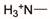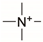Abstract
Bacterial adhesion and the subsequent formation of biofilms and biofouling have significant economic and health impacts across all sectors. They are especially impactful in industrial corrosion, healthcare, food processing, agriculture, and waste and drinking water. Synthetic polymers that resist bacterial adhesion are adaptable to a wide range of applications in all of these fields. While there are many bacteria-resistant polymers, some of the best performing include polyethylene glycol (PEG), poly(oxazoline) (POZ), and zwitterionic polymers, with zwitterionic polymers showing the most promise with reductions in bacteria adhesion up to 99% over controls. This review summarizes the demonstrated bacterial resistance performance of these polymer coatings based on literature published over the last ten years. It also identifies the front runners for preventing bacterial adhesion while providing the critical next steps for widespread adoption of this technology.
Keywords:
biofilm; biofouling; bacterial adhesion; hydrogels; polyampholytes; zwitterions; poly(oxazoline); PEG; nonfouling 1. Introduction
Every year significant resources are devoted to combating biofilms as an expansive challenge for human life and industry [1]. The estimated annual financial burden reported in 2019 was in excess of $5 trillion USD worldwide [1] with anti-microbial films reaching a global market of $4.28 billion USD in 2021 [2]. Reported financial costs imposed by biofilms do not include the costs for constant research and development which is necessary as bacteria become resistant to biocides and antimicrobials [3,4,5].
Traditionally, biofilms have been defined as bacterium contained within a three-dimensional, excreted extracellular polymeric matrix attached to a surface [5]. However, advancements in the scientific understanding of how biofilms form and function have expanded that definition to include not only surface-attached microbial aggregates, but also non-surface-attached aggregated bacteria [6]. In this review, references to biofilms are specific to surface-attached biofilms. Biofilms have a complex life cycle that leads to enhanced resistance to antimicrobial agents, tolerance to desiccation, shear stress, and protozoan grazing, and the increased capture of nutrients compared to free-living bacterial cells [4,6,7]. In addition to these benefits, surface-attached biofilms condition the surfaces they interact with by altering the physicochemical properties of the substratum surface, making repeat growth virtually impossible to prevent or eliminate [8,9]. Given the inherent challenges of treating biofilms with a reactive process, significant research efforts are dedicated towards the prevention of initial biofilm formation. Most prevention techniques utilize a surface modification or coating that impedes initial bacterial surface attachment, which in turn prevents surface-attached biofilm formation.
While the focus of this review is not on the impacts of biofouling, a few pertinent examples are provided to better frame its widespread impact. Biofouling in industrial systems not only causes the corrosion or degradation of surfaces and reductions in product quality, but also potentially exposes workers to aerosolized particles during processing or cleaning [10]. Any processes containing membranes are also significantly affected by biofilm formation; this biofilm then acts as a secondary membrane, imposing an increased pressure drop and reducing flux through the affected membrane [9]. Healthcare and the medical field are impacted in multiple ways, from dentistry and implants to chronic wound control, which have been the focus of many recent reviews [11,12,13,14,15,16,17,18]. Foodborne illnesses are a highly publicized result of biofilm formation in every stage of the food supply chain [19,20,21,22]. Agricultural irrigation systems are susceptible to biofouling, and a study in 2015 found that even the residual water remaining in the pipelines between irrigation events fosters significant bacterial growth [23]. Additionally, irrigation water and pipelines have been investigated for their role in disseminating antibiotic-resistant bacteria into the environment [23]. Biofilms have significant implications for wastewater and potable water, including corrosion and blockages of components, leading to mechanical failure within the systems [5,24,25,26,27,28]. Potable water systems contaminated with biofilm also cause at least 7 million incidents annually in the United States alone [6,29,30,31,32,33,34,35]. A common thread throughout the afflicted systems, excluding the complicated systems within the medical field, is the presence of pipelines and stagnation points that are susceptible to biofilm formation [5,9,10].
Across all sectors afflicted with the impacts of biofilm formation, the current mode of mitigation is treatment and control, generally including forms of UV/radiation treatment [36,37,38], antimicrobial biocides [3,39,40,41,42,43,44], biocidal-impregnated surfaces and coatings [45,46,47,48,49,50,51,52,53,54], signal-disrupting chemicals or enzymes [55,56,57,58,59], oxidants [60,61], or physical cleaning including aeration [62,63,64,65]. Each of these processes reduces bacterial loads within systems, but none reach total eradication. Mechanical scrubbing and other physical cleaning can also be impossible or impractical in confined spaces [66]. Biofilms inherently act as barriers for the bacteria contained within, which means the innermost layers of the biofilm are exposed to sublethal doses of antimicrobials, leading to acquired resistance [8]. In all these treatments, the inherent flaw is that even if 99% of the micro-organisms within the system are eliminated, the remainder will still recolonize the surface-conditioned interior of the system [67]. As an example, out of all waterborne pathogen outbreaks in the United States, the CDC reports that 54% of the contaminated systems were previously treated with disinfectants [29]. Therefore, the focus of the remainder of this review will be developing approaches for preventing the initial stages of bacterial adhesion to prevent biofilm formation from initiating.
2. Prevention
While there are many approaches to prevent bacterial adhesion, including surface hydrophobicity modifications by hydrophobic coatings or micropatterning [68,69,70,71,72,73] and surface topography modifications [69,74,75,76,77,78,79,80,81,82,83,84], these approaches are not the focus of this review due to the technical difficulties of adapting surface topological modifications to large-scale production. Further, the use of nanomaterials to enhance antibiofilm performance has been the subject of recent reviews by others [85].
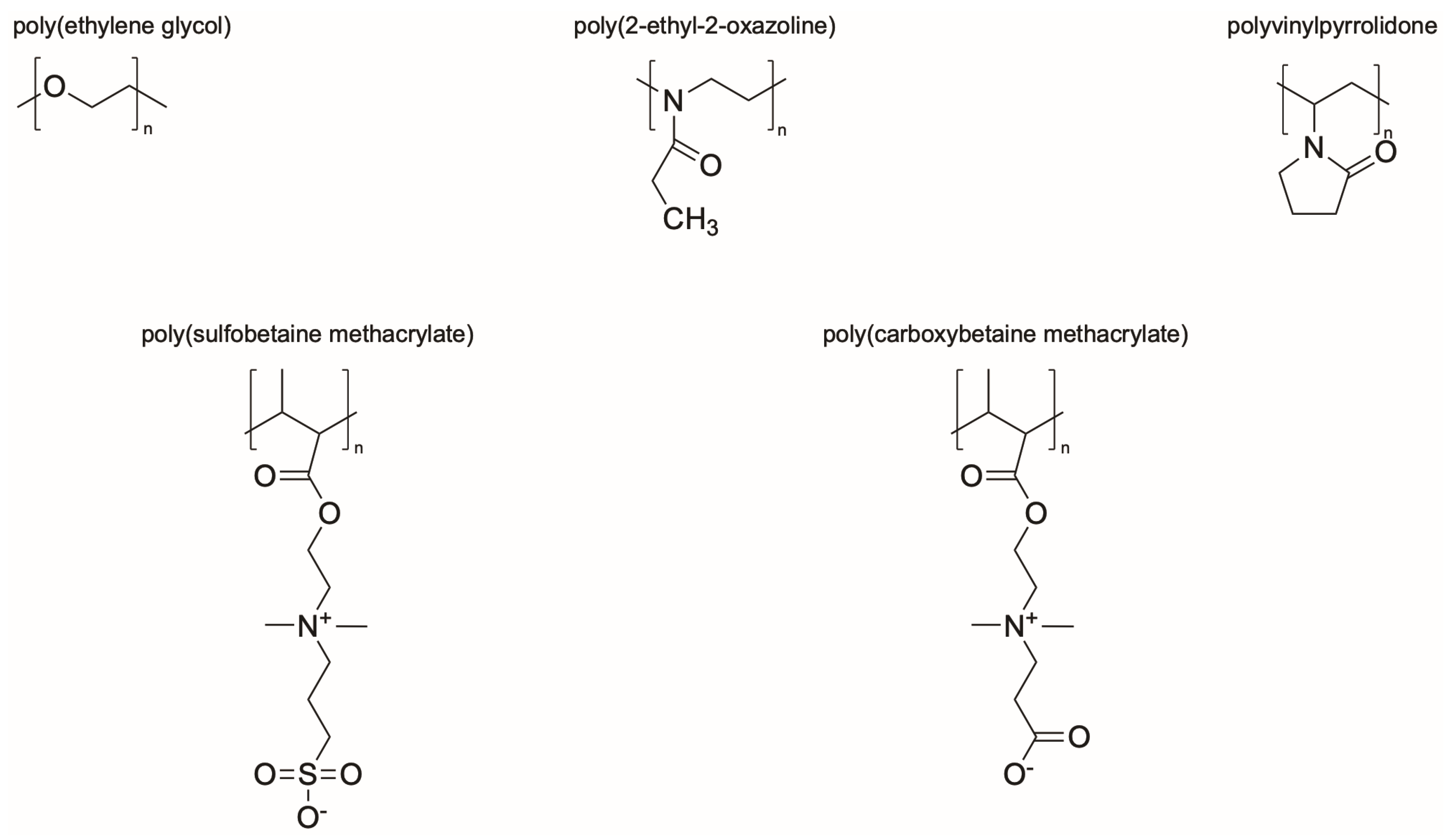
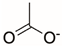
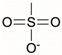
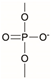
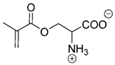
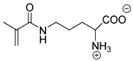
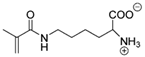
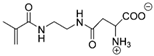
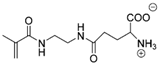
Synthetic polymer coatings are by nature more feasible for large-scale applications and their adaptation for biofilm prevention has made significant progress in recent years. Emerging synthetic polymer coatings believed to have significant antifouling capabilities include polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), poly(oxazoline) (POZ), and zwitterionic polymers, as shown in Figure 1.
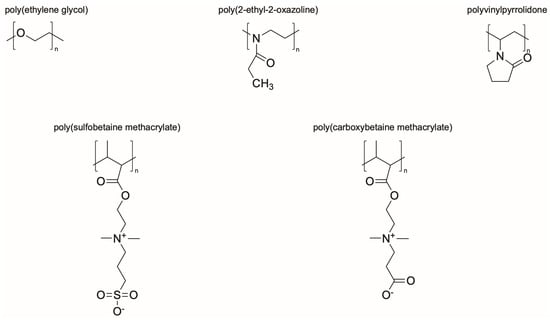
Figure 1.
Representative structures of synthetic polymer coatings employed as nonfouling or bacteria-resistant coatings.
The hypothesized mechanism by which these hydrophilic polymers resist bacterial adhesion has evolved over the years as study into their properties has continued. The properties that have been observed as common among all of these polymers include being hydrophilic, electrically neutral, and a hydrogen bond acceptor but not a hydrogen bond donor [86,87]. Although these properties have been observed among these polymers, the main mechanism of resistance is theorized to be osmotic repulsion from the tightly bound water layers adjacent to the polymers [87,88,89,90]. In addition to this tightly bound water layer, it is important that the polymers have a net neutral charge. Any positively or negatively charged polymers, or localized regions of charge within a net neutral polymer, will absorb oppositely charged protein or bacteria via electrostatic interactions, leading to failure [87].
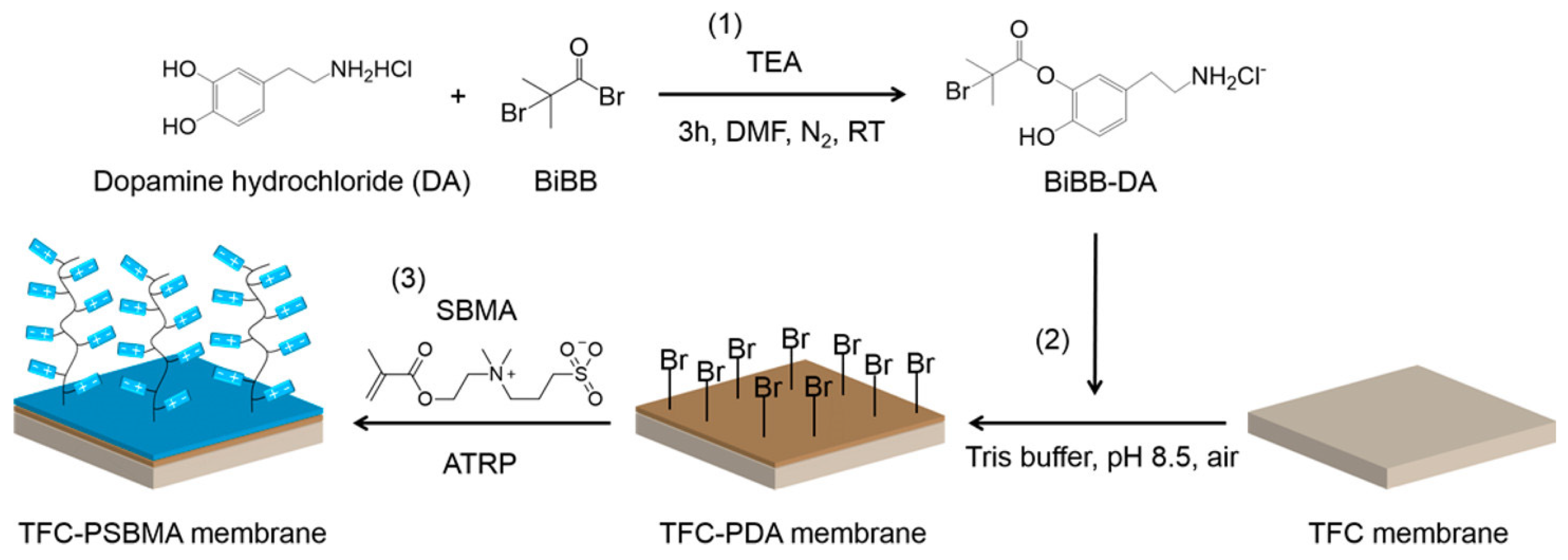
Prior to applying the bacteria-resistant polymer layer, most surfaces require modification to tightly anchor the polymer to the surface. Polymer brushes utilize surface-initiated atom transfer radical polymerization (ATRP), which commonly uses bromine-terminated surface-bound species [91]. Figure 2 is a schematic example of how the surface initiation process facilitates the formation of the subsequent polymer film using a dopamine-based surface-bound initiator. Other commonly used surface initiation techniques include silanes [92] or thiols [93,94,95], which contain the same terminal bromine-reactive group [96,97,98].
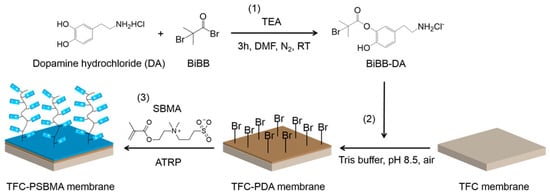
Figure 2.
Coupling of initiator and dopamine hydrochloride (1), immobilization of initiators on the TFC membrane (2), and ATRP grafting of zwitterionic thin film (3). Reprinted with permission from [99]. Copyright 2017 American Chemical Society.
This review will focus on advances in these coatings over the last ten years. Two prior reviews by Banerjee et al. [46] and Yu et al. [100] effectively cover progress prior to 2015. Further, while many bacteria-resistant coatings have been combined with impregnated antimicrobial species, antimicrobials are not included in this review because of bacteria’s innate ability to develop resistance. Finally, polymers such as polyvinylpyrrolidone and poly (hydroxy functional acrylates) will not be discussed due to their limited investigation in the literature for bacteria prevention [101,102,103].
2.1. Polyethylene Glycol (PEG)
PEG has long been considered the gold standard for nonfouling coatings and is widely used in the manufacture of biofilm-resistant coatings. As such, it is also frequently used as the control standard in nonfouling experiments [97,104,105,106], which is why it is included in this review. While nonfouling polymers specifically refer to the ability to withstand exposure to 100% pure concentrations of plasma, serum, or blood with a nonspecific protein adsorption rate of less than 5 ng/cm2 [107], these polymers have also shown resistance to bacteria adhesion [108,109,110,111].
Several groups have reported demonstrations of the bacteria-resistant properties of PEG coatings on glass or silicon wafers using Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and Pseudomonas aeruginosa (P. aeruginosa). Additional commonly investigated bacteria discussed later in this review include Staphylococcus epidermidis (S. epidermidis), Bacillus subtilis (B. subtilis), and Pseudomonas fluorescens (P. fluorescens). E. coli, P. aeruginosa, and P. fluorescens represent Gram-negative strains, while S. aureus, S. epidermidis, and B. subtilis are Gram-positive. These bacteria are commonly selected due to their prevalence as the main pathogenetic infection sources in hospitals, biomedical implants, and water systems. Experimental findings, including the underlying substrate, the polymer coating process, the bacterial species, the experimental duration, and the results for PEG-based systems, are summarized in Table 1.

Table 1.
Summary of recent investigations into bacterial resistance of PEG coatings.
Dang et al. [96], Xing et al. [97], and Duanis-Assaf and Reches [98] all tested zwitterionic moieties alongside the PEG coatings and all three groups concluded that the zwitterionic coatings showed superior performances compared to the PEG coatings. These results are discussed in more detail below. Xing et al. [97] additionally found that the PEG coating exhibited fouling when tested with fluorescently labeled bovine serum albumin, whereas the zwitterionic coating did not. Buxadera-Palomero et al. [112] found that the pulsed electrodeposition had clearly superior, statistically different results compared to standard continuous electrodeposition for S. aureus. However, a significant decrease in E. coli adhesion was only observed for two of the five pulsed electrodeposition conditions.
Liu et al. [104] focused on the increasing grafting density of PEG, which has been shown to increase the subsequent desired bacterial adhesion resistance. Current methods of grafting from surfaces require reactions that make industrial applications unlikely [113], so the group investigated the efficacy of coatings produced by creating metal-polyphenol networks (MPNs) and attaching hexameric lysine PEG to the network (K6-PEG). Liu et al. reported that the procedures successfully increased grafting density to 4.06 chains/nm2, compared to previously reported grafting densities of only 0.79–1.9 chains/nm2 [106,114]. Grafting density is known to be directly related to nonfouling performance, so increasing grafting density in turn increases resistance to bacterial adhesion [113,115]. Liu et al. [104] reported a hundred-fold decrease in bacterial adhesion to the high-density PEG coating compared to the bare glass control.
Although there has been limited success using PEG coatings to reduce bacteria adhesion, this success is often dependent upon the underlying substrate. Many of the successful experiments utilize glass or silicon substrates, which unfortunately have limited applied uses. Stainless steel is a more practical substrate, but covering stainless steel with PEG coatings is ineffective in terms of resisting bacterial adhesion [116] and even further limited for preventing long-term biofilm formation [117]. Another shortcoming of the successful demonstrations of PEG coatings is their short experimental time frames, static experimental conditions, and lack of coating characterization. As shown in Table 1, most of the published studies are less than 24 h. These studies also rarely involve flowing bacteria species due to their short duration. Finally, PEG has shown susceptibility to autooxidation, especially in the presence of oxygen and transition metal ions, which is relevant for most applications [86,118,119,120]. Additional studies need to be pursued to demonstrate that PEG coatings resist bacterial adhesion on a wider range of substrates and for extended periods of time. With the limitations of PEG-based coatings, other chemistries are emerging with better ability to resist bacterial adhesion without having the same susceptibility to degradation.
2.2. Polyoxazoline (POZ)
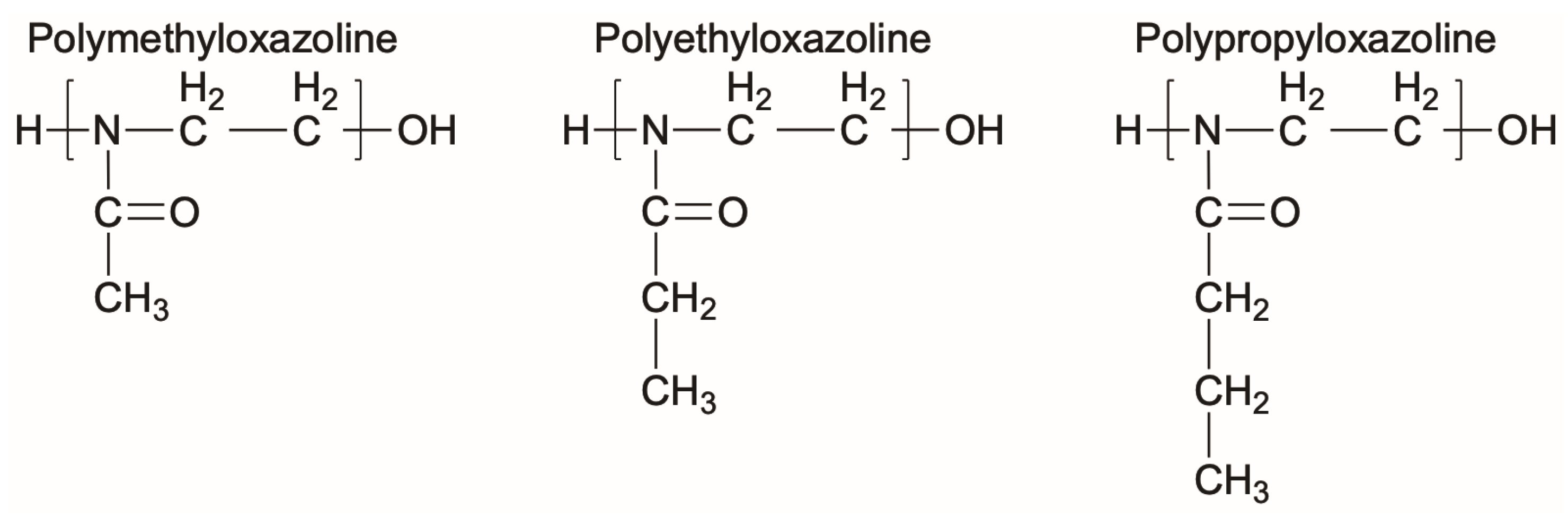
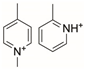
Polyoxazolines (POZs) are one family of chemistries that are not susceptible to the same oxidation that PEG suffers from [121], while also displaying effective resistance to bacteria adhesion. POZs are nonionic, stable, and have high solubilities in both water and organic solvents, making them well suited for many different applications [121]. In a similar timeline to PEG, POZs were first synthesized in the 1960s. However, their nonfouling or bacteria-resistant properties were not fully explored until the early 2000s due to their long reaction times and perceived limitations in terms of applications [122,123]. Recently, POZs have been employed for many biomedical applications including surface coatings that can control fouling and bacterial adhesion [122,123,124,125]. A few examples of the chemical structures of POZs are shown in Figure 3.
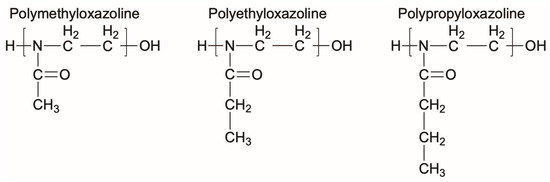
Figure 3.
Representative chemical structures of three different polyoxazolines.
POZs have quickly risen in popularity and have become a frontrunner as bacteria-resistant polymer coatings. A recent review providing a significantly broader view on research into POZs was published by Arsenie and Lapinte [126]. A summary of key experimental results, specifically demonstrating POZ’s resistance to bacterial adhesion, is provided in Table 2, including the underlying substrate, the polymer coating process, the bacterial species, the experimental duration, and the results.

Table 2.
Summary of recent investigations into bacterial resistance of POZ coatings.
While these studies all demonstrate promise, the performance is not as good as that of PEG-based coatings and there are additional similar concerns to those raised for PEG-based coatings. For example, there is a large gap in research into bacterial adhesion over extended exposure times, as the longest study was only 24 h. Additionally, there are limited investigations under flowing conditions. Finally, there is an even greater lack of diversity of underlying substrates than was seen for PEG, and multiple studies lack quantification of bacterial adhesion and the coating physical characteristics. POZs show potential, but considerable additional advancements are necessary before they can be applied broadly.
2.3. Zwitterions and Polyampholytes
Zwitterionic hydrogels and coatings have emerged among the highest-performing subsets of nonfouling polymers. Zwitterionic refers to chemistries which contain an equal number of closely spaced cationic and anionic groups. Polyampholytes are a subset of zwitterions that combine co-localized anionic and cationic monomers to create a net neutral system that behaves similar to their zwitterionic analogs [134]. Zwitterionic polymers exhibit superior hydrophilicity to other polymers due to their large densities of anionic and cationic groups [135,136,137,138]. Additionally, electrostatic interactions allow for tunable control of desired mechanical properties [135,139]. Zwitterionic chemistries have also proven to not have susceptibility to oxidation degradation like PEG-based polymers [119,120]. The most common zwitterionic polymers include polyphosphorylcholine, polysulfobetaine, polycarboxybetaine, and polyampholyte chemistries, although others including pseudo-zwitterions do exist. The Jiang group, among others, has made significant advances, demonstrating bacterial adhesion-resistant zwitterionic coatings that predate the scope of this review, but which are worth noting [91,118,140,141,142]. There is little recently published work utilizing polyampholytes as bacteria-resistant coatings, but there is current research in progress [108,109]. Table 3 summarizes the most common cationic and anionic groups found within most zwitterionic monomers [135].

Table 3.
Common cationic and anionic substituents found within zwitterions. This table was reproduced under an Elsevier Creative Commons license from [135].
Only one study of polyphosphorylcholine since 2015 was located, excluding those that also incorporate bactericides. However, phosphorylcholine research dates to the 1990s and there are many articles that predate this review [135]. Qian et al. [143] applied polyphosphorylcholine coatings to polyurethane-based uretal stents using immersion approaches, followed by UV curing. These were then challenged for 24 h against E. coli and S. aureus to evaluate bacterial adhesion resistance. The resistance was found to be 92.16% and 99.14%, respectively, indicating a strong performance.
Zwitterionic betaines, including phosphobetaine, carboxybetaine, and sulfobetaine, have received some of the most significant research efforts. Table 4 provides a summary of investigations of zwitterionic polymers including the underlying substrate, the polymer coating process, the bacterial species, the experimental duration, and the results.

Table 4.
Summary of recent investigations into the bacterial resistance of zwitterionic coatings including carboxybetaine (CB), sulfobetaine (SB), and phosphobetaine (PB) coatings.
As with the chemistries discussed above, critical gaps in zwitterionic coating research are the lack of detailed coating characterizations and the lack of diversity in the substrates that have been coated. More specifically, there is a lack of evaluations of films applied to metals, even though these substrates are widespread in applications where bacteria adhesion is problematic. However, two recent studies utilizing a zwitterionic thin film applied to metal were completed. Chen et al. [154] used 316L stainless steel substrates and prepared them with a one-step simultaneous polymerization and co-deposition of dopamine and poly(sulfobetaine methacrylate) (PSB) to create a polydopamine (PDA)/PSB coating. P. aeruginosa and B. subtilis adhesion over 24-h were investigated and the group reported a 99% reduction in the adhesion density of both species compared to bare stainless steel [154]. Sae-ung et al. [147] tested the adhesion of S. aureus to copolymers of 2-methacryloyloxyethyl phosphorylcholine (MPC) and methacrylate-substituted dihydrolipoic acid (DHLA) (poly(MPC-DHLA)) coated onto titanium. Sae-ung reported a reduction in adhered bacteria and biofilm formation after 1, 2, and 7 days for the poly(MPC-DHLA) compared to the uncoated titanium standard, although this was not quantified.
Other gaps in the zwitterionic research include the limited number of investigations involving flowing conditions and the lack of long-term studies. Many bacterial adhesion studies involve only 24–48 h of exposure to bacteria, with the majority using time points under 24 h. One recent study completed 14-day investigations, excluding the in vivo study discussed in the following section, but no studies beyond 14 days were discovered. Liu et al. [145] investigated bacterial adhesion to amino acid-based zwitterionic polymers at time points up to 14 days. The polymer brushes studied were composed of the amino acid-based monomers listed in Table 5 and results were compared to those obtained using a PEG coating.

Table 5.
Chemical structures of amino acid zwitterionic monomers. Reprinted with permission from [145]. Copyright 2016 American Chemical Society.
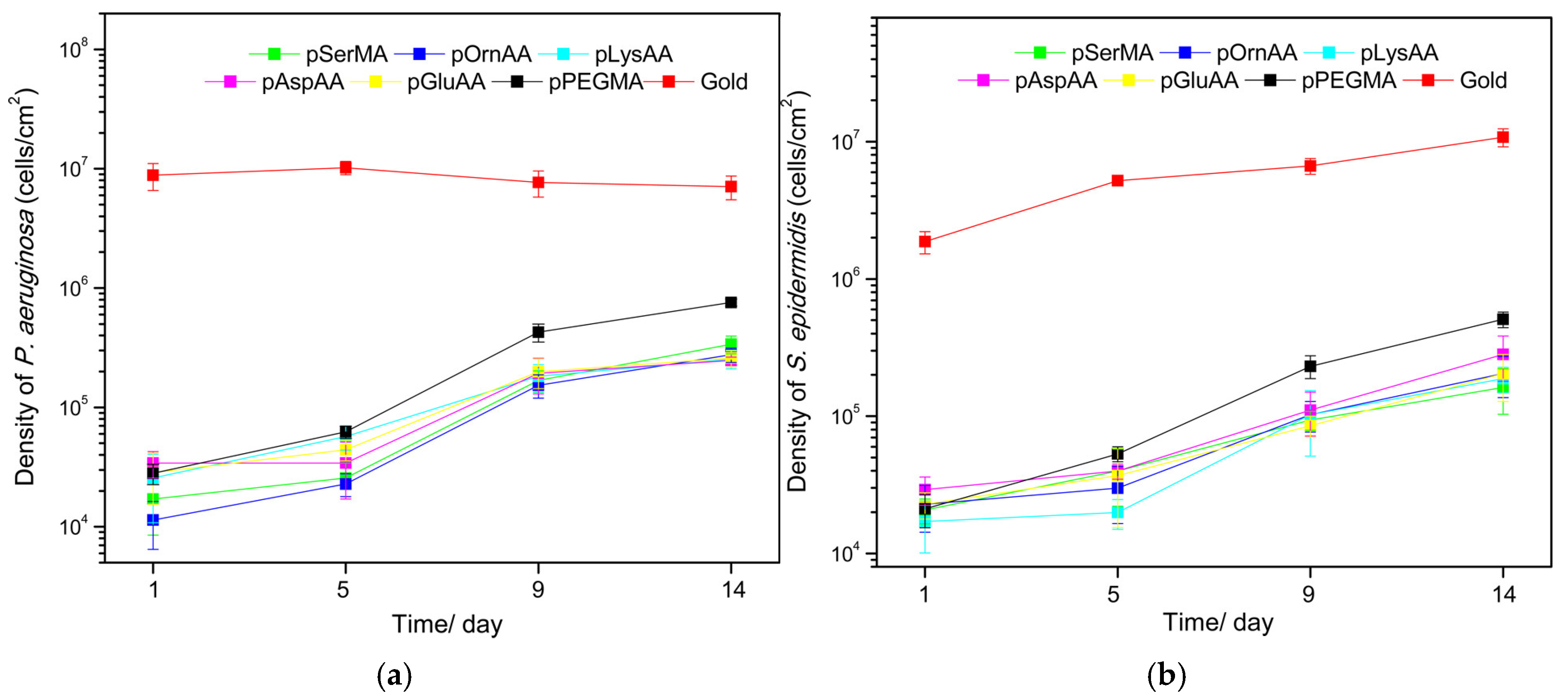
Polymer brushes were applied to gold surfaces and exposed to either S. epidermidis or P. aeruginosa through a parallel flow chamber system. Samples were assessed for bacterial coverage and biofilm formation at time points of 1, 5, 9, and 14 days. After one day, the PEG and zwitterionic coatings had similar coverage and resistance to bacteria. However, by 14 days, the PEG coating displayed more bacteria than the zwitterionic coating. The results observed are reproduced with permission below in Figure 4a,b. Figure 4a shows the density of P. aeruginosa cells observed and Figure 4b shows the density of S. epidermidis cells observed per square cm.
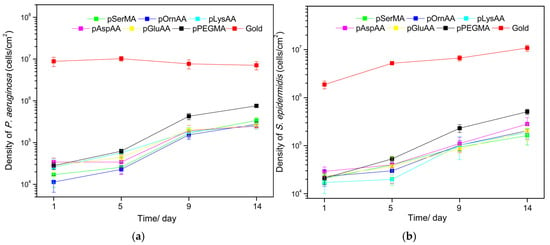
Figure 4.
(a) Density of P. aeruginosa cells observed in cells/cm2 at time points of 1, 5, 9, and 14 days. (b) Density of S. epidermidis cells observed in cells/cm2 at time points of 1, 5, 9, and 14 days. Reprinted with permission from [145]. Copyright 2016 American Chemical Society.
In Figure 4a,b, it is obvious that at the longer time points all of the polymers’ performances decrease. At the 14-day time point, the group reported that the PEG coating had 10.7% and 4.7% surface coverage accumulations of P. aeruginosa and S. epidermidis, respectively. These values were significantly greater than those of the zwitterionic coatings, which all had less than 2.6% surface coverage for both bacteria species. These results also highlight the need for studies beyond the 24–48-h time points given the increases in bacterial surface coverage over time.
A third gap, not identified earlier, but applicable to all of the chemistries covered in this review, is the significant lack of in vivo studies involving bacteria. Shen et al. [156] tested zwitterionic coatings, both in vitro and in vivo, against S. aureus and S. epidermidis. The group used a photo-grafting technique, which simultaneously polymerized sulfobetaine methacrylate (SBMA) or carboxybetaine methacrylate (CBMA) with a PEG crosslinker onto a poly(dimethyl) siloxane (PDMS) substrate. 2-Hydroxyethyl methacrylate (pHEMA) was used as the non-zwitterionic control coating.
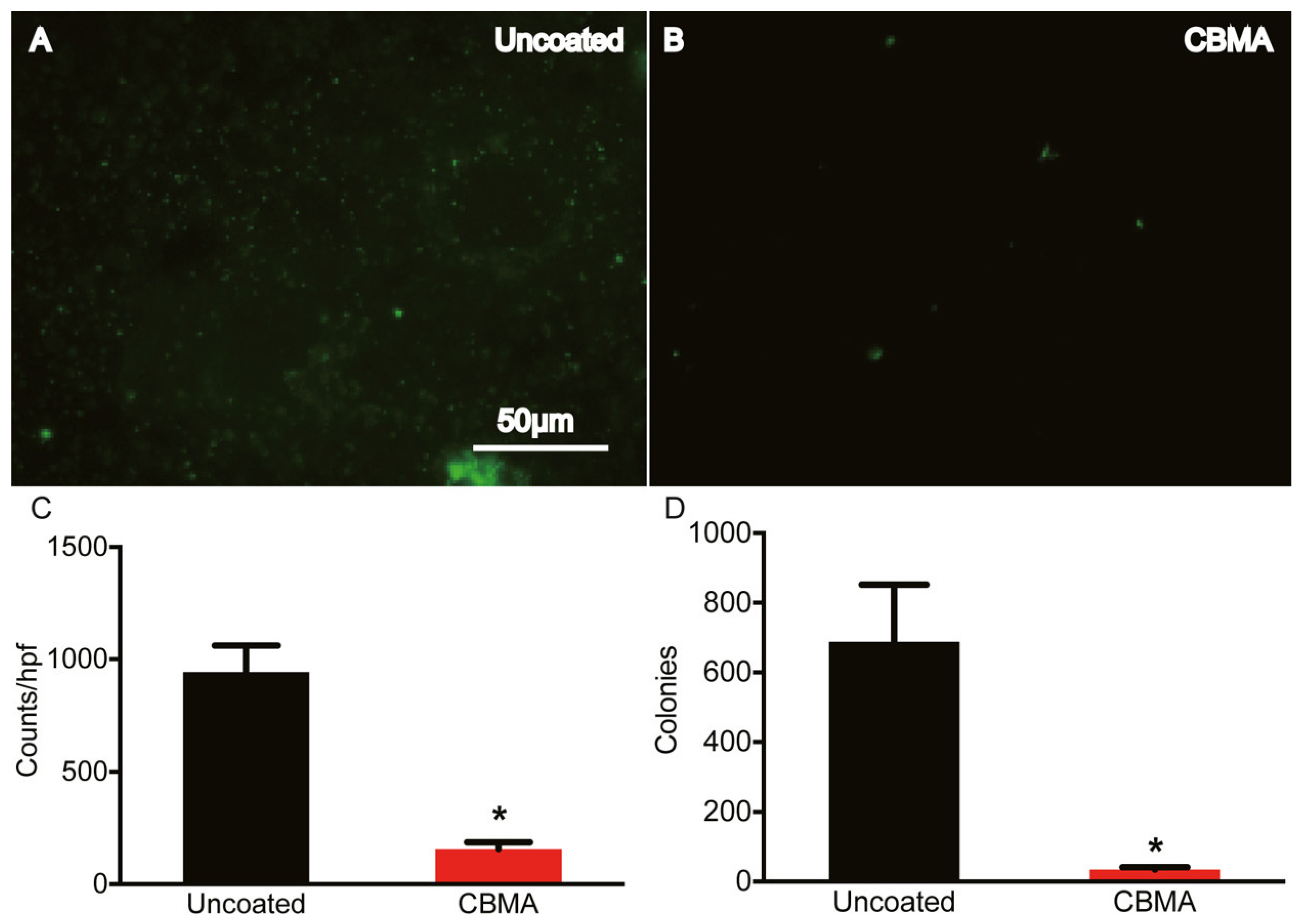
Two in vitro tests were conducted. The first immersed the coated samples into a 108 bacterial suspension (wet) and the second sprayed the suspension onto the substrate to imitate inoculation of an implant (droplet). For both conditions, samples were incubated for periods of both 24 and 48 h. It was found that there was a statistically significant decrease in both S. aureus and S. epidermidis adhesion to the CBMA polymer compared to the controls for both tests. However, the SBMA polymer only showed a statistically significant reduction in S. aureus adhesion, but not for S. epidermidis, under both wet and droplet conditions. Because the CBMA coatings were more reliable at preventing S. aureus and S. epidermidis adhesion compared to the SBMA coatings, they were the only coatings evaluated in vivo. The in vivo test consisted of inoculating the implant with S. aureus at the site of implantation. After 21 days, the samples were explanted and it was found that there was a statistically significant reduction in S. aureus compared to uncoated implants. This can be seen in Figure 5 [156].
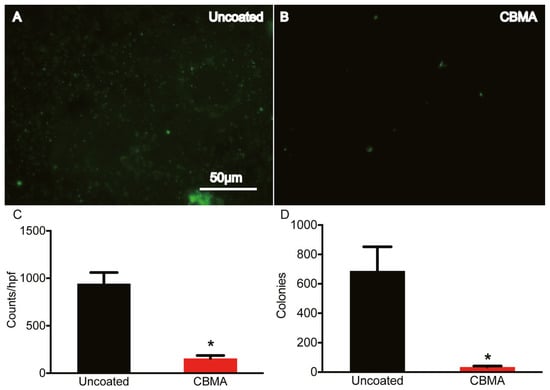
Figure 5.
Microscopic images with analysis on uncoated (A) and CBMA coated (B) implants at 21 days with quantitative analysis in (C,D). The scale bar in (A) is also representative of (B). * indicates statistically significant results (p < 0.0001). Reprinted with permission from [156]. Copyright 2021 American Chemical Society.
While all of these studies reported successes, it is also worth noting that the best results were typically reported with sulfobetaine-based coatings. Table 4 shows that each experiment that reported a reduction in adhesion of over 98% was for thin films composed of a sulfobetaine monomer. This could, in part, be attributed to its good chemical stability and less sensitive pH-dependent properties than other zwitterionic betaine species [135,157,158,159,160]. However, the in vivo results contradict this, suggesting that further investigations are still necessary. Further complicating our ability to directly compare the results obtained in different studies to identify the highest-performing chemistry is the lack of consistent experimental parameters. Across the sixteen studies summarized in Table 4, there are fifteen different coated substrates evaluated with six different bacteria strains over thirteen varying time points. As such, side-by-side comparisons between different chemistries are not possible unless they are directly compared within a study.
3. Conclusions
There have been many advancements in the use of polymer coatings to resist bacteria adhesion. In particular, zwitterionic coatings have demonstrated the strongest capacity to prevent bacterial adhesion across the widest variety of bacteria species, especially sulfobetaine-based systems. However, despite the significant advancements, there are still systematic shortages of long-term studies, evaluations under flowing conditions, detailed characterizations of the coating properties, and assessments for a diversity of underlying substrate compositions. To successfully address these shortcomings, different mechanical properties, film thickness, and coating approaches may be necessary. However, these variables must also be balanced with the performance requirements for the intended industrial and biomedical applications. The development and demonstration of techniques capable of coating large-scale systems is also necessary.
Author Contributions
Writing—original draft preparation, A.S.; writing—review and editing, A.S., M.T.B.; supervision, M.T.B.; funding acquisition, M.T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Aeronautics and Space Administration (NASA) under Federal Awards 80NSSC22M0240 and 80NSSC22M0120. Additional student support (A.S.) was provided by the Idaho Space Grant Consortium, a NASA-funded program under Federal Award 80NSSC20M0108. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. npj Biofilms Microbiomes 2022, 8, 42. [Google Scholar] [CrossRef]
- Shafi, H.Z.; Wang, M.; Gleason, K.K.; Khan, Z. Synthesis of surface-anchored stable zwitterionic films for inhibition of biofouling. Mater. Chem. Phys. 2020, 239, 121971. [Google Scholar] [CrossRef]
- Maillard, J.-Y. Resistance of Bacteria to Biocides. Microbiol. Spectr. 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qu, Y.; Yang, H.; Zhou, X.; Xiao, P.; Shao, T. Combatting biofilms in potable water systems: A comprehensive overview to ensuring industrial water safety. Environ. Microbiol. Rep. 2023, 15, 445–454. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef]
- Geesey, G.G.; Lewandowski, Z.; Flemming, H.-C. Biofouling and Biocorrosion in Industrial Water Systems; Lewis Publishers: Boca Raton, FL, USA, 1994. [Google Scholar]
- Walker, J.; Surman, S.; Jass, J. Industrial Biofouling: Detection, Prevention, and Control; Wiley: Chichester, UK, 2000. [Google Scholar]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Metcalf, D.G.; Bowler, P.G. Biofilm delays wound healing: A review of the evidence. Burn. Trauma 2013, 1, 2321–3868.113329. [Google Scholar] [CrossRef]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef]
- Fastenberg, J.H.; Hsueh, W.D.; Mustafa, A.; Akbar, N.A.; Abuzeid, W.M. Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies. World J. Otorhinolaryngol.-Head Neck Surg. 2016, 2, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Fiehn, N.-E. Dental biofilm infections—An update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef]
- Lamagni, T. Epidemiology and burden of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69, i5–i10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Bai, X.; Nakatsu, C.H.; Bhunia, A.K. Bacterial Biofilms and Their Implications in Pathogenesis and Food Safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef]
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States-major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Blaustein, R.A.; Shelton, D.R.; Van Kessel, J.A.S.; Karns, J.S.; Stocker, M.D.; Pachepsky, Y.A. Irrigation waters and pipe-based biofilms as sources for antibiotic-resistant bacteria. Environ. Monit. Assess. 2015, 188, 56. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A. Infection Prevention and Control During Prolonged Human Space Travel. Clin. Infect. Dis. 2013, 56, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Klintworth, R.; Reher, H.J.; Viktorov, A.N.; Bohle, D. Biological induced corrosion of materials II: New test methods and experiences from mir station. Acta Astronaut. 1999, 44, 569–578. [Google Scholar] [CrossRef]
- Erdei-Tombor, P.; Kiskó, G.; Taczman-Brückner, A. Biofilm Formation in Water Distribution Systems. Processes 2024, 12, 280. [Google Scholar] [CrossRef]
- Li, W.; Zheng, T.; Ma, Y.; Liu, J. Current status and future prospects of sewer biofilms: Their structure, influencing factors, and substance transformations. Sci. Total Environ. 2019, 695, 133815. [Google Scholar] [CrossRef]
- Liu, H.; Walski, T.; Fu, G.; Zhang, C. Failure Impact Analysis of Isolation Valves in a Water Distribution Network. J. Water Resour. Plan. Manag. 2017, 143, 04017019. [Google Scholar] [CrossRef]
- Collier, S.; Deng, L.; Adam, E.; Benedict, K.; Beshearse, E.; Blackstock, A.; Bruce, B.; Derado, G.; Edens, C.; Fullerton, K.; et al. Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States. Emerg. Infect. Dis. 2021, 27, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Learbuch, K.L.G.; Smidt, H.; van der Wielen, P.W.J.J. Influence of pipe materials on the microbial community in unchlorinated drinking water and biofilm. Water Res. 2021, 194, 116922. [Google Scholar] [CrossRef] [PubMed]
- Boe-Hansen, R.; Martiny, A.C.; Arvin, E.; Albrechtsen, H.J. Monitoring biofilm formation and activity in drinking water distribution networks under oligotrophic conditions. Water Sci. Technol. 2003, 47, 91–97. [Google Scholar] [CrossRef]
- Chaves Simões, L.; Simões, M. Biofilms in drinking water: Problems and solutions. RSC Adv. 2013, 3, 2520–2533. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El-Taweel, G.E.; Goswami, P.; Pant, D.; Sevda, S. The role of biofilm in the development and dissemination of ubiquitous pathogens in drinking water distribution systems: An overview of surveillance, outbreaks, and prevention. World J. Microbiol. Biotechnol. 2021, 37, 36. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.; Kohnen, W.; Jansen, B.; Obst, U. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol. Ecol. 2003, 43, 325–335. [Google Scholar] [CrossRef]
- Wingender, J.; Flemming, H.C. Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg. Environ. Health 2011, 214, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Drake, J.; Karney, B. UV Disinfection of Wastewater and Combined Sewer Overflows. Adv. Exp. Med. Biol. 2017, 996, 267–275. [Google Scholar] [CrossRef]
- Lui, G.Y.; Roser, D.; Corkish, R.; Ashbolt, N.J.; Stuetz, R. Point-of-use water disinfection using ultraviolet and visible light-emitting diodes. Sci. Total Environ. 2016, 553, 626–635. [Google Scholar] [CrossRef]
- Shuryak, I. Review of microbial resistance to chronic ionizing radiation exposure under environmental conditions. J. Environ. Radioact. 2019, 196, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef]
- Simões, M.; Pereira, M.O.; Vieira, M.J. Effect of mechanical stress on biofilms challenged by different chemicals. Water Res. 2005, 39, 5142–5152. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Machado, I.; Pereira, M.O.; Vieira, M.J. Control of Flow-Generated Biofilms with Surfactants: Evidence of Resistance and Recovery. Food Bioprod. Process. 2006, 84, 338–345. [Google Scholar] [CrossRef]
- Zea, L.; McLean, R.J.C.; Rook, T.A.; Angle, G.; Carter, D.L.; Delegard, A.; Denvir, A.; Gerlach, R.; Gorti, S.; McIlwaine, D.; et al. Potential biofilm control strategies for extended spaceflight missions. Biofilm 2020, 2, 100026. [Google Scholar] [CrossRef]
- Wong, W.C.; Dudinsky, L.A.; Garcia, V.M.; Ott, C.M.; Castro, V.A. Efficacy of various chemical disinfectants on biofilms formed in spacecraft potable water system components. Biofouling 2010, 26, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.L.; Morris, J.C. Elemental Iodine as a Disinfectant for Drinking Water. Ind. Eng. Chem. 1953, 45, 1009–1012. [Google Scholar] [CrossRef]
- Mettler, M.K.; Parker, C.W.; Venkateswaran, K.; Peyton, B.M. Antimicrobial Coating Efficacy for Prevention of Pseudomonas aeruginosa Biofilm Growth on ISS Water System Materials. Front. Microbiol. 2022, 13, 874236. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11, 1719. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Onaizi, S.A.; Leong, S.S. Tethering antimicrobial peptides: Current status and potential challenges. Biotechnol. Adv. 2011, 29, 67–74. [Google Scholar] [CrossRef]
- Nazi, N.; Humblot, V.; Debiemme-Chouvy, C. A New Antibacterial N-Halamine Coating Based on Polydopamine. Langmuir 2020, 36, 11005–11014. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Si, Y.; Huang, K.; Nitin, N.; Sun, G. Rechargeable Antibacterial N-Halamine Films with Antifouling Function for Food Packaging Applications. ACS Appl. Mater. Interfaces 2019, 11, 17814–17822. [Google Scholar] [CrossRef]
- Hui, F.; Debiemme-Chouvy, C. Antimicrobial N-Halamine Polymers and Coatings: A Review of Their Synthesis, Characterization, and Applications. Biomacromolecules 2013, 14, 585–601. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, F.; Wen, J.; Lv, W.; Porteous, N.; Deng, Y.; Sun, Y. Fluorinated and un-fluorinated N-halamines as antimicrobial and biofilm-controlling additives for polymers. Polymer 2015, 68, 92–100. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.; Porteous, N.; Sun, Y. An N-halamine-based rechargeable antimicrobial and biofilm controlling polyurethane. Acta Biomater. 2012, 8, 1498–1506. [Google Scholar] [CrossRef]
- Lee, K.; Yu, H.; Zhang, X.; Choo, K.-H. Quorum sensing and quenching in membrane bioreactors: Opportunities and challenges for biofouling control. Bioresour. Technol. 2018, 270, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Givskov, M. Chemical Biology Strategies for Biofilm Control. Microbiol. Spectr. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Tolker-Nielsen, T.; Givskov, M. Bacterial Biofilm Control by Perturbation of Bacterial Signaling Processes. Int. J. Mol. Sci. 2017, 18, 1970. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, R.; Elias, M. Quorum quenching enzymes and their effects on virulence, biofilm, and microbiomes: A review of recent advances. Expert Rev. Anti-Infect. Ther. 2020, 18, 1221–1233. [Google Scholar] [CrossRef]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Gassie, L.W.; Englehardt, J.D. Advanced oxidation and disinfection processes for onsite net-zero greywater reuse: A review. Water Res. 2017, 125, 384–399. [Google Scholar] [CrossRef]
- Koenig, D.W.; Mishra, S.K.; Pierson, D.L. Removal of Burkholderia cepacia biofilms with oxidants. Biofouling 1995, 9, 51–62. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Leiknes, T. Extractive biofilm membrane bioreactor with energy recovery from excess aeration and new membrane fouling control. Bioresour. Technol. 2011, 102, 2301–2307. [Google Scholar] [CrossRef]
- Menesses, M.; Belden, J.; Dickenson, N.; Bird, J. Measuring a critical stress for continuous prevention of marine biofouling accumulation with aeration. Biofouling 2017, 33, 703–711. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT-Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, S.C.; Wang, X.; Muhammad, T.; Song, P.; Zhou, B.; Zhou, Y.; Li, Y. Mitigation of biofouling in agricultural water distribution systems with nanobubbles. Environ. Int. 2020, 141, 105787. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; An, T.; Li, X.; Zou, J.; Lin, Z.; Gu, J.; Hu, R.; Fang, Z. Genomic analysis of Ralstonia pickettii reveals the genetic features for potential pathogenicity and adaptive evolution in drinking water. Front. Microbiol. 2024, 14, 1272636. [Google Scholar] [CrossRef]
- Flemming, H.C. Biofouling in water systems—Cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.F.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Brennan, A.B. Engineered antifouling microtopographies—Effect of feature size, geometry, and roughness on settlement of zoospores of the green alga Ulva. Biofouling 2007, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Plasma-assisted surface modification of organic biopolymers to prevent bacterial attachment. Acta Biomater. 2011, 7, 2015–2028. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 2010, 31, 3674–3683. [Google Scholar] [CrossRef]
- Chang, Y.-R.; Weeks, E.R.; Ducker, W.A. Surface Topography Hinders Bacterial Surface Motility. ACS Appl. Mater. Interfaces 2018, 10, 9225–9234. [Google Scholar] [CrossRef] [PubMed]
- Lutey, A.H.A.; Gemini, L.; Romoli, L.; Lazzini, G.; Fuso, F.; Faucon, M.; Kling, R. Towards Laser-Textured Antibacterial Surfaces. Sci. Rep. 2018, 8, 10112. [Google Scholar] [CrossRef]
- Rizzello, L.; Cingolani, R.; Pompa, P. Nanotechnology Tools for Antibacterial Materials. Nanomedicine 2013, 8, 807–821. [Google Scholar] [CrossRef]
- Rizzello, L.; Galeone, A.; Vecchio, G.; Brunetti, V.; Sabella, S.; Pompa, P.P. Molecular response of Escherichia coli adhering onto nanoscale topography. Nanoscale Res. Lett. 2012, 7, 575. [Google Scholar] [CrossRef]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.A.; Verran, J. The Effect of Surface Topography on the Retention of Microorganisms. Food Bioprod. Process. 2006, 84, 253–259. [Google Scholar] [CrossRef]
- Taylor, R.L.; Verran, J.; Lees, G.C.; Ward, A.J. The influence of substratum topography on bacterial adhesion to polymethylmethacrylate. J. Mater. Sci. Mater. Med. 1998, 9, 17–22. [Google Scholar] [CrossRef]
- Mitik-Dineva, N.; Wang, J.; Stoddart, P.R.; Crawford, R.J.; Ivanova, E.P. Nano-structured surfaces control bacterial attachment. In Proceedings of the 2008 International Conference on Nanoscience and Nanotechnology, Melbourne, Australia, 25–29 February 2008; pp. 113–116. [Google Scholar]
- Mitik-Dineva, N.; Wang, J.; Truong, V.K.; Stoddart, P.; Malherbe, F.; Crawford, R.J.; Ivanova, E.P. Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr. Microbiol. 2009, 58, 268–273. [Google Scholar] [CrossRef]
- Desrousseaux, C.; Sautou, V.; Descamps, S.; Traoré, O. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. J. Hosp. Infect. 2013, 85, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-H.; Cai, W.-J.; He, X.-W.; Song, H.-L.; Gao, J.; Yang, Y.-L.; Zhou, J. A review of advances & potential of applying nanomaterials for biofilm inhibition. npj Clean Water 2024, 7, 131. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Wagner, W.R. Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Academic Press: London, UK, 2020. [Google Scholar]
- Gombotz, W.R.; Wang, G.H.; Horbett, T.A.; Hoffman, A.S. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 1991, 25, 1547–1562. [Google Scholar] [CrossRef]
- Antonsen, K.P.; Hoffman, A.S. Water Structure of PEG Solutions by Differential Scanning Calorimetry Measurements. In Poly(Ethylene Glycol) Chemistry: Biotechnical and Biomedical Applications; Harris, J.M., Ed.; Springer: Boston, MA, USA, 1992; pp. 15–28. [Google Scholar]
- Heuberger, M.; Drobek, T.; Spencer, N.D. Interaction forces and morphology of a protein-resistant poly(ethylene glycol) layer. Biophys. J. 2005, 88, 495–504. [Google Scholar] [CrossRef]
- Bernards, M.T.; Cheng, G.; Zhang, Z.; Chen, S.; Jiang, S. Nonfouling Polymer Brushes via Surface-Initiated, Two-Component Atom Transfer Radical Polymerization. Macromolecules 2008, 41, 4216–4219. [Google Scholar] [CrossRef]
- Khlyustova, A.; Kirsch, M.; Ma, X.; Cheng, Y.; Yang, R. Surfaces with antifouling-antimicrobial dual function via immobilization of lysozyme on zwitterionic polymer thin films. J. Mater. Chem. B 2022, 10, 2728–2739. [Google Scholar] [CrossRef]
- Khire, V.S.; Harant, A.W.; Watkins, A.W.; Anseth, K.S.; Bowman, C.N. Ultrathin Patterned Polymer Films on Surfaces Using Thiol−Ene Polymerizations. Macromolecules 2006, 39, 5081–5086. [Google Scholar] [CrossRef]
- Khire, V.S.; Lee, T.Y.; Bowman, C.N. Synthesis, Characterization and Cleavage of Surface-Bound Linear Polymers Formed Using Thiol−Ene Photopolymerizations. Macromolecules 2008, 41, 7440–7447. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, H.J.; Jamison, A.C.; Lee, T.R. Robust Thick Polymer Brushes Grafted from Gold Surfaces Using Bidentate Thiol-Based Atom-Transfer Radical Polymerization Initiators. ACS Appl. Mater. Interfaces 2016, 8, 5586–5594. [Google Scholar] [CrossRef]
- Dang, Y.; Quan, M.; Xing, C.-M.; Wang, Y.-B.; Gong, Y.-K. Biocompatible and antifouling coating of cell membrane phosphorylcholine and mussel catechol modified multi-arm PEGs. J. Mater. Chem. B 2015, 3, 2350–2361. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.-M.; Meng, F.-N.; Quan, M.; Ding, K.; Dang, Y.; Gong, Y.-K. Quantitative fabrication, performance optimization and comparison of PEG and zwitterionic polymer antifouling coatings. Acta Biomater. 2017, 59, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Duanis-Assaf, T.; Reches, M. Factors influencing initial bacterial adhesion to antifouling surfaces studied by single-cell force spectroscopy. iScience 2024, 27, 108803. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lee, J.; Ma, J.; Elimelech, M. Antifouling Thin-Film Composite Membranes by Controlled Architecture of Zwitterionic Polymer Brush Layer. Environ. Sci. Technol. 2017, 51, 2161–2169. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, Z.; Chen, H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomater. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Vatsha, B.; Ngila, J.C.; Moutloali, R.M. Preparation of antifouling polyvinylpyrrolidone (PVP 40K) modified polyethersulfone (PES) ultrafiltration (UF) membrane for water purification. Phys. Chem. Earth Parts A/B/C 2014, 67–69, 125–131. [Google Scholar] [CrossRef]
- Guo, H.; Chen, P.; Tian, S.; Ma, Y.; Li, Q.; Wen, C.; Yang, J.; Zhang, L. Amphiphilic Marine Antifouling Coatings Based on a Hydrophilic Polyvinylpyrrolidone and Hydrophobic Fluorine–Silicon-Containing Block Copolymer. Langmuir 2020, 36, 14573–14581. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Li, L.; Cheng, G.; Gong, X.; Zheng, J. Dual Functionality of Antimicrobial and Antifouling of Poly(N-hydroxyethylacrylamide)/Salicylate Hydrogels. Langmuir 2013, 29, 1517–1524. [Google Scholar] [CrossRef]
- Liu, W.; He, S.; Liu, H.; Shou, Z.; Huo, K.; Xiang, H.; Feng, A.; Lu, W.; Li, N. A green, versatile, and facile strategy for anti-biofouling surface with ultra-high graft density polyethylene glycol. J. Nanobiotechnol. 2024, 22, 746. [Google Scholar] [CrossRef]
- Shin, E.; Lim, C.; Kang, U.J.; Kim, M.; Park, J.; Kim, D.; Choi, W.; Hong, J.; Baig, C.; Lee, D.W.; et al. Mussel-Inspired Copolyether Loop with Superior Antifouling Behavior. Macromolecules 2020, 53, 3551–3562. [Google Scholar] [CrossRef]
- Liu, S.; Chen, L.; Tan, L.; Cao, F.; Bai, L.; Wang, Y. A high efficiency approach for a titanium surface antifouling modification: PEG-o-quinone linked with titanium via electron transfer process. J. Mater. Chem. B 2014, 2, 6758–6766. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-B.; Grunkemeier, J.M.; Horbett, T.A. Human plasma fibrinogen adsorption and platelet adhesion to polystyrene. J. Biomed. Mater. Res. 1999, 44, 130–139. [Google Scholar] [CrossRef]
- Shea, A.; Harvey, K.; Keeley, A.; Johnson, H.; Hansen, N.; McCormack, R.; Stelck, K.; Lindsay, T.; Bryant, A.; Bernards, M.T. Payload Design and Evaluation of Staphylococcus epidermidis Adhesion to Nonfouling Polyampholyte Coatings Onboard the International Space Station. Molecules 2025, 30, 836. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.; Bryant, A.; McCormack, R.; Johnson, H.; Lindsay, T.; Stelck, K.; Bernards, M.T. Assessment of the performance of nonfouling polymer hydrogels utilizing citizen scientists. PLoS ONE 2021, 16, e0261817. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, S.C.; McGrath, D.E.; Bernards, M.T. Nonfouling Hydrogels Formed from Charged Monomer Subunits. J. Phys. Chem. B 2012, 116, 14346–14352. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S. Integrated Antimicrobial and Nonfouling Zwitterionic Polymers. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Albó, K.; Gil, F.J.; Mas-Moruno, C.; Rodríguez, D. Polyethylene Glycol Pulsed Electrodeposition for the Development of Antifouling Coatings on Titanium. Coatings 2020, 10, 456. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Kálosi, A.; Halahovets, Y.; Romanenko, I.; Slabý, J.; Homola, J.; Svoboda, J.; de los Santos Pereira, A.; Pop-Georgievski, O. Grafting density and antifouling properties of poly[N-(2-hydroxypropyl) methacrylamide] brushes prepared by “grafting to” and “grafting from”. Polym. Chem. 2022, 13, 3815–3826. [Google Scholar] [CrossRef]
- Kizhakkedathu, J.N.; Janzen, J.; Le, Y.; Kainthan, R.K.; Brooks, D.E. Poly(oligo(ethylene glycol)acrylamide) Brushes by Surface Initiated Polymerization: Effect of Macromonomer Chain Length on Brush Growth and Protein Adsorption from Blood Plasma. Langmuir 2009, 25, 3794–3801. [Google Scholar] [CrossRef]
- Svoboda, J.; Sedláček, O.; Riedel, T.; Hrubý, M.; Pop-Georgievski, O. Poly(2-oxazoline)s One-Pot Polymerization and Surface Coating: From Synthesis to Antifouling Properties Out-Performing Poly(ethylene oxide). Biomacromolecules 2019, 20, 3453–3463. [Google Scholar] [CrossRef]
- Wei, J.; Ravn, D.B.; Gram, L.; Kingshott, P. Stainless steel modified with poly(ethylene glycol) can prevent protein adsorption but not bacterial adhesion. Colloids Surf. B Biointerfaces 2003, 32, 275–291. [Google Scholar] [CrossRef]
- Roosjen, A.; van der Mei, H.C.; Busscher, H.J.; Norde, W. Microbial Adhesion to Poly(ethylene oxide) Brushes: Influence of Polymer Chain Length and Temperature. Langmuir 2004, 20, 10949–10955. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Haag, S.L.; Bernards, M.T. Enhanced Biocompatibility of Polyampholyte Hydrogels. Langmuir 2020, 36, 3292–3299. [Google Scholar] [CrossRef]
- Carr, L.R.; Xue, H.; Jiang, S. Functionalizable and nonfouling zwitterionic carboxybetaine hydrogels with a carboxybetaine dimethacrylate crosslinker. Biomaterials 2011, 32, 961–968. [Google Scholar] [CrossRef]
- Viegas, T.X.; Bentley, M.D.; Harris, J.M.; Fang, Z.; Yoon, K.; Dizman, B.; Weimer, R.; Mero, A.; Pasut, G.; Veronese, F.M. Polyoxazoline: Chemistry, Properties, and Applications in Drug Delivery. Bioconjug. Chem. 2011, 22, 976–986. [Google Scholar] [CrossRef]
- Zahoranova, A.; Luxenhofer, R. Poly(2-oxazoline)- and Poly(2-oxazine)-Based Self-Assemblies, Polyplexes, and Drug Nanoformulations-An Update. Adv. Healthc. Mater. 2021, 10, e2001382. [Google Scholar] [CrossRef]
- Hoogenboom, R. Poly(2-oxazoline)s: A Polymer Class with Numerous Potential Applications. Angew. Chem. Int. Ed. 2009, 48, 7978–7994. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Hoogenboom, R. Poly(2-oxazoline)s: Alive and Kicking. Macromol. Chem. Phys. 2007, 208, 18–25. [Google Scholar] [CrossRef]
- Arsenie, L.V.; Lapinte, V. A recent state of art on polyoxazoline-containing antifouling coatings for biological and environmental applications. Prog. Org. Coat. 2024, 186, 107993. [Google Scholar] [CrossRef]
- Cavallaro, A.A.; Macgregor-Ramiasa, M.N.; Vasilev, K. Antibiofouling Properties of Plasma-Deposited Oxazoline-Based Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 6354–6362. [Google Scholar] [CrossRef] [PubMed]
- Ramiasa, M.N.; Cavallaro, A.A.; Mierczynska, A.; Christo, S.N.; Gleadle, J.M.; Hayball, J.D.; Vasilev, K. Plasma polymerised polyoxazoline thin films for biomedical applications. Chem. Commun. 2015, 51, 4279–4282. [Google Scholar] [CrossRef] [PubMed]
- Al-Bataineh, S.A.; Cavallaro, A.A.; Michelmore, A.; Macgregor, M.N.; Whittle, J.D.; Vasilev, K. Deposition of 2-oxazoline-based plasma polymer coatings using atmospheric pressure helium plasma jet. Plasma Process. Polym. 2019, 16, 1900104. [Google Scholar] [CrossRef]
- He, T.; Jańczewski, D.; Jana, S.; Parthiban, A.; Guo, S.; Zhu, X.; Lee, S.S.-C.; Parra-Velandia, F.J.; Teo, S.L.-M.; Vancso, G.J. Efficient and robust coatings using poly(2-methyl-2-oxazoline) and its copolymers for marine and bacterial fouling prevention. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 275–283. [Google Scholar] [CrossRef]
- He, T.; Jańczewski, D.; Guo, S.; Man, S.M.; Jiang, S.; Tan, W.S. Stable pH responsive layer-by-layer assemblies of partially hydrolysed poly(2-ethyl-2-oxazoline) and poly(acrylic acid) for effective prevention of protein, cell and bacteria surface attachment. Colloids Surf. B Biointerfaces 2018, 161, 269–278. [Google Scholar] [CrossRef]
- Li, Y.; Pan, T.; Ma, B.; Liu, J.; Sun, J. Healable Antifouling Films Composed of Partially Hydrolyzed Poly(2-ethyl-2-oxazoline) and Poly(acrylic acid). ACS Appl. Mater. Interfaces 2017, 9, 14429–14436. [Google Scholar] [CrossRef]
- Portier, É.; Azemar, F.; Benkhaled, B.T.; Bardeau, J.-F.; Faÿ, F.; Réhel, K.; Lapinte, V.; Linossier, I. Poly(oxazoline) for the design of amphiphilic silicone coatings. Prog. Org. Coat. 2021, 153, 106116. [Google Scholar] [CrossRef]
- Bernards, M.; He, Y. Polyampholyte polymers as a versatile zwitterionic biomaterial platform. J. Biomater. Sci. Polym. Ed. 2014, 25, 1479–1488. [Google Scholar] [CrossRef]
- Qu, K.; Yuan, Z.; Wang, Y.; Song, Z.; Gong, X.; Zhao, Y.; Mu, Q.; Zhan, Q.; Xu, W.; Wang, L. Structures, properties, and applications of zwitterionic polymers. ChemPhysMater 2022, 1, 294–309. [Google Scholar] [CrossRef]
- Kondo, T.; Nomura, K.; Gemmei-Ide, M.; Kitano, H.; Noguchi, H.; Uosaki, K.; Saruwatari, Y. Structure of water at zwitterionic copolymer film–liquid water interfaces as examined by the sum frequency generation method. Colloids Surf. B Biointerfaces 2014, 113, 361–367. [Google Scholar] [CrossRef]
- Nomura, K.; Mikuni, S.; Nakaji-Hirabayashi, T.; Gemmei-Ide, M.; Kitano, H.; Noguchi, H.; Uosaki, K. Water structure at the interfaces between a zwitterionic self-assembled monolayer/liquid water evaluated by sum-frequency generation spectroscopy. Colloids Surf. B Biointerfaces 2015, 135, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Fang, L.-F.; Matsuyama, H. Electrostatic Adsorption Behavior of Zwitterionic Copolymers on Negatively Charged Surfaces. Langmuir 2019, 35, 9152–9160. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wei, X.; Liu, S.; Zhang, J.; Yang, T.; Chen, S. Recent Advances in Mechanical Reinforcement of Zwitterionic Hydrogels. Gels 2022, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Xue, H.; Zhang, Z.; Chen, S.; Jiang, S. A switchable biocompatible polymer surface with self-sterilizing and nonfouling capabilities. Angew. Chem. Int. Ed. 2008, 47, 8831–8834. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Li, G.; Xue, H.; Chen, S.; Bryers, J.D.; Jiang, S. Zwitterionic carboxybetaine polymer surfaces and their resistance to long-term biofilm formation. Biomaterials 2009, 30, 5234–5240. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, J.; Liu, L.; Hu, H.; Wang, B.; Zhang, H. Bioinspired Phosphorylcholine Coating for Surface Functionalization of Interventional Biomedical Implants with Bacterial Resistance and Anti-Encrustation Properties. Langmuir 2022, 38, 3597–3606. [Google Scholar] [CrossRef]
- Hassani, M.; Kamankesh, M.; Rad-Malekshahi, M.; Rostamizadeh, K.; Rezaee, F.; Haririan, I.; Daghighi, S.M. Biomaterials coated with zwitterionic polymer brush demonstrated significant resistance to bacterial adhesion and biofilm formation in comparison to brush coatings incorporated with antibiotics. Colloids Surf. B Biointerfaces 2024, 234, 113671. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Wang, H.; Newby, B.-m.Z.; Cheng, F.; Liu, L. Amino Acid-Based Zwitterionic Polymer Surfaces Highly Resist Long-Term Bacterial Adhesion. Langmuir 2016, 32, 7866–7874. [Google Scholar] [CrossRef]
- Karthäuser, J.F.; Kopecz, R.; Schönemann, E.; Martínez Guajardo, A.; Laschewsky, A.; Rosenhahn, A. Spacer Effects in Sulfo- and Sulfabetaine Polymers on Their Resistance against Proteins and Pathogenic Bacteria. Adv. Mater. Interfaces 2024, 11, 2300873. [Google Scholar] [CrossRef]
- Sae-ung, P.; Wijitamornloet, A.; Iwasaki, Y.; Thanyasrisung, P.; Hoven, V.P. Clickable Zwitterionic Copolymer as a Universal Biofilm-Resistant Coating. Macromol. Mater. Eng. 2019, 304, 1900286. [Google Scholar] [CrossRef]
- Venault, A.; Hsu, C.-H.; Ishihara, K.; Chang, Y. Zwitterionic bi-continuous membranes from a phosphobetaine copolymer/poly(vinylidene fluoride) blend via VIPS for biofouling mitigation. J. Membr. Sci. 2018, 550, 377–388. [Google Scholar] [CrossRef]
- Yin, M.; Li, J.; Wang, H.; Xu, X.; Wang, Y.; Ma, Z.; Chen, J.; Li, X. Development of anti-bacterial adhesion and antibacterial sulfobetaines modified chitosan/polyvinyl alcohol composite films as packaging materials. Int. J. Biol. Macromol. 2024, 260, 129465. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Lin, W.; Wang, Z.; Zhang, J.; Ji, F.; Ma, G.; Yuan, Z.; Chen, S. Development of Robust and Recoverable Ultralow-Fouling Coatings Based on Poly(carboxybetaine) Ester Analogue. ACS Appl. Mater. Interfaces 2015, 7, 16938–16945. [Google Scholar] [CrossRef]
- Cao, Q.; Wu, S.; Wang, L.; Shi, X.; Li, G. Effects of the morphology of sulfobetaine zwitterionic layers grafted onto a silicone surface on improving the hydrophilic stability, anti-bacterial adhesion properties, and biocompatibility. J. Appl. Polym. Sci. 2018, 135, 46860. [Google Scholar] [CrossRef]
- Texidó, R.; Cabanach, P.; Kaplan, R.; García-Bonillo, C.; Pérez, D.; Zhang, S.; Borrós, S.; Pena-Francesch, A. Bacteriophobic Zwitterionic/Dopamine Coatings for Medical Elastomers. Adv. Mater. Interfaces 2022, 9, 2201152. [Google Scholar] [CrossRef]
- Ran, B.; Jing, C.; Yang, C.; Li, X.; Li, Y. Synthesis of efficient bacterial adhesion-resistant coatings by one-step polydopamine-assisted deposition of branched polyethylenimine-g-poly(sulfobetaine methacrylate) copolymers. Appl. Surf. Sci. 2018, 450, 77–84. [Google Scholar] [CrossRef]
- Chen, S.; Shi, J.; Zhao, Y.; Wang, W.; Liao, H.; Liu, G. Rapid fabrication of zwitterionic coating on 316L stainless steel surface for marine biofouling resistance. Prog. Org. Coat. 2021, 161, 106552. [Google Scholar] [CrossRef]
- Venault, A.; Chou, Y.-N.; Wang, Y.-H.; Hsu, C.-H.; Chou, C.-J.; Bouyer, D.; Lee, K.-R.; Chang, Y. A combined polymerization and self-assembling process for the fouling mitigation of PVDF membranes. J. Membr. Sci. 2018, 547, 134–145. [Google Scholar] [CrossRef]
- Shen, N.; Cheng, E.; Whitley, J.W.; Horne, R.R.; Leigh, B.; Xu, L.; Jones, B.D.; Guymon, C.A.; Hansen, M.R. Photograftable Zwitterionic Coatings Prevent Staphylococcus aureus and Staphylococcus epidermidis Adhesion to PDMS Surfaces. ACS Appl. Bio Mater. 2021, 4, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Birkner, M.; Ulbricht, M. Ultrafiltration membranes with markedly different pH- and ion-responsivity by photografted zwitterionic polysulfobetain or polycarbobetain. J. Membr. Sci. 2015, 494, 57–67. [Google Scholar] [CrossRef]
- Shao, Q.; Mi, L.; Han, X.; Bai, T.; Liu, S.; Li, Y.; Jiang, S. Differences in Cationic and Anionic Charge Densities Dictate Zwitterionic Associations and Stimuli Responses. J. Phys. Chem. B 2014, 118, 6956–6962. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Zhang, P.-B.; Sun, J.; Liu, C.-J.; Zhu, L.-P.; Xu, Y.-Y. Electrolyte-responsive polyethersulfone membranes with zwitterionic polyethersulfone-based copolymers as additive. J. Membr. Sci. 2016, 510, 306–313. [Google Scholar] [CrossRef]
- Xiang, T.; Lu, T.; Zhao, W.-F.; Zhao, C.-S. Ionic strength- and thermo-responsive polyethersulfone composite membranes with enhanced antifouling properties. New J. Chem. 2018, 42, 5323–5333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).