Chemical Composition and Biological Activities of Pelargonium sp.: A Review with In Silico Insights into Potential Anti-Inflammatory Mechanism
Abstract
1. Introduction
2. Results
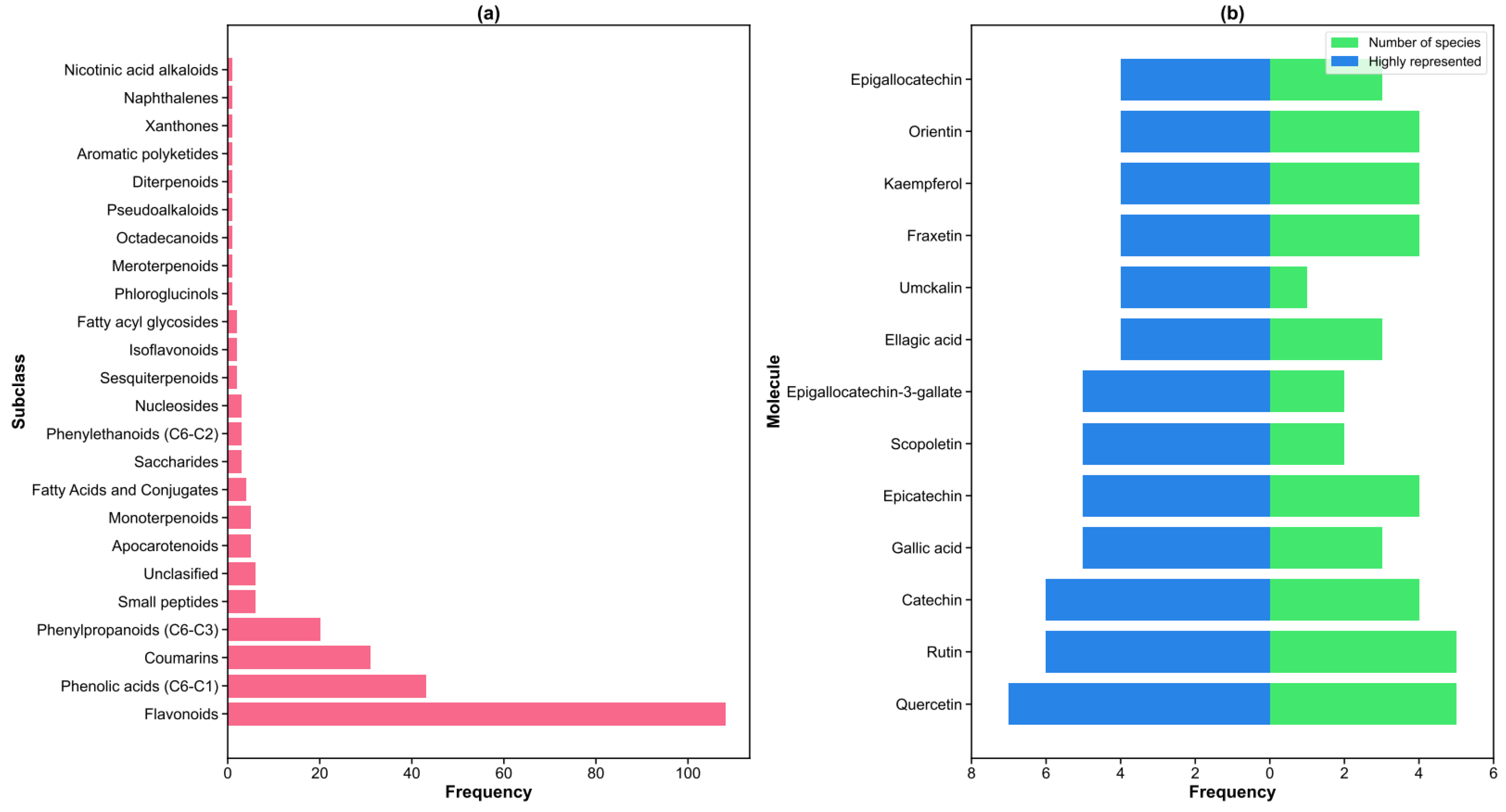
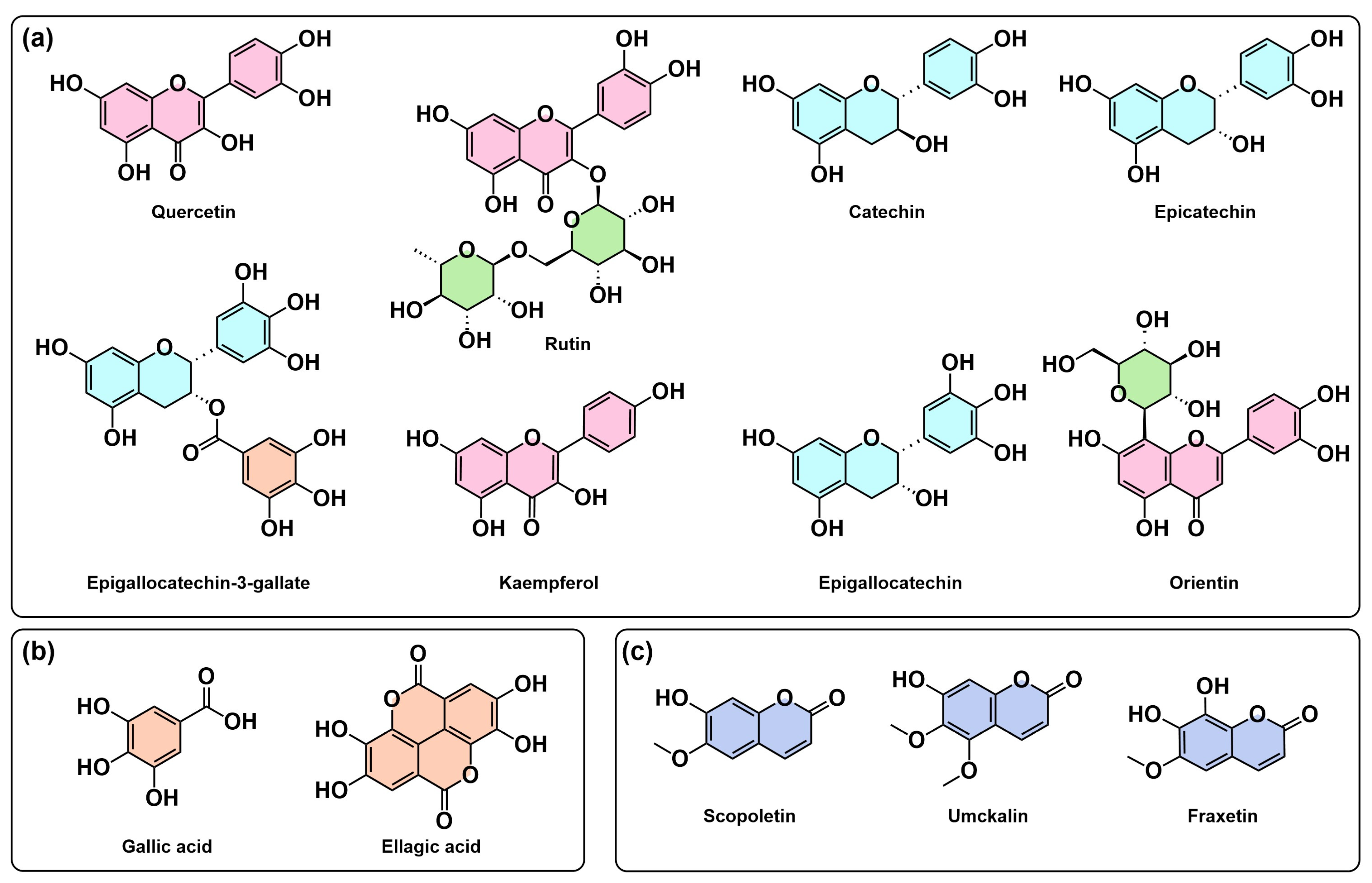
2.1. Chemical Composition
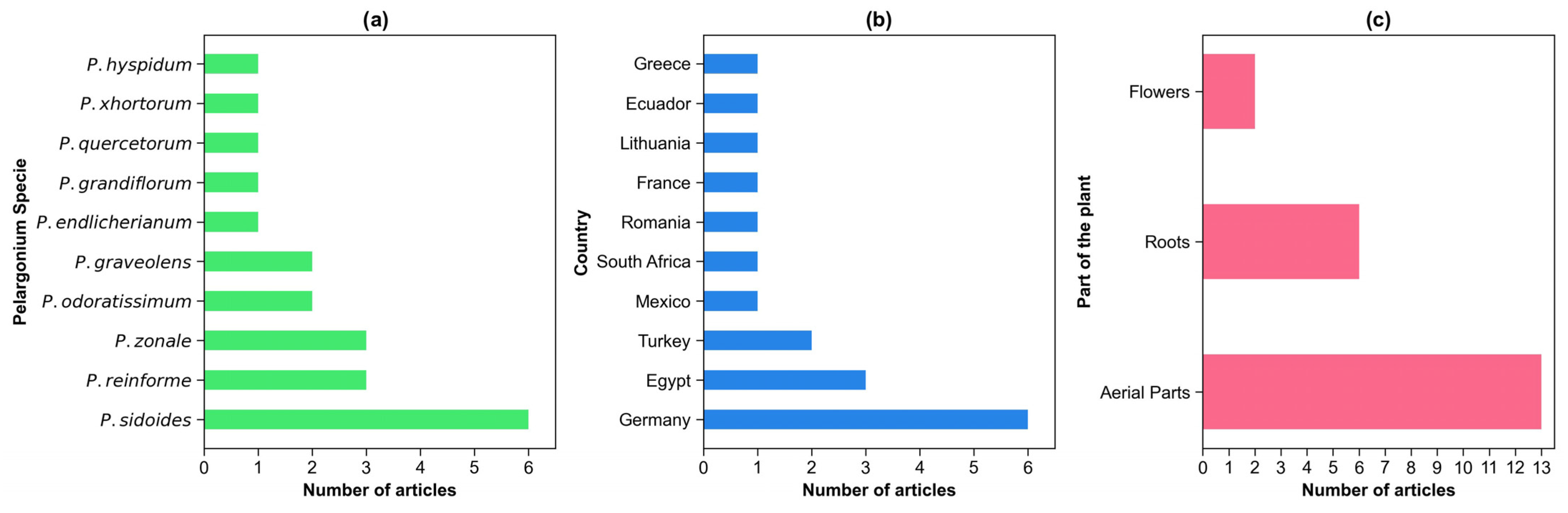
2.2. Distribution of Studies on Pelargonium Species, Countries, and Plant Parts
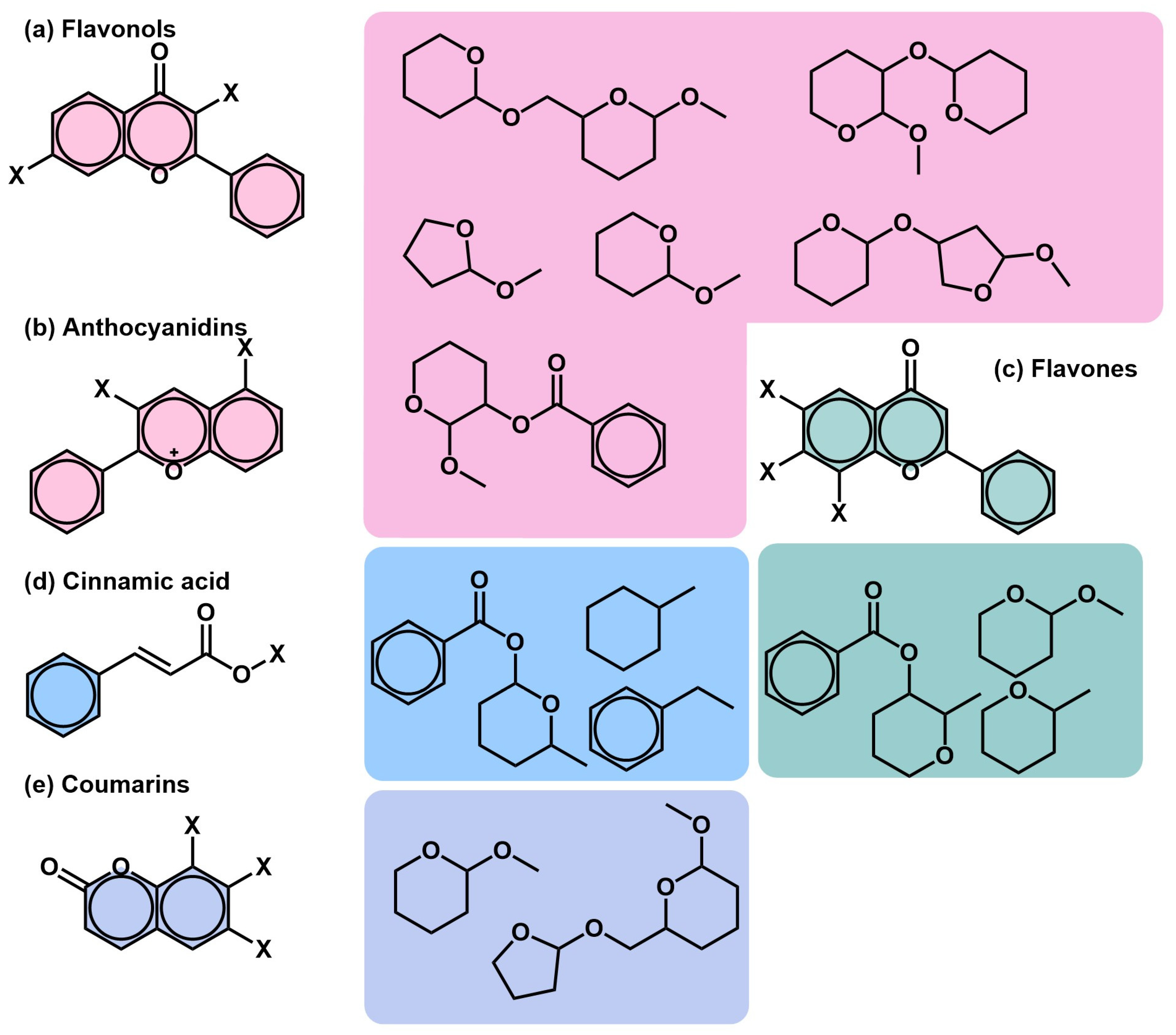
2.3. Analytical Techniques and Chemical Diversity
2.4. Biological Activities from Literature Analysis
2.5. In Silico Analysis
3. Discussion
4. Materials and Methods
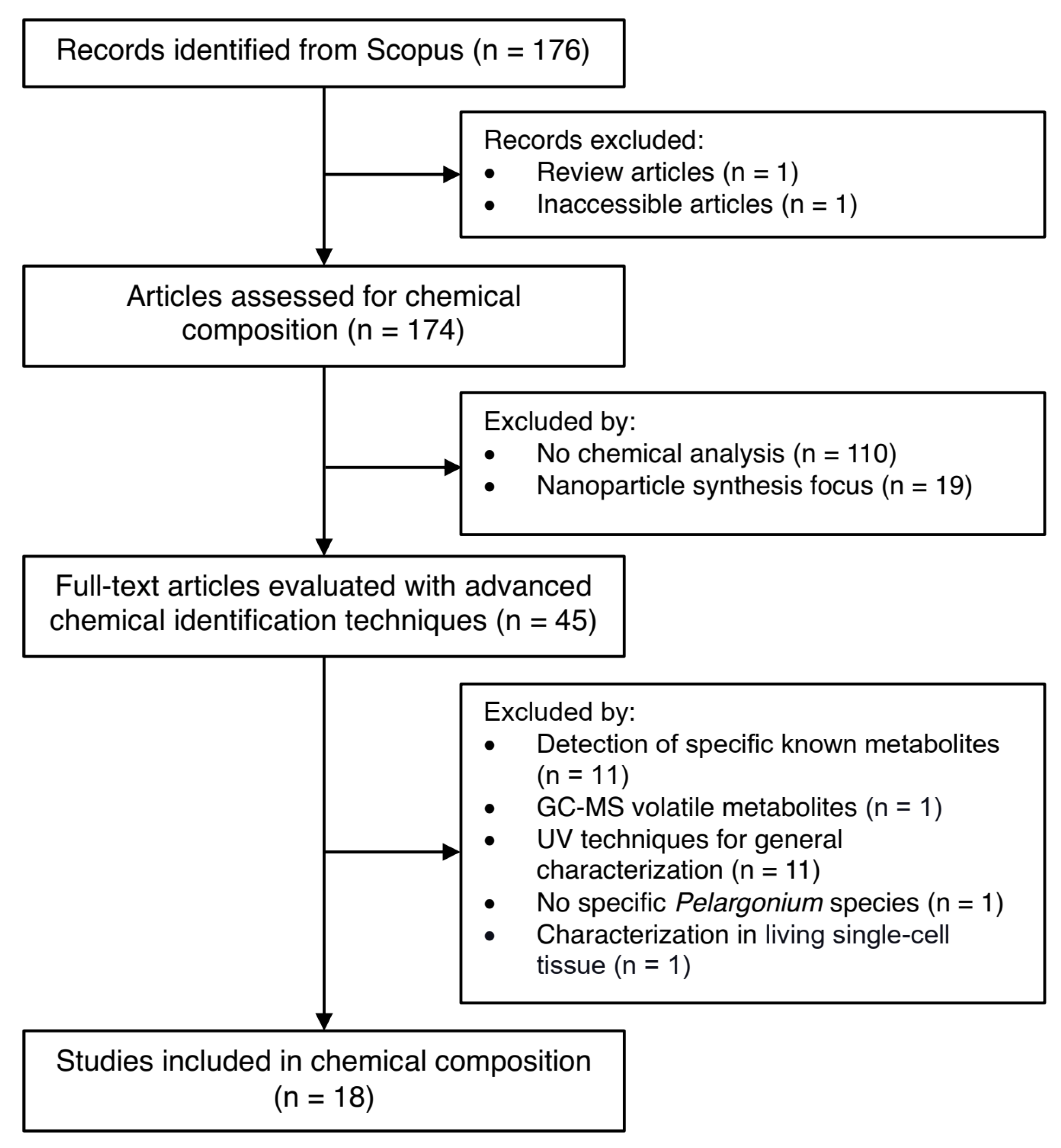
4.1. Literature Search for Chemical Composition Studies
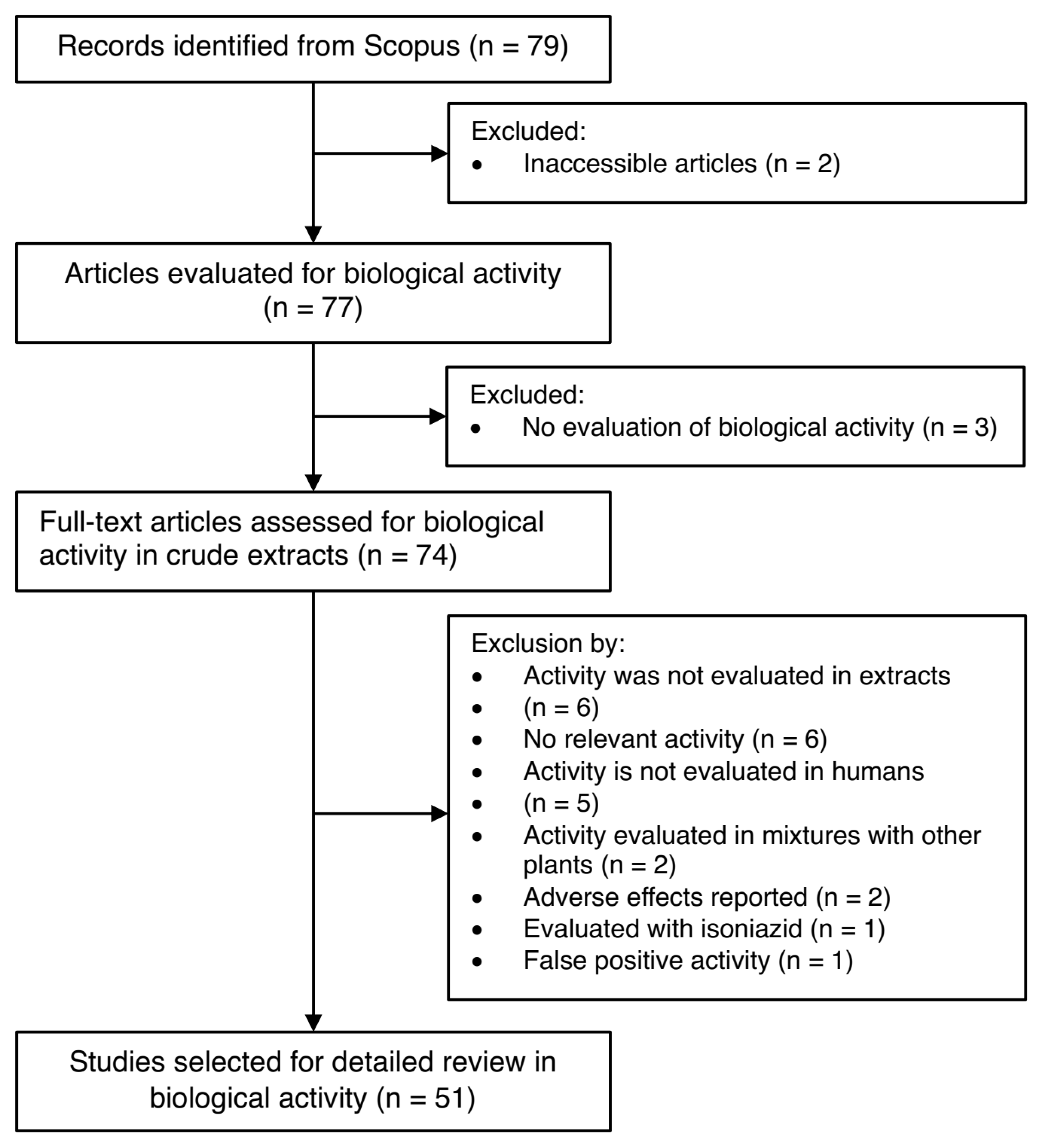
4.2. Literature Search for Biological Activity Studies
4.3. Chemical Data Curation and Molecular Classification
4.4. Compounds–Target Protein Interaction Prediction and Analysis
4.5. Functional Annotation of Targets and Biological Process Analysis
5. Conclusions
6. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brendler, T.; van Wyk, B.-E. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae). J. Ethnopharmacol. 2008, 119, 420–433. [Google Scholar] [CrossRef]
- Manning, J.C.; Strlič, M. Pelargonium elizabethiae and P. geophyllum, two new species in section Hoarea (Geraniaceae) from the Greater Cape Floristic Region, South Africa. S. Afr. J. Bot. 2024, 167, 301–309. [Google Scholar] [CrossRef]
- van der Walt, J.J.A. A taxonomic revision of the type section of Pelargonium L’Hérit. (Geraniaceae). Bothalia 1985, 15, 345–385. [Google Scholar] [CrossRef]
- Kolodziej, H. Antimicrobial, antiviral and immunomodulatory activity studies of Pelargonium sidoides (EPs® 7630) in the context of health promotion. Pharmaceuticals 2011, 4, 1295–1314. [Google Scholar] [CrossRef]
- van Wyk, B.-E.; Gericke, N. People’s Plants: A Guide to Useful Plants of Southern Africa, 1st ed.; Briza Publications: Pretoria, South Africa, 2000; ISBN 1875093192. [Google Scholar]
- Mativandlela, S.P.N.; Lall, N.; Meyer, J.J.M. Antibacterial, antifungal and antitubercular activity of (the roots of) Pelargonium reniforme (CURT) and Pelargonium sidoides (DC) (Geraniaceae) root extracts. S. Afr. J. Bot. 2006, 72, 232–237. [Google Scholar] [CrossRef]
- van Wyk, B.-E. A review of Khoi-San and Cape Dutch medical ethnobotany. J. Ethnopharmacol. 2008, 119, 331–341. [Google Scholar] [CrossRef]
- Sezik, E.; Yes, E. Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J. Ethnopharmacol. 2001, 75, 95–115. [Google Scholar] [CrossRef]
- Scott, G.; Springfield, E.P.; Coldrey, N. A Pharmacognostical study of 26 South African plant species used as traditional medicines. Pharm. Biol. 2004, 42, 186–213. [Google Scholar] [CrossRef]
- Demarne, F.E.; van der Walt, J.J.A. Pelargonium tomentosum: A potential source of peppermint-scented essential oil. S. Afr. J. Plant Soil 1990, 7, 36–39. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M. Antioxidant activity of essential oil and aqueous extract of Pelargonium graveolens L’Her. Food Control 2012, 23, 263–267. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Kameli, A.; Saidi, F. Essential oil of Algerian rose-scented geranium (Pelargonium graveolens): Chemical composition and antimicrobial activity against food spoilage pathogens. Food Control 2013, 34, 208–213. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Hamdi, N. Phytochemical composition and antimicrobial activities of the essential oils and organic extracts from Pelargonium graveolens growing in Tunisia. Lipids Health Dis. 2012, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H. Fascinating metabolic pools of Pelargonium sidoides and Pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine Umckaloabo®. Phytomedicine 2007, 14, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Ramírez, J.; Vidari, G. Poorly investigated ecuadorian medicinal plants. Plants 2022, 11, 1590. [Google Scholar] [CrossRef]
- Pietta, P.; Simonetti, P.; Mauri, P. Antioxidant Activity of Selected Medicinal Plants. J. Agric. Food Chem. 1998, 46, 4487–4490. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2014, 147, 59–110. [Google Scholar] [CrossRef]
- Van Wyngaard, J.; Famuyide, I.M.; Invernizzi, L.; Ndivhuwo, K.K.; Tordiffe, A.S.W.; Maharaj, V.J.; McGaw, L.J. Optimizing extraction of Pelargonium sidoides roots: Impact of ethanol concentration on biological activity of extracts. S. Afr. J. Bot. 2023, 162, 667–679. [Google Scholar] [CrossRef]
- Rezaizadehnajafi, L.; Wink, M. EPs7630® from Pelargonium sidoides increases stress resistance in Caenorhabditis elegans probably via the DAF-16/FOXO pathway. Phytomedicine 2014, 21, 547–550. [Google Scholar] [CrossRef]
- Lewu, F.B.; Grierson, D.S.; Afolayan, A.J. Extracts from Pelargonium sidoides inhibit the growth of bacteria and fungi. Pharm. Biol. 2006, 44, 279–282. [Google Scholar] [CrossRef]
- Samie, S.; Trollope, K.M.; Joubert, L.M.; Makunga, N.P.; Volschenk, H. The antifungal and Cryptococcus neoformans virulence attenuating activity of Pelargonium sidoides extracts. J. Ethnopharmacol. 2019, 235, 122–132. [Google Scholar] [CrossRef]
- Alossaimi, M.A.; Alzeer, M.A.; Abdel Bar, F.M.; ElNaggar, M.H. Pelargonium sidoides root extract: Simultaneous HPLC separation, determination, and validation of selected biomolecules and evaluation of SARS-CoV-2 inhibitory activity. Pharmaceuticals 2022, 15, 1184. [Google Scholar] [CrossRef]
- Lukman, V.; Odeyemi, S.W.; Roth, R.L.; Mbabala, L.; Tshililo, N.; Vlok, N.M.; Dewar, M.J.B.; Kenyon, C.P. Novel kinase platform for the validation of the anti-tubercular activities of Pelargonium sidoides (Geraniaceae). BMC Biotechnol. 2020, 20, 50. [Google Scholar] [CrossRef]
- Schnitzler, P.; Schneider, S.; Stintzing, F.C.; Carle, R.; Reichling, J. Efficacy of an aqueous Pelargonium sidoides extract against herpesvirus. Phytomedicine 2008, 15, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H.; Kayser, O.; Radtke, O.A.; Kiderlen, A.F.; Koch, E. Pharmacological profile of extracts of Pelargonium sidoides and their constituents. Phytomedicine 2003, 10, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Gao, Y.; Koch, E.; Pan, X.; Jin, Y.; Cui, X. Evaluation of pharmacodynamic activities of EPs® 7630, a special extract from roots of Pelargonium sidoides, in animals models of cough, secretolytic activity and acute bronchitis. Phytomedicine 2015, 22, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Janecki, A.; Conrad, A.; Engels, I.; Frank, U.; Kolodziej, H. Evaluation of an aqueous-ethanolic extract from Pelargonium sidoides (EPs® 7630) for its activity against group A-streptococci adhesion to human HEp-2 epithelial cells. J. Ethnopharmacol. 2011, 133, 147–152. [Google Scholar] [CrossRef]
- Conrad, A.; Hansmann, C.; Engels, I.; Daschner, F.D.; Frank, U. Extract of Pelargonium sidoides (EPs® 7630) improves phagocytosis, oxidative burst, and intracellular killing of human peripheral blood phagocytes in vitro. Phytomedicine 2007, 14, 46–51. [Google Scholar] [CrossRef]
- Trun, W.; Kiderlen, A.F.; Kolodziej, H. Nitric oxide synthase and cytokines gene expression analyses in Leishmania-infected RAW 264.7 cells treated with an extract of Pelargonium sidoides (Eps® 7630). Phytomedicine 2006, 13, 570–575. [Google Scholar] [CrossRef]
- Thäle, C.; Kiderlen, A.; Kolodziej, H. Anti-infective Activities of Pelargonium sidoides (EPS® 7630): Effects of Induced NO Production on Leishmania major in Infected Macrophages and Antiviral Effects as Assessed in a Fibroblast-Virus Protection Assay. Planta Med. 2011, 77, 718–725. [Google Scholar] [CrossRef]
- Mtimkulu, Y.; Lewu, M.N.; Mulidzi, A.R.; Lewu, F. Cultivation and beneficial uses of Pelargonium sidoides DC.—A review. J. Med. Plants Econ. Dev. 2024, 8, 246. [Google Scholar] [CrossRef]
- Zengin, G.; Leyva-Jiménez, F.J.; Fernández-Ochoa, Á.; Bouyahya, A.; Yildiztugay, E.; Carretero, A.S.; Mahomoodally, M.F.; Ponniya, S.K.M.; Nilofar; Koyuncu, I.; et al. UHPLC-ESI-QTOF-MS metabolite profiles of different extracts from Pelargonium endlicherianum parts and their biological properties based on network pharmacological approaches. Arch. Pharm. 2024, 357, e2300728. [Google Scholar] [CrossRef]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Yumrutas, O.; Sokmen, A. Screening of antioxidative properties of the methanolic extracts of Pelargonium endlicherianum Fenzl., Verbascum wiedemannianum Fisch. & Mey., Sideritis libanotica Labill. subsp. linearis (Bentham) Borm., Centaurea mucronifera DC. and Hieracium cappadocicum Freyn from Turkish flora. Food Chem. 2006, 98, 9–13. [Google Scholar] [CrossRef]
- Ozbilge, H.; Kaya, E.G.; Taskin, O.M.; Kosar, M. Antimicrobial activity of Pelargonium endlicherianum Fenzl. (Geraniaceae) roots against some microorganisms. J. Med. Plants Res. 2010, 4, 2647–2650. [Google Scholar] [CrossRef][Green Version]
- Cumaoğlu, A.; Karatoprak, G.Ş.; Yerer, M.B.; Koşar, M. Anti-inflammatory effects of Pelargonium endlicherianum Fenzl. extracts in lipopolysaccharide-stimulated macrophages. Turk. J. Pharm. Sci. 2018, 15, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kozan, E.; Küpeli Akkol, E.; Süntar, I. Potential anthelmintic activity of Pelargonium endlicherianum Fenzl. J. Ethnopharmacol. 2016, 187, 183–186. [Google Scholar] [CrossRef]
- Karatoprak, G.Ş.; Göger, F.; Yerer, M.B.; Koşar, M. Chemical composition and biological investigation of Pelargonium endlicherianum root extracts. Pharm. Biol. 2017, 55, 1608–1618. [Google Scholar] [CrossRef]
- Salem, M.A.; Radwan, R.A.; Mostafa, E.S.; Alseekh, S.; Fernie, A.R.; Ezzat, S.M. Using an UPLC/MS-based untargeted metabolomics approach for assessing the antioxidant capacity and anti-aging potential of selected herbs. RSC Adv. 2020, 10, 31511–31524. [Google Scholar] [CrossRef]
- Neagu, A.F.; Costea, T.; Nencu, I.; Duţu, L.E.; Popescu, M.L.; Olaru, O.T.; Gîrd, C.E. Obtaining and characterization of a selective Pelargonium graveolens L’Hér. Dry extract with potential therapeutic activity in metabolic diseases. Farmacia 2018, 66, 592–596. [Google Scholar] [CrossRef]
- El Ouadi, Y.; Bendaif, H.; Mrabti, H.N.; Elmsellem, H.; Kadmi, Y.; Shariati, M.A.; Abdel-Rahman, I.; Hammouti, B.; Bouyanzer, A. Antioxidant activity of phenols and flavonoids contents of aqueous extract of Pelargonium graveolens orgin in the North-East Morocco. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1218–1220. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Martiskainen, O.; Seif el-Din, S.H.; Sabra, A.-N.A.; Hammam, O.A.; El-Lakkany, N.M. Protective effect of Pelargonium graveolens against carbon tetrachloride-induced hepatotoxicity in mice and characterization of its bioactive constituents by HPLC–PDA–ESI–MS/MS analysis. Med. Chem. Res. 2015, 24, 1438–1448. [Google Scholar] [CrossRef]
- Saraswathi, J.; Prem, K.; Venkatesh, K.; Chakrapani, P.; Arun, J.B.; Amarashwari, P.; Sudhakar, C.; Roja Rani, A. Evalution of qualitative analysis and antibacterial activity of Pelargonium graveolens L’Herit. Int. J. Phytomed. 2016, 8, 58–61. [Google Scholar]
- Pradeepa, M.; Kalidas, V.; Geetha, N. Qualitative and quantitative phytochemical analysis and bactericidal activity of Pelargonium graveolens L’Her. Int. J. Appl. Pharm. 2016, 8, 7–11. [Google Scholar]
- Mahboubi, M.; Kazempour, N.; Farzin, N. Antimicrobial activity of Pelargonium graveolens and Oliveria decumbens extracts against clinical isolates of Staphylococcus aureus. J. Biol. Act. Prod. Nat. 2011, 1, 105–111. [Google Scholar] [CrossRef]
- Bayoub, K.; Baibai, T.; Mountassif, D.; Retmane, A.; Soukri, A. Antibacterial activities of the crude ethanol extracts of medicinal plants against Listeria monocytogenes and some other pathogenic strains. Afr. J. Biotechnol. 2010, 9, 4251–4258. [Google Scholar]
- Amel, H.A.; Kamel, H.; Meriem, F.; Abdelkader, K. Traditional Uses, Botany, Phytochemistry, and Pharmacology of Pelargonium graveolens: A Comprehensive Review. Trop. J. Nat. Prod. Res. 2022, 6, 1547–1569. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Ramezanloo, F. An overview on phytopharmacology of Pelargonium graveolens L. Indian J. Tradit. Knowl. 2015, 14, 558–563. [Google Scholar]
- Ben Hsouna, A.; Chahdoura, H.; Generalić Mekinić, I.; Maisto, M.; Kukula-Koch, W.; Ćavar Zeljković, S.; Koch, W.; Ben Akacha, B.; Taieb Bouteraa, M.; Ben Belgacem, A.; et al. A comprehensive review on traditional uses, chemical composition, pharmacological effects and applications in the food industry of Pelargonium odoratissimum (L.) L’Hér. in comparison to other Pelargonium spp. S. Afr. J. Bot. 2024, 174, 456–467. [Google Scholar] [CrossRef]
- Andreou, E.; Triantafyllou, A.K.; Mountsaki, S.; Rallis, E.; Lamari, F.N.; Hatziantoniou, S.; Kefala, V. Permanent make-up (PMU) inks decolorization using plant origin materials. Cosmetics 2022, 9, 48. [Google Scholar] [CrossRef]
- Iancu, C.; Cioancă, O.; Mircea, C.; Mocanu, M.; Hăncianu, M. Pelargonium sp.: Characterization of the polyphenols and their biological potential. Farmacia 2016, 64, 333–338. [Google Scholar]
- Iancu, C.; Filip, N.; Mocanu, M.; Dehelean, C.A.; Danciu, C.; Cioancă, O.; Hăncianu, M.; Corciovă, A.; Burlec, F.; Mircea, C.; et al. Antioxidant and anticancer effect of some Pelargonium species extracts. Farmacia 2023, 71, 312–321. [Google Scholar] [CrossRef]
- Onduru Okeyo, G.; Charimbu, M.K.; Nyaanga, J.; Mendes, T. Antibacterial activity of guava, moringa, camphor bush and pelargonium extracts against bacterial wilt (Ralstonia pseudosolanacearum sp. nov.) of potato. Saudi J. Biol. Sci. 2022, 29, 103438. [Google Scholar] [CrossRef]
- Iancu, C.; Cioancă, O.; Gaiddon, C.; Mircea, C.; Munteanu, A.; Filip, N.; Hanganu, B.; Manoilescu, I.; Hăncianu, M. Cytoprotective and antiinflamatory activity evaluation of some Pelargonium extracts. Farmacia 2017, 65, 891–895. [Google Scholar]
- Naz, R.; Saqib, F.; Latif, M.F.; Sajer, B.H.; Misarca, C.; Toma, S.I.; Podasca, P.C.; Andreescu, O. Pharmacodynamic Elucidation of Pelargonium zonale (L.) L’Hér. ex Aiton for its Folkloric Claims in Diarrhea and Asthma via in Vitro, in Vivo and in Silico Methods. Nat. Prod. Commun. 2025, 20, 1–16. [Google Scholar] [CrossRef]
- Páez, X.; Hernández, L. Topical hemostatic effect of a common ornamental plant, the geraniaceae Pelargonium zonale. J. Clin. Pharmacol. 2003, 43, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Adewusi, E.A.; Afolayan, A.J. Effect of Pelargonium reniforme roots on alcohol-induced liver damage and oxidative stress. Pharm. Biol. 2010, 48, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Adewusi, E.A.; Afolayan, A.J. Antibacterial, antifungal and antioxidant activity of the roots and leaves of Pelargonium reniforme Curtis (Geraniaceae). Afr. J. Biotechnol. 2009, 8, 6425–6433. [Google Scholar] [CrossRef][Green Version]
- Kim, C.E.; Griffiths, W.J.; Taylor, P.W. Components derived from Pelargonium stimulate macrophage killing of Mycobacterium species. J. Appl. Microbiol. 2009, 106, 1184–1193. [Google Scholar] [CrossRef]
- Kolodziej, H.; Kiderlen, A.F. In vitro evaluation of antibacterial and immunomodulatory activities of Pelargonium reniforme, Pelargonium sidoides and the related herbal drug preparation EPs® 7630. Phytomedicine 2007, 14, 18–26. [Google Scholar] [CrossRef]
- Iancu, C.; Mircea, C.; Pietrariu, F.; Cioancă, O.; Stan, C.; Corciovă, A.; Murărașu, A.; Filip, N.; Hăncianu, M. The evaluation of normo-glycemic and cyto-regenerative effects of Pelargonium species extracts. Farmacia 2020, 68, 135–141. [Google Scholar] [CrossRef]
- Coronado-López, S.; Caballero-García, S.; Aguilar-Luis, M.A.; Mazulis, F.; del Valle-Mendoza, J. Antibacterial activity and cytotoxic effect of Pelargonium peltatum (Geranium) against Streptococcus mutans and Streptococcus sanguinis. Int. J. Dent. 2018, 2018, 2714350. [Google Scholar] [CrossRef]
- Guerrero, J.; Ortiz, Z.; Peralta, L.; Pérez, F. Antibacterial activity of Pelargonium peltatum (L.) L’Her. against Streptococcus mutans, Streptococcus sanguis and Streptococcus mitis versus chlorhexidine. Rev. Cuba. Plantas Med. 2013, 18, 224–236. [Google Scholar]
- Rungqu, P.; Oyedeji, O.; Gondwe, M.; Oyedeji, A. Chemical Composition, Analgesic and Anti-Inflammatory Activity of Pelargonium peltatum Essential Oils from Eastern Cape, South Africa. Molecules 2023, 28, 5294. [Google Scholar] [CrossRef]
- Maroufi, Y.; Hoseini, S.R.; Alavi, M. Antiparasitic effect of leaf extract and major metabolites of Pelargonium quercetorum Agnew. against Leishmania Major: In Vitro and In Silico studies. J. Appl. Biotechnol. Rep. 2022, 9, 817–830. [Google Scholar] [CrossRef]
- Aztopal, N.; Cevatemre, B.; Sarimahmut, M.; Ari, F.; Dere, E.; Ozel, M.Z.; Firat, M.; Ulukaya, E. Pelargonium quercetorum Agnew induces apoptosis without PARP or cytokeratin 18 cleavage in non-small cell lung cancer cell lines. Oncol. Lett. 2016, 12, 1429–1437. [Google Scholar] [CrossRef]
- Cock, I.E.; Orchard, A.; Booi, L.; van Vuuren, S.F. Southern African traditional herbal medicinal plants used to treat cardiovascular disease and related medical conditions: Traditional use and scientific evidence. S. Afr. J. Bot. 2024, 173, 1–18. [Google Scholar] [CrossRef]
- Pranskuniene, Z.; Dauliute, R.; Pranskunas, A.; Bernatoniene, J. Ethnopharmaceutical knowledge in Samogitia region of Lithuania: Where old traditions overlap with modern medicine. J. Ethnobiol. Ethnomed. 2018, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Kaval, I.; Behçet, L.; Cakilcioglu, U. Ethnobotanical study on medicinal plants in Geçitli and its surrounding (Hakkari-Turkey). J. Ethnopharmacol. 2014, 155, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Klepacki, P.; Łuczaj, Ł. Fischer’s Plants in folk beliefs and customs: A previously unknown contribution to the ethnobotany of the Polish-Lithuanian-Belarusian borderland. J. Ethnobiol. Ethnomed. 2017, 13, 20. [Google Scholar] [CrossRef]
- Mbhele, N.; Ncube, B.; Ndhlala, A.R.; Moteetee, A. Pro-inflammatory enzyme inhibition and antioxidant activity of six scientifically unexplored indigenous plants traditionally used in South Africa to treat wounds. S. Afr. J. Bot. 2022, 147, 119–129. [Google Scholar] [CrossRef]
- Koutelidakis, A.E.; Serafini, M.; Komaitis, M.; Kapsokefalou, M. Oxidative activity of some iron compounds on colon tissue homogenates from mice after administration of green tea, white tea and Pelargonium purpureum. Food Chem. 2010, 120, 895–901. [Google Scholar] [CrossRef]
- Fernandez-Soto, P.; Celi, D.; Tejera, E.; Alvarez-Suarez, J.M.; Machado, A. Cinnamomum sp. and Pelargonium odoratissimum as the main contributors to the antibacterial activity of the medicinal drink horchata: A study based on the antibacterial and chemical analysis of 21 plants. Molecules 2023, 28, 693. [Google Scholar] [CrossRef]
- Izuegbuna, O.; Otunola, G.; Bradley, G. Estimation of phytochemical, vitamins composition and antioxidant activity of Pelargonium inquinans leaves. Pharmacogn. J. 2019, 11, 237–244. [Google Scholar] [CrossRef]
- Tembeni, B.; Oyedeji, O.O.; Manene, C.N.; Oyemitan, I.A.; Oyedeji, A.O. Anti-inflammatory, Analgesic Activity and Toxicity of Two Pelargonium inquinans Ait Essential Oils: Wild and Cultivated. J. Essent. Oil-Bear. Plants 2019, 22, 1252–1264. [Google Scholar] [CrossRef]
- Petlevski, R.; Flajs, D.; Kalodera, Z.; Zovko Končić, M. Composition and antioxidant activity of aqueous and ethanolic Pelargonium radula extracts. S. Afr. J. Bot. 2013, 85, 17–22. [Google Scholar] [CrossRef]
- Pepeljnjak, S.; Kalodera, Z.; Zovko, M. Investigation of antimicrobial activity of Pelargonium radula (Cav.) L’Hérit. Acta Pharm. 2005, 55, 409–415. [Google Scholar]
- Fayoumi, L.; Khalil, M.; Ghareeb, D.; El-Dakdouki, M.H. Chemical composition and therapeutic activity of lebanese rose geranium (Pelargonium hybrid) extracts. Farmacia 2022, 70, 477–490. [Google Scholar] [CrossRef]
- Jantová, S.; Nagy, M.; Ružeková, Ĺ.; Grančai, D. Antibacterial activity of plant extracts from the families Fabaceae, Oleaceae, Philadelphaceae, Rosaceae and Staphyleaceae. Phytother. Res. 2000, 14, 601–603. [Google Scholar] [CrossRef]
- Beltrán-Noboa, A.; Jordan-Álvarez, A.; Guevara-Terán, M.; Gallo, B.; Berrueta, L.A.; Giampieri, F.; Battino, M.; Álvarez-Suarez, J.M.; Tejera, E. Exploring the chemistry of Ocimum species under specific extractions and chromatographic methods: A systematic review. ACS Omega 2023, 8, 10747–10756. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M.H.A. Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-inflammatory activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Alqahtani, A.A.; El Raey, M.A.; Abdelsalam, E.; Ibrahim, A.M.; Alqahtani, O.; Torky, Z.A.; Attia, H.G. The biosynthesized zinc oxide nanoparticles’ antiviral activity in combination with Pelargonium zonale extract against the human Corona 229E virus. Molecules 2022, 27, 8362. [Google Scholar] [CrossRef]
- Venter, A.; Fisher, H.; Stafford, G.I.; Duodu, K.G. Pigmented flower extracts of plant species from the Geraniaceae and Lamiaceae families as natural food colourants: Anthocyanin composition, thermal and oxidative stability. Int. J. Food Sci. Technol. 2022, 57, 4347–4355. [Google Scholar] [CrossRef]
- Williams, C.A.; Newman, M.; Gibby, M. The application of leaf phenolic evidence for systematic studies within the genus Pelargonium (Geraniaceae). Biochem. Syst. Ecol. 2000, 28, 119–132. [Google Scholar] [CrossRef]
- Judge, M.T.; Ebbels, T.M.D. Problems, principles and progress in computational annotation of NMR metabolomics data. Metabolomics 2022, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Chaleckis, R.; Meister, I.; Zhang, P.; Wheelock, C.E. Challenges, progress and promises of metabolite annotation for LC–MS-based metabolomics. Curr. Opin. Biotechnol. 2019, 55, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P.; Lewis II, J.S.; Bobenchik, A.M.; Campeau, S.; Cullen, S.K.; Galas, M.F.; Gold, H.; Humphries, R.M.; Kirn, T.J., Jr.; Limbago, B.; et al. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; ISBN 978-1-68440-066-9. [Google Scholar]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef]
- Aoac International. AOAC SMPR 2011.011 Standard Method Performance Requirements for in vitro Determination of Total Antioxidant Activity in Foods, Beverages, Food Ingredients, and Dietary Supplements. J. AOAC Int. 2012, 95, 1557. [Google Scholar] [CrossRef]
- Scott, K.A.; Ropek, N.; Melillo, B.; Schreiber, S.L.; Cravatt, B.F.; Vinogradova, E.V. Stereochemical diversity as a source of discovery in chemical biology. Curr. Res. Chem. Biol. 2022, 2, 100028. [Google Scholar] [CrossRef]
- Careddu, D.; Pettenazzo, A. Pelargonium sidoides extract EPs 7630: A review of its clinical efficacy and safety for treating acute respiratory tract infections in children. Int. J. Gen. Med. 2018, 11, 91–98. [Google Scholar] [CrossRef]
- Moyo, M.; Van Staden, J. Medicinal properties and conservation of Pelargonium sidoides DC. J. Ethnopharmacol. 2014, 152, 243–255. [Google Scholar] [CrossRef]
- Wopker, P.M.; Schwermer, M.; Sommer, S.; Längler, A.; Fetz, K.; Ostermann, T.; Zuzak, T.J. Complementary and alternative medicine in the treatment of acute bronchitis in children: A systematic review. Complement. Ther. Med. 2020, 49, 102217. [Google Scholar] [CrossRef]
- Nöldner, M.; Schötz, K. Inhibition of lipopolysaccharide-induced sickness behavior by a dry extract from the roots of Pelargonium sidoides (EPs® 7630) in mice. Phytomedicine 2007, 14, 27–31. [Google Scholar] [CrossRef]
- Duarte, J.; Francisco, V.; Perez-Vizcaino, F. Modulation of nitric oxide by flavonoids. Food Funct. 2014, 5, 1653–1668. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, G.H. Evaluation of antioxidant and inhibitory activities for different subclasses flavonoids on enzymes for rheumatoid arthritis. J. Food Sci. 2010, 75, H212–H217. [Google Scholar] [CrossRef]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: Structure-activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, S.; Lee, W.; Hou, W.; Yang, L.; Lee, T.J.F. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expressions by rutin, quercetin, and quercetin pentaacetate in RAW 264.7 macrophages. J. Cell Biochem. 2001, 82, 537–548. [Google Scholar] [CrossRef]
- Maldonado-Rojas, W.; Olivero-Verbel, J. Food-related compounds that modulate expression of inducible nitric oxide synthase may act as its inhibitors. Molecules 2012, 17, 8118–8135. [Google Scholar] [CrossRef] [PubMed]
- Larit, F.; León, F.; Benyahia, S.; Cutler, S.J. Total phenolic and flavonoid content and biological activities of extracts and isolated compounds of Cytisus villosus pourr. Biomolecules 2019, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W.; Murugan, D.; Leong, X.-F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear Factor-Kappa B (NFκB) signaling in cardiovascular diseases: A Mini Review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef]
- Sudarshan, K.; Aidhen, I.S. Convenient Synthesis of 3-Glycosylated Isocoumarins. Eur. J. Org. Chem. 2017, 2017, 34–38. [Google Scholar] [CrossRef]
- Khodzhaieva, R.S.; Gladkov, E.S.; Kyrychenko, A.; Roshal, A.D. Progress and Achievements in Glycosylation of Flavonoids. Front. Chem. 2021, 9, 637994. [Google Scholar] [CrossRef]
- Tang, S.; Wang, B.; Liu, X.; Xi, W.; Yue, Y.; Tan, X.; Bai, J.; Huang, L. Structural insights and biological activities of flavonoids: Implications for novel applications. Food Front. 2025, 6, 218–247. [Google Scholar] [CrossRef]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Bijsman, M.N.C.P.; van Gameren, Y.; Cnossen, E.P.J.; de Vries, J.H.M.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Huang, Q.; Xiao, J.; Teng, H. Absorption, metabolism and bioavailability of flavonoids: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7730–7742. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Vasantha Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Chen, L.; Lin, X.; Fan, X.; Qian, Y.; Lv, Q.; Teng, H. Sonchus oleraceus Linn extract enhanced glucose homeostasis through the AMPK/Akt/ GSK-3β signaling pathway in diabetic liver and HepG2 cell culture. Food Chem. Toxicol. 2020, 136, 111072. [Google Scholar] [CrossRef]
- Silva, P.; Bonifácio, B.; Ramos, M.; Negri, K.; Maria Bauab, T.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2013, 9, 1–15. [Google Scholar] [CrossRef]
- Caddeo, C.; Gabriele, M.; Fernàndez-Busquets, X.; Valenti, D.; Fadda, A.M.; Pucci, L.; Manconi, M. Antioxidant activity of quercetin in Eudragit-coated liposomes for intestinal delivery. Int. J. Pharm. 2019, 565, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Ciaraldi, T.P.; Nikoulina, S.E.; Bandukwala, R.A.; Carter, L.; Henry, R.R. Role of glycogen synthase kinase-3α in insulin action in cultured human skeletal muscle cells. Endocrinology 2007, 148, 4393–4399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Li, J.; Di, L. Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases. Med. Res. Rev. 2022, 42, 946–982. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Simsek Papur, O.; Dirkx, E.; Wong, L.; Sips, T.; Wang, S.; Strzelecka, A.; Nabben, M.; Glatz, J.F.C.; Neumann, D.; et al. Phosphatidylinositol 4-kinase IIIβ mediates contraction-induced GLUT4 translocation and shows its anti-diabetic action in cardiomyocytes. Cell. Mol. Life Sci. 2021, 78, 2839–2856. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Wang, L.; Liu, Y.; Chen, H.; Hu, Q.; Xie, C.; Meng, X.; Shen, X. Scutellarein alleviates chronic obstructive pulmonary disease through inhibition of ferroptosis by chelating iron and interacting with arachidonate 15-lipoxygenase. Phytother. Res. 2023, 37, 4587–4606. [Google Scholar] [CrossRef]
- Hosseinymehr, M.; Matin, M.M.; Sadeghian, H.; Bahrami, A.R.; Kaseb-Mojaver, N. 8-Farnesyloxycoumarin induces apoptosis in PC-3 prostate cancer cells by inhibition of 15-lipoxygenase-1 enzymatic activity. Anticancer Drugs 2016, 27, 854–862. [Google Scholar] [CrossRef]
- Sun, Q.; Lv, M.; Wang, Z. Delphinidin inhibits the ALOX15-mediated ferroptosis in rats to alleviate myocardial ischemia and reperfusion injury. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2025, 1872, 120006. [Google Scholar] [CrossRef]
- Arita, M.; Philipov, S.; Galabov, A.S. Phosphatidylinositol 4-kinase III beta is the target of oxoglaucine and pachypodol (Ro 09-0179) for their anti-poliovirus activities, and is located at upstream of the target step of brefeldin A. Microbiol. Immunol. 2015, 59, 338–347. [Google Scholar] [CrossRef]
- Kim, H.W.; Wang, M.; Leber, C.A.; Nothias, L.-F.; Reher, R.; Kang, K.B.; van der Hooft, J.J.J.; Dorrestein, P.C.; Gerwick, W.H.; Cottrell, G.W. NPClassifier: A deep neural network-based structural classification tool for natural products. J. Nat. Prod. 2021, 84, 2795–2807. [Google Scholar] [CrossRef]
- Beltrán-Noboa, A.; Proaño-Ojeda, J.; Guevara, M.; Gallo, B.; Berrueta, L.A.; Giampieri, F.; Perez-Castillo, Y.; Battino, M.; Álvarez-Suarez, J.M.; Tejera, E. Metabolomic profile and computational analysis for the identification of the potential anti-inflammatory mechanisms of action of the traditional medicinal plants, Ocimum basilicum and Ocimum tenuiflorum. Food Chem. Toxicol. 2022, 164, 113039. [Google Scholar] [CrossRef]
- Jimenes-Vargas, K.; Pazos, A.; Munteanu, C.R.; Perez-Castillo, Y.; Tejera, E. Prediction of compound-target interaction using several artificial intelligence algorithms and comparison with a consensus-based strategy. J. Cheminform 2024, 16, 27. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Petersen, B.; Egert, S.; Bosy-Westphal, A.; Müller, M.J.; Wolffram, S.; Hubbermann, E.M.; Rimbach, G.; Schwarz, K. Bioavailability of quercetin in humans and the influence of food matrix comparing quercetin capsules and different apple sources. Food Res. Int. 2016, 88, 159–165. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Supek, F.; Bošnjak, M.; Škunca, N.; Šmuc, T. Revigo summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]

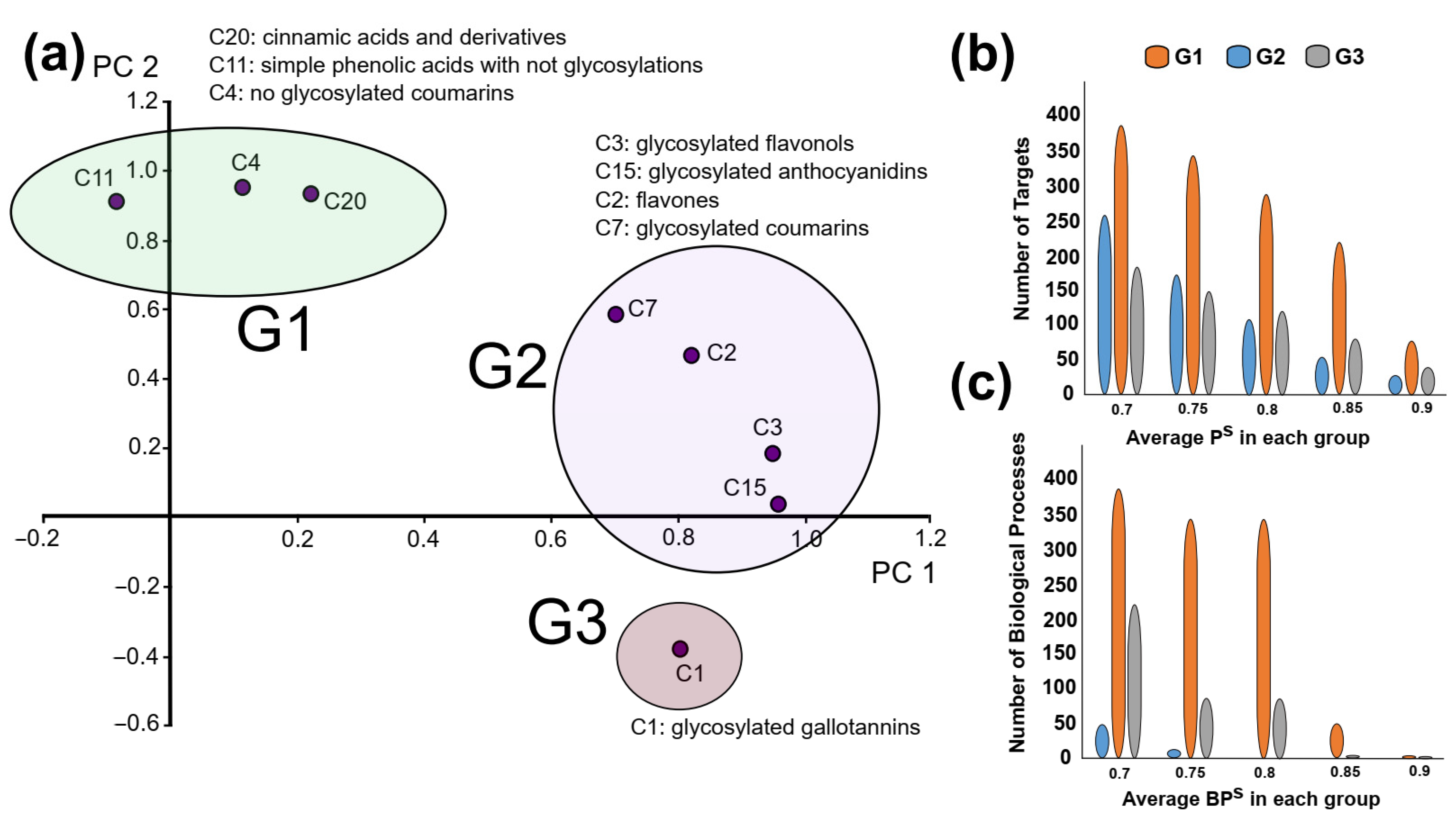
| Pelargonium Species | Biological Activities | Traditional Uses |
|---|---|---|
| P. sidoides | Antioxidant [18,19], antimicrobial (including antiviral, antifungal, and antibacterial) [6,18,20,21,22,23,24,25], antibiofilm [18], anti-inflammatory [26,27], immunomodulatory [25,28,29,30], and antitussive [26] | Respiratory infections, gastrointestinal disorders, fever, wound healing, immune support [1,14,25,27,31] |
| P. endlicherianum | Antioxidant [32,33], antibacterial [34], anti-inflammatory [35], antidiabetic, anticholinesterase, anticancer, anti-tyrosinase [32], and anthelmintic [36] | Respiratory infections, menstrual pain, gastrointestinal infections, skin diseases, anthelmintic [32,37] |
| P. graveolens | Antioxidant [38,39,40,41], antibacterial [42,43,44,45], anti-aging [38], and hepatoprotective [41] | Respiratory infections, gastrointestinal disorders, dermatological conditions, gynecological disorders, nervous system disorders [46,47,48] |
| P. zonale | Antioxidant [49,50,51], antibacterial [52], anti-inflammatory [53], and anticancer [51] | Respiratory disorders, gastrointestinal conditions, cardiovascular support, hemostatic effects, wound healing [54,55] |
| P. reniforme | Antioxidant [56,57] and antimicrobial (antibacterial and antifungal) [6,57,58] | Gastrointestinal disorders, respiratory infections, genitourinary conditions [1,14,57,59] |
| P. hispidum | Antioxidant [50], anti-inflammatory [53], and antidiabetic [60] | No specific ethnobotanical information available |
| P. peltatum | Antibacterial [61,62] | Respiratory and oral conditions, dermatological uses, cosmetic applications [61,63] |
| P. radens | Antioxidant, anticancer [51], and anti-inflammatory [53] | No specific ethnobotanical information available |
| P. odoratissimum | Antiparasitic [64] and anticancer [65] | Cardiac stimulant, cold and inflammation treatment, air disinfection, respiratory tract disorders [66,67,68] |
| P. grandiflorum | Anti-inflammatory [53] and antidiabetic [60] | Pneumonia treatment [69] |
| P. alchemilloides | Antioxidant and anti-inflammatory [70] | Wounds, sores, and abscesses [70] |
| P. purpureum | Antioxidant [71] | No specific ethnobotanical information available |
| P. quercetorum | Antioxidant and antibacterial [72] | Respiratory tract disorders |
| P. inquinans | Antioxidant [73] | Cold and bronchitis treatment [74] |
| P. radula | Antioxidant [75], antibacterial, and antifungal [76] | Diabetes treatment [75,76] |
| P. hybrid | Antioxidant, anti-inflammatory, anticholinesterase, and anti-haemolytic [77] | No specific ethnobotanical information available |
| P. tabulare | Antibacterial [78] | No specific ethnobotanical information available |
| Biological Processes | BPmean | GO Frequency | BPG2 |
|---|---|---|---|
| G protein-coupled acetylcholine receptor signaling pathway | 0.81 | 0.01 | 0.89 |
| Type II interferon-mediated signaling pathway | 0.76 | 0.00 | 0.88 |
| Regulation of cell adhesion mediated by integrin | 0.77 | 0.01 | 0.88 |
| Tachykinin receptor signaling pathway | 0.82 | 0.01 | 0.87 |
| Hormone catabolic process | 0.79 | 0.00 | 0.87 |
| Bradykinin catabolic process | 0.79 | 0.00 | 0.87 |
| Cellular response to virus | 0.75 | 0.01 | 0.87 |
| Positive regulation of the apoptotic signaling pathway | 0.77 | 0.03 | 0.86 |
| Positive regulation of the fatty acid metabolic process | 0.76 | 0.01 | 0.86 |
| Positive regulation of vasoconstriction | 0.77 | 0.01 | 0.86 |
| Response to pain | 0.76 | 0.00 | 0.86 |
| Cellular defense response | 0.76 | 0.00 | 0.86 |
| Response to dexamethasone | 0.75 | 0.00 | 0.85 |
| Smooth muscle contraction | 0.76 | 0.02 | 0.85 |
| Regulation of protein binding | 0.77 | 0.00 | 0.85 |
| Negative regulation of myosin-light-chain-phosphatase activity | 0.75 | 0.00 | 0.85 |
| Positive regulation of nitric oxide synthase biosynthetic process | 0.73 | 0.00 | 0.85 |
| Positive regulation of telomere capping | 0.74 | 0.00 | 0.85 |
| Regulation of inflammatory response | 0.73 | 0.06 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celi, D.; Jimenes-Vargas, K.; Machado, A.; Álvarez-Suárez, J.M.; Tejera, E. Chemical Composition and Biological Activities of Pelargonium sp.: A Review with In Silico Insights into Potential Anti-Inflammatory Mechanism. Molecules 2025, 30, 3198. https://doi.org/10.3390/molecules30153198
Celi D, Jimenes-Vargas K, Machado A, Álvarez-Suárez JM, Tejera E. Chemical Composition and Biological Activities of Pelargonium sp.: A Review with In Silico Insights into Potential Anti-Inflammatory Mechanism. Molecules. 2025; 30(15):3198. https://doi.org/10.3390/molecules30153198
Chicago/Turabian StyleCeli, Diana, Karina Jimenes-Vargas, António Machado, José Miguel Álvarez-Suárez, and Eduardo Tejera. 2025. "Chemical Composition and Biological Activities of Pelargonium sp.: A Review with In Silico Insights into Potential Anti-Inflammatory Mechanism" Molecules 30, no. 15: 3198. https://doi.org/10.3390/molecules30153198
APA StyleCeli, D., Jimenes-Vargas, K., Machado, A., Álvarez-Suárez, J. M., & Tejera, E. (2025). Chemical Composition and Biological Activities of Pelargonium sp.: A Review with In Silico Insights into Potential Anti-Inflammatory Mechanism. Molecules, 30(15), 3198. https://doi.org/10.3390/molecules30153198








