Abstract
The photocatalytic reduction of CO2 into valuable hydrocarbons presents significant potential. In this research, a ZnO/ZnAl2O4 composite photocatalyst was synthesized using the hydrothermal method, resulting in a marked enhancement in CO yield—approximately three times greater than that achieved with pure ZnAl2O4 nanoparticles. The formation of a Z-scheme heterojunction between ZnO and ZnAl2O4 was observed, characterized by low interfacial charge transfer resistance, an abundance of reaction sites, and optimized charge transport pathways. Within this composite, ZnO contributes additional vacancies, thereby increasing active sites and enhancing the separation and migration of photogenerated carriers. In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) analysis indicates that ZnAl2O4 facilitates the formation of key intermediates, such as *COOH and HCO3−, thus promoting the conversion of CO2 to CO. This study offers valuable insights into the design of heterogeneous catalysts with diverse active components to enhance the performance of CO2 photocatalytic reduction through synergistic effects.
1. Introduction
With the rapid growth of urbanization and industrialization brought about by global economic and technological advancement, human society has entered a new era. A number of ecological issues, including global warming, sea level rise, ocean acidification, faster permafrost thawing, and glacier retreat, have been brought on by excessive use of fossil fuels, which has increased CO2 emissions [1,2,3]. These issues pose a major threat to ecosystem stability and human health. As a result, reducing CO2 emissions has become an urgent global task, with efforts focused on sustainable development solutions [4,5]. Among the various solutions for mitigating the greenhouse effect, catalytic conversion of CO2 stands out for its lack of secondary pollution and high economic efficiency [6,7,8,9]. Because solar energy is inexhaustible and does not cause any pollution to the environment, photocatalytic CO2 reduction has a broad prospect [10,11,12]. A great deal of effort has been put in by researchers to find suitable materials to reduce CO2 into valuable products [13,14,15]. Out of all the photocatalyst possibilities, semiconductors are the most popular and promising [16,17,18].
ZnAl2O4 is a wide bandgap zinc-based spinel material with a forbidden bandwidth of about 3.8–3.9 eV, which shows better optical, electrical, magnetic, and catalytic properties under light excitation, and has been widely applied to various fields such as photocatalytic degradation of organic pollutants [19], sterilization and deodorization [20], catalytic reduction of CO2 [21], and so on. However, the wider bandgap structure greatly limits its application. The electrons and holes (e−, h+) generated by ZnAl2O4 are easily recombined, resulting in poor adsorption of CO2 and poor absorption of visible light. To solve these problems, researchers have tried various methods to modify it, such as elemental doping [22], morphology modulation [23], and construction of heterojunctions [24].
The creation of heterojunctions has proven to be an effective strategy for improving photocatalytic performance [25,26,27]. Composite materials can make up for the shortcomings of single materials, can not only maintain certain characteristics of single-phase catalyst components, but also through the composite structure to play a new performance. With a direct band gap of 3.2 eV, ZnO has been recognized as a high-quality material for photocatalytic processes due to its low synthesis cost, controllable morphology, and good thermal stability [28,29,30]. ZnO-based photocatalysts are even more effective than TiO2 in photodegradation applications [31]. ZnO and ZnAl2O4 are coupled to form a heterojunction through the energy band structure, which can induce the migration of photogenerated e− and h+ between the conduction and valence bands and thus enhance the survival lifetime of photogenerated carriers [32]. It also increases the surface active sites of the material, which contributes to the photocatalyst reduction capacity [33,34].
In this study, we successfully synthesized ZnO/ZnAl2O4 composites via the hydrothermal method and employed them for photocatalytic CO2 reduction. Compared to individual ZnO and ZnAl2O4, the composites exhibited not only superior stability but also enhanced photocatalytic activity. The formation of Z-scheme heterojunctions facilitated the development of an internal electric field at the interface, which in turn promoted efficient space charge separation and provided high redox potentials for photogenerated carriers. The synergistic interaction between ZnO and ZnAl2O4 was elucidated through various characterization techniques. To further clarify the mechanism underlying the photocatalytic reduction of CO2, we utilized in situ DRIFTS to propose potential reaction pathways.
2. Results and Discussion
2.1. Morphology and Structural Characterization
The XRD patterns of all the samples are shown in Figure 1a. The peaks of ZnO located at 31.8°, 34.4°, 36.3°, 47.6°, 56.6°, and 62.9° correspond to the (100), (002), (101), (102), (110), and (103) crystal planes of fibrillar zinc ore ZnO (JCPDS No. 36-1451). No other peaks were found, which proves the high purity of ZnO in the sample. And the sharp diffraction peaks indicate that the synthesized crystals are highly crystalline. The diffraction peaks of sample ZnAl2O4 at 31.2°, 36.8°, 59.3°, and 65.2° correspond to the (220), (311), (511), and (440) crystallographic planes of cubic ZnAl2O4 (JCPDS No. 05-0669). ZnO/ZnAl2O4 showed ZnO and ZnAl2O4 characteristic diffraction peaks, demonstrating the successful synthesis of the composite structure. It is noteworthy that the diffraction peaks of ZnO are significantly broadened and crystallinity is reduced after the composite, indicating a decrease in grain size or distortion of the lattice [35]. The disruption of the crystal lattice results in the formation of defects that increase the number of active sites on its surface, which in turn improves the catalytic performance.
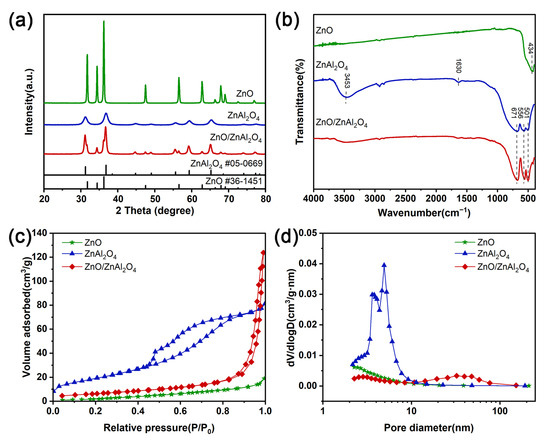
Figure 1.
(a) XRD patterns, (b) FTIR spectra, (c) N2 adsorption–desorption isotherms, and (d) pore distribution of ZnO, ZnAl2O4, and ZnO/ZnAl2O4.
The systematic molecular structure characterization of the samples was carried out using FTIR spectroscopy to resolve the functional group composition and chemical bonding structural features. As shown in Figure 1b shows that the absorption peaks at 3453 and 1630 cm−1 are caused by the stretching vibration of adsorbed water O-H or the bending vibration of adsorbed hydroxyl H-O-H on the surface of the sample material [20]. ZnO has a strong transmission peak near 434 cm−1, which is attributed to the stretching vibration of the Zn-O bond. The characteristic peaks at 671, 556, and 501 cm−1 can be attributed to Al-O stretching vibration, Al-O bending vibration, and Al-O asymmetric stretching vibration [36]. For the ZnO/ZnAl2O4 composites, the characteristic peaks of ZnO and ZnAl2O4 were observed in the low wavelength range of 400 to 1000 cm−1. Following the earlier XRD findings, the FTIR spectra thus show that the ZnO/ZnAl2O4 composite structure was successfully formed.
The samples’ N2 adsorption-desorption isotherms are displayed in Figure 1c. The mesoporous structure of each sample is demonstrated by type IV isotherms with H3-type hysteresis loops [37]. Figure 1d depicts the pore size distribution, and when combined with Table 1, it is clear that the addition of ZnO reduces the specific surface area of ZnAl2O4 while increasing pore volume and size. The smaller surface area of ZnO/ZnAl2O4 compared to ZnAl2O4 may be due to the fact that ZnO nanoparticles cover the surface and disordered pores are generated inside.

Table 1.
Textural property of samples ZnO, ZnAl2O4, and ZnO/ZnAl2O4.
TEM and HRTEM were used to study the microstructure of all samples. As can be seen from Figure 2a, ZnO behaves as irregular nanosquares with a wide range of lateral sizes, from 30 nm to 600 nm, whereas Figure 2b shows that ZnAl2O4 is a nano-microsphere structure with a sphere diameter of about 400–500 nm. Its edges are plush, increasing the contact area, so the specific surface area is the largest. Figure 2c shows that the circular ZnAl2O4 in ZnO/ZnAl2O4 is closely covered by massive ZnO nanoparticles with significantly reduced particle size. Their intimate contact makes it easier for charges to move between them and for the appropriate internal electric field to form [38]. Notably, no significant porosity was observed in the TEM images. This may be because the pore structure is not obvious or the TEM sample preparation process changed the pore structure. The HRTEM image of Figure 2d displays two distinct lattice fringes with lattice spacings of 0.281 and 0.243 nm, which correspond to the (100) and (311) crystal planes in ZnO and ZnAl2O4, respectively. This further confirms the successful preparation of ZnO/ZnAl2O4 heterojunction photocatalysts.
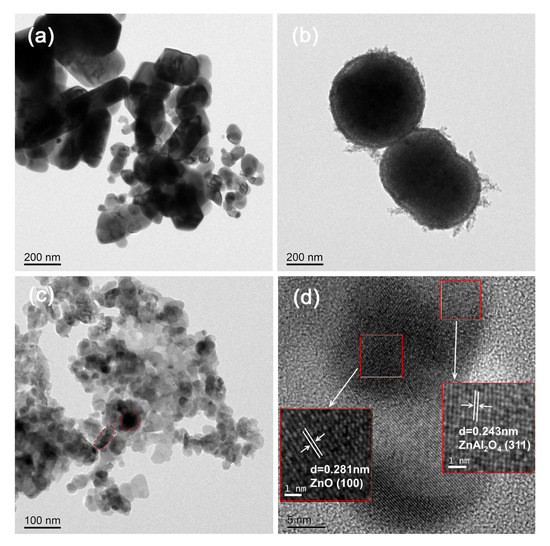
Figure 2.
TEM images of ZnO (a), ZnAl2O4 (b), and ZnO/ZnAl2O4 (c); HRTEM image (d) of ZnO/ZnAl2O4.
2.2. Surface Chemical States and Electron Distribution
The combination of two materials has an unavoidable effect on the structure and charge state. The elemental composition and valence states of the samples are studied by X-ray photoelectron spectroscopy (XPS). The full spectrum (Figure 3a) shows that the prepared catalysts all contain Zn 2p and O 1s orbitals, indicating that they are all composed of Zn and O elements. Al 2p orbitals were also observed in ZnAl2O4 and ZnO/ZnAl2O4, which is consistent with the conclusions of the previous characterization. Figure 3b shows the detailed spectrum of Al 2p for different samples. From the figure, it can be seen that the Al 2p binding energy of ZnO/ZnAl2O4 is shifted towards a lower electric field compared to ZnAl2O4. These slight shifts prove a charge transfer between ZnO and ZnAl2O4, with ZnAl2O4 gaining electrons [39]. The material’s Zn valence state is +2, as indicated by the two different characteristic peaks in the Zn 2p XPS spectrum (Figure 3c), Zn 2p3/2 and Zn 2p1/2 [40]. In the ZnO/ZnAl2O4 heterostructure, there is a significant broadening of the FWHM of Zn 2p3/2 (1.55 eV for ZnO, 1.74 eV for ZnZnAl2O4, and 1.88 eV for the composite structure), which is due to the presence of Zn in two different chemical environments. The Zn 2p binding energy in ZnO/ZnAl2O4 composite photocatalysts is shifted to a higher energy direction compared to ZnO. And compared to ZnAl2O4, the binding energy is shifted to the low-energy direction. This again suggests the existence of strong interaction and charge transfer between ZnO and ZnAl2O4, as well as electron transfer from ZnO to ZnAl2O4 [41]. The O 1s XPS spectra (Figure 3d) show two morphologies of O, with the peak around 531 eV assigned to lattice oxygen (Olatt) bonded to the metal, and the peak around 532 eV due to adsorbed oxygen (Oads) on the catalyst surface [42]. The decrease in binding energy of O 1s in ZnO/ZnAl2O4 is consistent with that of Zn 2p, demonstrating that the reaction process causes an increase in electron density, which promotes CO2 conversion. Oads is commonly used to indicate the concentration of oxygen vacancies (Ov) [43]. As can be seen from Table 2, the Ov concentration was enhanced from 13.20% to 21.95% after compounding with ZnO. The adsorption and activation of CO2 molecules, the provision of catalytically active sites for CO2 reduction, and the improvement of light energy use are all made possible by the presence of vacancies.
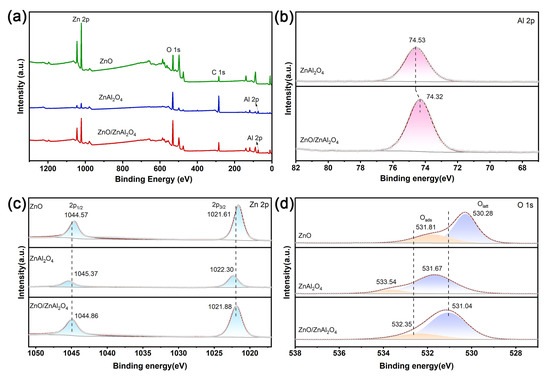
Figure 3.
XPS of (a) survey spectra (b) Al 2p, (c) Zn 2p, and (d) O 1s for ZnO, ZnAl2O4, and ZnO/ZnAl2O4.

Table 2.
The chemical state of Zn, Al, and O in the catalysts.
2.3. Optical and Photoelectrochemical Properties
Sample vacancies were analyzed by electron paramagnetic resonance (EPR) spectroscopy. At the g = 2.009 position, a symmetric EPR signal appears for all specimens (Figure 4a). The symmetric signal corresponding to this value of g can be attributed to the trapped electrons on Ov [44]. The sharper EPR signal of ZnO/ZnAl2O4 compared to ZnO and ZnAl2O4 indicates a higher Ov content, suggesting that ZnO introduces vacancies again. A strong signal was also observed for ZnO and ZnO/ZnAl2O4 at g = 1.966, which is an indicator of zinc vacancies (Znv) [45,46]. The separation and migration of photogenerated e− and h+ are facilitated by more vacancies, which enhances the photocatalytic performance for CO2 reduction.
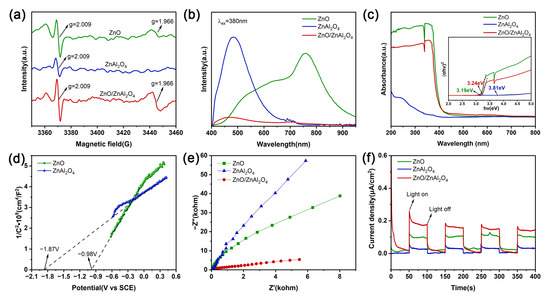
Figure 4.
EPR spectra (a) and PL spectra (b) UV-vis DRS and Tauc plots (c) of ZnO, ZnAl2O4, and ZnO/ZnAl2O4; Mott–Schottky plots (d) of ZnO and ZnAl2O4; EIS Nyquist plots (e) and Transient photocurrent responses (f) of ZnO, ZnAl2O4, and ZnO/ZnAl2O4.
Using steady-state photoluminescence (PL) under 380 nm optical excitation, the complexation and separation characteristics of photogenerated e− and h+ were examined (Figure 4b). ZnAl2O4 shows an emission peak at 480 nm, which is greatly attenuated when the complex is formed, implying that the formation of the complex inhibits the complexation of photogenerated carriers and favors the migration of photogenerated charges [47]. More photogenerated carriers are maintained when a heterojunction forms between ZnO and ZnAl2O4, passivating the non-radiative complex sites.
Samples were analyzed by UV-vis. As shown in Figure 4c, all three displayed significant light absorption in the UV spectrum. The corresponding forbidden bandwidths of the samples were calculated from Tauc plots [48]. The forbidden bandwidths of ZnO, ZnAl2O4, and ZnO/ZnAl2O4 are 3.19 eV, 3.81 eV, and 3.24 eV, respectively. It can be seen that the forbidden band range of the composite structure is mainly determined by ZnO, with enhanced light trapping compared to ZnAl2O4.
The location of the conduction zone was calculated by obtaining the Mott–Schottky (M-S) curve. The M-S curves for both ZnO and ZnAl2O4 have positive slopes, indicating that these samples are n-type semiconductors. For n-type semiconductors, the flat band potential is very close to the bottom of the conduction band position. Fitting the linear part yields slopes of 4.435 and 1.974 for ZnO and ZnAl2O4, respectively. Based on the slope = 2/Nqε0εr, where q = 1.602 × 10−19 C, ε0 = 8.854 × 10−12 F/m, ZnOεr ≈ 9, and ZnAl2O4εr ≈ 8.5, their charge carrier densities (N) can be estimated to be 3.533 × 1022 cm−3 and 8.404 × 1022 cm−3, respectively, which are tremendous values. In this case, the flat band potential can be approximated as the conduction band potential. The equivalent flat band potentials of ZnO and ZnAl2O4 are around −0.98 V and −1.87 V (vs. SCE), respectively, as Figure 4d illustrates. According to the calculation formula ENHE = ESCE + 0.244 V [49], the conduction band (CB) potentials of ZnO and ZnAl2O4 can be estimated as −0.74 V and −1.63 V (vs. NHE), respectively. Since EVB = ECB + Eg [50], ZnO and ZnAl2O4 have valence band (VB) potentials of 2.45 V and 2.18 V (vs. NHE), respectively. The CB and VB positions of the two are interleaved to form a Z-scheme heterojunction, which allows efficient electron transfer through the contact interface.
Interfacial charge transport resistance and carrier mobility were investigated using electrochemical impedance spectroscopy (EIS). The ZnO/ZnAl2O4 composites had the shortest Nyquist semicircle, according to the results, which are displayed in Figure 4e. This suggests that the combination of ZnO and ZnAl2O4 improves electron transfer kinetics and lowers interfacial charge transfer resistance [51]. The capacity of produced photocatalysts to separate charges has also been assessed using photochemical experiments. A higher charge separation efficiency is generally indicated by a stronger photocurrent response signal [52]. ZnO/ZnAl2O4 has the maximum photocurrent density and remains stable across four cycles of light and dark, as seen in Figure 4f.
2.4. Photocatalytic Performance
The photocatalytic activities for CO2 reduction are measured in a solid-gas phase reaction consisting of CO2 and water vapor under a 300 W xenon lamp. As can be seen in Figure 5a, CO is the main product and ZnO and ZnO/ZnAl2O4 are accompanied by traces of CH4 by-products during the irradiation time. The optimal CO production rate of ZnO/ZnAl2O4 was 3.47 μmol g−1h−1, which was 1.57 times higher than that of ZnO (2.21 μmol g−1h−1) and 3.02 times higher than that of ZnAl2O4 (1.15 μmol g−1h−1). It can be found that after the formation of a heterojunction, the catalyst yield of CO is significantly increased. The main reason is that the layered structure of the complex can realize multiple light reflections. Meanwhile, the Z-scheme charge transfer can effectively inhibit carrier complexation and retain the strong redox capacity of photogenerated e− and h+. The introduction of Ov and Znv in ZnO provides abundant active sites that enhance the adsorption and activation of CO2 molecules. The variation of CO production with reaction time was examined. Figure 5b shows that the CO production increased essentially linearly after 2 h of reaction. The CO yield of ZnO/ZnAl2O4 was significantly higher than the other two after 1 h of reaction and showed good stability.
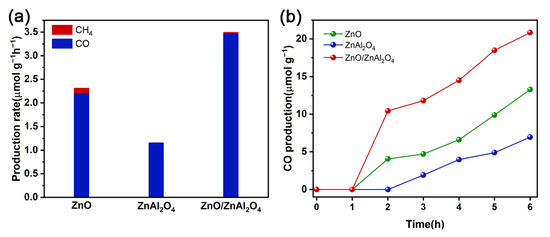
Figure 5.
The production rate (a) and photocatalytic CO evolution performance (b) for CO2 reduction of ZnO, ZnAl2O4, and ZnO/ZnAl2O4.
2.5. Mechanism of Photocatalytic CO2 Reduction
To identify important intermediates linked to the CO2 reduction reaction, in situ DRIFTS of the samples were obtained under light irradiation (Figure 6a–c). For all samples, the strong absorption peaks in the 3750–3550 cm−1 region were ascribed to both O-H stretching vibrations from H2O and adsorbed CO2 molecules [53]. Monodentate carbonate (m-CO32−) was identified as the source of the apparent signals at 1432 and 1431 cm−1. The characteristic spectral bands at 1387, 1374, 1334, and 1328 cm−1 are attributed to bidentate carbonate (b-CO32−) [41]. A characteristic peak of activated carbon dioxide molecules (*CO2) appears at 1682 cm−1 [54]. These peaks become more intense with time and their existence signifies that the adsorbed water molecules have dissociated and that the catalyst has been effectively activated by CO2. Signals for *COOH, an important intermediate in the conversion of CO2 to CO, were detected at 1630, 1613, and 1603 cm−1 [55]. It is noteworthy that the *COOH signals are stronger in ZnAl2O4 and ZnO/ZnAl2O4 compared to ZnO, and the characteristic peaks of bicarbonate (HCO3−) appear at 1472 and 1045 cm−1. ZnO and ZnO/ZnAl2O4 have weak signals of *CHO and *CH3O in the range of 1056–1108 cm−1, which leads them to generate small amounts of CH4 products [56].
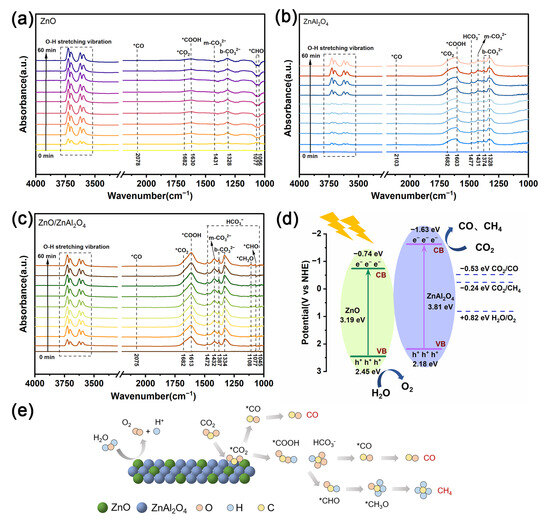
Figure 6.
In situ DRIFTS spectra of CO2 reduction under light irradiation from 0 to 60 min for ZnO (a), ZnAl2O4 (b), and ZnO/ZnAl2O4 (c); (d) schematic illustration on the energy band structures and charge transfer pathways of ZnO/ZnAl2O4 under light irradiation; (e) the possible processes for CO2 reduction of ZnO/ZnAl2O4.
The energy band structure and charge transfer diagram of ZnO/ZnAl2O4 can be created using the band gap and potential computation findings in 3.3 (Figure 6d). The misaligned energy band arrangement constitutes a Z-scheme heterojunction, which enhances the interfacial electric field, reduces the charge transfer resistance, and accelerates the charge separation. When exposed to visible light, e− in ZnO and ZnAl2O4 VBs were stimulated into their corresponding CBs. The photogenerated electrons are then moved to the VB of ZnAl2O4 from the CB of ZnO in the heterojunction. This prolongs the lifetime of the photogenerated electrons and successfully prevents ZnAl2O4’s natural photogenerated e− and h+ recombined. On the surfaces of ZnO and ZnAl2O4, H2O oxidation and CO2 reduction occur separately, realizing high redox potentials.
Three paths of CO2 reduction are presented in Figure 6e. The direct dissociation of CO2 is Path I. Following adsorption on the catalyst surface, CO2 transforms into *CO2. On the zinc surface’s carbon segment, the adsorption is more robust. The oxygen end binds to the proton and removes the O to produce *CO directly, and finally desorbs to produce CO [57]. In ZnO, CO2 reduction is mainly through this path. Path II is the formation of *CO2 after hydrogenation to form *COOH and HCO3−. On the catalyst surface, it breaks down into *CO and *OH, and *CO desorbs to create CO [58,59]. ZnAl2O4 provides a large amount of *COOH and HCO3− intermediates for ZnO/ZnAl2O4 to promote the efficient desorption of CO. Path III is the continuation of the hydrogenation reaction of *CO to produce *CHO and *CH3O, and finally the formation of CH4 [60].
3. Experimental Section
3.1. Materials
The chemicals used in this work include zinc nitrate hexahydrate (Zn(NO3)2·6H2O, A.R.), aluminum nitrate nonahydrate (Al(NO3)3·9H2O, A.R.), ammonia (25% NH3·H2O, A.R.), and anhydrous ethanol (C2H6O, A.R.) and were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). In this experiment, all chemicals were utilized as received without any other treatment.
3.2. Synthesis of Photocatalysts
A specific amount of distilled water was used to dissolve Zn(NO3)2·6H2O and Al(NO3)3·9H2O in accordance with the Zn:Al = 1:2 ratio. Ammonia (25% NH3·H2O) solution was added dropwise to the mixture while being magnetically stirred until the pH reached 9.0. The reaction solution was moved to an autoclave lined with polytetrafluoroethylene after being stirred for 1 hour. After being sealed and maintained at 80 °C for 10 h, the autoclave naturally cooled to ambient temperature. Following many filter washes with ethanol and distilled water, the final sample was dried for a whole night at 80 °C in an oven. To achieve pure ZnAl2O4, the material was then crushed and calcined for 6 hours at 700 °C in a muffle furnace.
ZnO/ZnAl2O4 was prepared by a procedure similar to the one described above, with the difference that the amount of substances Zn/Al = 1:1. An excess of zinc nitrate was added to make the composite.
The same procedure was used to create pure ZnO for comparison; however, aluminum nitrate was not added.
3.3. Catalyst Characterization
The catalysts were characterized by X-ray diffraction (XRD) using a Rigaku MiniFlex600-C X-ray powder diffractometer at room temperature (Tokyo, Japan), Cu Kα, λ = 0.15418 nm, scanning range 20–80°. The catalysts were characterized by FTIR using a Shimadzu IRAffinity-1S Fourier infrared spectrometer (Kyoto, Japan), using the KBr pressing method. The catalysts were characterized by BET using a JW-BK200C specific surface area, pore volume, and pore size analyzer of Jingmicro Gobo Company (Beijing, China), the samples were treated in a vacuum at 300 °C for 8 h, and the nitrogen adsorption and desorption curves were obtained in a liquid nitrogen environment at −196 °C. The specific surface area of the samples was calculated using the multi-point BET method and the pore volume and pore size of the samples were analyzed using the BJH method. The morphological structure and grain size of the prepared samples were observed by a JEOL JEM-2100F transmission electron microscope (TEM) manufactured by Hitachi (Tokyo, Japan), and information such as lattice fringing of the samples was obtained by using the high magnification function of the microscope with an accelerating voltage of 200 kV. The catalysts were characterized by XPS using an ESCALAB 250 X-ray photoelectron spectrometer from Thermo Fisher (Waltham, MA, USA), and the binding energy was calibrated by the C1s peak of carbon (284.8 eV). The Electron Paramagnetic Resonance (EPR, BRUKER MS5000, Billerica, MA, USA) technique was used to detect and analyze free radicals or species with unpaired electrons in the samples. The photoluminescence (PL) characteristics of all the samples were obtained by a HORIBA Fluoromax-4 (Kyoto, Japan). The light response range of the sample was analyzed by UV-vis diffuse reflectance spectra (DRS) spectra (Shimadzu UV-3600, Kyoto, Japan) and Mott–Schottky (M-S) plots. Electrochemical impedance spectroscopy (EIS) and chronoamperometry (I-t) curves were measured on a CHI660E electrochemical workstation (Shanghai, China). The tests were carried out in a standard three-electrode configuration with the electrolyte as 0.1 mol/L Na2SO4 solution, the counter and reference electrodes as platinum and a saturated calomel electrode (SCE), respectively, and the working electrode as a thin ITO conductive glass coated with the catalyst sample. M-S curves were tested at 1000 Hz under light conditions. For the EIS test, the working electrode was tested over the frequency range of 0.1 to 100,000 Hz at 0 V (vs. SCE). The I-t curves were measured under the irradiation of a 300 W xenon lamp (PLS-SXE 300 C, Beijing Perfectlight, Beijing, China) at 0 V (vs. SCE), with the light source alternately turned on and off for 50 s during the test. For in situ DRIFTS, the dynamic evolution process was monitored using a Fourier in situ infrared spectrometer (Nicoletis10, Thermo Fisher, Waltham, MA, USA) at different time points. Prior to measurement, the samples were purged with N2 for 20 min in the sample chamber to eliminate surface contaminants and achieve stabilization. After collecting the background spectrum at t = 0 min, which was subsequently subtracted from all measurements, the gas source was switched to CO2: N2 mixture (2:8 v/v) with simultaneous light illumination (300 W xenon lamp). Samples were collected every 6 min for a total duration of 60 min.
3.4. Photocatalytic CO2 Reduction Reaction
The photocatalytic reduction of CO2 is carried out in a stainless steel reactor with a light-transmitting and high-pressure resistant quartz glass at the top, a heating device at the bottom, a thermocouple inside the reactor, and a sample stage for the catalyst. The light source for the photocatalytic reaction test was a 300W xenon lamp (PLS-SXE 300 C, Beijing Perfectlight, Beijing, China). For the experiment, 20 mg of catalyst powder was evenly spread in the reactor tray and placed in the reactor perpendicular to the beam. The temperature was raised to 120 °C. The reactor was sealed and leak tested to ensure the tightness of the reactor, then 0.3 mL of deionized water was injected into the reactor, the light source was turned on, and the reaction was carried out for 6 h. The gas product content was measured once every hour. The reaction products were analyzed using a gas chromatograph (GC9790II PLF-01, Ar as carrier gas) equipped with a thermal conductivity detector (TCD) and a hydrogen flame ionization detector (FID).
4. Conclusions
In this work, composite catalysts for the photocatalytic CO2 reduction reaction were successfully produced. Compared to individual ZnO and ZnAl2O4, the ZnO/ZnAl2O4 catalysts demonstrated higher photocatalytic performance under light. The Z-scheme heterojunction’s optimized charge-transfer pathways, increased CO2 reduction reaction sites, and decreased interfacial charge-transfer resistance were all credited with the activity boost. The separation and migration of photogenerated carriers were improved by the addition of ZnO, which greatly enhanced the quantity of oxygen vacancies (Ov) and zinc vacancies (Znv), which served as electron traps. And based on in situ DRIFTS, it was concluded that the addition of ZnAl2O4 enhanced the adsorption and activation of CO2, promoted the generation of the intermediate products *COOH and HCO3−, and significantly enhanced the CO yield. This study demonstrated that the synergistic effect of different active components could effectively promote the photocatalytic reduction performance of CO2.
Author Contributions
Conceptualization, M.P. and W.W.; experiment and characterization, L.Z. and C.C.; data curation, M.P. and L.Z.; writing—original draft preparation, M.P. and L.Z.; writing—review and editing, M.P. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
Key Projects of Fujian Provincial Department of Education (JZ230070); Provincial Young and Middle-aged Teachers Education and Research Project of Fujian (Science and Technology) (JAT241231); Provincial First-class Undergraduate Major Construction Project for “Environmental Science” in 2021 (SJZY-2022-02); Provincial Education and Teaching Research of Fujian in 2024 (FBJY20240020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation—A review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Jeffry, L.; Ong, M.Y.; Nomanbhay, S.; Mofijur, M.; Mubashir, M.; Show, P.L. Greenhouse gases utilization: A review. Fuel 2021, 301, 121017. [Google Scholar] [CrossRef]
- Fan, W.; Leung, M.K.H. Recent Development of Plasmonic Resonance-Based Photocatalysis and Photovoltaics for Solar Utilization. Molecules 2016, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research progress on CO2 capture and utilization technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Valluri, S.; Claremboux, V.; Kawatra, S. Opportunities and challenges in CO2 utilization. J. Environ. Sci. 2022, 113, 322–344. [Google Scholar] [CrossRef]
- Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, H.; An, K.; Lee, J.S. Recycling Carbon Dioxide through Catalytic Hydrogenation: Recent Key Developments and Perspectives. ACS Catal. 2020, 10, 11318–11345. [Google Scholar] [CrossRef]
- Song, Q.-W.; Zhou, Z.-H.; He, L.-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Wang, D.; Li, Y. Recent advances of single-atom catalysts in CO2 conversion. Energy Environ. Sci. 2023, 16, 2759–2803. [Google Scholar] [CrossRef]
- Yergaziyeva, G.; Kuspanov, Z.; Mambetova, M.; Khudaibergenov, N.; Makayeva, N.; Daulbayev, C. Advancements in catalytic, photocatalytic, and electrocatalytic CO2 conversion processes: Current trends and future outlook. J. CO2 Util. 2024, 80, 102682. [Google Scholar] [CrossRef]
- Liu, X.; Chen, T.; Xue, Y.; Fan, J.; Shen, S.; Hossain, M.S.A.A.; Amin, M.A.; Pan, L.; Xu, X.; Yamauchi, Y. Nanoarchitectonics of MXene/semiconductor heterojunctions toward artificial photosynthesis via photocatalytic CO2 reduction. Coord. Chem. Rev. 2022, 459, 214440. [Google Scholar] [CrossRef]
- Wang, J.; Guo, R.-T.; Bi, Z.-X.; Chen, X.; Hu, X.; Pan, W.-G. A review on TiO2-x based materials for photocatalytic CO2 reduction. Nanoscale 2022, 14, 11512–11528. [Google Scholar] [CrossRef]
- Zhong, K.; Sun, P.; Xu, H. Advances in Defect Engineering of Metal Oxides for Photocatalytic CO2 Reduction. Small 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.-Q.; Jiang, H.-L. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- Rhimi, B.; Zhou, M.; Yan, Z.; Cai, X.; Jiang, Z. Cu-Based Materials for Enhanced C2+ Product Selectivity in Photo-/Electro-Catalytic CO2 Reduction: Challenges and Prospects. Nano-Micro Lett. 2024, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yu, L.; Kang, P.; Chu, Z.; Li, Y. Modifications and Applications of Metal-Organic-Framework-Based Materials for Photocatalysis. Molecules 2024, 29, 5834. [Google Scholar] [CrossRef]
- Albero, J.; Peng, Y.; Garcia, H. Photocatalytic CO2 Reduction to C2+Products. ACS Catal. 2020, 10, 5734–5749. [Google Scholar] [CrossRef]
- Fang, S.; Rahaman, M.; Bharti, J.; Reisner, E.; Robert, M.; Ozin, G.A.; Hu, Y.H. Photocatalytic CO2 reduction. Nat. Rev. Methods Primers 2023, 3, 61. [Google Scholar] [CrossRef]
- Marszewski, M.; Cao, S.; Yu, J.; Jaroniec, M. Semiconductor-based photocatalytic CO2 conversion. Mater. Horiz. 2015, 2, 261–278. [Google Scholar] [CrossRef]
- Somraksa, W.; Suwanboon, S.; Amornpitoksuk, P.; Randorn, C. Physical and Photocatalytic Properties of CeO2/ZnO/ZnAl2O4 Ternary Nanocomposite Prepared by Co-precipitation Method. Mater. Res. 2020, 23, e20190627. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, X.; Wei, S. Comparative study on sol-gel combined with a hydrothermal synthesis of ZnAl2O4 and ZnO/ZnAl2O4 nanocomposites and its photoluminescence properties and antibacterial activity. Optik 2021, 242, 167151. [Google Scholar] [CrossRef]
- Song, L.; Liu, G.; Qu, Z. Cation rearrangement at tetrahedral sites in the Cu/ZnAl2O4 spinel enhancing CO2 hydrogenation to methanol. Appl. Catal. B Environ. Energy 2025, 362, 124742. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S.; Liang, Y.; Sun, Y.; Xiong, H. B-modified Pd/ZnAl2O4 Catalyst for Enhancing CO2 Hydrogenation to Methanol. Catal. Lett. 2024, 154, 4805–4813. [Google Scholar] [CrossRef]
- Liu, C.; Xu, J.; Zhiani, R. Synthesis of nanofibrous ZnAl2O4 for hydrogenation of CO2 to formate. Inorg. Chem. Commun. 2022, 139, 109392. [Google Scholar] [CrossRef]
- Yuan, X.; Cheng, X.; Jing, Q.; Niu, J.; Peng, D.; Feng, Z.; Wu, X. ZnO/ZnAl2O4 Nanocomposite with 3D Sphere-like Hierarchical Structure for Photocatalytic Reduction of Aqueous Cr(VI). Materials 2018, 11, 1624. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, B.; Zhang, J.; Ghasemi, J.B.; Mousavi, M.; Yu, J. S-scheme heterojunction photocatalysts for CO2 reduction. Matter 2022, 5, 4187–4211. [Google Scholar] [CrossRef]
- Zhang, W.; Mohamed, A.R.; Ong, W.-J. Z-Scheme Photocatalytic Systems for Carbon Dioxide Reduction: Where Are We Now? Angew. Chem. Int. Ed. 2020, 59, 22894–22915. [Google Scholar] [CrossRef]
- Lin, G.; Sun, L.; Huang, G.; Chen, Q.; Fang, S.; Bi, J.; Wu, L. Direct Z-scheme copper cobaltite/covalent triazine-based framework heterojunction for efficient photocatalytic CO2 reduction under visible light. Sustain. Energy Fuels 2021, 5, 732–739. [Google Scholar] [CrossRef]
- Guo, S.-T.; Tang, Z.-Y.; Du, Y.-W.; Liu, T.; Ouyang, T.; Liu, Z.-Q. Chlorine anion stabilized Cu2O/ZnO photocathode for selective CO2 reduction to CH4. Appl. Catal. B Environ. 2023, 321, 122035. [Google Scholar] [CrossRef]
- Dhiman, P.; Rana, G.; Kumar, A.; Sharma, G.; Vo, D.-V.N.; Naushad, M. ZnO-based heterostructures as photocatalysts for hydrogen generation and depollution: A review. Environ. Chem. Lett. 2022, 20, 1047–1081. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef]
- Wang, G.; Lv, S.; Shen, Y.; Li, W.; Lin, L.; Li, Z. Advancements in heterojunction, cocatalyst, defect and morphology engineering of semiconductor oxide photocatalysts. J. Mater. 2024, 10, 315–338. [Google Scholar] [CrossRef]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloys Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Gao, W.; Chi, H.Q.; Xiong, Y.J.; Ye, J.H.; Zou, Z.G.; Zhou, Y. Comprehensive Insight into Construction of Active Sites toward Steering Photocatalytic CO2 Conversion. Adv. Funct. Mater. 2024, 34, 2312056. [Google Scholar] [CrossRef]
- Qorbani, M.; Sabbah, A.; Lai, Y.-R.; Kholimatussadiah, S.; Quadir, S.; Huang, C.-Y.; Shown, I.; Huang, Y.-F.; Hayashi, M.; Chen, K.-H.; et al. Atomistic insights into highly active reconstructed edges of monolayer 2H-WSe2 photocatalyst. Nat. Commun. 2022, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Eskandari Azar, B.; Ramazani, A.; Taghavi Fardood, S.; Morsali, A. Green synthesis and characterization of ZnAl2O4@ZnO nanocomposite and its environmental applications in rapid dye degradation. Optik 2020, 208, 164129. [Google Scholar] [CrossRef]
- Moradipour, P.; Dabirian, F.; Moradipour, M. Ternary ZnO/ZnAl2O4/Al2O3 composite nanofiber as photocatalyst for conversion of CO2 and CH4. Ceram. Int. 2020, 46, 5566–5574. [Google Scholar] [CrossRef]
- Shahmirzaee, M.; Shafiee Afarani, M.; Arabi, A.M.; Iran Nejhad, A. In situ crystallization of ZnAl2O4/ZnO nanocomposite on alumina granule for photocatalytic purification of wastewater. Res. Chem. Intermed. 2016, 43, 321–340. [Google Scholar] [CrossRef]
- Suwanboon, S.; Amornpitoksuk, P.; Rattana, T.; Randorn, C. Investigation of g-C3N4/ZnAl2O4 and ZnO/ZnAl2O4 nanocomposites: From synthesis to photocatalytic activity of pollutant dye model. Ceram. Int. 2020, 46, 21958–21977. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; He, Z.; Zheng, J.; Zhu, M.; Zeng, Z.; Li, H.; Liu, Y.; Yang, S. Selective Photoconversion of CO2 to C2H4 on Asymmetrical CeO2–Cu2O Interfaces Driven by Oxygen Vacancies. Adv. Funct. Mater. 2025. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, T.; Li, J.; Li, C.; Lu, W.; Liu, J.; Zhang, D.; Dong, Y.; Yang, M. Electron-Deficient Coδ+ Induced by Synergistic Co–ZnO–ZnAl2O4 Interface Interactions for Enhanced N-Propylcarbazole Hydrogenation. ACS Catal. 2025, 15, 5490–5502. [Google Scholar] [CrossRef]
- Wang, D.-D.; Xu, M.-Y.; Lin, Z.-X.; Wu, J.-H.; Yang, W.-T.; Li, H.-J.; Su, Z.-M. One-pot synthesis of MIL-68(In)-derived CdIn2S4/In2S3 tubular heterojunction for highly selective CO2 photoreduction. Rare Met. 2025, 44, 3956–3969. [Google Scholar] [CrossRef]
- Deng, H.; Fei, X.; Yang, Y.; Fan, J.; Yu, J.; Cheng, B.; Zhang, L. S-scheme heterojunction based on p-type ZnMn2O4 and n-type ZnO with improved photocatalytic CO2 reduction activity. Chem. Eng. J. 2021, 409, 127377. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zeng, Z.; Hu, J.; Hou, Y.; Huang, Z. Synergically engineering Cu+ and oxygen vacancies in CuMn2O4 catalysts for enhanced toluene oxidation performance. Mol. Catal. 2022, 517, 112043. [Google Scholar] [CrossRef]
- Ling, W.; Ma, J.; Hong, M.; Sun, R. Enhance photocatalytic CO2 reduction and biomass selective oxidation via sulfur vacancy-enriched S-scheme heterojunction of MoS2@GCN. Chem. Eng. J. 2024, 493, 152729. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, L.; Wang, G.; Cui, J.; Guan, G.L.; Miao, Y.T.; Wu, J.F.; Gao, P.; Yang, F.; Ling, Y.; et al. Spinel Nanostructures for the Hydrogenation of CO2 to Methanol and Hydrocarbon Chemicals. J. Am. Chem. Soc. 2024, 146, 14528–14538. [Google Scholar] [CrossRef]
- Sun, M.; Chen, Y.; Fan, X.; Li, D.; Song, J.; Yu, K.; Zhao, Z. Electronic asymmetry of lattice oxygen sites in ZnO promotes the photocatalytic oxidative coupling of methane. Nat. Commun. 2024, 15, 9900. [Google Scholar] [CrossRef]
- Shan, C.; Su, Z.; Liu, Z.; Xu, R.; Wen, J.; Hu, G.; Tang, T.; Fang, Z.; Jiang, L.; Li, M. One-Step Synthesis of Ag2O/Fe3O4 Magnetic Photocatalyst for Efficient Organic Pollutant Removal via Wide-Spectral-Response Photocatalysis-Fenton Coupling. Molecules 2023, 28, 4155. [Google Scholar] [CrossRef]
- Liang, J.; Chai, Y.; Li, L.; Li, D.; Shen, J.; Zhang, Y.; Wang, X. Germanium and iron double-substituted ZnGa2O4 solid-solution photocatalysts with modulated band structure for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B Environ. 2020, 265, 118551. [Google Scholar] [CrossRef]
- Wang, C.; Hu, C.; Chen, F.; Li, H.; Zhang, Y.; Ma, T.; Huang, H. Polar Layered Bismuth-Rich Oxyhalide Piezoelectrics Bi4O5X2 (X(sic)Br, I): Efficient Piezocatalytic Pure Water Splitting and Interlayer Anion-Dependent Activity. Adv. Funct. Mater. 2023, 33, 2301144. [Google Scholar] [CrossRef]
- Chen, W.; Kang, T.; Du, F.; Han, P.; Gao, M.; Hu, P.; Teng, F.; Fan, H. A new S-scheme heterojunction of 1D ZnGa2O4/ZnO nanofiber for efficient photocatalytic degradation of TC-HCl. Environ. Res. 2023, 232, 116388. [Google Scholar] [CrossRef]
- Guo, C.; Tang, Y.; Yang, Z.; Zhao, T.; Liu, J.; Zhao, Y.; Wang, F. Reinforcing the Efficiency of Photothermal Catalytic CO2 Methanation through Integration of Ru Nanoparticles with Photothermal MnCo2O4 Nanosheets. ACS Nano 2023, 17, 23761–23771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhuang, G.L.; Zhang, J.W.; Luo, F.; Cheng, X.; Sun, F.L.; Fu, S.S.; Lu, T.B.; Zhang, Z.M. Co-Dissolved Isostructural Polyoxovanadates to Construct Single-Atom-Site Catalysts for Efficient CO2 Photoreduction. Angew. Chem. Int. Ed. Engl. 2023, 62, e202216592. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wen, J.; Wang, Y.; Chen, J.; Lu, C.-Z. Vacancies induce the enhancement of CO2 photothermal reduction with water vapor via ZrO2/ZnS composite catalysts. Appl. Surf. Sci. 2025, 686, 162209. [Google Scholar] [CrossRef]
- Su, B.; Wang, S.; Xing, W.; Liu, K.; Hung, S.-F.; Chen, X.; Fang, Y.; Zhang, G.; Zhang, H.; Wang, X. Synergistic Ru Species on Poly(heptazine imide) Enabling Efficient Photocatalytic CO2 reduction with H2O Beyond 800 nm. Angew. Chem. Int. Ed. Engl. 2025. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, Y.; Liu, X.; Li, H.; Lu, Z. NH2-UIO-66 Based Hydrophobic Porous Liquid with High Mass Transfer and Affinity Surface for Enhancing CO2 Photoreduction. Acta Phys. Chim. Sin. 2024, 40, 2403032. [Google Scholar] [CrossRef]
- Lai, K.; Sun, Y.; Li, N.; Gao, Y.; Li, H.; Ge, L.; Ma, T. Photocatalytic CO2-to-CH4 Conversion with Ultrahigh Selectivity of 95.93% on S-Vacancy Modulated Spatial In2S3/In2O3 Heterojunction. Adv. Funct. Mater. 2024, 34, 2409031. [Google Scholar] [CrossRef]
- Singhvi, C.; Sharma, G.; Verma, R.; Paidi, V.K.; Glatzel, P.; Paciok, P.; Patel, V.B.; Mohan, O.; Polshettiwar, V. Tuning the electronic structure and SMSI by integrating trimetallic sites with defective ceria for the CO2 reduction reaction. Proc. Natl. Acad. Sci. USA 2025, 122, e2411406122. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, E.; Tang, J. Insight on Reaction Pathways of Photocatalytic CO2 Conversion. ACS Catal. 2022, 12, 7300–7316. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Yang, Y.; Chen, J.G.; Liu, P. Optimizing Binding Energies of Key Intermediates for CO2 Hydrogenation to Methanol over Oxide-Supported Copper. J. Am. Chem. Soc. 2016, 138, 12440–12450. [Google Scholar] [CrossRef]
- Oni, B.A.; Sanni, S.E.; Tomomewo, O.S.; Bade, S.O. Cu2O/SiC photocatalytic reduction of carbon dioxide to methanol using visible light on InTaO4. Mater. Sci. Semicond. Process. 2024, 174, 108235. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).