Abstract
To enhance the performance of photocatalytic CO2 reduction, the development of suitable cocatalysts represents an effective strategy. Cocatalysts can interact with photocatalysts to improve light absorption capabilities and facilitate the separation and transfer of photogenerated electrons and holes. Moreover, they provide highly active surface sites that promote the adsorption and activation of CO2, which leads to acceleration of photocatalytic reduction. Herein, WO3 is employed as a cocatalyst to promote the CO2 photoreduction performance of a g-C3N4-TiO2 heterojunction through a facile and scalable calcination method. In pure water, optimal WO3/g-C3N4-TiO2 (WCT) delivers high selectivity CO and CH4 formation of 48.31 µmol·g−1 and 77.18 µmol·g−1 in the absence of a sacrificial reagent and extra photosensitizer, roughly 13.9 and 45.7 times higher than that of g-C3N4-TiO2 (CT). WO3 can strongly interact with g-C3N4-TiO2 electronically, guiding electrons across the interface to the surface. The oxygen vacancies in WO3, as electron-enriched centers, not only enhance charge separation and form efficient charge transfer channels but also capture photogenerated electrons to suppress charge recombination. This strong interaction and oxygen vacancies in WO3 jointly improve photocatalytic CO2 reduction activity and selectivity, offering a feasible way to design efficient cocatalysts.
1. Introduction
Utilizing excess CO2 as a carbon raw material to generate value-added compounds via catalytic reactions is one of the viable strategies for reducing CO2 emissions. Thus, exploring photocatalytic CO2 reduction into C1 solar fuels such as CO and CH4, which can decrease CO2 content in the environment and reduce humanity’s reliance on petroleum and other products, holds great promise in curbing global warming [1,2,3,4]. The predominant limitations of reported photocatalysts for CO2 reduction include low efficiency, high cost, and toxicity [5,6]. In particular, slow charge dynamics including insufficient light absorption and high recombination rates of electron–hole pairs, together with high activation energies, severely restrict photocatalytic CO2 activity [7,8,9]. Consequently, approaches like band-gap engineering through metal or non-metal element doping, deposition of noble metals, or the incorporation of cocatalysts [10,11] have been proposed to tackle these problems.
The incorporation of cocatalysts into photocatalysts is an effective way to enhance CO2 photoreduction performance. Cocatalysts can promote surface charge separation, crucial for efficient charge transfer; boost the quantity of active sites by modifying the photocatalyst’s surface structure and electronics; reduce CO2 conversion activation energy, making the reaction more thermodynamically favorable and accelerating product formation; enhance selectivity toward target molecules like CO, methane, or formic acid, and improve the photocatalytic system’s stability by alleviating excess charge accumulation; optimize the reaction pathway, guiding reactants through beneficial steps to lower the energy barrier and minimize side reactions; and some with unique optical properties can enhance light harvesting by absorbing specific wavelengths of light and transferring energy to photocatalysts, expanding the light-response range. This multifaceted role of cocatalysts shows great potential for developing more efficient and sustainable photocatalytic CO2 utilization systems [12,13]. Among the reported cocatalysts, tungsten trioxide (WO3), as an oxide of transition metal elements, exhibits distinctive properties. Characterized by a relatively wide band-gap energy spanning from 2.4 to 2.8 eV, WO3 is capable of absorbing a broader spectrum of visible light, which endows it with enhanced attractiveness [14]. The synergistic effect with other materials can not only improve the light absorption performance and carrier separation efficiency of the photocatalyst but also adjust the surface properties and active site distribution of the catalyst, thereby improving the overall performance of the photocatalyst in CO2 adsorption and activation processes, and thereby enhancing catalytic efficiency. Moreover, WO3 emerges as a highly prospective candidate for photocatalysts on account of its non-toxicity, physicochemical stability, and remarkable resistance to photo-corrosion [15].
Concurrently, graphite carbon nitride (g-C3N4), due to its unique band structure and ease of fabrication, has become a prominent material in the field of photocatalysis [16,17,18]. This particular material exhibits a low band gap of 2.7 eV, minimal particle presence, non-toxicity, cost-effectiveness, favorable controllability of the structure, and superior electronic and optical properties [19]. This material also enhances the photocatalytic activity of the composite by extending the absorption band. Nevertheless, the low electrical conductivity, relatively small surface area of g-C3N4, as well as the rapid recombination of electrons and holes, limit its photocatalytic activity [20]. A promising aspect is that the integration of g-C3N4 and TiO2 in a heterojunction configuration has demonstrated remarkable photocatalytic efficiency [21]. The photoreduction of CO2 based on a g-C3N4/TiO2 heterojunction was studied to produce solar fuel. For instance, Wang et al. [22] fabricated g-C3N4–based composite photocatalysts modified with titanium dioxide (TiO2) through ball-milling and calcination for the photoreduction of CO2 to methane and carbon monoxide. Zhang et al. [23] constructed 3D/2D TiO2/p-g-C3N4 micro-nano heteroarchitectures through a facile acid hydrothermal route, which improved the performance of photocatalytic CO2 reduction. Although the g-C3N4-TiO2 heterostructure has shown good stability in CO2 reduction, the technology based on the g-C3N4-TiO2 heterostructure photocatalyst is not mature enough to obtain the ideal product possessing a high yield along with high selectivity.
In this study, the incorporation of a WO3 cocatalyst into a g-C3N4-TiO2 heterojunction was attempted to fabricate WO3/g-C3N4-TiO2 photocatalysts for enhanced photocatalytic CO2 reduction. A detailed synthesis process was elucidated; a comprehensive characterization of the catalysts was performed; and the mechanisms underlying the enhanced photocatalytic activity were revealed. These materials demonstrated excellent high photocatalytic activity and chemical stability for carbon dioxide reduction due to enhanced charge separation and transfer.
2. Results and Discussion
2.1. Characterizations of WO3/g-C3N4-TiO2
WO3/g-C3N4-TiO2 samples were synthesized by the calcining method as depicted in Figure 1a. The Zeta potential of TiO2 is 1.09 mV and that of WO3 is −55.41 mV, which favors the formation of the interfaces between TiO2 and WO3, and consequently, a strong interaction among these semiconductors, along with more light absorption pathways. The morphology of WO3/g-C3N4-TiO2 was probed further via SEM, showing a two-dimensional layered structure of g-C3N4 and a sphere structure of TiO2, which are shown in Figure 1b. WO3 synthesized by the hydrothermal method has a cubic configuration, and uniformly disperses over TiO2. Some g-C3N4 sheets are also observed to be attached to WO3. In this regard, WO3/g-C3N4-TiO2 with a good interaction to construct composite is achieved. More interestingly, the HRTEM images in Figure 1c provide further evidence of the strong interaction existing among these three components. The microspheres of TiO2 with multiple channels were seen as well as WO3 possessing a cubic structure, which facilitates the formation of oxygen vacancies [24]. Furthermore, the combined structure of these components is characterized by enhanced compactness, facilitating the separation of charge carriers and ensuring efficient electron transport. Thus, incorporation of a WO3 cocatalyst into the g-C3N4-TiO2 heterojunction resulted in better optical radiation penetration accompanied by an efficient adsorption process to maximize photoactivity. Further characterization of the catalyst’s elemental composition was carried out using energy-dispersive X-ray spectroscopy (EDS). These results presented in Figure 1d reveal that C, N, O, Ti, and W are dispersed throughout the composite photocatalyst. Elemental mapping of WO3/g-C3N4-TiO2 further indicates the uniform distribution. Moreover, they demonstrate the successful binding of WO3 to the surface of g-C3N4-TiO2. Based on these findings, the WCT heterojunction has been successfully synthesized, which is conducive to efficient light absorption and charge carrier separation.
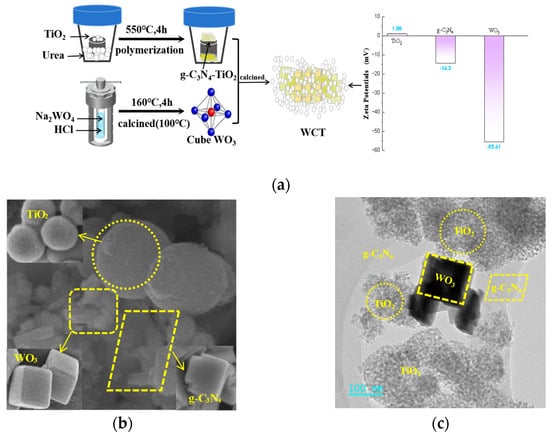
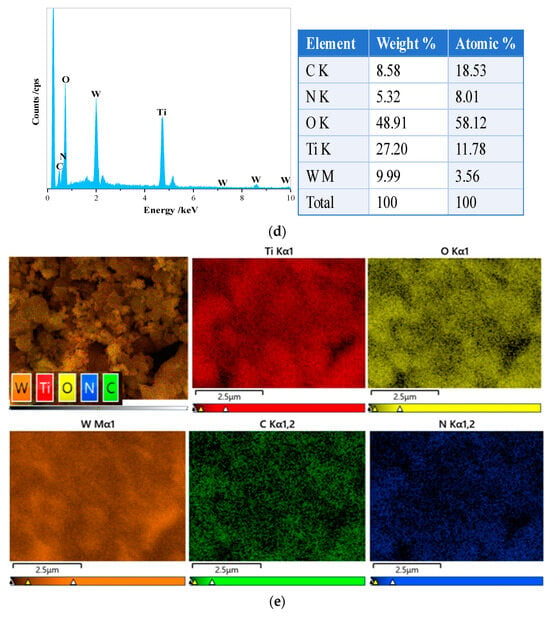
Figure 1.
Material preparation process and the Zeta potential of TiO2, WO3, and g−C3N4 (a); SEM image of WO3/g−C3N4-TiO2 and TiO2, WO3, and g−C3N4 (b); HRTEM image of WO3/g−C3N4−TiO2 (c); SEM image of WO3/g−C3N4-TiO2 with corresponding EDS (d); elemental mapping of WO3/g−C3N4−TiO2 (e).
The results of XRD are displayed in Figure 2 to characterize the phase structure of the prepared photocatalysts. The characteristic peaks of pristine WO3 are detected at 2θ of 23.10°, 23.62°, 24.38°, 26.54°, 28.72°, 33.32°, and 34.22°. These peaks are associated with the monoclinic phase of WO3 according to the JCPDS, Card No. 83-0951 [25]. For g-C3N4, the peaks at 13.4° and 27.3° correspond to (100) and (002) crystal planes of g-C3N4 (JCPDS, Card No.87-1526). In g-C3N4, the (002) plane is attributed to interplanar stacking of the aromatic system, whereas the (100) plane is associated with the in-plane structural packing motif [26]. The peaks of TiO2 match those of the standard PDF card (JCPDS, Card No.21-1272), which provides an indication that the experimental specimens are constituted purely of anatase TiO2 [27]. Regarding the WO3/g-C3N4-TiO2 composite, characteristic peaks belonging to TiO2, g-C3N4, and WO3 are clearly visible. Notably, the characteristic (002) diffraction peak of g-C₃N₄ shifted from 27.3° to 26.5° upon composite formation with WO3/g-C3N4-TiO2, demonstrating lattice expansion and interfacial electronic coupling effects [24]. Moreover, no impurity-related peaks are detected in the XRD pattern, confirming the high purity of the as-synthesized samples. This result further demonstrates successful fabrication of the composite without significant distortion of the original crystal structures of the individual components.
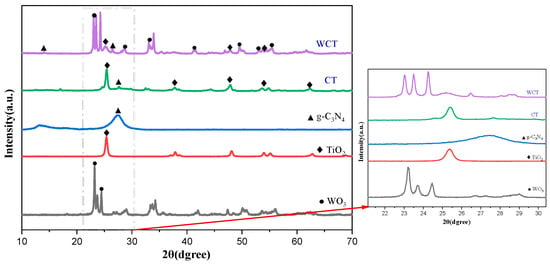
Figure 2.
XRD patterns of WO3, TiO2, g-C3N4, CT, and WCT.
As depicted in Figure 3, to study the structural features of the samples, Raman spectra were obtained. For pure WO3, characteristic bands are clearly discernible at 131.92, 272.46, 716.92, and 807.41 cm−1. The peak at 272.46 cm−1 is attributable to the bending vibration of the W–O bond, while the peaks at 716.92 and 807.41 cm−1 are indicative of the stretching vibration mode of the O–W–O bands. These results are consistent with the previous ones [28,29]. Analogously, the Raman peaks at 360.1 and 1229.6 cm−1 belong to the polymer-based structure of g-C3N4 [30]. The absence of D and G bands was probably accounted for by the relatively low content of g-C₃N₄ revealed by EDS, along with its poor crystallinity indicated by the indistinct XRD peaks [31]. Anatase TiO2 primarily consisted of peaks at 143.41, 199.35, 396.22, and 519.64 cm−1. Specifically, the peaks at 143.41 and 396.22 cm−1 correspond to vibration of the Ti–O bond, while the peak at 199.35 cm−1 is attributed to vibration of the Ti–O–Ti bond. The peak at 519.64 cm−1 is associated with vibration of the O–Ti–O bond [32,33]. In the distinctive characteristic patterns of the WO3/g-C3N4-TiO2 (WCT) composite, Raman modes appear at 142.21, 271.21, 716.17, and 808.95 cm−1. It is possible that due to the low content and poor crystallinity of g-C3N4, as well as it being encapsulated, the peaks of graphitic carbon nitride were not observed in the WCT composite. A slight shift was observed in the peaks of WO3 and TiO2, indicating an interaction between WO3 and TiO2 that results in the formation of a composite facilitating efficient charge carrier transfer. Furthermore, the distinct vibrational peak of WO3 in the Raman spectrum of the composite indicates that WO3 within the WCT composite possesses remarkable crystallinity.
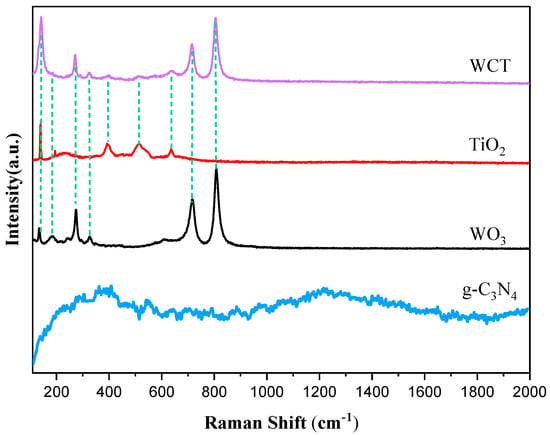
Figure 3.
Raman spectra of WO3, TiO2, g−C3N4, and WCT.
2.2. CO2 Photoreduction Efficiencies
To assess the effectiveness and catalytic activity of the WO3/g-C3N4-TiO2 photocatalyst, the CO2 reduction performance of the prepared materials was assessed under xenon lamp illumination in water without additional organic sacrificial agents (such as triethanolamine) or photosensitizers. And a series of conditional blank control experiments were carried out; the data can be found in Table S1 of the Supplementary File. The main reduction products were identified as CO and CH4, and nothing was detected in the dark or in the absence of photocatalysts or CO2. The evolution of CO and CH4 for all samples was observed in (Figure 4). The WO3/g-C3N4-TiO2 (WCT) samples exhibited boosted photocatalytic performance in reducing of CO2 to solar fuel C1 products, among which WCT displays a maximum CO production yield of 48.31 µmol·g−1and a maximum CH4 production yield of 77.18 µmol·g−1, roughly 13.9 and 45.7 times higher than that of CT (3.53 and 1.70 µmol·g−1, respectively). Furthermore, with increased loading of WO3, the photocatalytic performance of WCT is markedly enhanced. From Figure 4b,c, the yield of CO and CH4 for WCT samples are 1.25 and 1.36 times higher than 0.5 WCT samples. After carrying out the reaction for a four-cycle experiment, the activity decrease is negligible, revealing the good photocatalytic stability of WCT (Figure 4d). The apparent quantum efficiency (AQE) of CO2 photoreduction was measured at different monochromatic wavelengths including 380, 400, 420, 450, 480, and 500 nm over the WCT sample. Clearly, the trend of AQE matches well with the light absorption spectrum of WCT (Figure 4e) and the value of AQE reaches 4.41% at a 400 nm wavelength (Table S4 in Supplementary File), affirming the photocatalytic nature of CO2 reduction. In order to uncover the underlying reasons for the high production yield of CH4, in situ diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS) was explored. When CO2/H2O is introduced into the system in the dark, the chemisorption of CO2 on the catalyst surface is detected, evidenced by the appearance of peaks corresponding to HCO3−, m-CO32−, and b-CO32− species [34]. Under light irradiation, adsorption peaks associated with *COOH (at 1669 cm−1), *CHO (at 1012 cm−1), and *CH3O (at 1042 cm−1) emerge. These species are crucial intermediates in the process of converting CO2 into CH4.
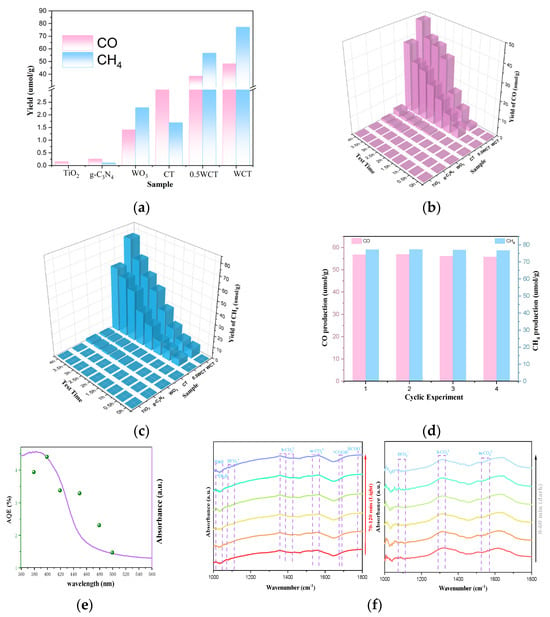
Figure 4.
(a) Yields of CO and CH4; (b) photocatalytic activity of CO; (c) photocatalytic activity of CH4; (d) the cyclic experiment results of WCT; (e) wavelength−dependent AQE and the UV−vis absorption spectrum of WCT; (f) in situ DRIFT spectra for photocatalytic CO2 reduction over WCT.
Additionally, a comparison of the photocatalytic performance between the WCT samples and other previously reported advanced photocatalysts in the literature is summarized in Table 1.

Table 1.
The photocatalytic activities of other previous advanced photocatalysts reported in the literature are summarized [34,35,36,37].
2.3. Insight into Increased Photocatalytic Activity
Figure 5a presents the XPS scan spectrum of WCT. As can be observed from this figure, WCT encompasses signals of the elements of tungsten, carbon, nitrogen, titanium, and oxygen. The signal peaks of these elements can be deconvoluted to conduct a more in-depth analysis of their chemical states. The high resolution W4f spectrum in Figure 5b shows binding energies of 34.8 and 37.0 eV for W4f7/2 and W4f5/2, respectively, corresponding to the W+6 oxidation state of WO3 [38]. From Figure 5c, the signals of Ti2P3/2 and Ti2P1/2 in TiO2 are located at the peaks of Ti2p at 458.2 and 464.1 eV. Figure 5d depicts the XPS spectrum of O1s with two peaks at 529.5 and 531.1 eV. The strong O1s peak at 529.5 eV is ascribed to the lattice O atom in the WCT composite. Owing to WO3 lattice O-defects and surface-adsorbed OH−/O2− [39], the intensity of peak at binding energy 531.1 eV decreases. Regarding Figure 5e, it reveals the C1s orbital spectrum of WCT, featuring three characteristic peaks at binding energies of 284.5 eV, 286.1 eV, and 288.4 eV. These peaks are each assigned to the sp2 C–C, C–N, and N–C=N bonds in g-C3N4. The N1s spectrum in Figure 5f, t can be split into peaks at 398.1 eV and 399.8 eV, which respectively correspond to the C=N–C and N–C bonds in g-C3N4 [40]. In conclusion, the successful synthesis of the WO3/g-C3N4-TiO2 (WCT) composites is further supported by X-ray photoelectron spectroscopy (XPS) analysis of the composite specimens. This analysis also provides additional evidence that aligns with the results obtained from other characterization methods.
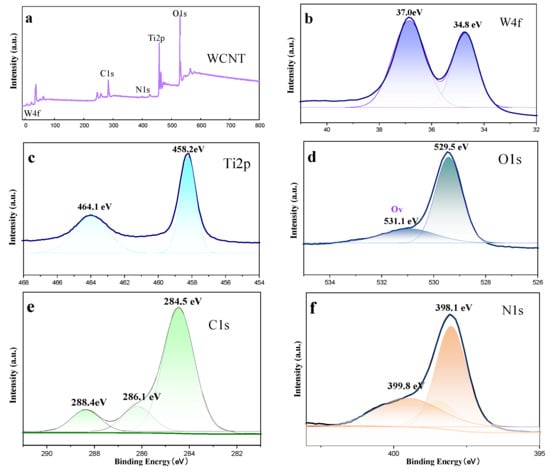
Figure 5.
The XPS survey spectra of the WCT sample (a) and XPS of W4f (b), Ti2p (c), O1s (d), C1s (e), and N1s (f) of the WCT sample.
To investigate light-harvesting capabilities and the band gap of the samples, UV–vis DRS spectra were obtained. Figure 6a depicts the UV–vis DRS spectra of TiO2, g-C3N4, WO3, CT, and WCT samples. The incorporation of WO3 significantly enhances the light absorption capacity of g-C3N4-TiO2 (CT), thereby exerting a considerable effect on the photocatalytic process. Moreover, narrower band-gap energies (Egs) can enhance the photoelectric conversion efficiency of composite materials, which is crucial for the photocatalytic process. The band gaps were determined by Tauc plots in Figure 6b. The band-gap energies (Egs) of pure WCT, CT, and WO3 were calculated to be 2.69, 2.77, and 2.81 eV, respectively [41]. This can be attributable to WO3–induced mid-gap states (XPS in Figure 5) and interfacial charge transfer. This enhanced light harvesting directly correlates with a 36% improvement in CH4 production (Figure 4c), confirming the critical role of band engineering.
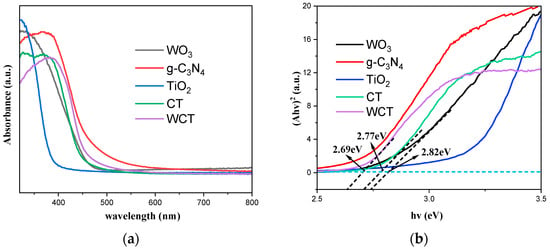
Figure 6.
(a) The spectra acquired from UV–vis DRS; (b) the plot of (αhν)2 versus the energy (hν) of the samples.
Photocatalytic activity depends mainly on efficient charge separation and transport. A higher photoluminescence (PL) intensity indicates a lower charge separation efficiency and leads to a higher electron–hole recombination rate. Photoluminescence carrier production and its recombination can be evaluated by photoluminescence (PL) analysis. Figure 7 shows the charge separation efficiency of the TiO2, g-C3N4, WO3, CT, and WCT composite samples. Using pristine TiO2, a strong emission peak appears, owning to faster recombination of photogenerated electron–hole pairs on the catalyst surface [42]. When coupling TiO2 with g-C3N4, the CT sample’s PL intensity experiences a gradual yet significant decrease, which is attributed to superior charge carrier separation [43,44]. Moreover, the incorporation of WO3 in a g-C3N4-TiO2 heterojunction to construct the WCT composite is found to be efficient for the separation and transfer of electrons; analogous discoveries have been presented in the relevant literature [45]. Consequently, the construction of a WO3/g-C3N4-TiO2 heterojunction can facilitate the efficient generation and separation of charge carriers, which is advantageous for the effective reduction of CO2 under visible light.
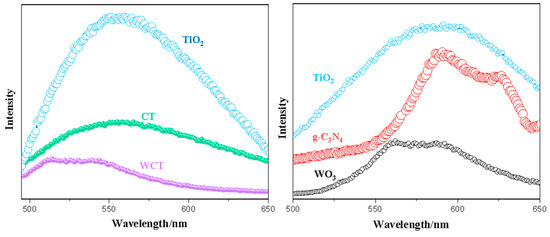
Figure 7.
PL analysis of TiO2, g-C3N4, WO3, CT, and WCT samples.
To gain deep insights into the electron excitation processes, charge migration mechanisms, and kinetic characteristics such as charge transfer rates of the as-prepared catalysts, measurements of photocurrent responses and analyses of electrochemical impedance spectroscopy (EIS) were carried out. A Nyquist plot of TiO2, WO3, g-C3N4, CT, and WCT is depicted in Figure 8a. In electrochemical impedance spectroscopy (EIS), there is generally a positive correlation between the arc diameter in the Nyquist plot and the electron transport resistance exhibited by the catalyst. The arc radius corresponding to the WCT–based electrode is smaller in comparison to TiO2, g-C3N4, and CT, resulting from the incorporation of WO3 into g-C3N4-TiO2. Therefore, due to the higher photocatalytic activity of WCT compared to CT, this signifies the lowest charge transfer resistance (Rct) among WCT catalysts. This facilitates the separation of charge carriers and enables the rapid separation of photogenerated electron–hole pairs [46,47]. Moreover, the electronic interactions of the prepared photocatalysts were studied through photocurrent measurements using an electrochemical workstation. As depicted in Figure 8b, the chronoamperometric current–time (I–t) curve is presented. The photocurrent measurements were obtained under multiple light-on and light-off conditions. In comparison to CT and WO3, the photocurrent density of WCT is the highest, manifesting the highest separation efficiency of carriers for WCT. Consistent with the PL results is the fact that the photocurrent response of WCT is higher than that of CT and pure WO3. The WCT composites exhibit a significantly higher photocurrent, providing compelling evidence of enhanced charge separation efficiency (e−-h+). This improvement enables the generation of a greater number of photogenerated electrons, thereby substantially augmenting photocatalytic activity. The aforementioned results unequivocally demonstrate that the integration of WO3 into the g-C3N4-TiO2 heterojunction is conducive to promoting photocatalytic performance by facilitating the separation and transfer of carriers.
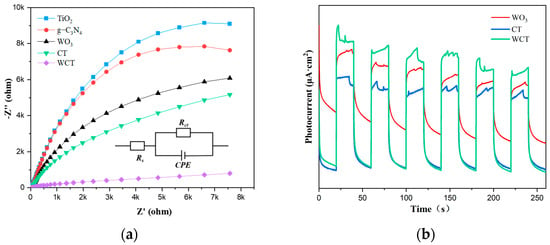
Figure 8.
(a) Electrochemical impedance spectroscopy and (b) the transient photocurrent response of the prepared photocatalysts.
Oxygen vacancies (Ovs), as intrinsic defects, can modulate the local geometric structure of metal oxides, thereby tailoring their physical and chemical properties [48]. This modulation influences the band alignment of metal oxides and introduces defect levels, which significantly enhances light absorption. Additionally, the existence of Ovs alters the charge separation and transfer processes within photocatalysts [49]. To investigate the unpaired electrons in WO3 with oxygen vacancies, electron paramagnetic resonance (EPR) measurements were conducted. As can be seen from Figure 9a, the signals of different samples (WCT, WO3, TiO2, CT, and g-C3N4) exhibit varying intensity around g = 2.003, which corresponds to unpaired electrons located at the Ovs sites, indicating differences in their unpaired-electron environments, likely related to distinct electronic structures or surface properties. It is evident that after the calcination process, the signal intensity at g = 2.003 of WCT markedly increases, confirming the substantial introduction of Ovs into WO3 through calcination. The trap states induced by Ovs effectively promote charge separation and facilitate rapid charge transport channels at the heterointerface. Moreover, Ovs enhance light absorption, leading to an increase in photogenerated carriers.
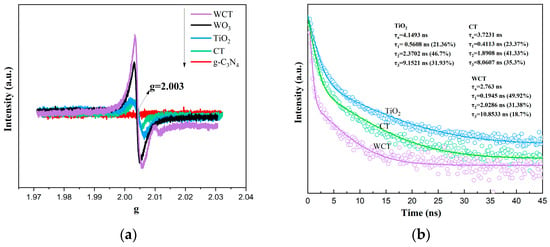
Figure 9.
EPR spectra of samples (a); TRPL spectra of TiO2, CT, and WCT (b).
Furthermore, time-resolved photoluminescence (TRPL) spectra can be utilized to explore the dynamic photogenerated charge transfer process. This approach provides more in-depth information, which is beneficial for further validating the underlying mechanism. The TRPL spectra clearly demonstrate that WO3/g-C3N4-TiO2 enables ultrafast electron extraction (τ1 = 0.19 ns) and promotes long-lived charge separation (with the contribution of τ3 reduced to 18.7%). The presence of oxygen vacancies can change the charge distribution on a material’s surface, which in turn affects the separation of electron–hole pairs. On one hand, these oxygen vacancies act as electron-trapping centers, capturing photogenerated electrons from the area near the holes. This process increases the distance between the electrons and holes, reducing their recombination and promoting long-lived charge separation. On the other hand, oxygen vacancies can create a specific electric field distribution on the material’s surface. This electric field helps drive the electrons and holes to move in opposite directions, further enhancing the efficiency of charge separation. Consequently, this reduces the contribution of τ3 to 18.7% and supports the achievement of sustained charge separation. These features are crucial for CO2 reduction processes, where multi-electron transfer often limits the reaction rate. The 58% reduction in τ compared to TiO2 implies that WO3 not only effectively suppresses electron–hole recombination but also efficiently channels electrons to the active sites for CO2 adsorption and protonation, which are key steps in the formation of CH4 or CO [49].
Based on the above research, we propose the following possible mechanisms in Figure 10: under visible light irradiation in a WO3/g-C₃N4-TiO2 (WCT) composite catalyst, photogenerated electrons from WO3 are effectively separated from holes in the TiO2 valence band (VB), while electrons from the TiO2 conduction band (CB) are separated from holes in g-C3N4 VB, resulting in increased charge carrier separation efficiency. The incorporation of a WO3 cocatalyst into g-C3N4-TiO2 reduces recombination rates, leading to enhanced production of hydroxyl radicals, thereby improving the overall photocatalytic activity of the prepared ternary composite catalysts. The optimized photocatalyst demonstrates efficient performance under visible light illumination with a prolonged lifetime suitable for industrial applications as well.
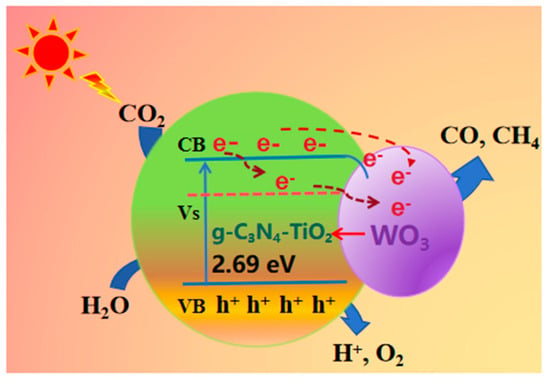
Figure 10.
Possible schematics of the mechanisms for charge transfer and photocatalysis.
3. Materials and Methods
3.1. Materials
Sodium tungstate hydrate, hydrochloric acid, anhydrous ethanol, urea, anatase titanium dioxide, and other chemicals were purchased from Shanghai Maklin Biochemical (Shanghai, China). The experimental water used in this study was deionized water sourced from the laboratory facilities.
3.2. Material Preparation
The g-C3N4-TiO2 sample was fabricated by a well-documented one-step vapor deposition method in the presence of urea and anatase titanium dioxide [50]; WO3 was obtained through a hydrothermal process [51]. The WO3/g-C3N4-TiO2 samples were prepared via a facile calcination method, in particular, as-synthesized WO3 and g-C3N4-TiO2 were put into an alumina crucible at a mole ratio of (0.1–1):1 to modulate the quantity of WO3, which was subsequently placed in a vacuum tube furnace, calcined for 4 h, cooled to room temperature, and ground into fine powders. The resultant products were denoted as (0.1–1) WCT composite photocatalysts.
3.3. Characterization
Characterization methods including powder X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), Raman measurements, scanning electron microscopy (SEM) images, a zeta sizer, high-resolution transmission electron microscopy (HRTEM), UV–vis diffuse reflectance spectroscopy (DRS), X-ray photoelectron spectroscopy (XPS), electrochemical impedance spectra (EIS), transient photocurrent responses, electron paramagnetic resonance (EPR), time-resolved photoluminescence (TRPL) spectra, and in situ diffuse reflectance infrared Fourier-transform spectroscopy (DRIFTS) were employed. Information about the instruments used for these tests is provided in the Supplementary File.
3.4. Photoreduction of CO2
Photocatalytic CO2 reduction was investigated using an automatic online trace gas analysis apparatus (Labsolar-6A, Beijing Perfect Light, Beijing, China). Irradiation was carried out using a 300 W xenon lamp equipped with an AM 1.5 filter and operating at a current of 20 A, positioned 2 cm away from the reactor. The gaseous products were then introduced into a gas chromatograph (GC, GC-9790II, Fuli Instruments, Wenling, China; with argon as the carrier gas and a methanizer attached) for detection. Carbon-containing products, including methane, carbon monoxide, methanol, and multi-carbon compounds, were analyzed by a flame ionization detector (FID), while hydrogen, oxygen, and nitrogen were quantified using thermal conductivity detection (TCD). Error bars indicated the standard deviation derived from three independent measurements conducted with 10 mg of fresh sample for each trial.
4. Conclusions
In summary, through a facile and scalable calcination method, we have successfully synthesized a WO3/g-C3N4-TiO2 nanohybrid characterized by a unique 3D/2D-3D hierarchical heterostructure, accompanied by the presence of oxygen vacancies. A comprehensive suite of advanced characterization techniques, including UV-DRS, PL, EIS, and EPR, was employed to elucidate the underlying mechanisms. The band-gap energy (Eg) of pure WCT was calculated to be 2.69 eV; compared to TiO2, g-C3N4, and CT, the arc radius corresponding to the WCT–based electrode is smaller; the photocurrent density of WCT is the highest; and the signal intensity at g = 2.003 of WCT corresponds to unpaired electrons located at the oxygen vacancy (Ovs) sites. The photocatalytic performance of the WO3/g-C3N4-TiO2 (WCT) composite for CO2 reduction was evaluated under simulated solar light irradiation without any external photosensitizer or sacrificial agent. Impressively, the WCT composite exhibited outstanding CO2 photoreduction activity, yielding CO and CH4 with formation rates reaching up to 48.31 µmol·g−1 and 77.18 µmol·g−1, respectively. These values represent a remarkable improvement over previously reported g-C3N4-TiO2–based photocatalysts for CO2 reduction photocatalysts. The presence of oxygen vacancies plays a pivotal role in this process. Oxygen vacancies in WCT act as active sites that enhance the adsorption and activation of CO2 molecules, which facilitate the electron transfer processes to enable more efficient conversion of CO2 to intermediates. Specifically, the unpaired electrons around oxygen vacancies interact with CO2 molecules, weakening C–O bonds and thus promoting the formation of *COOH. Subsequently, the reaction pathways leading to *CHO and *CH3O are also accelerated due to the modified electronic environment created by oxygen vacancies. Thus, the reaction rate is significantly increased, while selectivity for CH4 is simultaneously improved, attributed to the essential role of oxygen vacancies in the CO2 photoreduction process. This research opens up novel prospects for the rational design of other high-efficiency cocatalysts aiming to enhance CO2 photoreduction performance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30112317/s1, Characterization methods information; the data of a series of conditional blank control experiments; production yield of CO and CH4, and the calculated AQE data.
Author Contributions
Conceptualization, Y.H. and Z.C.; methodology, Y.H. and B.D.; validation, Y.H. and Z.W.; formal analysis, Y.H.; investigation, Y.H. and B.D.; resources, Y.Y. and Z.C.; data curation, Y.H.; writing—original draft preparation, Y.H.; writing—review and editing, Z.C. and Y.Y.; visualization, Y.H.; supervision, Z.C.; project administration, Z.C.; funding acquisition, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (22469001) and Collaborative Innovation Center for Utilization of Coal-based Silica-Aluminum Resources, Ordos Institute of Technology-Ordos Carbon Neutralization Research Institute (2023-07), and the Natural Science Research Project of Ordos Institute of Technology (ZRYB2024019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CT | g-C3N4-TiO2 |
| WCT | WO3/g-C3N4-TiO2 |
References
- Wang, H.; Guo, Q.; Zhang, H.; Zuo, C. Developments and challenges on enhancement of photocatalytic CO2 reduction through photocatalysis. Carbon Resour. Convers. 2024, 7, 100263. [Google Scholar] [CrossRef]
- Fang, S.; Rahaman, M.; Bharti, J.; Reisner, E.; Robert, M.; Ozin, G.A.; Hu, Y.H. Photocatalytic CO2 reduction. Nat. Rev. Methods Primers. 2023, 3, 61. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, C.; Wang, W.; Sheng, H.; Zhao, J. Achieving Almost 100% Selectivity in Photocatalytic CO2 Reduction to Methane via In-Situ Atmosphere Regulation Strategy. Adv. Mater. 2024, 36, 2405825. [Google Scholar] [CrossRef]
- Nahar, S.; Zain, M.F.M.; Kadhum, A.A.H.; Abu Hasan, H.; Hasan, R. Advances in photocatalytic CO2 reduction with water: A review. Materials 2017, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: Achievements, challenges, and opportunities. Adv. Mater. 2018, 30, 1704649. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Tong, L.; Wang, Y.; Zhang, P.; Zhu, M.; Dong, H. Recent progress and challenges of photocatalytic CO2 conversion into value-added multi-carbon products. Coord. Chem. Rev. 2024, 502, 215623. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Yin, S.; Sun, L.; Zhou, Y.; Li, X.; Li, J.; Song, X.; Huo, P.; Wang, H.; Yan, Y. Enhanced electron–hole separation in SnS2/Au/g-C3N4 embedded structure for efficient CO2 photoreduction. Chem. Eng. J. 2021, 406, 126776. [Google Scholar] [CrossRef]
- Yin, S.; Liu, Y.; Zhou, W.; Wang, H.; Song, X.; Wang, H.; Huo, P. Nanocluster-mediated electron–hole separation for efficient CO2 photoreduction. Chem. Eng. J. 2023, 477, 147292. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Bonchio, M.; Bonin, J.; Ishitani, O.; Lu, T.-B.; Morikawa, T.; Morris, A.J.; Reisner, E.; Sarkar, D.; Toma, F.M.; Robert, M. Best practices for experiments and reporting in photocatalytic CO2 reduction. Nat. Catal. 2023, 6, 657–665. [Google Scholar] [CrossRef]
- Khan, M.D.; Fareed, I.; Farooq, M.U.H.; Akram, M.; Rehman, S.U.; Ali, Z.; Tariq, Z.; Irshad, M.; Li, C.; Butt, F.K. Methodologies for enriched photocatalytic CO2 reduction: An overview. Int. J. Environ. Sci. Technol. 2024, 21, 3489–3526. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, P.; Sambyal, S.; Sharma, R.; Mandyal, P.; Fang, B. Properties, optimized morphologies, and advanced strategies for photocatalytic applications of WO3 based photocatalysts. J. Hazard. Mater. 2022, 428, 128218. [Google Scholar] [CrossRef] [PubMed]
- Dutta, V.; Sharma, S.; Raizada, P.; Thakur, V.K.; Khan, A.A.P.; Saini, V.; Asiri, A.M.; Singh, P. An overview on WO3 based photocatalyst for environmental remediation. J. Environ. Chem. Eng. 2021, 9, 105018. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Nawawi, M.G.M. Highly stable 3D/2D WO3/g-C3N4 Z-scheme heterojunction for stimulating photocatalytic CO2 reduction by H2O/H2 to CO and CH4 under visible light. J. CO2 Util. 2020, 41, 101270. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, J.; Wei, Z.; Guo, Q.; Jin, H.; Wang, S. Recent advances in solar-driven CO2 reduction over g-C3N4-based photocatalysts. Carbon Energy 2023, 5, e205. [Google Scholar] [CrossRef]
- Ghosh, U.; Majumdar, A.; Pal, A. Photocatalytic CO2 reduction over g-C3N4 based heterostructures: Recent progress and prospects. J. Environ. Chem. Eng. 2021, 9, 104631. [Google Scholar] [CrossRef]
- Hussien, M.K.; Sabbah, A.; Qorbani, M.; Elsayed, M.H.; Raghunath, P.; Lin, T.-Y.; Quadir, S.; Wang, H.-Y.; Wu, H.-L.; Tzou, D.-L.M.; et al. Metal-free four-in-one modification of g-C3N4 for superior photocatalytic CO2 reduction and H2 evolution. Chem. Eng. J. 2022, 430, 132853. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, D.; Yan, J.; Lee, L.Y.S. Photocatalytic CO2 reduction enabled by interfacial S-scheme heterojunction between ultrasmall copper phosphosulfide and g-C3N4. ACS Appl. Mater. Interfaces 2021, 13, 9762–9770. [Google Scholar] [CrossRef]
- Zhang, X. Layered g-C3N4/TiO2 nanocomposites for efficient photocatalytic water splitting and CO2 reduction: A review. Mater. Today Energy 2022, 23, 100904. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Chen, Z.; Li, J.; Li, X.; Huo, P.; Wang, Q. TiO2 modified g-C3N4 with enhanced photocatalytic CO2 reduction performance. Solid State Sci. 2020, 100, 106099. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Guo, Y.; Shen, M.; Wang, M.; Li, B.; Shi, J. Multifunctional 2D Porous g-C3N4 Nanosheets Hybridized with 3D Hierarchical TiO2 Micro-flowers for Selective Dye Adsorption, Antibiotic Degradation and CO2 Reduction. Chem. Eng. J. 2020, 396, 125347. [Google Scholar] [CrossRef]
- Ibrahim, Y.O.; Gondal, M.A. Visible-light-driven photocatalytic performance of a Z-scheme based TiO2/WO3/g-C3N4 ternary heterojunctions. Mol. Catal. 2021, 505, 111494. [Google Scholar] [CrossRef]
- Huo, Y.; Chang, Z.; Zhang, X.; Dong, B. Reuse of TiO2 from Waste SCR Catalyst to Synthesis g-C3N4/TiO2 for Photocatalytic CO2 Reduction. Waste Biomass Valoriz. 2024, 15, 6775–6785. [Google Scholar] [CrossRef]
- Saad, M.; Bahadur, A.; Iqbal, S.; Mahmood, S.; Tayyab, M.; Alshalwi, M.; Shah, M. Development of stable S-scheme 2D-2D g-C3N4/CdS nanoheterojunction arrays for enhanced visible light photomineralisation of nitrophenol priority water pollutants. Sci. Rep. 2024, 14, 2897. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K.; Mane, G.P.; Rane, V.; Tripathi, A.K.; Tyagi, A.K. Selective CO2 photoreduction with Cu-doped TiO2 photocatalyst: Delineating the crucial role of Cu-oxidation state and oxygen vacancies. J. Phys. Chem. C 2021, 125, 1793–1810. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A.; Sun, L.; Liu, R.; Cui, S. Studies of Z-scheme WO3-TiO2/Cu2ZnSnS4 ternary nanocomposite with enhanced CO2 photoreduction under visible light irradiation. J. CO2 Util. 2020, 37, 260–271. [Google Scholar] [CrossRef]
- Li, B.; Sun, L.; Bian, J.; Sun, N.; Sun, J.; Chen, L.; Li, Z.; Jing, L. Controlled synthesis of novel Z-scheme iron phthalocyanine/porous WO3 nanocomposites as efficient photocatalysts for CO2 reduction. Appl. Catal. B Environ. 2020, 270, 118849. [Google Scholar] [CrossRef]
- Linh, P.H.; Chung, P.D.; Van Khien, N.; Oanh, L.T.M.; Thu, V.T.; Bach, T.N.; Hang, L.T.; Hung, N.M.; Lam, V.D. A simple approach for controlling the morphology of g-C3N4 nanosheets with enhanced photocatalytic properties. Diam. Relat. Mater. 2021, 111, 108214. [Google Scholar] [CrossRef]
- He, F.; Zhu, B.; Cheng, B.; Cheng, B.; Yu, J.; Ho, W.; Macyk, W. 2D/2D/0D TiO2/C3N4/Ti3C2 MXene composite S-scheme photocatalyst with enhanced CO₂ reduction activity. Appl. Catal. B Environ. 2020, 272, 119006. [Google Scholar] [CrossRef]
- Ekoi, E.; Gowen, A.; Dorrepaal, R.; Dowling, D. Characterisation of titanium oxide layers using Raman spectroscopy and optical profilometry: Influence of oxide properties. Results Phys. 2019, 12, 1574–1585. [Google Scholar] [CrossRef]
- Feng, J.; Dong, Y.; Yan, Y.; Zhao, W.; Yang, T.; Zheng, J.; Li, Z.; Wu, M. Extended lattice space of TiO2 hollow nanocubes for improved sodium storage. Chem. Eng. J. 2019, 373, 565–571. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, Y.; Fan, H.; Liu, X.; Wang, F.; Qu, X.; Cao, J.; Wei, M. WO3 nanosheet/ZnIn2S4 S-scheme heterojunctions for enhanced CO2 photoreduction. ACS Appl. Nano Mater. 2024, 7, 3488–3498. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Wu, X.; Liu, Z.; Zhao, Z.; Liu, Y.; Dou, S.; Xiao, Y. Selective CO2 Photoreduction into CH4 Triggered by the Synergy between Oxygen Vacancy and Ru Substitution under Near-Infrared Light Irradiation. Adv. Sci. 2024, 11, 2405668. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, Y.; Liu, X.; Li, J.; Liu, B. Visible-light-driven CO2 photoreduction over atomically strained indium sites in ambient air. Nat. Commun. 2025, 16, 2094. [Google Scholar] [CrossRef]
- Zhao, F.; Feng, Y.; Wang, Y.; Zhang, X.; Liang, X.; Li, Z.; Gong, J.; Feng, W. Two-dimensional gersiloxenes with tunable bandgap for photocatalytic H2 evolution and CO2 photoreduction to CO. Nat. Commun. 2020, 11, 1443. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Vasanthakumar, V.; Shanavas, S.; Karthikeyan, S.; Anbarasan, P. Crumpled sheet like graphene based WO3-Fe2O3 nanocomposites for enhanced charge transfer and solar photocatalysts for environmental remediation. Appl. Surf. Sci. 2019, 470, 114–128. [Google Scholar] [CrossRef]
- Fu, M.; Liao, J.; Dong, F.; Li, H.; Liu, H. Growth of g-C3N4 Layer on Commercial TiO2 for Enhanced Visible Light Photocatalytic Activity. J. Nanomater. 2014, 2014, 869094. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, X.; Wu, Y.; Li, Z.; Han, Y.; Shen, X. A study of spherical TiO2/g-C3N4 photocatalyst: Morphology, chemical composition and photocatalytic performance in visible light. Mol. Catal. 2017, 432, 232–241. [Google Scholar] [CrossRef]
- Kadi, M.W.; Mohamed, R.M. Increasing visible light water splitting efficiency through synthesis route and charge separation in measoporous g-C3N4 decorated with WO3 nanoparticles. Ceram. Int. 2019, 45, 3886–3893. [Google Scholar] [CrossRef]
- Mhalsekar, M.; Kole, P.; Borker, V. Synthesis, characterization and application of MWCNT/TiO2 composite for photocatalytic reduction of benzophenone to benzopinacol. J. Environ. Chem. Eng. 2024, 12, 114681. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Fang, J.; Lu, C. TiO2@ g-C3N4 heterojunction with directional charge migration behavior for photodegradation of tetracycline antibiotics. Mater. Lett. 2019, 236, 622–624. [Google Scholar] [CrossRef]
- Yu, X.; Xie, J.; Liu, Q.; Dong, H.; Li, Y. The origin of enhanced photocatalytic activity in g-C3N4/TiO2 heterostructure revealed by DFT calculations. J. Colloid Interface Sci. 2021, 593, 133–141. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, H.; Yang, P. Microstructure driven charge carrier separation in superior thin g-C3N4 nanosheets towards enhanced photocatalytic activities. Colloids Surf. A 2024, 689, 133759. [Google Scholar] [CrossRef]
- Alghasham, H.A.; Alzahrani, S.O.; Alfi, A.A.; Alkhamis, K.M.; Alaysuy, O.; Mogharbel, R.T.; Alkhatib, F.M.; El-Metwaly, N.M. TiO2-MWCNTs/PVDF: Photophysical properties, photocatalytic activity, and recycling in industrial effluent wastewater treatment by different photocatalytic sources. J. Water Process. Eng. 2024, 65, 105905. [Google Scholar] [CrossRef]
- Bo, Y.; Wang, H.; Lin, Y.; Yang, T.; Ye, R.; Li, Y.; Hu, C.; Du, P.; Hu, Y.; Liu, Z.; et al. Altering hydrogenation pathways in photocatalytic nitrogen fixation by tuning local electronic structure of oxygen vacancy with dopant. Angew. Chem. Int. Ed. 2021, 60, 16085–16092. [Google Scholar] [CrossRef]
- Xu, F.; He, Y.; Zhang, J.; Liang, G.; Liu, C.; Yu, J. Prolonging Charge Carrier Lifetime via Intraband Defect Levels in S-Scheme Heterojunctions for Artificial Photosynthesis. Angew. Chem. Int. Ed. 2025, 64, e202414672. [Google Scholar] [CrossRef]
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Wang, L.; Hu, H.; Xu, J.; Zhu, S.; Ding, A.; Deng, C. WO3 nanocubes: Hydrothermal synthesis, growth mechanism, and photocatalytic performance. J. Mater. Res. 2019, 34, 2955–2963. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).