Rose Bengal Conjugated to Lectins for Targeted Antibacterial Photodynamic Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Microscopic Analysis of ConA and WGA Binding with Gram-Positive and Gram-Negative Bacteria
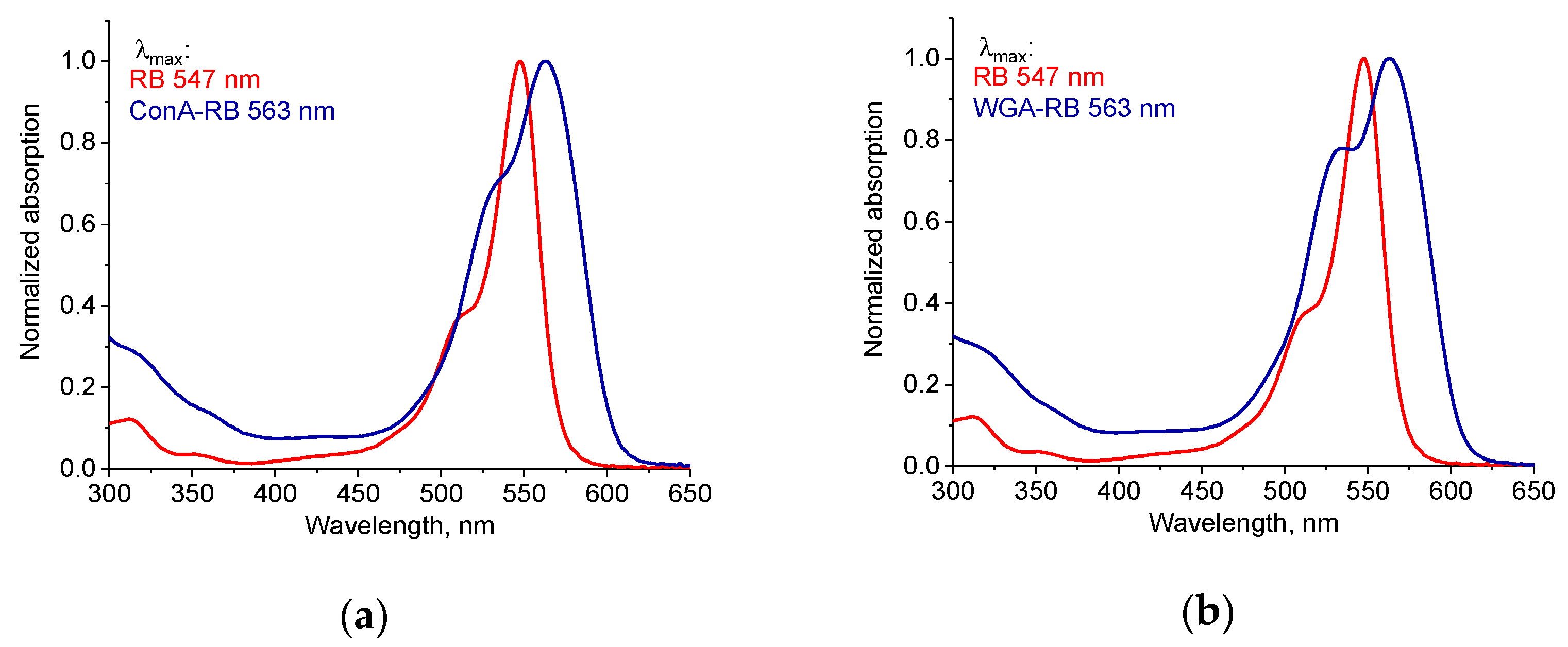
2.2. Synthesis and Characterization of Lectin–RB Conjugates
2.3. Photodynamic Activity of RB Conjugated with Lectins
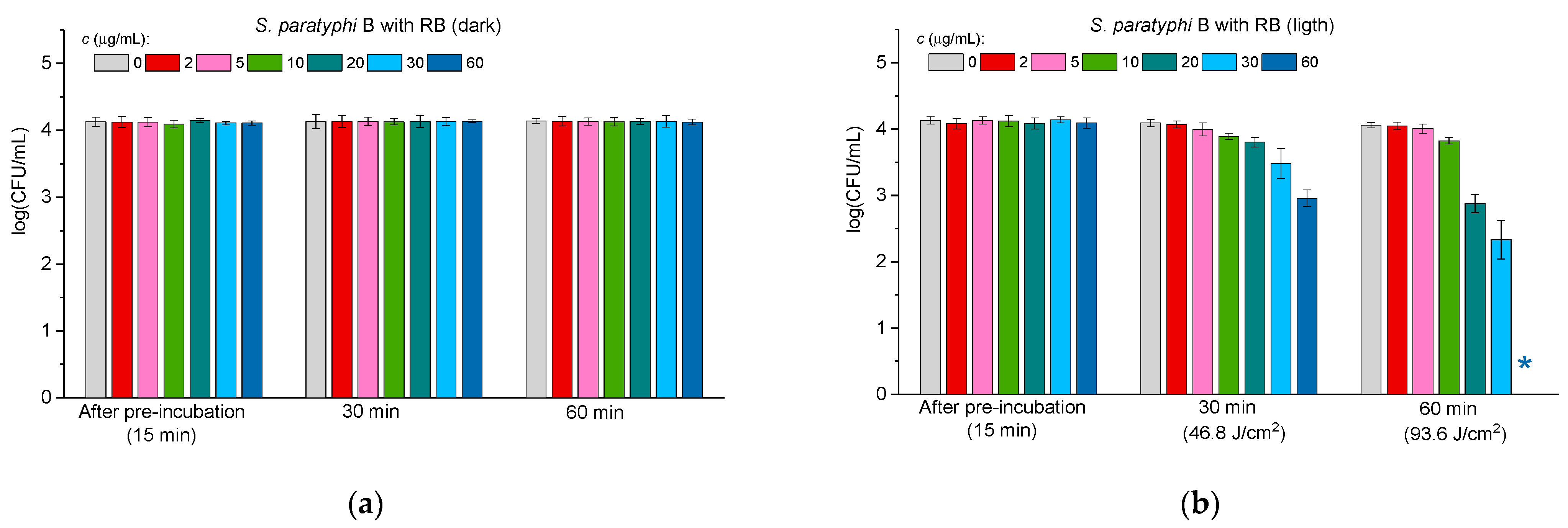
2.3.1. Antibacterial Activity of Lectin–RB Conjugates Against Gram-Positive Bacteria
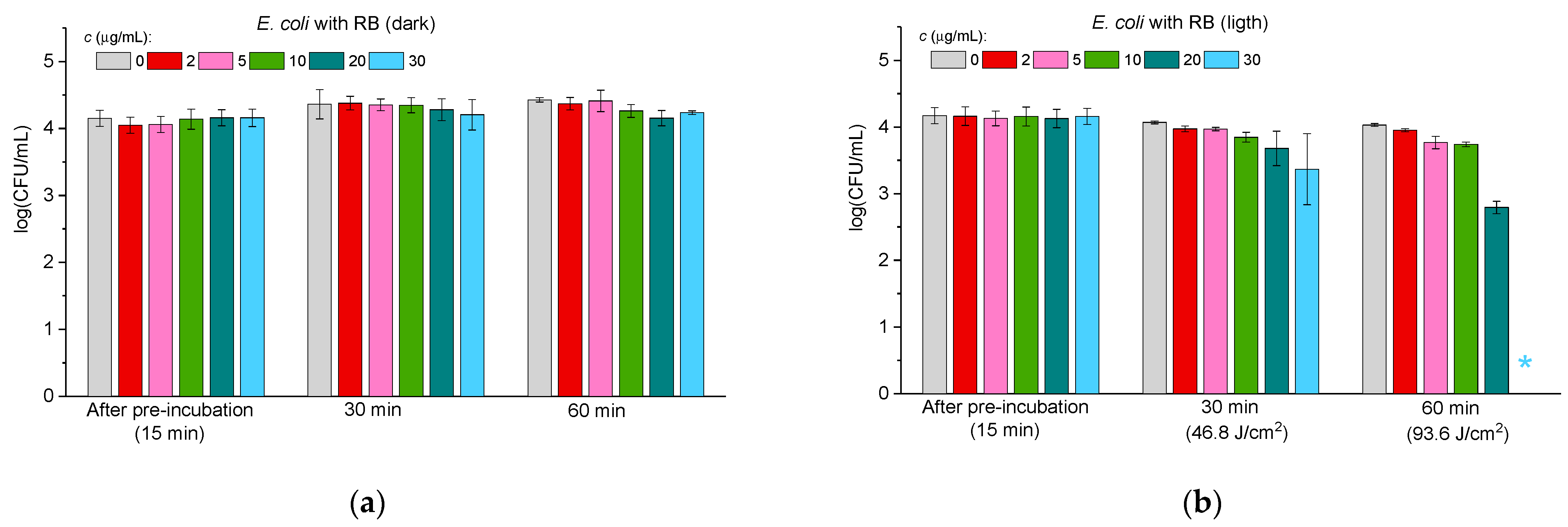
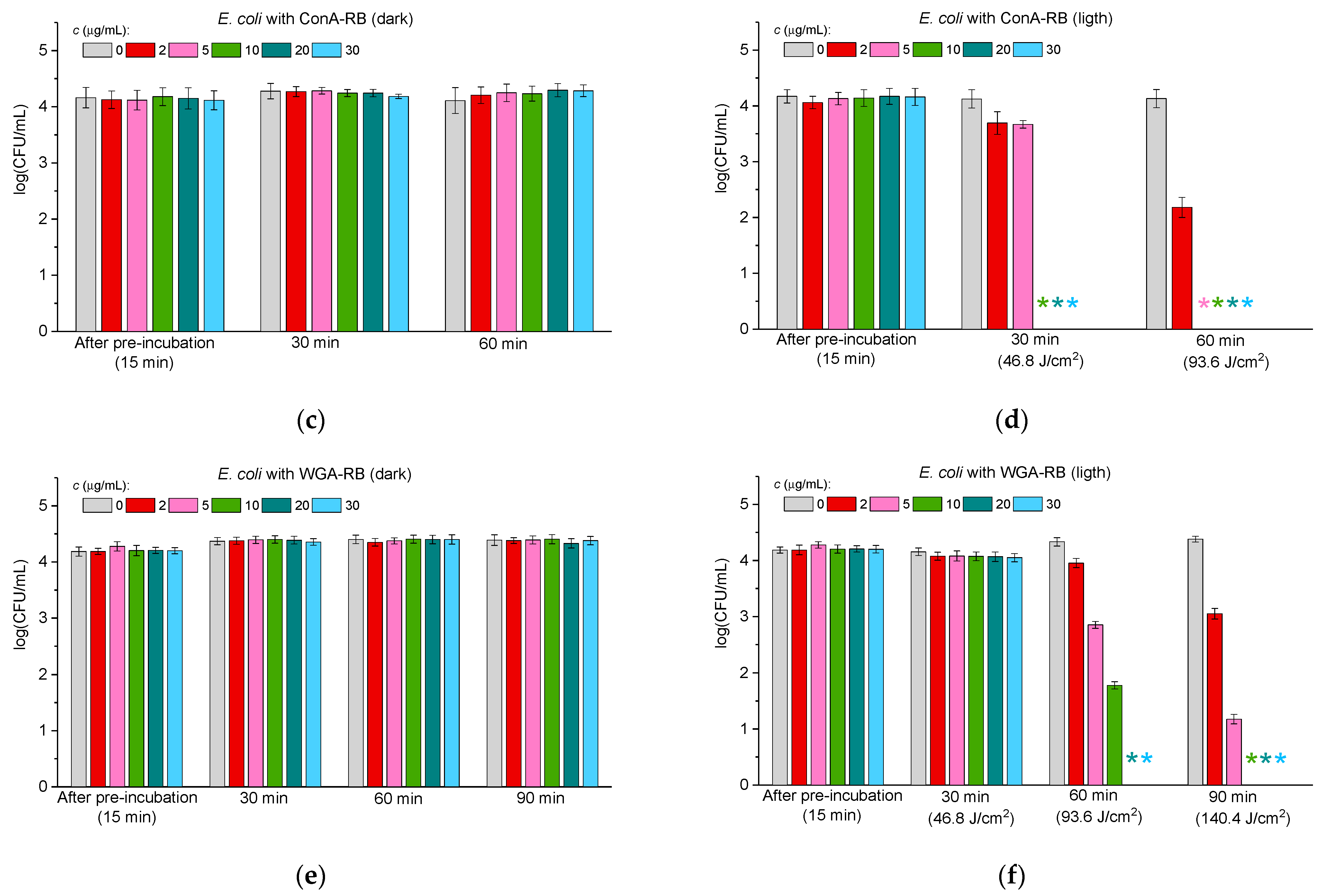
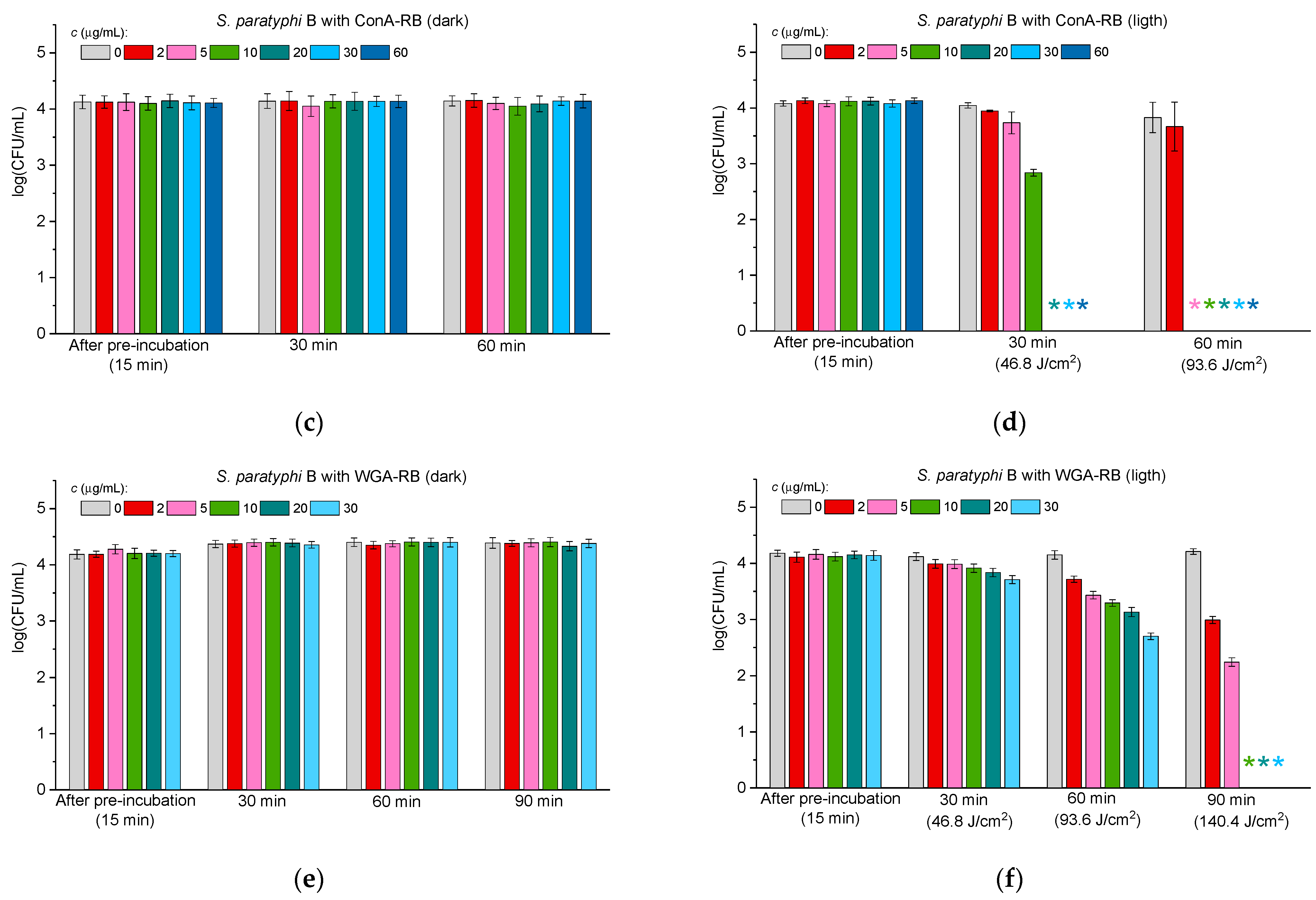
2.3.2. Antibacterial Activity Against Gram-Negative Bacteria
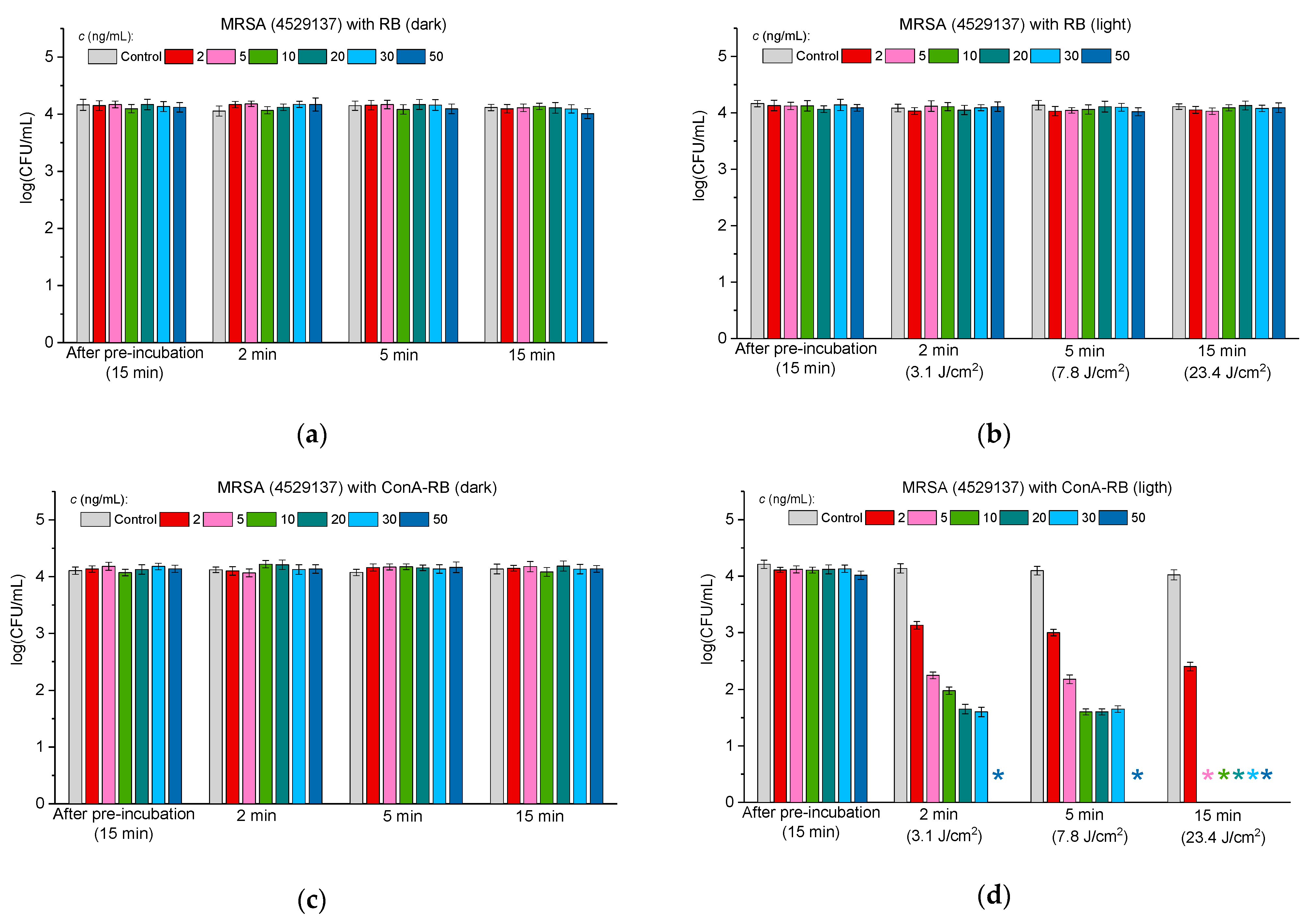
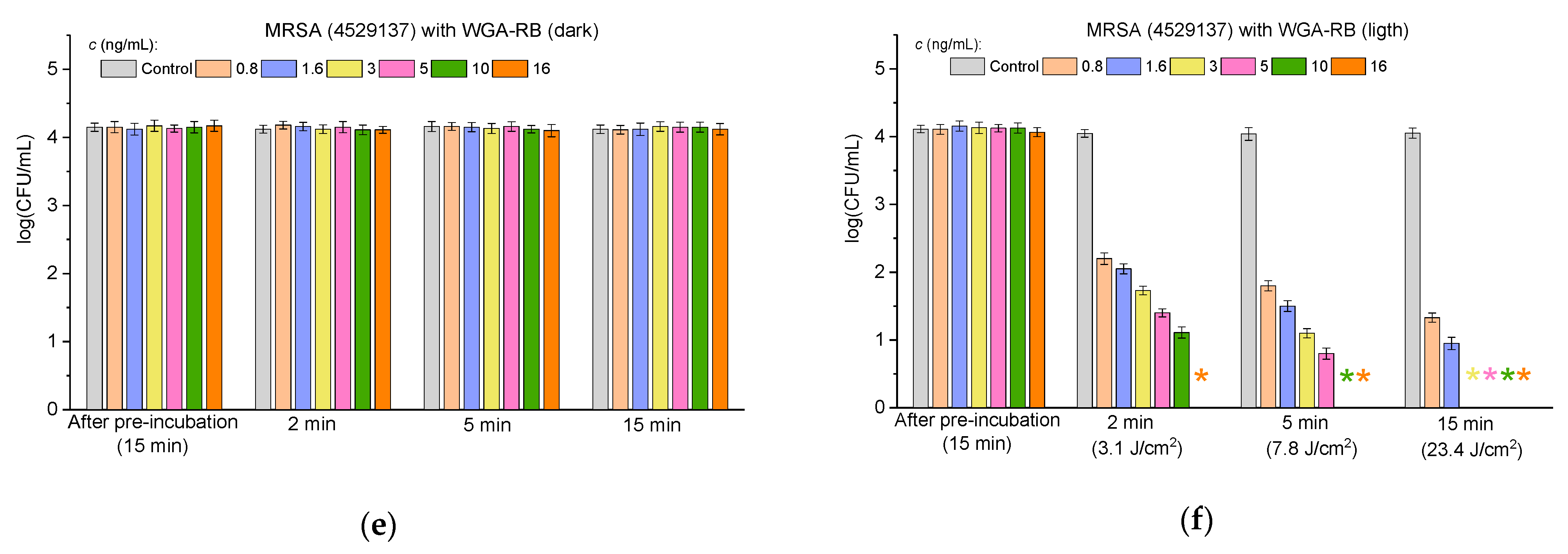
2.3.3. Antibacterial Activity Against Multidrug-Resistant Bacteria
3. Materials and Methods
3.1. Materials
3.2. Bacterial Growth
3.3. Agglutination Study
3.4. Synthesis and Characterization of Lectin–RB Conjugates
3.5. Determination of Rose Bengal to Lectin Ratios in the Conjugates
3.6. Antimicrobial Activity Test
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peleg, A.Y.; Hooper, D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010, 362, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.-P.; Chen, Y.-C.; Chen, S.-Y.; Chang, S.-C. Risk for subsequent infection and mortality after hospitalization among patients with multidrug-resistant gram-negative bacteria colonization or infection. Antimicrob. Resist. Infect. Control 2018, 7, 93. [Google Scholar] [CrossRef]
- Nelson, R.E.; Slayton, R.B.; Stevens, V.W.; Jones, M.M.; Khader, K.; Rubin, M.A.; Jernigan, J.A.; Samore, M.H. Attributable Mortality of Healthcare-Associated Infections Due to Multidrug-Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 2017, 38, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Bakaletz, L.O. Viral–bacterial co-infections in the respiratory tract. Curr. Opin. Microbiol. 2017, 35, 30–35. [Google Scholar] [CrossRef]
- Grassi, G.G. Infections by Gram-positive bacteria: An overview. J. Antimicrob. Chemother. 1988, 21 (Suppl. C), 1–7. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of antibiotic resistance mechanisms in gram-negative bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef]
- Giamarellou, H. Multidrug-resistant Gram-negative bacteria: How to treat and for how long. Int. J. Antimicrob. Agents 2010, 36, S50–S54. [Google Scholar] [CrossRef]
- Mehrad, B.; Clark, N.M.; Zhanel, G.G.; Lynch, J.P., III. Antimicrobial resistance in hospital-acquired gram-negative bacterial infections. Chest 2015, 147, 1413–1421. [Google Scholar] [CrossRef]
- Badger-Emeka, L.; Al Rashed, A.S.; Aljindan, R.Y.; Emeka, P.M.; Quadri, S.A.; Almutairi, H.H. Incidence of drug-resistant hospital-associated Gram-negative bacterial infections, the accompanying risk factors, and clinical outcomes with treatment. Antibiotics 2023, 12, 1425. [Google Scholar] [CrossRef]
- Watkins, R.R.; Bonomo, R.A. Overview: Global and local impact of antibiotic resistance. Infect. Dis. Clin. 2016, 30, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.L.; Fajardo, A.; Garmendia, L.; Hernandez, A.; Linares, J.F.; Martínez-Solano, L.; Sánchez, M.B. A global view of antibiotic resistance. FEMS Microbiol. Rev. 2008, 33, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Pallecchi, L.; Bartoloni, A.; Paradisi, F.; Rossolini, G.M. Antibiotic resistance in the absence of antimicrobial use: Mechanisms and implications. Expert. Rev. Anti-Infect. Ther. 2008, 6, 725–732. [Google Scholar] [CrossRef]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef]
- Acar, J.; Rostel, B. Antimicrobial resistance: An overview. Rev. Sci. Tech. 2001, 20, 797–810. [Google Scholar] [CrossRef]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Allen, H.K. Alternatives to antibiotics: Why and how. NAM Perspect. 2017, 7, 1–4. [Google Scholar] [CrossRef]
- Singha, B.; Singh, V.; Soni, V. Alternative therapeutics to control antimicrobial resistance: A general perspective. Front. Drug Discov. 2024, 4, 1385460. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- MacNair, C.R.; Rutherford, S.T.; Tan, M.W. Alternative therapeutic strategies to treat antibiotic-resistant pathogens. Nat. Rev. Microbiol. 2024, 22, 262–275. [Google Scholar] [CrossRef]
- Wainwright, M. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Anas, A.; Sobhanan, J.; Sulfiya, K.M.; Jasmin, C.; Sreelakshmi, P.K.; Biju, V. Advances in photodynamic antimicrobial chemotherapy. J. Photochem. Photobiol. C Photochem. Rev. 2021, 49, 100452. [Google Scholar] [CrossRef]
- Lanka, S.; Sharif, S.H.; Pandrangi, S.L. Photodynamic Antimicrobial Chemotherapy: Advancements and Applications. In Antimicrobial Photodynamic Therapy; Busi, S., Prasad, R., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 185–202. [Google Scholar]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Neckers, D.C. Rose Bengal. J. Photochem. Photobiol. A Chem. 1989, 47, 1–29. [Google Scholar] [CrossRef]
- Nakonechny, F.; Barel, M.; David, A.; Koretz, S.; Litvak, B.; Ragozin, E.; Etinger, A.; Livne, O.; Pinhasi, Y.; Gellerman, G.; et al. Dark antibacterial activity of Rose Bengal. Int. J. Mol. Sci. 2019, 20, 3196. [Google Scholar] [CrossRef]
- Nakonechny, F.; Nisnevitch, M.; Nitzan, Y.; Nisnevitch, M. Sonodynamic excitation of Rose Bengal for eradication of Gram-positive and Gram-negative bacteria. BioMed Res. Int. 2013, 2013, 684930. [Google Scholar] [CrossRef]
- Kurosu, M.; Mitachi, K.; Yang, J.; Pershing, E.V.; Horowitz, B.D.; Wachter, E.A.; Lacey, J.W., III; Ji, Y.; Rodrigues, D.J. Antibacterial activity of pharmaceutical-grade Rose Bengal: An application of a synthetic dye in antibacterial therapies. Molecules 2022, 27, 322. [Google Scholar] [CrossRef]
- Johnson, A.; Wu, J.; Zhou, Z.; Li, Y.; Yin, Y.; Ponder, M.A.; Kim, Y.-T.; Shuai, D.; Huang, H. Efficacy of a Rose Bengal-Embedded Antimicrobial Packaging Film in Inactivating Escherichia coli under Visible Light Irradiation. ACS Food Sci. Technol. 2024, 4, 561–566. [Google Scholar] [CrossRef]
- Silva, T.; Lunardi, A.J.L.; Barros, A.C.S.M.; Mandetta, A.R.H.; Grudzien, E.; San-Martín, M.; Horliana, A.C.R.T.; Bussadori, S.K.; Motta, L.J. Application of Photodynamic Therapy in Pediatric Dentistry: Literature Review. Pharmaceutics 2023, 15, 2335. [Google Scholar] [CrossRef]
- Hirose, M.; Yoshida, Y.; Horii, K.; Hasegawa, Y.; Shibuya, Y. Efficacy of antimicrobial photodynamic therapy with Rose Bengal and blue light against cariogenic bacteria. Arch. Oral Biol. 2020, 122, 105024. [Google Scholar] [CrossRef]
- Naranjo, A.; Arboleda, A.; Martinez, J.D.; Durkee, H.; Aguilar, M.C.; Relhan, N.; Nikpoor, N.; Galor, A.; Dubovy, S.R.; Leblanc, R.; et al. Rose Bengal Photodynamic Antimicrobial Therapy for Patients with Progressive Infectious Keratitis: A Pilot Clinical Study. Am. J. Ophthalmol. 2019, 208, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Edelman, G.M.; Cunningham, B.A.; Reeke, G.N., Jr.; Becker, J.W.; Waxdal, M.J.; Wang, J.L. The covalent and three-dimensional structure of concanavalin A. Proc. Natl. Acad. Sci. USA 1972, 69, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Hardman, K.D.; Ainsworth, C.F. Structure of concanavalin A at 2.4-Ang resolution. Biochemistry 1972, 11, 4910–4919. [Google Scholar] [CrossRef]
- Chowdhury, T.K.; Weiss, A.K. (Eds.) Concanavalin A. Advances in Experimental Medicine and Biology; Plenum Press: New York, NY, USA; London, UK, 1975; Volume 55. [Google Scholar]
- Baintner, K.; Kocsis, B.; Kovács, K.; Péterfi, Z.; Kökény, G.; Hamar, P. Interaction of concanavalin a with bacterial lipopolysaccharides in agarose gel. Acta Microbiol. Immunol. Hung. 2011, 58, 201–209. [Google Scholar] [CrossRef]
- Doyle, R.J.; Birdsell, D.C. Interaction of concanavalin A with the cell wall of Bacillus subtilis. J. Bacteriol. 1972, 109, 652–658. [Google Scholar] [CrossRef]
- Lin, S.; Yang, L.; Chen, G.; Li, B.; Chen, D.; Li, L.; Xu, Z. Pathogenic features and characteristics of foodborne pathogens biofilm: Biomass, viability, and matrix. Microb. Pathog. 2017, 111, 285–291. [Google Scholar] [CrossRef]
- Yang, K.; Gitter, B.; Rüger, R.; Albrecht, V.; Wieland, G.D.; Fahr, A. Wheat germ agglutinin modified liposomes for the photodynamic inactivation of bacteria. Photochem. Photobiol. 2012, 88, 548–556. [Google Scholar] [CrossRef]
- Cantelli, A.; Piro, F.; Pecchini, P.; Di Giosia, M.; Danielli, A.; Calvaresi, M. Concanavalin A-Rose Bengal bioconjugate for targeted Gram-negative antimicrobial photodynamic therapy. J. Photochem. Photobiol. B Biol. 2020, 206, 111852. [Google Scholar] [CrossRef]
- Mussini, A.; Delcanale, P.; Berni, M.; Pongolini, S.; Jordà-Redondo, M.; Agut, M.; Steinbach, P.J.; Nonell, S.; Abbruzzetti, S.; Viappiani, C. Concanavalin A delivers a photoactive protein to the bacterial wall. Int. J. Mol. Sci. 2024, 25, 5751. [Google Scholar] [CrossRef]
- Payne, M.J.; Campbell, S.; Patchett, R.A.; Kroll, R.G. The use of immobilized lectins in the separation of Staphylococcus aureus, Escherichia coli, Listeria and Salmonella spp. from pure cultures and foods. J. Appl. Bacteriol. 1992, 73, 41–52. [Google Scholar] [CrossRef]
- Serra, B.; Gamella, M.; Reviejo, A.J.; Pingarrón, J.M. Lectin-modified piezoelectric biosensors for bacteria recognition and quantification. Anal. Bioanal. Chem. 2008, 391, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Lotan, R.; Sharon, N.; Mirelman, D. Interaction of wheat-germ agglutinin with bacterial cells and cell-wall polymers. Eur. J. Biochem. 1975, 55, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Park, K. Glucose-binding property of pegylated concanavalin A. Pharm. Res. 2001, 18, 794–799. [Google Scholar] [CrossRef]
- Nagata, Y.; Burger, M. Wheat Germ Agglutinin. J. Biol. Chem. 1974, 249, 3116–3122. [Google Scholar] [CrossRef]
- Haque, M.; Forte, N.; Baker, J.R. Site-selective lysine conjugation methods and applications towards antibody-drug conjugates. Chem. Commun. 2021, 14, 10689–10702. [Google Scholar] [CrossRef]
- Hovor, I.V.; Kolosova, O.S.; Sanin, E.V.; Obukhova, O.M.; Tatarets, A.L.; Terpetschnig, E.A.; Patsenker, L.D. Water-soluble norsquaraine dyes for protein labeling and pH-sensing applications. Dye. Pigment. 2019, 170, 107567. [Google Scholar] [CrossRef]
- Alarcon, E.I.; Poblete, H.; Roh, H.; Couture, J.F.; Comer, J.; Kochevar, I.E. Rose Bengal Binding to Collagen and Tissue Photobonding. ACS Omega 2017, 2, 6646–6657. [Google Scholar] [CrossRef]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Characterization of a Conjugate between Rose Bengal and Chitosan for Targeted Antibiofilm and Tissue Stabilization Effects as a Potential Treatment of Infected Dentin. Antimicrob. Agents Chemother. 2012, 56, 4876–4884. [Google Scholar] [CrossRef]
- Nisnevitch, M.; Nakonechny, F.; Nitzan, Y. Photodynamic antimicrobial chemotherapy by liposome-encapsulated water-soluble photosensitizers. Russ. J. Bioorganic Chem. 2010, 36, 363–369. [Google Scholar] [CrossRef]
- Dahl, T.A.; Midden, W.R.; Neckers, D.C. Comparison of photodynamic action by Rose Bengal in gram-positive and gram-negative bacteria. Photochem. Photobiol. 1988, 48, 607–612. [Google Scholar] [CrossRef]
- Greczek-Stachura, M.; Różanowski, B.; Kania, A. The effect of rose bengal activated with green diode laser light on selected Gram-positive and Gram-negative bacterial strains. Ann. Univ. Paedagog. Cracoviensis Stud. Naturae 2023, 8, 53–67. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Atrash, M.; Hovor, I.; Gurianov, Y.; Barel, M.; Semenova, O.; Brider, T.; Nisnevitch, M.; Nakonechny, F. Antibacterial properties of Rose Bengal conjugated to hyaluronic acid. Int. J. Mol. Sci. 2024, 25, 3330. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Cassidy, C.M.; Elborn, J.S.; Tunney, M.M. Delivery of photosensitizers and light through mucus: Investigations into the potential use of photodynamic therapy for the treatment of Pseudomonas aeruginosa cystic fibrosis pulmonary infection. J. Control. Release 2007, 117, 217–226. [Google Scholar] [CrossRef]
- Jori, G.; Camerin, M.; Soncin, M.; Guidolin, L.; Coppellotti, O. Photodynamic inactivation of microbial pathogens: Medical and environmental applications. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Applications; Hamblin, M.R., Jori, G., Eds.; RSC Publishing: London, UK, 2011; pp. 1–18. [Google Scholar]
- Hammond, J.B.; Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1988, 3, 25–32. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atrash, M.; Hovor, I.; Nisnevitch, M.; Nakonechny, F. Rose Bengal Conjugated to Lectins for Targeted Antibacterial Photodynamic Treatment. Molecules 2025, 30, 2381. https://doi.org/10.3390/molecules30112381
Atrash M, Hovor I, Nisnevitch M, Nakonechny F. Rose Bengal Conjugated to Lectins for Targeted Antibacterial Photodynamic Treatment. Molecules. 2025; 30(11):2381. https://doi.org/10.3390/molecules30112381
Chicago/Turabian StyleAtrash, Melad, Iryna Hovor, Marina Nisnevitch, and Faina Nakonechny. 2025. "Rose Bengal Conjugated to Lectins for Targeted Antibacterial Photodynamic Treatment" Molecules 30, no. 11: 2381. https://doi.org/10.3390/molecules30112381
APA StyleAtrash, M., Hovor, I., Nisnevitch, M., & Nakonechny, F. (2025). Rose Bengal Conjugated to Lectins for Targeted Antibacterial Photodynamic Treatment. Molecules, 30(11), 2381. https://doi.org/10.3390/molecules30112381






