The Effects of Glyphosate and Roundup® Herbicides on the Kidneys’ Cortex and the Medulla and on Renal Tubular Cells’ Mitochondrial Respiration and Oxidative Stress
Abstract
1. Introduction
2. Results
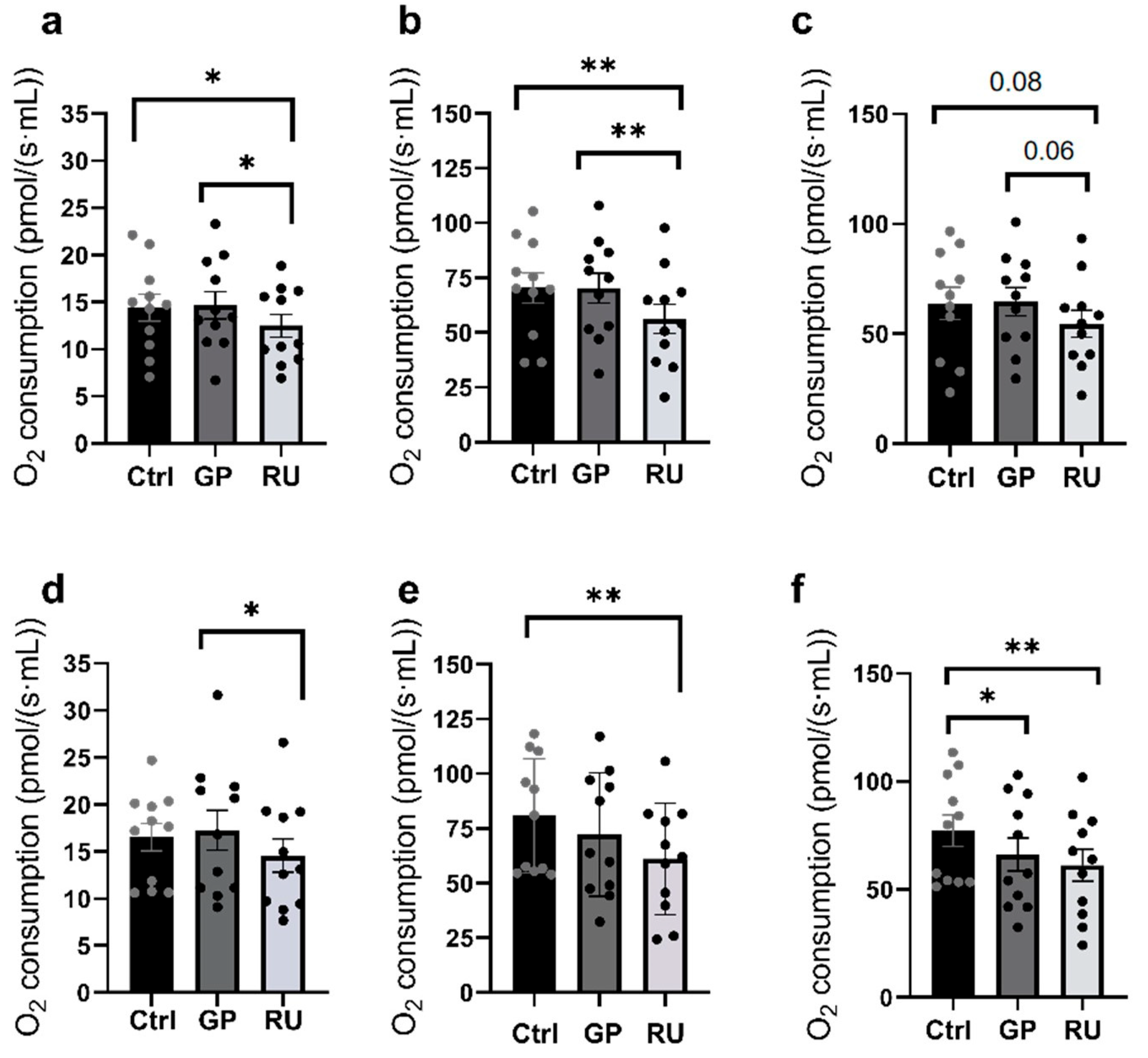
2.1. More than Glyphosate Alone, Roundup® Significantly Impairs Kidneys’ Mitochondrial Respiration
2.1.1. Renal Cortex Mitochondrial Respiration
2.1.2. Renal Medulla Mitochondrial Respiration
2.1.3. Comparison Between Cortex and Medulla Mitochondrial Respiration
2.1.4. HK2 Cells Mitochondrial Respiration
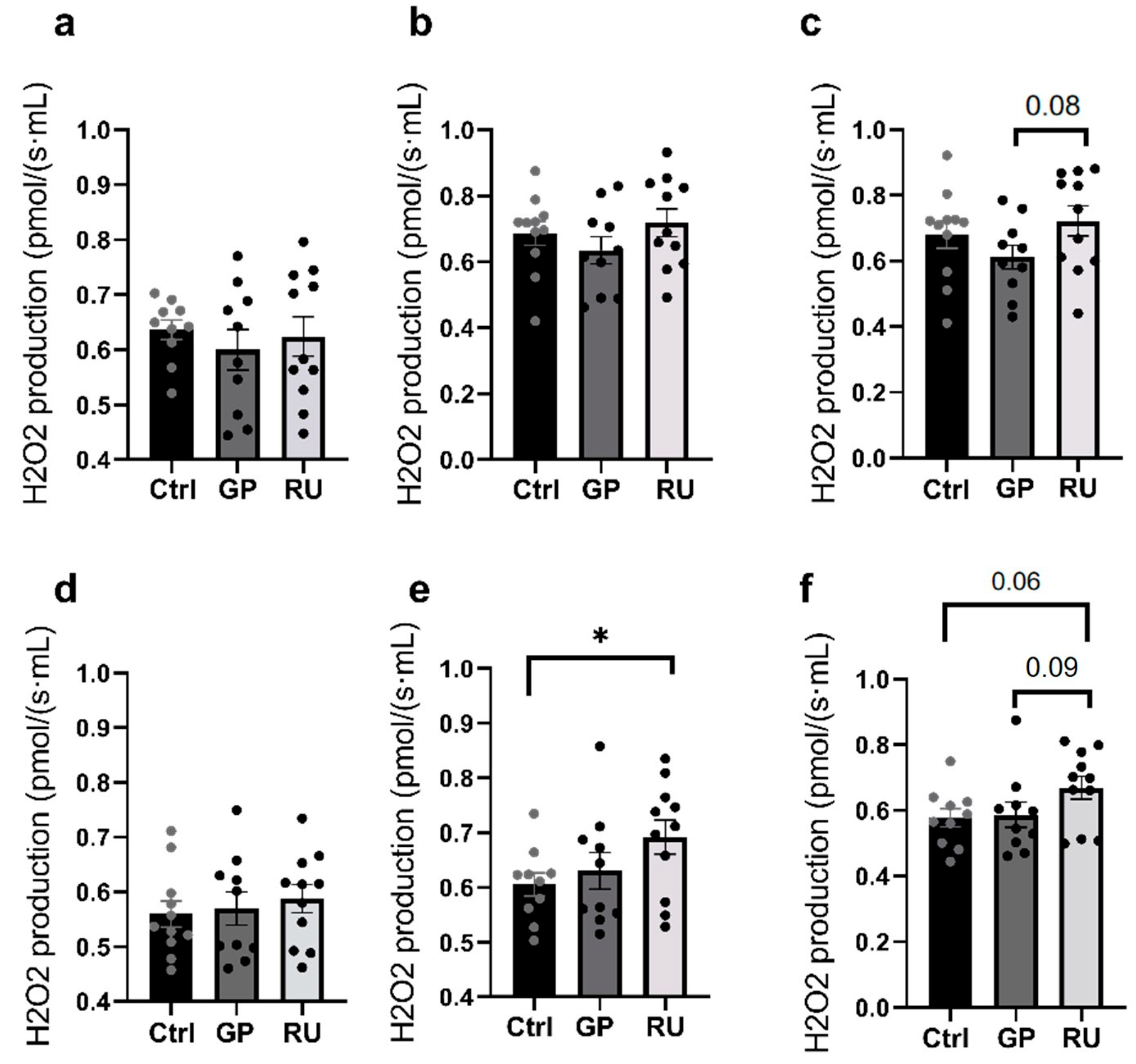
2.2. Roundup® Enhances Oxidative Stress in Kidneys’ Medulla Mainly at OXPHOS CI + II State
2.2.1. Hydrogen Peroxide Production by Renal Cortex Mitochondria
2.2.2. Hydrogen Peroxide Production by Renal Medulla Mitochondria
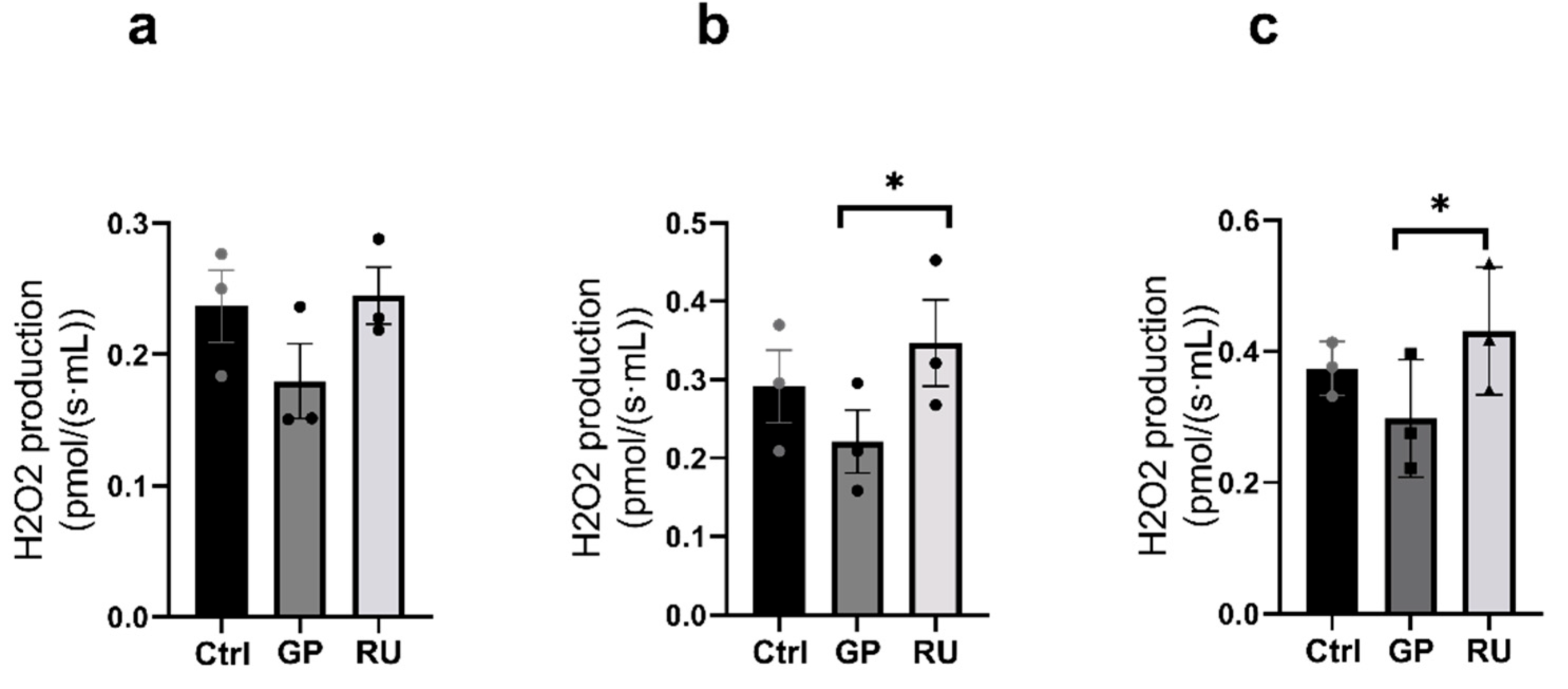
2.2.3. HK2 Cells’ Production of Hydrogen Peroxide
3. Discussion
3.1. Effects of Glyphosate Alone on Kidneys’ Mitochondrial Respiration and Oxidative Stress
3.2. Roundup® Decreased both Renal Cortex and Medulla Mitochondrial Respiration and Impaired Tubular Cell Mitochondria with Enhanced Hydrogen Peroxide Production
3.2.1. Effects of Roundup® on Renal Cortex and Medulla
3.2.2. Effects of Roundup® on HK2 Cell Line
4. Material and Methods
4.1. Study Design
4.1.1. Experimental Design
4.1.2. Cell Culture
4.2. Solutions Used
4.3. Mitochondrial Extraction
4.4. Mitochondrial Respiration
4.4.1. Isolated Mitochondria
4.4.2. HK-2 Cells
4.5. Mitochondrial H2O2 Production
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Environmental Implication of Herbicide Use. Molecules 2024, 29, 5965. [Google Scholar] [CrossRef] [PubMed]
- Konrad, B.N.L.; Pinheiro, S.C.; Ferreira, C.C.; Hoffmann, E.K.; Albertino, S.M.F. Using Glyphosate on Guarana Seedlings in the Amazon. Molecules 2023, 28, 5193. [Google Scholar] [CrossRef] [PubMed]
- Shumilina, E.; Andreasen, C.; Bitarafan, Z.; Dikiy, A. Determination of Glyphosate in Dried Wheat by 1H-NMR Spectroscopy. Molecules 2020, 25, 1546. [Google Scholar] [CrossRef]
- Fontanella, M.C.; Lamastra, L.; Beone, G.M. Determination of Glyphosate in White and Brown Rice with HPLC-ICP-MS/MS. Molecules 2022, 27, 8049. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Li, A. Review of the Occurrence of Herbicides in Environmental Waters of Taihu Lake Basin and Its Potential Impact on Submerged Plants. Water 2024, 16, 726. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and Health Effects of the Herbicide Glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide Glyphosate: Toxicity and Microbial Degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef]
- Soares, D.; Silva, L.; Duarte, S.; Pena, A.; Pereira, A. Glyphosate Use, Toxicity and Occurrence in Food. Foods 2021, 10, 2785. [Google Scholar] [CrossRef]
- Alarape, S.A.; Fagbohun, A.F.; Ipadeola, O.A.; Adeigbo, A.A.; Adesola, R.O.; Adeyemo, O.K. Assessment of Glyphosate and Its Metabolites’ Residue Concentrations in Cultured African Catfish Offered for Sale in Selected Markets in Ibadan, Oyo State, Nigeria. Front. Toxicol. 2023, 5, 1250137. [Google Scholar] [CrossRef]
- Gunatilake, S.; Seneff, S.; Orlando, L. Glyphosate’s Synergistic Toxicity in Combination with Other Factors as a Cause of Chronic Kidney Disease of Unknown Origin. Int. J. Environ. Res. Public Health 2019, 16, 2734. [Google Scholar] [CrossRef]
- Qiang, S.; Mohamed, F.; Raubenheimer, J.; Buckley, N.A.; Roberts, M.S.; Mackenzie, L. Clinical Toxicology of Acute Glyphosate Self-Poisoning: Impact of Plasma Concentrations of Glyphosate, Its Metabolite and Polyethoxylated Tallow Amine Surfactants on the Toxicity. Clin. Toxicol. 2024, 62, 483–496. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Benbrook, C.; Antoniou, M.N. Insight into the Confusion over Surfactant Co-Formulants in Glyphosate-Based Herbicides. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 128, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Novotny, E. Glyphosate, Roundup and the Failures of Regulatory Assessment. Toxics 2022, 10, 321. [Google Scholar] [CrossRef]
- European Food Safety Authority. Request for the Evaluation of the Toxicological Assessment of the Co-Formulant POE-Tallowamine. EFSA J. 2015, 13, 4303. [Google Scholar]
- Hu, L.; Chen, M.; Xue, X.; Zhao, M.; He, Q. Effect of Glyphosate on Renal Function: A Study Integrating Epidemiological and Experimental Evidence. Ecotoxicol. Environ. Saf. 2025, 290, 117758. [Google Scholar] [CrossRef]
- Noor, M.I.; Rahman, M.S. Effects of Environmentally Relevant Concentrations of Roundup on Oxidative-Nitrative Stress, Cellular Apoptosis, Prooxidant-Antioxidant Homeostasis, Renin and CYP1A Expressions in Goldfish: Molecular Mechanisms Underlying Kidney Damage During Roundup Exposure. Environ. Toxicol. 2025, 40, 817–834. [Google Scholar] [CrossRef]
- Omote, D.; Makino, S.-I.; Okunaga, I.; Ishii, M.; Tatsumoto, N.; Aizawa, M.; Asanuma, K. Unusual Course of Glyphosate-Induced Acute Kidney Injury: A Case Report of Tubulointerstitial Nephritis Treated with Steroids. CEN Case Rep. 2024, 14, 128–134. [Google Scholar] [CrossRef]
- de Bravo, M.I.S.; Medina, J.; Marcano, S.; Finol, H.J.; Boada-Sucre, A. Effects of Herbicide on the Kidneys of Two Venezuelan Cultured Fish: Caquetaia Kraussii and Colossoma Macropomum (Pisces: Ciclidae and Characeae). Rev. Biol. Trop. 2005, 53 (Suppl. S1), 55–60. [Google Scholar]
- Samanta, P.; Kumari, P.; Pal, S.; Mukherjee, A.K.; Ghosh, A.R. Histopathological and Ultrastructural Alterations in Some Organs of Oreochromis Niloticus Exposed to Glyphosate-Based Herbicide, Excel Mera 71. J. Microsc. Ultrastruct. 2018, 6, 35–43. [Google Scholar] [CrossRef]
- Docea, A.O.; Cirstea, A.E.; Cercelaru, L.; Drocas, A.I.; Dinca, V.; Mesnage, R.; Marginean, C.; Radu, A.; Popa, D.G.; Rogoveanu, O.; et al. Effect of Perinatal Exposure to Glyphosate and Its Mixture with 2,4-D and Dicamba on Rat Dam Kidney and Thyroid Function and Offspring’s Health. Environ. Res. 2023, 237, 116908. [Google Scholar] [CrossRef]
- Roma, D.; Cecchini, M.E.; Tonini, M.P.; Capella, V.; Aiassa, D.; Rodriguez, N.; Mañas, F. Toxicity Assessment and DNA Repair Kinetics in HEK293 Cells Exposed to Environmentally Relevant Concentrations of Glyphosate (Roundup® Control Max). Toxicol. Res. 2023, 12, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, F. Comparative Effects of the Roundup and Glyphosate on Mitochondrial Oxidative Phosphorylation. Chemosphere 2005, 61, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Tomar, N.; Zhang, X.; Kandel, S.M.; Sadri, S.; Yang, C.; Liang, M.; Audi, S.H.; Cowley, A.W.; Dash, R.K. Substrate-Dependent Differential Regulation of Mitochondrial Bioenergetics in the Heart and Kidney Cortex and Outer Medulla. Biochim. Biophys. Acta Bioenerg. 2022, 1863, 148518. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, Q.; Wu, Y.; Gao, Z.; Kobayashi, S.; Patel, J.; Li, C.; Cai, F.; Zhang, Y.; Liang, C.; et al. Comprehensive Lipidomic Profiling in Serum and Multiple Tissues from a Mouse Model of Diabetes. Metabolomics Off. J. Metabolomic Soc. 2020, 16, 115. [Google Scholar] [CrossRef]
- Astiz, M.; de Alaniz, M.J.T.; Marra, C.A. Effect of Pesticides on Cell Survival in Liver and Brain Rat Tissues. Ecotoxicol. Environ. Saf. 2009, 72, 2025–2032. [Google Scholar] [CrossRef]
- Babich, R.; Ulrich, J.C.; Ekanayake, E.M.D.V.; Massarsky, A.; De Silva, P.M.C.S.; Manage, P.M.; Jackson, B.P.; Ferguson, P.L.; Di Giulio, R.T.; Drummond, I.A.; et al. Kidney Developmental Effects of Metal-Herbicide Mixtures: Implications for Chronic Kidney Disease of Unknown Etiology. Environ. Int. 2020, 144, 106019. [Google Scholar] [CrossRef]
- Pfaller, W.; Gstraunthaler, G.; Willinger, C.C. Morphology of Renal Tubular Damage from Nephrotoxins. Toxicol. Lett. 1990, 53, 39–43. [Google Scholar] [CrossRef]
- Manucha, W. Mitochondria and oxidative stress participation in renal inflammatory process. Medicina 2014, 74, 254–258. [Google Scholar]
- Zhang, W.; Yang, Y.; Gao, H.; Zhang, Y.; Jia, Z.; Huang, S. Inhibition of Mitochondrial Complex I Aggravates Folic Acid-Induced Acute Kidney Injury. Kidney Blood Press. Res. 2019, 44, 1002–1013. [Google Scholar] [CrossRef]
- Stallons, L.J.; Whitaker, R.M.; Schnellmann, R.G. Suppressed Mitochondrial Biogenesis in Folic Acid-Induced Acute Kidney Injury and Early Fibrosis. Toxicol. Lett. 2014, 224, 326–332. [Google Scholar] [CrossRef]
- Ugarte, R. Interaction between Glyphosate and Mitochondrial Succinate Dehydrogenase. Comput. Theor. Chem. 2014, 1043, 54–63. [Google Scholar] [CrossRef]
- Burchfield, S.L.; Bailey, D.C.; Todt, C.E.; Denney, R.D.; Negga, R.; Fitsanakis, V.A. Acute Exposure to a Glyphosate-Containing Herbicide Formulation Inhibits Complex II and Increases Hydrogen Peroxide in the Model Organism Caenorhabditis Elegans. Environ. Toxicol. Pharmacol. 2019, 66, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, R.; Ferguson, S.; Brandsma, I.; Moelijker, N.; Zhang, G.; Mazzacuva, F.; Caldwell, A.; Halket, J.; Antoniou, M.N. The surfactant co-formulant POEA in the glyphosate-based herbicide RangerPro but not glyphosate alone causes necrosis in Caco-2 and HepG2 human cell lines and ER stress in the ToxTracker assay. Food Chem Toxicol. 2022, 168, 113380. [Google Scholar] [CrossRef]
- Nacano, B.R.M.; Convento, M.B.; de Oliveira, A.S.; Castino, R.; Castino, B.; Razvickas, C.V.; Bondan, E.; Borges, F.T. Effects of Glyphosate Herbicide Ingestion on Kidney Function in Rats on a Balanced Diet. J. Bras. Nefrol. 2024, 46, e20230043. [Google Scholar] [CrossRef]
- Kim, Y.; Hong, J.; Gil, H.; Song, H.; Hong, S. Mixtures of Glyphosate and Surfactant TN20 Accelerate Cell Death via Mitochondrial Damage-Induced Apoptosis and Necrosis. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2013, 27, 191–197. [Google Scholar] [CrossRef]
- Charles, A.-L.; Charloux, A.; Vogel, T.; Raul, J.-S.; Kindo, M.; Wolff, V.; Geny, B. Cumulative Deleterious Effects of Tetrahydrocannabinoid (THC) and Ethanol on Mitochondrial Respiration and Reactive Oxygen Species Production Are Enhanced in Old Isolated Cardiac Mitochondria. Int. J. Mol. Sci. 2024, 25, 1835. [Google Scholar] [CrossRef]
- Makame, K.M.; Masese, S.N.; Adam, B.; Nagy, K. Oxidative Stress and Cytotoxicity Induced by Co-Formulants of Glyphosate-Based Herbicides in Human Mononuclear White Blood Cells. Toxics 2023, 11, 976. [Google Scholar] [CrossRef]
- Zouaoui, K.; Dulaurent, S.; Gaulier, J.M.; Moesch, C.; Lachâtre, G. Determination of Glyphosate and AMPA in Blood and Urine from Humans: About 13 Cases of Acute Intoxication. Forensic Sci. Int. 2013, 226, e20–e25. [Google Scholar] [CrossRef]
- Guillot, M.; Charles, A.-L.; Chamaraux-Tran, T.N.; Bouitbir, J.; Meyer, A.; Zoll, J.; Schneider, F.; Geny, B. Oxidative Stress Precedes Skeletal Muscle Mitochondrial Dysfunction during Experimental Aortic Cross-Clamping but Is Not Associated with Early Lung, Heart, Brain, Liver, or Kidney Mitochondrial Impairment. J. Vasc. Surg. 2014, 60, 1043–1051.e5. [Google Scholar] [CrossRef]
- Quenardelle, V.; Charles, A.-L.; Charloux, A.; Raul, J.-S.; Wolff, V.; Geny, B. Young Age and Concomitant Cannabis (THC) and Ethanol (EtOH) Exposure Enhances Rat Brain Damage Through Decreased Cerebral Mitochondrial Respiration. Molecules 2025, 30, 918. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rihani, R.; Charles, A.-L.; Oulehri, W.; Geny, B. The Effects of Glyphosate and Roundup® Herbicides on the Kidneys’ Cortex and the Medulla and on Renal Tubular Cells’ Mitochondrial Respiration and Oxidative Stress. Molecules 2025, 30, 2335. https://doi.org/10.3390/molecules30112335
Rihani R, Charles A-L, Oulehri W, Geny B. The Effects of Glyphosate and Roundup® Herbicides on the Kidneys’ Cortex and the Medulla and on Renal Tubular Cells’ Mitochondrial Respiration and Oxidative Stress. Molecules. 2025; 30(11):2335. https://doi.org/10.3390/molecules30112335
Chicago/Turabian StyleRihani, Rayhana, Anne-Laure Charles, Walid Oulehri, and Bernard Geny. 2025. "The Effects of Glyphosate and Roundup® Herbicides on the Kidneys’ Cortex and the Medulla and on Renal Tubular Cells’ Mitochondrial Respiration and Oxidative Stress" Molecules 30, no. 11: 2335. https://doi.org/10.3390/molecules30112335
APA StyleRihani, R., Charles, A.-L., Oulehri, W., & Geny, B. (2025). The Effects of Glyphosate and Roundup® Herbicides on the Kidneys’ Cortex and the Medulla and on Renal Tubular Cells’ Mitochondrial Respiration and Oxidative Stress. Molecules, 30(11), 2335. https://doi.org/10.3390/molecules30112335





