Andrographolide and Fucoidan Induce a Synergistic Antiviral Response In Vitro Against Infectious Pancreatic Necrosis Virus
Abstract
1. Introduction
2. Results
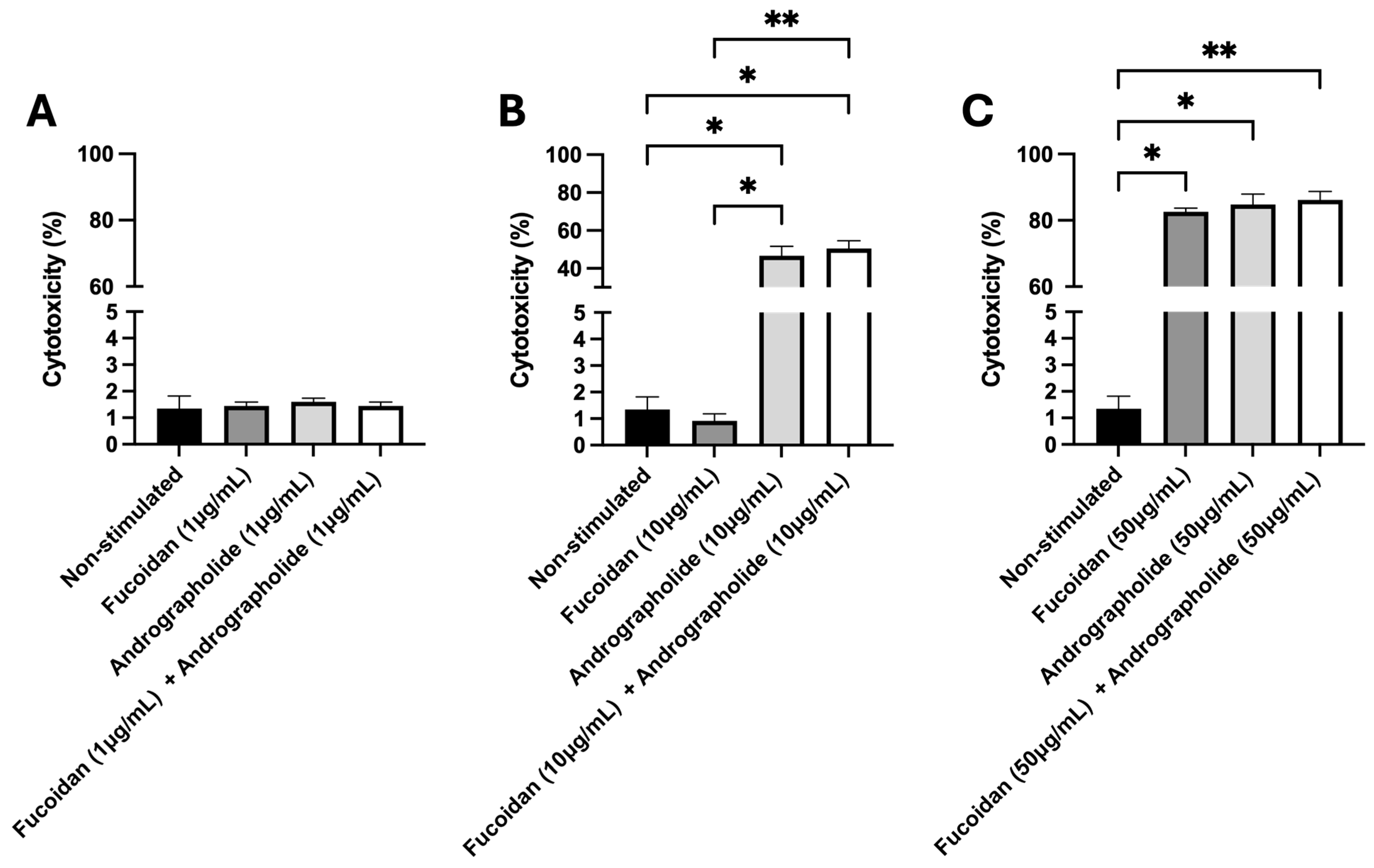
2.1. Quantification of Cytotoxicity Induced by Andrographolide, Fucoidan, or Their Mixture in SHK-1 Cells
2.2. Induction of the Transcript Expression of IFNα1 and Interferon-Stimulated Genes (ISGs) by Andrographolide, Fucoidan, and Their Mixture
2.3. Synergistic Activity of Andrographolide/Fucoidan Mixture Against IPNV
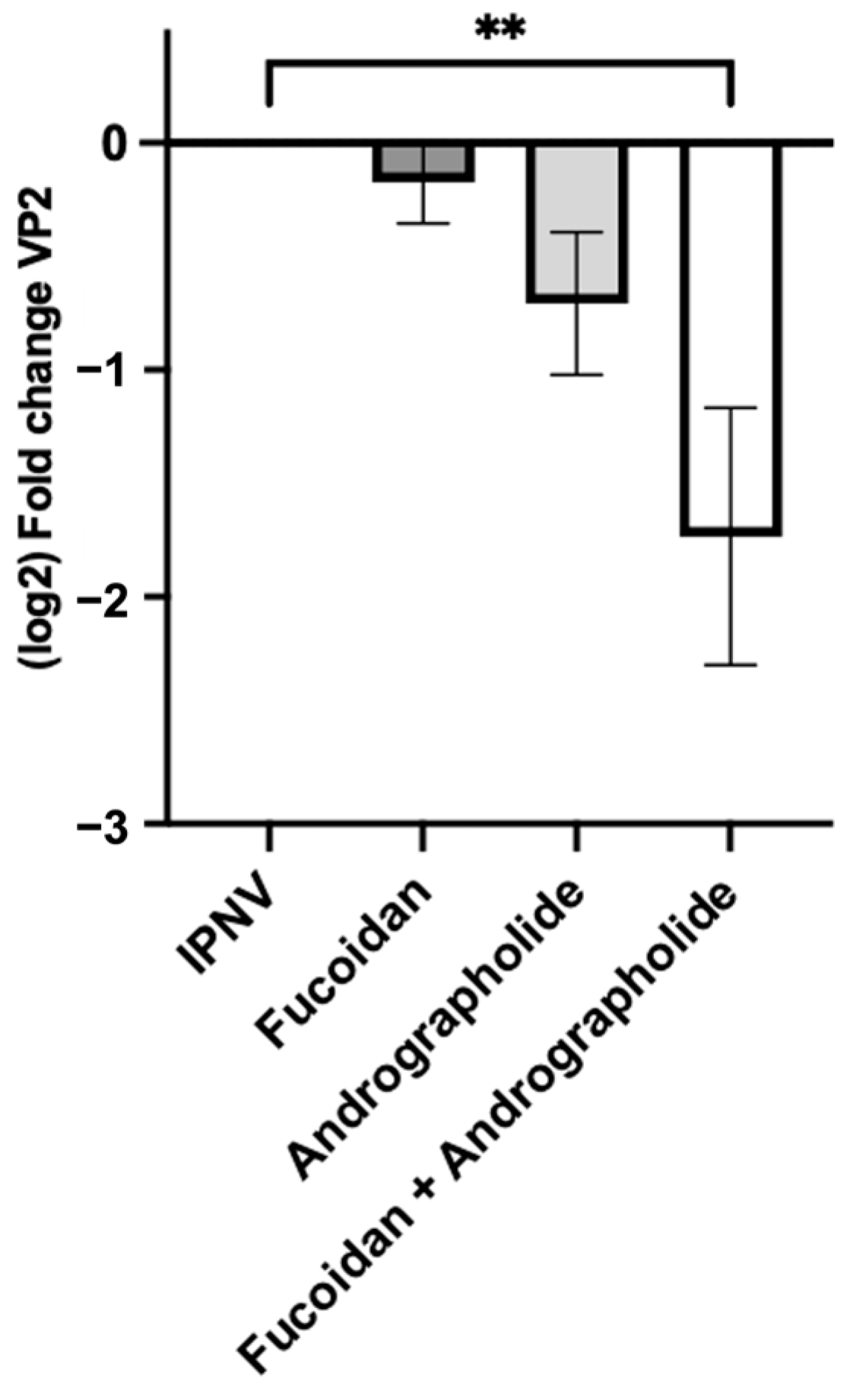
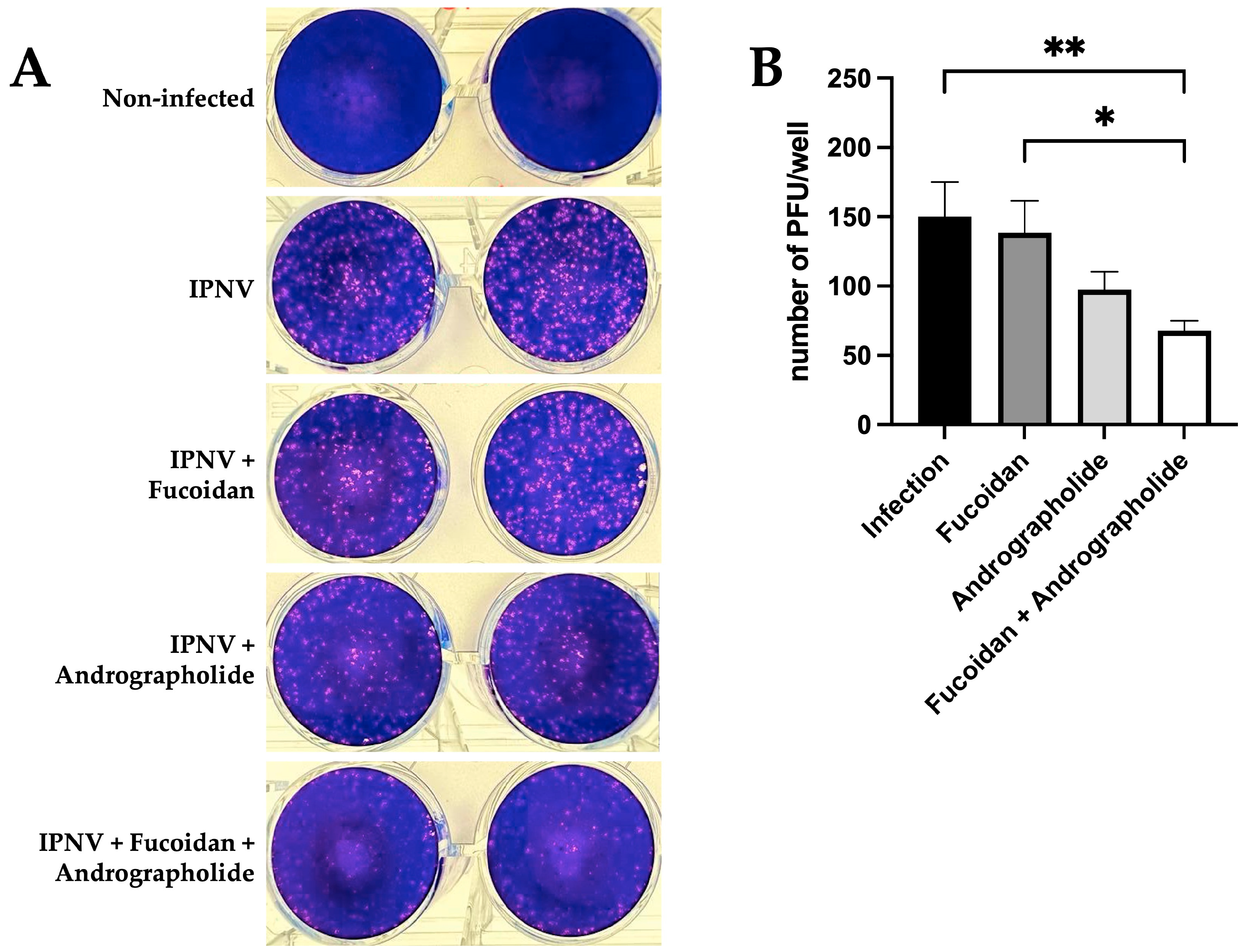
2.4. Andrographolide/Fucoidan Mixture Exhibits Potent and Synergistic Antiviral Activity Against IPNV
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Propagation and Titration of the IPN Virus
4.3. Evaluation of the Cytotoxicity Induced by Andrographolide, Fucoidan, and a Mixture of Andrographolide and Fucoidan
4.4. Evaluation of Antiviral Transcripts Expression Induced by Andrographolide, Fucoidan, or Their Mixture in SHK-1 Cells
4.5. Evaluation of Antiviral Transcripts Expression in SHK-1 Cells Infected by IPNV Pre-Treated with Andrographolide, Fucoidan, or Their Mixture
4.6. Quantification of IPNV Viral Protein 2 (VP2) Gene by Quantitative PCR (qPCR) in SHK-1 Cells Pre-Treated with Andrographolide, Fucoidan, or Their Mixture
4.7. Determination of the Antiviral Activity of Fucoidan and Andrographolide Pre-Treatment via Plaque Reduction Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture—Blue Transformation in Action; Food and Agriculture Organization: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, distribution, impacts and spatial assessments—A review. Ocean. Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Vallejos-Vidal, E.; Reyes-Lopez, F.; Teles, M.; MacKenzie, S. The response of fish to immunostimulant diets. Fish Shellfish Immunol. 2016, 56, 34–69. [Google Scholar] [CrossRef] [PubMed]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Cerpa, S.; Vallejos-Vidal, E.; Gonzalez-Bown, M.J.; Morales-Reyes, J.; Perez-Stuardo, D.; Vargas, D.; Imarai, M.; Cifuentes, V.; Spencer, E.; Sandino, A.M.; et al. Effect of yeast (Xanthophyllomyces dendrorhous) and plant (Saint John’s wort, lemon balm, and rosemary) extract based functional diets on antioxidant and immune status of Atlantic salmon (Salmo salar) subjected to crowding stress. Fish Shellfish Immunol. 2018, 74, 250–259. [Google Scholar] [CrossRef]
- Rocha, S.D.C.; Morales-Lange, B.; Montero, R.; Okbayohanese, D.T.; Kathiresan, P.; Press, M.C.; Mydland, L.T.; Øverland, M. Norway spruce extracts (NSEs) as bioactive compounds in novel feeds: Effect on intestinal immune-related biomarkers, morphometry and microbiota in Atlantic salmon pre-smolts. J. Funct. Foods 2023, 111, 105888. [Google Scholar] [CrossRef]
- Verma, M.; Hontecillas, R.; Abedi, V.; Leber, A.; Tubau-Juni, N.; Philipson, C.; Carbo, A.; Bassaganya-Riera, J. Modeling-Enabled Systems Nutritional Immunology. Front. Nutr. 2016, 3, 5. [Google Scholar] [CrossRef][Green Version]
- Firmino, J.P.; Vallejos-Vidal, E.; Balebona, M.C.; Ramayo-Caldas, Y.; Cerezo, I.M.; Salomon, R.; Tort, L.; Estevez, A.; Morinigo, M.A.; Reyes-Lopez, F.E.; et al. Diet, Immunity, and Microbiota Interactions: An Integrative Analysis of the Intestine Transcriptional Response and Microbiota Modulation in Gilthead Seabream (Sparus aurata) Fed an Essential Oils-Based Functional Diet. Front. Immunol. 2021, 12, 625297. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.A. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2017, 10, 950–974. [Google Scholar] [CrossRef]
- Kuhlwein, H.; Emery, M.J.; Rawling, M.D.; Harper, G.M.; Merrifield, D.L.; Davies, S.J. Effects of a dietary beta-(1,3)(1,6)-D-glucan supplementation on intestinal microbial communities and intestinal ultrastructure of mirror carp (Cyprinus carpio L.). J. Appl. Microbiol. 2013, 115, 1091–1106. [Google Scholar] [CrossRef]
- Kuhlwein, H.; Merrifield, D.L.; Rawling, M.D.; Foey, A.D.; Davies, S.J. Effects of dietary beta-(1,3)(1,6)-D-glucan supplementation on growth performance, intestinal morphology and haemato-immunological profile of mirror carp (Cyprinus carpio L.). J. Anim. Physiol. Anim. Nutr. 2014, 98, 279–289. [Google Scholar] [CrossRef]
- Figueiredo, F.; Kristoffersen, H.; Bhat, S.; Zhang, Z.; Godfroid, J.; Peruzzi, S.; Praebel, K.; Dalmo, R.A.; Xu, X. Immunostimulant Bathing Influences the Expression of Immune- and Metabolic-Related Genes in Atlantic Salmon Alevins. Biology 2021, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Eslamloo, K.; Caballero-Solares, A.; Katan, T.; Umasuthan, N.; Taylor, R.G.; Fast, M.D.; Andreassen, R.; Rise, M.L. Characterization of the impact of dietary immunostimulant CpG on the expression of mRNA biomarkers involved in the immune responses in Atlantic salmon (Salmo salar). Fish Shellfish Immunol. 2024, 153, 109840. [Google Scholar] [CrossRef] [PubMed]
- Thépot, V.; Campbell, A.H.; Rimmer, M.A.; Paul, N.A. Meta-analysis of the use of seaweeds and their extracts as immunostimulants for fish: A systematic review. Rev. Aquac. 2020, 13, 907–933. [Google Scholar] [CrossRef]
- Bahi, A.; Ramos-Vega, A.; Angulo, C.; Monreal-Escalante, E.; Guardiola, F. Microalgae with immunomodulatory effects on fish. Rev. Aquac. 2023, 15, 1522–1539. [Google Scholar] [CrossRef]
- Vijayaram, S.; Sun, Y.Z.; Zuorro, A.; Ghafarifarsani, H.; Van Doan, H.; Hoseinifar, S.H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol. 2022, 130, 294–308. [Google Scholar] [CrossRef]
- Rivals, F.; Gardeisen, A.; Cantuel, J. Domestic and wild ungulate dietary traits at Kouphovouno (Sparta, Greece): Implications for livestock management and paleoenvironment in the Neolithic. J. Archaeol. Sci. 2011, 38, 528–537. [Google Scholar] [CrossRef]
- Li, F.; Sun, H.; Li, Y.; He, D.; Ren, C.; Zhu, C.; Lv, G. Effects of fucoidan on growth performance, immunity, antioxidant ability, digestive enzyme activity, and hepatic morphology in juvenile common carp (Cyprinus carpio). Front. Mar. Sci. 2023, 10, 1167400. [Google Scholar] [CrossRef]
- Prabu, D.L.; Sahu, N.P.; Pal, A.K.; Dasgupta, S.; Narendra, A. Immunomodulation and interferon gamma gene expression in sutchi cat fish, Pangasianodon hypophthalmus: Effect of dietary fucoidan rich seaweed extract (FRSE) on pre and post challenge period. Aquac. Res. 2016, 47, 119–218. [Google Scholar] [CrossRef]
- Saeed, M.; Arain, M.A.; Fazlani, A.; Marghazani, I.B.; Umar, M.; Soomro, J.; Alagawany, M. A comprehensive review on the health benefits and nutritional significance of fucoidan polysaccharide derived from brown seaweeds in human, animals and aquatic organisms. Aquac. Nutr. 2021, 27, 633–654. [Google Scholar] [CrossRef]
- Hsu, C.H.; Chen, J.C.; Lin, Y.C.; Chen, Y.Y.; Liu, P.C.; Lin, B.W.; Hsieh, J.F. White shrimp Litopenaeus vannamei that have received mixtures of heat-killed and formalin-inactivated Vibrio alginolyticus and V. harveyi exhibit recall memory and show increased phagocytosis and resistance to Vibrio infection. Fish Shellfish Immunol. 2021, 112, 151–158. [Google Scholar] [CrossRef]
- Van Hai, N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Thomas, T.B.; Thirumalaikumar, E.; Sathishkumar, R.; Rajeswari, M.V.; Vimal, S.; Uma, G.; Jones, R.D.S.; Citarasu, T. Effects of dietary Andrographis paniculata extract on growth, haematological, immune responses, immune-related genes expression of ornamental goldfish (Carassius auratus) and its susceptibility to Aeromonas hydrophila infection. Aquac. Rep. 2023, 33, 101850. [Google Scholar] [CrossRef]
- Palanikani, R.; Chanthini, K.M.; Soranam, R.; Thanigaivel, A.; Karthi, S.; Senthil-Nathan, S.; Murugesan, A.G. Efficacy of Andrographis paniculata supplements induce a non-specific immune system against the pathogenicity of Aeromonas hydrophila infection in Indian major carp (Labeo rohita). Environ. Sci. Pollut. Res. Int. 2020, 27, 23420–23436. [Google Scholar] [CrossRef]
- Hernández, A.J.; Romero, A.; Gonzalez-Stegmaier, R.; Dantagnan, P. The effects of supplemented diets with a phytopharmaceutical preparation from herbal and macroalgal origin on disease resistance in rainbow trout against Piscirickettsia salmonis. Aquaculture 2016, 454, 109–117. [Google Scholar] [CrossRef]
- Miranda Campos, P.; Rabuco Jeraldino, C. Veterinary composition of marine algae and Andrographis sp extracts, which can be used to treat infections in fish. In World Intellectual Property Organization (WIPO); European Patent Office: Munich, Germany, 2016. [Google Scholar]
- Rodriguez Saint-Jean, S.; Borrego, J.J.; Perez-Prieto, S.I. Infectious pancreatic necrosis virus: Biology, pathogenesis, and diagnostic methods. Adv. Virus Res. 2003, 62, 113–165. [Google Scholar] [CrossRef]
- Kibenge, F.S.; Godoy, M.G.; Fast, M.; Workenhe, S.; Kibenge, M.J. Countermeasures against viral diseases of farmed fish. Antivir. Res. 2012, 95, 257–281. [Google Scholar] [CrossRef]
- Dopazo, C.P. The Infectious Pancreatic Necrosis Virus (IPNV) and its Virulence Determinants: What is Known and What Should be Known. Pathogens 2020, 9, 94. [Google Scholar] [CrossRef]
- Espinoza, D.; Laporte, D.; Martinez, F.; Sandino, A.M.; Valdes, N.; Moenne, A.; Imarai, M. Lambda carrageenan displays antiviral activity against the infectious pancreatic necrosis virus (IPNV) by inhibiting viral replication and enhancing innate immunity in salmonid cells. Int. J. Biol. Macromol. 2024, 282 Pt 2, 136875. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Reyes-Lopez, F.; Toro-Ascuy, D.; Montero, R.; Maisey, K.; Acuna-Castillo, C.; Sunyer, J.O.; Parra, D.; Sandino, A.M.; Imarai, M. Induction of anti-inflammatory cytokine expression by IPNV in persistent infection. Fish Shellfish Immunol. 2014, 41, 172–182. [Google Scholar] [CrossRef]
- Julin, K.; Johansen, L.H.; Sommer, A.I.; Jorgensen, J.B. Persistent infections with infectious pancreatic necrosis virus (IPNV) of different virulence in Atlantic salmon, Salmo salar L. J. Fish. Dis. 2015, 38, 1005–1019. [Google Scholar] [CrossRef]
- Julin, K.; Mennen, S.; Sommer, A.I. Study of virulence in field isolates of infectious pancreatic necrosis virus obtained from the northern part of Norway. J. Fish. Dis. 2013, 36, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.; Kibenge, M.J.T.; Montes de Oca, M.; Pontigo, J.P.; Coca, Y.; Caro, D.; Kusch, K.; Suarez, R.; Burbulis, I.; Kibenge, F.S.B. Isolation of a New Infectious Pancreatic Necrosis Virus (IPNV) Variant from Genetically Resistant Farmed Atlantic Salmon (Salmo salar) during 2021–2022. Pathogens 2022, 11, 1368. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma Biomath. 2013, 3, 71–85. [Google Scholar]
- Reyes-Lopez, F.E.; Romeo, J.S.; Vallejos-Vidal, E.; Reyes-Cerpa, S.; Sandino, A.M.; Tort, L.; Mackenzie, S.; Imarai, M. Differential immune gene expression profiles in susceptible and resistant full-sibling families of Atlantic salmon (Salmo salar) challenged with infectious pancreatic necrosis virus (IPNV). Dev. Comp. Immunol. 2015, 53, 210–221. [Google Scholar] [CrossRef]
- Duan, K.; Tang, X.; Zhao, J.; Ren, G.; Shao, Y.; Lu, T.; He, B.; Xu, L. An inactivated vaccine against infectious pancreatic necrosis virus in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2022, 127, 48–55. [Google Scholar] [CrossRef]
- Li, L.; Liu, W.; Zhang, Z.; Zhao, J.; Lu, T.; Shao, Y.; Xu, L. IPNV inactive vaccine supplemented with GEL 02 PR adjuvant: Protective efficacy, cross-protection, and stability. Fish Shellfish Immunol. 2025, 158, 110167. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, I.; Bhat, R.H.A.; Tandel, R.S.; Dash, P.; Chandra, S.; Dubey, M.K.; Ganie, P.A. Comprehensive review on infectious pancreatic necrosis virus. Aquaculture 2023, 574, 739737. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Zhang, X.; Hao, C.; Zhao, X.; Jiao, G.; Shan, X.; Tai, W.; Yu, G. Inhibition of Influenza A Virus Infection by Fucoidan Targeting Viral Neuraminidase and Cellular EGFR Pathway. Sci. Rep. 2017, 7, 40760. [Google Scholar] [CrossRef]
- Mandal, P.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007, 18, 153–162. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The anti-cancer effects of fucoidan: A review of both in vivo and in vitro investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef]
- Oliveira, C.; Neves, N.M.; Reis, R.L.; Martins, A.; Silva, T.H. A review on fucoidan antitumor strategies: From a biological active agent to a structural component of fucoidan-based systems. Carbohydr. Polym. 2020, 239, 116131. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Nikolova, M.; Iliev, I.; Peychev, L.; Trica, B.; Oancea, F.; Delattre, C.; et al. Immunomodulatory and Anti-Inflammatory Effects of Fucoidan: A Review. Polymers 2020, 12, 2338. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Herath, K.; Yang, H.W.; Choi, C.S.; Jeon, Y.J. Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review. Mar. Drugs 2021, 19, 678. [Google Scholar] [CrossRef]
- Adiguna, S.P.; Panggabean, J.A.; Atikana, A.; Untari, F.; Izzati, F.; Bayu, A.; Rosyidah, A.; Rahmawati, S.I.; Putra, M.Y. Antiviral Activities of Andrographolide and Its Derivatives: Mechanism of Action and Delivery System. Pharmaceuticals 2021, 14, 1102. [Google Scholar] [CrossRef]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, a Natural Antioxidant: An Update. Antioxidants 2019, 8, 571. [Google Scholar] [CrossRef]
- Banerjee, M.; Parai, D.; Chattopadhyay, S.; Mukherjee, S.K. Andrographolide: Antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiol. 2017, 62, 237–244. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, B.; Bao, M.; Cheng, Y.; Mahmood, T.; Yang, W.; Chen, Q.; Lv, L.; Li, L.; Yi, J.; et al. Andrographolide Sulfonate Is a Promising Treatment to Combat Methicillin-resistant Staphylococcus aureus and Its Biofilms. Front. Pharmacol. 2021, 12, 720685. [Google Scholar] [CrossRef]
- Latif, R.; Wang, C.Y. Andrographolide as a potent and promising antiviral agent. Chin. J. Nat. Med. 2020, 18, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Low, M.; Suresh, H.; Zhou, X.; Bhuyan, D.J.; Alsherbiny, M.A.; Khoo, C.; Munch, G.; Li, C.G. The wide spectrum anti-inflammatory activity of andrographolide in comparison to NSAIDs: A promising therapeutic compound against the cytokine storm. PLoS ONE 2024, 19, e0299965. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Luz-Crawford, P.; Soto-Rifo, R.; Reyes-Cerpa, S.; Toro-Ascuy, D. The Landscape of IFN/ISG Signaling in HIV-1-Infected Macrophages and Its Possible Role in the HIV-1 Latency. Cells 2021, 10, 2378. [Google Scholar] [CrossRef]
- Li, Q.; Sun, B.; Zhuo, Y.; Jiang, Z.; Li, R.; Lin, C.; Jin, Y.; Gao, Y.; Wang, D. Interferon and interferon-stimulated genes in HBV treatment. Front. Immunol. 2022, 13, 1034968. [Google Scholar] [CrossRef]
- Ye, J.; Chen, J. Interferon and Hepatitis B: Current and Future Perspectives. Front. Immunol. 2021, 12, 733364. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Jeon, S.A.; Heo, B.Y.; Kang, J.G.; Jung, Y.; Duong, P.T.T.; Song, I.C.; Kim, J.H.; Kim, S.Y.; Kwon, J. Gene Set Enrichment Analysis Reveals That Fucoidan Induces Type I IFN Pathways in BMDC. Nutrients 2022, 14, 2242. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Tang, Y.; Lin, L.; Xie, Z.; Zhou, J.; Zhang, L.; Zhang, X.; Zhao, X.; Chen, Z.; et al. Fucoidan from Fucus vesiculosus suppresses hepatitis B virus replication by enhancing extracellular signal-regulated Kinase activation. Virol. J. 2017, 14, 178. [Google Scholar] [CrossRef]
- Reynolds, D.; Huesemann, M.; Edmundson, S.; Sims, A.; Hurst, B.; Cady, S.; Beirne, N.; Freeman, J.; Berger, A.; Gao, S. Viral inhibitors derived from macroalgae, microalgae, and cyanobacteria: A review of antiviral potential throughout pathogenesis. Algal Res. 2021, 57, 102331. [Google Scholar] [CrossRef]
- Nosik, M.N.; Krylova, N.V.; Usoltseva, R.V.; Surits, V.V.; Kireev, D.E.; Shchelkanov, M.Y.; Svitich, O.A.; Ermakova, S.P. In Vitro Anti-HIV-1 Activity of Fucoidans from Brown Algae. Mar. Drugs 2024, 22, 355. [Google Scholar] [CrossRef]
- Claus-Desbonnet, H.; Nikly, E.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S.; Pierre, G.; Benbassat, N.; Katsarov, P.; Michaud, P.; Lukova, P.; et al. Polysaccharides and Their Derivatives as Potential Antiviral Molecules. Viruses 2022, 14, 426. [Google Scholar] [CrossRef]
- Sanniyasi, E.; Venkatasubramanian, G.; Anbalagan, M.M.; Raj, P.P.; Gopal, R.K. In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana J.V.Lamouroux and Turbinaria decurrens Bory). Sci. Rep. 2019, 9, 12185. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.; Ly, B.M.; Van, T.T.; Quang, N.V.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Gonzalez, R.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle Disease Virus. Virol. J. 2012, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Lauksund, S.; Greiner-Tollersrud, L.; Chang, C.J.; Robertsen, B. Infectious pancreatic necrosis virus proteins VP2, VP3, VP4 and VP5 antagonize IFNa1 promoter activation while VP1 induces IFNa1. Virus Res. 2015, 196, 113–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santi, N.; Vakharia, V.N.; Evensen, O. Identification of putative motifs involved in the virulence of infectious pancreatic necrosis virus. Virology 2004, 322, 31–40. [Google Scholar] [CrossRef]
- Marjara, I.S.; Thu, B.J.; Evensen, O. Differentially expressed genes following persistent infection with infectious pancreatic necrosis virus in vitro and in vivo. Fish Shellfish Immunol. 2010, 28, 845–853. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Teng, Y.; Zheng, Y.; Zhang, M.; Wang, X.; Cheng, H.; Xu, J.; Chen, X.; Zhao, Z.; et al. Enhancement of seaweed polysaccharides (fucoidan and laminarin) on the phagocytosis of macrophages via activation of intelectin in blunt snout bream (Megalobrama amblycephala). Front. Mar. Sci. 2023, 10, 1124880. [Google Scholar] [CrossRef]
- Caipang, C.M.; Lazado, C.C.; Berg, I.; Brinchmann, M.F.; Kiron, V. Influence of alginic acid and fucoidan on the immune responses of head kidney leukocytes in cod. Fish. Physiol. Biochem. 2011, 37, 603–612. [Google Scholar] [CrossRef]
- Sheeja, K.; Kuttan, G. Modulation of natural killer cell activity, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by andrographolide in normal and Ehrlich ascites carcinoma-bearing mice. Integr. Cancer Ther. 2007, 6, 66–73. [Google Scholar] [CrossRef]
- Sheeja, K.; Kuttan, G. Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by Andrographis paniculata extract and andrographolide. Immunopharmacol. Immunotoxicol. 2007, 29, 81–93. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, K.P.; Gupta, R.; Singh, S.B. Andrographolide—A prospective remedy for chikungunya fever and viral arthritis. Int. Immunopharmacol. 2021, 99, 108045. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Tseng, C.K.; Young, K.C.; Sun, H.Y.; Wang, S.W.; Chen, W.C.; Lin, C.K.; Wu, Y.H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014, 171, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Zhou, N.; Liu, Y.; Xie, J.; Liu, E. Andrographolide exerts anti-respiratory syncytial virus activity by up-regulating heme oxygenase-1 independent of interferon responses in human airway epithelial cells. Mol. Biol. Rep. 2023, 50, 4261–4272. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Dong, S.F.; Liu, C.H.; Italiani, P.; Sun, S.H.; Xu, J.; Boraschi, D.; Ma, S.P.; Qu, D. Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacol. Sin. 2010, 31, 191–201. [Google Scholar] [CrossRef]
- Chaopreecha, J.; Phueakphud, N.; Suksatu, A.; Krobthong, S.; Manopwisedjaroen, S.; Panyain, N.; Hongeng, S.; Thitithanyanont, A.; Wongtrakoongate, P. Andrographolide attenuates SARS-CoV-2 infection via an up-regulation of glutamate-cysteine ligase catalytic subunit (GCLC). Phytomedicine 2025, 136, 156279. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, K.P.; Dash, P.K.; Parida, M.; Ganju, L.; Singh, S.B. Andrographolide inhibits chikungunya virus infection by up-regulating host innate immune pathways. Asian Pac. J. Trop. Med. 2018, 11, 214–221. [Google Scholar] [CrossRef]
- Helbig, K.J.; Beard, M.R. The role of viperin in the innate antiviral response. J. Mol. Biol. 2014, 426, 1210–1219. [Google Scholar] [CrossRef]
- Mattijssen, S.; Pruijn, G.J. Viperin, a key player in the antiviral response. Microbes Infect. 2012, 14, 419–426. [Google Scholar] [CrossRef]
- Rivera-Serrano, E.E.; Gizzi, A.S.; Arnold, J.J.; Grove, T.L.; Almo, S.C.; Cameron, C.E. Viperin Reveals Its True Function. Annu. Rev. Virol. 2020, 7, 421–446. [Google Scholar] [CrossRef]
- Dannevig, B.H.; Brudeseth, B.E.; Gjoen, T.; Rode, M.; Wergeland, H.I.; Evensen, O.; Press, C. Characterisation of a long-term cell line (SHK-1) developed from the head kidney of Atlantic salmon (Salmo salar L.). Fish Shellfish Immunol. 1997, 7, 213–226. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Reyes-Lopez, F.E.; Toro-Ascuy, D.; Ibanez, J.; Maisey, K.; Sandino, A.M.; Imarai, M. IPNV modulation of pro and anti-inflammatory cytokine expression in Atlantic salmon might help the establishment of infection and persistence. Fish Shellfish Immunol. 2012, 32, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Levican-Asenjo, J.; Soto-Rifo, R.; Aguayo, F.; Gaggero, A.; Leon, O. Salmon cells SHK-1 internalize infectious pancreatic necrosis virus by macropinocytosis. J. Fish. Dis. 2019, 42, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Orpetveit, I.; Gjoen, T.; Sindre, H.; Dannevig, B.H. Binding of infectious pancreatic necrosis virus (IPNV) to membrane proteins from different fish cell lines. Arch. Virol. 2008, 153, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Jashes, M.; Gonzalez, M.; Lopez-Lastra, M.; De Clercq, E.; Sandino, A. Inhibitors of infectious pancreatic necrosis virus (IPNV) replication. Antivir. Res. 1996, 29, 309–312. [Google Scholar] [CrossRef]
- Velasquez, F.; Frazao, M.; Diez, A.; Villegas, F.; Álvarez-Bidwell, M.; Rivas-Pardo, J.A.; Vallejos-Vidal, E.; Reyes-lopez, F.E.; Toro-Ascuy, D.; Ahumada, M.; et al. Salmon-IgM Functionalized-PLGA Nanosystem for Florfenicol Delivery as an Antimicrobial Strategy against Piscirickettsia salmonis. Nanomaterials 2024, 14, 1658. [Google Scholar] [CrossRef]


| Gene | Sequence 5′→3′ | GenBank Accession No. | Reference |
|---|---|---|---|
| PKR | CCCTCCTGTCCGAGCAGTTA | EF523422 | This study |
| AGCCTCCTTCTTCGTGTTCC | |||
| Mx | CGATGCCCTCTCGAGCTGAA | NM_001139918 | This study |
| TGAGTGTGAGGTCTGGGACG | |||
| Viperin | CTGTACGCTGGAAGGTGTTC | NM_001140939 | This study |
| GCCAACATCAAGGATGGACTT | |||
| IFNα1 | GGACAAGAAAAACCTGGACG | AY216594 | [31] |
| CTTTCCTGATGAGCTCCCAC | |||
| VP2 | GACCAAGTTCGACTTCCAGC | FN257531 | [31] |
| ATCGGCTTGGTGATGTTCTC | |||
| 18S | CCTTAGATGTCCGGGGCT | AJ427629 | [36] |
| CTCGGCGAAGGGTAGACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazao, M.; Espinoza, D.; Canales-Muñoz, S.; Millán-Hidalgo, C.; Ulloa-Sarmiento, B.; Orellana, I.; Rivas-Pardo, J.A.; Imarai, M.; Vallejos-Vidal, E.; Reyes-López, F.E.; et al. Andrographolide and Fucoidan Induce a Synergistic Antiviral Response In Vitro Against Infectious Pancreatic Necrosis Virus. Molecules 2025, 30, 2443. https://doi.org/10.3390/molecules30112443
Frazao M, Espinoza D, Canales-Muñoz S, Millán-Hidalgo C, Ulloa-Sarmiento B, Orellana I, Rivas-Pardo JA, Imarai M, Vallejos-Vidal E, Reyes-López FE, et al. Andrographolide and Fucoidan Induce a Synergistic Antiviral Response In Vitro Against Infectious Pancreatic Necrosis Virus. Molecules. 2025; 30(11):2443. https://doi.org/10.3390/molecules30112443
Chicago/Turabian StyleFrazao, Mateus, Daniela Espinoza, Sergio Canales-Muñoz, Catalina Millán-Hidalgo, Benjamín Ulloa-Sarmiento, Ivana Orellana, J. Andrés Rivas-Pardo, Mónica Imarai, Eva Vallejos-Vidal, Felipe E. Reyes-López, and et al. 2025. "Andrographolide and Fucoidan Induce a Synergistic Antiviral Response In Vitro Against Infectious Pancreatic Necrosis Virus" Molecules 30, no. 11: 2443. https://doi.org/10.3390/molecules30112443
APA StyleFrazao, M., Espinoza, D., Canales-Muñoz, S., Millán-Hidalgo, C., Ulloa-Sarmiento, B., Orellana, I., Rivas-Pardo, J. A., Imarai, M., Vallejos-Vidal, E., Reyes-López, F. E., Toro-Ascuy, D., & Reyes-Cerpa, S. (2025). Andrographolide and Fucoidan Induce a Synergistic Antiviral Response In Vitro Against Infectious Pancreatic Necrosis Virus. Molecules, 30(11), 2443. https://doi.org/10.3390/molecules30112443







