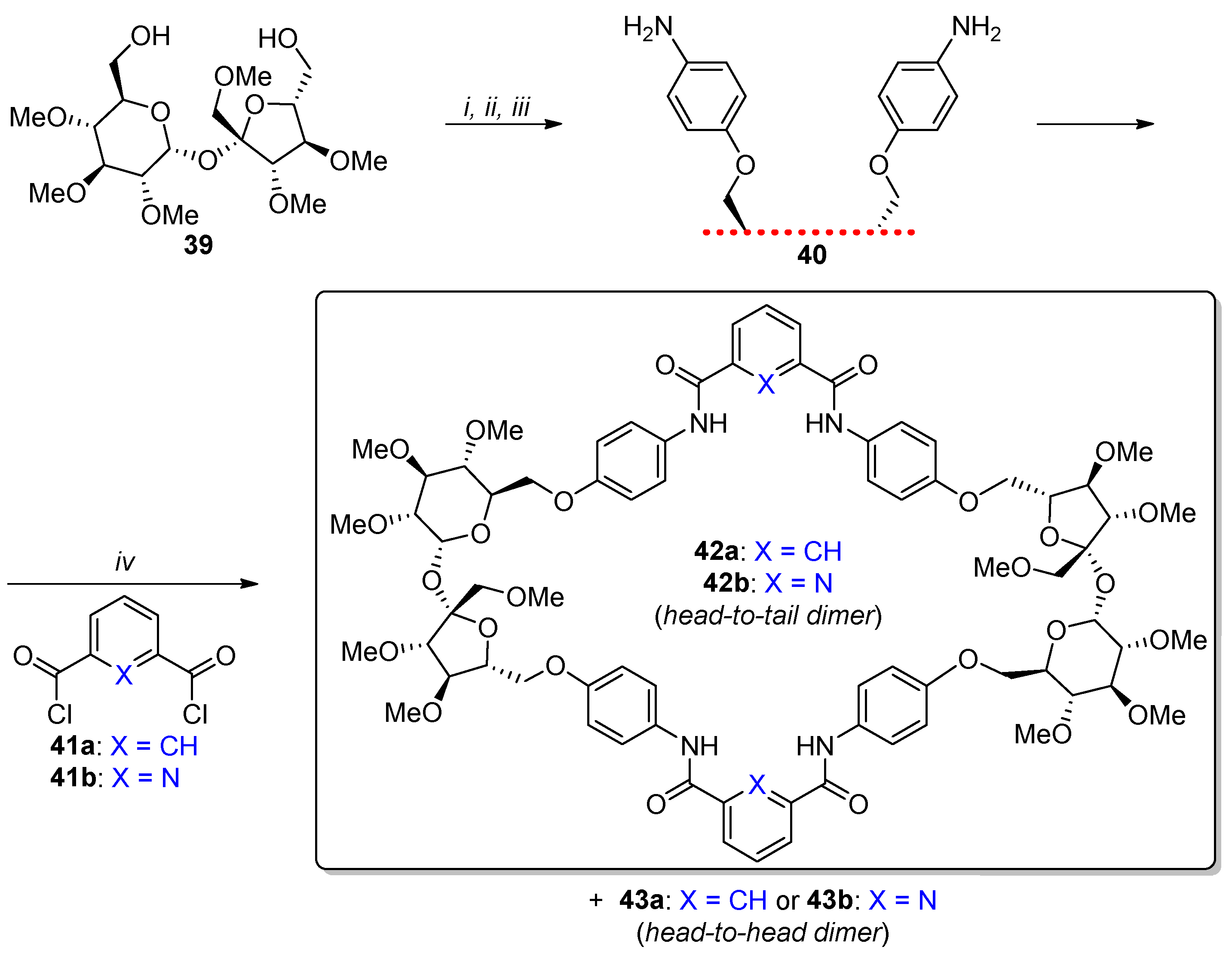
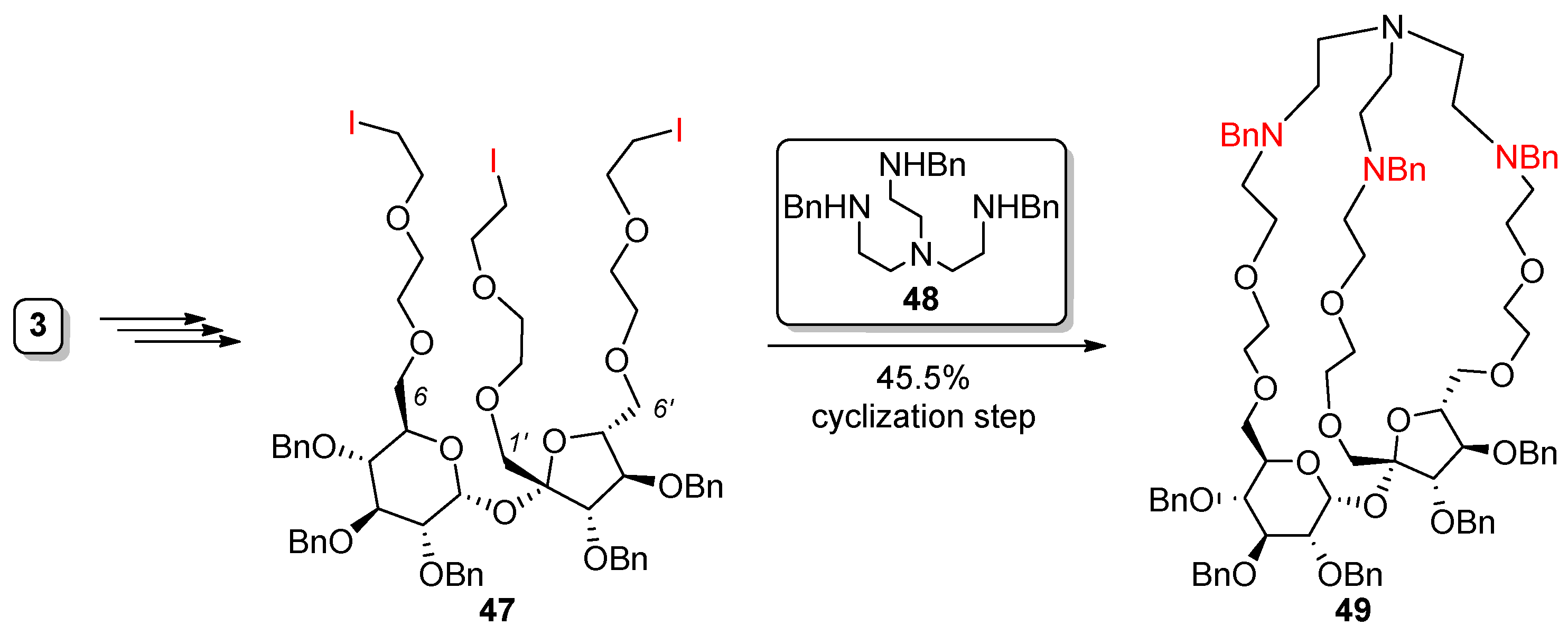
2.3. Synthesis of Macrocyclic Derivatives from “Sucrose Triol” 3
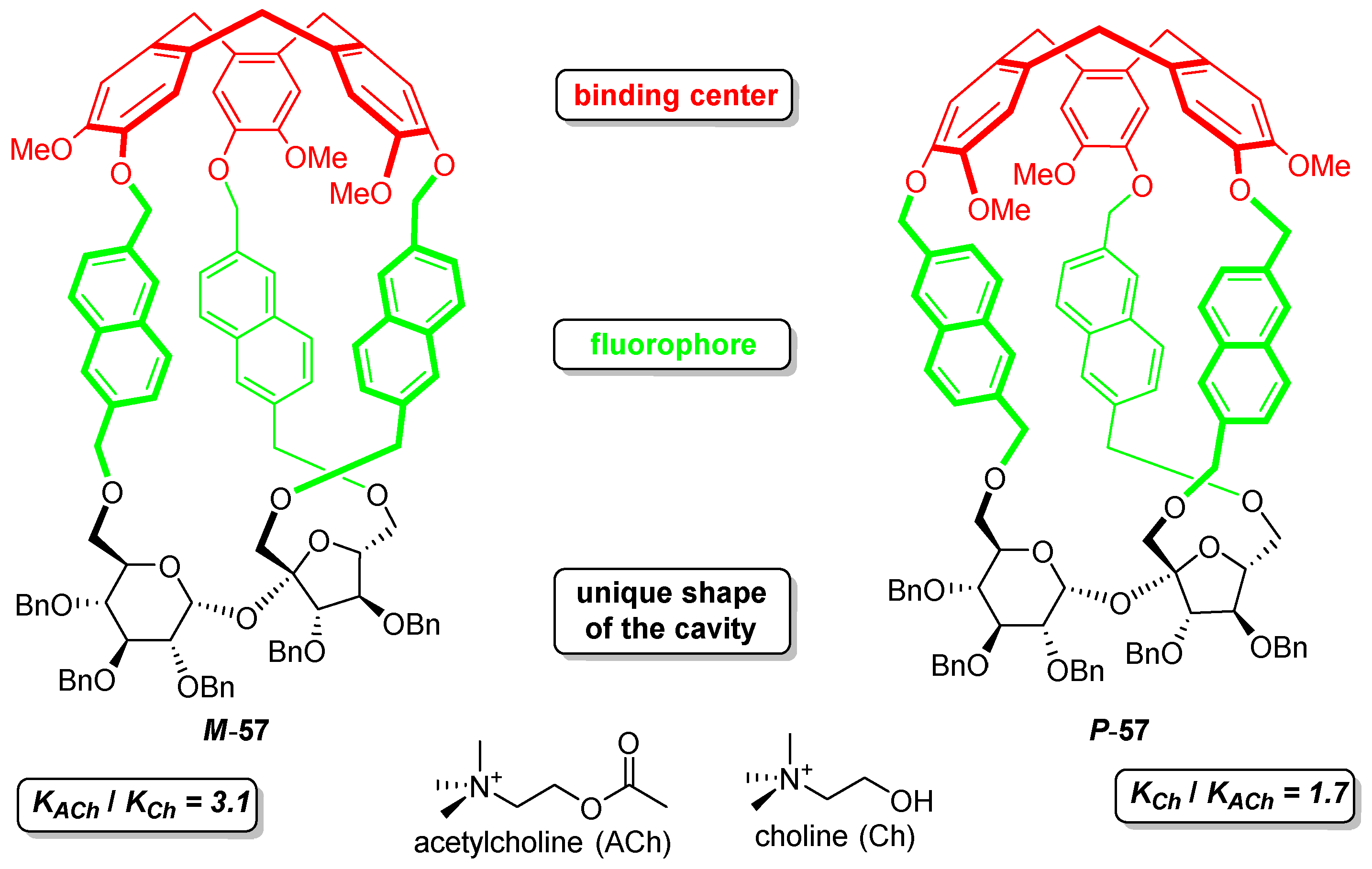
3. Complexing Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTV | cycloveratrylene |
| tert-BDPS-Cl | tert-butyldiphenyl silyl chloride |
| TrCl | triphenylmethyl chloride (trityl chloride) |
References
- Queneau, Y.; Jarosz, S.; Lewandowski, B.; Fitremann, J. Sucrose chemistry and applications of sucrochemicals. Adv. Carbohydr. Chem. Biochem. 2008, 61, 217–292. [Google Scholar] [CrossRef]
- Jarosz, S.; Pakulski, Z. The roadmap for sucrose—A very inexpensive raw product. Adv. Carbohydr. Chem. Biochem. 2024, 86, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, S.; Sokołowska, P.; Szyszka, Ł. Synthesis of fine chemicals with high added value from sucrose: Towards sucrose-based macrocycles. Tetrahedron Lett. 2020, 61, 151888. [Google Scholar] [CrossRef]
- Lichtenthaler, F.; Immel, W.; Kreis, S.U. Old roots-new branches: Evolution of the structural representation of sucrose. In Carbohydrates as Organic Raw Materials; VCH Verlagsgesellschaft: Weinheim, Germany, 1991; pp. 1–32. ISBN 3-527-28280-7. [Google Scholar]
- Karl, H.; Lee, C.K.; Khan, R. Synthesis and reactions of tert-butyldiphenylsilyl ethers of sucrose. Carbohydr. Res. 1982, 101, 31–38. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1758. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Xie, J.; Bogliotti, N. Synthesis and applications of carbohydrate-derived macrocyclic compounds. Chem. Rev. 2014, 114, 7678–7739. [Google Scholar] [CrossRef]
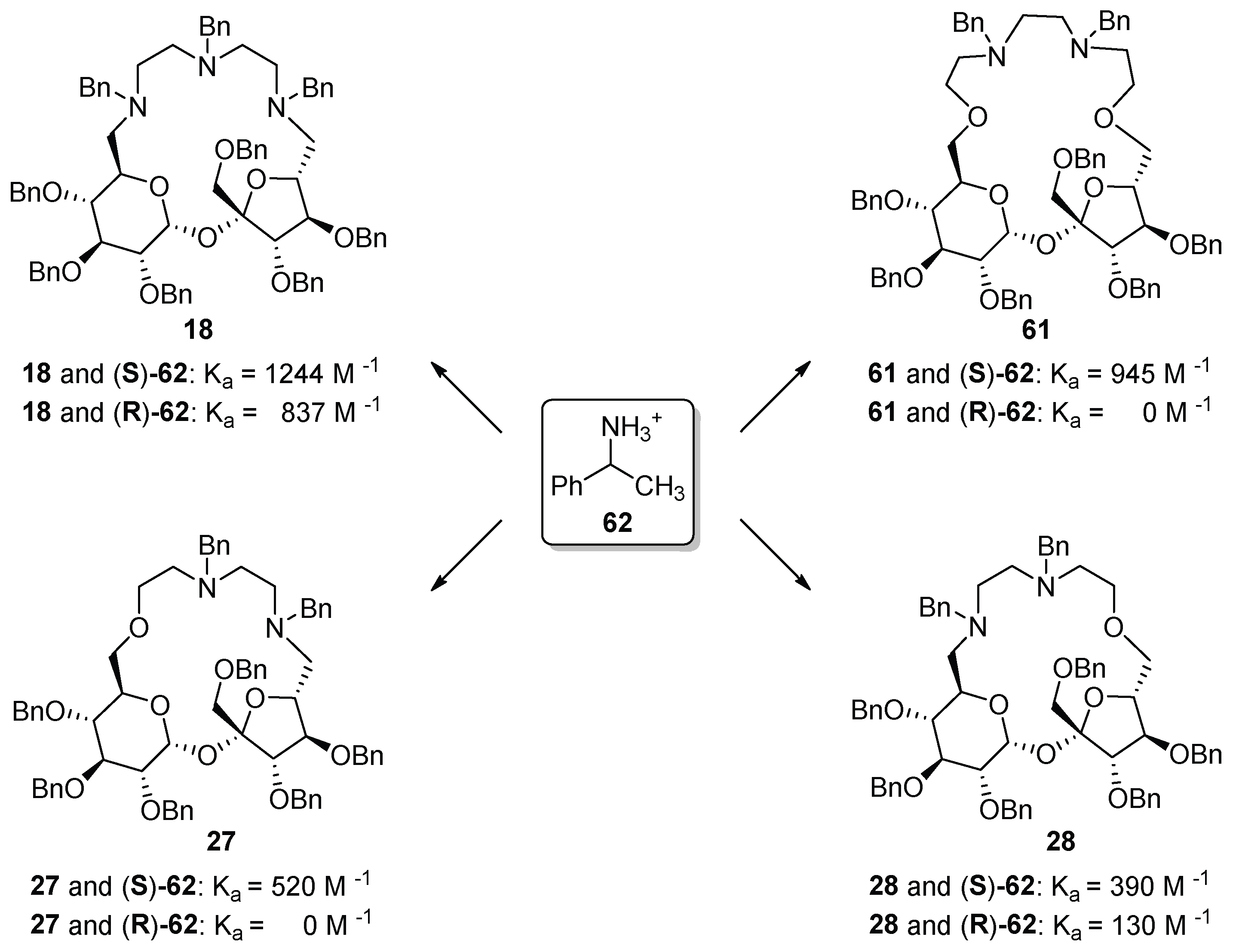
- Lewandowski, B.; Jarosz, S. Chiral recognition of α-phenylethylamine by sucrose-based macrocyclic receptors. Chem. Comm. 2008, 47, 6399–6401. [Google Scholar] [CrossRef]
- Jarosz, S.; Lewandowski, B. Synthesis and complexation properties towards the ammonium cation of aza-coronand analogues containing sucrose. Carbohydr. Res. 2008, 343, 965–969. [Google Scholar] [CrossRef]
- Potopnyk, M.A.; Lewandowski, B.; Jarosz, S. Novel sucrose-based macrocyclic receptors for enantioselective recognition of chiral ammonium cations. Tetrahedron Asymmetry 2012, 23, 1474–1479. [Google Scholar] [CrossRef]
- Potopnyk, M.A.; Jarosz, S. Synthesis and complexing properties of “unsymmetrical” sucrose-based receptors. Eur. J. Org. Chem. 2013, 2013, 5117–5126. [Google Scholar] [CrossRef]
- Whistler, R.L.; Anisuzzaman, A.K.M. Preferential halogenation of the primary hydroxyl group. Methods Carbohydr. Chem. 1980, 8, 227–231. [Google Scholar]
- Jarosz, S.; Listkowski, A. Synthesis of macrocyclic derivatives containing sucrose unit. J. Carbohydr. Chem. 2003, 22, 753–763. [Google Scholar] [CrossRef]
- Gajewska, A.; Osuch-Kwiatkowska, A.; Jarosz, S. Synthesis of sulfur containing macrocycles with sucrose scaffold. Carbohydr. Res. 2019, 486, 107825. [Google Scholar] [CrossRef]
- Lewandowski, B.; Jarosz, S. Amino-acid templated assembly of sucrose-derived macrocycles. Org. Lett. 2010, 12, 2532–2535. [Google Scholar] [CrossRef]
- Potopnyk, M.A.; Cmoch, P.; Jarosz, S. Short synthesis of diamide-linked sucrose macrocycles. Org. Lett. 2012, 14, 4258–4261. [Google Scholar] [CrossRef]
- Łęczycka-Wilk, K.; Dąbrowa, K.; Cmoch, P.; Jarosz, S. Chloride-templated macrocyclization and anion-binding properties of C2-symmetric macrocyclic ureas from sucrose. Org. Lett. 2017, 19, 4596–4599. [Google Scholar] [CrossRef]
- Łęczycka-Wilk, K.; Ulatowski, F.; Cmoch, P.; Jarosz, S. “Choose-a-size” control in the synthesis of sucrose based urea and thiourea macrocycles. Org. Biomol. Chem. 2018, 16, 6063–6069. [Google Scholar] [CrossRef]
- Sokołowska, P.; Kowalski, M.; Jarosz, S. First synthesis of cryptands with sucrose scaffold. Beilstein J. Org. Chem. 2019, 15, 210–217. [Google Scholar] [CrossRef]
- Szyszka, Ł.; Cmoch, P.; Butkiewicz, A.; Potopnyk, M.A.; Jarosz, S. Synthesis of cyclotriveratrylene-sucrose-based capsules. Org. Lett. 2019, 21, 6523–6528. [Google Scholar] [CrossRef]
- Szyszka, Ł.; Cmoch, P.; Górecki, M.; Ceborska, M.; Potopnyk, M.A.; Jarosz, S. Chiral molecular cages based on cyclotriveratrylene and sucrose units connected with p-phenylene linkers. Eur. J. Org. Chem. 2021, 2021, 897–906. [Google Scholar] [CrossRef]
- Szyszka, Ł.; Górecki, M.; Cmoch, P.; Jarosz, S. Fluorescent molecular cages with sucrose and cyclotriveratrylene units for the selective recognition of choline and acetylcholine. J. Org. Chem. 2021, 86, 5129–5141. [Google Scholar] [CrossRef] [PubMed]
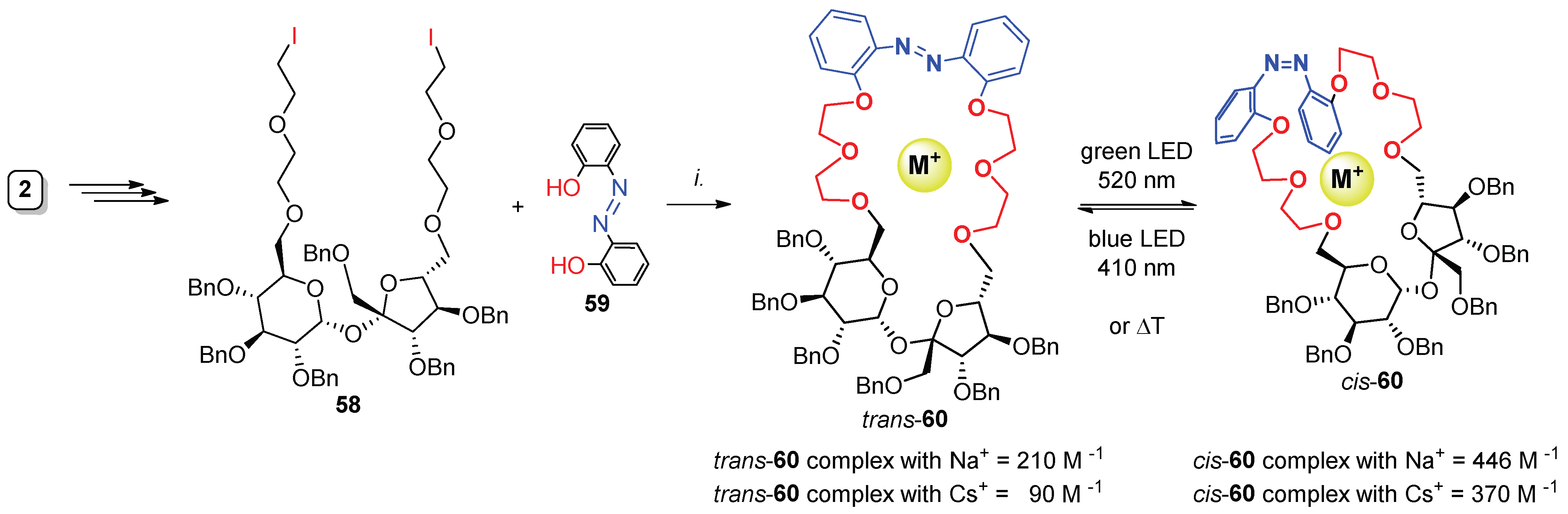
- Sokołowska, P.; Dąbrowa, K.; Jarosz, S. Visible-light responsive sucrose-containing macrocyclic host for cations. Org. Lett. 2021, 23, 2687–2692. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosz, S.; Pakulski, Z. Sucrose-Based Macrocycles: An Update. Molecules 2025, 30, 2721. https://doi.org/10.3390/molecules30132721
Jarosz S, Pakulski Z. Sucrose-Based Macrocycles: An Update. Molecules. 2025; 30(13):2721. https://doi.org/10.3390/molecules30132721
Chicago/Turabian StyleJarosz, Sławomir, and Zbigniew Pakulski. 2025. "Sucrose-Based Macrocycles: An Update" Molecules 30, no. 13: 2721. https://doi.org/10.3390/molecules30132721
APA StyleJarosz, S., & Pakulski, Z. (2025). Sucrose-Based Macrocycles: An Update. Molecules, 30(13), 2721. https://doi.org/10.3390/molecules30132721






