Phospholipase A2—A Significant Bio-Active Molecule in Honeybee (Apis mellifera L.) Venom
Abstract
1. Introduction
2. PLA2—A Common Molecule in Different Organisms
3. PLA2 Is a Major Molecule of HBV
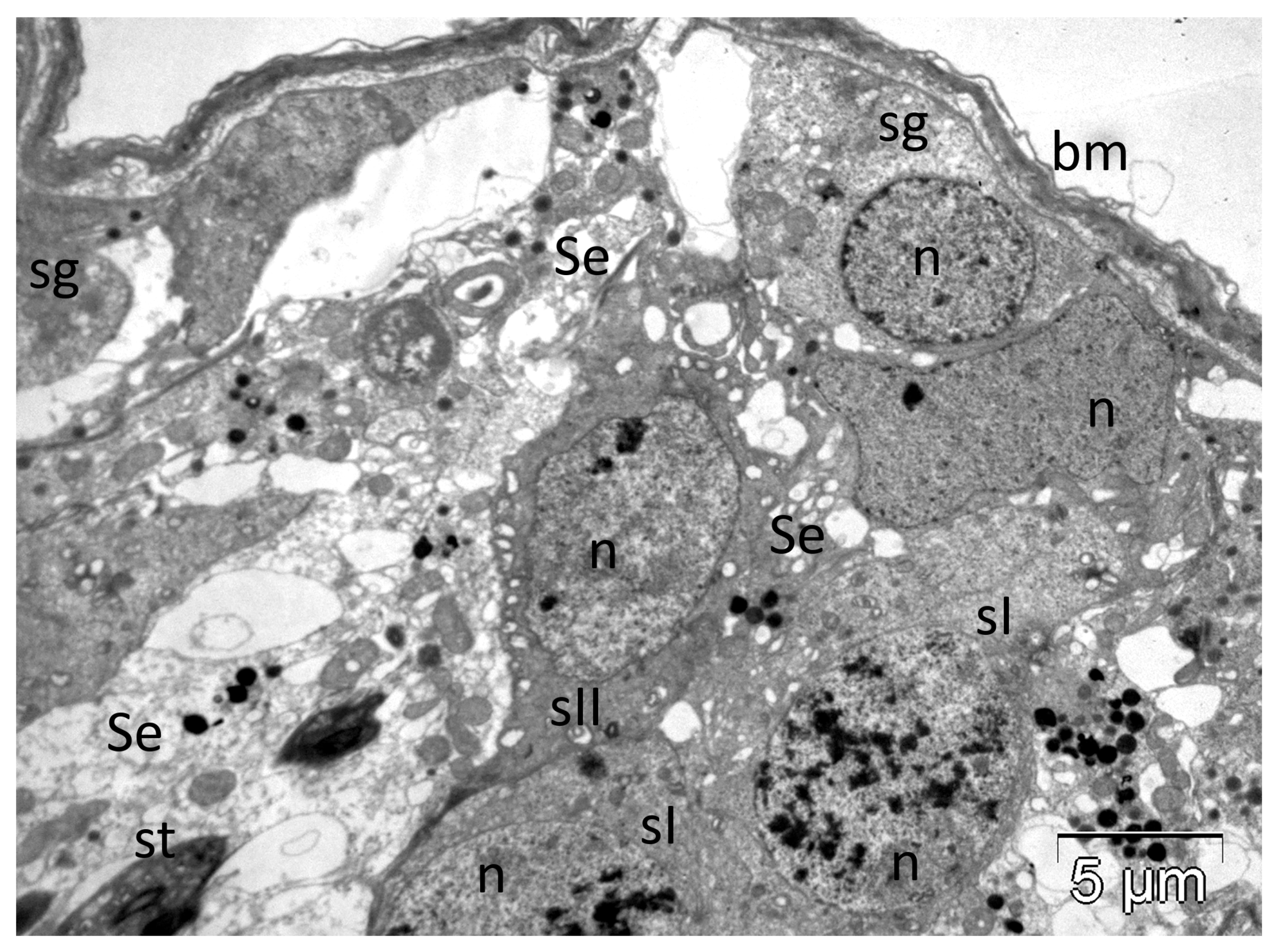
3.1. Bees and Their Venoms
3.2. hvPLA2—Differences
3.3. hvPLA2—Synthesis and Proteolysis
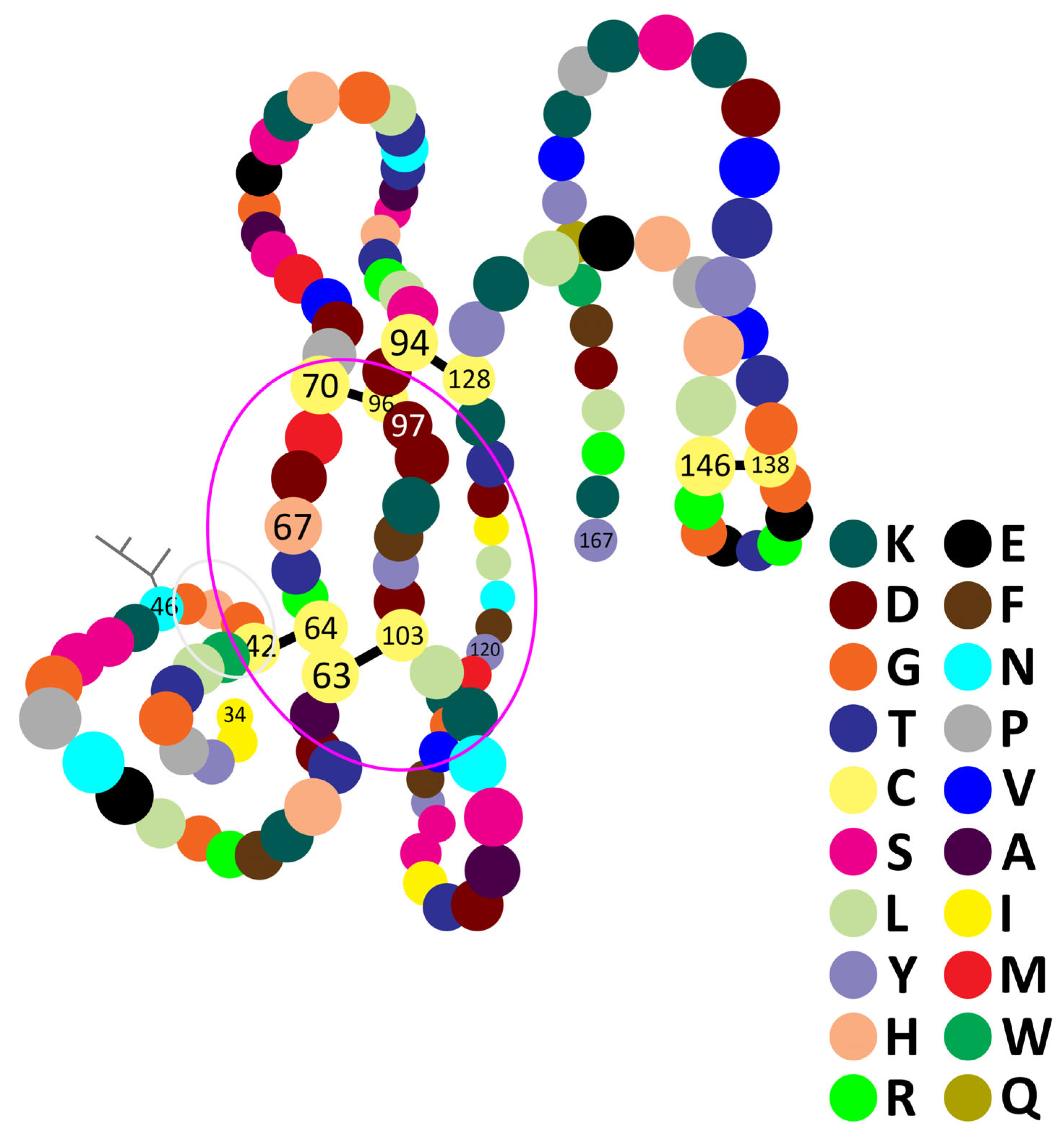
- MQVVLGSLFL10 LLLSTSHGWQ20 IRDRIGDNEL30 EERIIYPGTL40 WCGHGNKSSG50 PNELGRFKHT60 DACCRTHDMC70 PDVMSAGESK80 HGLTNTASHT90 RLSCDCDDKF100 YDCLKNSADT110 ISSYFVGKMY120 FNLIDTKCYK130 LEHPVTGCGE140 RTEGRCLHYT150 VDKSKPKVYQ160 WFDLRKY167
3.4. hvPLA2—Glycosylation
3.5. hvPLA2—Folding
3.6. Availability
4. Effects of hvPLA2
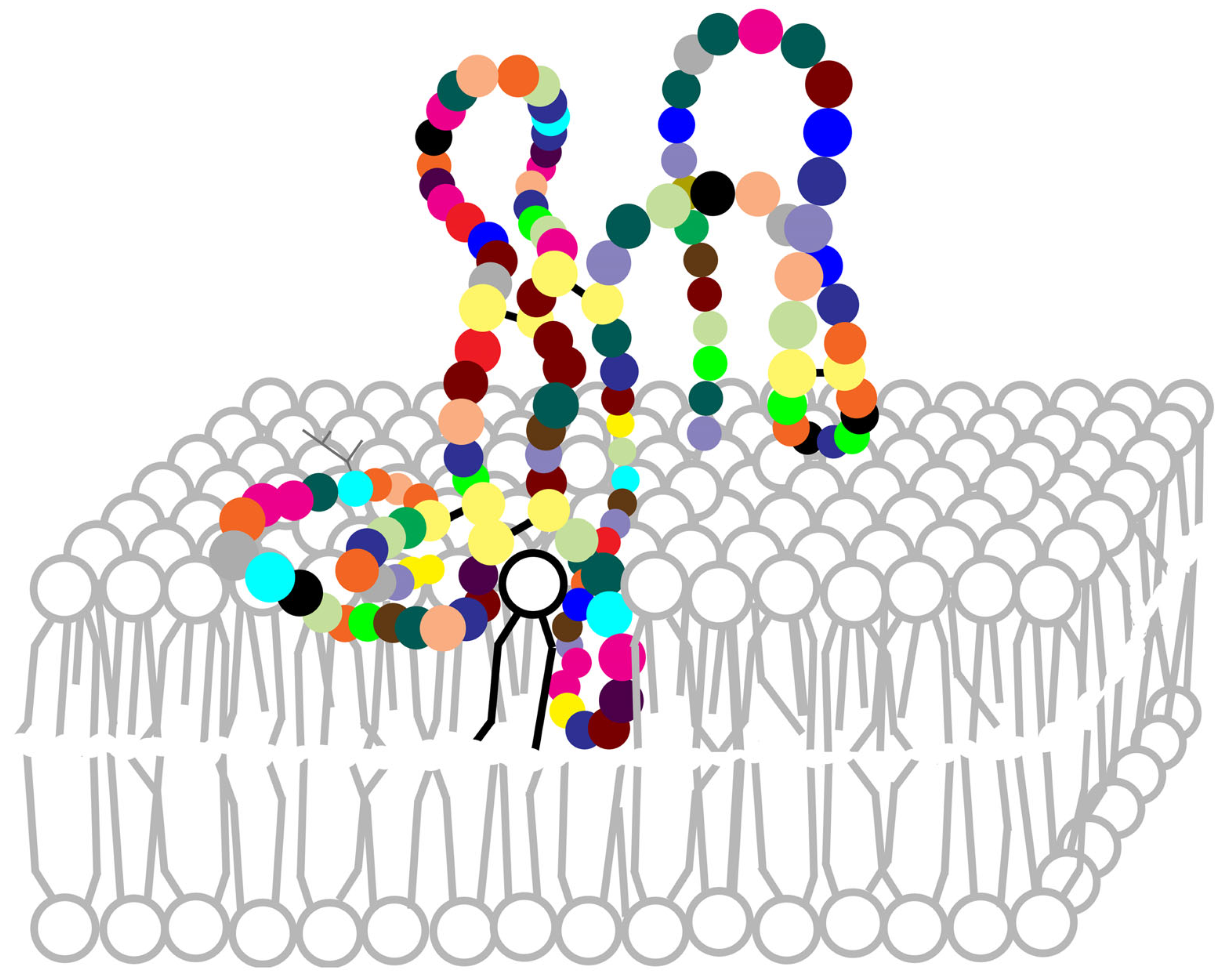
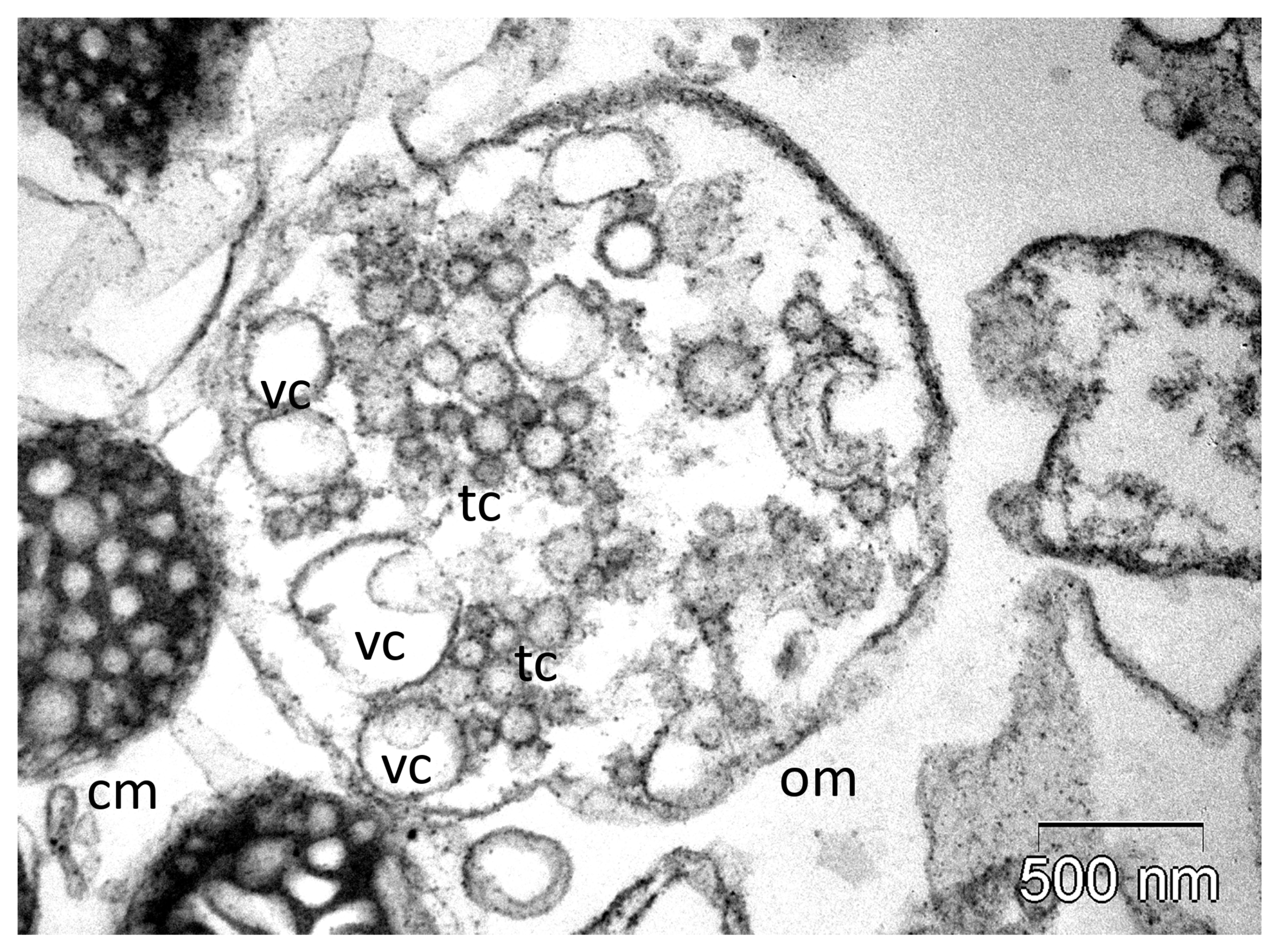
4.1. Breakdown of Membranes
4.2. Interaction with Membranes
4.3. Mechanism of Phospholipids Hydrolysis
4.4. Pro-Inflammatory Effect
4.5. PLA2—Allergen of Bee Venom
4.6. Anticoagulant Effect
4.7. PLA2 and Apoptosis
4.8. Antiviral, Antibiotic and Antitumoral Effects of hvPLA2
4.9. Toxicity of hvPLA2
5. Immunotherapy
6. Anti-PLA2 Molecules
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A.m. | Apis mellifera |
| HBV | honeybee venom |
| hv PLA2 | honeybee venom phospholipase A2 |
| HPLC | high performance liquid chromatography |
| PLA2 | phospholipase A2 |
| TNF | tumour necrosis factor |
References
- Bücherl, W. Introduction. In Venomous Animals and Their Venoms; Bücherl, W., Buckley, E., Eds.; Academic Press: New York, NY, USA, 1971; Volume 1, pp. xix–xxii. [Google Scholar] [CrossRef]
- Maschwitz, U.W.J.; Kloft, W. Morphology and function of the venom apparatus of insects—Bees, wasps, ants and caterpillars. In Venomous Animals and Their Venoms; Bücherl, W., Buckley, E., Eds.; Academic Press: New York, NY, USA, 1971; Volume 1, pp. 1–60. [Google Scholar] [CrossRef]
- Veena, V.; Mehta, H.; Dhingra, P.; Ballani, A. Multiple bee stings and acute kidney injury: A case report. Cureus 2024, 16, e66488. [Google Scholar] [CrossRef]
- Rahimian, R.; Shirazi, F.M.; Schmidt, J.O.; Klotz, S.A. Honeybee stings in the era of killer bees: Anaphylaxis and toxic envenomation. Am. J. Med. 2020, 133, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Neumann, W.; Habermann, E.; Amend, G. Zur Papierelektrophoresis den Fraktionierung tierischer Gifte. Naturwissenschaften 1952, 39, 286–287. [Google Scholar] [CrossRef]
- Habermann, E.; Neumann, W. Reinigung der Phosphalipase A des Bienegiftes. Biochem. Z. 1957, 328, 465–473. [Google Scholar]
- Habermann, E. Bee and wasp venoms. The biochemistry and pharmacology of their peptides and enzymes are reviewed. Science 1972, 177, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Förster, E.; Dudler, T.; Gmachl, M.; Aberer, W.; Urbanek, R.; Suter, M. Natural and recombinant enzymatically active or inactive bee venom phospholipase A2 has the same potency to release histamine from basophils in patients with Hymenoptera allergy. J. Allergy Clin. Immunol. 1995, 95, 1229–1235. [Google Scholar] [CrossRef]
- Yaacoub, C.; Wehbe, R.; Roufayel, R.; Fajloun, Z.; Coutard, B. Bee venom and its two main components—Melittin and phospholipase A2—As promising antiviral drug candidates. Pathogens 2023, 12, 1354. [Google Scholar] [CrossRef]
- Gajski, G.; Leonova, E.; Sjakste, N. Bee venom: Composition and anticancer properties. Toxins 2024, 16, 117. [Google Scholar] [CrossRef]
- Sadek, K.M.; Shib, N.A.; Taher, E.S.; Rashed, F.; Shukry, M.; Atia, G.A.; Taymour, N.; El-Nablaway, M.; Ibrahim, A.M.; Ramadan, M.M.; et al. Harnessing the power of bee venom for therapeutic and regenerative medical applications: An updated review. Front. Pharmacol. 2024, 18, 1412245. [Google Scholar] [CrossRef]
- El-Arabey, A.A.; Ghramh, H.A. Bee venom: Yesterday’s enemy becomes modern medicine for skin cancer. Exp. Cell Res. 2025, 445, 114435. [Google Scholar] [CrossRef]
- Tilinca, M.; Florea, A. Ultrastructural analysis of early toxic effects produced by bee venom phospholipase A2 and melittin in Sertoli cells in rats. Toxicon 2018, 141, 94–103. [Google Scholar] [CrossRef]
- Harfmann, D.; Florea, A. Experimental envenomation with honeybee venom melittin and phospholipase A2 induced multiple ultrastructural changes in adrenocortical mitochondria. Toxicon 2023, 229, 107136. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Varga, A.P.; Matei, H.V. Ultrastructural variability of mitochondrial cristae induced in vitro by bee (Apis mellifera) venom and its derivatives, melittin and phospholipase A2, in isolated rat adrenocortical mitochondria. Micron 2018, 112, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Bókay, A. Ueber die Verdaulichkeit des Nucleins und Lecithins. Biol. Chem. 1878, 1, 157–164. [Google Scholar] [CrossRef]
- Dennis, E.A. Phospholipases. In The Enzymes, 3rd ed.; Boyer, P.D., Ed.; Academic Press: New York, NY, USA, 1983; Volume 16, pp. 307–353. [Google Scholar]
- Six, D.A.; Dennis, E.A. The expanding superfamily of phospholipase A2 enzymes: Classification and characterisation. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2000, 1488, 1–19. [Google Scholar] [CrossRef]
- Wilton, D.C.; Waite, M. Phospholipases. In Biochemistry of Lipids, Lipoproteins and Membranes, 4th ed.; Vance, D.E., Vance, J.E., Eds.; Elsevier Science: Oxford, UK, 2002; pp. 291–314. [Google Scholar]
- Apis mellifera (Honey Bee): 100577717. Available online: https://www.genome.jp/entry/AME+100577717+406141+409277+409307+409614+410570+552242+724436+725668 (accessed on 8 April 2025).
- Thiyagaraj, A.; Gupta, A.; Salunkhe, S.; Sharathkumar, R.M. Role of phospholipases in chronic inflammatory diseases: A systems biology perspective (Chapter 13). In Phospholipases in Physiology and Pathology; Chakraborti, S., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 6, pp. 219–236. [Google Scholar] [CrossRef]
- Schaloske, R.H.; Dennis, E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2006, 1761, 1246–1259. [Google Scholar] [CrossRef]
- Valentin, E.; Lambeau, G. What can venom phospholipases A2 tell us about the functional diversity of mammalian secreted phospholipases A2? Biochimie 2000, 82, 815–831. [Google Scholar] [CrossRef]
- Ho, I.C.; Arm, J.P.; Bingham, C.O., 3rd; Choi, A.; Austen, K.F.; Glimcher, L.H. A novel group of phospholipase A2s preferentially expressed in type 2 helper T cells. J. Biol. Chem. 2001, 276, 18321–18326. [Google Scholar] [CrossRef]
- Murakami, M.; Kudo, I. Phospholipase A2. J. Biochem. 2002, 131, 285–292. [Google Scholar] [CrossRef]
- Rouault, M.; Bollinger, J.G.; Lazdunski, M.; Gelb, M.H.; Lambeau, G. Novel mammalian group XII secreted phospholipase A2 lacking enzymatic activity. Biochemistry 2003, 42, 11494–11503. [Google Scholar] [CrossRef]
- Ackermann, E.J.; Dennis, E.A. Mammalian calcium-independent phospholipase A2. Biochim. Biophys. Acta-Lipids Lipid Metab. 1995, 1259, 125–136. [Google Scholar] [CrossRef]
- Berg, O.G.; Gelb, M.H.; Tsai, M.D.; Jain, M.K. Interfacial enzymology: The secreted phospholipase A2-paradigm. Chem. Rev. 2001, 101, 2613–2653. [Google Scholar] [CrossRef]
- Bon, C.; Choumet, V.; Delot, E.; Faure, G.; Robbe-Vincent, A.; Saliou, B. Different evolution of phospholipase A2 neurotoxins (β-neurotoxins) from Elapidae and Viperidae snakes. Ann. N. Y. Acad. Sci. 1994, 710, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994, 269, 13057–13060. [Google Scholar] [CrossRef] [PubMed]
- Michener, C.D. The Bees of the World, 2nd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Crane, E. Apis species (Honey bees). In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: San Diego, CA, USA, 2009; pp. 31–32. [Google Scholar]
- Schmidt, J.O. Toxicology of venom from the honeybee genus Apis. Toxicon 1995, 33, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E. Chemistry, pharmacology and toxicology of bee, wasp and hornet venoms. In Venomous Animals and Their Venoms; Bücherl, W., Buckley, E.E., Eds.; Academic Press: New York, NY, USA, 1971; pp. 61–94. [Google Scholar] [CrossRef]
- Kang, S.S.; Pak, S.C.; Choi, S.H. The effect of whole bee venom on arthritis. Am. J. Chin. Med. 2002, 30, 73–84. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Yoon, O.B.; Beitz, A.J.; Lee, J.H. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci. 2002, 71, 191–204. [Google Scholar] [CrossRef]
- Bücherl, W.; Buckley, E. Venomous Animals and Their Venoms; Academic Press: New York, NY, USA, 1971; pp. 2–38. [Google Scholar] [CrossRef]
- Habermann, E. Chimie, pharmacologie et toxicologie du venin. In Traité de Biologie de L’abeille; Chauvin, R., Ed.; Masson ETC: Paris, France, 1968; pp. 363–387. [Google Scholar]
- Shipolini, R.A. Biochemistry of bee venom. In Handbook of Natural Toxins; Tu, A.T., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1984; Volume 2, pp. 49–85. [Google Scholar]
- Banks, B.E.C.; Shipolini, R.A. Chemistry and pharmacology of honey-bee venom. In Venoms of the Hymenoptera: Biochemical, Pharmacological and Behavioural Aspects; Piek, T., Ed.; Academic Press: Orlando, FL, USA, 1986; pp. 329–416. [Google Scholar]
- Schmidt, J.O. Bee products: Chemical composition and application. In Bee Products; Mizrahi, A., Lensky, Y., Eds.; Plenum Press: New York, NY, USA, 1996; pp. 15–26. [Google Scholar]
- Chauvin, R. Action du venin sur l’homme. In Traité de Biologie de L’abeille; Chauvin, R., Ed.; Masson ETC: Paris, France, 1968; pp. 160–161. [Google Scholar]
- Oh, M.M.; Power, J.M.; Thompson, L.T.; Disterhoft, J.F. Apamin increases excitability of CA1 hippocampal pyramidal neurons. Neurosci. Res. Commun. 2000, 27, 135–141. [Google Scholar] [CrossRef]
- Bridges, A.R.; Owen, M.D. The morphology of the honey bee (Apis mellifera L.) venom gland and reservoir. J. Morphol. 1984, 181, 69–86. [Google Scholar] [CrossRef]
- Vetter, R.S.; Visscher, P.K. Bites and stings of medically important venomous arthropods. Int. J. Dermatol. 1998, 37, 481–496. [Google Scholar] [CrossRef]
- Owen, M.D.; Bridges, A.R. Aging in the venom glands of queen and worker honey bees (Apis mellifera L.): Some morphological and chemical observations. Toxicon 1976, 14, 1–5. [Google Scholar] [CrossRef]
- Abreu, R.M.M.; Silva de Moraes, R.L.M.; Malaspina, O. Histological aspects and protein content of Apis mellifera L. worker venom glands: The effect of electrical shocks in summer and winter. J. Venom. Anim. Toxins 2000, 6, 87–98. [Google Scholar] [CrossRef]
- Owen, D.M.; Pfaff, L.A.; Reisman, R.E.; Wypych, J. Phospholipase A2 in venom extracts from honey bees (Apis mellifera L.) of different ages. Toxicon 1990, 28, 813–820. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, C.X.; Shen, L.R.; Tang, Z.H.; Cheng, J.A. Expression and regulation of phospholipase A2 in venom gland of the Chinese honeybee, Apis cerana cerana. Arch. Insect Biochem. Physiol. 2005, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.J.; Schmidt, J.O.; Egen, N.B.; Lowry, J.E. Quantity, analysis and lethality of European and Africanized honey bee venoms. Am. J. Trop. Med. Hyg. 1990, 43, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.S.; Brochetto-Braga, M.R. Biochemical variability between venoms from different honey-bee (Apis mellifera) races. Comp. Biochem. Physiol. Part. C Pharmacol. Toxicol. Endocrinol. 1993, 106, 423–427. [Google Scholar] [CrossRef]
- Schumacher, M.J.; Schmidt, J.O.; Egen, N.B.; Dilon, K.A. Biochemical variability of venoms from individual European and Africanized honey bees. J. Allergy Clin. Immunol. 1992, 90, 59–65. [Google Scholar] [CrossRef]
- Benton, A.W.; Patton, R.L. A qualitative analysis of the proteins in the venom of honey bees. J. Insect Physiol. 1965, 11, 1359–1364. [Google Scholar] [CrossRef]
- Ferreira Junior, R.S.; Sciani, J.M.; Marques-Porto, R.; Junior, A.L.; Orsi, R.O.; Barraviera, B.; Pimenta, D.C. Africanized honey bee (Apis mellifera) venom profiling: Seasonal variation of melittin and phospholipase A2 levels. Toxicon 2010, 56, 355–362. [Google Scholar] [CrossRef]
- Owen, M.D. Relationship between age and hyaluronidase activity in the venoms of queen and worker honey bees (Apis mellifera L.). Toxicon 1979, 17, 94–98. [Google Scholar] [CrossRef]
- Marz, R.; Mollay, C.; Kreil, G. Queen bee venom contains much less phospholipase than worker bee venom. Insect Biochem. 1981, 11, 685–690. [Google Scholar] [CrossRef]
- Shen, L.S.; Zhang, C.Z.; Cheng, J.A. Cloning and sequencing of gene encoding phospholipase A2 from the venom of Apis cerana cerana and A. mellifera. J. Agric. Biotechnol. 2002, 10, 29–32. [Google Scholar]
- Ryu, Y.; Oh, Y.; Yoon, J.; Cho, W.; Baek, K. Molecular characterization of a gene encoding the Drosophila melanogaster phospholipase A2. Biochim. Biophys. Acta-Gene Struct. Expr. 2003, 1628, 206–210. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Walker, A.A.; Robinson, S.D.; Yeates, D.K.; Jin, J.; Baumann, K.; Dobson, J.; Fry, B.G.; King, G.F. Entomo-venomics: The evolution, biology and biochemistry of insect venoms. Toxicon 2018, 154, 15–27. [Google Scholar] [CrossRef]
- Phospholipase A2—Apis mellifera (Honeybee) | UniProtKB. Available online: http://www.uniprot.org/uniprot/P00630 (accessed on 18 March 2025).
- King, T.P. Insect venom allergens. In Molecular Approaches to the Study of Allergens; Baldo, B.A., Ed.; Karger: Basel, Switzerland, 1990; Volume 28, pp. 84–100. [Google Scholar]
- Annand, R.R.; Kontoyianni, M.; Penzotti, J.E.; Dudler, T.; Lybrand, T.P.; Gelb, M.H. Active site of bee venom phospholipase A2: The role of histidine-34, aspartate-64 and tyrosine-87. Biochemistry 1996, 35, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Shipolini, R.A.; Callewaert, G.L.; Cottrell, R.C.; Vernon, C.A. The amino-acid sequence and carbohydrate content of phospholipase A2 from bee venom. Eur. J. Biochem. 1974, 48, 465–476. [Google Scholar] [CrossRef]
- Kuchler, K.; Gmachl, M.; Sippl, M.J.; Kreil, G. Analysis of the cDNA for phospholipase A2 from honeybee venom glands. The deduced amino acid sequence reveals homology to the corresponding vertebrate enzymes. Eur. J. Biochem. 1989, 184, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.L.; Otwinowski, Z.; Gelb, M.H.; Sigler, P.B. Crystal structure of bee-venom phospholipase A2 in a complex with a transition-state analogue. Science 1990, 250, 1563–1566. [Google Scholar] [CrossRef]
- Valentin, E.; Ghomashchi, F.; Gelb, M.H.; Lazdunski, M.; Lambeau, G. Novel human secreted phospholipase A2 with homology to the group III bee venom enzyme. J. Biol. Chem. 2000, 275, 7492–7496. [Google Scholar] [CrossRef]
- Hanasaki, K.; Arita, H. Biological and pathological functions of novel types of secretory phospholipase A2s and their receptors (Review). Ann. Rep. Shionogi Res. Lab. 2002, 52, 1–22. [Google Scholar]
- Sun, G.Y.; Shelat, P.B.; Jensen, M.B.; He, Y.; Sun, A.Y.; Simonyi, A. Phospholipases A2 and inflammatory responses in the central nervous system. Neuromol. Med. 2010, 12, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Lotan, A.; Fishman, L.; Loya, Y.; Zlotkin, E. Delivery of a nematocyst toxin. Nature 1995, 375, 456. [Google Scholar] [CrossRef]
- Weber, A.; Marz, L.; Altmann, F. Characteristics of the asparagine-linked oligosaccharide from honey-bee venom phospholipase A2. Evidence for the presence of terminal N-acetylglucosamine and fucose in an insect glycoprotein. Comp. Biochem. Phys. Part B Comp. Biochem. 1986, 83, 321–324. [Google Scholar] [CrossRef]
- Staudacher, E.; Altmann, F.; Marz, L.; Hard, K.; Kamerling, J.P.; Vliegenthart, J.F. Alpha 1-6(alpha 1-3)-difucosylation of the asparagine-bound N-acetylglucosamine in honeybee venom phospholipase A2. Glycoconj. J. 1992, 9, 82–85. [Google Scholar] [CrossRef]
- Kubelka, V.; Altmann, F.; Staudacher, E.; Tretter, V.; Marz, L.; Hard, K.; Kamerling, J.P.; Vliegenthart, J.F. Primary structures of the N-linked carbohydrate chains from honeybee venom phospholipase A2. Eur. J. Biochem. 1993, 213, 1193–1204. [Google Scholar] [CrossRef]
- Haslam, S.M.; Reason, A.J.; Morris, H.R.; Dell, A. Core fucosylation of honeybee venom phospholipase A2. Glycobiology 1994, 4, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Altmann, F.; Kubelka, V.; Staudacher, E.; Uhl, K.; März, L. Characterization of the isoforms of phospholipase A2 from honeybee venom. Insect Biochem. 1991, 21, 467–472. [Google Scholar] [CrossRef]
- Peiren, N.; Vanrobaeys, F.; de Graaf, D.C.; Devreese, B.; Van Beeumenb, J.; Jacobs, F.J. The protein composition of honeybee venom reconsidered by a proteomic approach. Biochim. Biophys. Acta-Proteins Proteom. 2005, 1752, 1–5. [Google Scholar] [CrossRef]
- Murakami, M.; Sato, H.; Miki, Y.; Yamamoto, K.; Taketomi, Y. A new era of secreted phospholipase A2. J. Lipid Res. 2015, 56, 1248–1261. [Google Scholar] [CrossRef]
- Scott, D.L.; White, S.P.; Browning, J.L.; Rosa, J.J.; Gelb, M.H.; Sigler, P.B. Structures of free and inhibited human secretory phospholipase A2 from inflammatory exudate. Science 1991, 254, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Shipolini, R.A.; Doonan, S.; Vernon, C.A. The disulphide bridges of phospholipase A2 from bee venom. Eur. J. Biochem. 1974, 48, 477–483. [Google Scholar] [CrossRef]
- Arni, R.K.; Ward, R.J. Phospholipase A2—A structural review. Toxicon 1996, 34, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: Common aspects of their mechanisms of action. Cell. Mol. Life Sci. 2008, 65, 2897–2912. [Google Scholar] [CrossRef]
- Scott, D.L.; White, S.P.; Otwinowski, Z.; Yuan, W.; Gelb, M.H.; Sigler, P.B. Interfacial catalysis: The mechanism of phospholipase A2. Science 1990, 250, 1541–1546. [Google Scholar] [CrossRef]
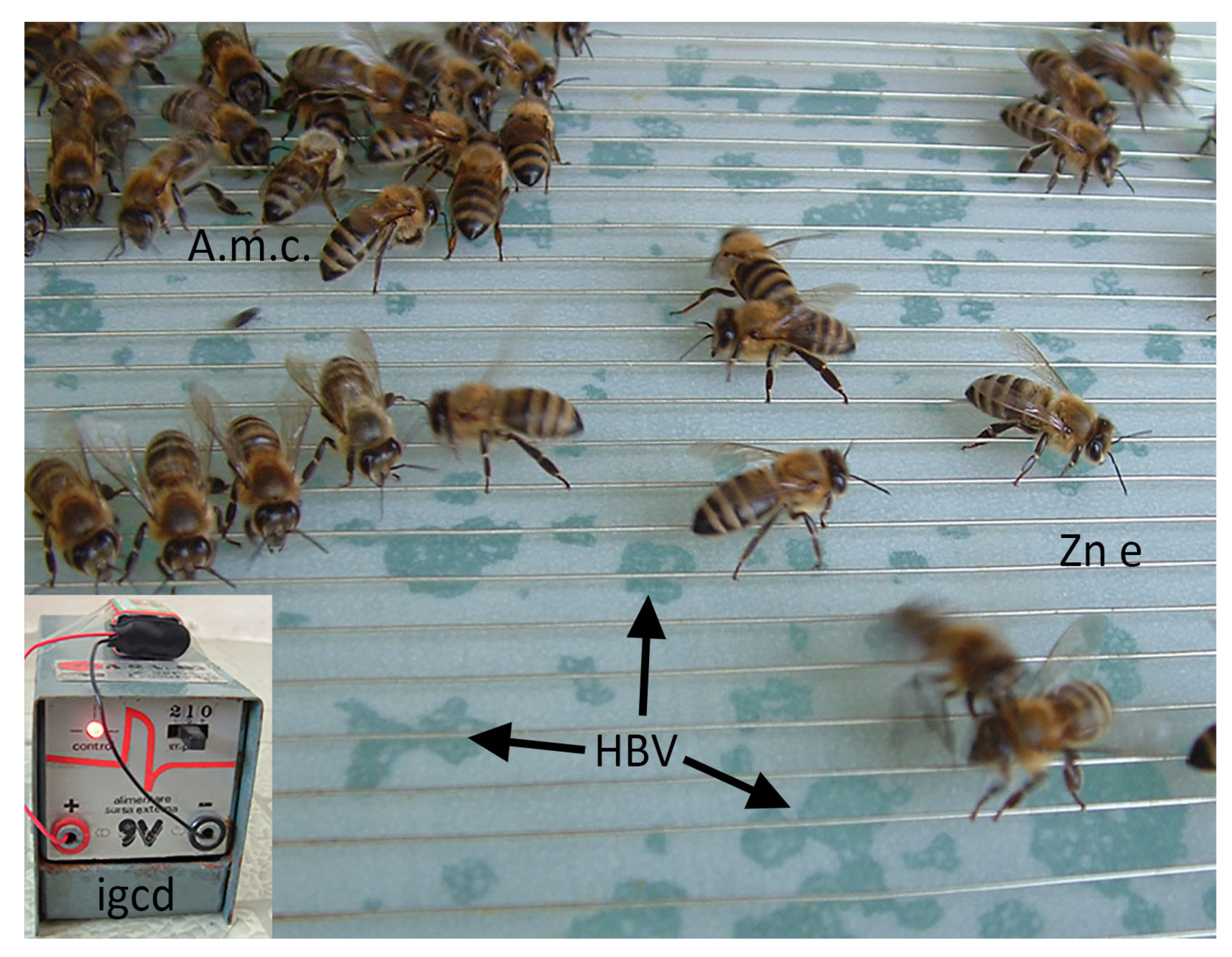
- Gelderblom, R. Collecting bee venom—The other way. Hivelights 1997, 13, 12–13. [Google Scholar]
- Abd El-Wahed, A.A.; Ghazala, N.A.; Elfeel, M.A.; Sayed, S.S. Bee venom extraction methods: A comparative study on quantity and honey bee performance. J. Apic. Res. 2025, 1–9. [Google Scholar] [CrossRef]
- Ali, E.A.; Saad, A.; Younis, M.S.; Metwally, A.A.A. Using sensing technology inside beehive to determine the optimal conditions affecting the demand for bee (Apis mellifera L.) venom collector device. Int. J. Eng. Invent. 2023, 12, 76–85. [Google Scholar]
- Scaccabarozzi, D.; Dods, K.; Le, T.T.; Gummer, J.P.A.; Lussu, M.; Milne, L.; Campbell, T.; Wafujian, B.P.; Priddis, C. Factors driving the compositional diversity of Apis mellifera bee venom from a Corymbia calophylla (marri) ecosystem, Southwestern Australia. PLoS ONE 2021, 16, e0253838. [Google Scholar] [CrossRef]
- P9279 Phospholipase A2 from Honey Bee Venom (Apis mellifera). Available online: https://www.sigmaaldrich.com/RO/en/product/sigma/p9279#product-documentation (accessed on 18 March 2025).
- Bee Venom PLA 2 Control. Available online: https://www.caymanchem.com/product/765016/bee-venom-pla2-control?srsltid=AfmBOopZ3xtGtm45Q2cojgo5jgoslc5LnhE7UmT0rsaNVbAnkUqL9fnX (accessed on 18 March 2025).
- Nair, C.; Hermans, J.; Munjal, D.; Elliott, W.B. Temperature stability of phospholipase A activity—I Bee (Apis mellifera) venom phospholipase A2. Toxicon 1976, 14, 35–42. [Google Scholar] [CrossRef]
- De Haas, G.H.; Postema, N.M.; Nieuwenhuizen, W.; Van Deenen, M. Purification and properties of an anionic zymogen of phospholipase a from porcine pancreas. Biochim. Biophys. Acta-Enzymol. 1968, 159, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Pruzanski, W.; Vadas, P. Phospholipase A2—A mediator between proximal and distal effectors of inflammation. Immunol. Today 1991, 12, 143–146. [Google Scholar] [CrossRef]
- Kudo, I.; Murakami, M.; Hara, S.; Inoue, K. Mammalian non-pancreatic phospholipases A2. Biochim. Biophys. Acta-Lipids Lipid Metab. 1993, 1170, 217–231. [Google Scholar] [CrossRef]
- Arita, H.; Hanasaki, K.; Nakano, T.; Oka, S.; Teraoka, H.; Matsumoto, K. Novel proliferative effect of phospholipase A2 in Swiss 3T3 cells via specific binding site. J. Biol. Chem. 1991, 266, 19139–19141. [Google Scholar] [CrossRef] [PubMed]
- Alder, G.M.; Arnold, W.M.; Bashford, C.L.; Drake, A.F.; Pasternak, C.A.; Zimmermann, U. Divalent cation-sensitive pores formed by natural and synthetic melittin and by Triton X-100. Biochim. Biophys. Acta-Biomembr. 1991, 1061, 111–120. [Google Scholar] [CrossRef]
- Blondelle, S.E.; Houghten, R.A. Probing the relationships between the structure and hemolytic activity of melittin with a complete set of leucine substitution analogs. Peptide Res. 1991, 4, 12–18. [Google Scholar]
- Diaz-Achirica, P. Permeabilization of the mitochondrial inner membrane by short cecropin-A-melittin hybrid peptides. Eur. J. Biochem. 1994, 224, 257–263. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef]
- Rivera, R.; Chun, J. Biological effects of lysophospholipids. Rev. Physiol. Biochem. Pharmacol. 2008, 160, 25–46. [Google Scholar] [CrossRef]
- Gelb, M.H.; Jain, M.K.; Hanel, A.M.; Berg, O.G. Interfacial enzymology of glycerolipid hydrolases: Lessons from secreted phospholipases A2. Annu. Rev. Biochem. 1995, 64, 653–688. [Google Scholar] [CrossRef]
- Scott, D.L.; Mandel, A.M.; Sigler, P.B.; Honig, B. The Electrostatic Basis for the Interfacial Binding of Secretory Phospholipases A2. Biophys. J. 1994, 67, 493–504. [Google Scholar] [CrossRef]
- Lin, Y.; Nielsen, R.; Murray, D.; Hubbell, W.L.; Mailer, C.; Robinson, B.H.; Gelb, M.H. Docking phospholipase A2 on membranes using electrostatic potential-modulated spin relaxation magnetic resonance. Science 1998, 279, 1925–1929. [Google Scholar] [CrossRef]
- Ball, A.; Nielsen, R.; Gelb, M.H.; Robinson, B.H. Interfacial membrane docking of cytosolic phospholipase A2 C2 domain using electrostatic potential-modulated spin relaxation magnetic resonance. Proc. Natl. Acad. Sci. USA 1999, 96, 6637–6642. [Google Scholar] [CrossRef]
- Ahmed, T.; Kelly, S.M.; Price, N.C.; Lawrence, A.J. Activation of phospholipase A2 by long chain fatty acyl groups involves a novel unstable linkage. J. Biochem. 2000, 127, 871–875. [Google Scholar] [CrossRef]
- Ghomashchi, F.; Lin, Y.; Hixon, M.S.; Yu, B.Z.; Annand, R.; Jain, M.K.; Gelb, M.H. Interfacial recognition by bee venom phospholipase A2: Insights into nonelectrostatic molecular determinants by charge reversal mutagenesis. Biochemistry 1998, 37, 6697–6710. [Google Scholar] [CrossRef] [PubMed]
- Koprivnjak, T.; Peschel, A.; Gelb, M.H.; Liang, N.S.; Weis, J.P. Role of charge properties of bacterial envelope in bactericidal action of human Group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 2002, 277, 47636–47644. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Cho, N.J.; Duran, R.S.; Frank, C.W. Interfacial binding dynamics of bee venom phospholipase A2 investigated by dynamic light scattering and quartz crystal microbalance. Langmuir 2010, 26, 4103–4112. [Google Scholar] [CrossRef]
- Maggio, B. Modulation of phospholipase A2 by electrostatic fields and dipole potential of glycosphingolipids in monolayers. J. Lipid Res. 1999, 40, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, J.G.; Diraviyam, K.; Ghomashchi, F.; Murray, D.; Gelb, M.H. Interfacial binding of bee venom secreted phospholipase A2 to membranes occurs predominantly by a nonelectrostatic mechanism. Biochemistry 2004, 43, 13293–13304. [Google Scholar] [CrossRef]
- Ahmed, T.; Kelly, S.M.; Lawrence, A.J.; Mezna, M.; Price, N.C. Conformational changes associated with activation of bee venom phospholipase A2. J. Biochem. 1996, 120, 1224–1231. [Google Scholar] [CrossRef]
- Wery, J.P.; Schevitz, R.W.; Clawson, D.K.; Bobbitt, J.L.; Dow, E.R.; Gamboa, G.; Goodson, T., Jr.; Hermann, R.B.; Kramer, R.M.; McClure, D.B. Structure of recombinant human rheumatoid arthritic synovial fluid phospholipase A2 at 2.2 A resolution. Nature 1991, 352, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, A.L.; Nonato, M.C.; de Araújo, H.S.; Arni, R.; Ward, R.J.; Ownby, C.L.; de Souza, D.H.; Garratt, R.C. A molecular mechanism for Lys49-phospholipase A2 activity based on ligand-induced conformational change. J. Biol. Chem. 2005, 280, 7326–7335. [Google Scholar] [CrossRef] [PubMed]
- Nabemoto, M.; Ohsawa, K.; Nakamura, H.; Hirabayashi, T.; Saito, T.; Okuma, Y.; Nomura, Y.; Murayama, T. Reversible activation of secretory phospholipase A2 by sulfhydryl reagents. Arch. Biochem. Biophys. 2005, 436, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.M.; Angelova, M.I.; Söderlund, T.; Kinnunen, P.K.J. Macroscopic consequences of the action of phospholipase C on giant unilamellar liposomes. Biophys. J. 2002, 83, 932–943. [Google Scholar] [CrossRef]
- Billy, D.; Speijer, H.; Zwaal, R.F.A.; Hack, E.C.; Hermens, W.T. Anticoagulant and membrane-degrading effects of secretory (non-pancreatic) phospholipase A2 are inhibited in plasma. Thromb. Haemost. 2002, 87, 978–984. [Google Scholar] [CrossRef]
- Thunnissen, M.M.G.M.; Ab, E.; Kalk, K.H.; Drenth, J.; Dijkstra, B.W.; Kuipers, O.P.; Dijkman, R.; De Haas, G.H.; Verheij, H.M. X-ray structure of phospholipase A2 complexed with a substrate-derived inhibitor. Nature 1990, 347, 689–691. [Google Scholar] [CrossRef]
- Canaan, S.; Nielsen, R.; Ghomashchi, F.; Robinson, B.H.; Gelb, M.H. Unusual mode of binding of human group IIA secreted phospholipase A2 to anionic interfaces as studied by continuous wave and time domain electron paramagnetic resonance spectroscopy. J. Biol. Chem. 2002, 277, 30984–30990. [Google Scholar] [CrossRef]
- Murakami, M.; Masuda, S.; Shimbara, S.; Bezzine, S.; Lazdunski, M.; Lambeau, G.; Gelb, M.H.; Matsukura, S.; Kokubu, F.; Adachi, M.; et al. Cellular arachidonate-releasing function of novel classes of secretory phospholipase A2s (Groups III and XII). J. Biol. Chem. 2003, 278, 10657–10667. [Google Scholar] [CrossRef]
- Verheij, H.M.; Volwerk, J.J.; Jansen, E.H.J.M.; Puyk, W.C.; Dijkistra, B.W.; Drenth, J.; de Haas, G.H. Methylation of Histidine-48 in Pancreatic Phospholipase A2: Role of Histidine and Calcium Ion in the Catalytic Mechanism. Biochemistry 1980, 19, 743–750. [Google Scholar] [CrossRef]
- Maraganore, J.M.; Merutka, G.; Cho, W.; Werlches, W.; Kezdy, F.J.; Heinrickson, R.L. A new class of phospholipases A2 with lysine in place of aspartate 49. Functional consequences for calcium and substrate binding. J. Biol. Chem. 1984, 259, 13839–13843. [Google Scholar] [CrossRef]
- de Araújo, H.S.; White, S.P.; Ownby, C.L. cDNA cloning and sequence analysis of a lysine-49 phospholipase A2 myotoxin from Agkistrodon contortrix laticinctus snake venom. Arch. Biochem. Biophys. 1996, 326, 21–30. [Google Scholar] [CrossRef]
- Schneider, T.; Lang, A.B.; Carballido, J.M.; Santamaria Babi, L.F.; Dudler, T.; Kägi, M.K.; Blaser, K.; Suter, M. Human monoclonal or polyclonal antibodies recognize predominantly discontinuous epitopes on bee venom phospholipase A2. J. Allergy Clin. Immunol. 1994, 94, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Yoshihara, K.; Shimbara, S.; Lambeau, G.; Gelb, M.H.; Singer, A.G.; Sawada, M.; Inagaki, N.; Nagai, H.; Ishihara, M.; et al. Cellular arachidonate-releasing function and inflammation-associated expression of group IIF secretory phospholipase A2. J. Biol. Chem. 2002, 277, 19145–19155. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.P.; Lin, Y.; Lambeau, G.; Ghomashchi, F.; Lazdunski, M.; Gelb, M.H. Localization of structural elements of bee venom phospholipase A2 involved in N-type receptor binding and neurotoxicity. J. Biol. Chem. 1997, 272, 7173–7181. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A. Excitatory amino acid receptors, neural membrane phospholipid metabolism and neurological disorders. Brain Res. Rev. 1991, 16, 171–191. [Google Scholar] [CrossRef]
- Berk, P.D.; Stump, D.D. Mechanisms of cellular uptake of long chain free fatty acids. Mol. Cell. Biochem. 1999, 192, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A. The regulation of eicosanoid production: Role of phospholipases and inhibitors. Bio/Technology 1987, 5, 1294–1300. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef]
- Austin, S.C.; Funk, C.D. Insight into prostaglandin, leukotriene, and other eicosanoid functions using mice with targeted gene disruptions. Prostaglandins Other Lipid Mediat. 1999, 58, 231–252. [Google Scholar] [CrossRef]
- Fourcade, O.; Simon, M.F.; Viodé, C.; Rugani, N.; Leballe, F.; Ragab, A.; Fournié, B.; Sarda, L.; Chap, H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell 1995, 80, 919–927. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Yang, H.C.; Rosenberger, T.A.; Horrocks, L.A. Phospholipase A2 and its role in brain tissue. J. Neurochem. 1997, 69, 889–901. [Google Scholar] [CrossRef]
- Sitprijaa, V.; Suteparak, S. Animal toxins: An overview. Asian Biomed. 2008, 2, 451–457. [Google Scholar]
- Okamoto, K.; Kim, J.S.; Rubin, B.K. Secretory phospholipases A2 stimulate mucus secretion, induce airway inflammation, and produce secretory hyperresponsiveness to neutrophil elastase in ferret trachea. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Arakawa, H.; Yamashita, S.; Sakamoto, K.; Ikei, S. Postoperative elevations of serum interleukin 6 and group II phospholipase A2: Group II phospholipase A2 in serum is an acute phase reactant. Res. Commun. Chem. Pathol. Pharmacol. 1992, 75, 109–115. [Google Scholar]
- Hoffman, D.R.; Shipman, W.H. Allergens in bee venom: I. Separation and identification of the major allergens. J. Allergy Clin. Immunol. 1976, 58, 551–562. [Google Scholar] [CrossRef] [PubMed]
- King, T.P.; Sobotka, A.K.; Kochoumian, L.; Lichtenstein, L.M. Allergens of honey bee venom. Arch. Biochem. Biophys. 1976, 172, 661–671. [Google Scholar] [CrossRef]
- Sobotka, A.K.; Franklin, R.M.; Adkinson, N.F., Jr.; Valentine, M.; Baer, H.; Lichtenstein, L.M. Allergy to insect stings: II. Phospholipase A: The major allergen in honeybee venom. J. Allergy Clin. Immunol. 1976, 57, 29–40. [Google Scholar] [CrossRef]
- Aalberse, R.C.; van der Gaag, R.; van Leeuwen, J. Serologic aspects of IgG4 antibodies. J. Immunol. 1983, 130, 722–726. [Google Scholar] [CrossRef]
- Müller, U.; Fricker, M.; Wymann, D.; Blaser, K.; Crameri, R. Increased specificity of diagnostic tests with recombinant major bee venom allergen phospholipase A2. Clin. Exp. Allergy 1997, 27, 915–920. [Google Scholar] [CrossRef]
- Müller, U.; Akdis, C.A.; Fricker, M.; Akdis, M.; Blesken, T.; Bettens, F.; Blaser, K. Successful immunotherapy with T-cell epitope peptides of bee venom phospholipase A2 induces specific T-cell anergy in patients allergic to bee venom. J. Allergy Clin. Immunol. 1998, 101, 747–754. [Google Scholar] [CrossRef]
- Carballido, J.M.; Carballido-Perrig, N.; Kägi, M.K.; Meloen, R.H.; Wüthrich, B.; Heusser, C.H.; Blaser, K. T cell epitope specificity in human allergic and nonallergic subjects to bee venom phospholipase A2. J. Immunol. 1993, 150 (8 Pt 1), 3582–3591. [Google Scholar] [CrossRef] [PubMed]
- Kämmerer, R.; Kettner, A.; Chvatchko, Y.; Dufour, N.; Tiercy, J.M.; Corradin, G.; Spertini, F. Delineation of PLA2 epitopes using short or long overlapping synthetic peptides: Interest for specific immunotherapy. Clin. Exp. Allergy 1997, 27, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Nishizaki, K.; Satoskar, A.R.; Yoshino, T.; Masuda, Y.; Harn, D.A. Involvement of carbohydrate on phospholipase A2, a bee-venom allergen, in in vivo antigenspecific IgE synthesis in mice. Allergy 1999, 54, 811–818. [Google Scholar] [CrossRef]
- Prenner, C.; Mach, L.; Glossl, J.; Marz, L. The antigenicity of the carbohydrate moiety of an insect glycoprotein, honey-bee (Apis mellifera) venom phospholipase A2. The role of alpha 1,3-fucosylation of the asparagine-bound Nacetylglucosamine. Biochem. J. 1992, 284, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Dudler, T.; Altmann, F.; Carballido, J.M.; Blaser, K. Carbohydrate-dependent, HLA class II-restricted, human T cell response to the bee venom allergen phospholipase A2 in allergic patients. Eur. J. Immunol. 1995, 25, 538–542. [Google Scholar] [CrossRef]
- Dudler, T.; Machado, D.C.; Kolbe, L.; Annand, R.R.; Rhodes, N.; Gelb, M.H.; Koelsch, K.; Suter, M.; Helm, B.A. A link between catalytic activity, IgE-independent mast cell activation, and allergenicity of bee venom phospholipase A2. J. Immunol. 1995, 155, 2605–2613. [Google Scholar] [CrossRef]
- Müller, U.; Dudler, T.; Schneider, T.; Crameri, R.; Fischer, H.; Skrbic, D.; Maibach, R.; Blaser, K.; Suter, M. Type I skin reactivity to native and recombinant phospholipase A2 from honey bee venom is similar. J. Allergy Clin. Immunol. 1995, 96, 395–402. [Google Scholar] [CrossRef]
- Perez-Riverol, A.; Lasa, A.M.; Dos Santos-Pinto, J.R.A.; Palma, M.S. Insect venom phospholipases A1 and A2: Roles in the envenoming process and allergy. Insect Biochem. Mol. Biol. 2019, 105, 10–24. [Google Scholar] [CrossRef]
- Blaser, K.; Carballido, J.M.; Faith, A.; Crameri, R.; Akdis, C.A. Determinants and mechanisms of human immune responses to bee venom phospholipase A2. Int. Arch. Allergy Immunol. 1998, 117, 1–10. [Google Scholar] [CrossRef]
- Petroianu, G.; Lin, J.; Helfrich, U.; Maleck, W.; Rufer, R. Phospholipase A2-induced coagulation abnormalities after bee sting. Ann. J. Emerg. Med. 2000, 18, 22–27. [Google Scholar] [CrossRef]
- Boffa, M.C.; Rothen, C.; Verheij, H.M.; Verger, R.; De Haas, G.H. Classification of phospholipases A2 based upon their anticoagulant activity and penetration ability into phospholipid monolayers. Nat. Toxins 1980, 131–138. [Google Scholar] [CrossRef]
- Kini, R.M.; Evans, H.J. Structure-function relationships of phospholipases. The anticoagulant region of phospholipases A. J. Biol. Chem. 1987, 262, 14402–14407. [Google Scholar] [CrossRef] [PubMed]
- Verheij, H.M.; Boffa, M.C.; Rothen, C.; Bryckaert, M.C.; Verger, R.; de Haas, G.H. Correlation of enzymatic activity and anticoagulant properties of phospholipase A2. Eur. J. Biochem. 1980, 112, 25–32. [Google Scholar] [CrossRef]
- Condrea, E.; Fletcher, J.E.; Rapuano, B.E.; Yang, C.C.; Rosenberg, P. Effect of modification of one histidine residue on the enzymatic and pharmacological properties of a toxic phospholipase A2 from Naja nigricollis snake venom and less toxic phospholipases A2 from Hemachatus haemachatus and Naja naja atra snake venoms. Toxicon 1981, 19, 61–71. [Google Scholar] [CrossRef]
- Condrea, E.; Rapuano, B.E.; Fletcher, J.E.; Yang, C.C.; Rosenberg, P. Ethoxyformylation and guanidination of snake venom phospholipases A2: Effects on enzymatic activity, lethality and some pharmacological properties. Toxicon 1983, 21, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Kalita, B.; Thakur, R. Two acidic, anticoagulant PLA2 isoenzymes purified from the venom of monocled cobra Naja kaouthia exhibit different potency to inhibit thrombin and factor Xa via phospholipids independent, non-enzymatic mechanism. PLoS ONE 2014, 9, e101334. [Google Scholar] [CrossRef]
- Kini, R.M. Structure-function relationships and mechanism of anticoagulant phospholipase A2 enzymes from snake venoms. Toxicon 2005, 45, 1147–1161. [Google Scholar] [CrossRef]
- Ouyang, C.; Lin, S.C.; Teng, C.M. Anticoagulant properties of Apis mellifera (honey bee) venom. Toxicon 1979, 17, 197–201. [Google Scholar] [CrossRef]
- Obeid, L.M.; Linardic, C.M.; Karolak, L.A.; Hannun, Y.A. Programmed cell death induced by ceramide. Science 1993, 259, 1769–1771. [Google Scholar] [CrossRef]
- MacEwan, D.J. Elevated cPLA2 levels as a mechanism by which the p70 TNF and p75 NGF receptors enhance apoptosis. FEBS Lett. 1996, 379, 77–81. [Google Scholar] [CrossRef]
- Wu, M.X.; Ao, Z.; Prasad, K.V.; Wu, R.; Schlossman, S.F. IEX-1L, an apoptosis inhibitor involved in NF-kappaB-mediated cell survival. Science 1998, 281, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.L.; Larkin, H.E.; Zaidi, S.I.; Mukhtar, H.; Oleinick, N.L. Phospholipase activation triggers apoptosis in photosensitized mouse lymphoma cells. Cancer Res. 1993, 53, 5897–5902. [Google Scholar]
- Jäättelä, M.; Benedict, M.; Tewari, M.; Shayman, J.A.; Dixit, V.M. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene 1995, 10, 2297–2305. [Google Scholar]
- Hatanaka, Y.; Suzuki, K.; Kawasaki, Y.; Endo, Y.; Taniguchi, N.; Takei, N. A role of peroxides in Ca2+ ionophore-induced apoptosis in cultured rat cortical neurons. Biochem. Biophys. Res. Commun. 1996, 227, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jang, H.J.; Leem, J.; Kim, G.M. Protective effects of bee venom-derived phospholipase A2 against cholestatic liver disease in mice. Biomedicines 2021, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Park, S.Y.; Ku, S.J.; Ryu, K.; Kim, Y.; Bae, H.; Lee, Y.S. Bee venom phospholipase A2 induces regulatory T cell populations by suppressing apoptotic signaling pathway. Toxins 2020, 12, 198. [Google Scholar] [CrossRef]
- Chen, M.; Bie, L.; Ying, J. Cancer cell-intrinsic PD-1: Its role in malignant progression and immunotherapy. Biomed. Pharmacother. 2023, 167, 115514. [Google Scholar] [CrossRef]
- Van Coillie, S.; Wiernicki, B.; Xu, J. Molecular and cellular functions of CTLA-4. Adv. Exp. Med. Biol. 2020, 1248, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Valentin, E.; Lefebvre, J.C.; Lazdunski, M.; Doglio, A. Secreted phospholipases A2, a new class of HIV inhibitors that block virus entry into host cells. J. Clin. Investig. 1999, 104, 611–618. [Google Scholar] [CrossRef]
- Fenard, D.; Lambeau, G.; Maurin, T.; Lefebvre, J.C.; Doglio, A. A peptide derived from bee venom-secreted phospholipase A2 inhibits replication of T-cell tropic HIV-1 strains via interaction with the CXCR4 chemokine receptor. Mol. Pharmacol. 2001, 60, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.A.; Shimizu, J.F.; de Oliveira, D.M.; Martins, D.O.S.; Cardoso-Sousa, L.; Cintra, A.C.O.; Aquino, V.H.; Sampaio, S.V.; Nicolau-Junior, N.; Sabino-Silva, R.; et al. Chikungunya virus entry is strongly inhibited by phospholipase A2 isolated from the venom of Crotalus durissus terrificus. Sci. Rep. 2021, 11, 8717. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Aoki-Utsubo, C.; Kameoka, M.; Deng, L.; Terada, Y.; Kamitani, W.; Sato, K.; Koyanagi, Y.; Hijikata, M.; Shindo, K.; et al. Broad-spectrum antiviral agents: Secreted phospholipase A2 targets viral envelope lipid bilayers derived from the endoplasmic reticulum membrane. Sci. Rep. 2017, 7, 15931. [Google Scholar] [CrossRef] [PubMed]
- Muller, V.D.; Russo, R.R.; Cintra, A.C.; Sartim, M.A.; Alves-Paiva, R.M.; Figueiredo, L.T.; Sampaio, S.V.; Aquino, V.H. Crotoxin and phospholipases A2 from Crotalus durissus terrificus showed antiviral activity against dengue and yellow fever viruses. Toxicon 2012, 59, 507–515. [Google Scholar] [CrossRef]
- Muller, V.D.; Soares, R.O.; dos Santos, N.N., Jr.; Trabuco, A.C.; Cintra, A.C.; Figueiredo, L.T.; Caliri, A.; Sampaio, S.V.; Aquino, V.H. Phospholipase A2 isolated from the venom of Crotalus durissus terrificus inactivates dengue virus and other enveloped viruses by disrupting the viral envelope. PLoS ONE 2014, 9, e112351. [Google Scholar] [CrossRef]
- Perumal Samy, R.; Gopalakrishnakone, P.; Thwin, M.M.; Chow, T.K.; Bow, H.; Yap, E.H.; Thong, T.W. Antibacterial activity of snake, scorpion and bee venoms: A comparison with purified venom phospholipase A2 enzymes. J. Appl. Microbiol. 2007, 102, 50–59. [Google Scholar] [CrossRef]
- Beers, S.A.; Buckland, A.G.; Koduri, R.S.; Cho, W.; Gelb, M.H.; Wilton, D.C. The antibacterial properties of secreted phospholipases A2. A major physiological role for the group IIa enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J. Biol. Chem. 2002, 277, 1788–1793. [Google Scholar] [CrossRef]
- Boutrin, M.C.F.; Foster, H.A.; Pentreath, V.W. The effects of bee (Apis mellifera) venom phospholipase A2 on Trypanosoma brucei brucei and enterobacteria. Exp. Parasitol. 2008, 119, 246–251. [Google Scholar] [CrossRef]
- Deregnaucourt, C.; Schrével, J. Bee venom phospholipase A2 induces stage-specific growth arrest of the intraerythrocytic Plasmodium falciparum via modifications of human serum components. J. Biol. Chem. 2000, 275, 39973–39980. [Google Scholar] [CrossRef]
- Guillaume, C.; Calzada, C.; Lagarde, M.; Schrével, J.; Deregnaucourt, C. Interplay between lipoproteins and bee venom phospholipase A2 in relation to their anti-Plasmodium toxicity. J. Lipid Res. 2006, 47, 1493–1506. [Google Scholar] [CrossRef]
- Moreira, L.A.; Ito, J.; Ghosh, A.; Devenport, M.; Zieler, H.; Abraham, E.G.; Crisanti, A.; Nolan, T.; Catteruccia, F.; Jacobs-Lorena, M. Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J. Biol. Chem. 2002, 277, 40839–40843. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T.J.; Martikainen, P.M. Different toxicity of pancreatic, snake and bee venom phospholipase A2 on MCF-7 cells grown in culture. Toxicon 1992, 30, 539–540. [Google Scholar]
- Putz, T.; Ramoner, R.; Gander, H.; Rahm, A.; Bartsch, G.; Bernardo, K.; Ramsay, S.; Thurnher, M. Bee venom secretory phospholipase A2 and phosphatidylinositol-homologues cooperatively disrupt membrane integrity, abrogate signal transduction and inhibit proliferation of renal cancer cells. Cancer Immunol. Immunother. 2007, 56, 627–640. [Google Scholar] [CrossRef]
- Putz, T.; Ramoner, R.; Gander, H.; Rahm, A.; Bartsch, G.; Thurnher, M. Antitumor action and immune activation through cooperation of bee venom secretory phospholipase A2 and phosphatidylinositol-(3,4)-bisphosphate. Cancer Immunol. Immunother. 2006, 55, 1374–1383. [Google Scholar] [CrossRef]
- Mollay, C.; Kreil, G. Enhancement of bee venom phospholipase A2 activity by melittin, direct lytic factor from cobra venom and polymyxin B. FEBS Lett. 1974, 46, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.W.; Wei, H.C.; Lin, J.P.; Kuo, H.M.; Liu, K.C.; Hsu, S.C.; Yang, J.S.; Mei, D.; Chiu, T.H.; Han, S.M.; et al. Bee venom induced cell cycle arrest and apoptosis in human cervical epidermoid carcinoma Ca Ski cells. Anticancer Res. 2008, 28, 833–842. [Google Scholar]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K.; Ho, D.; Johnstone, E.; Pfleger, K.; Redfern, A.; et al. Honeybee venom and melittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. NPJ Precis. Oncol. 2020, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Yaacoub, C.; Rifi, M.; El-Obeid, D.; Mawlawi, H.; Sabatier, J.M.; Coutard, B.; Fajloun, Z. The cytotoxic effect of Apis mellifera venom with a synergistic potential of its two main components-melittin and PLA2-on colon cancer HCT116 cell lines. Molecules 2021, 26, 2264. [Google Scholar] [CrossRef]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Shi, P.; Xie, S.; Yang, J.; Zhang, Y.; Han, S.; Su, S.; Yao, H. Pharmacological effects and mechanisms of bee venom and its main components: Recent progress and perspective. Front. Pharmacol. 2022, 13, 1001553. [Google Scholar] [CrossRef]
- de Vasconcelos Azevedo, F.V.P.; Zóia, M.A.P.; Lopes, D.S.; Gimenes, S.N.; Vecchi, L.; Alves, P.T.; Rodrigues, R.S.; Silva, A.C.A.; Yoneyama, K.A.G.; Goulart, L.R.; et al. Antitumor and antimetastatic effects of PLA2-BthTX-II from Bothrops jararacussu venom on human breast cancer cells. Int. J. Biol. Macromol. 2019, 135, 261–273. [Google Scholar] [CrossRef]
- Bazaa, A.; Luis, J.; Srairi-Abid, N.; Kallech-Ziri, O.; Kessentini-Zouari, R.; Defilles, C.; Lissitzky, J.C.; El Ayeb, M.; Marrakchi, N. MVL-PLA2, a phospholipase A2 from Macrovipera lebetina transmediterranea venom, inhibits tumor cells adhesion and migration. Matrix Biol. 2009, 28, 188–193. [Google Scholar] [CrossRef]
- Araya, C.; Lomonte, B. Antitumor effects of cationic synthetic peptides derived from Lys49 phospholipase A2 homologues of snake venoms. Cell Biol. Int. 2007, 31, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Yunes, R.; Goldhammer, A.R.; Garner, W.K.; Cordes, E.H. Phospholipases: Melittin facilitation of bee venom phospholipase A2-catalyzed hydrolysis of unsonicated lecithin liposomes. Arch. Biochem. Biophys. 1977, 183, 105–112. [Google Scholar] [CrossRef]
- Cajal, Y.; Jain, M.K. Synergism between mellitin and phospholipase A2 from bee venom: Apparent activation by intervesicle exchange of phospholipids. Biochemistry 1997, 36, 3882–3893. [Google Scholar] [CrossRef] [PubMed]
- Ownby, C.L.; Powell, J.R.; Jiang, M.S.; Fletcher, J.E. Melittin and phospholipase A2 from bee (Apis mellifera) venom cause necrosis of murine skeletal muscle in vivo. Toxicon 1997, 35, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Koumanov, K.; Momchilova, A.; Wolf, C. Bimodal regulatory effect of melittin and phospholipase A2-activating protein on human type II secretory phospholipase A2. Cell Biol. Int. 2003, 27, 871–877. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, H.S.; Lee, S.J.; Choi, M.U. Melittin exerts multiple effects on the release of free fatty acids from L1210 cells: Lack of selective activation of phospholipase A2 by melittin. Arch. Biochem. Biophys. 2001, 389, 57–67. [Google Scholar] [CrossRef]
- Schumacher, M.J.; Schmidt, J.O.; Egen, N.B. Lethality of “killer” bee stings. Nature 1989, 337, 413. [Google Scholar] [CrossRef]
- Bhat, M.K.; Gowda, T.V. Purification and characterization of a myotoxic phospholipase A2 from Indian cobra (Naja naja naja) venom. Toxicon 1989, 27, 861–873. [Google Scholar] [CrossRef]
- Nisenbom, H.E.; Perazzo, J.C.; Monserrat, A.J.; Vidal, J.C. Contribution of phospholipase A2 to the lethal potency of Bothrops alternatus (víbora de la cruz) venom. Toxicon 1986, 24, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Keyser, D.O.; Alger, B.E. Arachidonic acid modulates hippocampal calcium current via protein kinase C and oxygen radicals. Neuron 1990, 5, 545–553. [Google Scholar] [CrossRef]
- Rothschild, A.M. Histamine release by bee venom phospholipase A and mellitin in the rat. Br. J. Pharmacol. Chemother. 1965, 25, 59–66. [Google Scholar] [CrossRef]
- Noetzel, C.; Chandra, V.; Perbandt, M.; Rajashankar, K.; Singh, T.; Aleksiev, B.; Kalkura, N.; Genov, N.; Betzel, C. Enzymatic activity and inhibition of the neurotoxic complex vipoxin from the venom of Vipera ammodytes meridionalis. Z. Naturforsch 2002, 57, 1078–1083. [Google Scholar] [CrossRef][Green Version]
- Lambeau, G.; Lazdunski, M.; Barhanin, J. Properties of receptors for neurotoxic phospholipases A2 in different tissues. Neurochem. Res. 1991, 16, 651–658. [Google Scholar] [CrossRef]
- Lambeau, G.; Lazdunski, M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol. Sci. 1999, 20, 162–170. [Google Scholar] [CrossRef]
- Valentin, E.; Lambeau, G. Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2000, 1488, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Puică, C.; Vinţan, M.; Benga, I.; Crăciun, C. Electrophysiological and structural aspects in the frontal cortex after the bee (Apis mellifera) venom experimental treatment. Cell. Mol. Neurobiol. 2011, 31, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, G.; Lambeau, G.; Lazdunski, M.; Gottesmann, C. Effects on behaviour and EEG of single chain Phospholipases A2 from snake and bee venoms injected into rat brain: Search for a functional antagonism. Pharmacol. Toxicol. 1996, 78, 341–347. [Google Scholar] [CrossRef]
- Pungerčar, J.; Križaj, I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A2. Toxicon 2007, 50, 871–892. [Google Scholar] [CrossRef]
- Nicholls, D.; Snelling, R.; Dolly, J.O. Bioenergetic actions of β-bungarotoxin, dendrotoxin and bee-venom phospholipase A2 on guinea-pig synaptosomes. Biochem. J. 1985, 229, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Sribar, J.; Copic, A.; Paris, A.; Sherman, N.E.; Gubensek, F.; Fox, J.W.; Krizaj, I. A high affinity acceptor for phospholipase A2 with neurotoxic activity is a calmodulin. J. Biol. Chem. 2001, 276, 12493–12496. [Google Scholar] [CrossRef] [PubMed]
- Sorg, O.; Pellerin, L.; Stolz, M.; Beggah, S.; Magistretti, P.J. Adenosine triphosphate and arachidonic acid stimulate glycogenolysis in primary cultures of mouse cerebral cortical astrocytes. Neurosci. Lett. 1995, 188, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, H.; Okuda, S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog. Neurobiol. 1995, 46, 607–636. [Google Scholar] [CrossRef]
- Ham, H.J.; Han, J.H.; Lee, Y.S.; Kim, K.C.; Yun, J.; Kang, S.K.; Park, Y.; Kim, S.H.; Hong, J.T. Bee venom soluble phospholipase A2 exerts neuroprotective effects in a lipopolysaccharide-induced mouse model of Alzheimer’s disease via inhibition of nuclear factor-kappa B. Front. Aging Neurosci. 2019, 11, 287. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Ownby, C.L. Skeletal muscle degeneration induced by venom phospholipases A2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon 2003, 42, 915–931. [Google Scholar] [CrossRef]
- Lambeau, G.; Ancian, P.; Nicolas, J.P.; Beiboers, S.H.W.; Moinier, D.; Verheij, H.; Lazdunski, M. Structural elements of secretory phospholipases A2 involved in the binding to M-type receptors. J. Biol. Chem. 1995, 270, 5534–5540. [Google Scholar] [CrossRef]
- Kim, H.; Keum, D.J.; Kwak, J.W.; Chung, H.S.; Bae, H. Bee venom phospholipase A2 protects against acetaminophen-induced acute liver injury by modulating regulatory T cells and IL-10 in mice. PLoS ONE 2014, 9, e114726. [Google Scholar] [CrossRef]
- Kolecki, P. Delayed toxic reaction following massive bee envenomation. Ann. Emerg. Med. 1999, 33, 114–116. [Google Scholar] [CrossRef]
- Bresolin, N.L.; Carvalho, F.L.C.; Goes, J.E.C.; Fernandes, V.R.; Barotto, A.M. Acute renal failure following massive attack by Africanized bee stings. Pediatr. Nephrol. 2002, 17, 625–627. [Google Scholar] [CrossRef]
- Daher, E.D.; Silva, G.B.; Bezerra, G.P.; Pontes, L.B.; Martins, A.M.C.; Guimaraes, J.A. Acute renal failure after massive honeybee stings. Rev. Inst. Med. Trop. S. Paulo 2003, 45, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Odinaka, K.K.; Achigbu, K.; Ike, I.; Iregbu, F. Bee sting envenomation resulting in gross haematuria in an eight-year-old Nigerian male with sickle cell anaemia: A case report. Niger. Med. J. 2015, 56, 69–70. [Google Scholar] [CrossRef]
- Cowell, A.K.; Cowell, R.L.; Tyler, R.D.; Nieves, M.A. Severe systemic reactions to Hymenoptera stings in three dogs. J. Am. Vet. Med. Assoc. 1991, 198, 1014–1016. [Google Scholar] [CrossRef]
- Oliveira, E.C.; Pedroso, P.M.O.; Meirelles, A.E.W.B.; Pescador, C.A.; Gouvea, A.S.; Driemeier, D. Pathological findings in dogs after multiple Africanized bee stings. Toxicon 2007, 49, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, B.S.; Sood, N.K.; Gupta, K.; Singh, A. Honey bee (Apis mellifera) envenomation induced death in a mare. Ind. J. Vet. Pathol. 2011, 35, 77–79. [Google Scholar]
- Lewis, N.; Racklyeft, D.J. Mass envenomation of a mare and foal by bees. Aust. Vet. J. 2014, 92, 141–148. [Google Scholar] [CrossRef]
- França, F.O.; Benvenuti, L.A.; Fan, H.W.; Dos Santos, D.R.; Hain, S.H.; Picchi-Martins, F.R.; Cardoso, J.L.; Kamiguti, A.S.; Theakston, R.D.; Warrell, D.A. Severe and fatal mass attacks by ’killer’ bees (Africanized honey bees-Apis mellifera scutellata) in Brazil: Clinicopathological studies with measurement of serum venom concentrations. Q. J. Med. 1994, 87, 269–282. [Google Scholar]
- Schmidt, J.O. Clinical consequences of toxic envenomations by Hymenoptera. Toxicon 2018, 150, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Romero, O.; Bustos, G.J., Jr.; Bustos, G.D.; Azar, M.; Bustos, G.J. IgG1, IgG4 and IgE antibodies anti honey bee venom produced during honey bee venom immunotherapy. J. Allergy Clin. Immunol. 1991, 87, 198. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M.; Blesken, T.; Wymann, D.; Alkan, S.S.; Müller, U.; Blaser, K. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J. Clin. Investig. 1996, 98, 1676–1683. [Google Scholar] [CrossRef]
- Yunginger, J.W.; Dahlberg, M.E.; Jones, R.T.; Parker, J.L.; Carey, T.L. Diagnostic and therapeutic importance of individual honeybee venom allergens. J. Allergy Clin. Immunol. 1982, 69, 139. [Google Scholar] [CrossRef]
- Nair, B.C.; Nair, C.; Denne, S.; Wypych, J.; Arbesman, C.E.; Elliott, W.B. Immunologic comparison of phospholipases A present in Hymenoptera insect venoms. J. Allergy Clin. Immunol. 1976, 58, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jilek, S.; Barbey, C.; Spertini, F.; Corthésy, B. Antigen-independent suppression of the allergic immune response to bee venom phospholipase A2 by DNA vaccination in CBA/J mice. J. Immunol. 2001, 166, 3612–3621. [Google Scholar] [CrossRef] [PubMed]
- Potts, B.C.M.; Faulkner, D.J.; De Carvalho, S.M.; Jacobs, R.S. Chemical mechanism of inactivation of bee venom phospholipase A2 by the marine natural products manoalide, luffariellolide, and scalaradial. J. Am. Chem. Soc. 1992, 114, 5093–5100. [Google Scholar] [CrossRef]
- de Carvalho, M.S.; Jacobs, R.S. Two-step inactivation of bee venom phospholipase A2 by scalaradial. Biochem. Pharmacol. 1991, 42, 1621–1626. [Google Scholar] [CrossRef]
- De Rosa, S.; Crispino, A.; De Giulio, A.; Iodice, C.; Pronzato, R.; Zavodnik, N. Cacospongionolide B, a new sesterterpene from the sponge Fasciospongia cavernosa. J. Nat. Prod. 1995, 58, 1776–1780. [Google Scholar] [CrossRef]
- Cheung, A.K.; Snapper, M.L. Total syntheses of (+)- and (−)-cacospongionolide B: New insight into structural requirements for phospholipase A2 inhibition. J. Am. Chem. Soc. 2002, 124, 11584–11585. [Google Scholar] [CrossRef]
- Zhao, H.; Kinnunen, P.K. Modulation of the activity of secretory phospholipase A2 by antimicrobial peptides. Antimicrob. Agents Chemother. 2003, 47, 965–971. [Google Scholar] [CrossRef]
- Conricode, K.M.; Ochs, R.S. Mechanism for the inhibitory and stimulatory actions of proteins on the activity of phospholipase A2. Biochim. Biophys. Acta-Lipids Lipid Metab. 1989, 1003, 36–43. [Google Scholar] [CrossRef]
- Hains, P.G.; Sung, K.L.; Tseng, A.; Broady, K.W. Functional characteristics of a phospholipase A2 inhibitor from Notechis ater serum. J. Biol. Chem. 2000, 275, 983–991. [Google Scholar] [CrossRef]
- Shao, J.; Shen, H.; Havsteen, B. Purification, characterization and binding interactions of the Chinese-cobra (Naja naja atra) serum antitoxic protein CSAP. Biochem. J. 1993, 293, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, N.; Inoue, S.; Ikeda, K.; Hayashi, K. Isolation and characterization of a phospholipase A2 inhibitor from the blood plasma of the Thailand cobra Naja naja kaouthia. Biochem. Biophys. Res. Commun. 1994, 200, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Fortes-Dias, C.L.; Lin, Y.; Ewell, J.; Diniz, C.R.; Liou, T.Y. A phospholipase A2 inhibitor from the plasma of the South American rattlesnake (Crotalus durissus terrificus). J. Biol. Chem. 1994, 269, 15646–15651. [Google Scholar] [CrossRef]
- Perales, J.; Villela, C.; Domont, G.B.; Choumet, V.; Saliou, B.; Moussatché, H.; Bon, C.; Faure, G. Molecular structure and mechanism of action of the crotoxin inhibitor from Crotalus durissus terrificus serum. Eur. J. Biochem. 1995, 227, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Faure, G.; Villela, C.; Perales, J.; Bon, C. Interaction of the neurotoxic and nontoxic secretory phospholipases A2 with the crotoxin inhibitor from Crotalus serum. Eur. J. Biochem. 2000, 267, 4799–4808. [Google Scholar] [CrossRef]
- Ohkura, N.; Okuhara, H.; Inoue, S.; Ikeda, K.; Hayashi, K. Purification and characterization of three distinct types of phospholipase A2 inhibitors from the blood plasma of the Chinese mamushi, Agkistrodon blomhoffii siniticus. Biochem. J. 1997, 325, 527–531. [Google Scholar] [CrossRef]
- Landucci, E.C.T.; Toyama, M.H.; Marangoni, S.; Benedito, O.; Giuseppe, C.; Antunes, E.; de Nucci, G. Effect of crotapotin and heparin on the rat paw oedema induced by different secretory phospholipases A2. Toxicon 2000, 38, 199–208. [Google Scholar] [CrossRef]
- Francis, B.; Seebart, C.; Kaiser, I.I. Citrate is an endogenous inhibitor of snake venom enzymes by metal-ion chelation. Toxicon 1992, 30, 1239–1246. [Google Scholar] [CrossRef]
- Fenton, A.W.; West, P.R.; Odell, G.V.; Hudiburg, S.M.; Ownby, C.L.; Mills, J.N.; Scroggins, B.T.; Shannon, S.B. Arthropod venom citrate inhibits phospholipase A2. Toxicon 1995, 33, 763–770. [Google Scholar] [CrossRef]
- Dempsey, C.E. The actions of melittin on membranes. Biochim. Biophys. Acta-Rev. Biomembr. 1990, 1031, 143–161. [Google Scholar] [CrossRef]
- Yagami, T.; Ueda, K.; Asakura, K.; Hata, S.; Kuroda, T.; Sakaeda, T.; Takasu, N.; Tanaka, K.; Gemba, T.; Hori, Y. Human group IIA secretory phospholipase A2 induces neuronal cell death via apoptosis. Mol. Pharmacol. 2002, 61, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C.; Rose, S.P. Inhibitors of phospholipase A2 produce amnesia for a passive avoidance task in the chick. Behav. Neural Biol. 1994, 61, 225–232. [Google Scholar] [CrossRef]
- Yang, H.C.; Farooqui, A.A.; Horrocks, L.A. Effects of glycosaminoglycans and glycosphingolipids on cytosolic phospholipases A2 from bovine brain. Biochem. J. 1994, 299, 91–95. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Yang, H.C.; Horrocks, L.A. Plasmalogens, phospholipases A2, and signal transduction. Brain Res. Rev. 1995, 21, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.M.; Kim, D.K.; Bonventre, J.V.; Kish, S.J. Characterization of a novel phospholipase A2 activity in human brain. J. Neurochem. 1995, 64, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.; Dennis, E.A. Antisense inhibition of group II phospholipase A2 expression blocks the production of prostaglandin E2 by P388Dl cells. J. Biol. Chem. 1993, 268, 875–882. [Google Scholar] [CrossRef]
- Balsinde, J.; Barbour, S.E.; Bianco, I.D.; Dennis, E.A. Arachidonic acid mobilization in P388D1 macrophages is controlled by two distinct Ca2+-dependent phospholipase A2s. Proc. Natl. Acad. Sci. USA 1994, 91, 11060–11064. [Google Scholar] [CrossRef]
- Balsinde, J.; Dennis, E.A. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J. Biol. Chem. 1996, 271, 6758–6765. [Google Scholar] [CrossRef]
- Crous, C.; Petzer, A.; Petzer, J.P. Interactions of small molecule inhibitors with secreted phospholipase A2: A review of the structural data. Chem. Biol. Drug Des. 2024, 103, e14466. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntean, M.; Florea, A. Phospholipase A2—A Significant Bio-Active Molecule in Honeybee (Apis mellifera L.) Venom. Molecules 2025, 30, 2623. https://doi.org/10.3390/molecules30122623
Muntean M, Florea A. Phospholipase A2—A Significant Bio-Active Molecule in Honeybee (Apis mellifera L.) Venom. Molecules. 2025; 30(12):2623. https://doi.org/10.3390/molecules30122623
Chicago/Turabian StyleMuntean, Mara, and Adrian Florea. 2025. "Phospholipase A2—A Significant Bio-Active Molecule in Honeybee (Apis mellifera L.) Venom" Molecules 30, no. 12: 2623. https://doi.org/10.3390/molecules30122623
APA StyleMuntean, M., & Florea, A. (2025). Phospholipase A2—A Significant Bio-Active Molecule in Honeybee (Apis mellifera L.) Venom. Molecules, 30(12), 2623. https://doi.org/10.3390/molecules30122623





