Topical Application of RNAi Therapy Using Surface-Modified Liposomes for Treating Retinal-Vein Occlusion
Abstract
1. Introduction
2. Results and Discussion
2.1. PnkRNA-Loaded Liposomal Eye Drops Inhibit Retinal Edema in Retinal-Vein Occlusion Model Mice
2.1.1. Characterization of PnkRNA-Loaded Liposomes
2.1.2. Mouse Retinal Delivery of PnkRNA by Eye-Drop Administration
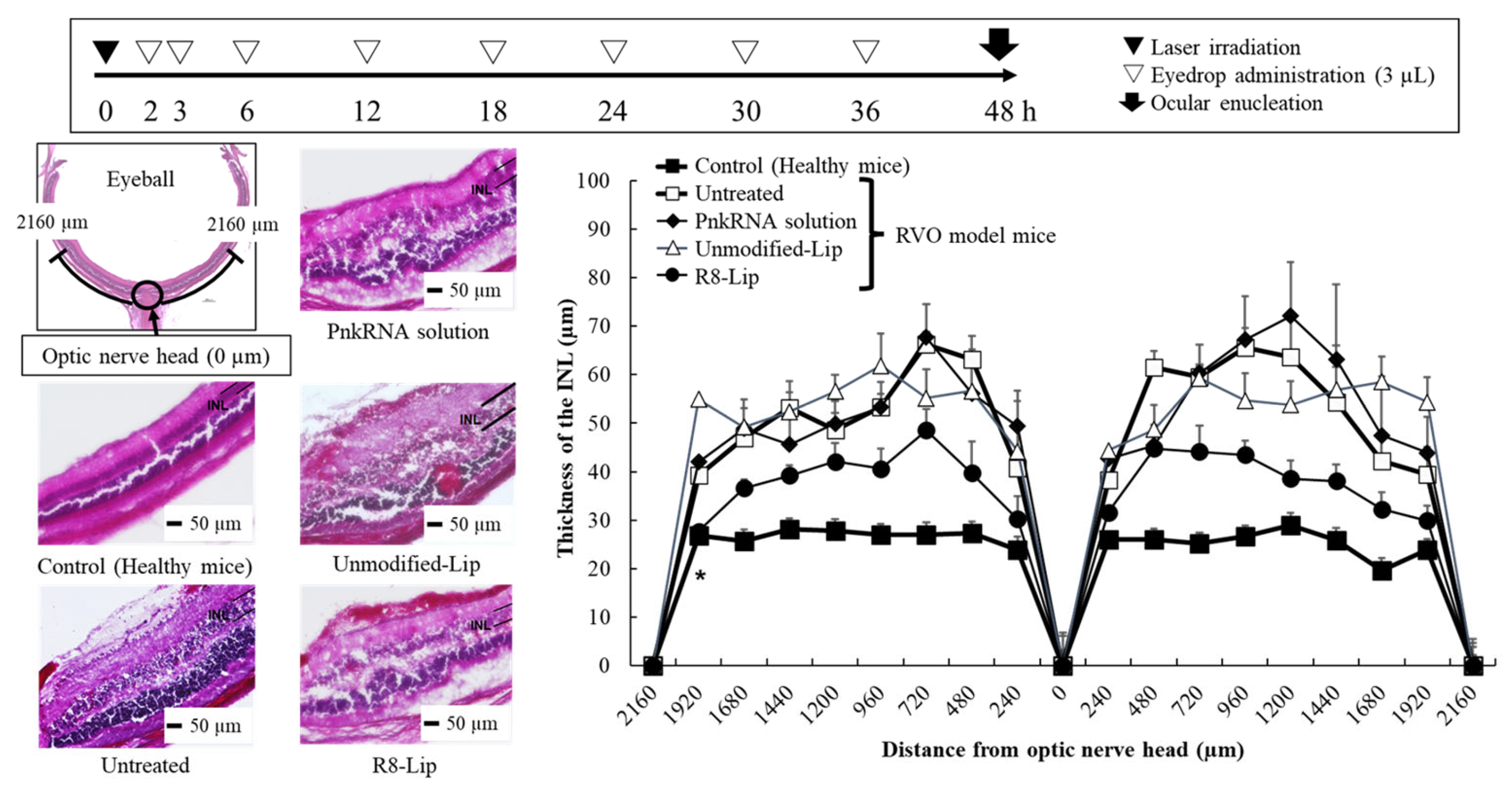
2.1.3. Retinal Edema Therapeutic Evaluation of a Retinal-Vein Occlusion Mouse Model Following PnkRNA-Loaded Liposome Eye-Drop Administration
2.2. Liposome-Loaded Thermoresponsive Gels Reduce the Number of PnkRNA Eye Drop Treatments
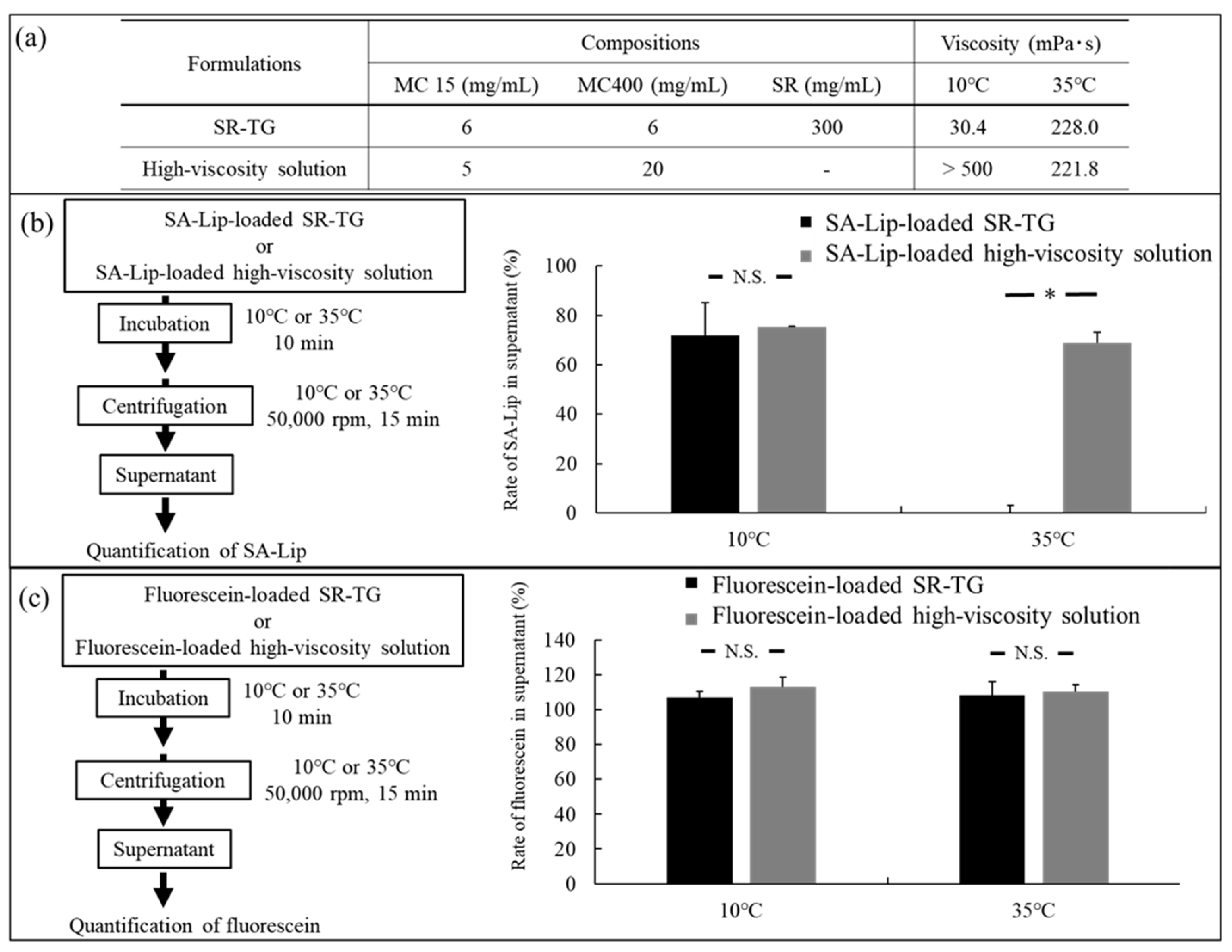
2.2.1. Characterization of the Liposome-Loaded Thermoresponsive Gels
2.2.2. Mouse Retinal Delivery of Coumarin 6 by Thermoresponsive Gel Eye-Drop Administration
2.2.3. Liposome Retention Evaluation of the Thermoresponsive Gels
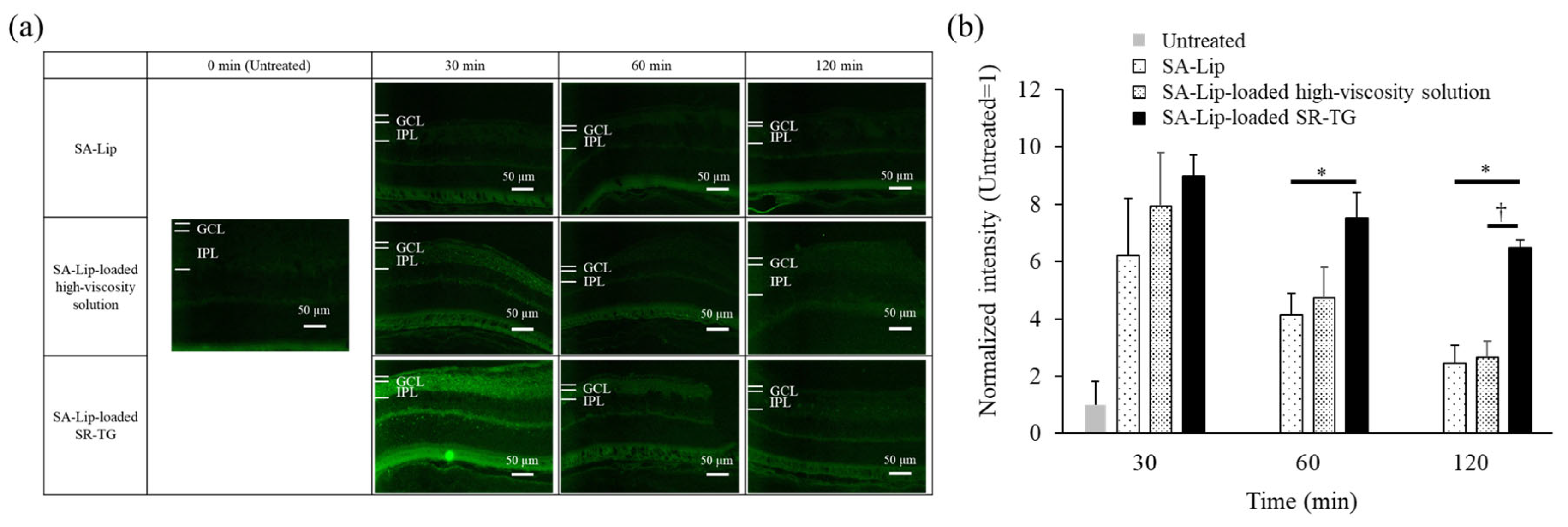
2.2.4. Effect of Liposome Retention of Thermoresponsive Gels on Mouse Retinal Delivery
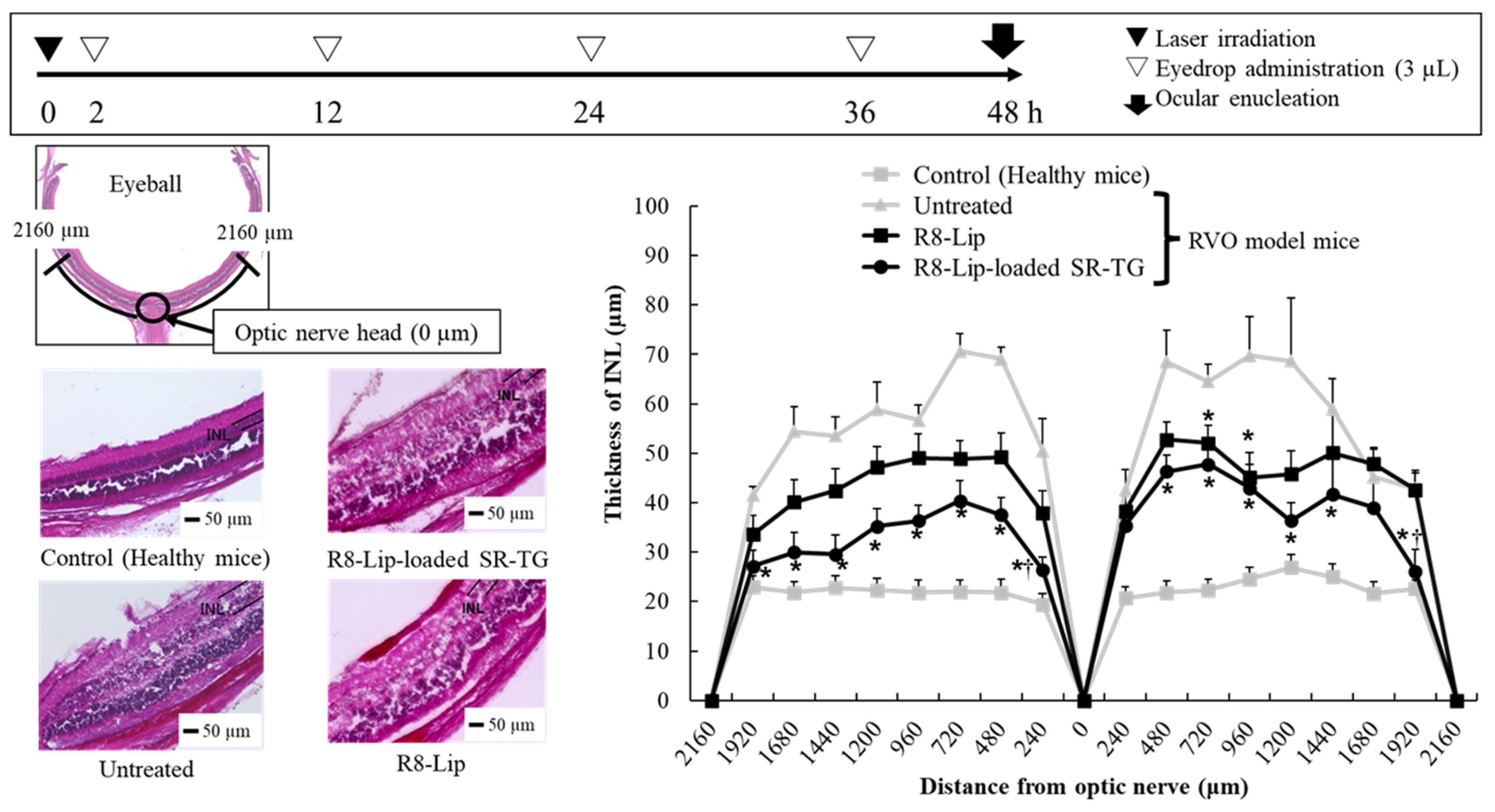
2.2.5. Retinal Edema Therapeutic Evaluation of Retinal-Vein Occlusion Model Mice After Liposome-Loaded Thermoresponsive Gel Eye-Drop Administration
3. Materials and Methods
3.1. Materials
3.2. PnkRNA-Loaded Liposomal Eye Drops Inhibit Retinal Edema in the Retinal-Vein Occlusion Model Mice
3.2.1. Preparation of PnkRNA-Loaded Liposomes by the Thin-Film Hydration Method
3.2.2. Retinal Drug-Delivery Test of TAMRA-Labeled PnkRNA-Loaded Liposomal Eye Drops
3.2.3. Creation of the Retinal-Vein Occlusion Model Mice
3.2.4. Retinal Observation of the Retinal-Vein Occlusion Model Mice After PnkRNA-Loaded Liposome Eye-Drop Administration
3.3. Development of Liposome-Loaded Thermoresponsive Gels
3.3.1. Preparation of Coumarin 6-Loaded Liposomes and Thermoresponsive Gels
3.3.2. Viscosity Test of the Thermoresponsive Gels
3.3.3. Coumarin 6 Retinal Delivery Test of Liposome-Loaded Thermoresponsive Gels
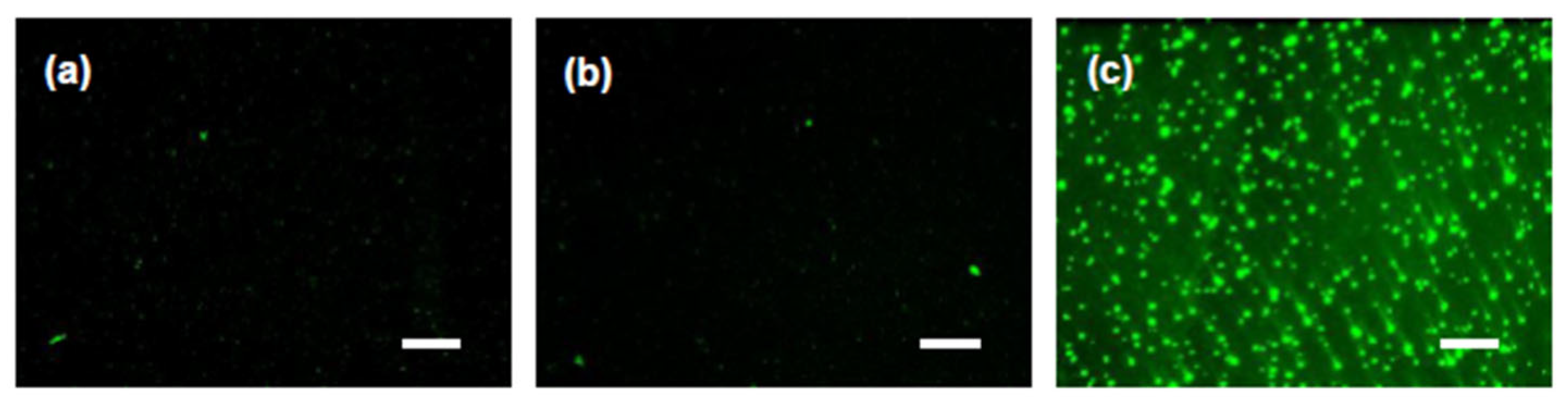
3.3.4. Images of the Liposomes in the Thermoresponsive Gels
3.3.5. Retention Study of Liposomes from the Thermoresponsive Gels
3.3.6. Retinal Observation of Retinal-Vein Occlusion Model Mice After Liposome-Loaded Thermoresponsive Gel Administration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| ANOVA | Analysis of variance |
| C6 | Coumarin 6 |
| COVID-19 | Coronavirus disease 2019 |
| DDS | Drug-delivery system |
| EPC | Egg phosphatidylcholine |
| GCL | Ganglion cell layer |
| HBSS | Hank’s balanced salt solution |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| MC | Methylcellulose |
| PBS | Phosphate-buffered saline |
| PEG | Polyethylene glycol |
| R8 | Stearoyl-octa-arginine |
| RVO | Retinal-vein occlusion |
| SA | Stearylamine |
| SC | Tri-sodium citrate dihydrate |
| SR | Sorbitol |
| TGF-β1 | Transforming growth factor-β1 |
| VEGF | Vascular endothelial growth factor |
References
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Vision loss expert group, magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Lamanna, F.; Gabrielle, P.H.; Teo, K.Y.C.; Parodi, M.B.; Iacono, P.; Fraser-Bell, S.; Cornish, E.E.; Nassisi, M.; Viola, F.; et al. Update on retinal vein occlusion. Asia-Pac. J. Ophthalmol. 2023, 12, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Noma, H.; Yasuda, K.; Shimura, M. Cytokines and pathogenesis of central retinal vein occlusion. J. Clin. Med. 2020, 9, 3457. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Flynn, H.W., Jr. Endophthalmitis after intravitreal injection: Prevention and management. Retina 2011, 31, 633–635. [Google Scholar] [CrossRef]
- Novack, G.D. Ophthalmic drug delivery: Development and regulatory considerations. Clin. Pharmacol. Ther. 2009, 85, 539–543. [Google Scholar] [CrossRef]
- Hori, M.; Sugano, K.; Kadowaki, T.; Inui, K.; Hayashi, M. Handbook of Clinical Drug 2024; Jiho, Inc.: Tokyo, Japan, 2024; ISBN 13 978-4840755467. [Google Scholar]
- Qi, Q.; Wei, Y.; Zhang, X.; Guan, J.; Mao, S. Challenges and strategies for ocular posterior diseases therapy via non-invasive advanced drug delivery. J. Control. Release 2023, 361, 191–211. [Google Scholar] [CrossRef]
- Dehghani, M.; Zahir-Jouzdani, F.; Shahbaz, S.; Andarzbakhsh, K.; Dinarvand, S.; Nasab, M.H.F.; Amoli, F.A.; Asgharian, R.; Atyabi, F. Triamcinolone-loaded self nano-emulsifying drug delivery systems for ocular use: An alternative to invasive ocular surgeries and injections. Int. J. Pharm. 2024, 653, 123840. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Gu, Y.; Cheng, Y.; Cao, F. Recent advance of nanoparticle-based topical drug delivery to the posterior segment of the eye. Expert. Opin. Drug Deliv. 2018, 15, 687–701. [Google Scholar] [CrossRef]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. Size effect of rebamipide ophthalmic nanodispersions on its therapeutic efficacy for corneal wound healing. Exp. Eye Res. 2016, 151, 47–53. [Google Scholar] [CrossRef]
- Hironaka, K.; Inokuchi, Y.; Tozuka, Y.; Shimazawa, M.; Hara, H.; Takeuchi, H. Design and evaluation of a liposomal delivery system targeting the posterior segment of the eye. J. Control. Release 2009, 136, 247–253. [Google Scholar] [CrossRef]
- Fujisawa, T.; Miyai, H.; Hironaka, K.; Tsukamoto, T.; Tahara, K.; Tozuka, Y.; Ito, M.; Takeuchi, H. Liposomal diclofenac eye drop formulations targeting the retina: Formulation stability improvement using surface modification of liposomes. Int. J. Pharm. 2012, 436, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Karasawa, K.; Hironaka, K.; Tahara, K.; Tozuka, Y.; Takeuchi, H. Retinal drug delivery using eyedrop preparations of poly-L-lysine-modified liposomes. Eur. J. Pharm. Biopharm. 2013, 83, 364–369. [Google Scholar] [CrossRef] [PubMed]
- McClements, M.E.; Elsayed, M.E.A.A.; Major, L.; de la Camara, C.M.; MacLaren, R.E. Gene therapies in clinical development to treat retinal disorders. Mol. Diagn. Ther. 2024, 28, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, V.; Mishra, G.P.; Mitra, A.K. Polymeric vectors for ocular gene delivery. Ther. Deliv. 2011, 2, 523–536. [Google Scholar] [CrossRef]
- Nishida, S.; Takashima, Y.; Udagawa, R.; Ibaraki, H.; Seta, Y.; Ishihara, H. A multifunctional hybrid nanocarrier for non-invasive siRNA delivery to the retina. Pharmaceutics 2023, 15, 611. [Google Scholar] [CrossRef]
- Takanashi, M.; Sudo, K.; Ueda, S.; Ohno, S.; Yamada, Y.; Osakabe, Y.; Goto, H.; Matsunaga, Y.; Ishikawa, A.; Usui, Y.; et al. Novel types of small RNA exhibit sequence- and target-dependent angiogenesis suppression without activation of Toll-like receptor 3 in an age-related macular degeneration (AMD) mouse model. Mol. Ther.-Nucleic Acids 2015, 4, e258. [Google Scholar] [CrossRef]
- Hamasaki, T.; Suzuki, H.; Shirohzu, H.; Matsumoto, T.; D’Alessandro-Gabazza, C.N.; Gil-Bernabe, P.; Boveda-Ruiz, D.; Naito, M.; Kobayashi, T.; Toda, M.; et al. Efficacy of a novel class of RNA interference therapeutic agents. PLoS ONE 2012, 7, e42655. [Google Scholar] [CrossRef]
- Igarashi, J.; Niwa, Y.; Sugiyama, D. Research and development of oligonucleotide therapeutics in Japan for rare diseases. Future Rare Dis. 2020, 2. [Google Scholar] [CrossRef]
- Taketani, Y.; Usui, T.; Toyono, T.; Shima, N.; Yokoo, S.; Kimakura, M.; Yamagami, S.; Ohno, S.; Onodera, R.; Tahara, K.; et al. Topical use of angiopoietin-like protein 2 RNAi-loaded lipid nanoparticles suppresses corneal neovascularization. Mol. Ther.-Nucleic Acids 2016, 5, e292. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Qi, Y.; Chen, Y.; Yang, X.; Li, Y.; Liu, S.; Lee, R.J. Delivery of siRNA using cationic liposomes incorporating stearic acid-modified octa-arginine. Anticancer Res. 2016, 36, 3271–3276. [Google Scholar]
- Khalil, I.; Kogure, K.; Futaki, S.; Hama, S.; Akita, H.; Ueno, M.; Kishida, H.; Kudoh, M.; Mishina, Y.; Kataoka, K.; et al. Octaarginine-modified multifunctional envelope-type nanoparticles for gene delivery. Gene Ther. 2007, 14, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, T.; Kashiwagi, K.; Suzuki, Y.; Yoshikawa, K.; Suzumura, H.; Maeda, T.; Takeda, R.; Saito, H.; Araie, M. A nationwide survey of factors influencing adherence to ocular hypotensive eyedrops in Japan. Int. Ophthalmol. 2019, 39, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Desbrieres, J.; Hirrien, M.; Ross-Murphy, S.B. Thermogelation of methylcellulose: Rheological considerations. Polymer 2000, 41, 2451–2461. [Google Scholar] [CrossRef]
- Fuma, S.; Nishinaka, A.; Inoue, Y.; Tsuruma, K.; Shimazawa, M.; Kondo, M.; Hara, H. A pharmacological approach in newly established retinal vein occlusion model. Sci. Rep. 2017, 7, 43509. [Google Scholar] [CrossRef]
- Yi, Y.; Pyun, S.H.; Kim, C.Y.; Yun, G.; Kang, E.; Heo, S.; Ullah, I.; Lee, S.K. Eye drop with Fas-blocking peptide attenuates age-related macular degeneration. Cells 2024, 13, 548. [Google Scholar] [CrossRef]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.E.; Wilson, C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef]
- Duke, S.L.; Kump, L.I.; Yuan, Y.; West, W.W.; Sachs, A.J.; Haider, N.B.; Margalit, E. The safety of intraocular linezolid in rabbits. Investig. Opthalmol. Vis. Sci. 2010, 51, 3115–3119. [Google Scholar] [CrossRef]
- Takeuchi, M. Farumashia; The Pharmaceutical Society of Japan: Tokyo, Japan, 2013; Volume 49, pp. 1194–1196. [Google Scholar]
- Itoh, K.; Hatakeyama, T.; Kimura, T.; Shimoyama, T.; Miyazaki, S.; D’Emanuele, A.; Attwood, D. Effect of D-sorbitol on the thermal gelation of methylcellulose formulations for drug delivery. Chem. Pharm. Bull. 2010, 58, 247–249. [Google Scholar] [CrossRef]
- Mohammadi, M.; Patel, K.; Alaie, S.P.; Shmueli, R.B.; Besirli, C.G.; Larson, R.G.; Green, J.J. Injectable drug depot engineered to release multiple ophthalmic therapeutic agents with precise time profiles for postoperative treatment following ocular surgery. Acta Biomater. 2018, 73, 90–102. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Kakino, Y.; Furukawa, H.; Tahara, K.; Takeuchi, H. Kobunshi Ronbunshu; Society of Polymer Science: Tokyo, Japan, 2015; Volume 72, pp. 57–63. [Google Scholar]
- Muniyandi, A.; Hartman, G.D.; Song, Y.; Mijit, M.; Kelley, M.R.; Corson, T.W. Beyond VEGF: Targeting inflammation and other pathways for treatment of retinal disease. J. Pharmacol. Exp. Ther. 2023, 386, 15–25. [Google Scholar] [CrossRef]
- Nishinaka, A.; Inoue, Y.; Fuma, S.; Hida, Y.; Nakamura, S.; Shimazawa, M.; Hara, H. Pathophysiological role of VEGF on retinal edema and nonperfused areas in mouse eyes with retinal vein occlusion. Investig. Opthalmol. Vis. Sci. 2018, 59, 4701–4713. [Google Scholar] [CrossRef]


| Formulations | Surface Modifier | Concentration of EPC (mM) | Composition of the Liposomes (Molar Ratio) | Concentration of PnkRNA (µM) | Concentration of TAMRA-Labeled PnkRNA (µM) |
|---|---|---|---|---|---|
| Unmodified-Lip | ― | 10.2 | EPC/Chol = 7/3 | 10 | ― |
| R8-Lip | R8 | 10.2 | EPC/Chol/R8 = 7/3/0.175 | 10 | ― |
| Unmodified-Lip | ― | 10.2 | EPC/Chol = 7/3 | ― | 10 |
| R8-Lip | R8 | 10.2 | EPC/Chol/R8 = 7/3/0.175 | ― | 10 |
| Formulations | Average Particle Size (nm) | Polydispersity | Zeta Potential (mV) | Loading Efficiency (%) |
|---|---|---|---|---|
| Unmodified-Lip | 140.7 | 0.062 | −12.0 | 18.8 |
| R8-Lip | 183.9 | 0.167 | 43.4 | 99.7 |
| Unmodified-Lip | 137.2 | 0.159 | −1.0 | 18.8 |
| R8-Lip | 190.9 | 0.222 | 40.3 | 99.9 |
| Formulation | Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Surface Modifier | Concentration of EPC (mM) | Liposomal Composition (Molar Ratio) | Concentration of C6 (mg/mL) | MC 15 (mg/mL) | MC400 (mg/mL) | SR (mg/mL) | SC (mg/mL) | PEG4000 (mg/mL) | |
| SA-Lip | SA | 10.2 | EPC/Chol/SA = 7/3/1 | 0.05 | - | - | - | - | - |
| SA-Lip-loaded SR-TG | SA | 10.2 | EPC/Chol/SA = 7/3/1 | 0.05 | 20 | - | 20 | - | - |
| SA-Lip-loaded SC-TG | SA | 10.2 | EPC/Chol/SA = 7/3/1 | 0.05 | 6 | 5 | - | 35 | 50 |
| Formulation | Particle Properties | Viscosity (mPa·s) | |||
|---|---|---|---|---|---|
| Average Particle Size (nm) | Polydispersity | Zeta Potential (mV) | 10 °C | 35 °C | |
| SA-Lip | 141.6 | 0.074 | 65.0 | <15 | <15 |
| SA-Lip-loaded SR-TG | 265.8 | 0.343 | 5.2 | 30.8 | 46.6 |
| SA-Lip-loaded SC-TG | 353.4 | 0.460 | −5.3 | 34.0 | 50.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiratori, T.; Ito, T.; Nishinaka, A.; Matsumiya, R.; Yamazoe, E.; Takeuchi, H.; Hara, H.; Tahara, K. Topical Application of RNAi Therapy Using Surface-Modified Liposomes for Treating Retinal-Vein Occlusion. Molecules 2025, 30, 2622. https://doi.org/10.3390/molecules30122622
Shiratori T, Ito T, Nishinaka A, Matsumiya R, Yamazoe E, Takeuchi H, Hara H, Tahara K. Topical Application of RNAi Therapy Using Surface-Modified Liposomes for Treating Retinal-Vein Occlusion. Molecules. 2025; 30(12):2622. https://doi.org/10.3390/molecules30122622
Chicago/Turabian StyleShiratori, Taishi, Takaaki Ito, Anri Nishinaka, Ryosuke Matsumiya, Eriko Yamazoe, Hirofumi Takeuchi, Hideaki Hara, and Kohei Tahara. 2025. "Topical Application of RNAi Therapy Using Surface-Modified Liposomes for Treating Retinal-Vein Occlusion" Molecules 30, no. 12: 2622. https://doi.org/10.3390/molecules30122622
APA StyleShiratori, T., Ito, T., Nishinaka, A., Matsumiya, R., Yamazoe, E., Takeuchi, H., Hara, H., & Tahara, K. (2025). Topical Application of RNAi Therapy Using Surface-Modified Liposomes for Treating Retinal-Vein Occlusion. Molecules, 30(12), 2622. https://doi.org/10.3390/molecules30122622









