Extracts, Fractions, and Subfractions from Purple-Orange Sweet Potato (Ipomoea batatas L.): Xanthine Oxidase Inhibitory Potential and Antioxidant Properties
Abstract
1. Introduction
2. Results
2.1. Bioactive Content, Inhibitory of Xanthine Oxidase and Antioxidant Activities of Ethanolic Extracts from Three Plant Parts
2.2. Thin Layer Chromatography, Bioactive Content, Xanthine Oxidase Inhibitory Activity, and Antioxidant Activity of Leaf Extracts
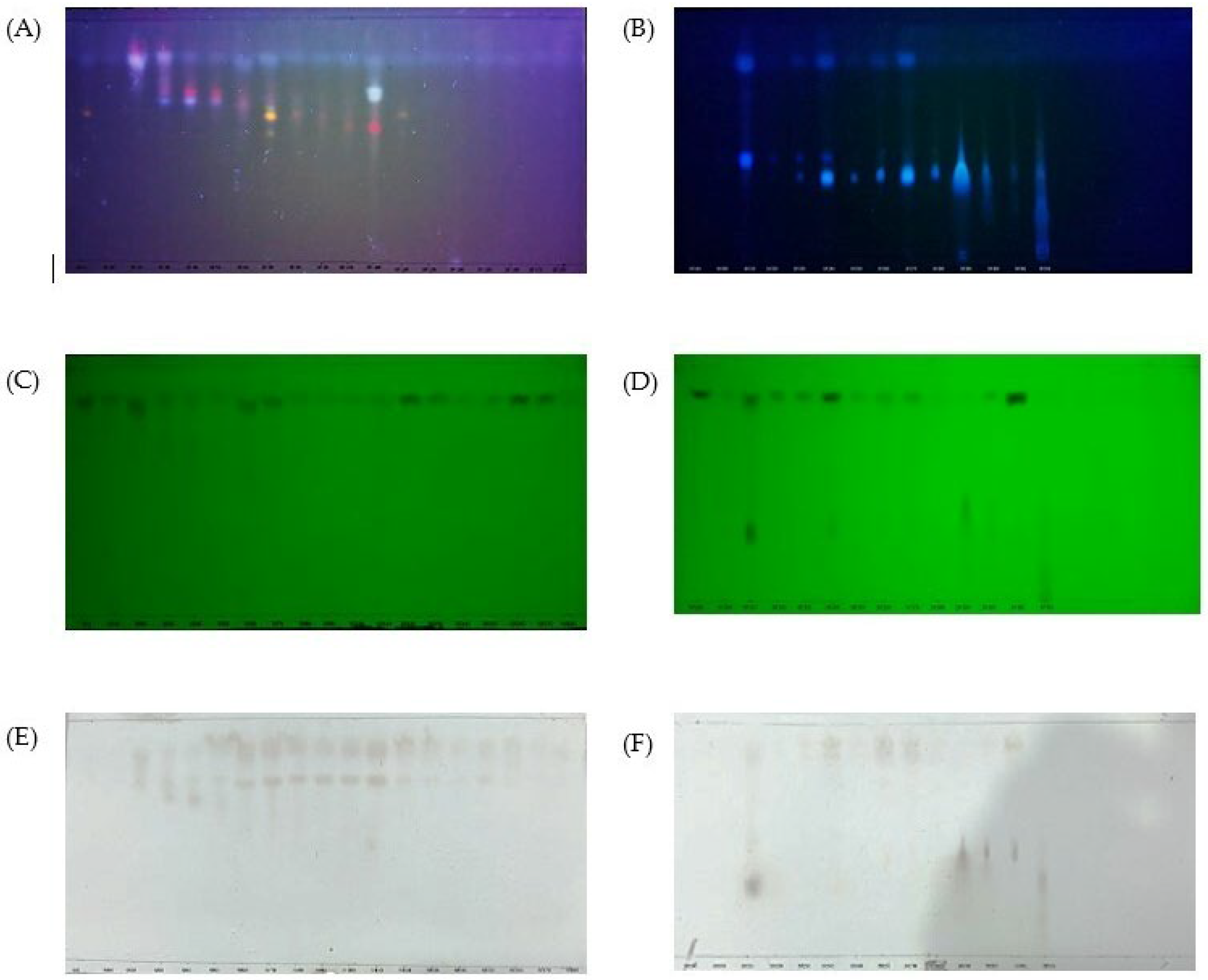
2.3. Thin Layer Chromatography, Bioactive Content, Xanthine Oxidase Inhibitory and Antioxidant Activities of Fractions
2.4. Bioactive Content, Xanthine Oxidase Inhibitory and Antioxidant Activities of Subfractions
2.5. Comparison of Xanthine Oxidase Inhibitory and Antioxidant Activities of ELE, EAE, CF5, and CSF3
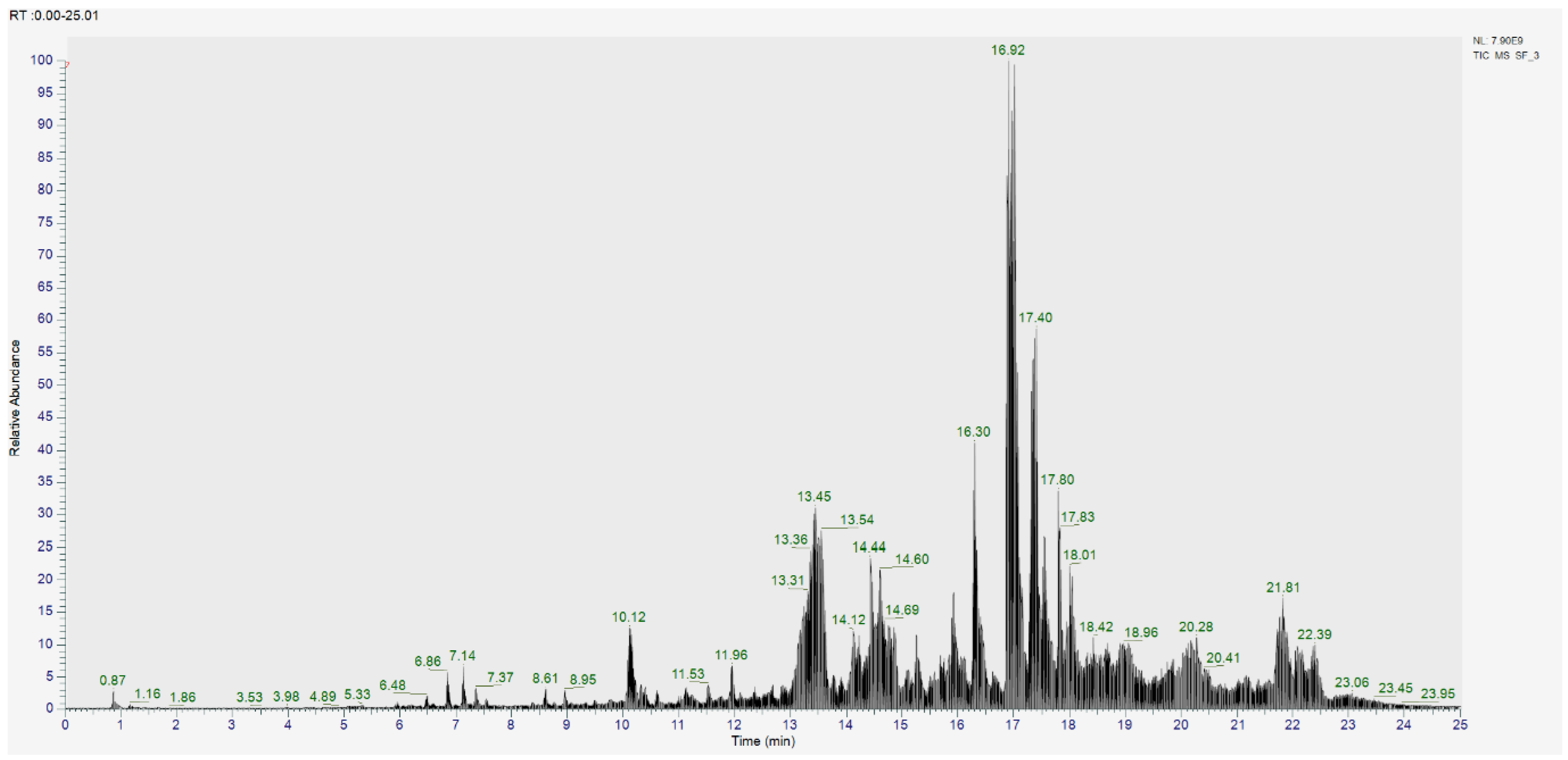
2.6. Analysis of Secondary Metabolites in CSF3 Using UPLC–MS/MS
3. Discussion
4. Materials and Methods
4.1. General Experiment Procedure
4.2. Material
4.3. Preparation of Crude Drugs and Extraction
4.4. Fractionation Using Vacuum Liquid Chromatography (VLC)
4.5. Subfractionation Using Classic Column Chromatography (CCC)
4.6. Thin Layer Chromatography (TLC) Procedure
4.7. Estimation of Total Phenol Content (TPC)
4.8. Quantification of Total Flavonoid Content (TFC)
4.9. DPPH (2,2-Diphenyl-1-picrylhydrazyl) Assay
4.10. FRAP (Ferric Reducing Antioxidant Power) Assay
4.11. CUPRAC (Cupric Ion Reducing Antioxidant Capacity)
4.12. Xanthine Oxidase Inhibitory (XOI) Activity
4.13. Identification LC-MS-MS
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunitha, D.A. Review on antioxidant methods. Asian J. Pharm. Clin. Res. 2016, 9, 14–32. [Google Scholar] [CrossRef]
- Palma, J.M.; Seiquer, I. To be or not to be… An antioxidant? That is the question. Antioxidants 2020, 9, 1234. [Google Scholar] [CrossRef] [PubMed]
- FAO: Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 12 May 2025).
- Pushpalatha, R.; Gangadharan, B. Climate resilience, yield and geographical suitability of sweet potato under the changing climate: A review. Nat. Resour. Forum 2024, 48, 106–119. [Google Scholar] [CrossRef]
- Ooi, S.F.; Sukri, S.A.M.; Zakaria, N.N.A.; Harith, Z.T. Carotenoids, phenolics and antioxidant properties of different sweet potatoes (Ipomoea batatas) varieties. In Proceedings of the Name of 3rd Asia Pacific Regional Conference on Food Security (ARCoFS 2021), Kelantan, Malaysia, 9 March 2021. [Google Scholar]
- Fidrianny, I.; Windyaswari, A.S.; Wirasutisna, K.R. Antioxidant capacities of various leaves extract from five colors varieties of sweet potatoes tubers using ABTS, DPPH assays and correlation with total flavonoid, phenolic, carotenoid content. Res. J. Med. Plant 2013, 7, 130–140. [Google Scholar] [CrossRef]
- Fu, Z.F.; Tu, Z.C.; Zhang, L.; Wang, H.; Wen, Q.H.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosc. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Fidrianny, I.; Suhendy, H.; Insanu, M. Correlation of phytochemical content with antioxidant potential of various sweet potato (Ipomoea batatas) in West Java, Indonesia. Asian Pac. J. Trop. Biomed. 2018, 8, 25–30. [Google Scholar] [CrossRef]
- Nabot, M.; Garcia, C.; Seguin, M.; Ricci, J.; Brabet, C.; Remize, F. Bioactive compound diversity in a wide panel of sweet potato (Ipomoea batatas L.) cultivars: A resource for nutritional food development. Metabolites 2024, 14, 523. [Google Scholar] [CrossRef] [PubMed]
- Iio, M.; Ono, Y.; Kai, S.; Fukumoto, M. Effects of flavonoids on xanthine oxidation as well as on cytochrome c reduction by milk xanthine oxidase. J. Nutr. Sci. Vitaminol. 1986, 32, 635–642. [Google Scholar] [CrossRef]
- Chang, W.S.; Lee, Y.J.; Lu, F.J.; Chiang, H.C. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993, 13, 2165–2170. [Google Scholar]
- Shoskes, D.A. Effect of bioflavonoids quercetin and curcumin on ischemic renal injury: A new class of renoprotective agents: 1. Transplantation 1998, 66, 147–152. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Berghe, D.V. Structure−activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Wang, H.B.; Zhou, Q.; Hu, B.; Wen, J.H.; Zhang, J.L. Screening of effective xanthine oxidase inhibitors in dietary anthocyanins from purple sweet potato (Ipomoea batatas L. Cultivar Eshu No.8) and deciphering of the underlying mechanisms in vitro. J. Funct. Foods 2017, 36, 102–111. [Google Scholar] [CrossRef]
- Yang, Z.W.; Tang, C.E.; Zhang, J.L.; Zhou, Q.; Zhang, Z.C. Stability and antioxidant activity of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) subjected to simulated in vitro gastrointestinal digestion. Int. J. Food Sci. Technol. 2019, 54, 2604–2614. [Google Scholar] [CrossRef]
- Tamokou, J.D.D.; Kuiate, J.R.; Gatsing, D.; Efouet, A.P.N.; Njouendou, A.J. Antidermatophytic and toxicological evaluations of dichloromethane-methanol extract, fractions and compounds isolated from Coula edulis. Iran. J. Med. Sci. 2011, 36, 111–121. [Google Scholar] [PubMed]
- Obonga, W.O.; Nnadi, C.O.; Chima, C.C.; Okafor, S.N.; Omeje, E.O. In- vitro antioxidant and in vivo anti-inflammatory potentials of Marantochloa leucantha (Marantaceae) extracts and fractions. Dhaka Univ. J. Pharm. Sci. 2019, 18, 233–240. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef]
- Telagari, M.; Hullatti, K. In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar] [PubMed]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic compounds of pomegranate byproducts (outer skin, mesocarp, divider membrane) and their antioxidant activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef]
- Algariri, K.; Atangwho, I.J.; Meng, K.Y.; Asmawi, M.Z.; Sadikun, A.; Murugaiyah, V. Antihyperglycaemic and toxicological evaluations of extract and fractions of Gynura procumbens leaves. Trop. Life Sci. Res. 2014, 25, 75–93. [Google Scholar]
- Khorasani Esmaeili, A.; Mat Taha, R.; Mohajer, S.; Banisalam, B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red Clover). Biomed. Res. Int. 2015, 1, 643285. [Google Scholar]
- Lubis, S.R.; Subandi, S.; Muntholib, M.; Abbas, J.; Mozef, T. Antioxidant activity, xanthine oxidase inhibitory activity, and compounds determination of Cipadessa baccifera leaf extract. In Proceedings of the Name of International Conference on Life Sciences and Technology (ICoLiST 2020), Malang, Indonesia, 29 September 2020. [Google Scholar]
- Singh, K.D.; Jena, S.; Patra, B.; Devi, T.B.; Chawla, S.; Bharali, R.; Rajashekar, Y. Safety evaluation of enriched fraction from leaves of Dillenia indica L. in BALB/c mice. Toxicol. Rep. 2022, 9, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Raji, R.O.; Muhammad, H.L.; Abubakar, A.; Maikai, S.S.; Raji, H.F. Acute and sub-acute toxicity profile of crude extract and fractions of Gymnema sylvestre. Clin. Phytosci. 2021, 7, 56. [Google Scholar] [CrossRef]
- Geleta, B.; Makonnen, E.; Debella, A. Toxicological evaluations of the crude extracts and fractions of Moringa stenopetala leaves in liver and kidney of rats. J. Cytol. Histol. 2015, 7, 383. [Google Scholar]
- Wang, M.; Guilbert, L.J.; Ling, L.; Li, J.; Wu, Y.; Xu, S.; Shan, J.J. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium). J. Pharm. Pharmacol. 2001, 53, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Kuo, Y.H.; Chang, H.N.; Kang, P.L.; Tsay, H.S.; Lin, K.F.; Shyur, L.F. Profiling and characterization antioxidant activities in Anoectochilus formosanus Hayata. J. Agric. Food Chem. 2002, 50, 1859–1865. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Lezoul, N.E.H.; Belkadi, M.; Habibi, F.; Guillén, F. Extraction processes with several solvents on total bioactive compounds in different organs of three medicinal plants. Molecules 2020, 25, 4672. [Google Scholar] [CrossRef]
- Okselni, T.; Santoni, A.; Dharma, A.; Efdi, M. Determination of antioxidant activity, total phenolic content, and total flavonoid content of roots, stem bark, and leaves of Elaeocarpus mastersii King. Rasayan J. Chem. 2018, 11, 1211–1216. [Google Scholar] [CrossRef]
- Sarikurkcu, C. Antioxidant activities of solvent extracts from endemic Cyclamen mirabile Hildebr. tubers and leaves. Afr. J. Biotechnol. 2011, 10, 831–839. [Google Scholar]
- Arawande, J.O.; Orimoloye, O.R.; Adeleke, A.R.; Olaide, F.; Afolabi, A.T.A.; Imoukhuede, B.; Ayodele, C.O. Study on solvent extraction values and antioxidant properties of bioactive extracts obtained from leaves, tuber peels and tubers of sweet potato. Biomed. J. Sci. Tech. Res. 2023, 48, 39856–39861. [Google Scholar] [CrossRef]
- Nana, F.W.; Hilou, A.; Millogo, J.F.; Nacoulma, O.G. Phytochemical composition, antioxidant and xanthine oxidase inhibitory activities of Amaranthus cruentus L. and Amaranthus hybridus L. extracts. Pharmaceuticals 2012, 5, 613–628. [Google Scholar] [CrossRef]
- Neupane, S.; Bajracharya, S.; Thada, S.; Bakabal, A.; Khadka, R.B.; Devkota, H.P.; Pandey, J. Total phenolic and flavonoid contents, and preliminary antioxidant, xanthine oxidase inhibitory and antibacterial activities of fruits of lapsi (Choerospondias axillaris Roxb.), an underutilized wild fruit of Nepal. Appl. Sci. 2023, 13, 8945. [Google Scholar] [CrossRef]
- Indarti, K.; Apriani, E.F.; Wibowo, A.E.; Simanjuntak, P. Antioxidant activity of ethanolic extract and various fractions from green tea (Camellia sinensis L.) leaves. Pharmacogn. J. 2019, 11, 771–776. [Google Scholar] [CrossRef]
- Indrayudha, P.; Cahyani, I.G. Cytotoxic activity of ethanol extract legundi leaf (Vitex trifolia L.) and n-hexane, ethyl acetate and ethanol-water fraction against MCF-7 breast cancer cells. Sys. Rev. Pharm. 2020, 11, 779–785. [Google Scholar]
- Abu-Niaaj, L.; Katampe, I. Isolation and Characterization of Flavones from Artemisia monosperma. Pharmacogn. J. 2018, 10, 1018–1023. [Google Scholar] [CrossRef]
- Juanda, D.; Fidrianny, I.; Wirasutisna, K.R.; Insanu, M. Evaluation of xanthine oxidase inhibitory and antioxidant activities of three organs of idat (Cratoxylum glaucum Korth.) and correlation with phytochemical content. Pharmacogn. J. 2021, 13, 971–976. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by ‘ferric reducing antioxidant power’ assay and cyclic voltammetry. Biochim. Biophys. Acta-Gen. Subj. 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary flavonoids as xanthine oxidase inhibitors: Structure–affinity and structure–activity relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Paya, M.; Rios, J.L.; Alcaraz, M.J. Structure-activity relationships of polymethoxyflavones and other flavonoids as inhibitors of non-enzymic lipid peroxidation. Biochem. Pharmacol. 1990, 40, 793–797. [Google Scholar] [CrossRef]
- Ratty, A.K.; Das, N.P. Effects of flavonoids on nonenzymatic lipid peroxidation: Structure-activity relationship. Biochem. Med. Metab. Biol. 1988, 39, 69–79. [Google Scholar] [CrossRef]
- Cholbi, M.R.; Paya, M.; Alcaraz, M.J. Inhibitory effects of phenolic compounds on CCl4-induced microsomal lipid peroxidation. Experientia 1991, 47, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, L.; Malterud, K.E.; Sund, R.B. Hydrogen bond formation as basis for radical scavenging activity: A structure–activity study of C-methylated dihydrochalcones from Myrica gale and structurally related acetophenones. Free Radic. Biol. Med. 1997, 22, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Dugas, A.J., Jr.; Castañeda-Acosta, J.; Bonin, G.C.; Price, K.L.; Fischer, N.H.; Winston, G.W. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: Structure− activity relationships. J. Nat. Prod. 2000, 63, 327–331. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, Y.W.; Hou, C.Y. Antioxidant and antibacterial activity of seven predominant terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- Shi, B.B.; Chen, J.; Bao, M.F.; Zeng, Y.; Cai, X.H. Alkaloids isolated from Tabernaemontana bufalina display xanthine oxidase inhibitory activity. Phytochemistry 2019, 66, 112060. [Google Scholar] [CrossRef]
- Alfaro Jiménez, M.A.; Zugasti Cruz, A.; Silva Belmares, S.Y.; Ascacio Valdés, J.A.; Sierra Rivera, C.A. Phytochemical and biological characterization of the fractions of the aqueous and ethanolic extracts of Parthenium hysterophorus. Separations 2022, 9, 359. [Google Scholar] [CrossRef]
- Awouafack, M.D.; McGaw, L.J.; Gottfried, S.; Mbouangouere, R.; Tane, P.; Spiteller, M.; Eloff, J.N. Antimicrobial activity and cytotoxicity of the ethanol extract, fractions and eight compounds isolated from Eriosema robustum (Fabaceae). BMC Complement. Altern. Med. 2013, 13, 289. [Google Scholar] [CrossRef]
- Mehmood, N.; Zubaır, M.; Rızwan, K.; Rasool, N.; Shahid, M.; Ahmad, V.U. Antioxidant, antimicrobial and phytochemical analysis of cichoriumintybus seeds extract and various organic fractions. Iran. J. Pharm. Res. 2012, 11, 1145–1151. [Google Scholar]
- Noor Hashim, N.H.; Abas, F.; Shaari, K.; Lajis Noordin, H. Antioxidant and xanthine oxidase inhibitory activities of Persicaria hydropiper. Int. J. Food Prop. 2013, 16, 1028–1036. [Google Scholar] [CrossRef]
- Zhang, W.W.; Duan, X.J.; Huang, H.L.; Zhang, Y.; Wang, B.G. Evaluation of 28 marine algae from the Qingdao coast for antioxidative capacity and determination of antioxidant efficiency and total phenolic content of fractions and subfractions derived from Symphyocladia latiuscula (Rhodomelaceae). J. Appl. Phycol. 2007, 19, 97–108. [Google Scholar] [CrossRef]
- Saeed, R.; Ahmed, D. Bioactive compounds from Carissa opaca roots and xanthine oxidase and alpha-amylase inhibitory activities of their methanolic extract and its fractions in different solvents. Pharmacogn. Res. 2015, 7, 295. [Google Scholar]
- Bordet, T.; Berna, P.; Abitbol, J.L.; Pruss, R.M. Olesoxime (TRO19622): A novel mitochondrial-targeted neuroprotective compound. Pharmaceuticals 2010, 3, 345–368. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.C.; Chen, Y.Y. Xanthine oxidase inhibitors from the roots of eggplant (Solanum melongena L.). J. Enzyme Inhib. 1993, 7, 225–235. [Google Scholar] [CrossRef]
- Koduru, S.; Jimoh, F.O.; Grierson, D.S.; Afolayan, A.J. Antioxidant activity of two steroid alkaloids extracted from Solanum aculeastrum. J. Pharmacol. Toxicol. 2007, 2, 160–167. [Google Scholar] [CrossRef]
- CHEBI:18946-δ-lactone. Available online: https://www.ebi.ac.uk/chebi/searchId.do?chebiId=18946 (accessed on 13 January 2025).
- Kim, J.W.; Yoo, I.D.; Kim, W.G. Free radical-scavenging δ-lactones from Boletus calopus. Planta Med. 2006, 72, 1431–1432. [Google Scholar] [CrossRef]
- Wichaidit, W.; Thongyoo, P. A novel γ-lactone isolated from the leaves of Pithecellobium dulce (Roxb.) Benth. and its xanthine oxidase activity. Nat. Prod. Res. 2023, 37, 1168–1176. [Google Scholar] [CrossRef]
- Fatima, A.; Khanam, S.; Jyoti, S.; Naz, F.; Rahul Beg, T.; Siddique, Y.H. Effect of tangeritin against cyclophosphamide-induced toxicity in the larvae of transgenic Drosophila melanogaster (hsp70-lac Z). J. Diet. Suppl. 2018, 15, 893–909. [Google Scholar] [CrossRef]
- Ghallab, D.S.; Mohyeldin, M.M.; Shawky, E.; Metwally, A.M.; Ibrahim, R.S. Chemical profiling of Egyptian propolis and determination of its xanthine oxidase inhibitory properties using UPLC–MS/MS and chemometrics. LWT-Food Sci. Technol. 2021, 136, 110298. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Girreser, U.; Kitanov, G. Benzophenones and flavonoids from Hypericum maculatum and their antioxidant activities. Nat. Prod. Res. 2012, 26, 1576–1583. [Google Scholar] [CrossRef]
- Castro, G.T.; Blanco, S.E.; Ferretti, F.H. Inhibition of xanthine–oxidase by 2, 4–dihydroxy–benzophenone and 2, 3, 4–trihydroxy–benzophenone. Internet Electron. J. Mol. Des. 2004, 3, 684–703. [Google Scholar]
- Sheu, S.Y.; Tsai, H.J.; Chiang, H.C. Benzophenones as xanthine oxidase inhibitors. Anticancer Res. 1999, 19, 1131–1135. [Google Scholar] [PubMed]
- Oriola, A.O. Turmeric–black cumin essential oils and their capacity to attenuate free radicals, protein denaturation, and cancer proliferation. Molecules 2024, 29, 3523. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Tsai, C.S.; Hwang, T.L.; Shieh, P.C.; Chen, J.F.; Sung, P.J. Sesquiterpenes from the rhizome of Curcuma longa with inhibitory activity on superoxide generation and elastase release by neutrophils. Food Chem. 2010, 119, 974–980. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Liu, T.W.; Hsu, S.J.; Huynh, Q.D.T.; Duong, T.L.T.; Chu, M.H.; Lee, C.K. Xanthine oxidase inhibition study of isolated secondary metabolites from Dolichandrone spathacea (Bignoniaceae): In vitro and in silico approach. Saudi Pharm. J. 2024, 32, 101980. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Rasheed, A.; Yasin, K.A.; Ahmed, M.N.; Ghous, T.; Andleeb, S. Evaluation of anti-acetylcholinesterase activity and antioxidant potential of ricinine (a central nervous system stimulant) isolated from Ricinius communis. J. Chem. Soc. Pak. 2016, 38, 326–332. [Google Scholar]
- Fachriyah, E.; Ghifari, M.A.; Anam, K. Isolation, identification, and xanthine oxidase inhibition activity of alkaloid compound from Peperomia pellucida. In Proceedings of the Name of the 12th Joint Conference on Chemistry, Semarang, Indonesia, 19–20 September 2018. [Google Scholar]
- Purnamasari, D.; Safithri, M.; Andrianto, D. In vitro evaluation of purple sweet potato leaf extract (Ipomoea batatas) as a tyrosinase inhibitor and malondialdehyde formation inhibitor. Int. J. Appl. Res. 2024, 5, 64–74. [Google Scholar] [CrossRef]
- Phongtongpasuk, S.; Poadang, S. Extraction of antioxidants from Peperomia pellucida L. Kunth. Sci. Technol. Asia 2014, 19, 38–43. [Google Scholar]
- Maulana, T.I.; Falah, S.; Andrianto, D. Total phenolic content, total flavonoid content, and antioxidant activity of water and ethanol extract from Surian (Toona sinensis) leaves. In Proceedings of the Name of the 5th International Seminar on Sciences, Bogor, Indonesia, 25 October 2018. [Google Scholar]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid content of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Celep, E.; Charehsaz, M.; Akyüz, S.; Acar, E.T.; Yesilada, E. Effect of in vitro gastrointestinal digestion on the bioavailability of phenolic components and the antioxidant potentials of some Turkish fruit wines. Food Res. Int. 2015, 78, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Bektasoglu, B.; Güclü, K.; Güngör, N.; Apak, R. Simultaneous total antioxidant capacity assay of lipophilic and hydrophilic antioxidants in the same acetone–water solution containing 2% methyl-β-cyclodextrin using the cupric reducing antioxidant capacity (CUPRAC) method. Anal. Chim. Acta 2008, 630, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Noro, T.; Oda, Y.; Miyase, T.; Ueno, A.; Fukushima, S. Inhibitors of xanthine oxidase from the flowers and buds of Daphne genkwa. Chem. Pharm. Bull. 1983, 31, 3984–3987. [Google Scholar] [CrossRef] [PubMed]
- Owen, P.L.; Johns, T. Xanthine oxidase inhibitory activity of northeastern North American plant remedies used for gout. J. Ethnopharmacol. 1999, 64, 149–160. [Google Scholar] [CrossRef]
- Duong, N.T.; Vinh, P.D.; Thuong, P.T.; Hoai, N.T.; Thanh, L.N.; Bach, T.T.; Nam, N.H.; Anh, N.H. Xanthine oxidase inhibitors from Archidendron clypearia (Jack.) I.C. Nielsen: Results from systematic screening of Vietnamese medicinal plants. Asian Pac. J. Trop. Med. 2017, 10, 549–556. [Google Scholar] [CrossRef]
 = there is a qualitative correlation between phenolic and flavonoid compounds with antioxidant activity.
= there is a qualitative correlation between phenolic and flavonoid compounds with antioxidant activity.
 = there is a qualitative correlation between phenolic and flavonoid compounds with antioxidant activity.
= there is a qualitative correlation between phenolic and flavonoid compounds with antioxidant activity.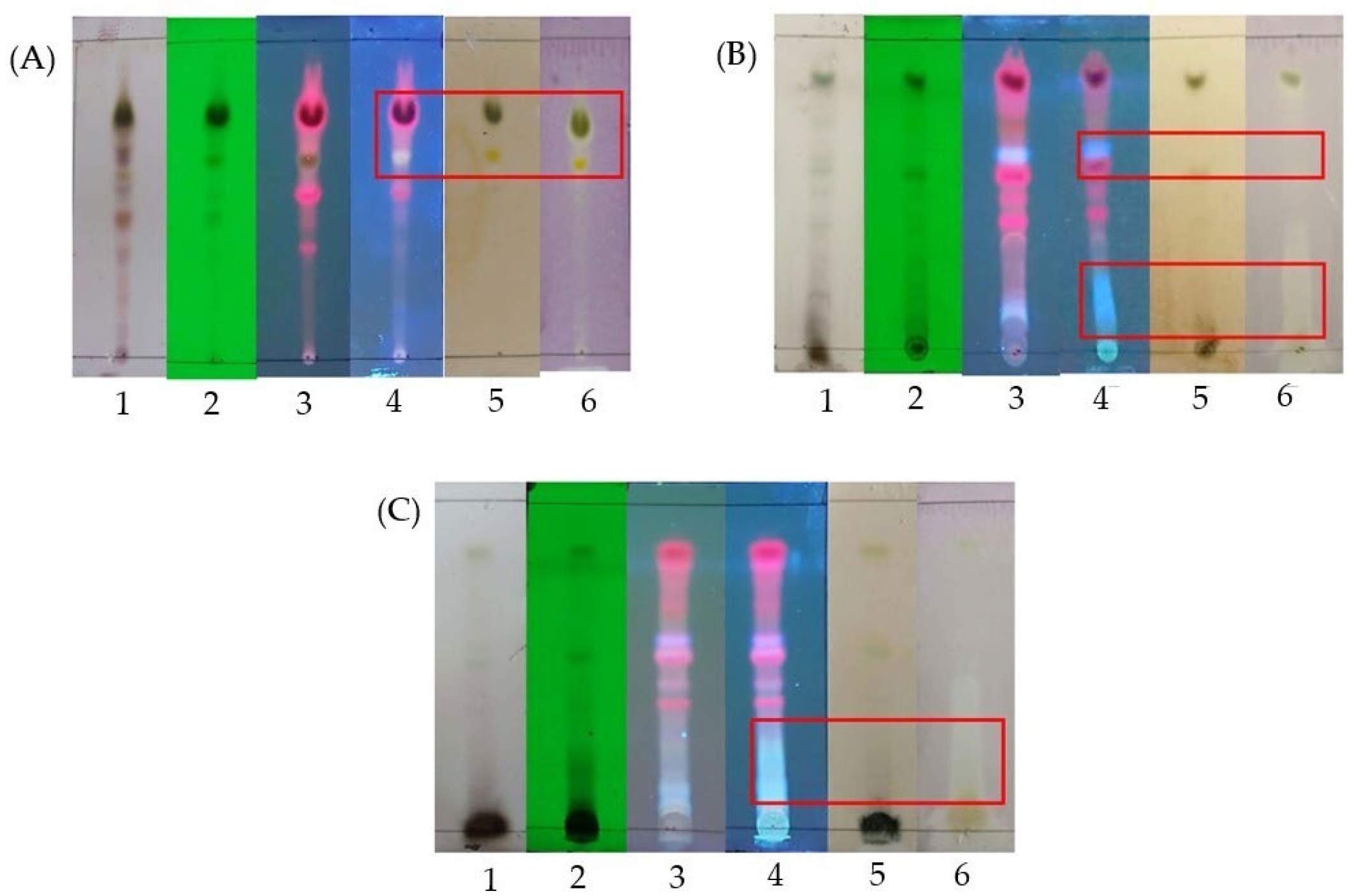

 = there is a qualitative correlation between phenolic and flavonoid compounds with antioxidant activity.
= there is a qualitative correlation between phenolic and flavonoid compounds with antioxidant activity.
 = there is a qualitative correlation between phenolic and flavonoid compounds with antioxidant activity.
= there is a qualitative correlation between phenolic and flavonoid compounds with antioxidant activity.
| Ethanol Extract | Yield (%) | Total Phenols (mg GAE/g) | Total Flavonoids (mg QE/g) | DPPH (mg AEAC/g) | FRAP (mg AEAC/g) | CUPRAC (mg AEAC/g) | XOI Activity (mg AEXIC/g) |
|---|---|---|---|---|---|---|---|
| Leaves | 24.120 | 131.187 ± 0.110 a | 149.959 ± 0.277 a | 333.047 ± 28.172 a | 452.609 ± 0.112 a | 982.728 ± 3.492 a | 69.131 ± 1.400 a |
| Stems | 19.960 | 27.184 ± 1.740 b | 15.469 ± 0.011 b | 21.117 ± 3.440 b | 23.395 ± 0.045 b | 92.120 ± 1.426 b | 8.329 ± 2.741 b |
| Tubers | 14.430 | 12.347 ± 0.027 c | 22.062 ± 0.118 c | 6.979 ± 2.905 c | 9.782 ± 0.079 c | 42.232 ± 0.889 c | 1.121 ± 0.729 c |
| Extract | Yield (%) | Total Phenols (mg GAE/g) | Total Flavonoids (mg QE/g) | DPPH (mg AEAC/g) | FRAP (mg AEAC/g) | CUPRAC (mg AEAC/g) | XOI Activity (mg AEXIC/g) |
|---|---|---|---|---|---|---|---|
| n-Hexane | 4.233 | 90.697 ± 0.373 a | 161.153 ± 0.445 a | 138.181 ± 0.094 a | 52.267 ± 0.607 a | 298.181 ± 1.221 a | 4.417 ± 2.393 a |
| Ethyl acetate | 10.478 | 142.776 ± 0.083 b | 76.591 ± 0.264 b | 511.212 ± 0.416 b | 90.837 ± 0.149 b | 237.944 ± 1.711 b | 45.192 ± 4.981 b |
| Ethanol | 6.787 | 182.803 ± 0.057 c | 65.786 ± 0.336 c | 1067.407 ± 4.173 c | 161.179 ± 0.268 c | 409.556 ± 1.623 c | 15.870 ± 6.523 c |
| Pearson Correlation Coefficient (r) | ||||
|---|---|---|---|---|
| DPPH | FRAP | CUPRAC | XOI | |
| Total phenols | 0.981 a | 0.970 a | 0.580 b | 0.339 c |
| Total flavonoids | −0.861 | −0.833 | −0.270 | −0.618 |
| Fraction | Yield (%) | Total Phenols (mg GAE/g) | Total Flavonoids (mg QE/g) | DPPH (mg AEAC/g) | FRAP (mg AEAC/g) | CUPRAC (mg AEAC/g) | XOI Activity (mg AEXIC/g) |
|---|---|---|---|---|---|---|---|
| CF1 | 11.804 | 27.965 ± 0.184 a | 135.297± 0.222 a | 30.844 ± 0.054 a | 3.658 ± 0.346 a | 222.410± 1.950 a | ND |
| CF2 | 8.773 | 36.242 ± 0.045 b | 107.731 ± 0.865 b | 145.416 ± 0.190 b | 22.910 ± 0.047 b | 384.054 ± 4.625 b | 6.377 ± 1.677 a |
| CF3 | 19.293 | 109.283 ± 0.613 c | 155.343± 0.477 c | 82.001 ± 0.150 c | 81.144 ± 0.220 c | 705.175 ± 3.308 c | ND |
| CF4 | 17.233 | 28.188 ± 0.098 d | 48.117 ± 0.111 d | 66.914 ± 0.417 d | 34.621 ± 0.018 d | ND | ND |
| CF5 | 19.005 | 62.453 ± 0.094 e | 56.926 ± 0.255 e | 158.475 ± 0.170 e | 86.849 ± 0.048 e | 1008.892 ± 1.620 d | 6.062 ± 1.730 a |
| Sample | Total Phenols (mg GAE/g) | Total Flavonoids (mg QE/g) | DPPH (mg AEAC/g) | FRAP (mg AEAC/g) | CUPRAC (mg AEAC/g) | XOI Activity (mg AEXIC/g) |
|---|---|---|---|---|---|---|
| CSF1 | 19.069 ± 0.065 a | 36.592 ± 0.185 a | 154.143 ± 4.593 a | ND | ND | 7.583 ± 1.29 a |
| CSF2 | 28.961 ± 0.056 b | 51.479 ± 0.032 b | 42.502 ± 0.150 b | ND | ND | ND |
| CSF3 | 21.942 ± 0.055 c | 28.388 ± 0.028 c | 51.878 ± 0.242 c | ND | ND | 9.620 ± 1.508 b |
| CSF4 | 6.980 ± 0.017 d | ND | 50.286 ± 0.205 c | ND | ND | 25.367 ± 0.559 c |
| CSF5 | 11.694 ± 0.01 e | 32.603 ± 0.074 d | 41.513 ± 0.239 b | ND | ND | ND |
| Sample | DPPH (mg AEAC/g) | FRAP (mg AEAC/g) | CUPRAC (mg AEAC/g) | XOI Activity (mg AEXIC/g) |
|---|---|---|---|---|
| ELE | 333.047 ± 28.172 a | 452.609 ± 0.112 a | 982.728 ± 3.492 a | 69.131 ± 1.400 a |
| EAE | 511.212 ± 0.416 b | 90.837 ± 0.149 b | 237.944 ± 1.711 b | 45.192 ± 4.981 b |
| CF5 | 158.475 ± 0.170 c | 86.849 ± 0.048 c | 1008.892 ± 1.620 c | 6.062 ± 1.730 c |
| CSF3 | 51.878 ± 0.242 d | ND | ND | 9.620 ± 1.508 d |
| No. | Rt (min.) | Identified Compounds | Molecular Formula | Molecular Weight | Concentration (%) |
|---|---|---|---|---|---|
| 1 | 18.419 | Cholest-4-en-3-one | C27H44O | 384.338 | 0.109 |
| 2 | 10.45 | 4-hydroxy-6-[2-(2-methyl-1,2,4a,5,6,7,8,8a-octahydronaphthalen-1-yl)ethyl]oxan-2-one | C18H28O3 | 292.204 | 0.021 |
| 3 | 11.759 | 4-methylbenzophenone | C14H12O | 196.089 | 0.014 |
| 4 | 11.063 | Benzophenone | C13H10O | 182.073 | 0.061 |
| 5 | 10.817 | Tangeritin | C20H20O7 | 372.121 | 0.011 |
| 6 | 12.969 | (+)-ar-Turmerone | C15H20O | 216.152 | 0.017 |
| 7 | 15.066 | 4-Methoxycinnamic acid | C10H10O3 | 178.063 | 0.494 |
| 8 | 4.63 | Ricinine | C8H8N2O2 | 164.059 | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhendy, H.; Insanu, M.; Fidrianny, I. Extracts, Fractions, and Subfractions from Purple-Orange Sweet Potato (Ipomoea batatas L.): Xanthine Oxidase Inhibitory Potential and Antioxidant Properties. Molecules 2025, 30, 2442. https://doi.org/10.3390/molecules30112442
Suhendy H, Insanu M, Fidrianny I. Extracts, Fractions, and Subfractions from Purple-Orange Sweet Potato (Ipomoea batatas L.): Xanthine Oxidase Inhibitory Potential and Antioxidant Properties. Molecules. 2025; 30(11):2442. https://doi.org/10.3390/molecules30112442
Chicago/Turabian StyleSuhendy, Hendy, Muhamad Insanu, and Irda Fidrianny. 2025. "Extracts, Fractions, and Subfractions from Purple-Orange Sweet Potato (Ipomoea batatas L.): Xanthine Oxidase Inhibitory Potential and Antioxidant Properties" Molecules 30, no. 11: 2442. https://doi.org/10.3390/molecules30112442
APA StyleSuhendy, H., Insanu, M., & Fidrianny, I. (2025). Extracts, Fractions, and Subfractions from Purple-Orange Sweet Potato (Ipomoea batatas L.): Xanthine Oxidase Inhibitory Potential and Antioxidant Properties. Molecules, 30(11), 2442. https://doi.org/10.3390/molecules30112442







