Measuring the Carboxypeptidase and γ-Glutamyltranspeptidase Activities of Lager and Ale Yeasts to Assess Their Impact on the Release of Odorant Polyfunctional Thiols Through Fermentation
Abstract
1. Introduction
2. Results and Discussion
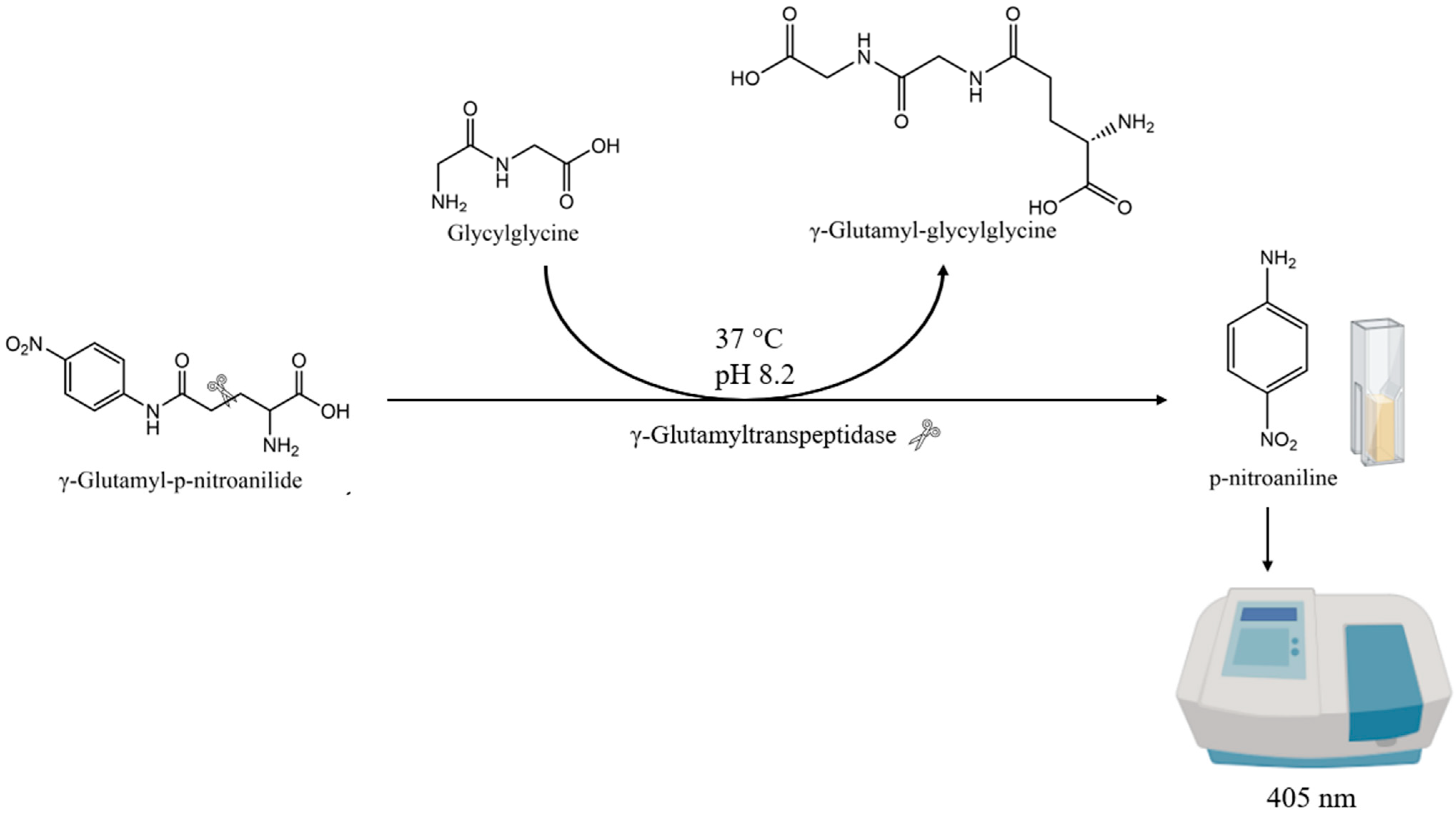
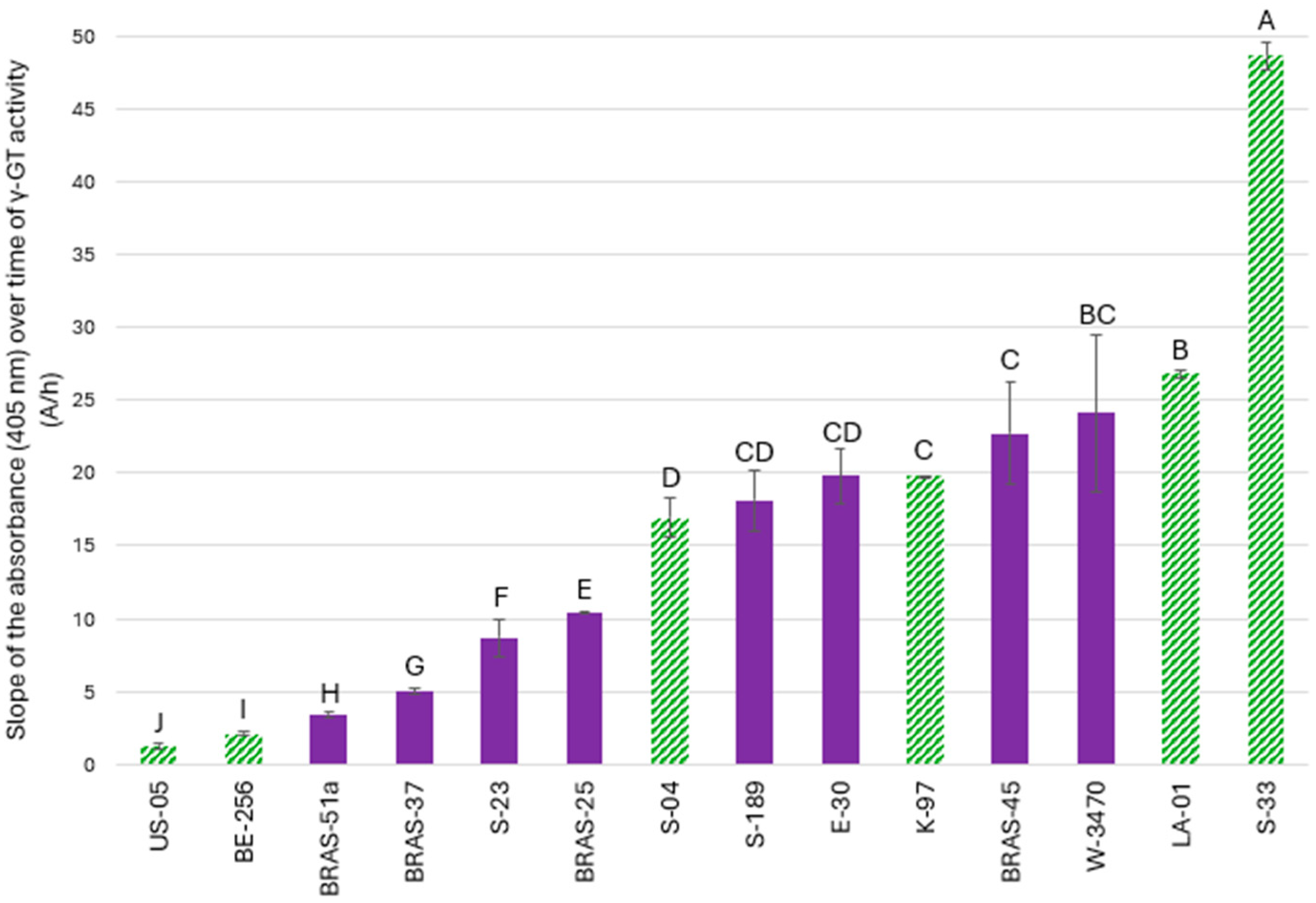
2.1. New Enzymatic Assay for Comparing γ-GT Activity in Various Lager and Ale Brewing Yeasts
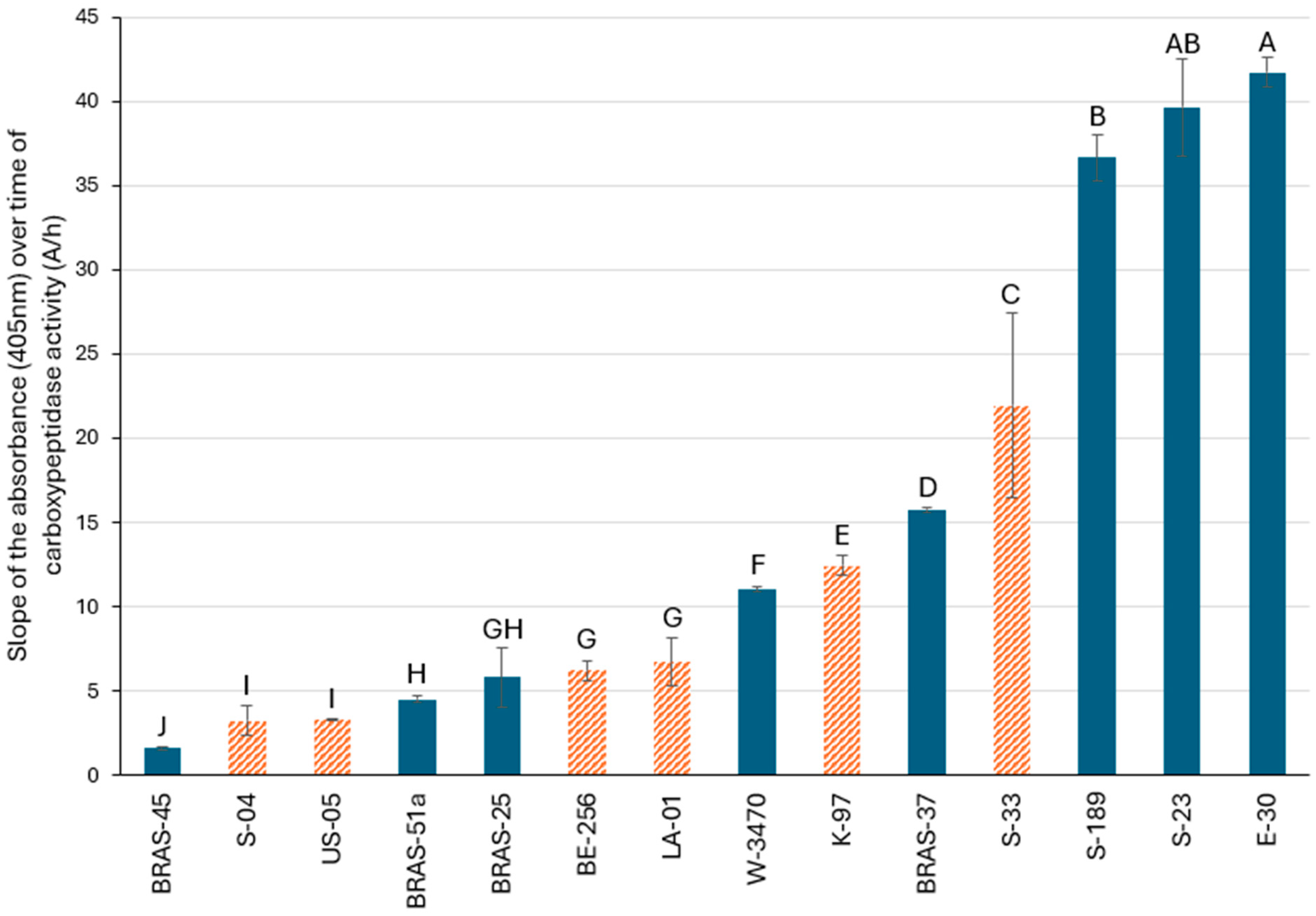
2.2. Enzymatic Assay for Comparing the Carboxypeptidase Activities of Various Lager and Ale Brewing Yeasts
2.3. Investigation of Whether, in Lager Yeasts, There Is a Relationship Between γ-GT or Carboxypeptidase Activity and the Ability to Release Free PFTs from S Conjugates, Especially γ-GluCys-Precursors
2.4. Investigation of Whether There Is a Relationship Between the γ-GT or Carboxypeptidase Activity of Ale Yeasts and Their Ability to Release Free PFTs from G-Conjugates
3. Materials and Methods
3.1. Chemicals
3.2. Yeasts
3.3. γ-GT Activity Measurement
3.3.1. γ-GT Extraction
3.3.2. γ-GT Enzymatic Assay
3.4. Carboxypeptidase Activity Measurement
3.4.1. Carboxypeptidase Enzymatic Extraction
3.4.2. Carboxypeptidase Enzymatic Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tominaga, T.; Dubourdieu, D. Identification of Cysteinylated Aroma Precursors of Certain Volatile Thiols in Passion Fruit Juice. J. Agric. Food Chem. 2000, 48, 2874–2876. [Google Scholar] [CrossRef] [PubMed]
- Chenot, C.; Collin, S.; Suc, L.; Roland, A. Unusual Profile of Thiol Precursors in Special Malts: First Evidence of Chemical Glutathione-/γGluCys- and CysGly-/Cys- Conversions. J. Am. Soc. Brew. Chem. 2024, 82, 15–22. [Google Scholar] [CrossRef]
- Chenot, C.; Haest, S.; Robiette, R.; Collin, S. Thiol S-Conjugate Profiles: A Comparative Investigation on Dual Hop and Grape Must with Focus on Sulfanylalkyl Aldehydes and Acetates Adducts. J. Am. Soc. Brew. Chem. 2023, 81, 23–32. [Google Scholar] [CrossRef]
- Gros, J.; Peeters, F.; Collin, S. Occurrence of Odorant Polyfunctional Thiols in Beers Hopped with Different Cultivars. First Evidence of an S -Cysteine Conjugate in Hop (Humulus Lupulus L.). J. Agric. Food Chem. 2012, 60, 7805–7816. [Google Scholar] [CrossRef]
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of New Volatile Thiols in the Aroma of Vitis vinifera L. Var. Sauvignon Blanc Wines. Flavour Fragr. J. 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Bonnaffoux, H.; Roland, A.; Schneider, R.; Cavelier, F. Spotlight on Release Mechanisms of Volatile Thiols in Beverages. J. Agric. Food Chem. 2021, 339, 127628. [Google Scholar] [CrossRef]
- Chenot, C.; Thibault De Chanvalon, E.; Janssens, P.; Collin, S. Modulation of the Sulfanylalkyl Acetate/Alcohol Ratio and Free Thiol Release from Cysteinylated and/or Glutathionylated Sulfanylalkyl Alcohols in Beer under Different Fermentation Conditions. J. Agric. Food Chem. 2021, 69, 6005–6012. [Google Scholar] [CrossRef]
- Chenot, C.; Donck, W.; Janssens, P.; Collin, S. Malt and Hop as Sources of Thiol S-Conjugates: Thiol-Releasing Property of Lager Yeast during Fermentation. J. Agric. Food Chem. 2022, 70, 3272–3279. [Google Scholar] [CrossRef]
- Schwartz, M.; Poirier, N.; Moreno, J.; Proskura, A.; Lelièvre, M.; Heydel, J.-M.; Neiers, F. Microbial β C-S Lyases: Enzymes with Multifaceted Roles in Flavor Generation. Int. J. Mol. Sci. 2024, 25, 6412. [Google Scholar] [CrossRef]
- Parker, M.; Capone, D.L.; Francis, I.L.; Herderich, M.J. Aroma Precursors in Grapes and Wine: Flavor Release during Wine Production and Consumption. J. Agric. Food Chem. 2018, 66, 2281–2286. [Google Scholar] [CrossRef]
- Li, Z.-S.; Szczypka, M.; Lu, Y.-P.; Thiele, D.J.; Rea, P.A. The Yeast Cadmium Factor Protein (YCF1) Is a Vacuolar Glutathione S-Conjugate Pump. J. Biol. Chem. 1996, 271, 6509–6517. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Mamnun, Y.M.; Eggmann, T.; Schüller, C.; Wolfger, H.; Martinoia, E.; Kuchler, K. The ATP-Binding Cassette (ABC) Transporter Bpt1p Mediates Vacuolar Sequestration of Glutathione Conjugates in Yeast. FEBS Lett. 2002, 520, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Capone, D.L.; Curtin, C.D. Unravelling Glutathione Conjugate Catabolism in Saccharomyces Cerevisiae: The Role of Glutathione/Dipeptide Transporters and Vacuolar Function in the Release of Volatile Sulfur Compounds 3-Mercaptohexan-1-Ol and 4-Mercapto-4-Methylpentan-2-One. Appl. Microbiol. Biotechnol. 2015, 99, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-Y.; Bae, Y.-H.; Kim, S.-K.; Seong, Y.-J.; Choi, S.-H.; Kim, K.H.; Park, Y.-C.; Seo, J.-H. Effects of Signal Sequences and Folding Accessory Proteins on Extracellular Expression of Carboxypeptidase Y in Recombinant Saccharomyces Cerevisiae. Bioprocess Biosyst. Eng. 2014, 37, 1065–1071. [Google Scholar] [CrossRef]
- Bonnaffoux, H.; Delpech, S.; Rémond, E.; Schneider, R.; Roland, A.; Cavelier, F. Revisiting the Evaluation Strategy of Varietal Thiol Biogenesis. Food Chem. 2018, 268, 126–133. [Google Scholar] [CrossRef]
- Donald Allison, R. γ-Glutamyl transpeptidase: Kinetics and mechanism. Methods Enzymol. 1985, 113, 419–437. [Google Scholar]
- Brett, C.L.; Kallay, L.; Hua, Z.; Green, R.; Chyou, A.; Zhang, Y.; Graham, T.R.; Donowitz, M.; Rao, R. Genome-Wide Analysis Reveals the Vacuolar pH-Stat of Saccharomyces cerevisiae. PLoS ONE 2011, 6, 17619. [Google Scholar] [CrossRef]
- Jung, J.; Ueno, H.; Hayashi, R. Carboxypeptidase Y: Structural Basis for Protein Sorting and Catalytic Triad. J. Biochem. 1999, 126, 1–6. [Google Scholar] [CrossRef]
- Simon, M.; Christiaens, R.; Janssens, P.; Collin, S. Unexpected Behavior of a Maltose-Negative Saccharomyces Cerevisiae Yeast: Higher Release of Polyfunctional Thiols from Glutathionylated Than from Cysteinylated S-Conjugates. Fermentation 2024, 10, 276. [Google Scholar] [CrossRef]
- Christiaens, R.; Simon, M.; Robiette, R.; Collin, S. Evidence in Lager Yeasts of β-Lyase Activity Breaking Down γ-GluCys-Conjugates More Efficiently Than Cys-Conjugates to Odorant Beer Polyfunctional Thiols. Molecules 2025, 30, 325. [Google Scholar] [CrossRef]
- Tate, S.S.; Meister, A. γ-Glutamyl Transpeptidase: Catalytic, Structural and Functional Aspects. Mol. Cell Biochem. 1981, 39, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Penninckx, M.; Jaspers, C.; Wiame, J.M. Glutathione Metabolism in Relation to the Amino-Acid Permeation Systems of the Yeast Saccharomyces Cerevisiae. Occurrence of Gamma-Glutamyltranspeptidase: Its Regulation and the Effects of Permeation Mutations on the Enzyme Cellular Level. Eur. J. Biochem. 1980, 104, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Easwaran, S.N.; Mohanakrishnan, A.S.; Santharam, L.; Adimoolam, S.R.; Mahadevan, S. Metabolic Heat Responses of Kluyveromyces Marxianus and Saccharomyces Cerevisiae during Carboxypeptidase Y Enzyme Production. Process Biochem. 2022, 112, 71–79. [Google Scholar] [CrossRef]
- Ramı́rez-Zavala, B.; Mercado-Flores, Y.; Hernández-Rodrı́guez, C.; Villa-Tanaca, L. Purification and Characterization of a Serine Carboxypeptidase from Kluyveromyces Marxianus. Int. J. Food Microbiol. 2004, 91, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Delhez, J.; Dufour, J.P.; Thines, D.; Goffeau, A. Comparison of the Properties of Plasma Membrane-Bound and Mitochondria-Bound ATPases in the Yeast Schizosaccharmoyces Pombe. Eur. J. Biochem. 1977, 79, 319–328. [Google Scholar] [CrossRef]

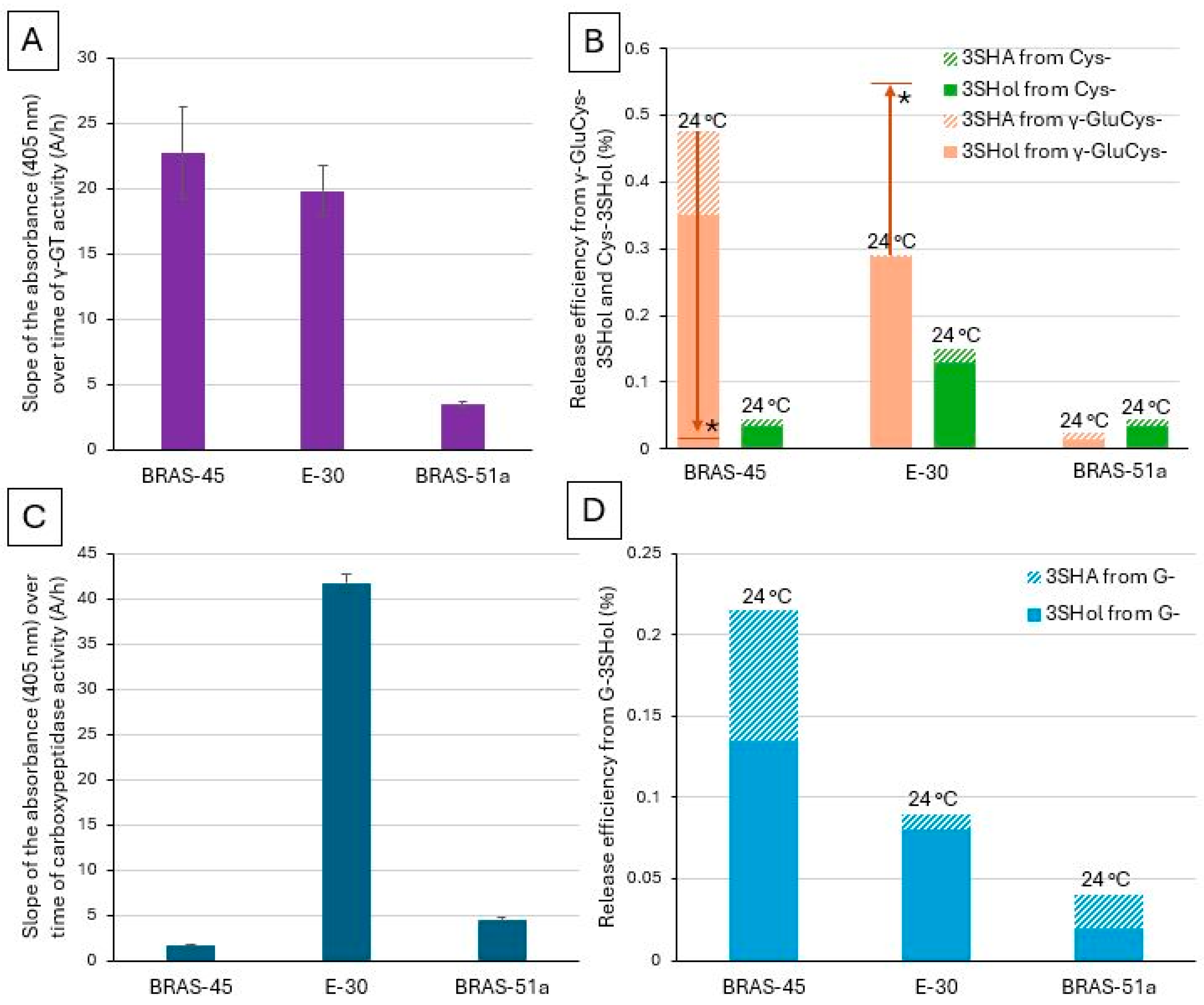
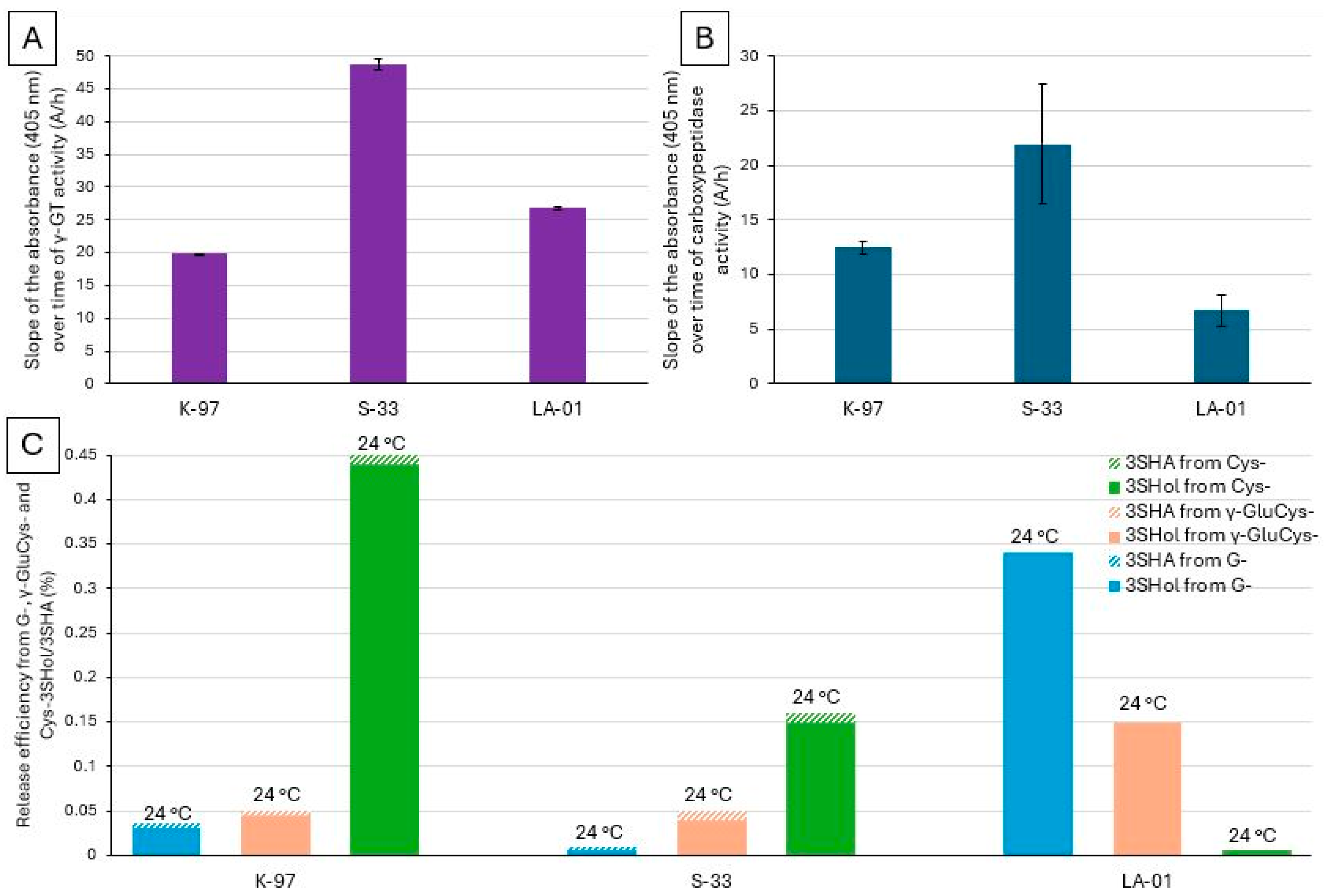
 ) and non-limiting factors (
) and non-limiting factors ( ) in the release, by brewing yeasts, of odorant PFTs from G, γ-GluCys-, and Cys- precursors.
) in the release, by brewing yeasts, of odorant PFTs from G, γ-GluCys-, and Cys- precursors.
 ) and non-limiting factors (
) and non-limiting factors ( ) in the release, by brewing yeasts, of odorant PFTs from G, γ-GluCys-, and Cys- precursors.
) in the release, by brewing yeasts, of odorant PFTs from G, γ-GluCys-, and Cys- precursors.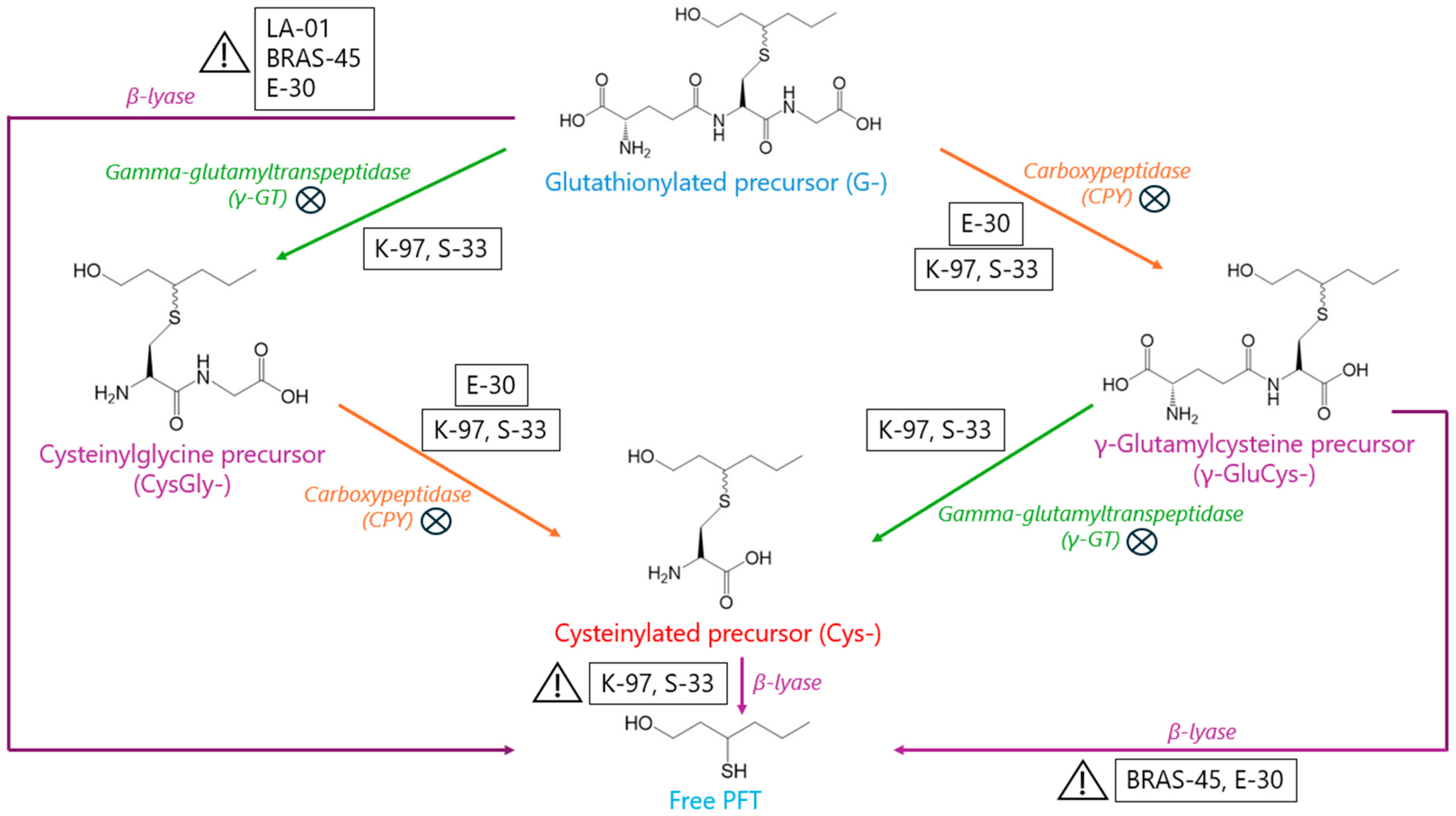
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calicis, C.; Christiaens, R.; Loquet, N.; Simon, M.; Collin, S. Measuring the Carboxypeptidase and γ-Glutamyltranspeptidase Activities of Lager and Ale Yeasts to Assess Their Impact on the Release of Odorant Polyfunctional Thiols Through Fermentation. Molecules 2025, 30, 2491. https://doi.org/10.3390/molecules30122491
Calicis C, Christiaens R, Loquet N, Simon M, Collin S. Measuring the Carboxypeptidase and γ-Glutamyltranspeptidase Activities of Lager and Ale Yeasts to Assess Their Impact on the Release of Odorant Polyfunctional Thiols Through Fermentation. Molecules. 2025; 30(12):2491. https://doi.org/10.3390/molecules30122491
Chicago/Turabian StyleCalicis, Coraline, Romain Christiaens, Natacha Loquet, Margaux Simon, and Sonia Collin. 2025. "Measuring the Carboxypeptidase and γ-Glutamyltranspeptidase Activities of Lager and Ale Yeasts to Assess Their Impact on the Release of Odorant Polyfunctional Thiols Through Fermentation" Molecules 30, no. 12: 2491. https://doi.org/10.3390/molecules30122491
APA StyleCalicis, C., Christiaens, R., Loquet, N., Simon, M., & Collin, S. (2025). Measuring the Carboxypeptidase and γ-Glutamyltranspeptidase Activities of Lager and Ale Yeasts to Assess Their Impact on the Release of Odorant Polyfunctional Thiols Through Fermentation. Molecules, 30(12), 2491. https://doi.org/10.3390/molecules30122491




