Facile One-Step Fabrication of 1T-Phase-Rich Bimetallic CoFe Co-Doped MoS2 Nanoflower: Synergistic Engineering for Bi-Functional Water Splitting Electrocatalysis
Abstract
1. Introduction
2. Results and Discussion
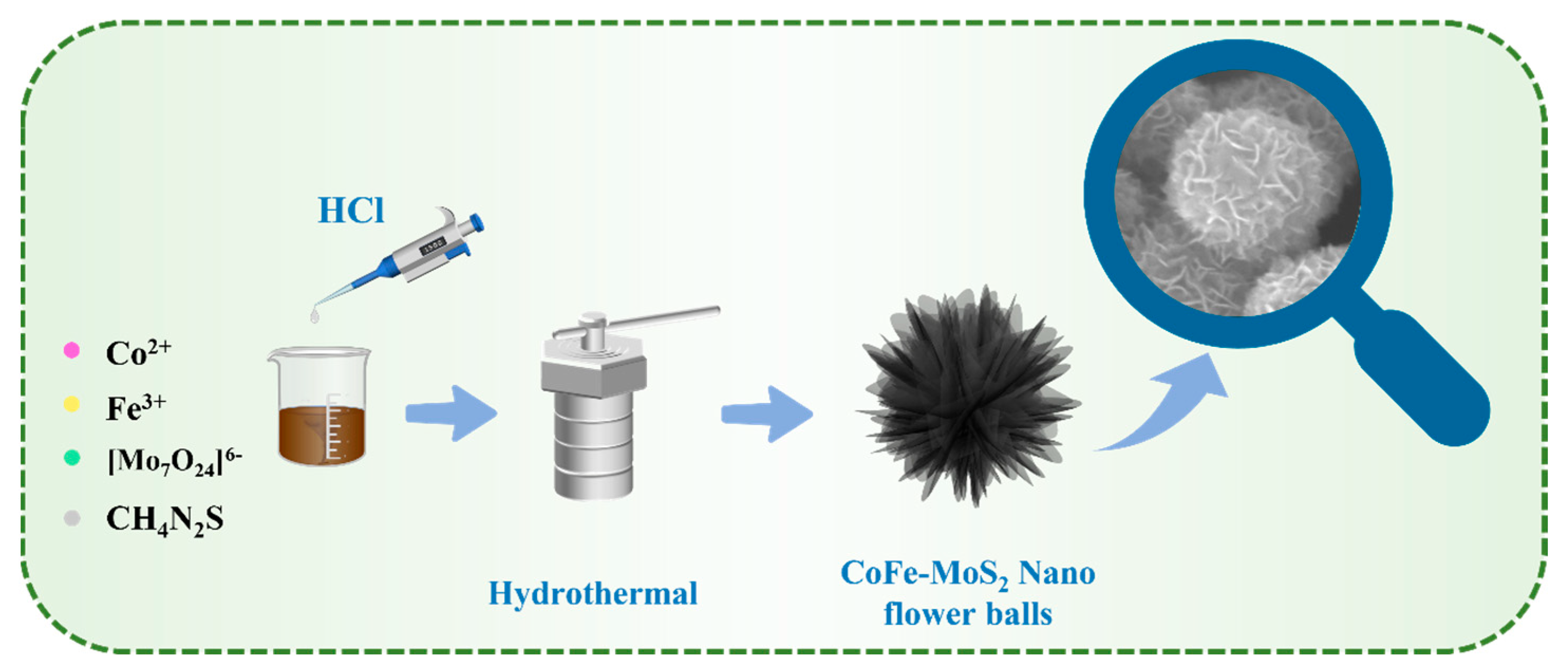
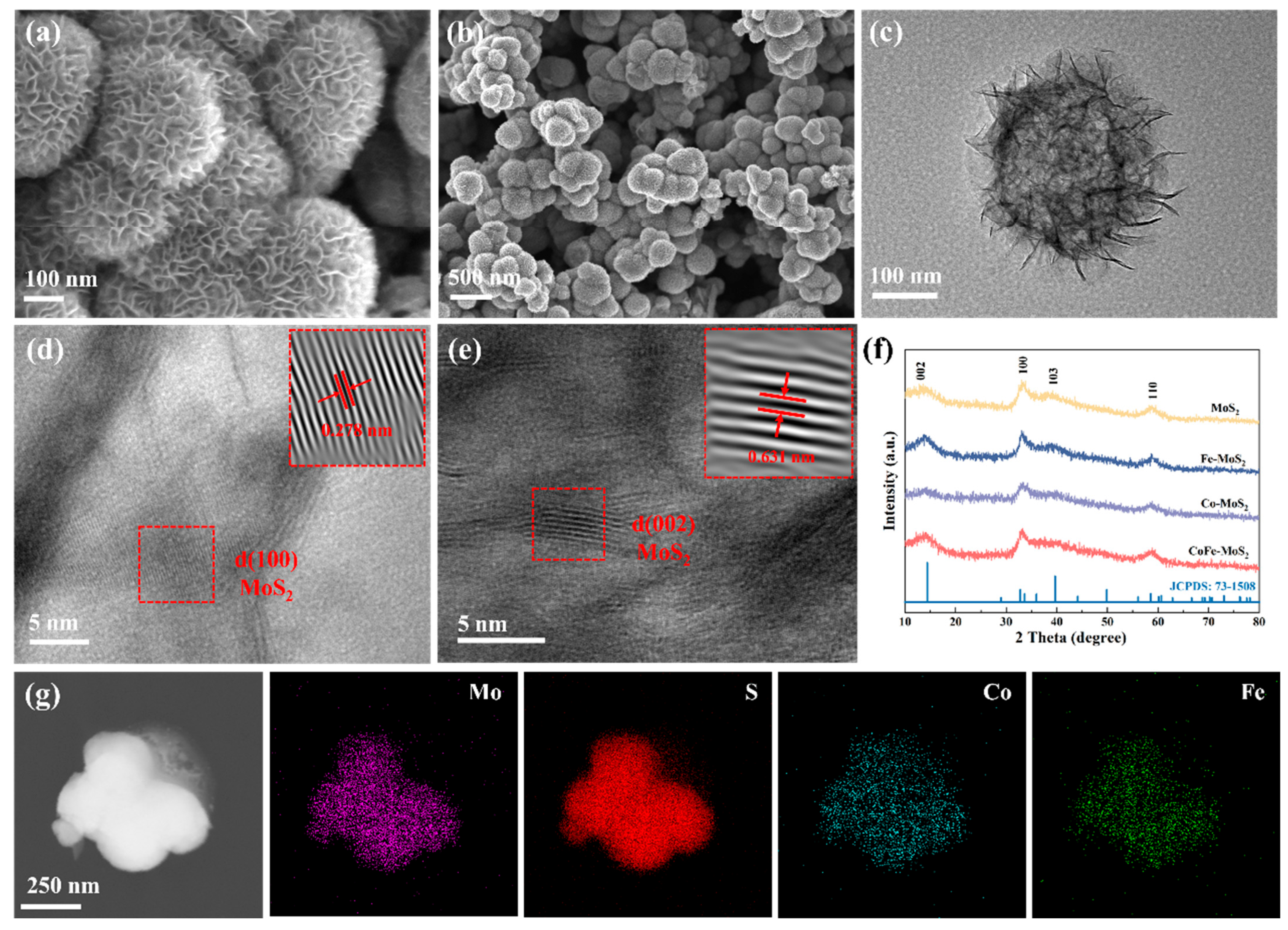
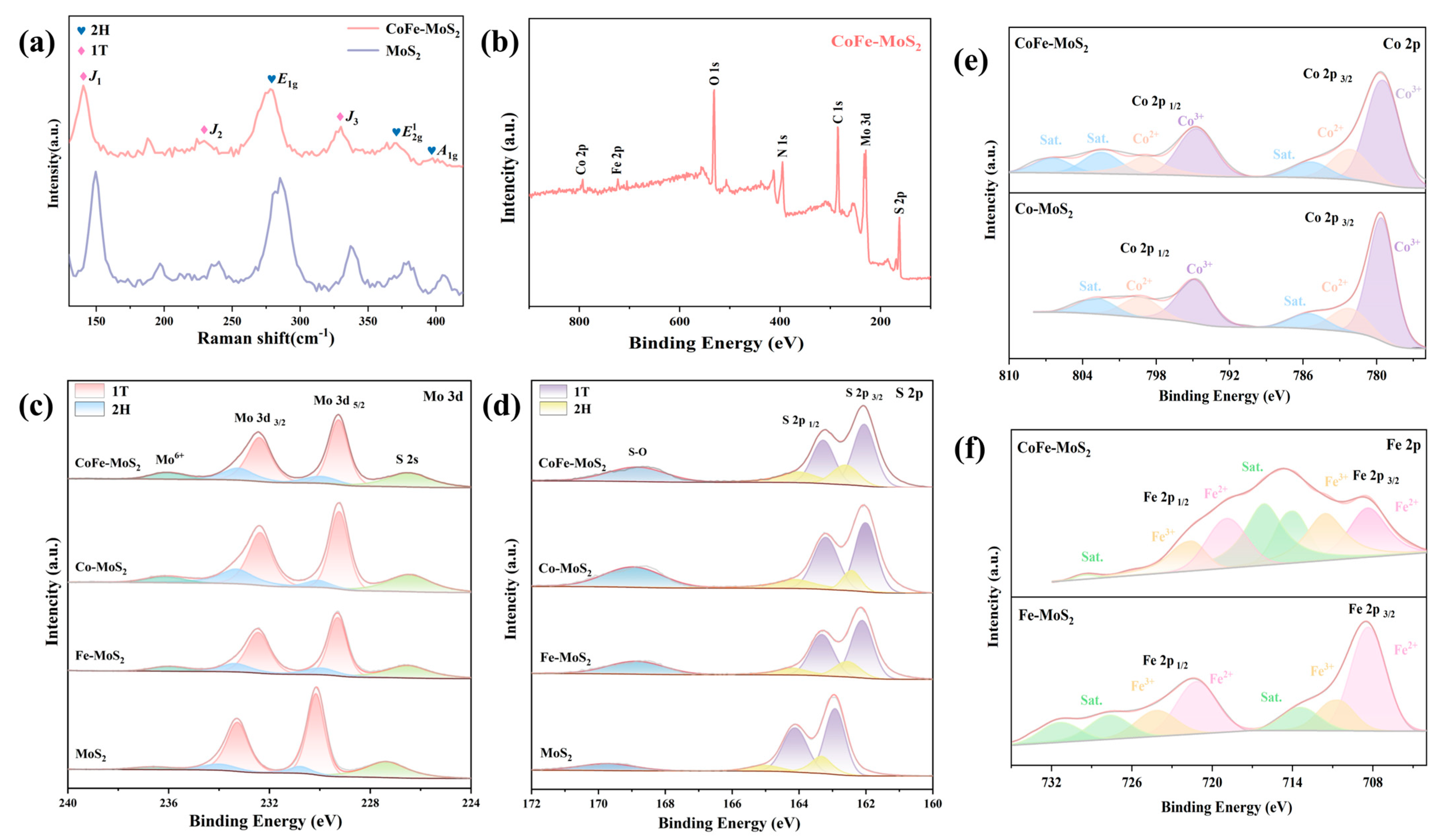
2.1. Structure and Morphology Characterizations
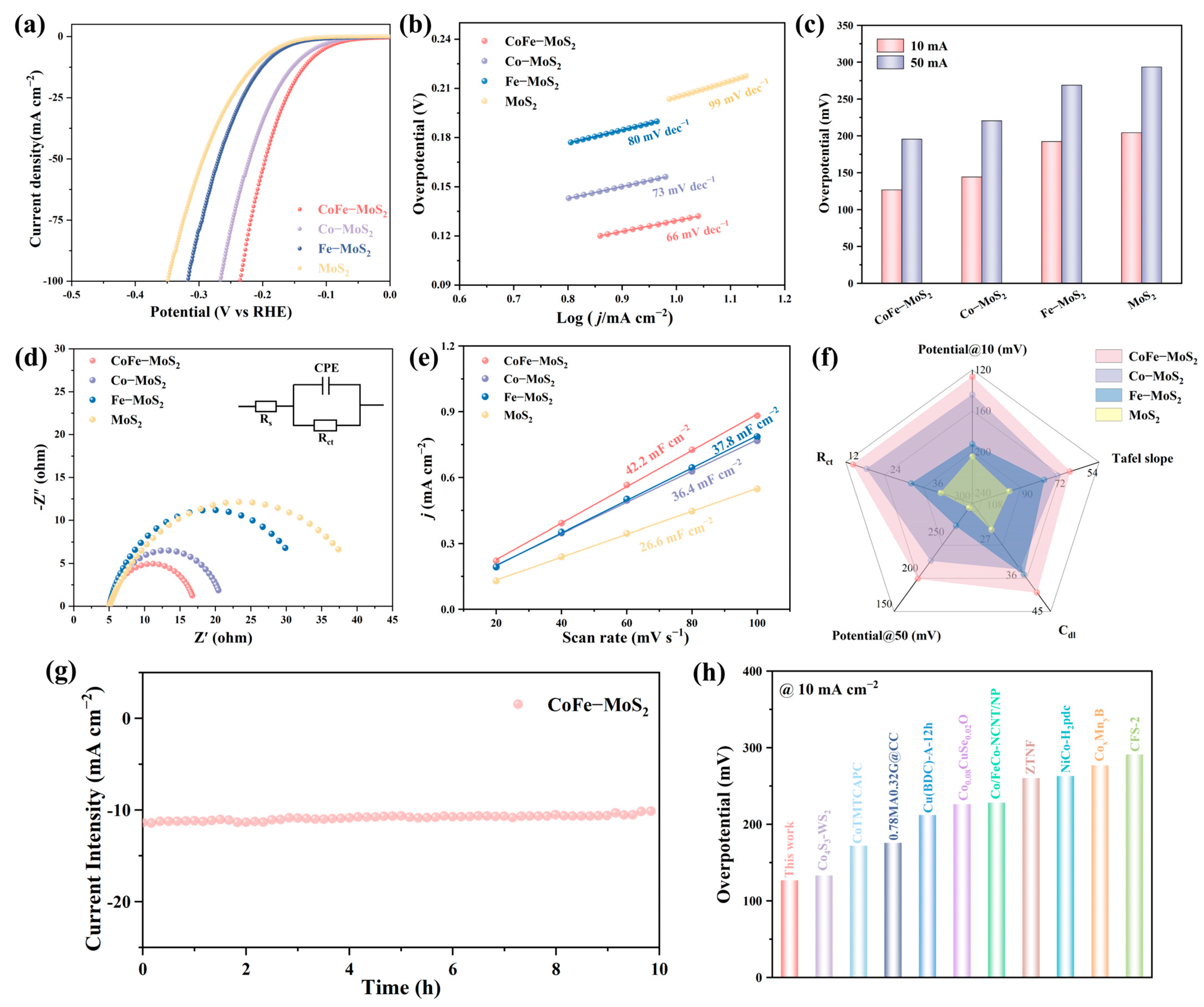
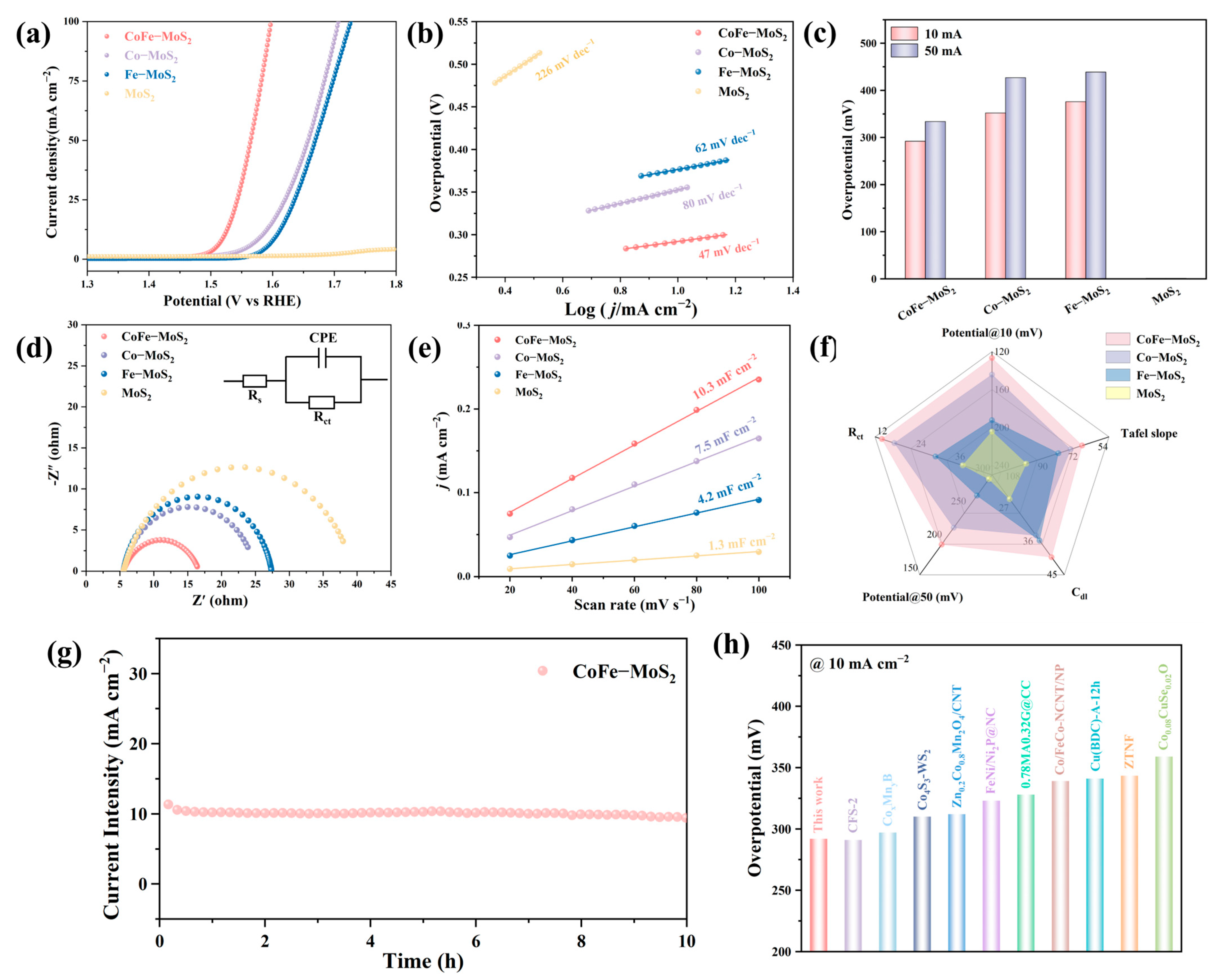
2.2. Electrocatalytic Performance Investigation
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of CoFe-MoS2
3.3. Electrochemical Characterization
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAA | Thioacetamide |
References
- Luo, Y.; Zhang, Z.; Chhowalla, M.; Liu, B. Recent Advances in Design of Electrocatalysts for High-Current-Density Water Splitting. Adv. Mater. 2022, 34, 2108133. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Duan, Y.; Feng, X.; Yu, X.; Gao, M.; Yu, S. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Deng, J.; Wang, Z.; Liang, Q.; Hu, L.; Ren, X.; Li, R.; Lin, Y.; Li, Y.; Wang, Q.; et al. Aggregate-Dominated Dilute Electrolytes with Low-Temperature-Resistant Ion-Conducting Channels for Highly Reversible Na Plating/Stripping. Adv. Mater. 2024, 36, 2408161. [Google Scholar] [CrossRef]
- Ge, B.; Hu, L.; Yu, X.; Wang, L.; Fernandez, C.; Yang, N.; Liang, Q.; Yang, Q. Engineering Triple-Phase Interfaces around the Anode toward Practical Alkali Metal–Air Batteries. Adv. Mater. 2024, 36, 2400937. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent Progress in Cobalt-Based Heterogeneous Catalysts for Electrochemical Water Splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef]
- Yu, Q. Boron Doping Activate Strong Metal-Support Interaction for Electrocatalytic Hydrogen Evolution Reaction in Full pH Range. Appl. Catal. B Environ. 2023, 324, 122297. [Google Scholar] [CrossRef]
- Luyen Doan, T.L.; Nguyen, D.C.; Kang, K.; Ponnusamy, A.; Eya, H.I.; Dzade, N.Y.; Kim, C.S.; Park, C.H. Advanced Mott-Schottky Heterojunction of Semi-Conductive MoS2 Nanoparticles/Metallic CoS2 Nanotubes as an Efficient Multifunctional Catalyst for Urea-Water Electrolysis. Appl. Catal. B Environ. 2024, 342, 123295. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, X.; Pan, Q.-M.; Zhang, L.-F.; Cao, Y.-F.; Qian, T. Bimetallic Active Site Nuclear-Shell Heterostructure Enables Efficient Dual-Functional Electrocatalysis in Alkaline Media. Rare Met. 2023, 42, 3024–3033. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, H.; Qian, X.; He, G.; Chen, H. Sulfur Vacancies Engineered Self-Supported Co3S4 Nanoflowers as an Efficient Bifunctional Catalyst for Electrochemical Water Splitting. Appl. Catal. B Environ. 2023, 322, 122104. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Luyen Doan, T.L.; Prabhakaran, S.; Tran, D.T.; Kim, D.H.; Lee, J.H.; Kim, N.H. Hierarchical Co and Nb Dual-Doped MoS2 Nanosheets Shelled Micro-TiO2 Hollow Spheres as Effective Multifunctional Electrocatalysts for HER, OER, and ORR. Nano Energy 2021, 82, 105750. [Google Scholar] [CrossRef]
- Sultan, S.; Tiwari, J.N.; Singh, A.N.; Zhumagali, S.; Ha, M.; Myung, C.W.; Thangavel, P.; Kim, K.S. Single Atoms and Clusters Based Nanomaterials for Hydrogen Evolution, Oxygen Evolution Reactions, and Full Water Splitting. Adv. Energy Mater. 2019, 9, 1900624. [Google Scholar] [CrossRef]
- Wang, P.; Han, X.; Bai, P.; Mu, J.; Zhao, Y.; He, J.; Su, Y. Utilizing an Electron Redistribution Strategy to Inhibit the Leaching of Sulfur from CeO2/NiCo2S4 Heterostructure for High-Efficiency Oxygen Evolution. Appl. Catal. B Environ. 2024, 344, 123659. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, S.; Lv, X.; Dai, S.; Wang, H.; Huang, M. Local-Reconstruction Enables Cobalt Phosphide Array with Bifunctional Hydrogen Evolution and Hydrazine Oxidation. Appl. Catal. B Environ. 2024, 345, 123661. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, L.; Zhao, Y.; Wang, C.; Xin, H.; Li, D.; Ma, F. Synergistic Effects of 1T MoS2 and Interface Engineering on Hollow NiCoP Nanorods for Enhanced Hydrogen Evolution Activity. J. Mater. Sci. Technol. 2023, 145, 165–173. [Google Scholar] [CrossRef]
- Wang, R.; Sun, X.; Zhong, J.; Wu, S.; Wang, Q.; (Ken) Ostrikov, K. Low-Temperature Plasma-Assisted Synthesis of Iron and Nitrogen Co-Doped CoFeP-N Nanowires for High-Efficiency Electrocatalytic Water Splitting. Appl. Catal. B Environ. Energy 2024, 352, 124027. [Google Scholar] [CrossRef]
- Chen, J.; Li, Z.; Li, Z.; Zhou, Y.; Lai, Y. Lattice-Matched Spinel/Layered Double Hydroxide 2D/2D Heterojunction towards Large-Current-Density Overall Water Splitting. Appl. Catal. B Environ. Energy 2024, 355, 124204. [Google Scholar] [CrossRef]
- Li, S.-M.; Liu, Z.; Li, X.-Y.; Ye, C.-F.; Li, Y.; Liu, J.-P.; Yu, S.; Sun, M.-H.; Chen, L.-H.; Su, B.-L.; et al. Synergistically Modulating D-Band Centers of Bimetallic Elements for Activating Cobalt Atoms and Promoting Water Dissociation toward Accelerating Alkaline Hydrogen Evolution. Appl. Catal. B Environ. Energy 2024, 351, 123972. [Google Scholar] [CrossRef]
- Naresh, B.; Sreekanth, T.V.M.; Yoo, K.; Kim, J. Electrocatalytic Behavior of Transition Metal Cr and Co Doped ZnO Nanoparticles for Oxygen Evolution Reaction. Mater. Lett. 2024, 373, 137095. [Google Scholar] [CrossRef]
- Chen, H.; Hu, M.; Jing, P.; Liu, B.; Gao, R.; Zhang, J. Constructing Heterostructure of CeO2/WS2 to Enhance Catalytic Activity and Stability toward Hydrogen Generation. J. Power Sources 2022, 521, 230948. [Google Scholar] [CrossRef]
- Zeng, P.; Meng, Y.; Liu, Z.; Sun, G.; Li, X.; Yang, X.; Ye, C.; Li, Y.; Liu, J.; Chen, L.; et al. N-Doping Coupled with Co-Vacancies Activating Sulfur Atoms and Narrowing Bandgap for CoS Toward Synergistically Accelerating Hydrogen Evolution. Small 2023, 19, 2301279. [Google Scholar] [CrossRef]
- Li, S.; Zhuo, Y.; Liu, D.; Pan, H.; Wang, Z. Anchoring Highly Surface-Exposed Pt Single Atoms on Ni3S2/Co9S8 with Abundant S Vacancies Triggers d-Orbital Electron Rearrangements for Boosted Seawater Hydrogen Evolution. Appl. Catal. B Environ. Energy 2024, 355, 124188. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, S.; Wang, H.; Yuan, W.; Fan, Y.; Cheng, N.; Xue, H.; Jiang, T.; Tian, J. Boron-Doping on the Surface Mediated Low-Valence Co Centers in Cobalt Phosphide for Improved Electrocatalytic Hydrogen Evolution. Appl. Catal. B-Environ. 2023, 320, 122014. [Google Scholar] [CrossRef]
- Cao, W.; Zhao, R.; Liu, G.; Wu, L.; Li, J. Three-Dimensional Ordered Macroporous Design of Heterogeneous Nickel-Iron Phosphide as Bifunctional Electrocatalyst for Enhanced Overall Water Splitting. Appl. Surf. Sci. 2023, 607, 154905. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S. Constructing Built-in Electric Field to Accelerate the Asymmetric Local Charge Distribution for Efficient Alkaline Overall Water/Seawater Splitting. Appl. Catal. B Environ. Energy 2024, 352, 124002. [Google Scholar] [CrossRef]
- He, C.; Yang, L.; Peng, X.; Liu, S.; Wang, J.; Dong, C.; Du, D.; Li, L.; Bu, L.; Huang, X. Alkylamine-Confined Thickness-Tunable Synthesis of Co(OH)2-CoO Nanosheets toward Oxygen Evolution Catalysis. ACS Nano 2023, 17, 5861–5870. [Google Scholar] [CrossRef]
- Wang, B.; Chen, X.; He, Y.; Liu, Q.; Zhang, X.; Luo, Z.; Kennedy, J.V.; Li, J.; Qian, D.; Liu, J.; et al. Fe2O3/P-Doped CoMoO4 Electrocatalyst Delivers Efficient Overall Water Splitting in Alkaline Media. Appl. Catal. B Environ. 2024, 346, 123741. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, A.; Yang, G.; Wang, J.; Liu, Y.; Yan, H.; Tian, C.; Fu, H. Lower-Temperature Synthesis of Nitrogen-Rich Molybdenum Nitride/Nickel (Cobalt) Heterojunctional Assembly for the Effective Water Electrolysis. Adv. Funct. Mater. 2024, 35, 2412979. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Yang, H.; Sivagurunathan, A.T.; Jeong, H.; Han, J.W.; Kim, D. Regulating Electronic Structure of Iron Nitride by Tungsten Nitride Nanosheets for Accelerated Overall Water Splitting. Small 2023, 19, 2300963. [Google Scholar] [CrossRef]
- Qiao, H.; Li, Z.; Liu, F.; Ma, Q.; Ren, X.; Huang, Z.; Liu, H.; Deng, J.; Zhang, Y.; Liu, Y.; et al. Au Nanoparticle Modification Induces Charge-Transfer Channels to Enhance the Electrocatalytic Hydrogen Evolution Reaction of InSe Nanosheets. ACS Appl. Mater. Interfaces 2022, 14, 2908–2917. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, S.; Liu, S.; Wu, J.; Guan, J.; Li, Q.; Wang, Y.; Tao, Y.; Hu, S.; Bai, Y.; et al. The Heterointerface between Fe1/NC and Selenides Boosts Reversible Oxygen Electrocatalysis. Adv. Funct. Mater. 2023, 33, 2300815. [Google Scholar] [CrossRef]
- Lu, B. The CoSe2 Hollow Cube/CoSe2 Nanosheet Interface Catalyst for Efficient Electrolysis of Urea–Assisted Hydrogen Production at Industrial–Grade Currents. Appl. Catal. B Environ. Energy 2024, 350, 123940. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Xin, P.; Wang, H.; Wu, Y.; Gao, C.; He, Q.; Jiang, Y.; Hu, Z.; Huang, S. Interface Engineering of NiS@MoS2 Core-Shell Microspheres as an Efficient Catalyst for Hydrogen Evolution Reaction in Both Acidic and Alkaline Medium. J. Alloys Compd. 2021, 853, 157352. [Google Scholar] [CrossRef]
- Gonzalez-Anota, D.E.; Castaneda-Morales, E.; Paredes-Carrera, S.P.; Manzo-Robledo, A. Modulating the HER-Overpotential at the Interface of Nanostructured MoS2 Synthesized via Hydrothermal Route: An in-Situ Mass-Spectroscopy Approach. Int. J. Hydrogen Energy 2023, 48, 17852–17867. [Google Scholar] [CrossRef]
- Chen, D.-R.; Muthu, J.; Guo, X.-Y.; Chin, H.-T.; Lin, Y.-C.; Haider, G.; Ting, C.-C.; Kalbac, M.; Hofmann, M.; Hsieh, Y.-P. Edge-Dominated Hydrogen Evolution Reactions in Ultra-Narrow MoS2 Nanoribbon Arrays. J. Mater. Chem. A 2023, 11, 15802–15810. [Google Scholar] [CrossRef]
- Alsabban, M.M.; Min, S.; Hedhili, M.N.; Ming, J.; Li, L.-J.; Huang, K.-W. Growth of Layered WS2 Electrocatalysts for Highly Efficient Hydrogen Production Reaction. ECS J. Solid State Sci. Technol. 2016, 5, Q3067–Q3071. [Google Scholar] [CrossRef]
- Agboola, P.O.; Shakir, I.; Almutairi, Z.A.; Shar, S.S. Hydrothermal Synthesis of Cu-Doped CoS2@NF as High Performance Binder Free Electrode Material for Supercapacitors Applications. Ceram. Int. 2022, 48, 8509–8516. [Google Scholar] [CrossRef]
- Gao, C.; Hua, H.; Du, M.; Liu, J.; Wu, X.; Pu, Y.; Li, X. Sulfurized Co-Mo Alloy Thin Films as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Appl. Surf. Sci. 2020, 515, 145842. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Ren, X.; Wang, W. Facile Synthesis of NiS2-MoS2 Heterostructured Nanoflowers for Enhanced Overall Water Splitting Performance. J. Mater. Sci. 2020, 55, 13892–13904. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, J.; Zhao, Y.; Zheng, Y.; Qiao, S.-Z. Engineering 2D Metal-Organic Framework/MoS2 Interface for Enhanced Alkaline Hydrogen Evolution. Small 2019, 15, 1805511. [Google Scholar] [CrossRef]
- Nie, K.; Qu, X.; Gao, D.; Li, B.; Yuan, Y.; Liu, Q.; Li, X.; Chong, S.; Liu, Z. Engineering Phase Stability of Semimetallic MoS2 Monolayers for Sustainable Electrocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2022, 14, 19847–19856. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, L.; Cao, H.; Zhang, X.; Li, G. Symbiosis of 1 T and 2H Phases in the Basal Plane of Defective MoS2 Nanoflowers for Efficient Hydrodesulfurization. Fuel 2022, 322, 124252. [Google Scholar] [CrossRef]
- Ghanashyam, G.; Kim, H. Co-Doped 1T-MoS2 Microspheres Embedded in N-Doped Reduced Graphene Oxide for Efficient Electrocatalysis toward Hydrogen and Oxygen Evolution Reactions. J. Power Sources 2024, 596, 234088. [Google Scholar] [CrossRef]
- Gurusamy, P.; Perumalsamy, S.V.; Pandian, T.; Vellingiri, G.; Kulanthaivel, J.; Sinthika, S.; Mithra, K.M. Effective Solar-Driven Overall Electrocatalytic Water-Splitting by Co-Doped 1T/2H MoS2 Nanoparticles. Int. J. Hydrogen Energy 2025, 130, 452–461. [Google Scholar] [CrossRef]
- Kong, L.; Gao, C.; Liu, Z.; Pan, L.; Yin, P.; Lin, J. Cerium-Doped 1 T Phase Enriched MoS2 Flower-like Nanoflakes for Boosting Hydrogen Evolution Reaction. Chem. Eng. J. 2024, 479, 147725. [Google Scholar] [CrossRef]
- Li, B.; Nie, K.; Zhang, Y.; Yi, L.; Yuan, Y.; Chong, S.; Liu, Z.; Huang, W. Engineering Single-Layer Hollow Structure of Transition Metal Dichalcogenides with High 1T-Phase Purity for Hydrogen Evolution Reaction. Adv. Mater. 2023, 35, 2303285. [Google Scholar] [CrossRef]
- Wu, K.; Wang, D.; Fu, Q.; Xu, T.; Xiong, Q.; Peera, S.G.; Liu, C. Co/Ce-MOF-Derived Oxygen Electrode Bifunctional Catalyst for Rechargeable Zinc–Air Batteries. Inorg. Chem. 2024, 63, 11135–11145. [Google Scholar] [CrossRef]
- Li, H.; Du, L.; Zhang, Y.; Liu, X.; Li, S.; Yang, C.C.; Jiang, Q. A Unique Adsorption-Diffusion-Decomposition Mechanism for Hydrogen Evolution Reaction towards High-Efficiency Cr, Fe-Modified CoP Nanorod Catalyst. Appl. Catal. B Environ. 2024, 346, 123749. [Google Scholar] [CrossRef]
- Saini, R.; Naaz, F.; El-Sheikh, M.A.; Farooq, U. Co-Doping-Assisted Systematic Evolution of a Truncated Octahedral from Tetrahedral Cu2O for Enhanced Electrochemical OER Activity. ACS Appl. Energy Mater. 2024, 7, 8882–8893. [Google Scholar] [CrossRef]
- Ajmal, M.; Zhang, S.; Guo, X.; Liu, X.; Shi, C.; Gao, R.; Huang, Z.-F.; Pan, L.; Zhang, X.; Zou, J.-J. Rapid Reconstruction of Nickel Iron Hydrogen Cyanamide with In-Situ Produced Proton Acceptor for Efficient Oxygen Evolution. Appl. Catal. B Environ. Energy 2025, 361, 124561. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Z.; Wang, K.; Ye, Q.; Shen, B.; Yang, F.; Cheng, Y. Dual Doping of B and Fe Activated Lattice Oxygen Participation for Enhanced Oxygen Evolution Reaction Activity in Alkaline Freshwater and Seawater. Adv. Funct. Mater. 2024, 34, 2402264. [Google Scholar] [CrossRef]
- Jung, S.; Senthil, R.A.; Min, A.; Kumar, A.; Moon, C.J.; Choi, M.Y. Laser-Synthesized Co-Doped CuO Electrocatalyst: Unveiling Boosted Methanol Oxidation Kinetics for Enhanced Hydrogen Production Efficiency by In Situ/Operando Raman and Theoretical Analyses. Small Methods 2024, 8, 2301628. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, A.; Jiao, Y.; Zheng, H.; Wang, X.; Xie, Y.; Wang, L.; Tian, C.; Fu, H. Two-Dimensional Porous Molybdenum Phosphide/Nitride Heterojunction Nanosheets for pH-Universal Hydrogen Evolution Reaction. Angew. Chem.-Int. Edit. 2021, 60, 6673–6681. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z.; Li, Y.; Guo, L.; Wang, Y.; Fan, C.; Wang, Y.; Li, R.; Zhang, X.; Li, F.; et al. Sulfur Vacancy MoS2 for Electrocatalytic Reduction of Nitrate to Ammonia with Enhanced Selectivity. J. Alloys Compd. 2023, 955, 170199. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Chai, Y.; Dong, B. Ligand Modulation of Active Sites to Promote Cobalt-Doped 1T-MoS2 Electrocatalytic Hydrogen Evolution in Alkaline Media. Angew. Chem. Int. Edtion 2023, 62, e202313845. [Google Scholar] [CrossRef]
- Dong, J.; An, B.; Liu, W.; Su, H.; Li, N.; Gao, Y.; Ge, L. Cation-Induced Interface Electric Field Redistribution and Molecular Orbital Coupling in Co-FeS/MoS2 for Boosting Electrocatalytic Overall Water Splitting. Chem. Eng. J. 2024, 498, 155102. [Google Scholar] [CrossRef]
- Sang, Y.; Xue, J.; Hu, J.; Chen, L. High-Current Density Alkaline Water/Seawater Splitting by Mo and Fe Co-Doped Ni3S2: Invariant Active Sites with Accelerated Water Dissociation Kinetics. Appl. Catal. B Environ. Energy 2025, 361, 124698. [Google Scholar] [CrossRef]
- Xu, X.; Guo, K.; Sun, J.; Yu, X.; Miao, X.; Lu, W.; Jiao, L. Interface Engineering of Mo-doped Ni2P/FexP-V Multiheterostructure for Efficient Dual-pH Hydrogen Evolution and Overall Water Splitting. Adv. Funct. Mater. 2024, 34, 2400397. [Google Scholar] [CrossRef]
- Cheng, X.; Tong, Y. Interface Coupling of Cobalt Hydroxide/Molybdenum Disulfide Heterostructured Nanosheet Arrays for Highly Efficient Hydrazine-Assisted Hydrogen Generation. ACS Sustain. Chem. Eng. 2023, 11, 3219–3227. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, Z.; Xie, Y.; Zheng, J.; Pan, X.; Li, L.; Zhao, G. Surface Self-Reconstruction of Fe-Ni3S2 Electrocatalyst for Value-Generating Nitrile Evolution Reaction to Drive Efficient Hydrogen Production. Adv. Funct. Mater. 2023, 33, 2301884. [Google Scholar] [CrossRef]
- Deng, Y.; Cao, Y.; Xia, Y.; Xi, X.; Wang, Y.; Jiang, W.; Yang, D.; Dong, A.; Li, T. Self-Templated Synthesis of CoFeP@C Cage-In-Cage Superlattices for Enhanced Electrocatalytic Water Splitting. Adv. Energy Mater. 2022, 12, 2202394. [Google Scholar] [CrossRef]
- Wang, J.; Ling, Q.; Yao, Y.; Zhu, D.; Shu, S.; Zhou, Z.; Wu, X.; Wu, P. Willow Catkin-like Co4S3–WS2 Nanostructured Electrocatalyst for Efficient Overall Alkaline Water Splitting. ACS Appl. Nano Mater. 2024, 7, 24408–24416. [Google Scholar] [CrossRef]
- Mounesh; Thippeswamy, B.A.; Shiralkar, P.; Balakrishna, R.G.; Nagaraja, B.M.; Pramoda, K. Non-Precious Tetra-(4-Methylthiazole)-Carboxamide Cobalt(II) Phthalocyanine Supported on Functionalized Carbon Nanotubes as an Efficient Electrocatalyst for a Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2024, 8, acsaem.4c01292. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, C.; Wen, M.; Pan, F.; Zhang, X.; Ma, H.; Hou, B.; Xin, X. Low-Cost Composite Electrodes by Active Fe3O4 (111) of Fly Ash Magnetic-Sphere for Efficient Electrochemical Overall Water Splitting. Int. J. Environ. Sci. Technol. 2025, 24, 1475–1496. [Google Scholar] [CrossRef]
- Shooshtari Gugtapeh, H.; Abbasi, M.; Hasanzadeh Moghadam, M.; Rezaei, M. Solvent-Exchange-Assisted Activation of Cu-1,4-Benzene Dicarboxylate Metal-Organic Framework for Use as a Bifunctional Water Splitting Electrocatalyst. Electrochim. Acta 2024, 508, 145224. [Google Scholar] [CrossRef]
- Rashid, U.; Zhu, Y.; Cao, C. Microwave Assisted Synthesis of Cobalt-Doped Copper Selenite Nanorice as Bifunctional Electrocatalyst for Overall Water Splitting. J. Electroanal. Chem. 2024, 962, 118267. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wan, Z.; Wang, Z.; Gao, F.; Xuan, C. Nitrogen-Doped Carbon Nanotubes/Nanoparticles Confined Co/FeCo Composites with Metal-Nitrogen Sites for Efficient Multifunctional Electrocatalysis. J. Environ. Chem. Eng. 2024, 12, 114326. [Google Scholar] [CrossRef]
- Huang, C.; Zhan, G.; Xiao, Z.; Lin, S. Synergistic Dual-Functional Full Deionization and Electrocatalysis of Water by ZnO/Ti3C2Tx Heterojunction Supported with Novel Template. Next Mater. 2024, 5, 100267. [Google Scholar] [CrossRef]
- Zahid, R.; Abdul Karim, M.R.; Khan, F.S.; Zeb, G.; Marwat, M.A.; Khan, M.Z.; Gohar, O.; Haq, E.U. Catalytically Active Bimetallic Nickel–Cobalt MOF Linked Via Pyridine 2, 6-Dicarboxylate for Electrochemical Water Splitting Applications. Arab. J. Sci. Eng. 2025, 50, 6625–6637. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Yang, Y.; Pan, S.; Pan, W.; Tang, M.; Liu, K. Electronic Modulation of MOF-Derived CoxMnyB Nanosheet Arrays toward Efficient Bifunctional Electrocatalysts for Water Splitting. J. Electroanal. Chem. 2024, 970, 118553. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Lv, T.; Tan, X.; Wang, Q.; Wang, Y.; Meng, C. Core-Shell Cobalt-Iron Silicide Electrocatalysts with Enhanced Bifunctional Performance in Hydrogen and Oxygen Evolution Reactions. J. Colloid Interface Sci. 2025, 682, 1–10. [Google Scholar] [CrossRef]
- Kang, H.; Liu, Y.; Wei, M.; Zhou, L.; Wang, C. Activating Spinel CoMn2O4 Supported on CNT via Zn Substitution for Bifunctional Oxygen Electrocatalysis. J. Alloys Compd. 2024, 1000, 175089. [Google Scholar] [CrossRef]
- Yu, T. FeNi/Ni2P Nanoparticles Encapsulated in Nitrogen-Doped Porous Carbon: Efficient Electrocatalysts for Oxygen Evolution Reaction. J. Mater. Sci. 2024, 59, 21710. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Song, Y.; Huang, Y.; Zhang, J.; Wu, S.; Zhang, W.; Wang, J.; Zhang, X. Facile One-Step Fabrication of 1T-Phase-Rich Bimetallic CoFe Co-Doped MoS2 Nanoflower: Synergistic Engineering for Bi-Functional Water Splitting Electrocatalysis. Molecules 2025, 30, 2343. https://doi.org/10.3390/molecules30112343
Li X, Song Y, Huang Y, Zhang J, Wu S, Zhang W, Wang J, Zhang X. Facile One-Step Fabrication of 1T-Phase-Rich Bimetallic CoFe Co-Doped MoS2 Nanoflower: Synergistic Engineering for Bi-Functional Water Splitting Electrocatalysis. Molecules. 2025; 30(11):2343. https://doi.org/10.3390/molecules30112343
Chicago/Turabian StyleLi, Xinyue, Yahui Song, Yiming Huang, Jihui Zhang, Siyu Wu, Wentao Zhang, Jin Wang, and Xian Zhang. 2025. "Facile One-Step Fabrication of 1T-Phase-Rich Bimetallic CoFe Co-Doped MoS2 Nanoflower: Synergistic Engineering for Bi-Functional Water Splitting Electrocatalysis" Molecules 30, no. 11: 2343. https://doi.org/10.3390/molecules30112343
APA StyleLi, X., Song, Y., Huang, Y., Zhang, J., Wu, S., Zhang, W., Wang, J., & Zhang, X. (2025). Facile One-Step Fabrication of 1T-Phase-Rich Bimetallic CoFe Co-Doped MoS2 Nanoflower: Synergistic Engineering for Bi-Functional Water Splitting Electrocatalysis. Molecules, 30(11), 2343. https://doi.org/10.3390/molecules30112343





