Abstract
Tanacetum parthenium L. (Asteraceae) is a perennial herbaceous plant with a long-standing historical use in traditional medicine. Recently Tanacetum parthenium L. essential oil has been associated with a promising potential for future applications in the pharmaceutical industry, in the cosmetics industry, and in agriculture. Investigations on the essential oil (EO) have indicated antimicrobial, antioxidant, and repellent activity. The present study aimed to evaluate the chemical composition of Bulgarian T. parthenium essential oil from two different regions, to compare the results to those reported previously in the literature, and to point out some of its future applications. The essential oils of the air-dried flowering aerial parts were obtained by hydrodistillation using a Clevenger-type apparatus. The chemical composition was evaluated using gas chromatography with mass spectrometry (GC-MS). It was established that the oxygenated monoterpenes were the predominant terpene class, followed by the monoterpene hydrocarbons. Significant qualitative and quantitative differences between both samples were revealed. Camphor (50.90%), camphene (16.12%), and bornyl acetate (6.05%) were the major constituents in the feverfew EO from the western Rhodope Mountains, while in the EO from the central Balkan mountains camphor (45.54%), trans-chrysanthenyl acetate (13.87%), and camphene (13.03%) were the most abundant components.
1. Introduction
Tanacetum parthenium L. (T. parthenium) is a perennial herbaceous plant belonging to the Asteraceae family with a long-standing historical use for traditional medicinal applications such as treatment of headache, fever, morning sickness, menstrual disorders, colic, skin conditions, arthritis, kidney pain, asthma etc. [1,2,3,4]. One of its common names is feverfew, presumably originating from its earlier name “featherfew” on account of its feather-like leaves [5]. It was reported that the prominent Greek physician Dioscorides recommended feverfew for “all hot inflammations” [1]. It is also suggested that the ancient Greeks called the herb “Parthenium,” because it was used as an important medicine for the treatment of workers who had fallen from the Parthenon during its construction in the 5th century AD [1]. The herb had an important role not only in ancient Greek traditional medicine but it was also used for the treatment of different disorders by the native peoples of Central and South America [1].
Tanacetum parthenium L. is a small, bushy, aromatic perennial plant. In general, its height is between 0.3 and 1 m [1]. The plant has yellow-green alternate leaves that are usually less than 8 cm in length. The leaves are described as almost hairless and chrysanthemum-like [1]. The diameter of its flowers is about 2 cm and they have a beautiful yellow colour. The flowers are arranged in a dense flat-topped cluster [1]. The bloom is from July to October. The flowers are sometimes compared to those of chamomile (Matricaria chamomilla), and both species could be confused for the other [1]. Tanacetum parthenium L. has a strong and bitter odour. Because of the similarities with chamomile, the plant is also known as wild chamomile, chamomile grande, grande chamomile, Matricaria capensis, Matricaria eximia hort, and Matricaria parthenium L. [1].
Other common names of Tanacetum parthenium L. are bachelor’s button, featherfoil, Chrysanthemum parthenium, altamisa, febrifuge plant, midsummer daisy, nosebleed, Santa Maria, wild quinine, chrysanthemum atricaire, federfoy, flirtwort, Leucanthemum parthenium, mother herb, Parthenium hysterophorus, parthenolide, Pyrenthrum parthenium L., feddygen fenyw, flirtroot, mutterkraut, and vetter-voo.
The species is indigenous to the Balkan Peninsula but is widespread in different areas all over the world, including in Europe, Asia, Australia, North Africa, and North America [1,6]. In the last decades, it has gained popularity as a remedy for migraine prophylaxis [7,8,9,10,11]. Recently, many efforts have been directed towards researching natural products with health-beneficial effects [12]. According to different studies, T. parthenium extracts exhibit anti-nociceptive [13,14,15], antioxidant [13,16,17,18,19,20,21], antibacterial [22], insecticidal [23], anti-inflammatory, neuromodulatory, antispasmodic, and uterine stimulant activity [24,25]. Investigations on feverfew EO have indicated antimicrobial [26,27,28,29,30,31], antioxidant [32], repellent [33], cytotoxic, and low anti-inflammatory [34] properties. Parthenolide, a sesquiterpene lactone isolated from the plant, has been reported to demonstrate anti-inflammatory [35,36,37,38,39,40,41,42,43,44,45], neuroprotective [46,47], cholesterol-lowering [48], antiviral [49], antileishmanial [50], and antitumor [51,52,53,54,55,56,57] effects. In a recent paper, Lakhera et al. suggested parthenolide as a candidate for the development of an anti-coronavirus drug [58]. Tanacetum parthenium contains various bioactive compounds including phenolic acids, flavonoids, coumarins, fatty acids, sesquiterpene lactones, and essential oil (EO) [4,59,60,61,62].
EOs are complex odoriferous mixtures of organic volatiles consisting of terpenes, terpenoids, phenylpropenes, and other compounds [63,64,65]. The number of individual constituents is usually around 20–60, but in some cases, it can even exceed 300 [66,67,68]. Generally, the main two or three that are present in higher concentrations are accountable for the biological activities of the EO. Nevertheless, those at minor concentrations could also affect the bioactivity by exhibiting additive effects, synergism, or antagonism [66,69,70]. Synergism occurs when the combined effect of multiple compounds is greater than the sum of their individual effects, while antagonism occurs when the combined effect is less than expected based on the individual effects of each compound. Understanding the complex interactions between the different compounds in the compositions of EOs is crucial for elucidating their biological activities and potential therapeutic applications. It also underscores the importance of considering the full chemical profile of EOs rather than focusing solely on individual compounds. Exploration of the full chemical profile of EOs plays an essential role in better understanding how EOs affect physiological processes and to potentially identify new therapeutic targets.
EOs exert various biological activities, such as anti-inflammatory, antimicrobial, and antioxidant, and have a multitude of applications [66,71,72], including the development of nutraceuticals and pharmaceuticals [73,74,75,76], cosmetic products (skin care, hair care, perfumery etc.) as active ingredients or as preservatives [71], and in aromatherapy [77,78]. As antibiotic resistance is becoming a major concern in modern medicine, EOs and their components have been widely investigated for antimicrobial effects alone or in combinations [79,80]. Furthermore, they are used in the food industry as flavouring agents, preservatives, and food packaging materials [75,81,82,83]. Recently, EOs have been associated with a promising potential for utilisation as alternatives for synthetic pesticides in agriculture [84,85].
The chemical composition of EOs varies depending on plant origin, plant organ, development stage, edaphic and climatic factors, as well as the method of extraction, drying method, etc. [29,66,86,87,88]. Literature data regarding the genus Tanacetum reveals that a significant variation in terms of EO constituents and chemovariability is observed on the species and subspecies levels [89]. Studies on the relationship between phytochemicals produced by plant metabolism and their effects are especially important for exploring the drug-discovery potential of the plant species [90,91].
The present study aimed to evaluate the chemical composition of Bulgarian T. parthenium EOs from two different locations and to expand the knowledge of its quantitative and qualitative differences, which could serve as a starting point in the selection of plant material for further cultivar development. Moreover, the results were compared with EOs from other geographical regions, highlighting some future application perspectives.
2. Results
The flowers of wild-grown T. parthenium L. were collected from two different mountain regions in Bulgaria: Tsigov Chark (41°56′23.2″ N 24°11′17.4″ E), western Rhodope Mountains (RM), and Gabrovo (42°49′39.7″ N 25°18′17.6″ E), central Balkan Mountains (BM) (Figure 1). The two locations differ in altitude, soil type, and climate (Table 1).

Figure 1.
Wild-growing populations of T. parthenium L.

Table 1.
Comparison between the plant collection locations.
Both obtained EOs were pale yellow in colour, with a distinct aroma. Their chemical composition was analysed by GC-MS, which resulted in the identification of 25 volatile components in the EO from the wild feverfew population in RM, representing 90.52% of the total oil, while 28 volatile compounds were detected in the EO extracted from plants collected in BM, representing 91.41% of the total oil. The predominant class terpenes in both samples were the oxygenated monoterpenes, accounting for 63.77% (RM sample) and 70.93% (BM sample), followed by the monoterpene hydrocarbons, representing 26.03% and 18.52%, respectively. The content of sesquiterpene hydrocarbons and other compounds was minimal, ranging between 0.05–1.33% and 0.63–0.67%, respectively, and oxygenated sesquiterpenes were absent in both EOs. The chromatograms of the EOs from wild-grown T. parthenium collected in the western Rhodope Mountains and the central Balkan Mountains are presented in Figure 2 and Figure 3, respectively.
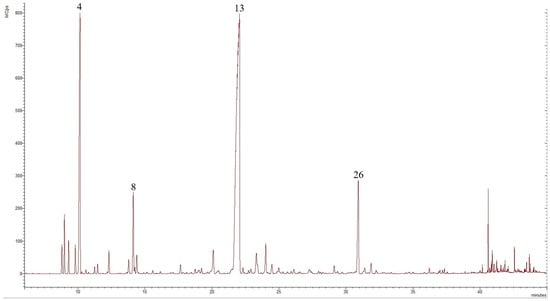
Figure 2.
Chromatogram of T. parthenium EO from the wild population in the western Rhodope Mountains, compounds derived from GC-MS analysis, in which the numbers refer to the following: 4—camphene, 8—p-cymene, 13—camphor, and 26—bornyl acetate.
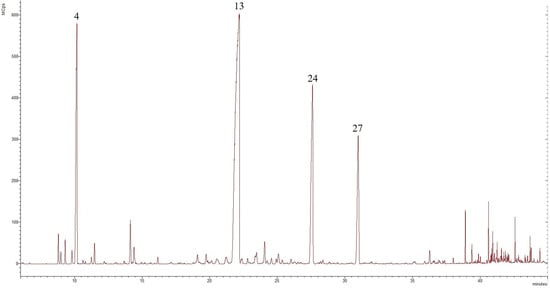
Figure 3.
Chromatogram of T. parthenium EO from the wild population in the central Balkan Mountains, compounds derived from GC-MS analysis, in which the numbers refer to the following: 4—camphene, 13—camphor, 24—trans-chrysanthenyl acetate, and 27—trans-verbenyl acetate.
The GC-MS analysis revealed some significant differences in the volatile constituents of the two EOs. Table 2 shows the chemical composition of the EOs with formulas, retention indices, class terpenes, and relative percentage amounts. Twenty-five different compounds were established in the EO obtained from plants from RM and 28 compounds in the EO from plants collected in BM. The oxygenated monoterpenes were the most abundant constituents in both of the samples (>60%), followed by the monoterpene hydrocarbons (26.03% in the RM EO and 18.52% in the BM EO).

Table 2.
Volatile organic compounds in essential oil obtained from wild populations of Tanacetum parthenium L. growing in the western Rhodope Mountains (RM) and in the central Balkan Mountains (BM), where “–‘’ = not detected.
3. Discussion
The main compound found in both samples was camphor, in concentrations between 45.54% and 50.90%. The camphene content was also similar (13.03–16.12%).
Camphor is a bicyclic monoterpene ketone that can be obtained from plants or can be produced synthetically, the difference being that natural camphor is dextrorotatory and synthetic camphor is optically inactive [96]. Major natural sources of this compound include the wood of Cinnamomum camphora L. (camphor tree, camphor laurel) (Lauraceae family) and the leaves from Ocimum kilimandscharicum Gürke (also known as camphor basil) (Lamiaceae family) [96,97]. Camphor is also a constituent of the EOs of several aromatic plant species such as Cinnamomum agasthyamalayanum, Ocimum canum, Salvia officnalis, Rosmarinus officinalis, Lavandula sp., Artemisia sp. etc. [98,99,100,101,102]. Camphor has a long-standing use as an antiseptic, antipruritic, abortifacient, aphrodisiac, counterirritant and rubefacient, heart stimulant, fumigant, and a fragrance and flavoring agent [96,103,104]. It has also been used to relieve nasal congestion, pain, and inflammation [96,105,106]. Furthermore, camphor exhibited neuroprotective effects [107], insecticidal activity [108], and skin penetration enhancing properties [109]. In addition, it can be utilised in the synthesis of new important molecules [104,106]. Despite these beneficial actions, the potential risks associated with the use of camphor and camphor containing products should not be overlooked, since there are many reports of intoxication due to irrational use, especially in children [96,110,111,112]. However, products with this substance are generally considered safe for topical application as long as the indications and dosage are followed [96].
Camphene belongs to the group of monoterpene hydrocarbons; it is present in the EOs of various aromatic plants, fruits, and spices and has been used in the food and cosmetics industries as a flavouring substance or fragrance ingredient [113,114]. It was reported to inhibit ROS generation, NO release, and decrease lipid peroxidation showing cytoprotective and antioxidant activity [114]. Quintans-Júnior et al. also reported high free radicals scavenging activity and strong antioxidant effect as well as modest antinociceptive activity [115]. In addition, camphene attenuates muscle atrophy by inhibiting oxidative stress [116]. Moreover, camphene demonstrates hypolipidemic [117,118], anti-inflammatory [119,120], anti-tumor [121], anti-hepatosteatotic [122], and insecticidal [123,124,125] effects. Camphene-based derivatives have shown to be promising agents against pathogens, such as Mycobacterium tuberculosis, Staphylococcus aureus, Enterococcus spp., and different viruses [126,127,128].
Despite the similarity in the content of the two main compounds, there are some important differences in the composition of the EO isolated from the plant material from Balkan Mountain and the plant material from Rhodope Mountains. Trans-Chrysanthenyl acetate was found only in the EO from Tanacetum parthenium L. growing in the central Balkan Mountains (13.87%). The presence or the absence of chrysanthenyl acetate in the different samples could affect the biological activity of the EO. Recently, it has been reported that chrysanthenyl acetate has an indirect antioxidant activity, increasing the activity of antioxidant enzymes [129]. Apart from members of the Tanacetum genus [130,131,132,133,134], it also occurs in high concentrations in species such as Anthemis maritime [135], Lamium amplexicalule [136], Zieria cytisoides [137], and Allium neapolitanum [138]. A content of 100% (E)-chrysanthenyl acetate was reported in oil obtained from Anthemis secundiramea Biv. subsp. secundiramea flowers [129]. The high amount of chrysanthenyl acetate is associated with potent phytotoxic, antioxidant, and antimicrobial activity [139,140].
Trans-Verbenyl acetate is another compound that is presented only in the composition of the EO from Tanacetum parthenium L. growing in the central Balkan Mountains. Trans-Verbenyl acetate was previously detected in feverfew EO obtained by steam distillation but the content was only 0.5% [141]. It has also been found in other members of the Tanacetum genus in relatively low concentrations [142,143]. The content of this compound in the BM sample was significant (8.93%). Trans-Verbenyl acetate belongs to the class of the oxygenated monoterpenes; however, data on its effects are very scarce. Nishino et al. reported on sex pheromonal activity in a study using the American cockroach (Periplaneta americana L.) [144].
Although the composition of the EO isolated from the plant material from the central Balkan Mountains was more abundant, bornyl acetate was found only in the sample from the Rhodope Mountains (6.05%). It is a bicyclic monoterpene with promising anti-inflammatory and immunomodulatory effects and low toxicity [145]. Due to its anti-oxidant and anti-inflammatory properties, it has been suggested as a potential therapeutic agent in the treatment of atherosclerosis [146], osteoarthritis [147], autoimmune demyelinating diseases (including multiple sclerosis) [148], and memory disorders [149]. Several studies pinpointed its insecticidal effects and possible use as an environmentally friendly biopesticide [124,125,150]. Inhalation of bornyl acetate in low doses causes a sedative effect without affecting vigilance [151]. Intravenous administration of this compound leads to vasorelaxation [152]. Furthermore, it exhibits analgesic [153,154], anti-proliferative [155,156], and anti-abortive [157] effects.
P-cymene was detected in both EOs, however the concentration in the RM sample was more notable (3.89%). It is an aromatic monoterpene with a distinctive woody, spicy scent, found in over 200 foods and spices, including cinnamon, nutmeg, carrots, raspberries, orange juice, grapefruit, tangerine, and is a major compound in EOs from members of the Thymus, Origanum, Ocimum, Artemisia, Protium, Eucalyptus, Hyptis, and Zataria genus with a plethora of health beneficial properties [158,159,160,161,162,163,164,165]. P-cymene demonstrates anti-inflammatory and anti-nociceptive effects [115,159,160,162,166,167]. Including it in a complex with β-cyclodextrin could even improve the analgesic and anti-inflammatory properties [163]. Additionally, p-cymene exhibits anti-oxidant activity and could be used as a neuroprotective agent [168]. The anti-oxidant and anti-inflammatory potential could attribute to the gastroprotective effect of the substance revealed in an ethanol-induced gastric ulcer in rats [169]. Moreover, p-cymene can prevent beta-amyloid-caused synaptic plasticity impairment in a rat model of Alzheimer’s disease [170] and is reported to possess vasorelaxant [171,172] and anti-tumor effects [161], the latter being mainly manifested when p-cymene is associated with metals in complexes such as ruthenium [173] and osmium [174]. As a natural antimicrobial component, p-cymene in low concentrations could increase the shelf life of un-pasteurised fruit juices [175]. It enhances the antimicrobial properties of other components and exerts an anti-biofilm activity [176].
In a study on tansy EO composition, Nurzyńska-Wierdak et al. assumed less environmental influence on camphor content compared to genetic factors [177]. A higher concentration was noted in plants in acidic sites. On the contrary, more trans-chrysanthenyl acetate was detected in plants from a location with alkaline and neutral soils [177]. Thus, a less acidic pH of the soil in BM could explain the lower amount of camphor and the higher share of trans-chrysanthenyl acetate compared to the RM sample. Estell et al. reported a positive effect of UV light restriction on camphene, bornyl acetate, and p-cymene content [178]. The more significant percentage of p-cymene in the RM sample could be linked to the altitude [179].
Tanacetum parthenium EO analyses have been conducted by several authors. Table 3 compares the main constituents of feverfew EOs obtained from several different geographical regions.

Table 3.
Comparison of the main volatile compounds of T. parthenium EO from different geographical areas.
Camphor has been recognised as the main compound identified in feverfew EOs from various locations, including Turkey, Egypt, Iran, Serbia, Italy, and Tajikistan. Typically, its content is around 45–65% [6,25,26,27,28,30,131,180,181,182,183], which is in agreement with our results. Sharopov et al. detected a concentration of up to 94% in EO from a wild population growing in Tajikistan [34]. Végh et al. determined the highest amount of camphor in the leaves of the plant during flowering [184]. However, considerable variations have been documented concerning the other predominant components. Trans-Chrysanthenyl acetate was noted as the second most abundant constituent in EOs extracted from leaves, flowers, and aerial parts in concentrations ranging from 21.12% to 33.8% [25,27]. In contrast, in other studies it was not detected at all [6,19,26,29,34,180,181,183] or was found in relatively small amounts/traces [27,30]. Camphene emerged as another characteristic compound of feverfew EO, with a fraction reaching up to 13.74% [6,19,25,26,27,28,29,30,34,131,180,181,182]. The present study supports these data, and the RM sample afforded an even higher camphene amount (16.12%). On the contrary, this substance was lacking in EO from T. parthenium cultivated in Northern Italy [183]. Unlike previous results, Giuliani et al. also reported a large content of farnesol (28.83%) belonging to the oxygenated sesquiterpenes [183], which were completely absent in this investigation. The amount of sesquiterpene hydrocarbons in the RM and BM samples was negligible (only 0.05 and 1.33%, respectively). In contrast to the Bulgarian feverfew EOs, the share of SH in the EO from a wild sample of T. parthenium from Iran was relatively large (14.9%), the major representative from this class being germacrene-D (9.2%) [181]. Shafaghat et al. detected SH as the second most abundant class of terpenes (after MO), at 16.1%, in EO from feverfew leaves, represented by considerable content of trans-β-farnesene (8.3%) and β-caryophyllene (5.9%) [180]. In regards to bornyl acetate, the data vary as well, from its total lack [25,27,183] to 18.35% detected in the EO from T. parthenium collected during flowering in Iran [29]. Mohsenzadeh et al. suggested a correlation between the concentration of the compounds and the developmental stage [29]; however, other factors could also affect the composition of EOs- geographical area, plant material, environmental factors, methods used for drying and extraction, etc. [6,27,182,185]. Shahhoseini et al. concluded a concentration dependent positive effect on the quantity and quality of feverfew EO by titanium dioxide-nanoparticles application [186].
The chemical profiles of both analysed EOs indicate a prominent potential for implementation in environmentally friendly pest control products, which are gaining importance since synthetic pesticides are subject to more and more restrictions due to their negative impact on human health and the environment [187]. The obtained results suggest the two T. parthenium EOs as suitable bioresources for incorporation in products for external application (creams, ointments, gels, patches, etc.) aimed at relieving joint and muscle pain, especially in patients with chronic inflammatory disorders (such as osteoarthritis and rheumatoid arthritis) since the use of synthetic drugs (for instance non-steroidal anti-inflammatory drugs) for long periods of time is associated with an increased risk of severe side effects, even when applied topically. Further in vitro and in vivo investigations are needed to evaluate these activities and to clarify the feverfew EO utilisation perspectives.
4. Materials and Methods
4.1. Plant Materials
The flowers of wild-grown T. parthenium L. were collected from two locations in Bulgaria: Tsigov Chark (41°56′23.2″ N 24°11′17.4″ E), western Rhodope Mountains, and Gabrovo (42°49′39.7″ N 25°18′17.6″ E), central Balkan Mountains. The plants were authenticated by Associate Professor Niko Benbassat in accordance with the European Pharmacopoeia [188]. Voucher specimens (No. 063400 from RM and No. 063395 from BM) were deposited in the Herbarium of the University of Agriculture, Plovdiv, Bulgaria. The two locations differ in altitude (RM 1100 m, BM 500 m), soil type (RM Dystric Cambisols, BM Haplic Luvisols), and climate (RM Middle Mountain region, BM Temperate Continental region) [92]. The plant material was collected in the phase of full flowering and was then dried at room temperature.
4.2. Chemicals and Reagents
For the determination of the retention indices (RI) of the separated compounds, the following hydrocarbons were used: nonane (≥99%), decane (≥99%), undecane (≥99%), dodecane (99%), tridecane (≥99%), tetradecane (≥99%), and hexadecane (≥99%) purchased from Merck KGaA (Darmstadt, Germany). Hexane (Thermo Fisher Scientific GmbH, Bremen, Germany) was used for the dilution of the EO.
4.3. Isolation of the Essential Oil
The essential oils of the air-dried flowering aerial parts were obtained by hydrodistillation for 4 h using a Clevenger-type apparatus. The collected essential oils were dried over anhydrous sodium sulfate and stored in dark glass vials at 4 °C until GC-MS analysis.
4.4. Chromatographic Conditions
The analysis of both EOs was carried out using gas chromatography with mass spectrometry (GC-MS). For the analysis, a Bruker Scion 436-GC SQ MS (Bremen, Germany)equipped with a Zebron ZB-5MSplus capillary column (0.25 µm film thickness and 30 m × 0.25 mm i.d.) was used. The carrier gas was helium with a constant flow rate of 1 mL/min. The volume of the injection was 1 µL, with the temperature of the injector set to 250 °C and split ratio of 1:20. The oven temperature was initially set at 50 °C for 1 min, then increased to 130 °C at a rate of 2 °C/min, and then increased to 240 °C at a rate of 15 °C/min and held for 1 min. The detector temperature was set to 300 °C. The mass spectra were collected in a full scan mode with a mass range of 50–350 m/z. The retention indices (RI) of the separated compounds were calculated from the retention times of the C8–C30 n-alkane series injected under the same conditions described above. The identification of the essential oil constituents was achieved by comparing their MS spectra and RI values with spectral data within the Wiley NIST11 Mass Spectral Library (NIST11/2011/EPA/NIH) and the literature data. The analyses were performed in triplicate. Standard deviations (SDs) did not exceed 2% of the obtained values of each component.
5. Conclusions
Studies on the phytochemicals produced by plant metabolism have significant importance for exploring the drug-discovery potential of the plant species. The present study focused on a comparative evaluation of the chemical composition of T. parthenium EO from two locations in Bulgaria. The occurrence of two chemotypes based on the predominant components—camphor/camphene/bornyl acetate chemotype (RM) and camphor/trans-chrysanthenyl acetate/camphene chemotype (BM) was established. Although there are some similarities in the composition, the two samples are characterised by important differences. For targeting some future applications and performing biological activity studies, it is important to consider the full chemical profile of EOs rather than focusing solely on individual compounds. Understanding the possible interactions between the different compounds found in the compositions of EOs is crucial for elucidating their biological activities and potential therapeutic applications. Exploration of the full chemical profile of EOs is essential for better understanding how EOs affect various physiological processes and potentially identify new therapeutic targets.
Author Contributions
Conceptualization, B.L. and S.I.; methodology, S.I., N.B. and L.P.; software, B.L.; validation, B.L., S.I., N.B. and L.P.; formal analysis, B.L.; investigation, B.L., S.D., S.I., D.K.-B., Y.G.-D. and K.I; resources, B.L. and S.I.; data curation, B.L. and S.I.; writing—original draft preparation, S.I., B.L., Z.P. and K.K.; writing—review and editing, S.I., K.I., N.B. and L.P.; visualization, B.L. and S.I.; supervision, S.I., D.K.-B., L.P., K.I. and N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors gratefully acknowledge the support of Medical University of Plovdiv, project DPDP-07/2023.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pareek, A.; Suthar, M.; Rathore, G.; Bansal, V. Feverfew (Tanacetum parthenium L.): A Systematic Review. Pharmacogn. Rev. 2011, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; Pittler, M. The Efficacy and Safety of Feverfew (Tanacetum parthenium L.): An Update of a Systematic Review. Public Health Nutr. 2000, 3, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Guarino, C. Ethnobotanical Study of the Sannio Area, Campania, Southern Italy. Ethnobot. Res. Appl. 2008, 6, 255. [Google Scholar] [CrossRef]
- Gevrenova, R.; Balabanova, V.; Zheleva-Dimitrova, D.; Momekov, G. The Most Promising Southeastern European Tanacetum Species: A Review of Chemical Composition and Biological Studies. Pharmacia 2023, 70, 1067–1081. [Google Scholar] [CrossRef]
- Chavez, M.L.; Chavez, P.I. Feverfew. Hosp. Pharm. 1999, 34, 436–461. [Google Scholar] [CrossRef]
- Akpulat, H.A.; Tepe, B.; Sokmen, A.; Daferera, D.; Polissiou, M. Composition of the Essential Oils of Tanacetum argyrophyllum (C. Koch) Tvzel. Var. Argyrophyllum and Tanacetum parthenium (L.) Schultz Bip. (Asteraceae) from Turkey. Biochem. Syst. Ecol. 2005, 33, 511–516. [Google Scholar] [CrossRef]
- Saranitzky, E.; White, C.M.; Baker, E.L.; Baker, W.L.; Coleman, C.I. Feverfew for Migraine Prophylaxis: A Systematic Review. J. Diet. Suppl. 2009, 6, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, T.; Davenport, W.J. Phytomedicines in the Treatment of Migraine. CNS Drugs 2019, 33, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.K.; Goldstein, J.; Nett, R.; Mitchell, R.; Beach, M.E.; Browning, R. A Double-Blind Placebo-Controlled Pilot Study of Sublingual Feverfew and Ginger (LipiGesic™M) in the Treatment of Migraine. Headache J. Head Face Pain 2011, 51, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Moscano, F.; Guiducci, M.; Maltoni, L.; Striano, P.; Ledda, M.G.; Zoroddu, F.; Raucci, U.; Villa, M.P.; Parisi, P. An Observational Study of Fixed-Dose Tanacetum parthenium Nutraceutical Preparation for Prophylaxis of Pediatric Headache. Ital. J. Pediatr. 2019, 45, 36. [Google Scholar] [CrossRef] [PubMed]
- Volta, G.D.; Zavarise, P.; Perego, L.; Savi, L.; Pezzini, A. Comparison of the Effect of Tanacethum Parthenium, 5-Hydroxy Tryptophan, and Magnesium (Aurastop) versus Magnesium Alone on Aura Phenomenon and Its Evolution. Pain Res. Manag. 2019, 2019, 6320163. [Google Scholar] [CrossRef] [PubMed]
- Lukova, P.; Apostolova, E.; Baldzhieva, A.; Murdjeva, M.; Kokova, V. Fucoidan from Ericaria Crinita Alleviates Inflammation in Rat Paw Edema, Downregulates Pro-Inflammatory Cytokine Levels, and Shows Antioxidant Activity. Biomedicines 2023, 11, 2511. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Calderon, O.; Bulege-Gutiérrez, W.; Javier-Silva, L.A.; Iparraguirre-Meza, M.; Sanchez-Araujo, V.G.; Melgar-Merino, E.J.; Tinco-Jayo, J.A.; Almeida-Galindo, J.S.; Bertha Pari-Olarte, J. Tanacetum parthenium (L.) Sch Bip From Peru: Antioxidant Profile and The Antinociceptive Effect in An Experimental Model. Pharmacogn. J. 2023, 15, 435–437. [Google Scholar] [CrossRef]
- Mannelli, L.D.-C.; Tenci, B.; Zanardelli, M.; Maidecchi, A.; Lugli, A.; Mattoli, L.; Ghelardini, C. Widespread Pain Reliever Profile of a Flower Extract of Tanacetum parthenium. Phytomedicine 2015, 22, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Asgari, A.; Parvin, N. The Analgesic Effect of Ethanolic Extract of Tanacetum parthenium in Acetic Acid Model. Zahedan J. Res. Med. Sci. 2013, 15, 22–25. [Google Scholar]
- Wu, C.; Chen, F.; Wang, X.; Kim, H.-J.; He, G.; Haley-Zitlin, V.; Huang, G. Antioxidant Constituents in Feverfew (Tanacetum parthenium) Extract and Their Chromatographic Quantification. Food Chem. 2006, 96, 220–227. [Google Scholar] [CrossRef]
- Nikolova, M.; Dzhurmanski, A. Evaluation of Free Radical Scavenging Capacity of Extracts from Cultivated Plants. Biotechnol. Biotechnol. Equip. 2009, 23, 109–111. [Google Scholar] [CrossRef]
- Hanganu, D.; Benedec, D.; Vlase, L.; Popica, I.; Bele, C.; Raita, O.; Gheldiu, A.-M.; Valentin, C. Polyphenolic Content and Antioxidant Activity of Chrysanthemum parthenium Extract. Farmacia 2016, 64, 498–501. [Google Scholar]
- Shahhoseini, R.; Azizi, M.; Asili, J.; Moshtaghi, N.; Samiei, L. Comprehensive Assessment of Phytochemical Potential of Tanacetum parthenium (L.): Phenolic Compounds, Antioxidant Activity, Essential Oil and Parthenolide. J. Essent. Oil Bear. Plants 2019, 22, 614–629. [Google Scholar] [CrossRef]
- Fa, Z.; Jianyun, Z.; Yiqun, S.; Ken, K. Identification of Antioxidative Ingredients from Feverfew (Tanacetum parthenium) Extract Substantially Free of Parthenolide and Other Alpha-Unsaturated Gamma-Lactones. Open J. Anal. Bioanal. Chem. 2019, 3, 076–082. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Şenkardes, I.; Dogan, A.; Ibrahime Sinan, K.; Uysal, S.; Aumeeruddy-Elalfi, Z.; et al. Modern and Traditional Extraction Techniques Affect Chemical Composition and Bioactivity of Tanacetum parthenium (L.) Sch. Bip. Ind. Crops Prod. 2020, 146, 112202. [Google Scholar] [CrossRef]
- Shiferaw, Z.; Jagathala Mahalingam, S.; Kebede, A.; Teju, E. Antibacterial Effects of Extracts from Tanacetum parthenium L. Leaves. Bact. Emp. 2022, 5, e394. [Google Scholar] [CrossRef]
- Pavela, R.; Sajfrtová, M.; Sovová, H.; Bárnet, M.; Karban, J. The Insecticidal Activity of Tanacetum parthenium (L.) Schultz Bip. Extracts Obtained by Supercritical Fluid Extraction and Hydrodistillation. Ind. Crops Prod. 2010, 31, 449–454. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; di Giacomo, V.; Antolini, M.D.; Acquaviva, A.; Leone, S.; Brunetti, L.; Menghini, L.; Ak, G.; Zengin, G.; et al. Anti-Inflammatory and Neuromodulatory Effects Induced by Tanacetum parthenium Water Extract: Results from In Silico, In Vitro and Ex Vivo Studies. Molecules 2020, 26, 22. [Google Scholar] [CrossRef] [PubMed]
- Rateb, M.E.M.; El-Gendy, A.-N.A.M.; El-Hawary, S.S.; El-Shamy, M. Phytochemical and Biological Investigation of Tanacetum parthenium (L.) Cultivated in Egypt. J. Med. Plants Res. 2007, 1, 18–26. [Google Scholar]
- Shafaghat, A.; Larijani, K.; Salimi, F. Composition and Antibacterial Activity of the Essential Oil of Chrysanthemum Parthenium Flower from Iran. J. Essent. Oil Bear. Plants 2009, 12, 708–713. [Google Scholar] [CrossRef]
- Izadi, Z.; Esna-Ashari, M.; Piri, K.; Davoodi, P. Chemical Composition and Antimicrobial Activity of Feverfew (Tanacetum parthenium) Essential Oil. Int. J. Agric. Biol. 2010, 12, 1560–8530. [Google Scholar]
- Izadi, Z.; Aghaalikhani, M.; Esna-Ashari, M.; Davoodi, P. Determining Chemical Composition and Antimicrobial Activity of Feverfew (Tanacetum parthenium L.) Essential Oil on Some Microbial Strains. Zahedan J. Res. Med. Sci. 2013, 15, 8–13. [Google Scholar]
- Mohsenzadeh, F.; Chehregani, A.; Amiri, H. Chemical Composition, Antibacterial Activity and Cytotoxicity of Essential Oils of Tanacetum parthenium in Different Developmental Stages. Pharm. Biol. 2011, 49, 920–926. [Google Scholar] [CrossRef]
- Polatoglu, K.; Demirci, F.; Demirci, B.; Gören, N.; Baser, K.H.C. Antibacterial Activity and the Variation of Tanacetum parthenium (L.) Schultz Bip. Essential Oils from Turkey. J. Oleo Sci. 2010, 59, 177–184. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Essential Oils from the Asteraceae Family Active against Multidrug-Resistant Bacteria. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Elsevier: Amsterdam, The Netherlands, 2013; pp. 205–221. ISBN 978-0-12-398539-2. [Google Scholar]
- Rezaei, F.; Jamei, R.; Heidari, R. Evaluation of the Phytochemical and Antioxidant Potential of Aerial Parts of Iranian Tanacetum parthenium. Pharm. Sci. 2017, 23, 136–142. [Google Scholar] [CrossRef]
- Lazarević, J.; Kostić, I.; Milanović, S.; Šešlija Jovanović, D.; Krnjajić, S.; Ćalić, D.; Stanković, S.; Kostić, M. Repellent Activity of Tanacetum parthenium (L.) and Tanacetum vulgare (L.) Essential Oils against Leptinotarsa decemlineata (Say). Bull. Entomol. Res. 2021, 111, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.S.; Setzer, W.N.; Isupov, S.J.; Wink, M. Composition and Bioactivity of the Essential Oil of Tanacetum parthenium from a Wild Population Growing in Tajikistan. Am. J. Essent. Oils Nat. Prod. 2015, 2, 32–34. [Google Scholar]
- Fukuda, K.; Hibiya, Y.; Mutoh, M.; Ohno, Y.; Yamashita, K.; Akao, S.; Fujiwara, H. Inhibition by Parthenolide of Phorbol Ester-Induced Transcriptional Activation of Inducible Nitric Oxide Synthase Gene in a Human Monocyte Cell Line THP-1. Biochem. Pharmacol. 2000, 60, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Sobota, R.; Szwed, M.; Kasza, A.; Bugno, M.; Kordula, T. Parthenolide Inhibits Activation of Signal Transducers and Activators of Transcription (STATs) Induced by Cytokines of the IL-6 Family. Biochem. Biophys. Res. Commun. 2000, 267, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.Y.; Chung, S.W.; Kim, T.S. Inhibition of Interleukin-12 Production in Lipopolysaccharide-Activated Mouse Macrophages by Parthenolide, a Predominant Sesquiterpene Lactone in Tanacetum parthenium: Involvement of Nuclear Factor-κB. Immunol. Lett. 2001, 77, 159–163. [Google Scholar] [CrossRef]
- Kwok, B.H.B.; Koh, B.; Ndubuisi, M.I.; Elofsson, M.; Crews, C.M. The Anti-Inflammatory Natural Product Parthenolide from the Medicinal Herb Feverfew Directly Binds to and Inhibits IκB Kinase. Chem. Biol. 2001, 8, 759–766. [Google Scholar] [CrossRef]
- Li-Weber, M.; Giaisi, M.; Treiber, M.; Krammer, P. The Anti-Inflammatory Sesquiterpene Lactone Parthenolide Suppresses IL-4 Gene Expression in Peripheral Blood T Cells. Eur. J. Immunol. 2002, 32, 3587–3597. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, H.; Takao, T.; Yamamoto, K.; Nakayama, J.; Miyagawa, Y.; Tsujimura, A.; Nonomura, N.; Okuyama, A. Sesquiterpene Lactone Parthenolide Ameliorates Bladder Inflammation and Bladder Overactivity in Cyclophosphamide Induced Rat Cystitis Model by Inhibiting Nuclear Factor-κB Phosphorylation. J. Urol. 2009, 181, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.; Gerstberger, R.; Roth, J.; Hübschle, T. Parthenolide Attenuates LPS-Induced Fever, Circulating Cytokines and Markers of Brain Inflammation in Rats. Cytokine 2011, 56, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, C.; Xiao, Y.; Mao, X. Anti-Inflammatory and Antiosteoclastogenic Activities of Parthenolide on Human Periodontal Ligament Cells In Vitro. Evid. Based Complement. Altern. Med. 2014, 2014, 546097. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Q. Parthenolide Could Become a Promising and Stable Drug with Anti-Inflammatory Effects. Nat. Prod. Res. 2015, 29, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, K.; Zhang, M.; Zhang, X.; Zhang, R. Parthenolide Suppresses T Helper 17 and Alleviates Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2022, 13, 856694. [Google Scholar] [CrossRef] [PubMed]
- Shou, D.-W.; Li, Y.-R.; Xu, X.-J.; Dai, M.-H.; Zhang, W.; Yang, X.; Tu, Y.-X. Parthenolide Attenuates Sepsis-Induced Acute Kidney Injury in Rats by Reducing Inflammation. Evid. Based Complement. Altern. Med. 2023, 2023, 8759766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Shi, L.; Zhao, Z.; Xu, H.; Liang, F.; Li, H.-B.; Zhao, Y.; Xu, X.; Yang, K.; et al. Parthenolide Attenuates Cerebral Ischemia/Reperfusion Injury via Akt/GSK-3β Pathway in PC12 Cells. Biomed. Pharmacother. 2017, 89, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, H.; Fischer, D. Parthenolide: A Novel Pharmacological Approach to Promote Nerve Regeneration. Neural Regen. Res. 2016, 11, 1566. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liang, B.; Zeng, J.; Meng, J.; Shi, L.; Yang, S.; Chang, J.; Wang, C.; Hu, X.; Wang, X.; et al. First Discovery of Cholesterol-Lowering Activity of Parthenolide as NPC1L1 Inhibitor. Molecules 2022, 27, 6270. [Google Scholar] [CrossRef] [PubMed]
- Benassi-Zanqueta, É.; Marques, C.F.; Nocchi, S.R.; Dias Filho, B.P.; Nakamura, C.V.; Ueda-Nakamura, T. Parthenolide Influences Herpes Simplex Virus 1 Replication in Vitro. Intervirology 2018, 61, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Tiuman, T.S.; Ueda-Nakamura, T.; Garcia Cortez, D.A.; Dias Filho, B.P.; Morgado-Díaz, J.A.; De Souza, W.; Nakamura, C.V. Antileishmanial Activity of Parthenolide, a Sesquiterpene Lactone Isolated from Tanacetum parthenium. Antimicrob. Agents Chemother. 2005, 49, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yuan, W.; Wen, G.; Yu, B.; Xu, F.; Gan, X.; Tang, J.; Zeng, Q.; Zhu, L.; Chen, C.; et al. Parthenolide Inhibits Human Lung Cancer Cell Growth by Modulating the IGF-1R/PI3K/Akt Signaling Pathway. Oncol. Rep. 2020, 44, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Xiao, Y.; Ma, J.; Ou, W.; Wang, H.; Wu, J.; Tang, J.; Zhang, B.; Liao, X.; Yang, D.; et al. Parthenolide Inhibits Angiogenesis in Esophageal Squamous Cell Carcinoma Through Suppression of VEGF. OncoTargets Ther. 2020, 13, 7447–7458. [Google Scholar] [CrossRef] [PubMed]
- Sufian, H.B.; Santos, J.M.; Khan, Z.S.; Munir, M.T.; Zahid, M.K.; Al-Harrasi, A.; Gollahon, L.S.; Hussain, F.; Rahman, S.M. Parthenolide Inhibits Migration and Reverses the EMT Process in Breast Cancer Cells by Suppressing TGFβ and TWIST1. Res. Sq. 2021. in review. [Google Scholar] [CrossRef]
- Karam, L.; Abou Staiteieh, S.; Chaaban, R.; Hayar, B.; Ismail, B.; Neipel, F.; Darwiche, N.; Abou Merhi, R. Anticancer Activities of Parthenolide in Primary Effusion Lymphoma Preclinical Models. Mol. Carcinog. 2021, 60, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.; Tan, X.; Cui, Q.; Gu, W.; Pan, Z.; Yang, L.; Wu, S.; Wang, X.; Li, D. Parthenolide Inhibits Proliferation of Cells Infected with Kaposi’s Sarcoma-Associated Herpesvirus by Suppression of the NF-κB Signaling Pathway. Arch. Virol. 2023, 168, 39. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, Z.; Huang, L.-T.; Wang, J.-H. Parthenolide Leads to Proteomic Differences in Thyroid Cancer Cells and Promotes Apoptosis. BMC Complement. Med. Ther. 2022, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Cancers 2023, 15, 3037. [Google Scholar] [CrossRef] [PubMed]
- Lakhera, S.; Devlal, K.; Ghosh, A.; Chowdhury, P.; Rana, M. Modelling the DFT Structural and Reactivity Study of Feverfew and Evaluation of Its Potential Antiviral Activity against COVID-19 Using Molecular Docking and MD Simulations. Chem. Pap. 2022, 76, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.; Sobeh, M.; Faraloni, C.; Bouissane, L. Tanacetum Species: Bridging Empirical Knowledge, Phytochemistry, Nutritional Value, Health Benefits and Clinical Evidence. Front. Pharmacol. 2023, 14, 1169629. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; JeszkaSkowron, M. A Systematic Review on the Ethnobotany Essential Oils Bioactive Compounds and Biological Activities of Tanacetum Species. Trends Phytochem. Res. 2023, 7, 1–29. [Google Scholar] [CrossRef]
- Abad, M.J.; Bermejo, P.; Villar, A. An Approach to the genus Tanacetum L. (Compositae): Phytochemical and Pharmacological Review. Phytother. Res. 1995, 9, 79–92. [Google Scholar] [CrossRef]
- Hordiei, K.; Gontova, T.; Trumbeckaite, S.; Yaremenko, M.; Raudone, L. Phenolic Composition and Antioxidant Activity of Tanacetum parthenium Cultivated in Different Regions of Ukraine: Insights into the Flavonoids and Hydroxycinnamic Acids Profile. Plants 2023, 12, 2940. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.; Padilla-González, G.; Phumthum, M. Fundamental Chemistry of Essential Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Zhelev, I.; Petkova, Z.; Kostova, I.; Damyanova, S.; Stoyanova, A.; Dimitrova-Dyulgerova, I.; Antova, G.; Ercisli, S.; Assouguem, A.; Kara, M.; et al. Chemical Composition and Antimicrobial Activity of Essential Oil of Fruits from Vitex Agnus-Castus L., Growing in Two Regions in Bulgaria. Plants 2022, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; De Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Andonova, T.; Dimitrova-Dyulgerova, I.; Slavov, I.; Muhovski, Y.; Stoyanova, A. A Comparative Study of Koelreuteria Paniculata Laxm. Aerial Parts Essential Oil Composition. J. Essent. Oil Bear. Plants 2020, 23, 1363–1370. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Zouari, M. Essential Oils Chemotypes: A Less Known Side. Med. Aromat. Plants 2013, 2, 1000e145. [Google Scholar] [CrossRef]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Goranova-Marinova, V.; Grekova, D.; Georgieva, V.; Andreevska, K.; Gvozdeva, Y.; Kassarova, M.; Grudeva-Popova, Z. Analysis of the Pharmacotherapeutic Effectiveness of the Tyrosine Kinase Inhibitors Therapy in Patients with Chronic Myeloid Leukemia in a Single Hematology Center in Plovdiv, Bulgaria. Pharmacia 2023, 70, 1355–1362. [Google Scholar] [CrossRef]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef] [PubMed]
- Pinto, G.B.; Dos Reis Corrêa, A.; Da Silva, G.N.C.; Da Costa, J.S.; Figueiredo, P.L.B. Drug Development from Essential Oils: New Discoveries and Perspectives. In Drug Discovery and Design Using Natural Products; Cruz, J.N., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 79–101. ISBN 978-3-031-35204-1. [Google Scholar]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Staynova, R.; Yanachkova, V. Weight Management Strategies and Food Supplement Intake among Bulgarian Adults: Results of a National Survey. Pharmacia 2023, 70, 1119–1126. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Padilla-González, G.F.; Leuner, O.; Melnikovova, I.; Fernandez-Cusimamani, E. Pharmacology of Natural Volatiles and Essential Oils in Food, Therapy, and Disease Prophylaxis. Front. Pharmacol. 2021, 12, 740302. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential Oils Used in Aromatherapy: A Systemic Review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar] [PubMed]
- Sahu, A.; Parai, D.; Choudhary, H.R.; Singh, D.D. Essential Oils as Alternative Antimicrobials: Current Status. Recent Adv. Anti-Infect. Drug Discov. 2024, 19, 56–72. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Essential Oils as Potential Antimicrobial Agents in Meat and Meat Products: A Review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Tsitlakidou, P.; Tasopoulos, N.; Chatzopoulou, P.; Mourtzinos, I. Current Status, Technology, Regulation and Future Perspectives of Essential Oils Usage in the Food and Drink Industry. J. Sci. Food Agric. 2023, 103, 6727–6751. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Shahzad, S.; Hussain, A.; Pradhan, R.A.; Arshad, M.; Ullah, A. Current Trends in the Utilization of Essential Oils for Polysaccharide- and Protein-Derived Food Packaging Materials. Polymers 2022, 14, 1146. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Casas, J.L.; Sagarduy-Cabrera, A.; López Santos-Olmo, M.; Marcos-García, M.Á. Essential Oils from Selected Mediterranean Aromatic Plants—Characterization and Biological Activity as Aphid Biopesticides. Life 2023, 13, 1621. [Google Scholar] [CrossRef] [PubMed]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of Essential Oil from Different Plants and Herbs by Supercritical Fluid Extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, G.; Papafotiou, M.; Daferera, D.J.; Tarantilis, P.A. Yield and Composition of the Essential Oil of Clinopodium Nepeta Subsp. Spruneri as Affected by Harvest Season and Cultivation Method, i.e., Outdoor, Greenhouse and In Vitro Culture. Plants 2023, 12, 4098. [Google Scholar] [CrossRef] [PubMed]
- Politeo, O.; Popović, M.; Veršić Bratinčević, M.; Koceić, P.; Ninčević Runjić, T.; Mekinić, I.G. Conventional vs. Microwave-Assisted Hydrodistillation: Influence on the Chemistry of Sea Fennel Essential Oil and Its By-Products. Plants 2023, 12, 1466. [Google Scholar] [CrossRef]
- Kumar, V.; Tyagi, D. Chemical Composition and Biological Activities of Essential Oils of Genus Tanacetum—A Review. J. Pharmacogn. Phytochem. 2013, 2, 155–159. [Google Scholar]
- Petkov, V.H.; Ardasheva, R.G.; Prissadova, N.A.; Kristev, A.D.; Stoyanov, P.S.; Argirova, M.D. Receptor-Mediated Biological Effects of Extracts Obtained from Three Asplenium Species. Z. Naturforschung C 2021, 76, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Babotă, M.; Pop, A.; Fizeșan, I.; Diuzheva, A.; Locatelli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R.; et al. Chemical Constituents and Biologic Activities of Sage Species: A Comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur ex Griseb. & Schenk) Schur. Antioxidants 2020, 9, 480. [Google Scholar] [CrossRef]
- Hristov, B.; Filcheva, E. Soil Organic Matter Content and Composition in Different Pedoclimatic Zones of Bulgaria. Eurasian J. Soil Sci. EJSS 2017, 6, 65. [Google Scholar] [CrossRef]
- Lazarova, M.; Tonkov, S.; Marinova, E.; Ivanov, D.; Bozilova, E. Western Rhodopes Mountains (Bulgaria): Peat Bog Beliya Kanton. Grana 2011, 50, 162–164. [Google Scholar] [CrossRef][Green Version]
- Malcheva, K.; Bocheva, L.; Marinova, T. Mapping Temperature and Precipitation Climate Normals over Bulgaria by Using ArcGIS Pro 2.4. Bulg. J. Meteorol. Hydrol. 2020, 2, 61–77. [Google Scholar]
- Pospíšilová, Ľ.; Uhlík, P.; Menšík, L.; Hlisnikovský, L.; Eichmeier, A.; Horáková, E.; Vlček, V. Clay Mineralogical Composition and Chemical Properties of Haplic Luvisol Developed on Loess in the Protected Landscape Area Litovelské Pomoraví. Eur. J. Soil Sci. 2021, 72, 1128–1142. [Google Scholar] [CrossRef]
- Zuccarini, P.; Soldani, G. Camphor: Benefits and Risks of a Widely Used Natural Product. Acta Biol. Szeged. 2009, 53, 77–82. [Google Scholar] [CrossRef]
- Dos Santos, E.; Leitão, M.M.; Aguero Ito, C.N.; Silva-Filho, S.E.; Arena, A.C.; Silva-Comar, F.M.D.S.; Nakamura Cuman, R.K.; Oliveira, R.J.; Nazari Formagio, A.S.; Leite Kassuya, C.A. Analgesic and Anti-Inflammatory Articular Effects of Essential Oil and Camphor Isolated from Ocimum Kilimandscharicum Gürke Leaves. J. Ethnopharmacol. 2021, 269, 113697. [Google Scholar] [CrossRef] [PubMed]
- Sriramavaratharajan, V.; Stephan, J.; Sudha, V.; Murugan, R. Leaf Essential Oil of Cinnamomum Agasthyamalayanum from the Western Ghats, India—A New Source of Camphor. Ind. Crops Prod. 2016, 86, 259–261. [Google Scholar] [CrossRef]
- Chagonda, L.S.; Makanda, C.D.; Chalchat, J.-C. The Essential Oils of Ocimum Canum Sims (Basilic Camphor) and Ocimum Urticifolia Roth from Zimbabwe. Flavour Fragr. J. 2000, 15, 23–26. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Arak, E. Composition of the Essential Oil of Salvia Officinalis L. from Various European Countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Karaca, N.; Şener, G.; Demirci, B.; Demirci, F. Synergistic Antibacterial Combination of Lavandula Latifolia Medik. Essential Oil with Camphor. Z. Naturforschung C 2021, 76, 169–173. [Google Scholar] [CrossRef]
- Diass, K.; Brahmi, F.; Mokhtari, O.; Abdellaoui, S.; Hammouti, B. Biological and Pharmaceutical Properties of Essential Oils of Rosmarinus officinalis L. and Lavandula officinalis L. Mater. Today Proc. 2021, 45, 7768–7773. [Google Scholar] [CrossRef]
- Singh, R.; Jawaid, T. Cinnamomum Camphora (Kapur): Review. Pharmacogn. J. 2012, 4, 1–5. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gan, Y.; Kang, T.; Zhao, Y.; Huang, T.; Chen, Y.; Liu, J.; Ke, B. Camphor Attenuates Hyperalgesia in Neuropathic Pain Models in Mice. J. Pain Res. 2023, 16, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Mahdy, A.-H.S.; Zayed, S.E.; Abo-Bakr, A.M.; Hassan, E.A. Camphor: Synthesis, Reactions and Uses as a Potential Moiety in the Development of Complexes and Organocatalysts. Tetrahedron 2022, 121, 132913. [Google Scholar] [CrossRef]
- Salama, A.; Mahmoud, H.A.-A.; Kandeil, M.A.; Khalaf, M.M. Neuroprotective Role of Camphor against Ciprofloxacin Induced Depression in Rats: Modulation of Nrf-2 and TLR4. Immunopharmacol. Immunotoxicol. 2021, 43, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Ofori, D.; Reichmuth, C.H.; Bekele, A.J.; Hassanali, A. Toxicity and Protectant Potential of Camphor, a Major Component of Essential Oil of Ocimum Kilimandscharicum, against Four Stored Product Beetles. Int. J. Pest Manag. 1998, 44, 203–209. [Google Scholar] [CrossRef]
- Xie, F.; Chai, J.; Hu, Q.; Yu, Y.; Ma, L.; Liu, L.; Zhang, X.; Li, B.; Zhang, D. Transdermal Permeation of Drugs with Differing Lipophilicity: Effect of Penetration Enhancer Camphor. Int. J. Pharm. 2016, 507, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.; Sarkar, S.; Dasgupta, M.K.; Das, A. Camphor Poisoning: An Unusual Cause of Seizure in Children. J. Pediatr. Neurosci. 2015, 10, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Khine, H.; Weiss, D.; Graber, N.; Hoffman, R.S.; Esteban-Cruciani, N.; Avner, J.R. A Cluster of Children With Seizures Caused by Camphor Poisoning. Pediatrics 2009, 123, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, A.N.; Var, C.; Grossman, F.; Oberhelman, R.A. Use of Camphor and Essential Oil Balms for Infants in Cambodia. J. Trop. Pediatr. 2017, 63, 65–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hachlafi, N.E.; Aanniz, T.; Menyiy, N.E.; Baaboua, A.E.; Omari, N.E.; Balahbib, A.; Shariati, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Bouyahya, A. In Vitro and in Vivo Biological Investigations of Camphene and Its Mechanism Insights: A Review. Food Rev. Int. 2023, 39, 1799–1826. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant Derived Antioxidants—Geraniol and Camphene Protect Rat Alveolar Macrophages against t-BHP Induced Oxidative Stress. Toxicol. In Vitro 2009, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.; Moreira, J.C.F.; Pasquali, M.A.B.; Rabie, S.M.S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.A.; Lima, P.S.S.; Cavalcanti, S.C.H.; et al. Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in Experimental Models. ISRN Toxicol. 2013, 2013, 459530. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Kim, J.; Moon, B.S.; Park, S.M.; Jung, D.E.; Kang, S.Y.; Lee, S.J.; Oh, S.J.; Kwon, S.H.; Nam, M.H.; et al. Camphene Attenuates Skeletal Muscle Atrophy by Regulating Oxidative Stress and Lipid Metabolism in Rats. Nutrients 2020, 12, 3731. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, I.; Hadzopoulou-Cladaras, M. Camphene, a Plant Derived Monoterpene, Exerts Its Hypolipidemic Action by Affecting SREBP-1 and MTP Expression. PLoS ONE 2016, 11, e0147117. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a Plant-Derived Monoterpene, Reduces Plasma Cholesterol and Triglycerides in Hyperlipidemic Rats Independently of HMG-CoA Reductase Activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef] [PubMed]
- Gadotti, V.M.; Huang, S.; Zamponi, G.W. The Terpenes Camphene and Alpha-Bisabolol Inhibit Inflammatory and Neuropathic Pain via Cav3.2 T-Type Calcium Channels. Mol. Brain 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological Activity of the Essential Oil of Kadsura longipedunculata (Schisandraceae) and Its Major Components. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.A.; Matsuo, A.L.; Travassos, L.R.; et al. Camphene Isolated from Essential Oil of Piper Cernuum (Piperaceae) Induces Intrinsic Apoptosis in Melanoma Cells and Displays Antitumor Activity in Vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef]
- Kim, S.; Choi, Y.; Choi, S.; Choi, Y.; Park, T. Dietary Camphene Attenuates Hepatic Steatosis and Insulin Resistance in Mice: Camphene Prevents Hepatic Steatosis. Obesity 2014, 22, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Govindarajan, M.; Rajeswary, M.; Vaseeharan, B.; Alyahya, S.A.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Maggi, F. Insecticidal Activity of Camphene, Zerumbone and α-Humulene from Cheilocostus Speciosus Rhizome Essential Oil against the Old-World Bollworm, Helicoverpa Armigera. Ecotoxicol. Environ. Saf. 2018, 148, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Wang, Y.; Geng, Z.-F.; Zhang, D.; Almaz, B.; Du, S.-S. Contact Toxicity and Repellent Efficacy of Valerianaceae Spp. to Three Stored-Product Insects and Synergistic Interactions between Two Major Compounds Camphene and Bornyl Acetate. Ecotoxicol. Environ. Saf. 2020, 190, 110106. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Wang, Y.; Chen, Z.-Y.; Guo, S.-S.; You, C.-X.; Du, S.-S. Efficacy of Bornyl Acetate and Camphene from Valeriana Officinalis Essential Oil against Two Storage Insects. Environ. Sci. Pollut. Res. 2019, 26, 16157–16165. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.R.P.; Coelho, N.P.; Baldin, V.P.; Scodro, R.B.L.; Cardoso, R.F.; Da Silva, C.C.; Vandresen, F. Synthesis of Novel (-)-Camphene-Based Thiosemicarbazones and Evaluation of Anti-Mycobacterium tuberculosis Activity. Nat. Prod. Res. 2019, 33, 3372–3377. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, B.C.; Queiroz, P.A.; Baldin, V.P.; Do Amaral, P.H.; Rodrigues, L.L.; Vandresen, F.; R Caleffi-Ferracioli, K.; De L Scodro, R.B.; Cardoso, R.F.; Siqueira, V.L. (-)-Camphene-Based Derivatives as Potential Antibacterial Agents against Staphylococcus aureus and Enterococcus Spp. Future Microbiol. 2020, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Putilova, V.P.; Yarovaya, O.I.; Zybkina, A.V.; Mordvinova, E.D.; Zaykovskaya, A.V.; Shcherbakov, D.N.; Orshanskaya, I.R.; Sinegubova, E.O.; Esaulkova, I.L.; et al. Synthesis and Antiviral Activity of Camphene Derivatives against Different Types of Viruses. Molecules 2021, 26, 2235. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Maresca, V.; Varcamonti, M.; Bruno, M.; Badalamenti, N.; Basile, A.; Zanfardino, A. (+)-(E)-Chrysanthenyl Acetate: A Molecule with Interesting Biological Properties Contained in the Anthemis secundiramea (Asteraceae) Flowers. Appl. Sci. 2020, 10, 6808. [Google Scholar] [CrossRef]
- Raal, A.; Orav, A.; Gretchushnikova, T. Essential Oil Content and Composition in Tanacetum Vulgare L. Herbs Growing Wild in Estonia. J. Essent. Oil Bear. Plants 2014, 17, 670–675. [Google Scholar] [CrossRef]
- Dajić Stevanović, Z.P.; Nastovski, T.L.; Ristić, M.S.; Radanović, D.S. Variability of Essential Oil Composition of Cultivated Feverfew (Tanacetum parthenium (L.) Schultz Bip.) Populations. J. Essent. Oil Res. 2009, 21, 292–294. [Google Scholar] [CrossRef]
- Javidnia, K.; Miri, R.; Soltani, M.; Khosravi, A.R. Composition of the Essential Oil of Tanacetum polycephalum Schultz Bip. Subsp. Farsicum Podl. from Iran. J. Essent. Oil Res. 2008, 20, 209–211. [Google Scholar] [CrossRef]
- Habibi, Z.; Yousefi, M.; Shahriari, F.; Khalafi, J.; As’habi, M.A. Chemical Composition of the Essential Oil of Tanacetum turcomanicum and T. canescens from Iran. Chem. Nat. Compd. 2009, 45, 93–95. [Google Scholar] [CrossRef]
- El-Shazly, A.; Dorai, G.; Wink, M. Composition and Antimicrobial Activity of Essential Oil and HexaneÐEther Extract of Tanacetum santolinoides (DC.) Feinbr. and Fertig. Z. Naturforschung C 2002, 57, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Darriet, F.; Desjobert, J.; Costa, J.; Muselli, A. Identification of Chrysanthenyl Esters from the Essential Oil of Anthemis maritima L. Investigated by GC/RI, GC-MS (EI and CI) and 13C-NMR Spectroscopy: Chemical Composition and Variability. Phytochem. Anal. 2009, 20, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Cioni, P.L.; Morelli, I. Composition of the Essential Oils and in Vivo Emission of Volatiles of Four Lamium Species from Italy: L. purpureum, L. hybridum, L. bifidum and L. amplexicaule. Food Chem. 2005, 91, 63–68. [Google Scholar] [CrossRef]
- Flynn, T.M.; Southwell, I.A. Essential Oil Constituents of the Genus Zieria. Phytochemistry 1987, 26, 1673–1686. [Google Scholar] [CrossRef]
- Casiglia, S.; Bruno, M.; Senatore, F.; Senatore, F. Composition of the Essential Oil of Allium neapolitanum Cirillo Growing Wild in Sicily and Its Activity on Microorganisms Affecting Historical Art Crafts. J. Oleo Sci. 2015, 64, 1315–1320. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Simsek, D.; Bostanlik, F.D.; Özbek, H.; Kiliç, C.S.; Güvenalp, Z.; Demirci, B.; Altanlar, N. Antioxidant, Antimicrobial and Anticholinesterase Activities of Ferulago pauciradiata Boiss. & Heldr. Growing in Turkey. J. Biol. Act. Prod. Nat. 2018, 8, 364–375. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Omer, E.A.; Dar, B.A.; Al-Taisan, W.A.; Elshamy, A.I. Essential Oil Enriched with Oxygenated Constituents from Invasive Plant Argemone Ochroleuca Exhibited Potent Phytotoxic Effects. Plants 2020, 9, 998. [Google Scholar] [CrossRef]
- HekmatSorush, I.; Kalkhorani, N.M.; Rezaee, M.B.; HeroAbadi, F.; Hamisi, M. Phytochemical Analysis of Essential Oil of Tanacetum parthenium L. with Hydro-Distillation and Steam Distillation. J. Med. Plants Prod. 2014, 1, 53–57. [Google Scholar]
- Pålsson, K.; Jaenson, T.G.T.; Bæckström, P.; Borg-Karlson, A.-K. Tick Repellent Substances in the Essential Oil of Tanacetum vulgare. J. Med. Entomol. 2008, 45, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Soleimani-Ahmadi, M.; Sanei-Dehkordi, A.; Turki, H.; Madani, A.; Abadi, Y.S.; Paksa, A.; Gorouhi, M.A.; Rashid, G. Phytochemical Properties and Insecticidal Potential of Volatile Oils from Tanacetum persicum and Achillea kellalensis against Two Medically Important Mosquitoes. J. Essent. Oil Bear. Plants 2017, 20, 1254–1265. [Google Scholar] [CrossRef]
- Nishino, C.; Takayanagi, H. Sex Pheromonal Activity of (+)-Trans-Verbenyl Acetate and Related Compounds to the American Cockroach, Periplaneta Americana L. J. Chem. Ecol. 1981, 7, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, Y.; Ruan, X. Bornyl Acetate: A Promising Agent in Phytomedicine for Inflammation and Immune Modulation. Phytomedicine 2023, 114, 154781. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, J.; Li, Y.; Qi, G. Bornyl Acetate Suppresses Ox-LDL-Induced Attachment of THP-1 Monocytes to Endothelial Cells. Biomed. Pharmacother. 2018, 103, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, R.; Chen, H.; Jia, P.; Bao, L.; Tang, H. Bornyl Acetate Has an Anti-inflammatory Effect in Human Chondrocytes via Induction of IL-11. IUBMB Life 2014, 66, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-I.; Choi, J.-H.; Kwon, T.-W.; Jo, H.-S.; Kim, D.-G.; Ko, S.-G.; Song, G.J.; Cho, I.-H. Neuroprotective Effects of Bornyl Acetate on Experimental Autoimmune Encephalomyelitis via Anti-Inflammatory Effects and Maintaining Blood-Brain-Barrier Integrity. Phytomedicine 2023, 112, 154569. [Google Scholar] [CrossRef] [PubMed]
- Alipour, H.-R.; Yaghmaei, P.; Ahmadian, S.; Ghobeh, M.; Ebrahim-Habibi, A. The Effect of Bornyl Acetate on Male Alzheimer’s Disease Wistar Rats and In Vitro Amyloid Fibrils and Investigating the Immobility Stress. J. Anim. Biol. 2022, 15, 205–220. [Google Scholar] [CrossRef]
- Song, H.-J.; Yong, S.-H.; Kim, H.-G.; Kim, D.-H.; Park, K.-B.; Shin, K.-C.; Choi, M.-S. Insecticidal Activity against Myzus Persicae of Terpinyl Acetate and Bornyl Acetate in Thuja Occidentalis Essential Oil. Horticulturae 2022, 8, 969. [Google Scholar] [CrossRef]
- Matsubara, E.; Fukagawa, M.; Okamoto, T.; Ohnuki, K.; Shimizu, K.; Kondo, R. (-)-Bornyl Acetate Induces Autonomic Relaxation and Reduces Arousal Level after Visual Display Terminal Work without Any Influences of Task Performance in Low-Dose Condition. Biomed. Res. 2011, 32, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-Y.; Ki, Y.-W.; Na, G.-M.; Kang, M.-J.; Kim, B.-C.; Kim, O.-M.; Hong, S.-P. Influence of Bornyl Acetate on Blood Pressure and Aortic Strips Contractility of the Rat. Biomol. Ther. 2003, 11, 119–125. [Google Scholar]
- Wu, X.; Li, X.; Xiao, F.; Zhang, Z.; Xu, Z.; Wang, H. Studies on the analgesic and anti-inflammatory effect of bornyl acetate in volatile oil from Amomum villosum. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2004, 27, 438–439. [Google Scholar]
- Wu, X.; Xiao, F.; Zhang, Z.; Li, X.; Xu, Z. Research on the analgesic effect and mechanism of bornyl acetate in volatile oil from Amomum villosum. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2005, 28, 505–507. [Google Scholar]
- Li, X.; Duan, Z.; Yue, J.; Zhang, Y.; Li, Y.; Liu, S.; Nie, Q.; Yang, D.; Zhang, L. Bornyl Acetate Extracted from Sharen (Fructus Amomi) Inhibits Proliferation, Invasion and Induces Apoptosis by Suppressing Phosphatidylinositol-3-Kinase/Protein Kinase B Signaling in Colorectal Cancer. J. Tradit. Chin. Med. 2023, 43, 1081–1091. [Google Scholar] [CrossRef]
- Karan, T.; Yildiz, I.; Aydin, A.; Erenler, R. Inhibition of Various Cancer Cells Proliferation of Bornyl Acetate and Essential Oil from Inula graveolens (Linnaeus) Desf. Rec. Nat. Prod. 2018, 12, 273–283. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Wang, X.D.; Shi, W.Y.; Zhong, X.H. Anti-Abortive Effect of Quercetin and Bornyl Acetate on Macrophages and IL-10 in Uterus of Mice. Afr. J. Biotechnol. 2011, 10, 8675–8682. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, Phytotoxic and Insecticidal Properties of Essential Oil Isolated from Turkish Origanum Acutidens and Its Three Components, Carvacrol, Thymol and p-Cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.F.; Quintans-Júnior, L.J.; Cavalcanti, S.C.H.; Oliveira, M.G.B.; Guimarães, A.G.; Cunha, E.S.; Melo, M.S.; Santos, M.R.V.; Araújo, A.A.S.; Bonjardim, L.R. P-Cymene Reduces Orofacial Nociceptive Response in Mice. Rev. Bras. Farmacogn. 2011, 21, 1138–1143. [Google Scholar] [CrossRef]
- Xie, G.; Chen, N.; Soromou, L.W.; Liu, F.; Xiong, Y.; Wu, Q.; Li, H.; Feng, H.; Liu, G. P-Cymene Protects Mice Against Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Inflammatory Cell Activation. Molecules 2012, 17, 8159–8173. [Google Scholar] [CrossRef] [PubMed]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health Beneficial and Pharmacological Properties of P-Cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- De Santana, M.F.; Guimarães, A.G.; Chaves, D.O.; Silva, J.C.; Bonjardim, L.R.; Lucca Júnior, W.D.; Ferro, J.N.D.S.; Barreto, E.D.O.; Santos, F.E.D.; Soares, M.B.P.; et al. The Anti-Hyperalgesic and Anti-Inflammatory Profiles of p-Cymene: Evidence for the Involvement of Opioid System and Cytokines. Pharm. Biol. 2015, 53, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- De Souza Siqueira Quintans, J.; Menezes, P.P.; Santos, M.R.V.; Bonjardim, L.R.; Almeida, J.R.G.S.; Gelain, D.P.; Araújo, A.A.D.S.; Quintans-Júnior, L.J. Improvement of P-Cymene Antinociceptive and Anti-Inflammatory Effects by Inclusion in β-Cyclodextrin. Phytomedicine 2013, 20, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, O.; Khatib, A.; Juhari, H.; Patiram, P.; Lasekan, S. Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry Determination of Volatile Compounds in Different Varieties of African Star Apple Fruit (Chrysophillum albidum). Food Chem. 2013, 141, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Pal Singh, H.; Kaur, S.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxicity of Major Constituents of the Volatile Oil from Leaves of Artemisia scoparia Waldst. & Kit. Z. Naturforschung C 2008, 63, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Bonjardim, L.R.; Cunha, E.S.; Guimarães, A.G.; Santana, M.F.; Oliveira, M.G.B.; Serafini, M.R.; Araújo, A.A.S.; Antoniolli, Â.R.; Cavalcanti, S.C.H.; Santos, M.R.V.; et al. Evaluation of the Anti-Inflammatory and Antinociceptive Properties of p-Cymene in Mice. Z. Naturforschung C 2012, 67, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Chi, G.; Jiang, L.; Soromou, L.W.; Chen, N.; Huo, M.; Guo, W.; Deng, X.; Feng, H. P-Cymene Modulates In Vitro and In Vivo Cytokine Production by Inhibiting MAPK and NF-κB Activation. Inflammation 2013, 36, 529–537. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.M.; De Carvalho, R.B.F.; Da Costa, I.H.F.; De Oliveira, G.A.L.; De Souza, A.A.; De Lima, S.G.; De Freitas, R.M. Evaluation of p -Cymene, a Natural Antioxidant. Pharm. Biol. 2015, 53, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Shareef, S.H.; Al-Medhtiy, M.H.; Ibrahim, I.A.A.; Alzahrani, A.R.; Jabbar, A.A.; Galali, Y.; Agha, N.F.S.; Aziz, P.Y.; Thabit, M.A.; Agha, D.N.F.; et al. Gastroprophylactic Effects of P-Cymene in Ethanol-Induced Gastric Ulcer in Rats. Processes 2022, 10, 1314. [Google Scholar] [CrossRef]
- Kazemi, S.; Safari, S.; Komaki, S.; Karimi, S.A.; Golipoor, Z.; Komaki, A. The Effects of Carvacrol and P-cymene on Aβ 1-42 -induced Long-term Potentiation Deficit in Male Rats. CNS Neurosci. Ther. 2023, 30, e14459. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.L.; Santos, J.H.; Cunha, P.S.; Quintans-Júnior, L.J.; Bonjardim, L.R.; De Souza Araújo, A.A. Vasorelaxant Activity of P-cymene in Superior Mesenteric Artery of Rats. FASEB J. 2013, 27, lb599. [Google Scholar] [CrossRef]
- Silva, M.T.M.; Ribeiro, F.P.R.A.; Medeiros, M.A.M.B.; Sampaio, P.A.; Silva, Y.M.S.; Silva, M.T.A.; Quintans, J.S.S.; Quintans-Júnior, L.J.; Ribeiro, L.A.A. The Vasorelaxant Effect of p -Cymene in Rat Aorta Involves Potassium Channels. Sci. World J. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pujante-Galián, M.A.; Pérez, S.A.; Montalbán, M.G.; Carissimi, G.; Fuster, M.G.; Víllora, G.; García, G. P-Cymene Complexes of Ruthenium(II) as Antitumor Agents. Molecules 2020, 25, 5063. [Google Scholar] [CrossRef] [PubMed]
- Păunescu, E.; Nowak-Sliwinska, P.; Clavel, C.M.; Scopelliti, R.; Griffioen, A.W.; Dyson, P.J. Anticancer Organometallic Osmium(II)-p-cymene Complexes. ChemMedChem 2015, 10, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Kiskó, G.; Roller, S. Carvacrol and P-Cymene Inactivate Escherichia Coli O157:H7 in Apple Juice. BMC Microbiol. 2005, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Arciola, C.; Barbieri, R.; Silva, A.; Nabavi, S.; Tsetegho Sokeng, A.; Izadi, M.; Jafari, N.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Nurzyńska-Wierdak, R.; Sałata, A.; Kniaziewicz, M. Tansy (Tanacetum vulgare L.)—A Wild-Growing Aromatic Medicinal Plant with a Variable Essential Oil Composition. Agronomy 2022, 12, 277. [Google Scholar] [CrossRef]
- Estell, R.E.; Fredrickson, E.L.; James, D.K. Effect of Light Intensity and Wavelength on Concentration of Plant Secondary Metabolites in the Leaves of Flourensia cernua. Biochem. Syst. Ecol. 2016, 65, 108–114. [Google Scholar] [CrossRef]
- Goyal, S.; Pathak, R.; Pandey, H.K.; Kumari, A.; Tewari, G.; Bhandari, N.S.; Bala, M. Comparative Study of the Volatile Constituents of Thymus serpyllum L. Grown at Different Altitudes of Western Himalayas. SN Appl. Sci. 2020, 2, 1208. [Google Scholar] [CrossRef]
- Shafaghat, A.; Ghorban-Dadras, O.; Mohammadhosseini, M.; Akhavan, M.; Shafaghatlonbar, M.; Panahi, A. A Comparative Study on Chemical Composition and Antimicrobial Activity of Essential Oils from Tanacetum parthenium (L.) Schultz. Bip. and Tanacetum punctatum (Desr.) Grierson. Leaves from Iran. J. Essent. Oil Bear. Plants 2017, 20, 1143–1150. [Google Scholar] [CrossRef]
- Mirjalili, M.H.; Salehi, P.; Sonboli, A.; Mohammadi Vala, M. Essential Oil Composition of Feverfew (Tanacetum parthenium) in Wild and Cultivated Populations from Iran. Chem. Nat. Compd. 2007, 43, 218–220. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Kabudani, M.; Tabibzadeh, Z. Effect of Drying Methods on the Essential Oil Content and Composition of Tanacetum parthenium (L.) Schultz Bip Cv. Zardband. J. Essent. Oil Bear. Plants 2007, 10, 26–30. [Google Scholar] [CrossRef]
- Giuliani, C.; Bottoni, M.; Milani, F.; Spada, A.; Falsini, S.; Papini, A.; Santagostini, L.; Fico, G. An Integrative Approach to Selected Species of Tanacetum L. (Asteraceae): Insights into Morphology and Phytochemistry. Plants 2024, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Végh, K.; Riethmüller, E.; Tóth, A.; Alberti, Á.; Béni, S.; Balla, J.; Kéry, Á. Convergence Chromatographic Determination of Camphor in the Essential Oil of Tanacetum parthenium L. Biomed. Chromatogr. 2016, 30, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.Y.W.; Chong, C.H.; Chua, B.L.; Figiel, A. Influence of Drying Methods on the Antibacterial, Antioxidant and Essential Oil Volatile Composition of Herbs: A Review. Food Bioprocess Technol. 2019, 12, 450–476. [Google Scholar] [CrossRef]
- Shahhoseini, R.; Daneshvar, H. Phytochemical and Physiological Reactions of Feverfew (Tanacetum parthenium (L.) Schultz Bip) to TiO2 Nanoparticles. Plant Physiol. Biochem. 2023, 194, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Leal Filho, W.; Azeiteiro, U.M.; Setti, A.F.F. (Eds.) Sustainability in Natural Resources Management and Land Planning; World Sustainability Series; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-76623-8. [Google Scholar]
- Council of Europe. European Pharmacopoeia; Council of Europe: Strasburg, France, 2019. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).