Impact of Ultrasound-Treated Emulsion Gels on the Structure of Purees for Oropharyngeal Dysphagia
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural and Stability Analysis of Emulsion Gels
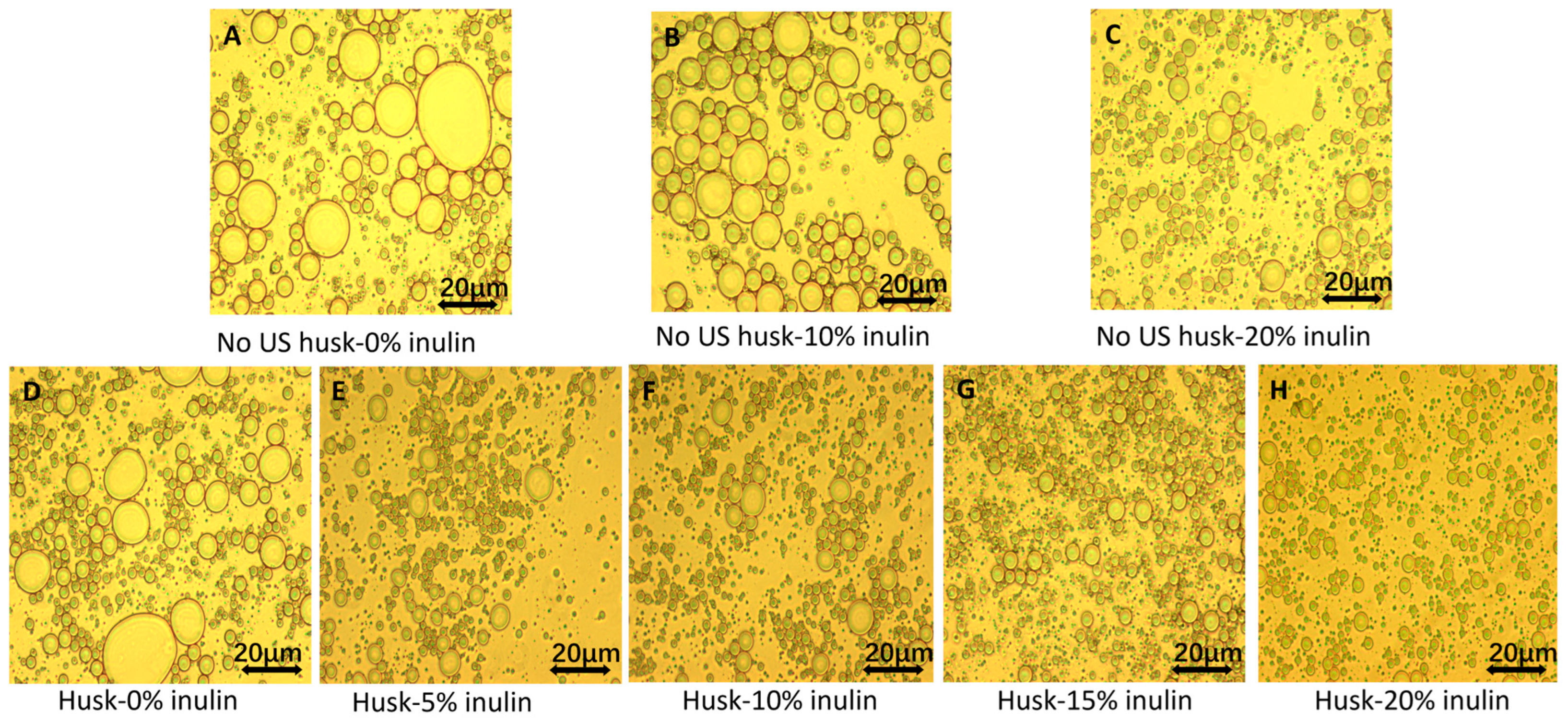
2.1.1. Polarized Light Microscopic Observation
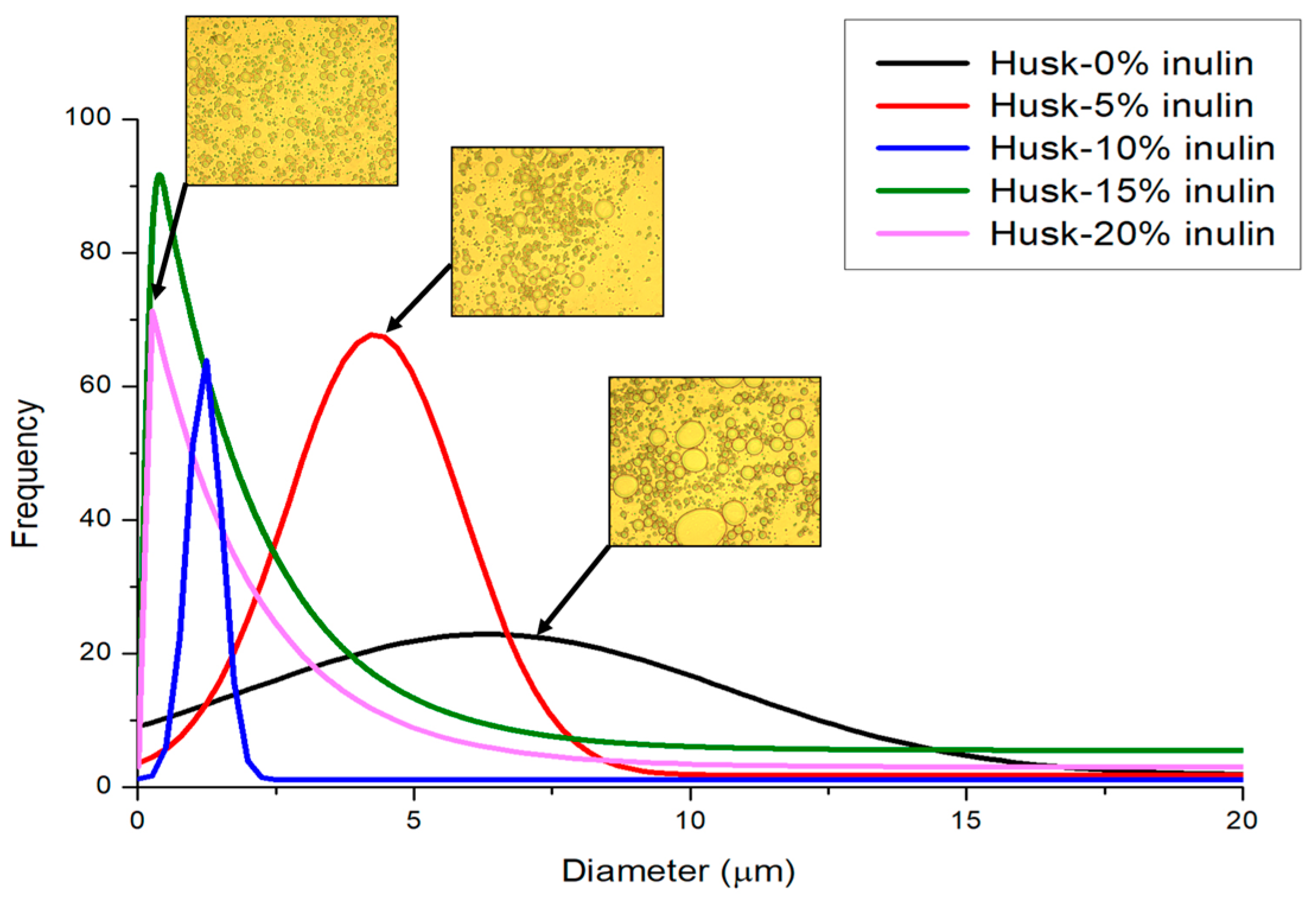
2.1.2. Particle Size Distribution
2.1.3. Cryo-Scanning Electron Microscopic Observation
2.2. Effect of Emulsion Gel Addition in Meat Puree
2.2.1. Microstructure Morphology
2.2.2. Total Expressible Fluid (TEF)
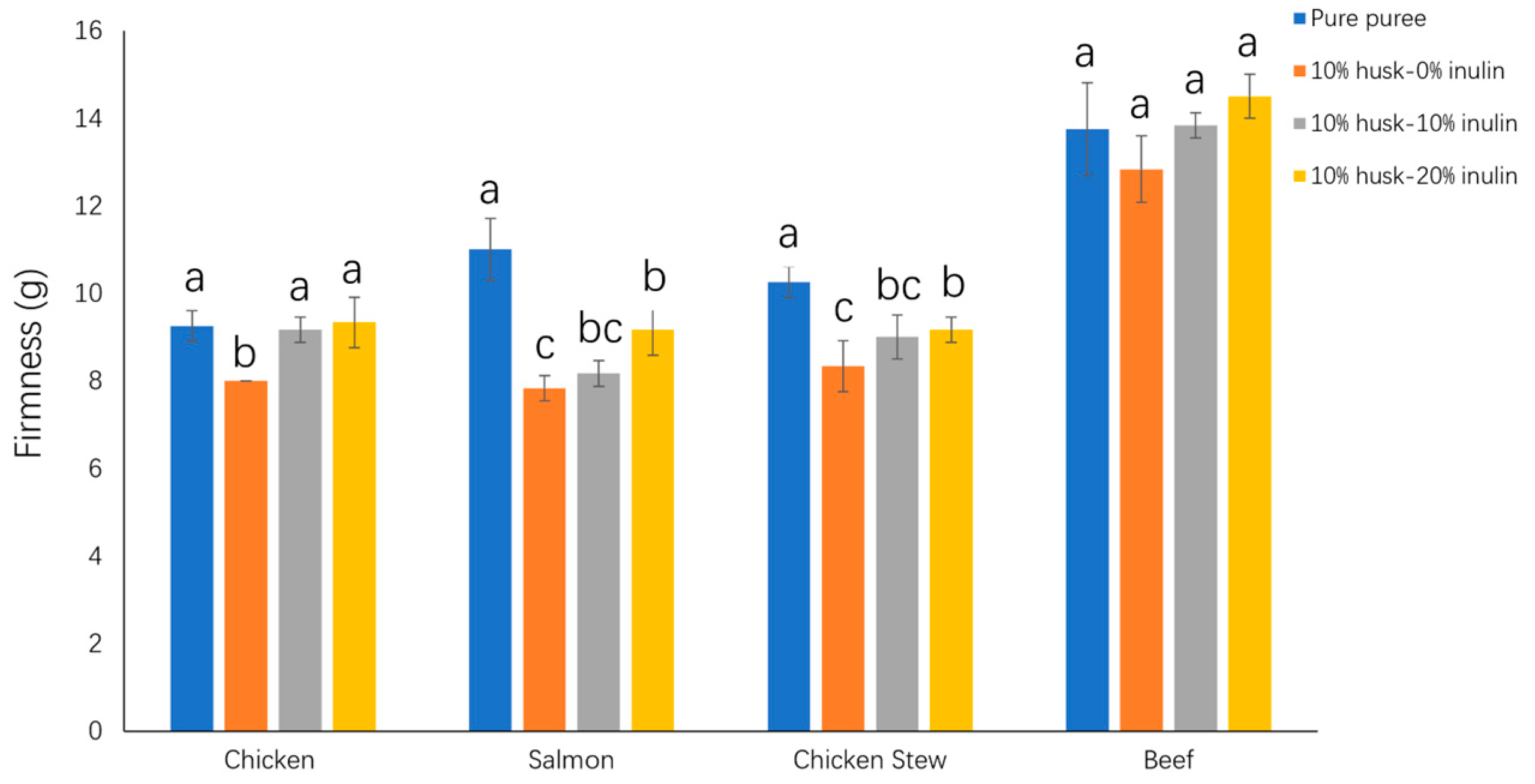
2.2.3. Effect of Emulsion Gel Addition on the Firmness of Puree Samples
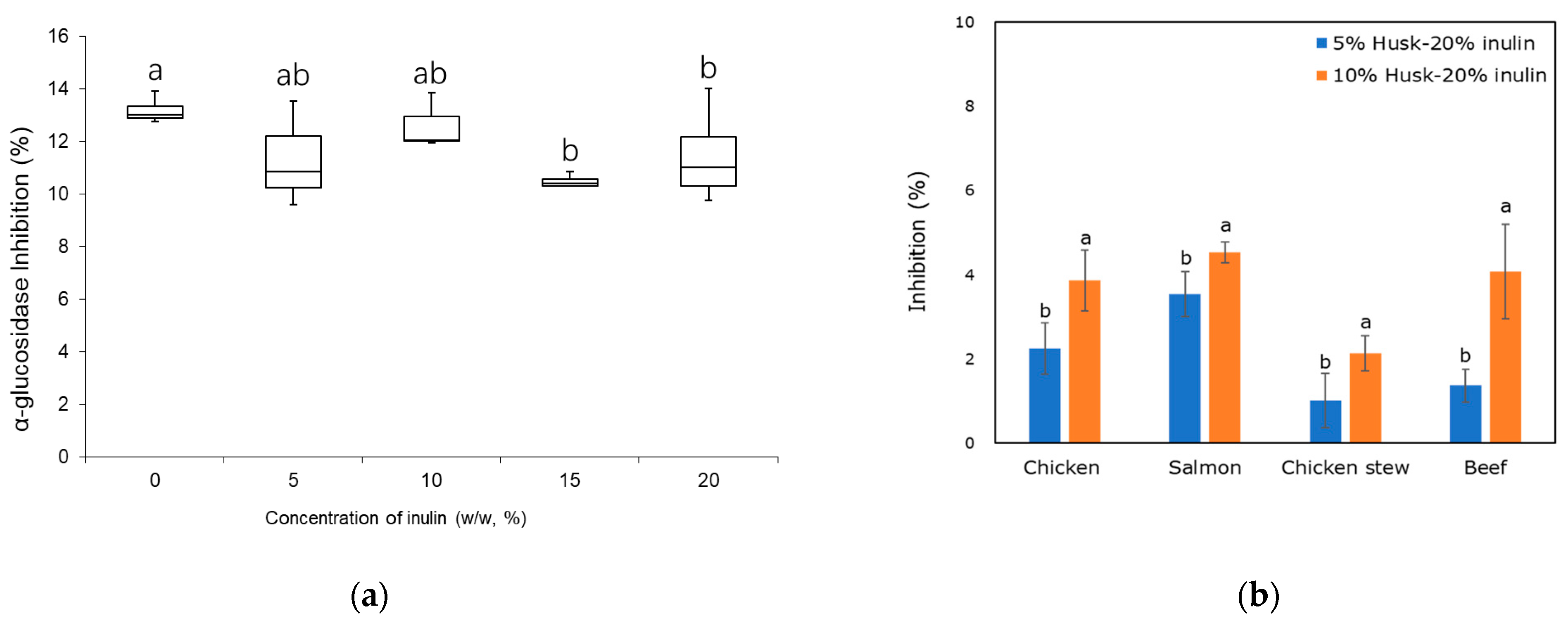
2.3. Enzyme Inhibition
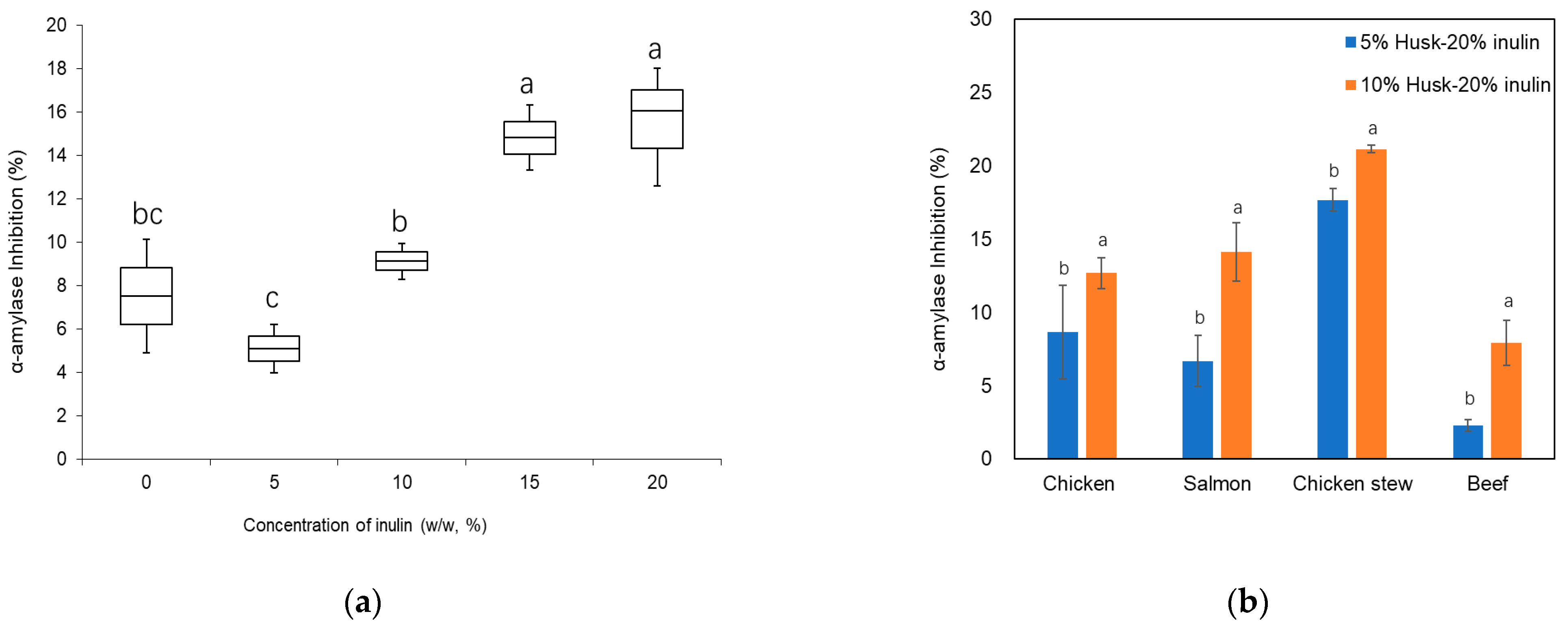
2.3.1. Alpha-Amylase Inhibition
2.3.2. Alpha-Glucosidase Inhibition
2.4. International Dysphagia Diet Standardization Initiative (IDDSI) Tests
3. Materials and Methods
3.1. Materials
3.2. Formulation and Preparation of Psyllium Husk Emulsion Gels
3.3. Ultrasound-Treated Emulsion Gel
3.4. Polarized Light Microscopic Observation of Emulsion Droplets and Size Measurement
3.5. Puree Sample Preparation
3.6. Cryo-Scanning Electron Microscopic Observation of Emulsion Gels and Puree Samples
3.7. Total Expressible Fluid (TEF) Determination
3.8. Texture Analysis
3.9. α-Amylase and α-Glucosidase Inhibitory Activities
3.10. International Dysphagia Diet Standardization Initiative (IDDSI) Tests
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sasegbon, A.; Hamdy, S. The Anatomy and Physiology of Normal and Abnormal Swallowing in Oropharyngeal Dysphagia. Neurogastroenterol. Motil. 2017, 29, e13100. [Google Scholar] [CrossRef] [PubMed]
- Werstuck, M.M.; Steel, C. Dysphagia Identification and Assessment in Adults in Primary Care Settings—A Canadian Study of Dietitians. Can. J. Diet. Pract. Res. 2021, 82, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.; Chambers, L.; Niezgoda, H.; Duizer, L. Issues Associated with the Use of Modified Texture Foods. J. Nutr. Health Aging 2012, 16, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Turcanu, M.; Nakauma, M.; Fang, Y. Role of Fluid Cohesiveness in Safe Swallowing. npj Sci. Food 2019, 3, 5. [Google Scholar] [PubMed]
- Sukkar, S.G.; Maggi, N.; Travalca Cupillo, B.; Ruggiero, C. Optimizing Texture Modified Foods for Oro-Pharyngeal Dysphagia: A Difficult but Possible Target? Front. Nutr. 2018, 5, 68. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, T.; Iskandar, M.M.; Baeghbali, V.; Ngadi, M.O.; Kubow, S. 3D Food Printing Applications Related to Dysphagia: A Narrative Review. Foods 2022, 11, 1789. [Google Scholar] [CrossRef]
- Dahl, W.J.; Whiting, S.J.; Isaac, T.M.; Weeks, S.J.; Arnold, C.J. Effects of Thickened Beverages Fortified with Inulin on Beverage Acceptance, Gastrointestinal Function, and Bone Resorption in Institutionalized Adults. Nutrition 2005, 21, 308–311. [Google Scholar] [CrossRef]
- Bernstein, A.; Titgemeier, B.; Kirkpatrick, K.; Golubic, M.; Roizen, M. Major Cereal Grain Fibers and Psyllium in Relation to Cardiovascular Health. Nutrients 2013, 5, 1471–1487. [Google Scholar] [CrossRef]
- White, N. A Guide to Recommending Fiber Supplements for Self-Care. Am. J. Lifestyle Med. 2020, 14, 589–591. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.; Hou, Z.; Miao, S.; Gao, Y. Development of Emulsion Gels for the Delivery of Functional Food Ingredients: From Structure to Functionality. Food Eng. Rev. 2019, 11, 245–258. [Google Scholar] [CrossRef]
- Zhou, P.; Wen, L.; Ai, T.; Liang, H.; Li, J.; Li, B. A Novel Emulsion Gel Solely Stabilized by the Hot Water Extracted Polysaccharide from Psyllium Husk: Self-Healing Plays a Key Role. Food Hydrocoll. 2022, 130, 107718. [Google Scholar] [CrossRef]
- Franco, E.A.N.; Sanches-Silva, A.; Ribeiro-Santos, R.; De Melo, N.R. Psyllium (Plantago Ovata Forsk): From Evidence of Health Benefits to Its Food Application. Trends Food Sci. Technol. 2020, 96, 166–175. [Google Scholar] [CrossRef]
- Ahmed, F.; Sairam, S.; Urooj, A. In Vitro Hypoglycemic Effects of Selected Dietary Fiber Sources. J. Food Sci. Technol. 2011, 48, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Perret, J.; Parker, T.; Allen, K.G.D. Enzymatic Modification to Improve the Water-Absorbing and Gelling Properties of Psyllium. Food Chem. 2003, 82, 243–248. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, Health Benefits and Food Applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Paglarini, C.D.S.; Vidal, V.A.S.; Ozaki, M.M.; Ribeiro, A.P.B.; Bernardinelli, O.D.; Câmara, A.K.F.I.; Herrero, A.M.; Ruiz-Capillas, C.; Sabadini, E.; Pollonio, M.A.R. Inulin Gelled Emulsion as a Fat Replacer and Fiber Carrier in Healthier Bologna Sausage. Food Sci. Technol. Int. 2022, 28, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xiong, Y.; Li, Z.; Luo, D.; Wang, Z.; Sun, Y.; Shah, B.R. Stability, Microstructural and Rheological Properties of Complex Prebiotic Emulsion Stabilized by Sodium Caseinate with Inulin and Konjac Glucomannan. Food Hydrocoll. 2020, 105, 105772. [Google Scholar] [CrossRef]
- Sarkar, A.; Ademuyiwa, V.; Stubley, S.; Esa, N.H.; Goycoolea, F.M.; Qin, X.; Gonzalez, F.; Olvera, C. Pickering Emulsions Co-Stabilized by Composite Protein/ Polysaccharide Particle-Particle Interfaces: Impact on in Vitro Gastric Stability. Food Hydrocoll. 2018, 84, 282–291. [Google Scholar] [CrossRef]
- Leong, T.S.H.; Zhou, M.; Kukan, N.; Ashokkumar, M.; Martin, G.J.O. Preparation of Water-in-Oil-in-Water Emulsions by Low Frequency Ultrasound Using Skim Milk and Sunflower Oil. Food Hydrocoll. 2017, 63, 685–695. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion Gels: The Structuring of Soft Solids with Protein-Stabilized Oil Droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.; Souki, N.; Moraes, I.; Pinho, S. Rheology of Emulsion-Filled Gels Applied to the Development of Food Materials. Gels 2016, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Grundy, M.M.L.; McClements, D.J.; Ballance, S.; Wilde, P.J. Influence of Oat Components on Lipid Digestion Using an in Vitro Model: Impact of Viscosity and Depletion Flocculation Mechanism. Food Hydrocoll. 2018, 83, 253–264. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Han, L.; Zhou, B.; Yang, J.; Li, S. Pomegranate Seed Oil Stabilized with Ovalbumin Glycated by Inulin: Physicochemical Stability and Oxidative Stability. Food Hydrocoll. 2020, 102, 105602. [Google Scholar] [CrossRef]
- Mao, L.; Miao, S. Structuring Food Emulsions to Improve Nutrient Delivery During Digestion. Food Eng. Rev. 2015, 7, 439–451. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Yuan, F.; Gao, Y.; Mao, L. Emulsion Gels with Different Proteins at the Interface: Structures and Delivery Functionality. Food Hydrocoll. 2021, 116, 106637. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, Q.; Miao, S.; Zhang, Y.; Zheng, B.; Zhang, L. Effect of Ultrasound on Physicochemical Properties of Emulsion Stabilized by Fish Myofibrillar Protein and Xanthan Gum. Innov. Food Sci. Emerg. Technol. 2019, 54, 225–234. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Li, D.S.; Ilavsky, J.; Kuzmenko, I.; Jeng, G.-S.; O’Donnell, M.; Pozzo, L.D. Ultrasound-Based Formation of Nano-Pickering Emulsions Investigated via in-Situ SAXS. J. Colloid Interface Sci. 2019, 536, 281–290. [Google Scholar] [CrossRef]
- Kim, Y.; Faqih, M.N.; Wang, S.S. Factors Affecting Gel Formation of Inulin. Carbohydr. Polym. 2001, 46, 135–145. [Google Scholar] [CrossRef]
- Dresselhuis, D.M.; Klok, H.J.; Stuart, M.A.C.; De Vries, R.J.; Van Aken, G.A.; De Hoog, E.H.A. Tribology of o/w Emulsions Under Mouth-like Conditions: Determinants of Friction. Food Biophys. 2007, 2, 158–171. [Google Scholar] [CrossRef]
- Steele, C.M.; Alsanei, W.A.; Ayanikalath, S.; Barbon, C.E.A.; Chen, J.; Cichero, J.A.Y.; Coutts, K.; Dantas, R.O.; Duivestein, J.; Giosa, L.; et al. The Influence of Food Texture and Liquid Consistency Modification on Swallowing Physiology and Function: A Systematic Review. Dysphagia 2015, 30, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Farjami, T.; Madadlou, A. An Overview on Preparation of Emulsion-Filled Gels and Emulsion Particulate Gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Galvão, M.T.E.L.; Picone, C.S.F.; Cunha, R.L.; Pollonio, M.A.R. Effect of Prebiotic Ingredients on the Rheological Properties and Microstructure of Reduced-Sodium and Low-Fat Meat Emulsions. LWT-Food Sci. Technol. 2015, 60, 148–155. [Google Scholar] [CrossRef]
- López-López, I.; Cofrades, S.; Yakan, A.; Solas, M.T.; Jiménez-Colmenero, F. Frozen Storage Characteristics of Low-Salt and Low-Fat Beef Patties as Affected by Wakame Addition and Replacing Pork Backfat with Olive Oil-in-Water Emulsion. Food Res. Int. 2010, 43, 1244–1254. [Google Scholar] [CrossRef]
- Morin, L.A.; Temelli, F.; McMullen, L. Interactions between Meat Proteins and Barley (Hordeum Spp.) β-Glucan within a Reduced-Fat Breakfast Sausage System. Meat Sci. 2004, 68, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, F.J.; Carballo, J.; Solas, M.T. The Effect of Use of Freeze-thawed Pork on the Properties of Bologna Sausages with Two Fat Levels. Int. J. Food Sci. Technol. 1995, 30, 335–345. [Google Scholar] [CrossRef]
- Abdullah; Liu, L.; Javed, H.U.; Xiao, J. Engineering Emulsion Gels as Functional Colloids Emphasizing Food Applications: A Review. Front. Nutr. 2022, 9, 890188. [Google Scholar] [CrossRef]
- Hashemi, B.; Assadpour, E.; Wang, Y.; Jafari, S.M. Pickering Emulsion Gels Stabilized by Protein and Polysaccharide-Based Particles: A Review of Stability, Synthesis, Applications and Prospective. Adv. Colloid Interface Sci. 2025, 343, 103564. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi Pure, A.; Yarmand, M.S.; Farhoodi, M.; Adedeji, A. Microwave Treatment to Modify Textural Properties of High Protein Gel Applicable as Dysphagia Food. J. Texture Stud. 2021, 52, 638–646. [Google Scholar] [CrossRef]
- Zhou, Y.; Dai, H.; Ma, L.; Yu, Y.; Zhu, H.; Wang, H.; Zhang, Y. Effect and Mechanism of Psyllium Husk (Plantago Ovata) on Myofibrillar Protein Gelation. LWT 2021, 138, 110651. [Google Scholar] [CrossRef]
- Huang, X.; Yang, H.; Lou, A.; Jiang, S.; Kang, K.; Wei, Y.; Li, X.; Wu, Y.; Yu, M.; Huang, Q. Effect of Psyllium Husk Powder on the Gelation Behavior, Microstructure, and Intermolecular Interactions in Myofibrillar Protein Gels from Andrias Davidianus. Food Chem. 2024, 458, 140266. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y. Development of Low-Fat/Reduced-Fat Processed Meat Products Using Fat Replacers and Analogues. Food Rev. Int. 2021, 37, 296–312. [Google Scholar] [CrossRef]
- Colmenero, F.J.; Ayo, M.J.; Carballo, J. Physicochemical Properties of Low Sodium Frankfurter with Added Walnut: Effect of Transglutaminase Combined with Caseinate, KCl and Dietary Fibre as Salt Replacers. Meat Sci. 2005, 69, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.J.; Xu, X.L.; Li, C.B.; Huang, M.; Liu, D.Y.; Zhou, G.H. A Comparison of heat-induced changes of intramuscular connective tissue and collagen of beef semitendinosus muscle during water bath and microwave heating. J. Food Process Eng. 2011, 34, 2233–2250. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Muhoza, B.; Feng, T.; Xia, S.; Zhang, X. Microwave Combined with Conduction Heating Effects on the Tenderness, Water Distribution, and Microstructure of Pork Belly. Innov. Food Sci. Emerg. Technol. 2020, 62, 102344. [Google Scholar] [CrossRef]
- Grigelmo-Miguel, N. Characterisation of Low-Fat High-Dietary ®bre Frankfurters. Meat Sci. 1999, 52, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Comer, F.W.; Dempster, S. Functionality of Fillers and Meat Ingredients in Comminuted Meat Products. Can. Inst. Food Sci. Technol. J. 1981, 14, 295–303. [Google Scholar] [CrossRef]
- Nourbehesht, N.; Shekarchizadeh, H.; Soltanizadeh, N. Investigation of Stability, Consistency, and Oil Oxidation of Emulsion Filled Gel Prepared by Inulin and Rice Bran Oil Using Ultrasonic Radiation. Ultrason. Sonochemistry 2018, 42, 585–593. [Google Scholar] [CrossRef]
- Zheng, M.; You, Q.; Lin, Y.; Lan, F.; Luo, M.; Zeng, H.; Zheng, B.; Zhang, Y. Effect of Guar Gum on the Physicochemical Properties and in Vitro Digestibility of Lotus Seed Starch. Food Chem. 2019, 272, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, S.; Ai, L. Physical Barrier Effects of Dietary Fibers on Lowering Starch Digestibility. Curr. Opin. Food Sci. 2022, 48, 100940. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, H.; Zhao, J.; Zhang, H.; Chen, S. Enzyme-Assisted Extraction of a Cup Plant (Silphium perfoliatum L.) Polysaccharide and Its Antioxidant and Hypoglycemic Activities. Process Biochem. 2020, 92, 17–28. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. Mini-Special Issue Paper Management of Diabetic Patients with Hypoglycemic Agents α-Glucosidase Inhibitors and Their Use in Clinical Practice. Arch. Med. Sci. 2012, 5, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Güler, N.; Sensoy, I. The Effect of Psyllium Fiber on the in Vitro Starch Digestion of Steamed and Roasted Wheat Based Dough. Food Res. Int. 2023, 168, 112797. [Google Scholar] [CrossRef] [PubMed]
- Palanuvej, C. In Vitro Glucose Entrapment and Alpha-Glucosidase Inhibition of Mucilaginous Substances from Selected Thai Medicinal Plants. Sci. Pharm. 2009, 77, 837–850. [Google Scholar] [CrossRef]
- Gibb, R.D.; McRorie, J.W.; Russell, D.A.; Hasselblad, V.; D’Alessio, D.A. Psyllium Fiber Improves Glycemic Control Proportional to Loss of Glycemic Control: A Meta-Analysis of Data in Euglycemic Subjects, Patients at Risk of Type 2 Diabetes Mellitus, and Patients Being Treated for Type 2 Diabetes Mellitus. Am. J. Clin. Nutr. 2015, 102, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hu, J.; Liang, Y.; Huang, X.; Seah, G.L.; Li, J.; Liu, D.; Tan, C. Protein Gel with Designed Network and Texture Regulated via Building Blocks to Study Dysphagia Diet Classifications. Food Hydrocoll. 2023, 140, 108640. [Google Scholar] [CrossRef]
- Malouh, M.A.; Cichero, J.A.Y.; Manrique, Y.J.; Crino, L.; Lau, E.T.L.; Nissen, L.M.; Steadman, K.J. Are Medication Swallowing Lubricants Suitable for Use in Dysphagia? Consistency, Viscosity, Texture, and Application of the International Dysphagia Diet Standardization Initiative (IDDSI) Framework. Pharmaceutics 2020, 12, 924. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Preparation of Fiber-Enriched Chicken Meat Constructs Using 3D Printing. J. Culin. Sci. Technol. 2023, 21, 127–138. [Google Scholar] [CrossRef]
- Mohammad Rashedi, I.F.; Ismail, N.A.; Bakar, J.; Sazili, A.Q. Effect of Different Levels of Fat, Sodium Chloride, and Sodium Tripolyphosphate on the Physicochemical and Microstructure Properties of Jamnapari Goat Meat Emulsion Modelling System. Int. Food Res. J. 2021, 28, 916–925. [Google Scholar] [CrossRef]
- Picot, C.M.N.; Subratty, A.H.; Mahomoodally, M.F. Inhibitory Potential of Five Traditionally Used Native Antidiabetic Medicinal Plants on α-Amylase, α-Glucosidase, Glucose Entrapment, and Amylolysis Kinetics In Vitro. Adv. Pharmacol. Sci. 2014, 2014, 739834. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Akoetey, W.; Martí, N.; Saura, D.; Hosseinian, F. Impact of Ultrasound-Treated Emulsion Gels on the Structure of Purees for Oropharyngeal Dysphagia. Molecules 2025, 30, 3933. https://doi.org/10.3390/molecules30193933
Luo M, Akoetey W, Martí N, Saura D, Hosseinian F. Impact of Ultrasound-Treated Emulsion Gels on the Structure of Purees for Oropharyngeal Dysphagia. Molecules. 2025; 30(19):3933. https://doi.org/10.3390/molecules30193933
Chicago/Turabian StyleLuo, Minfang, Winifred Akoetey, Nuria Martí, Domingo Saura, and Farah Hosseinian. 2025. "Impact of Ultrasound-Treated Emulsion Gels on the Structure of Purees for Oropharyngeal Dysphagia" Molecules 30, no. 19: 3933. https://doi.org/10.3390/molecules30193933
APA StyleLuo, M., Akoetey, W., Martí, N., Saura, D., & Hosseinian, F. (2025). Impact of Ultrasound-Treated Emulsion Gels on the Structure of Purees for Oropharyngeal Dysphagia. Molecules, 30(19), 3933. https://doi.org/10.3390/molecules30193933






