Antioxidant Activity and Microbial Quality of Freeze-Dried, Lactic Acid Fermented Peach Products
Abstract
1. Introduction
2. Results and Discussion
2.1. Microbiology of Freeze-Dried Peach Samples
2.2. Physical and Chemical Properties of Freeze-Dried Peach Samples
2.3. Antioxidant Properties of Freeze-Dried Peach Samples
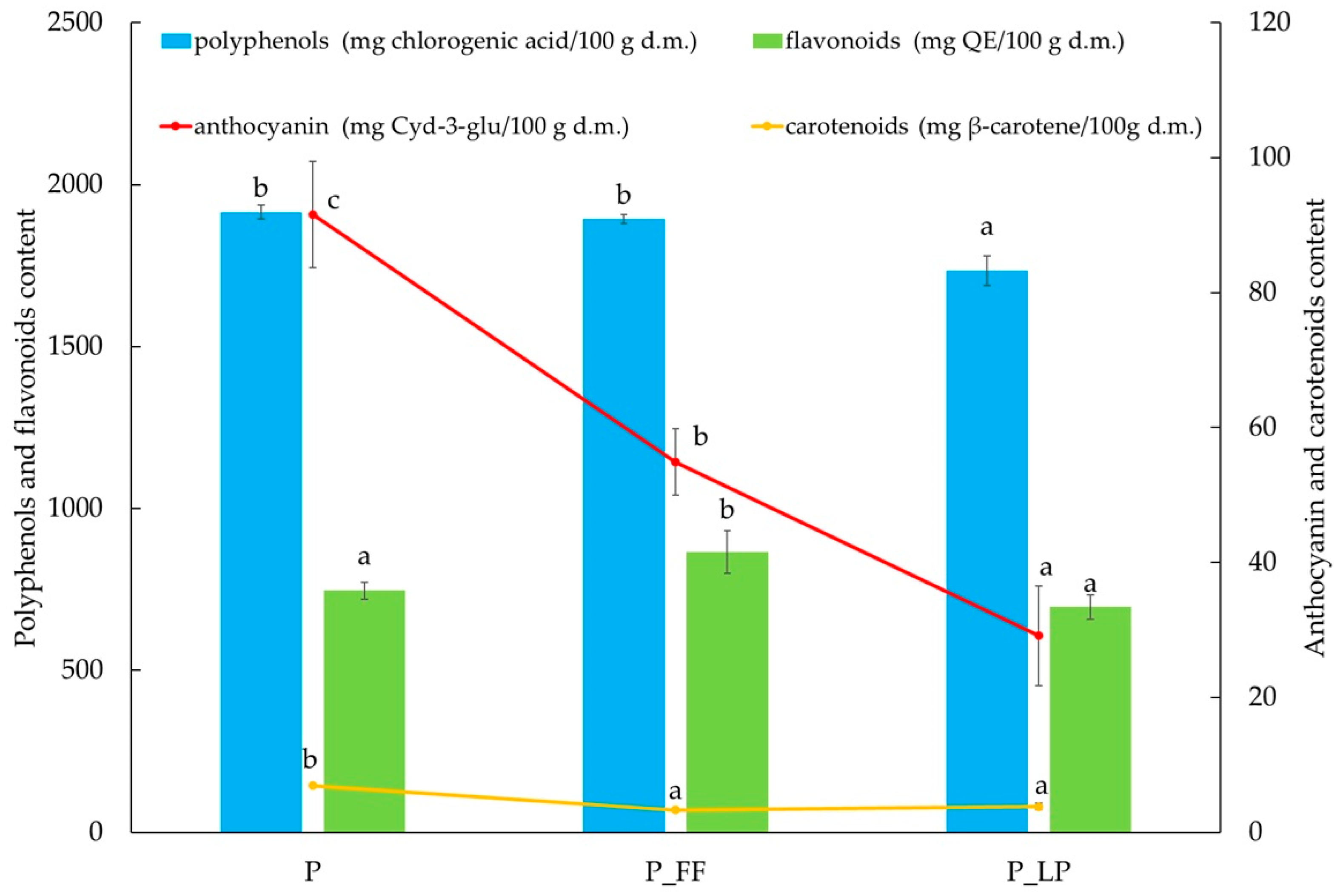
2.4. Active Substances Content in Freeze-Dried Peach Samples
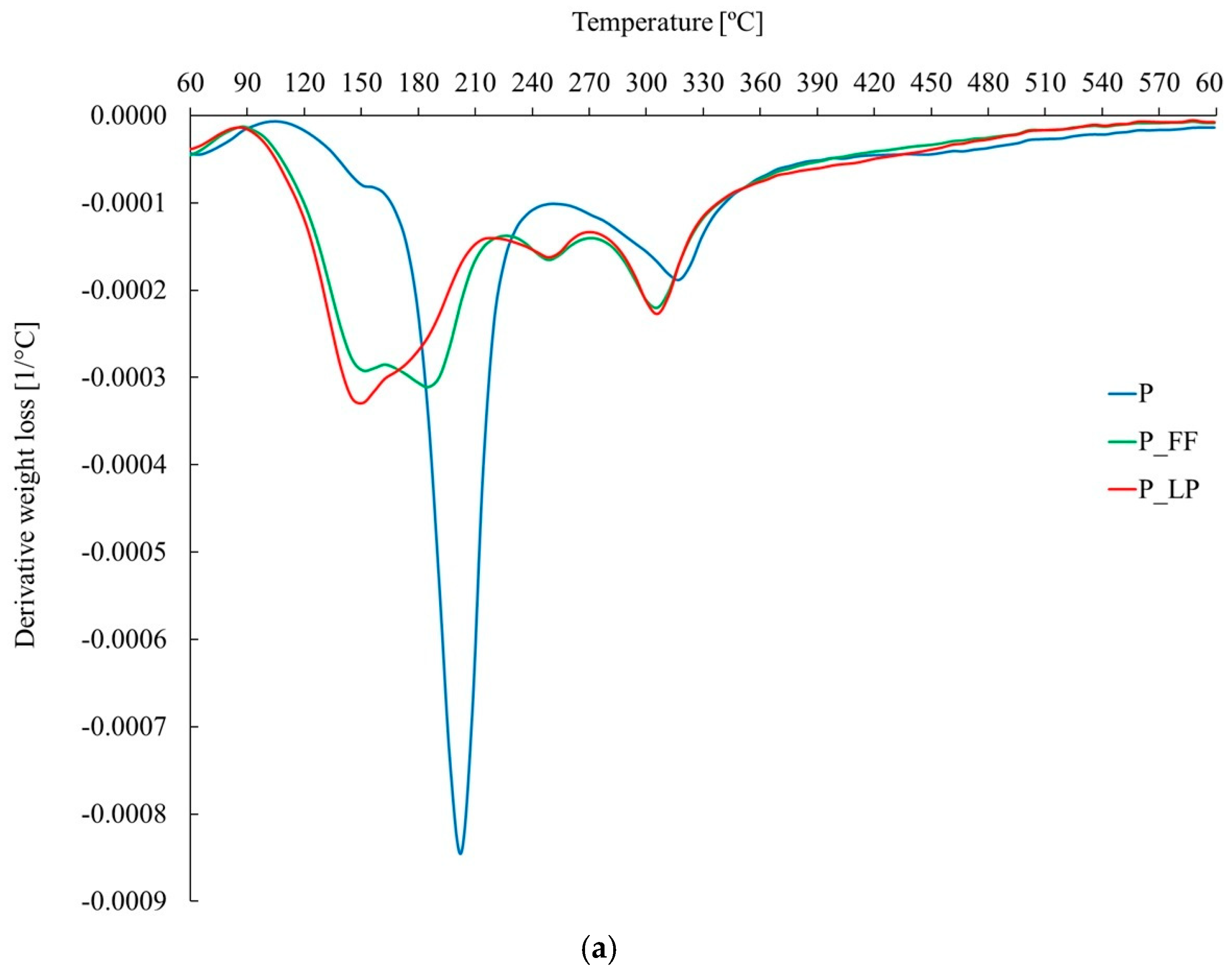
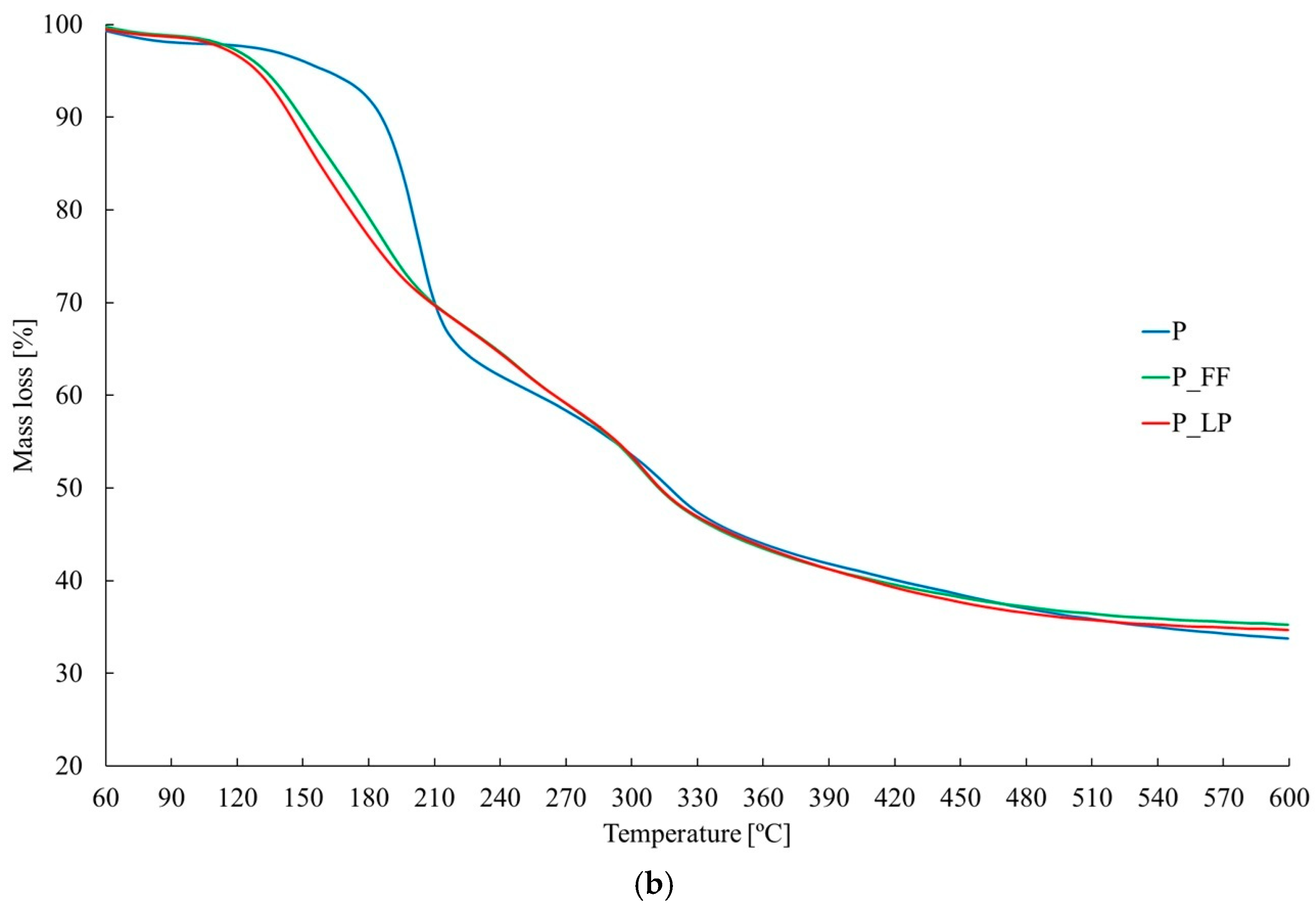
2.5. Thermal Stability and Chemical Structure of Freeze-Dried Peach Samples (TGA and FTIR Analysis)
2.6. TGA
3. Materials and Methods
3.1. Materials
3.2. Fermentation
3.3. Freeze-Drying
3.4. Microbiological Tests
3.5. Water Content
3.6. Chemical Compounds Analysis
3.6.1. The Total Sugar Content
3.6.2. Vitamin C
3.6.3. Antioxidant Properties
Extract Preparation
Total Phenolic Content (TPC)
Antioxidant Activity (ABTS and DPPH)
RP (Reducing Power)
Flavonoids
3.7. Pigments
3.7.1. Total Carotenoids Content (TCC)
- ABS is the absorbance at 450 nm;
- m2 is the total weight of the extract (g);
- 4.0 is the extinction coefficient of β-carotene in petroleum ether;
- m1 is the initial sample weight (g);
- and dm represents the dry matter content of the sample.
3.7.2. Anthocyanin
- ABS = (ABS510 − A70BS0) pH1 − (ABS510 − ABS700) pH4.5;
- MW = molecular weight of cyanidin-3-glucoside (449.2 g/mol);
- DF = dilution factor of the sample;
- ϵ = molar absorption coefficient of cyanidin-3-glucoside (26,900 L/mol·cm);
- L = optical path length of the solution in the well (0.173 cm).
3.8. Chemical Compounds—Fourier Infrared Spectroscopy (FT-IR)
3.9. Thermal Analysis (TGA)
3.10. Statistical Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Z.; Bie, H.; Li, M.; Xia, L.; Chen, L.; Chen, Y.; Wang, L.; Gan, Z.; Cao, K. Integrated GWAS, BSA-seq, and RNA-seq analyses to identify candidate genes associated with male fertility trait in peach. Plant Physiol. Biochem. 2025, 220, 109525. [Google Scholar] [CrossRef]
- Linyun, M.; Ya, L.; Jiale, M.; Shuwei, S.; Jianlong, L.; Ganpeng, L. Grafted modified chitosan/pullulan film loaded with essential oil/β-CD-MOFs for freshness preservation of peach. Int. J. Biol. Macromol. 2025, 306, 141491. [Google Scholar] [CrossRef]
- Yanming, R.; Shuifang, M.; Pin, C.; Xingqian, Y.; Jinhu, T. Metal ion-induced multi-layer coatings improves gastrointestinal resistance of Lactiplantibacillus plantarum and biosafety evaluation. Food Hydrocoll. 2025, 162, 110928. [Google Scholar] [CrossRef]
- Hsuan-Min, W.; Shwu-Jene, T.; Jin-Yuarn, L. Fermentation characteristics of lactofermented cucumbers using an isolated Lactiplantibacillus plantarum NCHU–FC1 strain. Appl. Food Res. 2025, 5, 100742. [Google Scholar] [CrossRef]
- Li, Q.; Gänzle, M.G. Host-adapted lactobacilli in food fermentations: Impact of metabolic traits of host adapted lactobacilli on food quality and human health. Curr. Opin. Food Sci. 2020, 31, 71–80. [Google Scholar] [CrossRef]
- Lu, J.; Sui, Y.; Liu, X.; Wang, J.; Li, J.; Kong, B.; Chen, Q.; Yang, W. Effect of exogenous DPD on fermentation characteristics and flavour formation of Lactiplantibacillus plantarum HRB1 in vitro and in a dry sausage model: Insights from the quorum sensing system. LWT 2025, 218, 117516. [Google Scholar] [CrossRef]
- Yang, X.; Hong, J.; Wang, L.; Cai, C.; Mo, H.; Wang, J.; Fang, X.; Liao, Z. Effect of Lactic Acid Bacteria Fermentation on Plant-Based Products. Fermentation 2024, 10, 48. [Google Scholar] [CrossRef]
- Qinggele, B.; Zhan, Y.; Kailonng, L.; Chengcong, Y.; Xiaoyuan, Z.; Guoqiang, Y. Comparative genomics and metabolite analysis of Lactiplantibacillus plantarum strains in dairy and vegetable fermentation environments. Food Biosci. 2025, 65, 106058. [Google Scholar] [CrossRef]
- Stephen, J.M.; Saleh, A.M. Homofermentative Lactobacilli isolated from organic sources exhibit potential ability of lactic acid production. Front. Microbiol. 2023, 14, 1297036. [Google Scholar] [CrossRef]
- Giuffrè, D.; Giuffrè, A.M. Fermentation technology and functional foods. Front. Biosci. Elite 2024, 16, 8. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Svetoslav Dimitrov, T.; Bernadette Dora Gombossy De Melo Franco, A. Lactobacillus Plantarum: Characterization of the Species and Application in Food Production. Food Rev. Int. 2010, 26, 205–229. [Google Scholar] [CrossRef]
- Maaike, C.; Elaine, E.V.; Michiel, K.; Willem, M. Lactobacillus plantarum—Survival, functional and potential probiotic properties in the human intestinal tract. Int. Dairy J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Widyastuti, Y.; Rohmatussolihat; Febrisiantosa, A. The Role of Lactic Acid Bacteria in Milk Fermentation. Food Nutr. Sci. 2014, 5, 435–442. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, S.; Fang, X.; Wang, Z.; Jiang, Y.; Guo, X.; Zhu, J.; Zhang, Y. The Effect of Lactiplantibacillus plantarum BX62 Alone or in Combination with Chitosan on the Qualitative Characteristics of Fresh-Cut Apples during Cold Storage. Microorganisms 2021, 9, 2404. [Google Scholar] [CrossRef]
- Yanlu, L.; Wenting, F.; Huiru, D.; Zhideng, Z.; Lei, L.; Zhigang, D.; Jun, W. Metabolic profile and antioxidant properties of grape juice subjected to long-fermentation with Lactiplantibacillus plantarum strain isolated from traditional pickled vegetables. Food Biosci. 2024, 62, 105455. [Google Scholar] [CrossRef]
- Rizzi, F.; Juan, B.; Espadaler-Mazo, J.; Capellas, M.; Huedo, P. Lactiplantibacillus plantarum KABP051: Stability in Fruit Juices and Production of Bioactive Compounds During Their Fermentation. Foods 2024, 13, 3851. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Gu, C.T. Lactobacillus homohiochii is a later heterotypic synonym of Lactobacillus fructivorans. Int. J. Syst. Evol. Microbiol. 2019, 69, 1720–1723. [Google Scholar] [CrossRef]
- Endo, A.; Maeno, S.; Tanizawa, Y.; Kneifel, W.; Arita, M.; Dicks, L.; Salminen, S. Fructophilic lactic acid bacteria, a unique group of fructose-fermenting microbes. Appl. Environ. Microbiol. 2018, 84, e01290-18. [Google Scholar] [CrossRef]
- Maeno, S.; Nishimura, H.; Tanizawa, Y.; Dicks, L.; Arita, M.; Endo, A. Unique niche-specific adaptation of fructophilic lactic acid bacteria and proposal of three Apilactobacillus species as novel members of the group. BMC Microbiol. 2021, 21, 41. [Google Scholar] [CrossRef]
- Liang, T.; Xie, X.; Wu, L.; Li, L.; Li, H.; Xi, Y.; Feng, Y.; Xue, L.; Chen, M.; Chen, X.; et al. Microbial Communities and Physiochemical Properties of Four Distinctive Traditionally Fermented Vegetables from North China and Their Influence on Quality and Safety. Foods 2022, 11, 21. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Rybak, K.; Witrowa-Rajchert, D.; Pobiega, K.; Wierzbicka, A.; Ossowski, S.; Sękul, J.; Kufel, A.; Wiśniewska, A.; Trych, U.; et al. Influence of Heat Treatment and Lactic Acid Fermentation on the Physical and Chemical Properties of Pumpkin Juice. Molecules 2024, 29, 4519. [Google Scholar] [CrossRef] [PubMed]
- Silvia Carballo, M.; Alejandro, M.-R.; Salvador, E.M.P.; Soledad Prats, M. 2—New Trends and Applications in Fermented Beverages. Fermented Beverages 2019, 5, 31–66. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on non-dairy probiotics and their use in non-dairy based products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Behera, S.S.; Panda, S.K. Ethnic and industrial probiotic foods and beverages: Efficacy and acceptance. Curr. Opin. Food Sci. 2020, 32, 29–36. [Google Scholar] [CrossRef]
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res. Int. 2021, 140, 110033. [Google Scholar] [CrossRef]
- Grom, L.C.; Coutinho, N.M.; Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Freitas, M.Q.; Duarte, M.C.K.; Pimentel, T.C.; Esmerino, E.A. Probiotic dairy foods and postprandial glycemia: A mini-review. Trends Food Sci. Technol. 2020, 101, 165–171. [Google Scholar] [CrossRef]
- Kiepś, J.; Dembczyński, R. Current Trends in the Production of Probiotic Formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Baines, S.K.; Balthazar, C.F.; Cruz, A.G.; Esmerino, E.A.; Freitas, M.Q.; Pimentel, T.C.; Wittwer, A.E.; Naumovski, N.; et al. Probiotics in Goat Milk Products: Delivery Capacity and Ability to Improve Sensory Attributes. Com. Rev. Food Sci. Food Saf. 2019, 18, 867–882. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Morais, S.G.G.; Borges, G.d.S.C.; dos Santos Lima, M.; Martín-Belloso, O.; Magnani, M. Effects of probiotics on the content and bioaccessibility of phenolic compounds in red pitaya pulp. Food Res. Int. 2019, 126, 108681. [Google Scholar] [CrossRef] [PubMed]
- Amandine, F.; Fabienne, R. Genetic and technological characterization of lactic acid bacteria isolated from tropically grown fruits and vegetables. Int. J. Food Microbiol. 2019, 301, 61–72. [Google Scholar] [CrossRef]
- Yang, W.; Liu, J.; Zhang, Q.; Liu, H.; Lv, Z.; Zhang, C.; Jiao, Z. Changes in nutritional composition, volatile organic compounds and antioxidant activity of peach pulp fermented by lactobacillus. Food Biosci. 2022, 49, 101894. [Google Scholar] [CrossRef]
- Cele, N.P.; Akinola, S.A.; Manhivi, V.E.; Shoko, T.; Remize, F.; Sivakumar, D. Influence of Lactic Acid Bacterium Strains on Changes in Quality, Functional Compounds and Volatile Compounds of Mango Juice from Different Cultivars during Fermentation. Foods 2022, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Verón, H.E.; Contreras, L.; Isla, M.I.; Torres, S. Assessment of technological and functional features of Lactiplantibacillus and Fructobacillus strains isolated from Opuntia ficus-indica fruits. NFS J. 2023, 31, 110–122. [Google Scholar] [CrossRef]
- Olaide Olawunmi, A.; Raymond, T.; Babatunde Femi, B. Selected fermented indigenous vegetables and fruits from Malaysia as potential sources of natural probiotics for improving gut health. Food Sci. Hum. Wellness 2023, 12, 1493–1509. [Google Scholar] [CrossRef]
- Xin, L.; Junpeng, Y.; Jian, H.; Jingyin, D.; Xu, D. Comparative evaluation of quality characteristics of fermented napa cabbage subjected to hot air drying, vacuum freeze drying, and microwave freeze drying. Lwt 2024, 192, 115740. [Google Scholar] [CrossRef]
- Leyva Salas, M.; Thierry, A.; Lemaître, M.; Garric, G.; Harel-Oger, M.; Chatel, M.; Lê, S.; Mounier, J.; Valence, F.; Coton, E. Antifungal Activity of Lactic Acid Bacteria Combinations in Dairy Mimicking Models and Their Potential as Bioprotective Cultures in Pilot Scale Applications. Front. Microbiol. 2018, 9, 1787. [Google Scholar] [CrossRef]
- Germer, S.P.M.; De Queiroz, M.R.; Aguirre, J.M. Sensory attributes of osmotically dehydrated peaches. In Proceedings of the 6th International CIGR Technical Symposium—Towards a Sustainable Food Chain: Food Process, Bioprocessing and Food Quality Management, Nantes, France, 18–20 April 2011. [Google Scholar]
- Louesdon, S.; Charlot-Rougé, S.; Juillard, V.; Tourdot-Maréchal, R.; Béal, C. Osmotic stress affects the stability of freeze-dried Lactobacillus buchneri R1102 as a result of intracellular betaine accumulation and membrane characteristics. J. Appl. Microbiol. 2014, 117, 196–207. [Google Scholar] [CrossRef]
- Swain, M.R.; Ray, R.C. Nutritional values and bioactive compounds in lactic acid fermented vegetables and fruits. Lact. Acid Ferment. Fruits Veg. 2016, 1, 37–52. [Google Scholar]
- Urbiené, S.; Mitkuté, D. Changes of vitamin C during milk fermentation. Milchwissenschaft 2007, 62, 130–132. [Google Scholar]
- Chen, R.; Chen, W.; Chen, H.; Zhang, G.; Chen, W. Comparative evaluation of the antioxidant capacities, organic acids, and volatiles of papaya juices fermented by Lactobacillus acidophilus and Lactobacillus plantarum. J. Food Qual. 2018, 2018, 9490435. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Lactic acid fermentation of guava juice: Evaluation of nutritional and bioactive properties, enzyme (α-amylase, α-glucosidase, and angiotensin-converting enzyme) inhibition abilities, and anti-inflammatory activities. Food Sci. Nutr. 2023, 11, 7638–7648. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lai, C.; Liang, Y.; Xiong, Q.; Chen, C.; Ju, Z.; Jiang, Y.; Zhang, J. Improving the functional components and biological activities of navel orange juice through fermentation with an autochthonous strain Lactiplantibacillus paraplantarum M23. Food Bioprod. Process 2025, 149, 249–260. [Google Scholar] [CrossRef]
- Minghao, C.; Hao, T.; Huan, L.; Jie, L.; Jian, D.; Yehan, T.; Yi, C.; Haisong, W. A cyclic process for enzymatic hydrolysis and fermentation of lactic acid pretreated reed. Ind. Crops Prod. 2022, 181, 114848. [Google Scholar] [CrossRef]
- Jabłońska-Ryś, E.; Sławińska, A.; Skrzypczak, K.; Goral, K. Dynamics of changes in pH and the contents of free sugars, organic acids and LAB in button mushrooms during controlled lactic fermentation. Foods 2022, 11, 1553. [Google Scholar] [CrossRef]
- Viesser, J.A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rogez, H.; Góes-Neto, A.; Azevedo, V.; Brenig, B.; Aburjaile, F.; Soccol, C.R. Co-culturing fructophilic lactic acid bacteria and yeast enhanced sugar metabolism and aroma formation during cocoa beans fermentation. Int. J. Food Microbiol. 2021, 339, 109015. [Google Scholar] [CrossRef]
- Viesser, J.A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Vandenberghe, L.P.D.S.; Azevedo, V.; Brenig, B.; Rogez, H.; Góes-Neto, A.; Soccol, C.R. Exploring the contribution of fructophilic lactic acid bacteria to cocoa beans fermentation: Isolation, selection and evaluation. Food Res. Int. 2020, 136, 109478. [Google Scholar] [CrossRef]
- Endo, A.; Dicks, L.M. The genus Fructobacillus. In Lactic Acid Bacteria: Biodiversity and Taxonomy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 381–389. [Google Scholar]
- Endo, A.; Futagawa-Endo, Y.; Dicks, L.M. Isolation and characterization of fructophilic lactic acid bacteria from fructose-rich niches. Syst. Appl. Microbiol. 2009, 32, 593–600. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Robles-Apodaca, S.M.; González-Vega, R.I.; Ruíz-Cruz, S.; Estrada-Alvarado, M.I.; Cira-Chávez, L.A.; Márquez-Ríos, E.; Del-Toro-Sánchez, C.L.; Ornelas-Paz, J.d.J.; Suárez-Jiménez, G.M.; Ocaño-Higuera, V.M. Optimization of Extraction Process for Improving Polyphenols and Antioxidant Activity from Papaya Seeds (Carica papaya L.) Using Response Surface Methodology. Processes 2024, 12, 2729. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Portocarrero, A.C.M.; Miranda López, J.M.; Lombardo, M.; Koch, W.; Raposo, A.; El-Seedi, H.R.; de Brito Alves, J.L.; Esatbeyoglu, T.; Karav, S.; et al. The Impact of Fermentation on the Antioxidant Activity of Food Products. Molecules 2024, 29, 3941. [Google Scholar] [CrossRef]
- Munawar, A.; Farhan, S.; Faqir Muhammad, A.; Muhammad, A.; Tabussam, T.; Muhammad Shakeel, B.; Adnan, I.; Shahzad, H.; Hafiz Ansar Rasul Suleria, A. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E. Mutual influence of polyphenols and Lactobacillus spp. bacteria in food: A review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Sackey, A.S.; Wu, M.; Xiao, L. Impact of ultrasonication and pulsed light treatments on phenolics concentration and antioxidant activities of lactic-acid-fermented mulberry juice. Lwt 2018, 92, 61–66. [Google Scholar] [CrossRef]
- Zhao, D.; Shah, N.P. Lactic acid bacterial fermentation modified phenolic composition in tea extracts and enhanced their antioxidant activity and cellular uptake of phenolic compounds following in vitro digestion. J. Funct. Foods 2016, 20, 182–194. [Google Scholar] [CrossRef]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, H.; Wang, Y.; Nussio, L.G.; Yang, F.; Ni, K. Insights into fermentation with lactic acid bacteria on the flavonoids biotransformation of alfalfa silage. Chem. Biol. Technol. Agric. 2024, 11, 73. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M.; Curiel, J.A.; Rodríguez, H.; De Las Rivas, B.; Muñoz, R. Aryl glycosidases from Lactobacillus plantarum increase antioxidant activity of phenolic compounds. J. Funct. Foods 2014, 7, 322–329. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Wang, Y.; Wang, X.; Ren, Y.; Yue, T.; Wang, Z.; Gao, Z. Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem. 2021, 363, 130351. [Google Scholar] [CrossRef]
- Custodio-Mendoza, J.A.; Aktaş, H.; Zalewska, M.; Wyrwisz, J.; Kurek, M.A. A Review of Quantitative and Topical Analysis of Anthocyanins in Food. Molecules 2024, 29, 1735. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, F.; Marinelli, A.; Toccaceli, M.; Tonelli, C.; Petroni, K. Anthocyanins: From mechanisms of regulation in plants to health benefits in foods. Front. Plant Sci. 2021, 12, 748049. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. Lwt 2021, 139, 110590. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Escudero-López, B.; Cerrillo, I.; Herrero-Martín, G.; Hornero-Méndez, D.; Gil-Izquierdo, A.; Medina, S.; Ferreres, F.; Berná, G.; Martín, F.; Fernández-Pachón, M.-S. Fermented Orange Juice: Source of Higher Carotenoid and Flavanone Contents. J. Agric. Food Chem. 2013, 61, 8773–8782. [Google Scholar] [CrossRef]
- Xu, Y.; Hlaing, M.M.; Glagovskaia, O.; Augustin, M.A.; Terefe, N.S. Fermentation by Probiotic Lactobacillus gasseri Strains Enhances the Carotenoid and Fibre Contents of Carrot Juice. Foods 2020, 9, 1803. [Google Scholar] [CrossRef]
- Quijano-Ortega, N.; Fuenmayor, C.A.; Zuluaga-Dominguez, C.; Diaz-Moreno, C.; Ortiz-Grisales, S.; García-Mahecha, M.; Grassi, S. FTIR-ATR Spectroscopy Combined with Multivariate Regression Modeling as a Preliminary Approach for Carotenoids Determination in Cucurbita spp. Appl. Sci. 2020, 10, 3722. [Google Scholar] [CrossRef]
- Shukla, R.K.; Kant, R. Assessment of phytochemical screening by Fourier Transform Infrared spectroscopic analysis of peach (Prunus persica) seed biomass from Uttarakhand region of India. J. Appl. Nat. Sci. 2020, 12, 519–524. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Fry, F.S.; McLaughlin, M.A.; Calvey, E.M. Rapid analysis of sugars in fruit juices by FT-NIR spectroscopy. Carbohydr. Res. 2001, 336, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Nizamlioglu, N.M.; Yasar, S.; Bulut, Y. Chemical versus infrared spectroscopic measurements of quality attributes of sun or oven dried fruit leathers from apple, plum and apple-plum mixture. Lwt 2022, 153, 112420. [Google Scholar] [CrossRef]
- Dhaulaniya, A.S.; Balan, B.; Yadav, A.; Jamwal, R.; Kelly, S.; Cannavan, A.; Singh, D.K. Development of an FTIR based chemometric model for the qualitative and quantitative evaluation of cane sugar as an added sugar adulterant in apple fruit juices. Food Addit. Contam. Part A 2020, 37, 539–551. [Google Scholar] [CrossRef]
- Geminiani, L.; Campione, F.P.; Corti, C.; Luraschi, M.; Motella, S.; Recchia, S.; Rampazzi, L. Differentiating between Natural and Modified Cellulosic Fibres Using ATR-FTIR Spectroscopy. Heritage 2022, 5, 4114–4139. [Google Scholar] [CrossRef]
- Janković, B.; Marinović-Cincović, M.; Janković, M. TG-DTA-FTIR analysis and isoconversional reaction profiles for thermal and thermo-oxidative degradation processes in black chokeberry (Aroniamelanocarpa). Chem. Pap. 2016, 70, 1094–1105. [Google Scholar] [CrossRef]
- Karwacka, M.; Ciurzyńska, A.; Galus, S.; Janowicz, M. Freeze-dried snacks obtained from frozen vegetable by-products and apple pomace–Selected properties, energy consumption and carbon footprint. Innov. Food Sci. Emerg. Technol. 2022, 77, 102949. [Google Scholar] [CrossRef]
- Tepe, T.K. Convective drying of golden delicious apple enhancement: Drying characteristics, artificial neural network modeling, chemical and ATR-FTIR analysis of quality parameters. Biomass Convers. Biorefinery 2024, 14, 13513–13531. [Google Scholar] [CrossRef]
- Choque-Quispe, D.; Ligarda-Samanez, C.A.; Huamán-Rosales, E.R.; Aguirre Landa, J.P.; Agreda Cerna, H.W.; Zamalloa-Puma, M.M.; Álvarez-López, G.J.; Barboza-Palomino, G.I.; Alzamora-Flores, H.; Gamarra-Villanueva, W. Bioactive Compounds and Sensory Analysis of Freeze-Dried Prickly Pear Fruits from an Inter-Andean Valley in Peru. Molecules 2023, 28, 3862. [Google Scholar] [CrossRef]
- Filip, M.; Vlassa, M.; Petean, I.; Țăranu, I.; Marin, D.; Perhaiță, I.; Prodan, D.; Borodi, G.; Dragomir, C. Structural Characterization and Bioactive Compound Evaluation of Fruit and Vegetable Waste for Potential Animal Feed Applications. Agriculture 2024, 14, 2038. [Google Scholar] [CrossRef]
- Niemira, J.; Galus, S. Valorization of Red Beetroot (Beta vulgaris L.) Pomace Combined with Golden Linseed (Lini semen) for the Development of Vegetable Crispbreads as Gluten-Free Snacks Rich in Bioactive Compounds. Molecules 2024, 29, 2105. [Google Scholar] [CrossRef]
- Morales-Quintana, L.; Tapia-Valdebenito, D.; Castro, R.I.; Rabert, C.; Larama, G.; Gutiérrez, A.; Ramos, P. Characterization of the cell wall component through thermogravimetric analysis and its relationship with an expansin-like protein in Deschampsia antarctica. Int. J. Mol. Sci. 2022, 23, 5741. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Falacińska, J.; Kowalska, H.; Kowalska, J.; Galus, S.; Marzec, A.; Domian, E. The effect of pre-treatment (blanching, ultrasound and freezing) on quality of freeze-dried red beets. Foods 2021, 10, 132. [Google Scholar] [CrossRef]
- Zlatanović, S.; Ostojić, S.; Micić, D.; Rankov, S.; Dodevska, M.; Vukosavljević, P.; Gorjanović, S. Thermal behaviour and degradation kinetics of apple pomace flours. Thermochim. Acta 2019, 673, 17–25. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 2016, 113, 325–333. [Google Scholar] [CrossRef]
- Cheng, X.-C.; Cui, X.-Y.; Qin, Z.; Liu, H.-M.; Wang, X.-D.; Liu, Y.-L. Effect of drying pretreatment methods on structural features and antioxidant activities of Brauns native lignin extracted from Chinese quince fruit. Process Biochem. 2021, 106, 70–77. [Google Scholar] [CrossRef]
- Iolanda, N.-L.; Maribel, A.; Gloria, B.; Ingrid, A.-A.; Tomás, L.; Inmaculada, V. Strawberry sanitization by peracetic acid washing and its effect on fruit quality. Food Microbiol. 2019, 83, 159–166. [Google Scholar] [CrossRef]
- Nalbant, A.; Ersoy Omeroglu, E. Lactic Acid Bacteria Isolation from Üçburun Peppers and Comparison of the Different Production Process for Pickled Pepper. Fermentation 2024, 10, 196. [Google Scholar] [CrossRef]
- Yang, S.; Meng, Z.; Li, Y.; Chen, R.; Yang, Y.; Zhao, Z. Evaluation of physiological characteristics, soluble sugars, organic acids and volatile compounds in ‘Orin’apples (Malus domestica) at different ripening stages. Molecules 2021, 26, 807. [Google Scholar] [CrossRef]
- Trych, U.; Buniowska, M.; Skąpska, S.; Kapusta, I.; Marszałek, K. Bioaccessibility of antioxidants in blackcurrant juice after treatment using supercritical carbon dioxide. Molecules 2022, 27, 1036. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Karwacka, M.; Ciurzyńska, A.; Galus, S.; Janowicz, M. The effect of storage time and temperature on quality changes in freeze-dried snacks obtained with fruit pomace and pectin powders as a sustainable approach for new product development. Sustainability 2024, 16, 4736. [Google Scholar] [CrossRef]
- Bochnak-Niedźwiecka, J.; Szymanowska, U.; Świeca, M. The Protein-Rich Powdered Beverages Stabilized with Flax Seeds Gum—Antioxidant and Antiproliferative Properties of the Potentially Bioaccessible Fraction. Appl. Sci. 2022, 12, 7159. [Google Scholar] [CrossRef]
- Tian, W.; Chen, G.; Tilley, M.; Li, Y. Changes in phenolic profiles and antioxidant activities during the whole wheat bread-making process. Food Chem. 2021, 345, 128851. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Antoniewska, A.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Skoczeń-Słupska, R. Bioactive components, volatile profile and in vitro antioxidative properties of Taxus baccata L. red arils. Molecules 2021, 26, 4474. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.; Wrolstad, R. Characterization and measurement with UV-visible spectroscopy. Unit F2. 2, Ch. 2. In Current Protocols in Food Analytical Chemistry; King, S., Gates, M., Scalettar, L., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Bednarska, M.A.; Janiszewska-Turak, E. The influence of spray drying parameters and carrier material on the physico-chemical properties and quality of chokeberry juice powder. J. Food Sci. Technol. 2020, 57, 564–577. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Petrovich, D.S.; Ilinichna, K.T.; Morton, D.W. HPTLC and FTIR Fingerprinting of Olive Leaves Extracts and ATR-FTIR Characterisation of Major Flavonoids and Polyphenolics. Molecules 2021, 26, 6892. [Google Scholar] [CrossRef]
- Tien, N.N.T.; Nguyen, H.T.; Le, N.L.; Khoi, T.T.; Richel, A. Biodegradable films from dragon fruit (Hylocereus polyrhizus) peel pectin and potato starches crosslinked with glutaraldehyde. Food Packag. Shelf Life 2023, 37, 101084. [Google Scholar] [CrossRef]


| Sample Code | P | P_FF | P_LP |
|---|---|---|---|
| Water content (%) | 3.1 ± 0.1 a | 10.3 ± 0.0 c | 9.1 ± 0.3 b |
| Vitamin C (mg/100 g d.m.) | 109.1 ± 3.5 b | 81.6 ± 5.5 a | 102.3 ± 8.7 b |
| Fructose (%) | 6.2 ± 0.1 a | 10.6 ± 0.6 b | 10.2 ± 0.3 b |
| Glucose (%) | 5.2 ± 0.3 a | 10.9 ± 0.2 c | 9.1 ± 0.3 b |
| Sucrose (%) | 30.9 ± 0.3 c | 7.6 ± 0.2 b | 4.6 ± 0.0 a |
| ABTS (mg TE/g d.m.) | 3.5 ± 0.1 b | 3.1 ± 0.2 a | 3.4 ± 0.0 ab |
| DPPH (mg TE/g d.m.) | 47.0 ± 2.5 b | 46.3 ± 2.3 ab | 40.1 ± 2.8 a |
| RP (mg TE/g d.m.) | 18.2 ± 0.5 b | 12.7 ± 1.0 a | 12.9 ± 1.5 a |
| Sample Code | Step 1 | Step 2 | Step 3 | Step 4 | Sum [%] | Decomposition Temperature [°C] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. Range (°C) | Mass Loss (%) | Temp. Range (°C) | Mass Loss (%) | Temp. Range (°C) | Mass Loss (%) | Temp. Range (°C) | Mass Loss (%) | 2 | 3 | ||||
| P | 30–95 | 2.0 | 95–245 | 36.0 | 245–380 | 19.6 | 380–600 | 8.7 | 66.2 | 199.7 | 314.6 | ||
| P_FF | 30–95 | 1.2 | 95–270 | 39.7 | 270–380 | 17.1 | 380–601 | 6.7 | 64.7 | 149.3 | 182.8 | 246.3 | 302.1 |
| P_LP | 30–90 | 1.4 | 90–220 | 30.7 | 220–380 | 26.2 | 380–602 | 7.3 | 65.6 | 146.9 | 246.3 | 304.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossowski, S.; Rybak, K.; Pobiega, K.; Sękul, J.; Domżalska, Z.; Gregorek, K.; Gramza-Michałowska, A.; Janiszewska-Turak, E. Antioxidant Activity and Microbial Quality of Freeze-Dried, Lactic Acid Fermented Peach Products. Molecules 2025, 30, 2360. https://doi.org/10.3390/molecules30112360
Ossowski S, Rybak K, Pobiega K, Sękul J, Domżalska Z, Gregorek K, Gramza-Michałowska A, Janiszewska-Turak E. Antioxidant Activity and Microbial Quality of Freeze-Dried, Lactic Acid Fermented Peach Products. Molecules. 2025; 30(11):2360. https://doi.org/10.3390/molecules30112360
Chicago/Turabian StyleOssowski, Szymon, Katarzyna Rybak, Katarzyna Pobiega, Joanna Sękul, Zuzanna Domżalska, Klaudia Gregorek, Anna Gramza-Michałowska, and Emilia Janiszewska-Turak. 2025. "Antioxidant Activity and Microbial Quality of Freeze-Dried, Lactic Acid Fermented Peach Products" Molecules 30, no. 11: 2360. https://doi.org/10.3390/molecules30112360
APA StyleOssowski, S., Rybak, K., Pobiega, K., Sękul, J., Domżalska, Z., Gregorek, K., Gramza-Michałowska, A., & Janiszewska-Turak, E. (2025). Antioxidant Activity and Microbial Quality of Freeze-Dried, Lactic Acid Fermented Peach Products. Molecules, 30(11), 2360. https://doi.org/10.3390/molecules30112360








