The Chemical Profiling and Immunological Activity of Polysaccharides from the Rhizome of Imperata cylindrica Using Hot Water Extraction
Abstract
1. Introduction
2. Results and Discussion
2.1. The Separation and Purification Results of IRPs-H
2.2. Structural Analysis of Different IRPs-H Polysaccharides
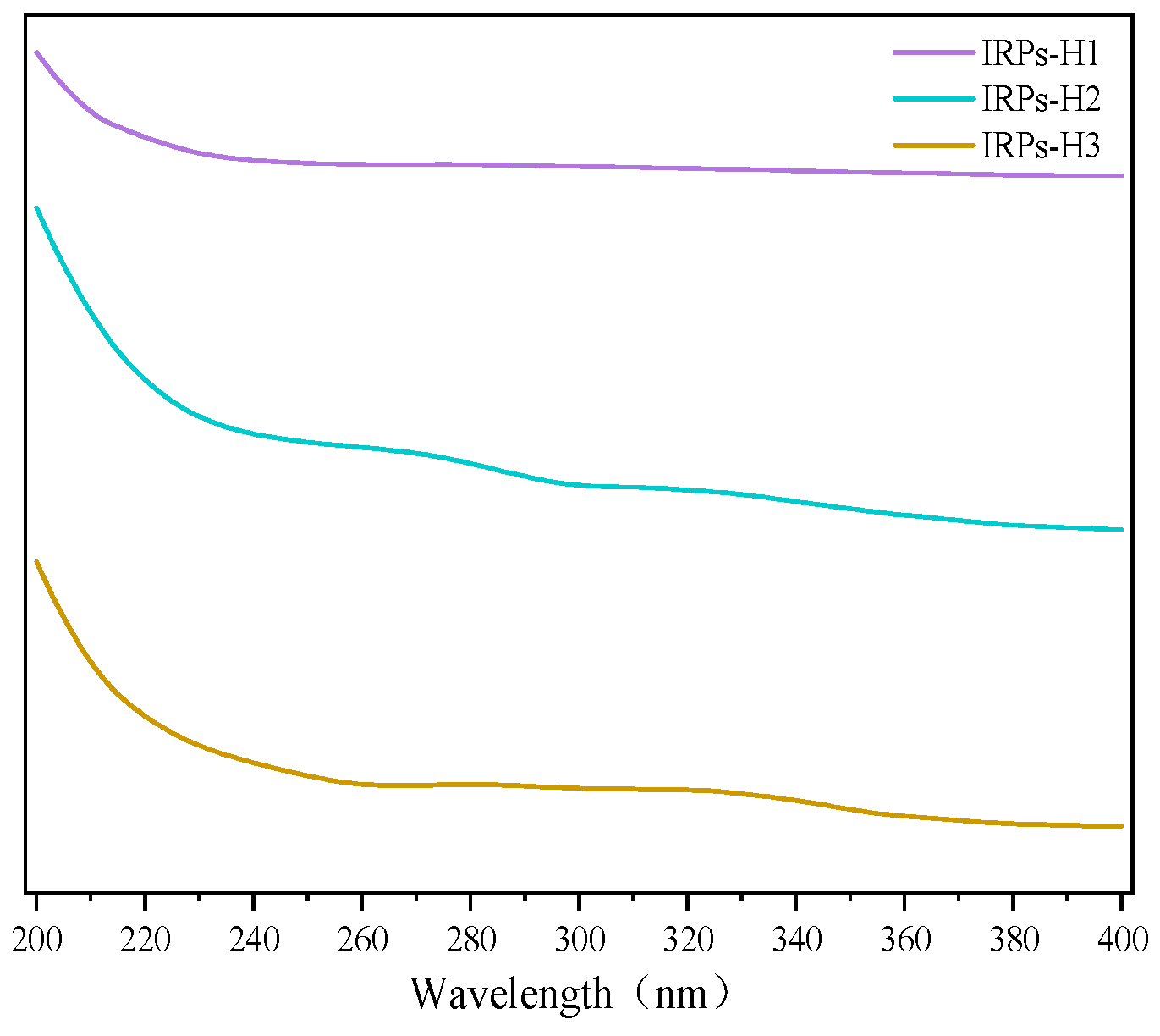
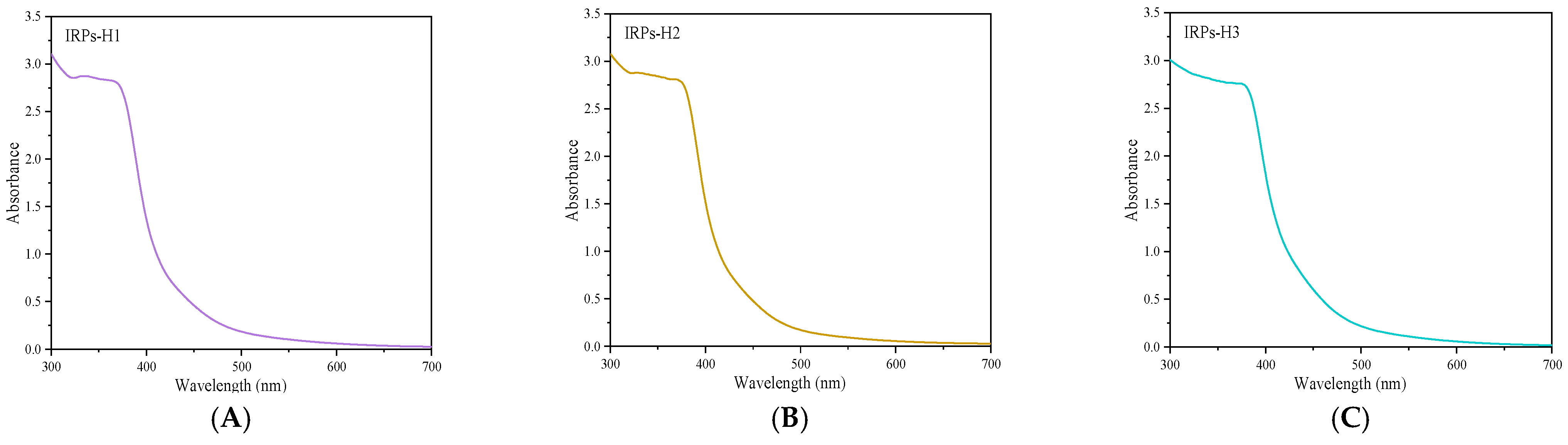
2.2.1. Analysis of UV Spectroscopy
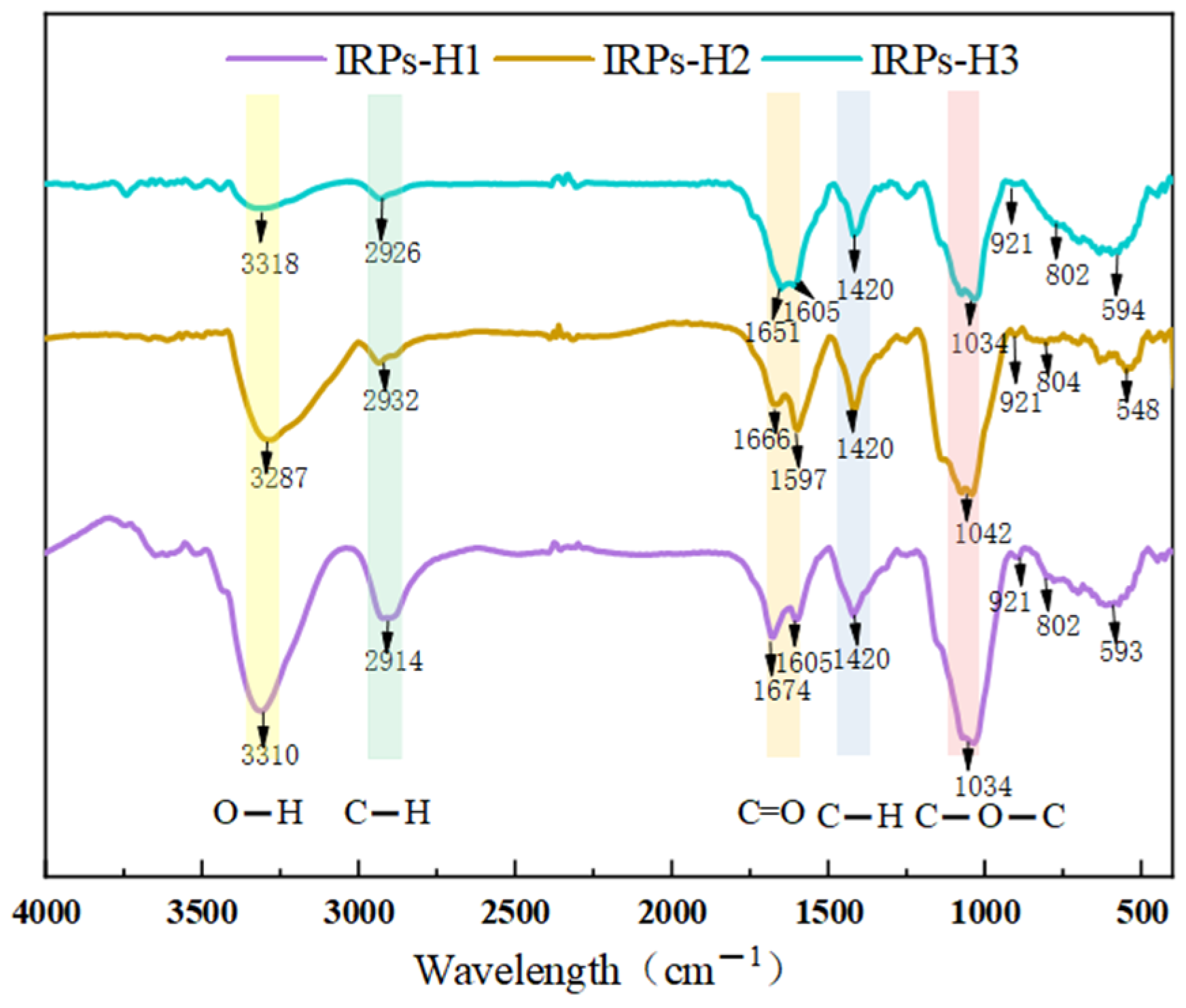
2.2.2. Analysis of FT-IR Spectroscopy
2.2.3. Analysis of Monosaccharide Composition
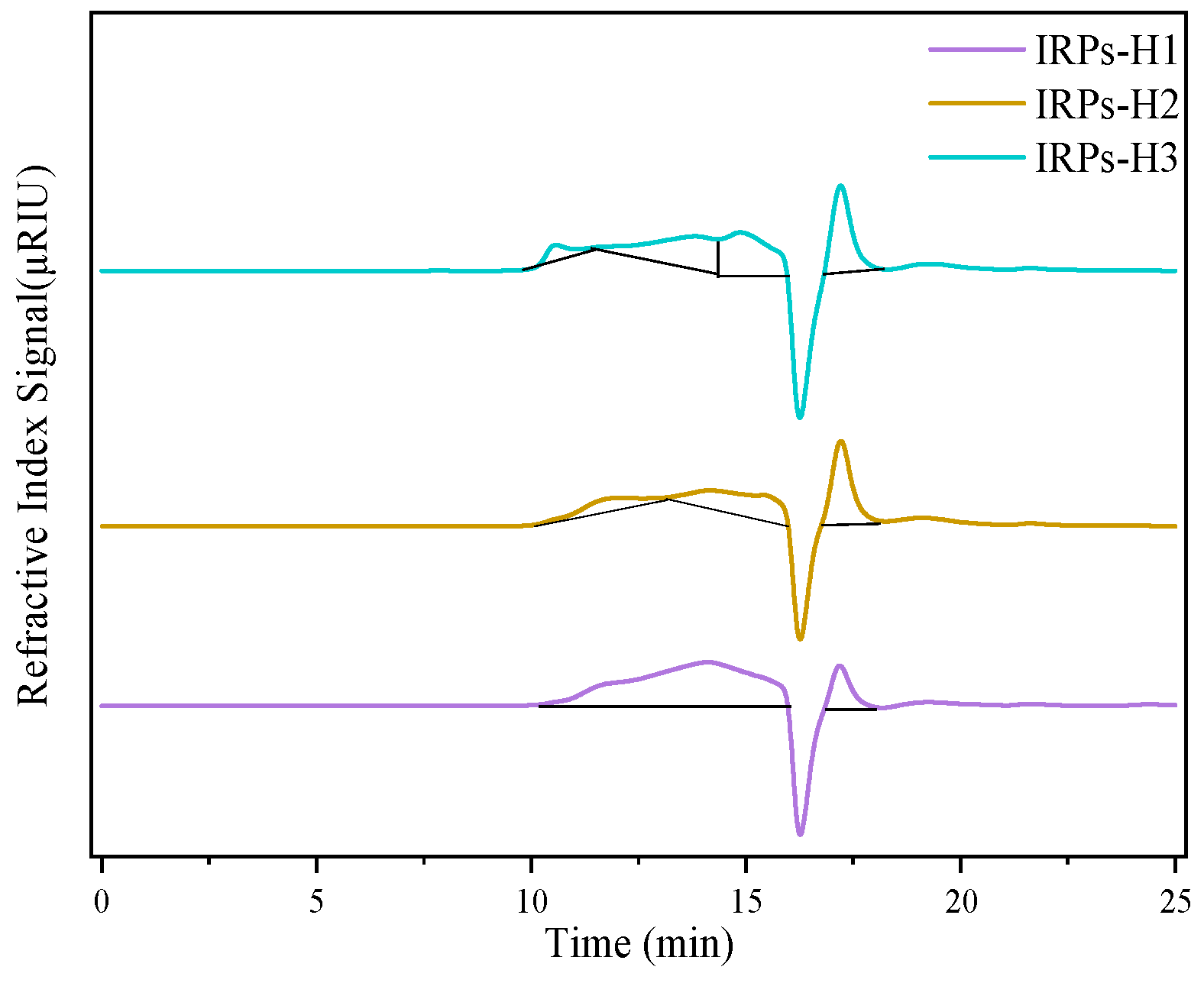
2.2.4. Determination of Molecular Weight
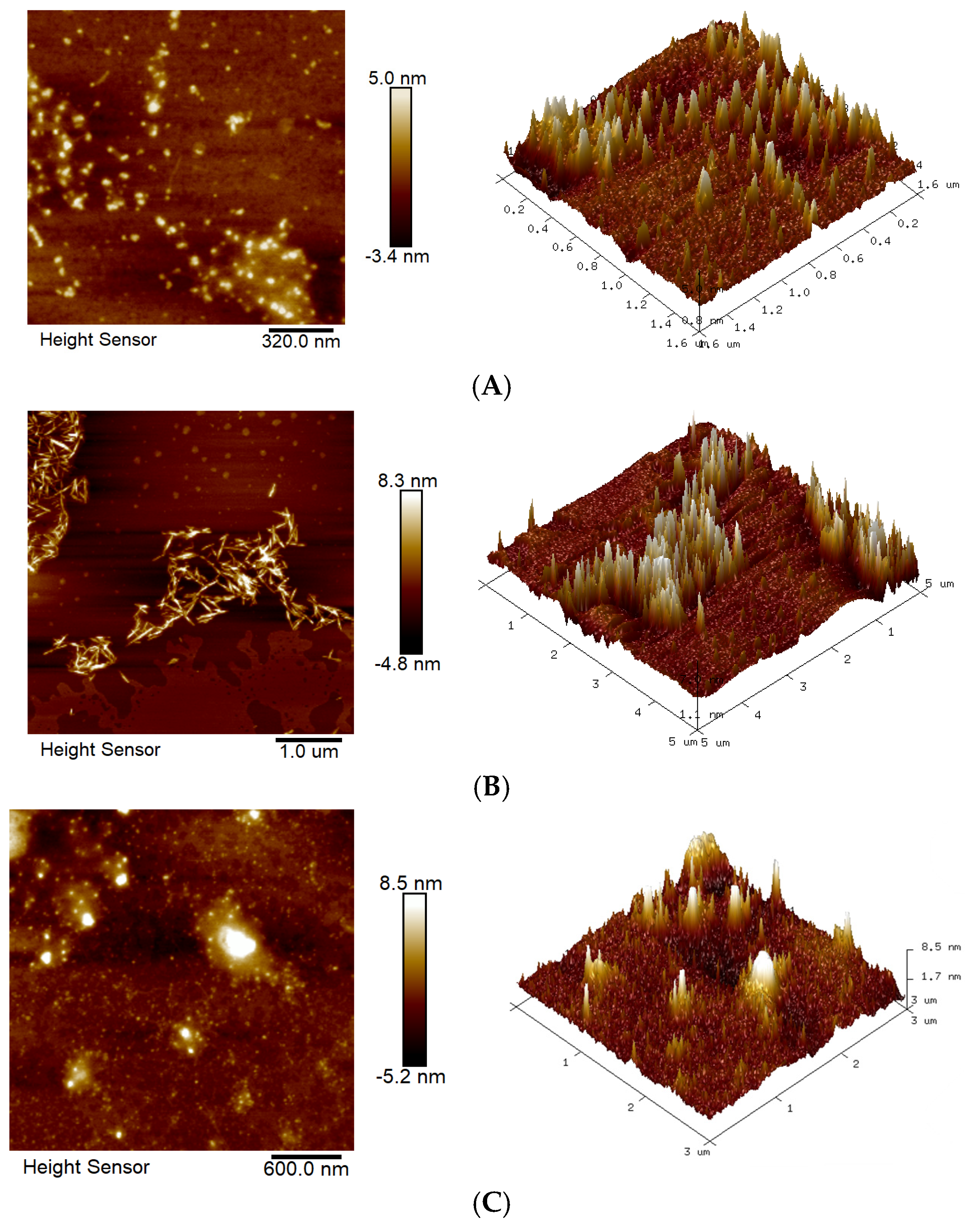
2.2.5. Analysis of AFM
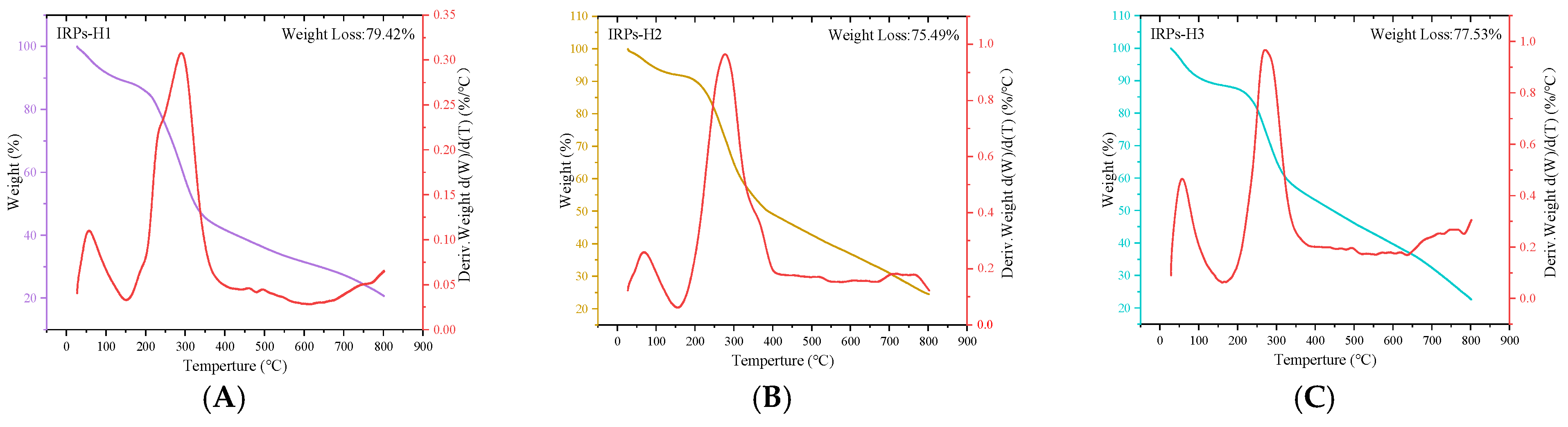
2.2.6. Analysis of Thermal Stability
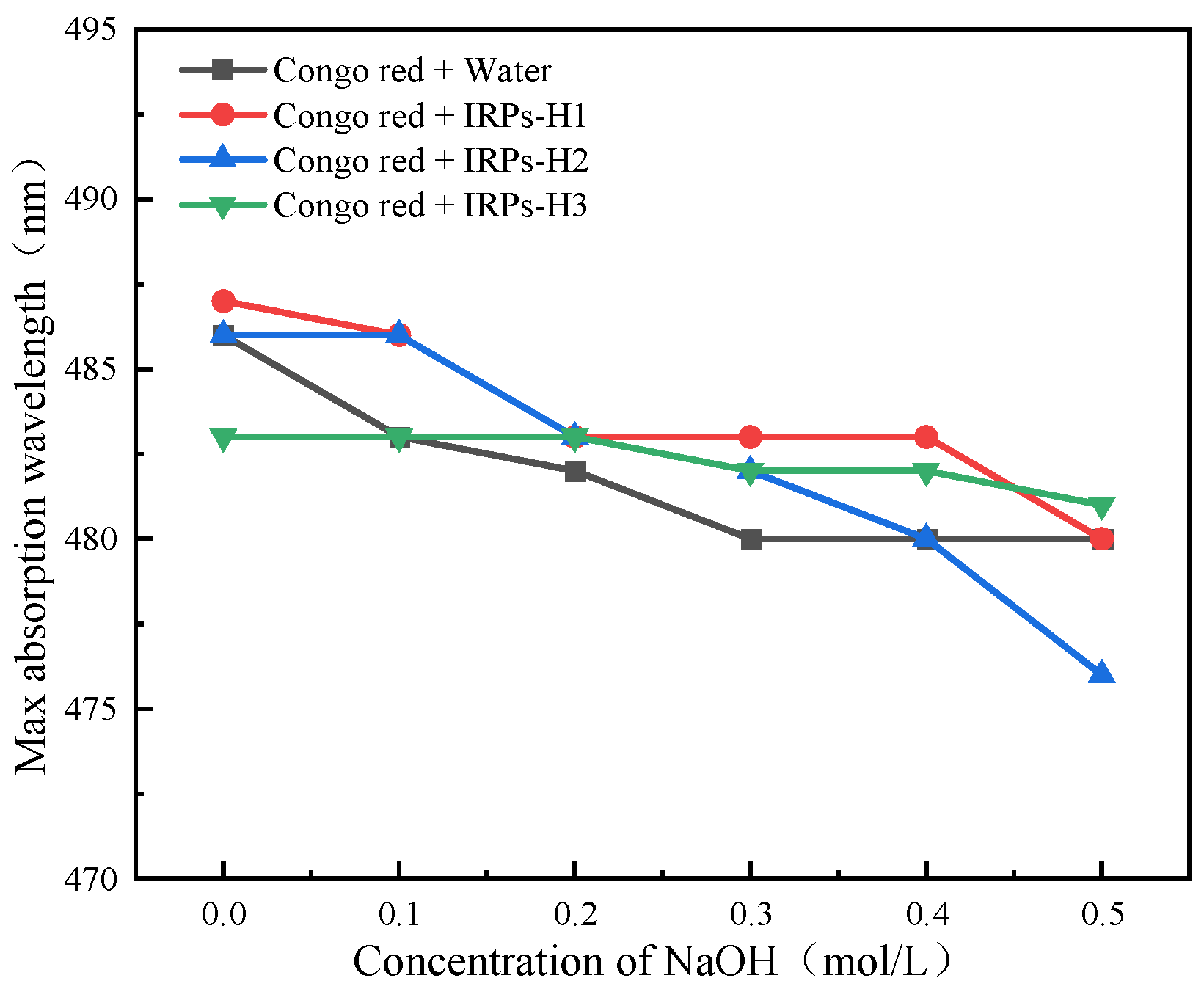
2.2.7. Analysis of Triple Helix Structure
2.2.8. Analysis of Branched Chain Structure
2.3. Immunological Activity of Different IRPs-H Polysaccharides
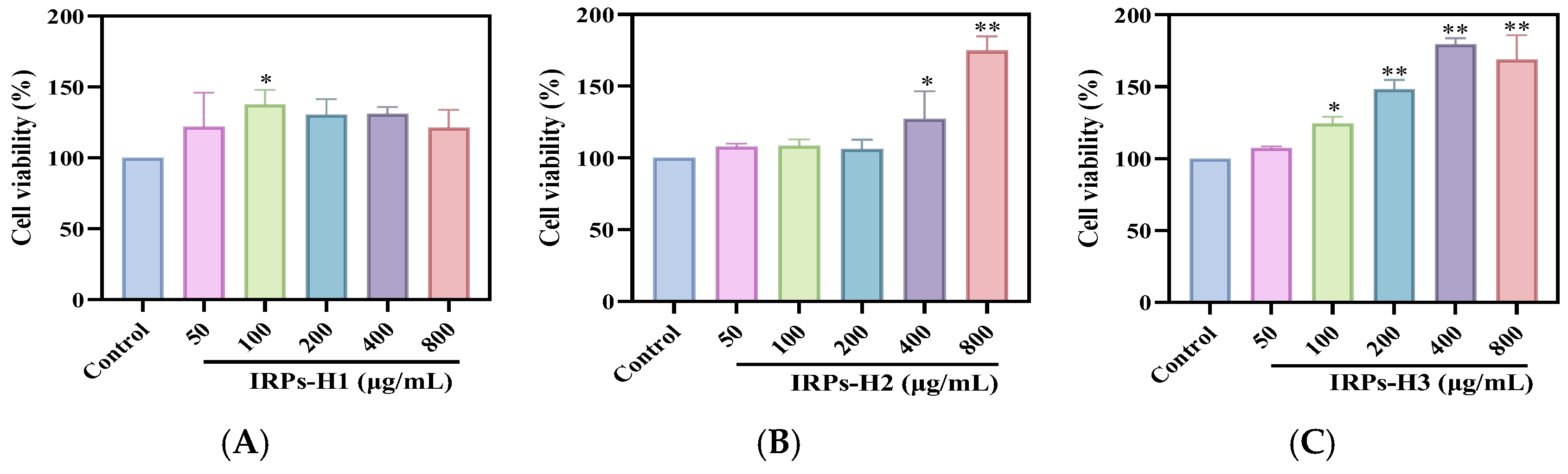
2.3.1. Proliferation of RAW264.7 Cells
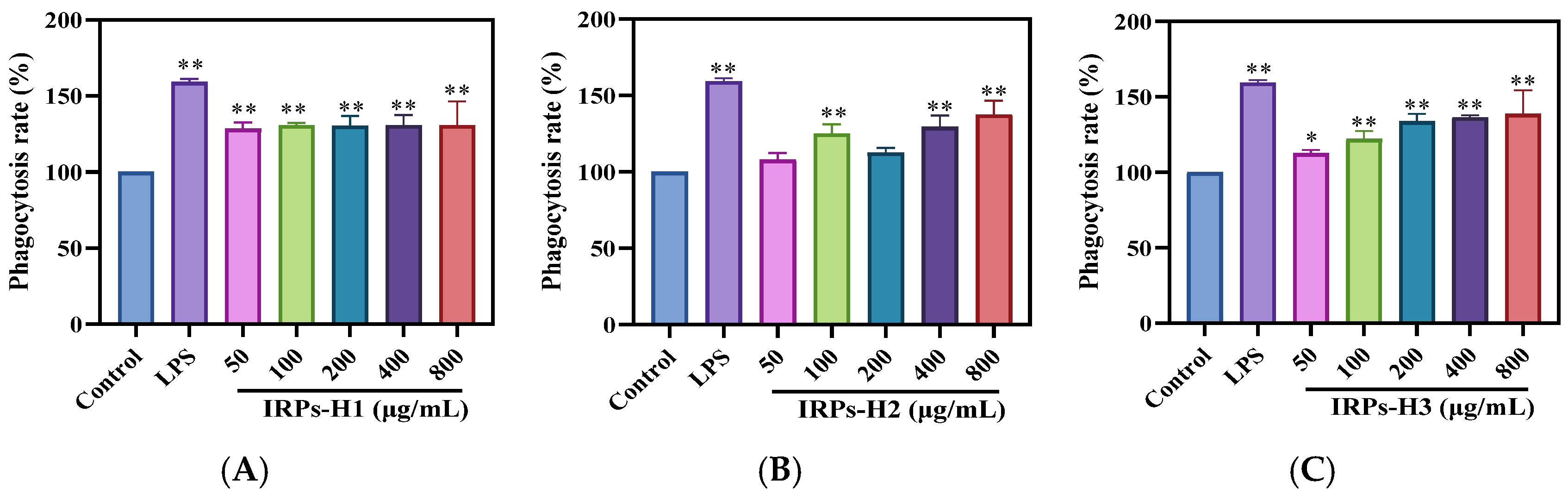
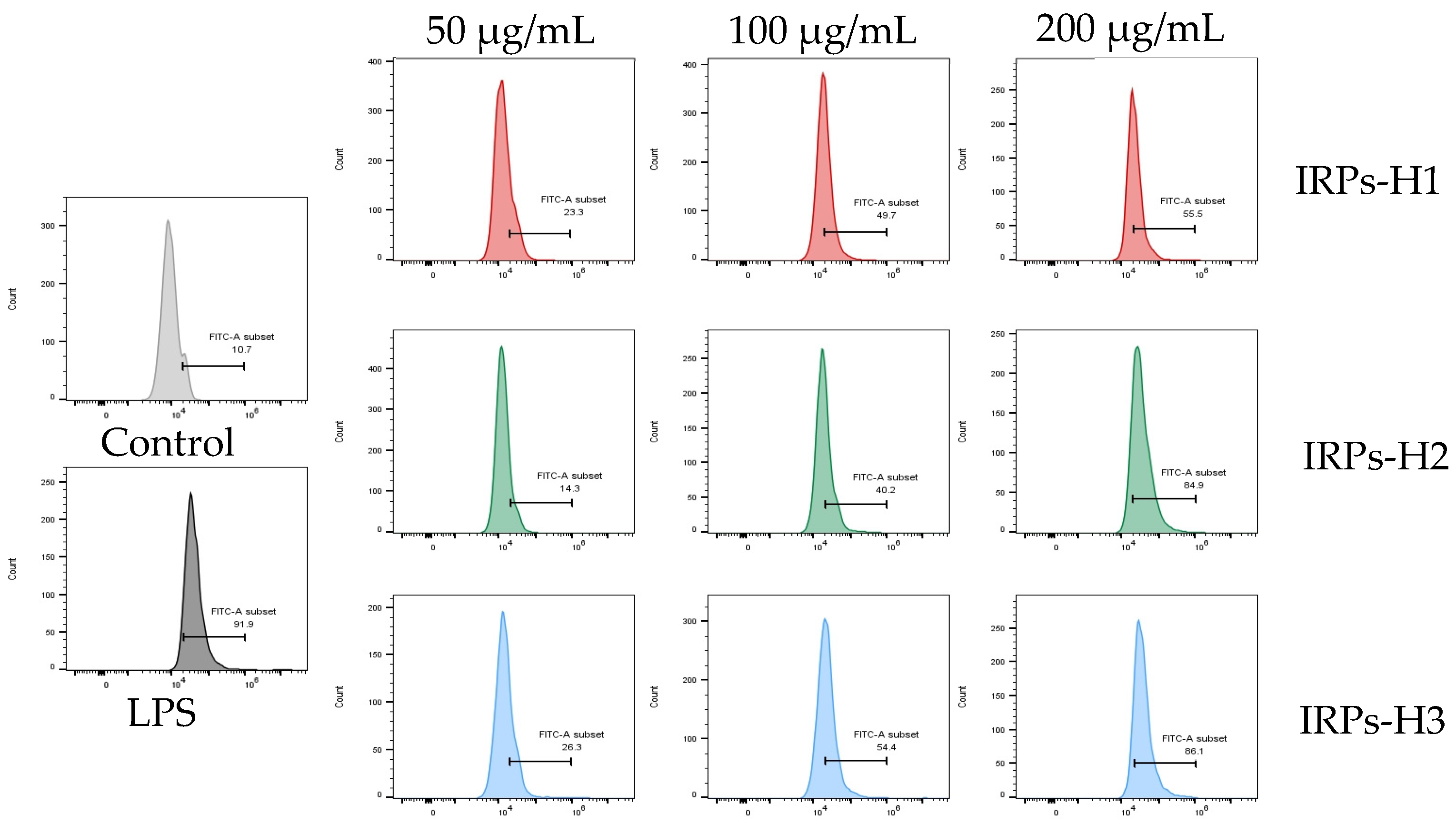
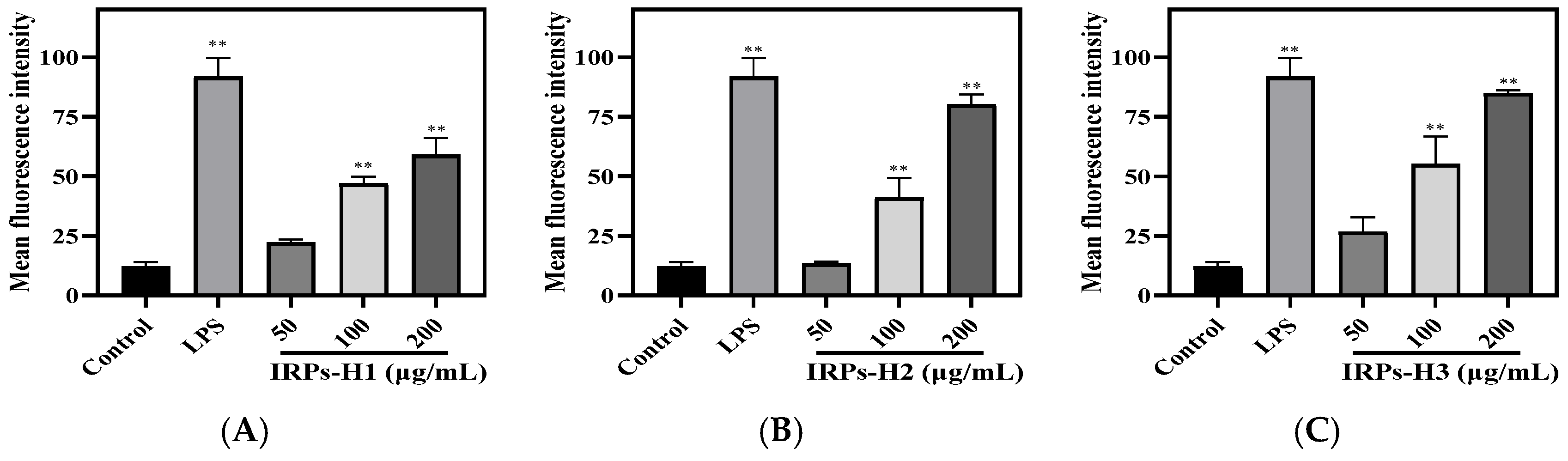
2.3.2. Analysis of Phagocytosis for RAW264.7 Cells
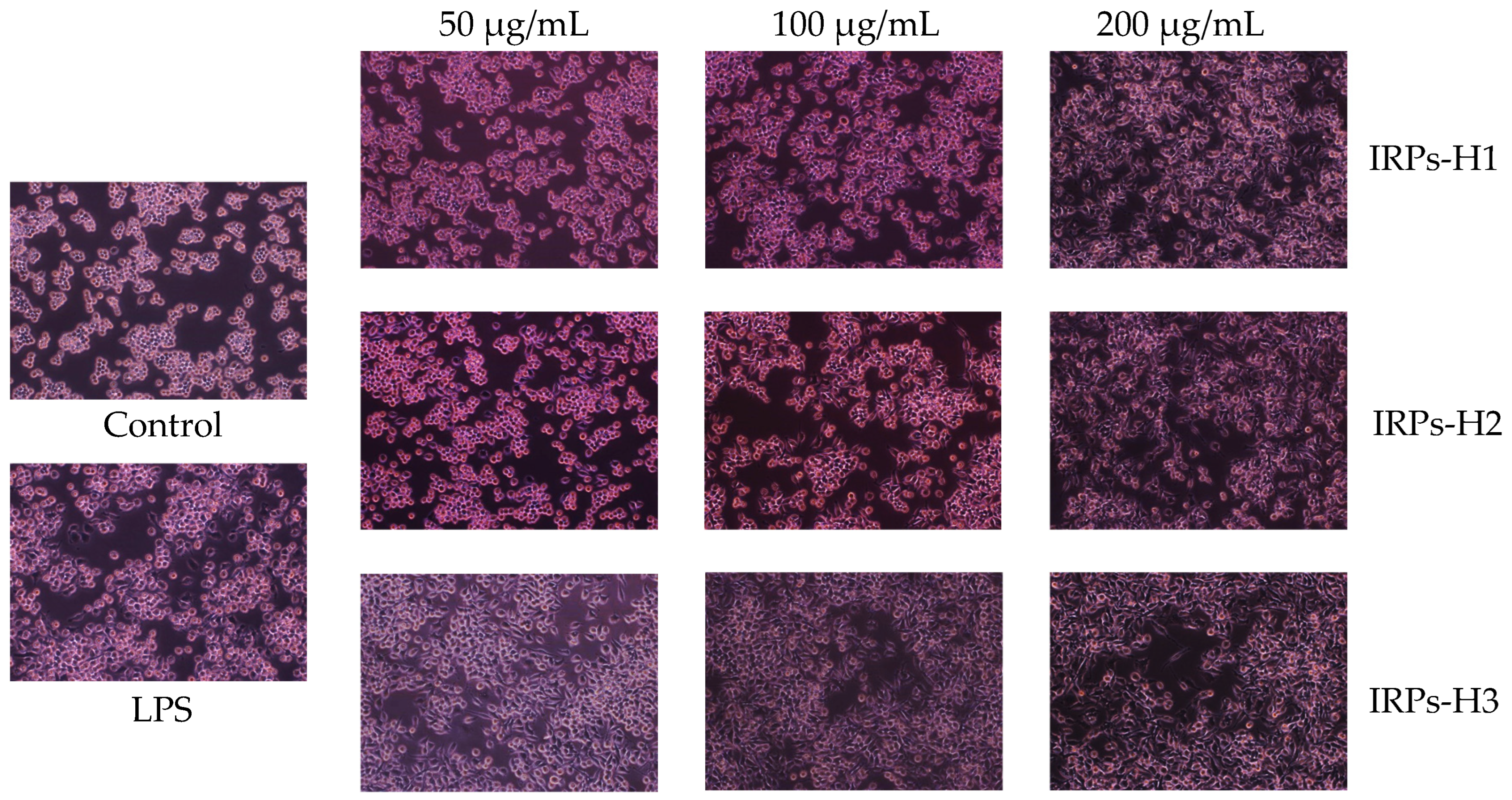
2.3.3. Morphological Changes in RAW264.7 Cells
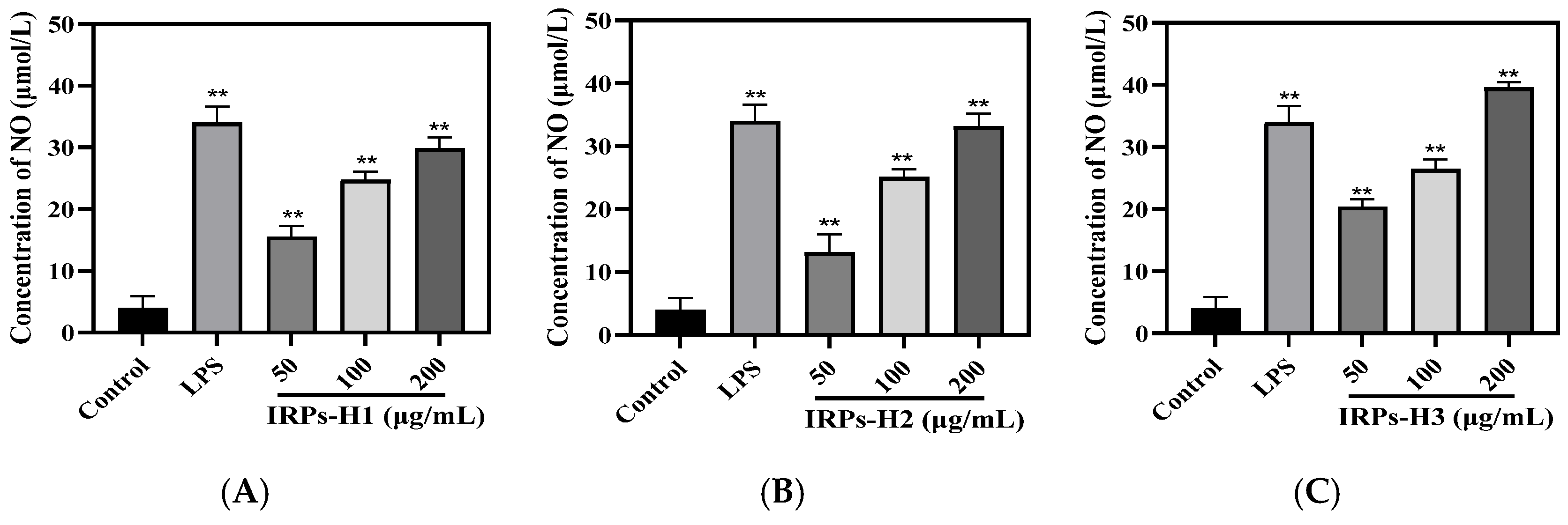
2.3.4. Analysis of Different IRPs-H on NO Secretion in RAW264.7 Cells
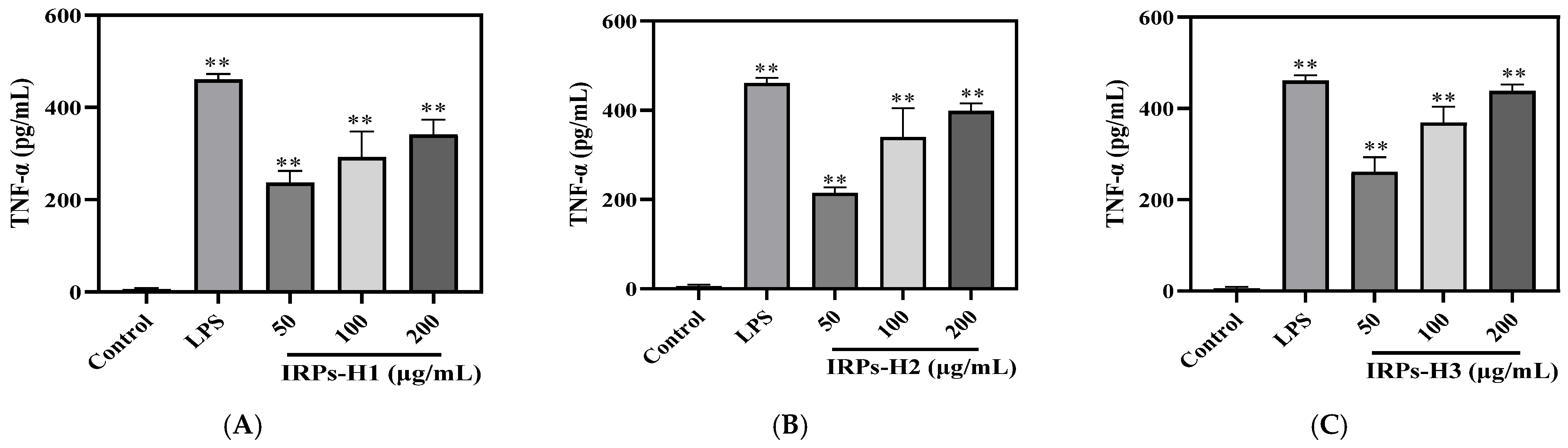
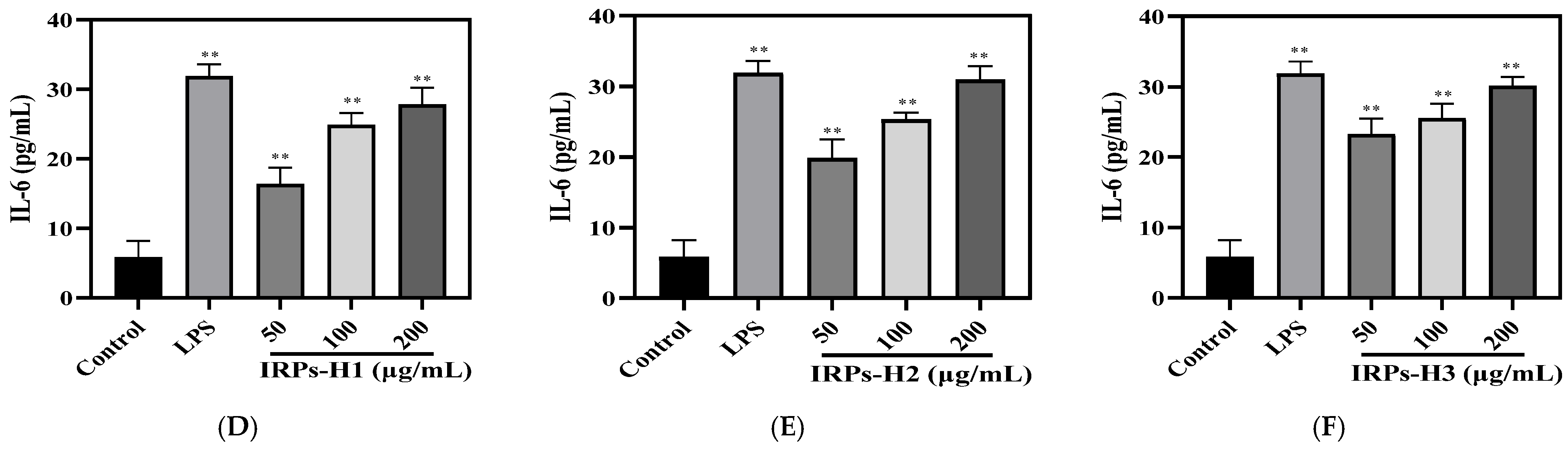
2.3.5. Analysis of Different IRPs-H on Cytokines in RAW264.7 Cells
2.3.6. Analysis of the Release of Reactive Oxygen Species from RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Isolation and Purification of IRPs-H
4.3. Structural Elucidation of Each IRPs-H
4.3.1. Ultraviolet Spectroscopy Characteristics of Each IRPs-H
4.3.2. Fourier-Transform Infrared Spectroscopy of Each IRPs-H
4.3.3. Analysis of the Monosaccharide Composition of Each IRPs-H
4.3.4. Molecular Weight Determination of Each IRPs-H
4.3.5. AFM Analysis of Each IRPs-H
4.3.6. Thermal Stability Analysis of Each IRPs-H
4.3.7. Determination of the Triple Helix Structure of Each IRPs-H
4.3.8. Branched Chain Determination of Each IRPs-H
4.4. Cell Culture
4.4.1. Determination of the Proliferation Capacity of Each Component of IRPs-H on RAW264.7 Cells
4.4.2. Effect of Each of the IRPs-H Components on the Phagocytic Capacity of RAW264.7 Cells
4.4.3. The Morphological Changes in RAW264.7 Cells Were Observed by Inverted Microscope
4.4.4. Determination of NO Release by IRPs-H Component in RAW264.7 Cells
4.4.5. Determination of Cytokines by Each IRPs-H Component in RAW264.7 Cells
4.4.6. Determination of the ROS from RAW264.7 Cells by Polysaccharides of Each Component of IRPs-H
4.5. Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The Structures and Biological Functions of Polysaccharides from Traditional Chinese Herbs. Prog. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Yang, M.; Ma, L.; Liu, X.; Ding, Q.; Chai, G.; Lu, Y.; Wei, H.; Zhang, S.; Ding, C. Structural Modification and Biological Activity of Polysaccharides. Molecules 2023, 28, 5416. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Quinn, M.T. Botanical Polysaccharides: Macrophage Immunomodulation and Therapeutic Potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Z.; Wang, Z.; Yu, S.; Long, T.; Zhou, X.; Bao, Y. Astragalus Polysaccharides Exerts Immunomodulatory Effects via TLR4-Mediated MyD88-Dependent Signaling Pathway in Vitro and in Vivo. Sci. Rep. 2017, 7, 44822. [Google Scholar] [CrossRef]
- Wu, J.; Yu, G.; Zhang, X.; Staiger, M.P.; Gupta, T.B.; Yao, H.; Wu, X. A Fructan-Type Garlic Polysaccharide Upregulates Immune Responses in Macrophage Cells and in Immunosuppressive Mice. Carbohydr. Polym. 2024, 344, 122530. [Google Scholar] [CrossRef]
- Sato, W.; Takeshita, K.; Tsuboi, M.; Kanamori, M.; Ishibashi, K.; Miura, N.N.; Adachi, Y.; Ohno, N. Specificity of the Immunomodulating Activity of Sasa Veitchii (Japanese Folk Medicine Kumazasa) to Fungal Polysaccharides. Int. J. Med. Mushrooms 2015, 17, 415–426. [Google Scholar] [CrossRef]
- Shin, M.-R.; Lee, J.H.; Lee, J.A.; Kim, M.J.; Park, H.-J.; Park, B.W.; Seo, S.B.; Roh, S.-S. Immunomodulatory and Anti-Inflammatory Effects of Phellinus Linteus Mycelium. BMC Complement. Med. Ther. 2021, 21, 269. [Google Scholar] [CrossRef]
- Jung, Y.-K.; Shin, D. Imperata cylindrica: A Review of Phytochemistry, Pharmacology, and Industrial Applications. Molecules 2021, 26, 1454. [Google Scholar] [CrossRef]
- Fu, L.-N.; Chen, L.-Y.; Liu, R.-H.; Chen, D.-F. [Chemical Constituents of Rhizoma Imperatae and Their Anti-Complementary Activity]. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2010, 33, 1871–1874. [Google Scholar]
- Zhou, C.; Yin, S.; Yu, Z.; Feng, Y.; Wei, K.; Ma, W.; Ge, L.; Yan, Z.; Zhu, R. Preliminary Characterization, Antioxidant and Hepatoprotective Activities of Polysaccharides from Taishan Pinus Massoniana Pollen. Molecules 2018, 23, 281. [Google Scholar] [CrossRef]
- Mi, S.; Yan, W.; Wei, L.; Xiong, X.; Tian, Y.; Lu, Q.; Mu, L. Structural Characterization and Hypoglycemic Activity of a Polysaccharide from Imperatae Rhizoma. Int. J. Biol. Macromol. 2025, 308, 142654. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Z.; Wang, C.; Luo, Y.; Luo, Y.; Duan, M.; Liu, R. [Protective Effects of Different Extracts of Imperatae Rhizoma in Rats with Adriamycin Nephrosis and Influence on Expression of TGF-Β1, and NF-κB P65]. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2015, 38, 2342–2347. [Google Scholar]
- Jee, W.; Ko, H.M.; Park, D.-I.; Park, Y.-R.; Park, S.-M.; Kim, H.; Na, Y.-C.; Jung, J.H.; Jang, H.-J. Momordicae Semen Inhibits Migration and Induces Apoptotic Cell Death by Regulating C-Myc and CNOT2 in Human Pancreatic Cancer Cells. Sci. Rep. 2023, 13, 12800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-H.; Ju, X.-Y.; Wang, K.-W.; Chen, X.-J.; Sun, H.-X.; Cheng, K.-J. Structure Characterization, Antioxidant and Immunomodulatory Activities of Polysaccharide from Pteridium aquilinum (L.) Kuhn. Foods 2022, 11, 1834. [Google Scholar] [CrossRef]
- Shen, Q.; He, Z.; Ding, Y.; Sun, L. Effect of Different Drying Methods on the Quality and Nonvolatile Flavor Components of Oudemansiella Raphanipes. Foods 2023, 12, 676. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Lai, Z.; Hu, X.; Wang, L.; Wang, X.; Li, Z.; Gao, M.; Yang, Y.; Wang, Q.; et al. Effect of Monosaccharide Composition and Proportion on the Bioactivity of Polysaccharides: A Review. Int. J. Biol. Macromol. 2024, 254, 127955. [Google Scholar] [CrossRef]
- Zhong, W.; Yang, C.; Zhang, Y.; Liu, Y.; Yang, D. The Chemical Profiling and Anticancer Potential of Functional Polysaccharides from Flos Sophorae Immaturus. Molecules 2022, 27, 5978. [Google Scholar] [CrossRef]
- Yu, W.; Xiong, Y.; Liu, M.; Zeng, D.; Zhao, H.; Liu, J.; Lu, W. Structural Analysis and Attenuates Hyperuricemic Nephropathy of Dextran from the Imperata cylindrica Beauv. Var. Major (Nees) C. E. Hubb. Carbohydr. Polym. 2023, 317, 121064. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, L.; Wang, Y.; Wan, P.; Zhou, H. Basic Characterization, Antioxidant and Immunomodulatory Activities of Polysaccharides from Sea Buckthorn Leaves. Fitoterapia 2023, 169, 105592. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Marette, A. Potential Health Benefits of Combining Yogurt and Fruits Based on Their Probiotic and Prebiotic Properties. Adv. Nutr. 2017, 8, 155S–164S. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Shu, X.; Du, H.; Li, N.; Wang, J. Extraction, Characterization and Immunological Activity of Polysaccharides from Rhizoma Gastrodiae. Int. J. Mol. Sci. 2016, 17, 1011. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; Qian, J.; He, Y.; Zheng, J.; Lu, Z.; Xu, Z.; Shi, J. Structural and Immunological Activity Characterization of a Polysaccharide Isolated from Meretrix Meretrix Linnaeus. Mar. Drugs 2016, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, H.; Wang, X.; Wu, S.; Wang, Y.; Wang, C.; Zhang, Y.; Zhao, H. Unraveling the Web of Defense: The Crucial Role of Polysaccharides in Immunity. Front. Immunol. 2024, 15, 1406213. [Google Scholar] [CrossRef] [PubMed]
- Sha, A.; Li, Y. Preparation, Structural Characterization, Bioactivities, and Potential Clinical Applications of the Polysaccharides from Paris Polyphylla: A Review. Front. Pharmacol. 2025, 16, 1539237. [Google Scholar] [CrossRef]
- Li, J.H.; Zhu, Y.Y.; Gu, F.T.; Wu, J.Y. Efficient Isolation of Immunostimulatory Polysaccharides from Lentinula Edodes by Autoclaving-Ultrasonication Extraction and Fractional Precipitation. Int. J. Biol. Macromol. 2023, 237, 124216. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Tan, H.-Y.; Li, S.; Feng, Y. Protective Actions of Acidic Hydrolysates of Polysaccharide Extracted from Mactra Veneriformis against Chemical-Induced Acute Liver Damage. Front. Pharmacol. 2020, 11, 446. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Sheng, L.; Zhang, D.; Zheng, X.; Pan, Y.; Yu, X.; Liang, X.; Wang, Q.; Wang, B.; et al. Effect of Ultrasonic Degradation on the Structural Feature, Physicochemical Property and Bioactivity of Plant and Microbial Polysaccharides: A Review. Int. J. Biol. Macromol. 2023, 236, 123924. [Google Scholar] [CrossRef]
- Yin, F.; Lin, P.; Yu, W.-Q.; Shen, N.; Li, Y.; Guo, S.-D. The Cordyceps Militaris-Derived Polysaccharide CM1 Alleviates Atherosclerosis in LDLR(-/-) Mice by Improving Hyperlipidemia. Front. Mol. Biosci. 2021, 8, 783807. [Google Scholar] [CrossRef]
- Kwansang, J.; Chen, C.-J.; Chaiprateep, E.-O. Optimization of Water-Based Ultrasonic-Microwave Assisted Extraction (UMAE) of Bioactive Compounds from Garcinia Mangostana Pericarp. J. Complement. Integr. Med. 2022, 19, 225–231. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Su, J.; Liang, G.; Tan, S.; Bi, Y.; Kong, F.; Wang, Z. Extraction of Pithecellobium Clypearia Benth Polysaccharides by Dual-Frequency Ultrasound-Assisted Extraction: Structural Characterization, Antioxidant, Hypoglycemic and Anti-Hyperlipidemic Activities. Ultrason. Sonochem. 2024, 107, 106918. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Q.; Lv, B.; Li, N.; Bian, X.; Chen, L.; Kong, M.; Shen, Y.; Zheng, W.; Zhang, J.; et al. Ganoderma Lucidum Polysaccharide Supplementation Significantly Activates T-Cell-Mediated Antitumor Immunity and Enhances Anti-PD-1 Immunotherapy Efficacy in Colorectal Cancer. J. Agric. Food Chem. 2024, 72, 12072–12082. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chen, L.; Huang, X.; Xiao, F.; Fu, L.; Jing, D.; Wang, J.; Zhang, H.; Sun, L.; Wu, Y. Structural Characteristics of Polysaccharide GP2a in Gardenia Jasminoides and Its Immunomodulatory Effect on Macrophages. Int. J. Mol. Sci. 2022, 23, 11279. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, Z.; Arifeen, M.Z.U.; Xue, Y.; Yuan, S.; Liu, C. Structure and Bioactivity of Polysaccharide from a Subseafloor Strain of Schizophyllum Commune 20R-7-F01. Int. J. Biol. Macromol. 2022, 222, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jia, Y.; Xue, Z.; Li, N.; Liu, J.; Chen, H. Recent Developments in Inonotus Obliquus (Chaga mushroom) Polysaccharides: Isolation, Structural Characteristics, Biological Activities and Application. Polymers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Liang, L.; Qiu, H.; Liu, Y.; Liu, Y.; Weng, L.; Zhong, W.; Meng, F. Exploring the Potential of Ume-Derived Proanthocyanidins: Novel Applications for Blueberry Preservation. Front. Microbiol. 2023, 14, 1265993. [Google Scholar] [CrossRef]
- Wang, L.; Lian, J.; Zheng, Q.; Wang, L.; Wang, Y.; Yang, D. Composition Analysis and Prebiotics Properties of Polysaccharides Extracted from Lepista Sordida Submerged Cultivation Mycelium. Front. Microbiol. 2022, 13, 1077322. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. Preparation, Structure and Activity of Polysaccharide Phosphate Esters. Biomed. Pharmacother. 2021, 144, 112332. [Google Scholar] [CrossRef]
- Qiu, Y.; Batool, Z.; Liu, R.; Sui, G.; Sheng, B.; Zheng, X.; Xu, D. Characterization and immunological activity of polysaccharides from Potentilla chinensis. Int. J. Biol. Macromol. 2020, 165, 683–690. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Wen, C.; Zhang, J.; He, Y.; Ma, H.; Duan, Y. Purification, Characterization, Antioxidant and Immunological Activity of Polysaccharide from Sagittaria sagittifolia L. Food Res. Int. 2020, 136, 109345. [Google Scholar] [CrossRef]
- Hao, W.; Wang, S.; Zhao, J.; Li, S. Effects of Extraction Methods on Immunology Activity and Chemical Profiles of Lycium barbarum Polysaccharides. J. Pharm. Biomed. Anal. 2020, 185, 113219. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Zhao, C.; Fu, X. Physicochemical Characterization, Antioxidative and Immunoregulatory Activity of Polysaccharides from the Flower of Hylocereus undatus (Haw.) Britton et Rose. Int. J. Biol. Macromol. 2023, 251, 126408. [Google Scholar] [CrossRef]
| Man | Rha | GlcA | GalA | Glc | Gal | Xyl | Rib | Fuc | |
|---|---|---|---|---|---|---|---|---|---|
| IRPs-H1 (%) | 3.18 | 1.42 | - | 1.47 | 46.43 | 21.29 | 25.41 | - | 0.57 |
| IRPs-H2 (%) | 1.61 | 3.74 | - | - | 34.75 | 5.8 | 42.88 | 6.54 | 4.53 |
| IRPs-H3 (%) | 0.47 | 16.85 | 3.11 | - | 48.01 | 4.05 | 17.04 | 2.33 | 8.13 |
| Polysaccharides | Peak | Time (min) | Mw (kDa) |
|---|---|---|---|
| IRPs-H1 | 1 | 14.094 | 6.9 |
| 2 | 17.231 | 0.3 | |
| IRPs-H2 | 1 | 11.742 | 66.55 |
| 2 | 14.114 | 6.8 | |
| 3 | 17.233 | 0.3 | |
| IRPs-H3 | 1 | 10.542 | 210.7 |
| 2 | 13.749 | 9.2 | |
| 3 | 14.87 | 3.3 | |
| 4 | 17.239 | 0.3 |
| Time (min) | A% | B% | Peak |
|---|---|---|---|
| 0 | 13 | 87 | 1 |
| 0.5 | 13 | 87 | 2 |
| 2 | 16 | 84 | 1 |
| 15 | 21 | 79 | 2 |
| 16 | 60 | 40 | 3 |
| 17 | 90 | 10 | 1 |
| 17.1 | 13 | 87 | 2 |
| 20 | 13 | 87 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.-G.; Chen, J.-J.; Xu, J.-M.; Chen, W.; Chen, X.-B.; Yang, D.-S. The Chemical Profiling and Immunological Activity of Polysaccharides from the Rhizome of Imperata cylindrica Using Hot Water Extraction. Molecules 2025, 30, 2635. https://doi.org/10.3390/molecules30122635
Sun M-G, Chen J-J, Xu J-M, Chen W, Chen X-B, Yang D-S. The Chemical Profiling and Immunological Activity of Polysaccharides from the Rhizome of Imperata cylindrica Using Hot Water Extraction. Molecules. 2025; 30(12):2635. https://doi.org/10.3390/molecules30122635
Chicago/Turabian StyleSun, Meng-Ge, Jia-Jie Chen, Jia-Min Xu, Wei Chen, Xiao-Bing Chen, and Dong-Sheng Yang. 2025. "The Chemical Profiling and Immunological Activity of Polysaccharides from the Rhizome of Imperata cylindrica Using Hot Water Extraction" Molecules 30, no. 12: 2635. https://doi.org/10.3390/molecules30122635
APA StyleSun, M.-G., Chen, J.-J., Xu, J.-M., Chen, W., Chen, X.-B., & Yang, D.-S. (2025). The Chemical Profiling and Immunological Activity of Polysaccharides from the Rhizome of Imperata cylindrica Using Hot Water Extraction. Molecules, 30(12), 2635. https://doi.org/10.3390/molecules30122635






