Alteration of m6A Methylation in Breast Cancer Cells by Kalanchoe pinnata Aqueous Extract
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization and Antioxidant Effects of K. pinnata Aqueous Extract
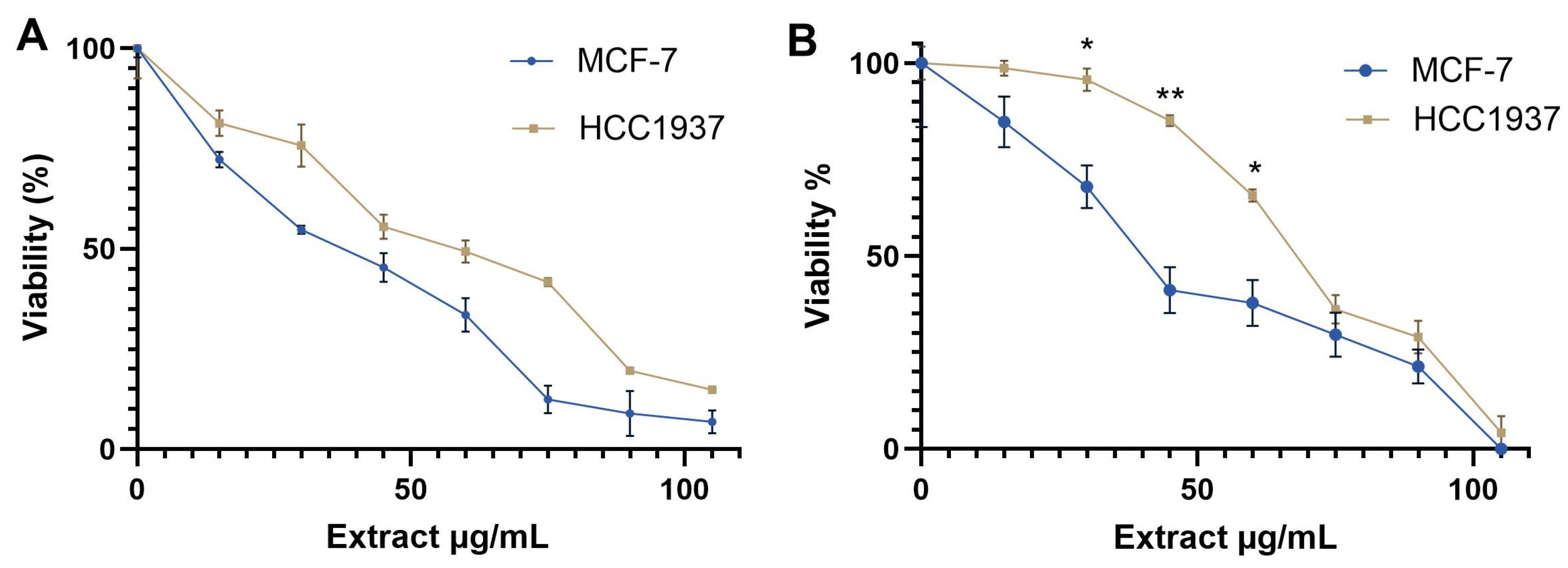
2.2. Cytotoxic Activity of Aqueous Extract from K. pinnata on MCF-7 and HCC1937 Cell Lines
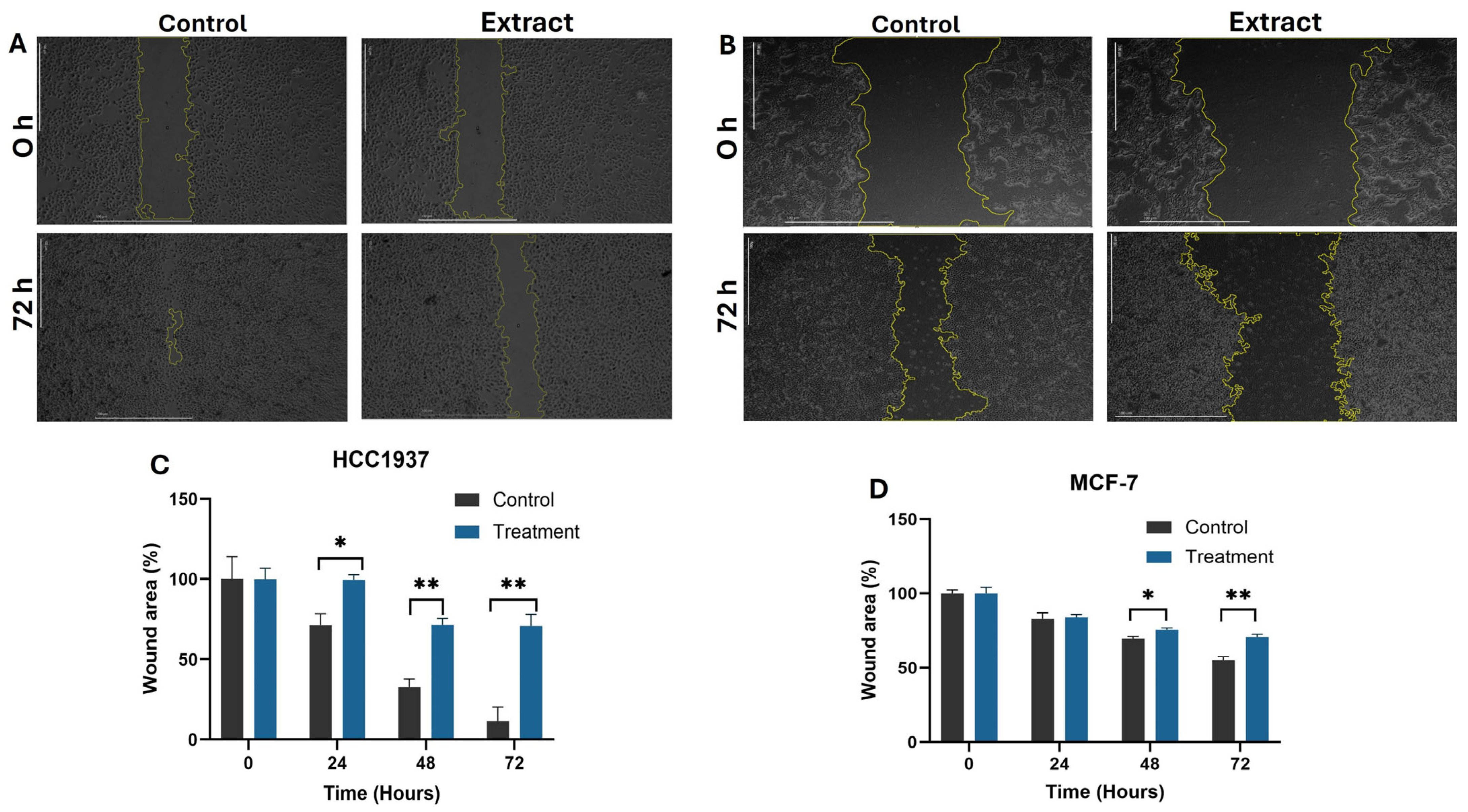
2.3. Inhibition of Cell Migration on MCF-7 and HCC1937 Cells by Aqueous Extract from K. pinnata
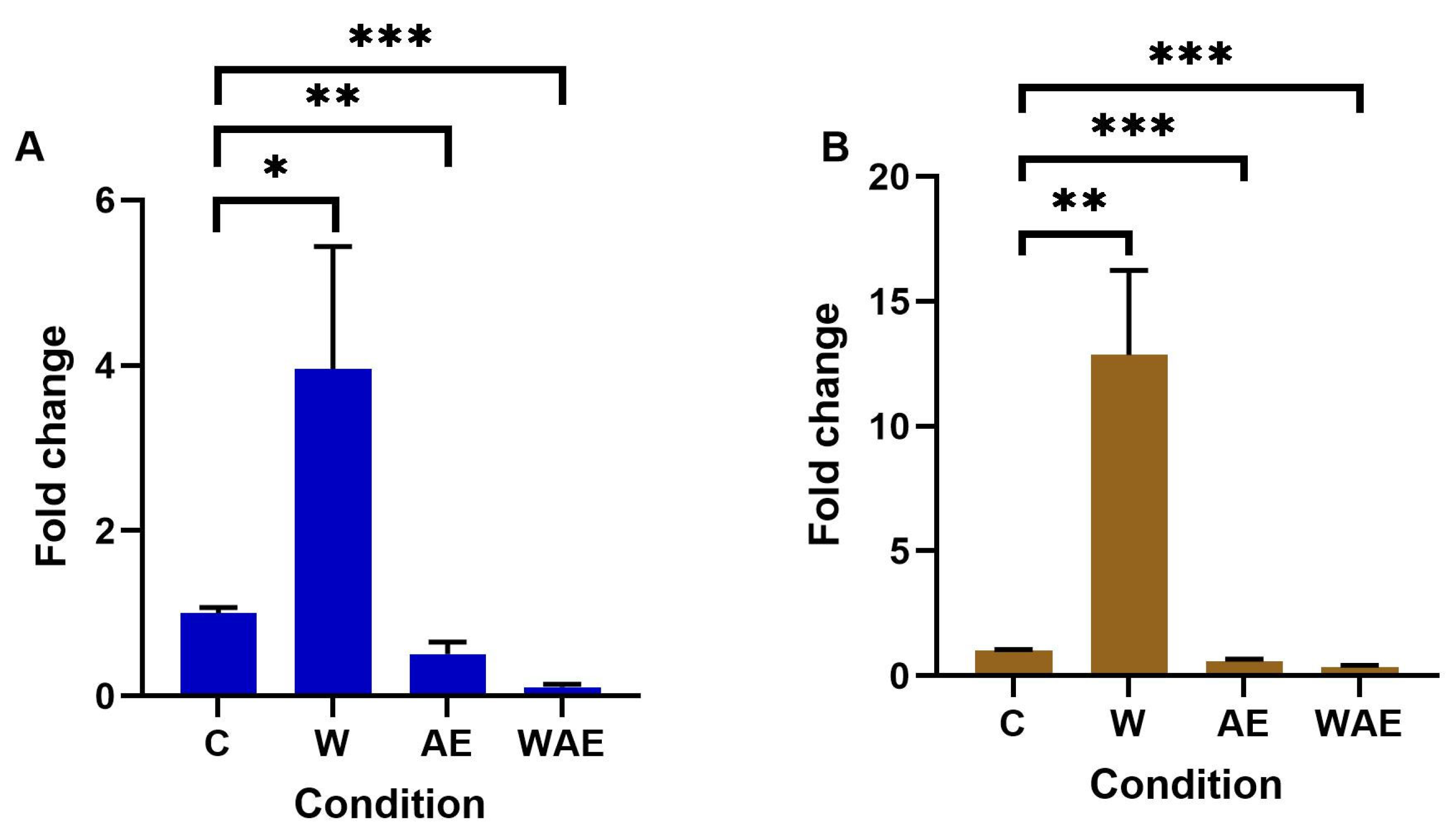
2.4. Effect on METTL3 Expression in HCC1937 and MCF-7 Cells by Aqueous Extract from K. pinnata and Mechanical Stimulus
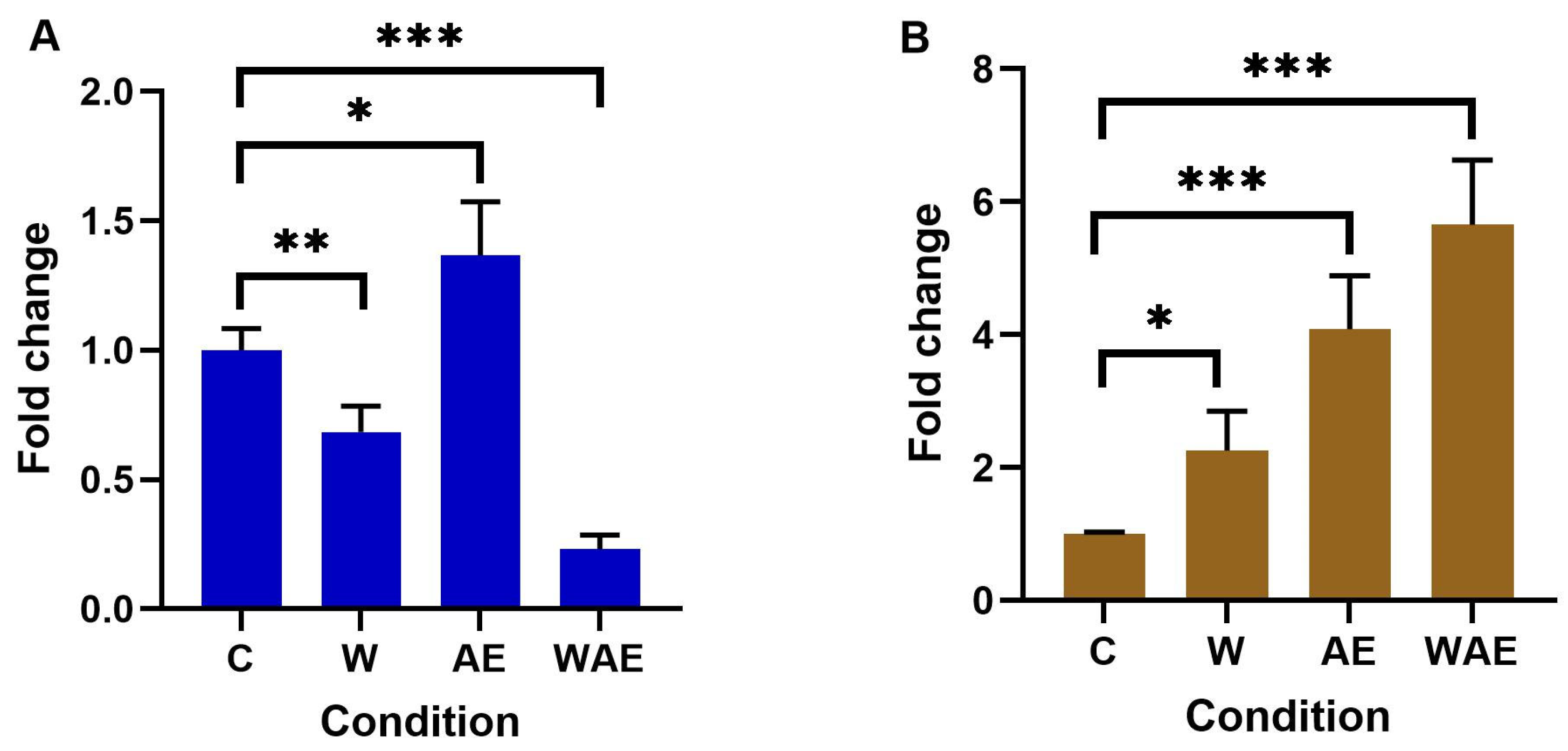
2.5. Effect on FTO Expression in HCC1937 and MCF-7 Cells by Aqueous Extract from K. pinnata and Mechanical Stimulus
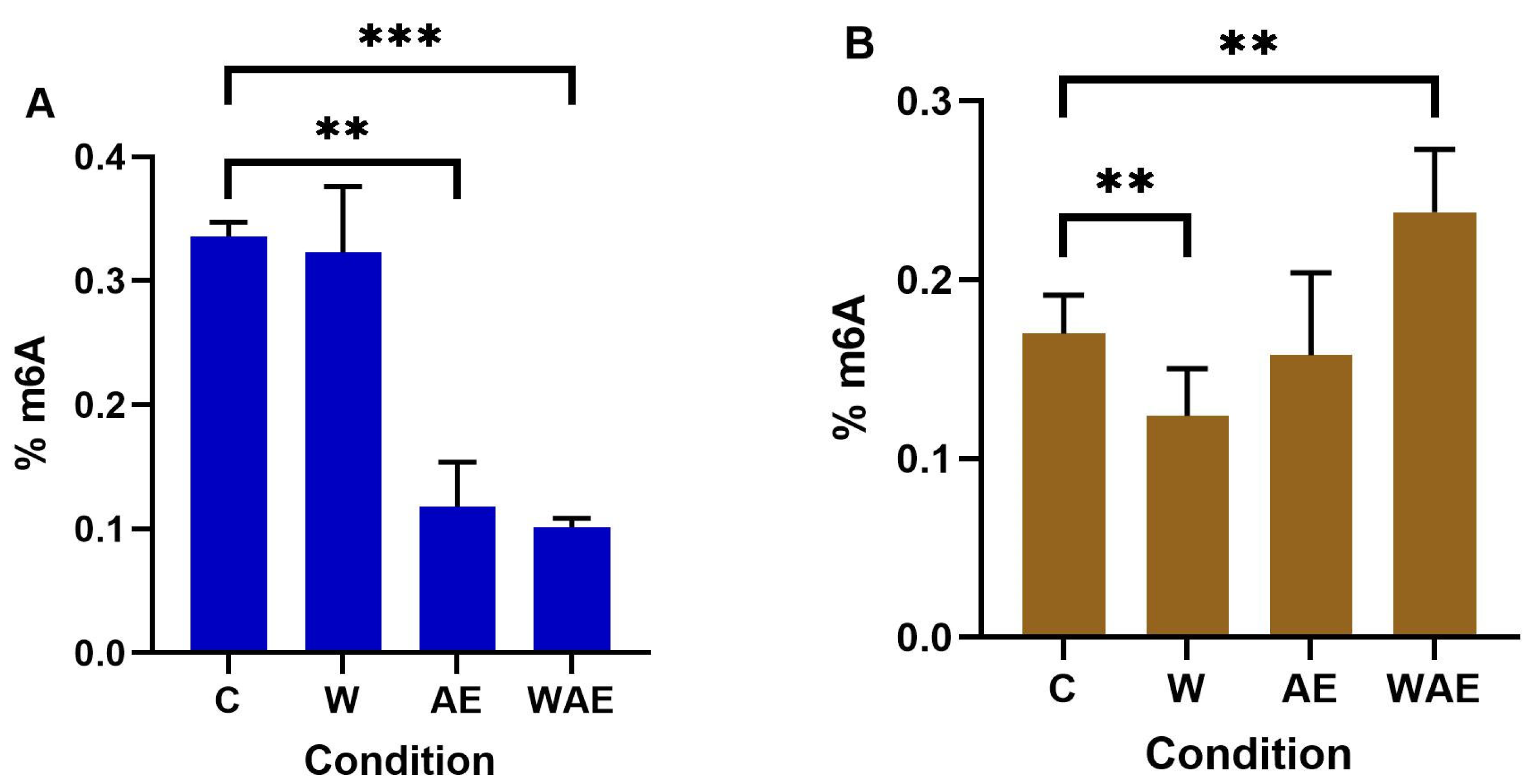
2.6. Effect on m6A Levels in MCF-7 and HCC1937 Cells by K. pinnata Extract
2.7. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of Plant Extract
4.1.1. Collection
4.1.2. Dry and Extraction
4.1.3. HPLC-QToF-MS Analysis
4.2. Antioxidant Tests
4.2.1. DPPH Assay
4.2.2. ABTS Assay
4.2.3. Folin–Ciocalteu Assay
4.3. Flavonoid Total Test
4.4. Cell Culture
4.5. Staining with Trypan Blue
4.6. MTT Assay
4.7. Wound Healing Assay
4.8. RNA Extraction and Quantitative Real-Time PCR
4.9. m6A Assay
4.10. Molecular Docking Study
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER-2 | Human epidermal growth factor receptor-2 |
| TNBC | Triple-negative breast cancer |
| METTL3 | Methyltransferase-like 3 |
| FTO | Fat and obesity-related protein |
| RCC | Renal cell carcinoma |
| SEM | Standard error of media |
| NT | Nontreated group |
| W | Wound healing group |
| AE | Aqueous extract group |
| WAE | Wound healing and aqueous extract group |
References
- Rajsekhar, P.; Bharani, R.; Ramachandran, M.; Angel, K.; Rajsekhar, S. The “Wonder Plant” Kalanchoe pinnata (Linn.) Pers.: A Review. J. Appl. Pharm. Sci. 2016, 6, 151–158. [Google Scholar] [CrossRef]
- Allorge-Boiteau, L. Madagascar Centre de Speciation et D’origine du Genre Kalanchoe (Crassulaceae). Biogéographie Madagasca 1996, 137–145. [Google Scholar]
- Fernandes, J.M.; Cunha, L.M.; Azevedo, E.P.; Lourenço, E.M.G.; Fernandes-Pedrosa, M.F.; Zucolotto, S.M. Kalanchoe laciniata and Bryophyllum pinnatum: An updated review about ethnopharmacology, phytochemistry, pharmacology and toxicology. Rev. Bras. Farmacogn. 2019, 29, 529–558. [Google Scholar] [CrossRef]
- Elufioye, T.O.; Oyedeji, A.O.; Habtemariam, S. A Review of the Traditional Uses, Phytochemistry and Pharmacology of Bryophyllum pinnatum (Lam.) (Crassulaceae). J. Biol. Act. Prod. Nat. 2022, 12, 190–222. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Johnson, K.S.; Conant, E.F.; Soo, M.S. Molecular Subtypes of Breast Cancer: A Review for Breast Radiologists. J. Breast Imaging 2021, 3, 12–24. [Google Scholar] [CrossRef]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef]
- Wang, P.; Doxtader, K.A.; Nam, Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol. Cell 2016, 63, 306–317. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Li, Z.; Zhang, N.; Zhou, C.; He, X.; Xue, D. METTL3 facilitates renal cell carcinoma progression by PLOD2 m6A-methylation under prolonged hypoxia. Cell Death Dis. 2024, 15, 62. [Google Scholar] [CrossRef]
- Gundert, L.; Strick, A.; von Hagen, F.; Schmidt, D.; Klümper, N.; Tolkach, Y.; Toma, M.; Kristiansen, G.; Ritter, M.; Ellinger, J. Systematic expression analysis of m6A RNA methyltransferases in clear cell renal cell carcinoma. BJUI Compass 2021, 2, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yan, K.; Liu, J.; Gao, L.; Jiang, X.; Fan, Y. FTO promotes tumour proliferation in bladder cancer via the FTO/miR-576/CDK6 axis in an m6A-dependent manner. Cell Death Discov. 2021, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhang, S.; Sun, D.; Bei, C.; Li, D.; Zheng, C.; Song, X.; Chen, M.; Tan, S.; Zhu, X.; et al. M6A Demethylase FTO Plays a Tumor Suppressor Role in Thyroid Cancer. DNA Cell Biol. 2020, 39, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.-H.; Fu, X.-H.; Li, G.-Q.; He, Q.; Qiu, X.-G. FTO Prevents Thyroid Cancer Progression by SLC7A11 m6A Methylation in a Ferroptosis-Dependent Manner. Front. Endocrinol. 2022, 13, 857765. [Google Scholar] [CrossRef]
- Niu, Y.; Zhao, X.; Wu, Y.-S.; Li, M.-M.; Wang, X.-J.; Yang, Y.-G. N6-methyl-adenosine (m6A) in RNA: An Old Modification with A Novel Epigenetic Function. Genom. Proteom. Bioinform. 2013, 11, 8–17. [Google Scholar] [CrossRef]
- Liu, Z.; Zou, H.; Dang, Q.; Xu, H.; Liu, L.; Zhang, Y.; Lv, J.; Li, H.; Zhou, Z.; Han, X. Biological and pharmacological roles of m6A modifications in cancer drug resistance. Mol. Cancer 2022, 21, 220. [Google Scholar] [CrossRef]
- Chen, G.; Lu, J.; Li, B.; Zhao, M.; Liu, D.; Yang, Z.; Liu, F. Efficacy and safety of Shenqi Fuzheng injection combined with chemotherapy for cancer: An overview of systematic reviews. Phytomedicine 2024, 125, 155293. [Google Scholar] [CrossRef]
- Liu, X.; Xiu, L.-J.; Jiao, J.-P.; Zhao, J.; Zhao, Y.; Lu, Y.; Shi, J.; Ye, M.; Gu, Y.-F.; Wang, X.-W.; et al. Traditional Chinese medicine integrated with chemotherapy for stage IV non-surgical gastric cancer: A retrospective clinical analysis. J. Integr. Med. 2017, 15, 469–475. [Google Scholar] [CrossRef]
- Tomlinson, G.E.; Chen, T.T.L.; Stastny, V.A.; Virmani, A.K.; Spillman, M.A.; Tonk, V.; Blum, J.L.; Schneider, N.R.; I Wistuba, I.; Shay, J.W.; et al. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res. 1998, 58, 3237–3242. [Google Scholar]
- Ramon, P.; Bergmann, D.; Abdulla, H.; Sparks, J.; Omoruyi, F. Bioactive Ingredients in K. pinnata Extract and Synergistic Effects of Combined K. pinnata and Metformin Preparations on Antioxidant Activities in Diabetic and Non-Diabetic Skeletal Muscle Cells. Int. J. Mol. Sci. 2023, 24, 6211. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tsai, M.; Wu, Y.; Lee, Y.; Yen, A.; Wang, C.; Kao, S. Gallic acid attenuates metastatic potential of human colorectal cancer cells through the miR-1247-3p-modulated integrin/FAK axis. Environ. Toxicol. 2024, 39, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Al Yassine, D.; El Massri, N.; Demircan, G.; Bulut, G.; Akin, D.; Tacer-Caba, Z. Total Antioxidant Potential, Total Phenolic Profile and Cytotoxic Activity Against Brain Cancer: Melocan and Galdirik. Food Technol. Biotechnol. 2023, 61, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Caballero, M.E.; Sierra-Ramírez, J.A.; Villalobos-Valencia, R.; Seseña-Méndez, E. Potential of Kalanchoe pinnata as a Cancer Treatment Adjuvant and an Epigenetic Regulator. Molecules 2022, 27, 6425. [Google Scholar] [CrossRef]
- Thiago, J.d.S.B.; Andréa, F.F.; Ana, C.d.P.R.I.; Norma, A. Cytotoxic, antibacterial and antibiofilm activities of aqueous extracts of leaves and flavonoids occurring in Kalanchoe pinnata (Lam.) Pers. J. Med. Plants Res. 2016, 10, 763–770. [Google Scholar] [CrossRef]
- Pereira, K.M.F.; Grecco, S.S.; Figueiredo, C.R.; Hosomi, J.K.; Nakamura, M.U.; Lago, J.H.G. Chemical Composition and Cytotoxicity of Kalanchoe pinnata Leaves Extracts prepared using Accelerated System Extraction (ASE). Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Hałasa, R.; Soluch, A.; Kowalczyk, M.; Stochmal, A.; Ochocka, R. Biological activities of leaf extracts from selected Kalanchoe species and their relationship with bufadienolides content. Pharm. Biol. 2020, 58, 732–740. [Google Scholar] [CrossRef]
- Quintero, E.J.; León EGDe Morán-Pinzón, J.; Mero, A.; León, E.; Cano, L.P.P. Evaluation of the Leaf Extracts of Kalanchoe pinnata and Kalanchoe daigremontiana Chemistry, Antioxidant and Anti-inflammatory Activity. Eur. J. Med. Plants 2021, 32, 45–54. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Hering, A.; Kowalczyk, M.; Hałasa, R.; Gucwa, M.; Ochocka, J.R. Kalanchoe sp. Extracts—Phytochemistry, Cytotoxic, and Antimicrobial Activities. Plants 2023, 12, 2268. [Google Scholar] [CrossRef]
- Schmidt, C.; Fronza, M.; Goettert, M.; Geller, F.; Luik, S.; Flores, E.; Bittencourt, C.; Zanetti, G.; Heinzmann, B.; Laufer, S.; et al. Biological studies on Brazilian plants used in wound healing. J. Ethnopharmacol. 2009, 122, 523–532. [Google Scholar] [CrossRef]
- Hsieh, Y.-J.; Yang, M.-Y.; Leu, Y.-L.; Chen, C.; Wan, C.-F.; Chang, M.-Y.; Chang, C.-J. Kalanchoe tubiflora extract inhibits cell proliferation by affecting the mitotic apparatus. BMC Complement. Altern. Med. 2012, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Badmann, S.; Kraus, F.; Topalov, N.E.; Mayr, D.; Kolben, T.; Hester, A.; Beyer, S.; Mahner, S.; Jeschke, U.; et al. PLA2G7/PAF-AH as Potential Negative Regulator of the Wnt Signaling Pathway Mediates Protective Effects in BRCA1 Mutant Breast Cancer. Int. J. Mol. Sci. 2023, 24, 882. [Google Scholar] [CrossRef] [PubMed]
- Varghese, E.; Samuel, S.; Abotaleb, M.; Cheema, S.; Mamtani, R.; Büsselberg, D. The “Yin and Yang” of Natural Compounds in Anticancer Therapy of Triple-Negative Breast Cancers. Cancers 2018, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Farghadani, R.; Naidu, R. The anticancer mechanism of action of selected polyphenols in triple-negative breast cancer (TNBC). Biomed. Pharmacother. 2023, 165, 115170. [Google Scholar] [CrossRef]
- Huang, C.; Lai, W.; Mao, S.; Song, D.; Zhang, J.; Xiao, X. Quercetin-induced degradation of RhoC suppresses hepatocellular carcinoma invasion and metastasis. Cancer Med. 2024, 13, e7082. [Google Scholar] [CrossRef]
- Li, L.; Chai, Q.; Guo, C.; Wei, J.; Qin, Y.; Liu, H.; Lu, Z. METTL3-mediated N6-methyladenosine modification contributes to vascular calcification. J. Mol. Cell Cardiol. 2025, 203, 22–34. [Google Scholar] [CrossRef]
- Wang, H.; Xu, B.; Shi, J. N6-methyladenosine METTL3 promotes the breast cancer progression via targeting Bcl-2. Gene 2020, 722, 144076. [Google Scholar] [CrossRef]
- Su, C.; Zhang, Y.; Chen, P.; Yang, W.; Du, J.; Zhang, D. Methyltransferase-like 3 induces the development of cervical cancer by enhancing insulin-like growth factor 2 mRNA-binding proteins 3-mediated apoptotic chromatin condensation inducer 1 mRNA stability. Bioengineered 2022, 13, 7034–7048. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Lin, M.; Jiang, Y.; Tan, G.; Huang, L.; Song, D. Silencing of METTL3 prevents the proliferation, migration, epithelial-mesenchymal transition, and renal fibrosis of high glucose-induced HK2 cells by mediating WISP1 in m6A-dependent manner. Aging 2024, 16, 1237–1248. [Google Scholar] [CrossRef]
- Jia, J.; Yu, L. METTL3-mediated m6A modification of EPPK1 to promote the development of esophageal cancer through regulating the PI3K/AKT pathway. Environ. Toxicol. 2024, 39, 2830–2841. [Google Scholar] [CrossRef]
- Du, Y.; Yuan, Y.; Xu, L.; Zhao, F.; Wang, W.; Xu, Y.; Tian, X. Discovery of METTL3 Small Molecule Inhibitors by Virtual Screening of Natural Products. Front. Pharmacol. 2022, 13, 878135. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Williams, A.; Wei, N. Quercetin ameliorated insulin resistance via regulating METTL3-mediated N6-methyladenosine modification of PRKD2 mRNA in skeletal muscle and C2C12 myocyte cell line. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2655–2668. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, Y.; Chen, K.; Zhang, M.; Jiang, Q.; Xu, T. Quercetin attenuated necroptosis and apoptosis caused by LPS-induced mitochondrial function dysfunction through the METTL3-mediated PTEN m6A methylation/PI3K/AKT signaling in broiler livers. Phytomedicine 2025, 139, 156551. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.A.; Ababneh, N.A.; Tarawneh, N.; Ismail, M.A.; Awidi, A.; Abdalla, S. Antitumor Effects of Quercetin and Luteolin in A375 Cutaneous Melanoma Cell Line Are Mediated by Upregulation of P-ERK, c-Myc, and the Upstream GPER. Life 2025, 15, 417. [Google Scholar] [CrossRef]
- Guo, C.; Chu, H.; Gong, Z.; Zhang, B.; Li, C.; Chen, J.; Huang, L. HOXB13 promotes gastric cancer cell migration and invasion via IGF-1R upregulation and subsequent activation of PI3K/AKT/mTOR signaling pathway. Life Sci. 2021, 278, 119522. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Wu, L.; Zhai, L.; Chen, L.; Yao, L.; Yu, K.; Tang, Z. N6-methyladenosine RNA demethylase FTO regulates extracellular matrix-related genes and promotes pancreatic cancer cell migration and invasion. Cancer Med. 2023, 12, 3731–3743. [Google Scholar] [CrossRef]
- Jeschke, J.; Collignon, E.; Al Wardi, C.; Krayem, M.; Bizet, M.; Jia, Y.; Garaud, S.; Wimana, Z.; Calonne, E.; Hassabi, B.; et al. Downregulation of the FTO m6A RNA demethylase promotes EMT-mediated progression of epithelial tumors and sensitivity to Wnt inhibitors. Nat. Cancer 2021, 2, 611–628. [Google Scholar] [CrossRef]
- Gilbert, W.V.; Bell, T.A.; Schaening, C. Messenger RNA modifications: Form, distribution, and function. Science 2016, 352, 1408–1412. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef]
- Soule, H.D.; Vazquez, J.; Long, A.; Albert, S.; Brennan, M. A human cell line from a pleural effusion derived from a breast carcinoma1,2. J. Natl. Cancer Inst. 1973, 51, 1409–1416. [Google Scholar] [CrossRef]
- Rosales-López, A.; López-Castillo, G.; Sandoval-Ramírez, J.; Terán, J.; Carrasco-Carballo, A. Correlation between Molecular Docking and the Stabilizing Interaction of HOMO-LUMO: Spirostans in CHK1 and CHK2, an In Silico Cancer Approach. Int. J. Mol. Sci. 2024, 25, 8588. [Google Scholar] [CrossRef]
| Name | Formula | [M + H]+ | Polarity |
|---|---|---|---|
| Apigenin | C15H10O5 | 271.0601 | Positive |
| Luteolin | C15H10O6 | 287.0550 | Positive |
| Diosmetin | C16H12O6 | 301.0707 | Positive |
| Quercetin–Methylester | C16H12O7 | 317.0656 | Positive |
| Astragalin | C21H20O11 | 449.1078 | Positive |
| Rutin | C27H30O16 | 611.1607 | Positive |
| [M − H]−. | |||
| p-coumaric acid | C9H8O3 | 163.0400 | Negative |
| Gallic acid | C7H6O5 | 169.0142 | Negative |
| Caffeic acid | C9H8O4 | 179.0350 | Negative |
| Syringic acid | C9H10O5 | 197.0455 | Negative |
| Kaempferol | C15H10O6 | 285.0405 | Negative |
| Quercetin | C15H10O7 | 301.0354 | Negative |
| Myricetin | C15H10O8 | 317.0303 | Negative |
| Bryophyllin B | C26H34O9 | 489.2130 | Negative |
| Concentration mg/mL | Inhibition % DPPH | Inhibition % ABTS |
|---|---|---|
| 10 | 77.275 ± 1.185 | 88.979 ± 0.533 |
| 8 | 75.170 ± 0.564 | 85.154 ± 0.705 |
| 6 | 64.699 ± 1.627 | 73.905 ± 1.924 |
| 4 | 48.995 ± 0.476 | 59.856 ± 2.394 |
| 2 | 34.073 ± 0.743 | 43.659 ± 2.128 |
| Molecule | METTL3 | FTO |
|---|---|---|
| Apigenin | −6.649 | −6.62 |
| Luteolin | −6.792 | −6.562 |
| Diosmetin | −6.872 | −7.324 |
| Astragalin | −5.262 | −5.69 |
| Rutin | −6.521 | −5.406 |
| p-coumaric acid | −5.701 | −7.395 |
| Gallic Acid | −4.076 | −8.21 |
| Caffeic Acid | −6.119 | −7.146 |
| Syringic Acid | −6.131 | −7.444 |
| Kaempferol | −4.008 | −6.919 |
| Quercetin | −6.970 | −7.500 |
| Myricetin | −3.933 | −8.163 |
| Bryophyllin B | −4.601 | −3.212 |
| Quercetin methyl | −6.43 | −7.601 |
| Adenosine | −7.553 | |
| 6MK | −8.259 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvizo-Rodríguez, C.R.; Calzada, F.; López-Vázquez, U.; Tiburcio, E.T.; Hernandez-Rivera, J.A.; Carrasco-Carballo, A.; Hernández-Caballero, M.E. Alteration of m6A Methylation in Breast Cancer Cells by Kalanchoe pinnata Aqueous Extract. Molecules 2025, 30, 2634. https://doi.org/10.3390/molecules30122634
Alvizo-Rodríguez CR, Calzada F, López-Vázquez U, Tiburcio ET, Hernandez-Rivera JA, Carrasco-Carballo A, Hernández-Caballero ME. Alteration of m6A Methylation in Breast Cancer Cells by Kalanchoe pinnata Aqueous Extract. Molecules. 2025; 30(12):2634. https://doi.org/10.3390/molecules30122634
Chicago/Turabian StyleAlvizo-Rodríguez, Carlos Rogelio, Fernando Calzada, Uriel López-Vázquez, Emmanuel Tomay Tiburcio, Juan A. Hernandez-Rivera, Alan Carrasco-Carballo, and Marta Elena Hernández-Caballero. 2025. "Alteration of m6A Methylation in Breast Cancer Cells by Kalanchoe pinnata Aqueous Extract" Molecules 30, no. 12: 2634. https://doi.org/10.3390/molecules30122634
APA StyleAlvizo-Rodríguez, C. R., Calzada, F., López-Vázquez, U., Tiburcio, E. T., Hernandez-Rivera, J. A., Carrasco-Carballo, A., & Hernández-Caballero, M. E. (2025). Alteration of m6A Methylation in Breast Cancer Cells by Kalanchoe pinnata Aqueous Extract. Molecules, 30(12), 2634. https://doi.org/10.3390/molecules30122634








