Abstract
The development of photoactive molecules for photothermal energy storage is a focus of research in solar energy utilization technology. Azobenzene photoswitch has emerged as a promising candidate for solar energy conversion and storage due to its unique photoisomerization characteristics. Nonetheless, a majority of azobenzene-based molecular photothermal systems have a significant drawback: they depend on ultraviolet light for E-to-Z isomerization to store photon energy rather than visible light, which seriously hinders the development of azobenzene photoswitch in practical solar energy utilization applications. In this study, an azobenzene photothermal molecule that can effectively store visible-light photon energy was design and synthesized, which includes a tetra-ortho-chlorinated azo structure as the “head” part and an alkyl chain at para-position as the “tail” part. The ultraviolet–visible and 1H NMR spectrum indicated that the obtained tetra-ortho-chlorinated azobenzene photothermal molecule could effectively absorb and store photon energy under 550 nm irradiation and release the stored energy upon 430 nm light irradiation. The storage energy density of the charged azobenzene photothermal molecule was determined to be 13.50 kJ/mol through differential scanning calorimetry and 28.21 kJ/mol via density functional theory theoretical calculations. This discrepancy was ascribed to the 64% Z-isomer yield harvesting during the charging process. Furthermore, the obtained tetra-ortho-chlorinated azobenzene exhibited long-term energy storage (approximately 11 days of half-life) and cyclic stability (100 cycles). Notably, the E-isomer of tetra-ortho-chlorinated azobenzene exhibited a high degree of supercooling, which may be advantageous for use in extremely low-temperature environments.
1. Introduction
Azobenzene-based molecular photothermal systems, also known as molecular solar thermal systems, offer a promising approach to solar energy conversion and storage, attributed to their distinctive photoisomerization characteristics [1,2]. Azobenzene photoswitch facilitates dynamic energy conversion and storage through trans-to-cis (E-to-Z) molecular configuration transitions. This process effectively captures photon energy, converting it into chemical bond energy for storage, which can be subsequently released as thermal energy on demand [3]. This innovative photo energy utilization strategy transcends traditional energy conversion methods, such as direct light-to-heat conversion, electricity-mediated thermal generation, and phase-change latent heat systems, significantly enhancing the efficiency of photo energy utilization [4]. This photothermal system boasts remarkable advantages, including closed-cycle operation, zero emissions, safety, cleanliness, and ease of transportation, establishing its potential for diverse applications encompassing personal thermal management [5,6,7,8], solar-thermal textiles [9,10,11,12], and anti-/de-icing technologies [13,14].
However, a critical limitation faced by current azobenzene-based molecular photothermal energy storage technology is that the E-to-Z switching usually requires ultraviolet irradiation (typically 365 nm), which comprises only 5% of solar energy and has limited penetration capabilities [15]. Furthermore, prolonged exposure to ultraviolet light may also cause material degradation, severely limiting the practical application of azobenzene in photothermal materials. In contrast, visible light, which constitutes approximately 50% of the solar spectrum and is the primary component of sunlight, presents an opportunity for enhanced solar energy utilization. An optimal azobenzene-based molecular photothermal system should effectively absorb a substantial portion of solar photons, especially from the intense visible-light region of the solar spectrum. Nevertheless, visible light generally triggers the Z-to-E isomerization of azobenzene molecules, predominantly serving as a stimulus for the heat release during the discharge process [16,17,18]. This poses a challenge in the design of an azobenzene photoswitch that can effectively store visible-light photon energy.
To address this challenge, scientists have proposed two primary strategies. The first strategy involves the integration of upconversion materials, which convert long-wavelength radiation from sunlight into short-wavelength radiation (such as UV light) that can subsequently be absorbed by azobenzene molecules [19,20,21]. The Moth–Poulsen research group demonstrated the first example of a far-red (740 nm) triplet sensitized Z-to-E photoswitching of azobenzene in a condensed phase through the co-assembly of a liquid azobenzene derivative with a liquid surfactant–protein film [22]. The novel bioplastics technology offers a sustainable platform for the development of solid-state photoswitching materials for energy harvesting. Yu et al. incorporated upconversion nanophosphors into a cross-linked liquid–crystal polymer film with azotolane, enabling rapid bending under continuous-wave near-infrared light at 980 nm [23]. However, the conversion efficiency of upconversion materials is often constrained by their low luminescence efficiency and complex preparation processes. The second strategy, which is the primary focus of the current research, aims to create azobenzene structures capable of efficiently storing photon energy from visible light through molecular design. This method focuses on adding specific substituents or modifying the molecular backbone to either separate the n–π* bands within the visible spectrum or shift the π–π* transition to longer wavelengths. Herges et al. [24,25] and Woolley et al. [26] synthesized ethylene-bridged and tetra-ortho-methoxy azobenzene derivatives to separate the n–π* transitions of the Z and E isomers. The distinct n–π* absorption bands in these azobenzene derivatives allow for E-to-Z and Z-to-E photoisomerization when exposed to visible light. Recent studies have shown that tetra-ortho-halogenate azobenzene derivatives exhibit significant potential for visible-light photon energy storage due to their capacity to induce a substantial red-shift in the E-isomer’s absorption spectrum, making them responsive to visible light [5,17,27,28,29,30,31]. For example, the Z-isomer of the tetra-ortho-fluorinated azobenzene exhibits a thermal half-life of 700 days and can be photoisomerized using green light for E-to-Z conversion and blue light for Z-to-E reversion [28]. In addition, several azopyrazole derivatives also show efficient E-to-Z and Z-to-E isomerization under 405 and 520/532 nm light irradiations, respectively [6,32,33]. In summary, molecular design engineering provides an innovative, efficient, and versatile method for the fabrication of azobenzene-based molecular photothermal storage system that effectively store visible-light photon energy.
We designed and synthesized an azobenzene photothermal molecule that stores visible-light photon energy through molecular engineering. The molecule consists of a tetra-ortho-chlorinated azo structure as the “head” part and an alkyl chain at para-position as the “tail” part. By grafting chlorine atoms on the ortho positions of azobenzene molecule, we red-shifted the π–π* transition to the green portion (520–550 nm) of the solar spectrum. The photoisomerization of tetra-ortho-chlorinated azobenzene was examined using ultraviolet–visible (UV-vis) spectroscopy, revealing that E-to-Z isomerization occurs under 550 nm light irradiation, demonstrating the molecule’s ability to absorb and store green photon energy. The Z-to-E isomerization under 430 nm light irradiation indicates that the molecule can release stored energy when exposed to blue light. In addition, the energy storage density, energy storage lifetime, and cyclic stability of the obtained azobenzene molecule were systematically evaluated via UV–vis analysis, differential scanning calorimetry (DSC) measurement, and theoretical DFT calculation, resulting in an energy density of 13.50 kJ/mol, half-life of 10.97 days, and charging–discharging cycling performance of 100 times. It is worth mentioning that the alkyl chain at the para-position endows the molecule with a controlled optical crystal-to-liquid phase transition and high supercooling performance, which may be beneficial for the charging process and its application in extremely low-temperature environments. This study adds a new member to the family of azobenzene-based photothermal storage materials capable of efficiently storing visible-light photon energy, further advancing this field towards practical applications.
2. Results and Discussion
2.1. Molecule Design and Synthesis
In recent years, it has been demonstrated that tetra-ortho-halogenate azobenzene exhibits a remarkable capacity to separate n→π* transitions, which allows for the generation of Z-isomers via irradiation with yellow or red light [17,27,29,34]. Therefore, tetra-ortho-chlorinated azobenzene was chosen as the photoswitching structure, and an alkyl chain was incorporated in para-positions to endow the azobenzene molecules with the photoinduced phase transition feature. As the photoinduced Z-liquid can facilitate a decline in the melting point of the E/Z eutectic mixture, the liquid-state Z-isomer will play the role of a “solvent” that supplies free volume and thereby allows for further E-crystal-to-Z-rich liquid photoisomerization [35,36,37,38,39,40]. The target product o-4ClAzo-C8 was obtained through the azo-coupling between substituted phenols and anilines [41], followed by the Williamson ether reaction [5,15]. The synthesis route is shown in Scheme 1. The characterization data, including the 1H and 13C NMR spectra of products, are included in the Supplementary Materials.
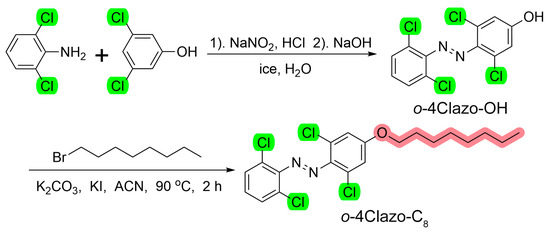
Scheme 1.
Synthetic route for the o-4ClAzo-C8 compound.
2.2. Light Source Optimization
We systematically investigated the photostationary state (PSS) behavior of the o-4ClAzo-C8 compound under various wavelengths of light by conducting UV–vis spectroscopic measurements (Figure 1a). Subsequently, the percentages of Z-isomers in the resulting photostationary states (PSS) were calculated using Equation (S1) (Figure 1b). As illustrated in Figure 1a, the initial E-rich state of o-4ClAzo-C8 compound exhibits two distinct absorption peaks: a π–π* transition peak at 326 nm and an n–π* transition peak at 457 nm. Upon conversion to the Z-rich state, the absorption intensity at 326 nm decreased significantly, and the n–π* transition peak exhibited a slight blue shift accompanied by a slight increase in intensity. The light sources at 365 nm, 520 nm, 530 nm, 550 nm, 590 nm, and 610 nm induced the E-to-Z photoisomerization of the o-4ClAzo-C8, corresponding to the charging process of the photothermal energy storage materials. In the visible-light range, despite the fact that orange light (590 nm and 610 nm) irradiation resulted in a PSS containing almost only the Z isomer, these wavelengths required more time (2 h) for photoisomerization. The 550 nm light-induced PSS could harvest a 64.9% Z-isomer, slightly less than that of 365 nm light irradiation (72.1%). Consequently, 550 nm was identified as the optimal visible-light source for the charging of o-4ClAzo-C8 based photothermal energy storage materials. Conversely, light sources at 395 nm, 420 nm, 430 nm, and 450 nm triggered the Z-to-E reverse isomerization corresponding to the discharging process. In the visible-light range, the PSS induced by blue light (430 nm) demonstrated the lowest Z-isomer content (12.2%), indicating that blue light can be effectively employed for back-isomerization (Figure 1b). Therefore, to release heat from the charged o-4ClAzo-C8 molecule, irradiation with 430 nm light is recommended.
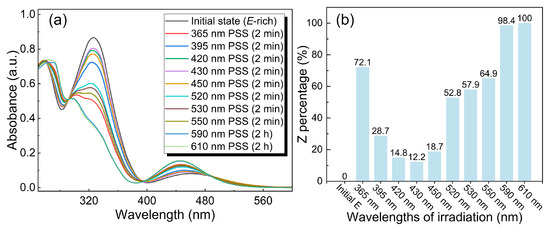
Figure 1.
(a) UV–vis absorption spectra of the o-4ClAzo-C8 molecule in dichloromethane (DCM) solution (1 × 10−4 mol L−1), irradiated with difference wavelengths of light sources. (b) The percentage content of the Z isomer in the o-4ClAzo-C8 molecule at PSS under different wavelengths of light irradiation.
2.3. Photoisomerization Performance
The photoisomerization of the o-4ClAzo-C8 molecule under 550 nm and 430 nm light irradiation was studied using UV–vis absorption spectra at room temperature (Figure 2a,c). As the irradiation time increased during the charging, the π–π* transition peak intensity significantly decreased, while the n–π* transition peak slightly increased and exhibited a blue shift from 457 nm to 447 nm, indicating isomerization from E to Z isomers (Figure 2a). This time-evolution ultimately led to the attainment of the Z-rich photostationary. During the discharging process, the π–π* transition peak increased, while the n–π* transition peak decreased due to the Z-to-E reversion (Figure 2c). To further investigate the efficiency of the charging and discharging processes, we monitored the isomeric compositions at various durations of light irradiation. The irradiated samples were characterized using UV–vis and 1H NMR spectroscopy. In the initial state, the E/Z mixture of o-4ClAzo-C8 compound was primarily composed of the E-isomer (E:Z = 96:4, calculated using Equation (S2)), as determined from the NMR spectrum (Figure 2e, top). During the photo-charging process, the sample was irradiated with 550 nm visible light, resulting in a fraction of 67.8% of the Z isomer within 70 s (Figure 2a,b). It is noteworthy that the Z-fraction calculated from the UV–vis spectroscopy data was slightly higher than that derived from the 1H NMR spectra (E:Z = 41:59) (Figure 2e, middle). This discrepancy may be attributable to the inherent error om the UV–vis spectroscopic data calculation method. In the photo-discharging process, the Z isomer content dropped to 13.4% after 6 s of 430 nm light illumination (Figure 2c,d), consistent with the NMR results (E:Z = 87:13) (Figure 2e, bottom). The rapid discharging process (6 s) under 430 nm blue light indicated the potential for rapid heat release within a short timeframe. Based on the above analysis, its efficient bidirectional photoisomerization, with excellent reversibility of o-4ClAzo-C8, renders it a promising candidate for energy harvesting applications.
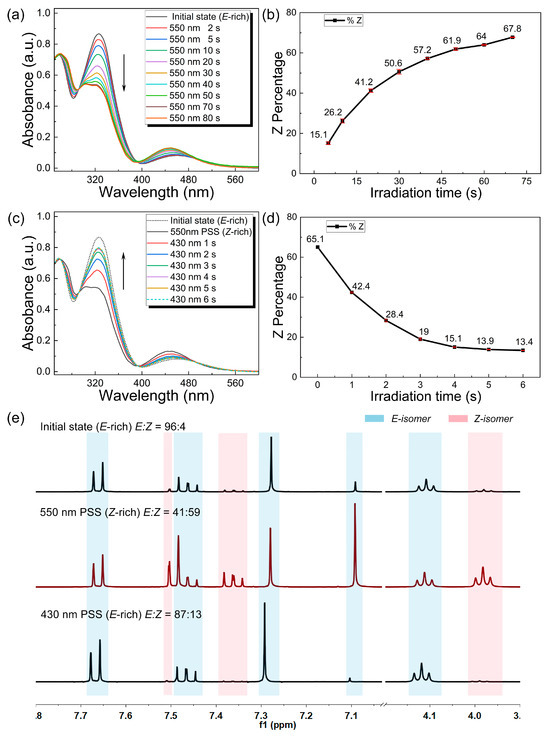
Figure 2.
Time-evolved UV–vis spectra of o-4ClAzo-C8 samples under irradiations of 550 nm (a) and 430 nm (c). The spectra were normalized with respect to the isosbestic point at 332 nm. The arrows indicate the order of tests performed. Z-isomer percentage of o-4ClAzo-C8 samples vs. 550 nm irradiation time (b) and 430 nm irradiation time (d), respectively. (The numerical values were calculated using Equation (S1).) (e) The 1H NMR spectrum and isomeric composition of the initial o-4ClAzo-C8 (top) after charging under 550 nm light (middle) and discharging under 430 nm light (bottom).
2.4. Energy Storage Lifetime
In addition to being triggered by blue light, the Z-rich sample exhibits spontaneous reversion back to the E-rich state in darkness. This dynamic thermal relaxation behavior provides insights into the isomerization energy storage time of the o-4ClAzo-C8 compound, which is crucial to improve the energy storage lifetime and controllable energy release. Figure 3a illustrates a continuous increase in the peak intensity of the π–π* transition for the 550 nm PSS sample over time, indicating that the Z-isomer in the molecule gradually reverts to the E form. Until day 21, the E-isomer content in the E/Z mixture of the o-4ClAzo-C8 compound reaches approximately 83% (calculated by Equation (S1)). This observation indicates that the thermal Z-to-E reversion occurs very slowly in darkness, which is beneficial to control the heat release of o-4ClAzo-C8-based molecular photothermal systems. According to Equation (S3), the first-order rate of the Z-to-E isomerization (4.39 × 10−6 s−1) is four orders of magnitude lower than that of pure azobenzene (4.15 × 10−2 s−1) [42]. The calculated half-life of the Z isomer is 10.97 days (Equation (S4)), more than twice that of the pure azobenzene molecule (Figure 3b) [43]. This result suggests that the o-4ClAzo-C8 photothermal molecule could be utilized in long-term azobenzene-based photothermal systems. In addition, the synergistic effect of chlorine’s steric distortion and the alkoxy group’s electronic modulation likely amplifies the kinetic instability of the Z-isomer compared to the pristine tetra-ortho-fluorinated azobenzene fluorinated analogs [28], and thereby significantly decrease the half-life of the Z-isomer for the o-4ClAzo-C8 compound.
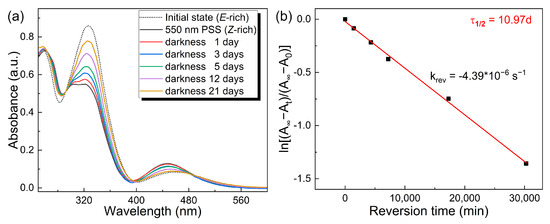
Figure 3.
(a) The UV–vis absorption spectra of the o-4ClAzo-C8 sample, which was charged with 550 nm light and naturally recovered in the dark at room temperature over time. (b) The first-order rate constants for Z-to-E reversion (krev) in darkness for o-4ClAzo-C8.
We investigated the cycling stability of energy charging and heat releasing in the o-4ClAzo-C8 molecule through repeated E-to-Z photoisomerization (charging process) and Z-to-E photoreversion (discharging processes). The initial sample was charged to its PSS (i.e., Z-rich state) under 550 nm light irradiation, and then discharged back to the E-rich state with 430 nm light exposure. Figure 4 depicts the UV–vis spectroscopy-recorded absorbance changes in the π–π* transition peak for E-rich and Z-rich states after 550 or 430 nm alternating-light irradiations. The results demonstrate that the o-4ClAzo-C8 photothermal molecule exhibits favorable cycling stability over 100 complete cycles without notable photodegradation.
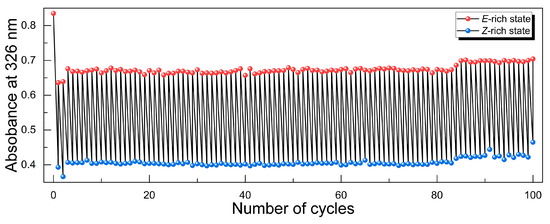
Figure 4.
The stability of cycling through alternating charging and discharging over 100 cycles.
2.5. Phase Transition Property
XRD analysis was performed to examine the phase transition of o-4ClAzo-C8 from an E-crystal to Z-liquid. As shown in Figure 5a, the initial state featured sharp peaks at 2θ values ranging from 5° to 25°, which correspond to the ordered stacking of the E-crystal. This XRD pattern is characteristic of the E configuration in azobenzene compounds [13,44,45]. Subsequently, these characteristic peaks vanished upon irradiation with 550 nm light, indicating that the Z-isomer was in an amorphous state, accompanied by the photo-melting of the E-crystal into a Z-liquid (Figure 5b inset). These observations clearly demonstrate that the E-to-Z photoisomerization of o-4ClAzo-C8 leads to its liquefaction into an isotropic liquid phase. However, the subsequent irradiation of the Z-liquid with 430 nm light did not cause the E-crystal to reappear, but instead produced E-liquid (Figure 5c), with no recovery of the initial XRD pattern. This intriguing result may be attributed to four factors: (1) the tetra-ortho-substituted chlorine atom likely impedes the ordered molecular arrangement through steric hindrance, which significantly increasing the difficulty of molecular packing and thereby reduces the crystallization ability of the Z-liquid [29]; (2) the introduction of the para-alkyl chain drastically alters the molecular symmetry, disrupting the π–π stacking of Azobenzene molecules and making it difficult for them to reorganize into ordered crystals after melting [13]; (3) the conjugated double bonds in azobenzene and the electron-withdrawing effect of the chlorine atom may enhance molecular rigidity, inhibiting molecular chain mobility and obstructing the formation of crystal nuclei [17,45]; (4) the liquid state of the E-isomer possesses a relatively high degree of supercooling.
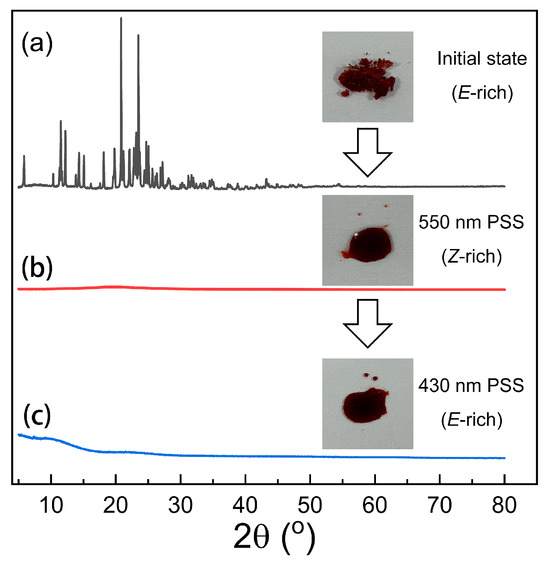
Figure 5.
X-ray diffraction patterns of o-4ClAzo-C8 molecule: (a) initial state sample, (b) after 550 nm irradiation, and (c) after 430 nm irradiation. A digital photograph of each sample is shown as an inset.
2.6. Energy Storage Density
The energy storage capacity of the o-4ClAzo-C8 photothermal molecule was thoroughly investigated. Figure 6a presents the DSC thermograms for the E-rich. The DSC trace of the E-isomer showcases a distinct endothermic melting peak at approximately 37 °C during the heating phase, indicative of a phase transition from a crystalline to a liquid state. Notably, the absence of a detectable exothermic crystallization peak during the cooling phase (temperature range: 80 °C to −60 °C) emphasizes the pronounced supercooling exhibited by the E-isomer of o-4ClAzo-C8 (consistent with previous XRD observations). In the subsequent reheating cycle, no endothermic melting peak in the first heating cycle was observed again (Figure S7), further confirming the E-configuration can maintain its liquid state across a wider temperature range, thereby effectively preserving the phase change’s latent heat. This distinctive thermal behavior could position the o-4ClAzo-C8 photothermal molecule as a promising candidate for advanced heat-preservation applications in extreme low-temperature environments.
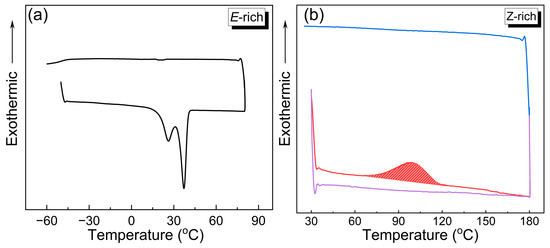
Figure 6.
DSC curves of o-4ClAzo-C8 for (a) initial state (E-rich) and (b) after charging under 550 nm light for 30 min (Z-rich), including heating (red), cooling (blue), and reheating (purple) segments.
Figure 6b presents the DSC thermograms of Z-rich o-4ClAzo-C8 photothermal molecules. In the heating stage, a broad exothermic peak was observed within the temperature range of 70 °C to 120 °C, with a ΔH value of 30.2 J/g (13.5 kJ/mol). The observed peak corresponds to thermal Z-to-E isomerization, which disappears during the reheating phase. This suggests that the exothermic heat flow observed in the first heating cycle originates from photo-induced energy storage. Due to the significant supercooling of the E-liquid, no crystallization peak was observed during the cooling phase either. Theoretical calculations indicated an energy gap of 28.21 kJ/mol between the Z-isomer and E-isomer (Figure 7a,b), which is twice the observed experimental value. This discrepancy is associated with the low degree of E-to-Z isomerization during the charging process (with only 64% of the Z-isomer yield achieved). Compared with the reported azobenzene photothermal molecule of the same type [17,30,34,38], this work has a relatively moderate energy density value. Although the Z-isomer shows a relatively moderate energy density, the significant supercooling of the E-isomer presents a promising opportunity for its use in temperature buffering systems in extreme low-temperature environments; it could potentially provide antifreeze protection for equipment used in polar or space exploration and future protective clothing.
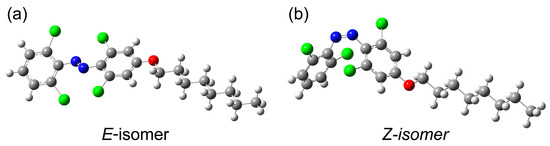
Figure 7.
The optimized geometric structure of o-4ClAzo-C8 with the E-isomer (a) and Z-isomer (b). Green, blue and red represent Cl, N, and O, respectively.
3. Materials and Methods
3.1. Materials
In this study, 3,5-dichlorophenol (98%), 2,6-dichloroaniline (99%), sodium hydroxide (NaOH, 98%), sodium nitrite (NaNO2, 99%), 1-bromooctane (99%), potassium iodide (KI, 99.8%), and potassium carbonate (K2CO3, 99.9%) were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Hexyl hydride (AR), ethyl acetate (AR), dichloromethane (DCM, AR and SP), acetonitrile (ACN, AR), and hydrochloric acid (HCl, AR) were purchased from Titan Scientific Co., Ltd. (Shanghai, China). All the reagents were used directly without further purification.
3.2. Synthesis
3.2.1. Synthesis of (E)-3,5-Dichloro-4-((2,6-dichlorophenyl)diazenyl)phenol (o-4ClAzo-OH)
A solution of 2,6-dichloroaniline (648 mg, 4 mmol) in 10 mL of distilled water was acidified with 1 mL of concentrated hydrochloric acid and cooled to 0 °C. A cold solution of sodium nitrite (304 mg, 4.4 mmol) was added dropwise to 5 mL distilled water. The orange suspension was then added dropwise into a chilled solution of 3,5-dichlorophenol (717 mg, 4.4 mmol) and sodium hydroxide (1.5 M, 8 mL). The mixture was stirred at 0 °C for 2 h before being acidified to pH 2 using 0.5 M hydrochloric acid. The product underwent extraction with DCM three times, followed by washing the organic layer with saturated sodium chloride solution. It was then dried using anhydrous sodium sulfate and concentrated using a rotary evaporator. Purification via flash silica gel chromatography (hexyl hydride–ethyl acetate = 7:2) yielded the desired orange solid product (590 mg, 44%). The 1H and 13C NMR spectrum are displayed in the Supporting Materials (Figures S1 and S2).
3.2.2. Synthesis of (E)-1-(2,6-Dichloro-4-(octyloxy)phenyl)-2-(2,6-dichlorophenyl)diazene (o-4ClAzo-C8)
o-4Clazo-OH (672 mg, 2 mmol), potassium iodide (664 mg, 4 mmol), and potassium carbonate (553 mg, 4 mmol) were dissolved in 10 mL acetonitrile, followed by the dropwise addition of 1-bromooctane (0.418 mL, 2.4 mmol). The mixture was stirred at 90 °C for two hours while monitoring the reaction with TLC. The reaction was halted, and the solution was cooled to room temperature, filtered, and concentrated via rotary evaporation. Using flash silica gel chromatography (hexyl hydride–ethyl acetate = 30:1), the residue was purified to produce an orange solid weighing 851 mg with a 95% yield. The 1H and 13C NMR spectrum are displayed in the Supporting Materials (Figures S3 and S6).
3.3. Characterization
Purification was performed using the SepaBean machine automated flash chromatography system by Santai Technologies, Inc. (Pointe-Claire, QC, USA). 1H and 13C NMR spectra were obtained on a 500 MHz INOVA spectrometer (Varian, Palo Alto, CA, USA), using dimethyl sulfoxide-d6 or Chloroform-d as the solvent and tetramethylsilane as the standard. The samples were charged/discharged under 550/430 nm light irradiation. The optical power meter (CEL-NP2000, Beijing China Education Au-light Co., Ltd., Beijing, China) was applied to measuring the intensity of light. The X-Ray Diffraction (XRD) patterns were measured on a Rigaku SmartLab SE diffractometer (Tokyo, Japan). Differential Scanning Calorimetry (DSC) measurements were conducted on a DSC 214 (Netzsch, Selb, Germany).
3.4. UV-Vis Absorption Spectroscopy
Light Source Optimization: The sample was dissolved in dichloromethane at a 0.0100 mg/mL concentration. The UV–vis spectra of the E-isomers were first documented in the dark. Subsequently, the samples were irradiated with the specified wavelengths of the light source until no absorbance changes were observed, typically after 120 s or 2 h. After the irradiation, the UV–vis spectra were recorded as the photostationary state (PSS) at the specified wavelengths of the light sources. The light sources included 365 nm (50 mW cm−2), 395 nm (50 mW cm−2), 420 nm (50 mW cm−2), 430 nm (50 mW cm−2), 520 nm (20 mW cm−2), 530 nm (20 mW cm−2), 550 nm (20 mW cm−2), 590 nm (20 mW cm−2), and 610 nm (20 mW cm−2).
Time-Evolved Photoisomerization: The samples were irradiated using a 550 nm or 430 nm wavelength light source. During irradiation, the UV–vis absorption spectra were recorded at regular intervals, and the data obtained were normalized using software.
3.5. Lifetime Measurements
Samples of the 550 nm PSS liquid Z isomer were placed in a dark room at room temperature. The UV–vis absorption spectra of the samples were measured periodically until they returned to their original state, and the data collected were normalized using software.
3.6. DSC Measurements
The liquid Z isomer was obtained from the solid E isomer after 30 min of exposure to a 550 nm LED lamp. The samples were then moved to a DSC pan to measure their exothermic properties. Unless specified otherwise in the figure captions, the cooling and heating rates during the DSC tests were maintained at 10 °C/min.
3.7. Computational Studies
Density functional theory (DFT) was employed to calculate the single-point energies of the Z and E forms of o-4Clazo-C8. The geometry of each structure was optimized using the B3LYP method and the 6-31+G(d,p) basis set. A frequency analysis was then performed at the same theoretical level on each optimized structure to obtain their thermodynamic parameters. For more accurate computational outcomes, single point energies were calculated using the wB97XD/6-311++G(d,p) method for optimized geometries. All computations were performed using Gaussian 16 software, and the 3D molecular structures were visualized with CYLview 6.0.
3.8. Statistical Analysis
The data obtained in this study were used without preprocessing, unless otherwise noted. All data are presented as mean ± standard deviation, with measurements from at least three independent experiments. All experiments were performed with multiple replicates (triplicate to quintuplicate).
4. Conclusions
This study reports the successful design and synthesis of an azobenzene photothermal molecule, o-4ClAzo-C8, featuring a tetra-ortho-chlorinated azo structure as the head and an alkyl chain at the para-position as the tail. The tetra-ortho-chlorinated structure endows the photothermal molecule with the ability to effectively convert visible-light photon energy into chemical energy and store it in an N=N bond, before it is released as heat energy when needed. The photoisomerization properties of o-4ClAzo-C8 were researched through the UV–vis and 1H NMR spectra. Specifically, 64.9% of Z-isomer was obtained via charging under a 550 nm wavelength of light irradiation, and 87.8% of E-isomer was achieved via discharging at a 430 nm wavelength of light irradiation. The o-4ClAzo-C8 photothermal molecule demonstrated long-term energy storage with a half-life of approximately 11 days and maintained cyclic stability for 100 cycles, likely due to the steric hindrance effects of the tetra-ortho-substituted chlorine atom. The experimental energy storage density of the Z-isomer (13.50 kJ/mol) is lower than the theoretical calculation value (28.21 kJ/mol) due to the relatively low degree of E-to-Z isomerization. Remarkably, the o-4ClAzo-C8 photothermal molecule showed an irreversible photoinduced phase transition from an E-crystal to Z-liquid and a high degree of supercooling through XRD and DSC experiments, which may be due to the para-alkyl chain of molecules and the steric hindrance effect of the chlorine atoms. In the future, this azobenzene photothermal molecule may have potential applications in temperature buffering systems such as antifreeze protection for equipment and clothing used in polar or space explorations in extremely cold weather.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30112333/s1, Figure S1. The 1H NMR spectrum of o-4Clazo-OH molecule. Figure S2. The 13C NMR spectrum of o-4Clazo-OH molecule. Figure S3. The 1H NMR spectrum of o-4Clazo-C8 molecule in its initial state. Figure S4. The 1H NMR spectrum of o-4Clazo-C8 molecule in its 550 nm PSS. Figure S5. The 1H NMR spectrum of o-4Clazo-C8 molecule in its 430 nm PSS. Figure S6. The 13C NMR spectrum of o-4Clazo-C8 molecule. Figure S7. DSC curves of o-4ClAzo-C8 for (a) initial state (E-rich) along with heating (red), cooling (blue) and reheating (purple) segments.
Author Contributions
Conceptualization, F.T.; methodology, L.D.; software, J.X.; validation, S.T., Y.Z. and F.T.; formal analysis, Y.Z. and S.T.; investigation, S.T.; resources, J.X. and J.Q.; data curation, S.T. and J.Q.; writing—original draft preparation, S.T.; writing—review and editing, L.D.; visualization, F.Z.; supervision, L.D.; project administration, L.D.; funding acquisition, L.D. and F.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52203106, the National Natural Science Foundation of Hangzhou, grant number 2024SZRYBF05003, the Science Fund of Shandong Laboratory of Advanced Materials and Green Manufacturing at Yantai, grant number AMGM2024F21, the National College Students Innovation and Entrepreneurship Training Program, grant number 202311842029X, and the Introduction of Talents and Scientific Research Project of Zhejiang Shuren University, grant number 2021R045.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors were grateful for the language editing services provided by the HOME for Researchers (https://www.home-for-researchers.com). The authors extend their gratitude to Chen from Scientific Compass (www.shiyanjia.com) for providing invaluable assistance with the DSC analysis.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| o-4ClAzo-OH | (E)-3,5-dichloro-4-((2,6-dichlorophenyl)diazenyl)phenol |
| o-4ClAzo-C8 | (E)-1-(2,6-dichloro-4-(octyloxy)phenyl)-2-(2,6-dichlorophenyl)diazene |
| UV–vis | Ultraviolet–visible |
| XRD | X-ray diffraction |
| DSC | Differential scanning calorimetry |
References
- Wang, Z.; Hölzel, H.; Moth-Poulsen, K. Status and challenges for molecular solar thermal energy storage system based devices. Chem. Soc. Rev. 2022, 51, 7313–7326. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.Q.; Feng, Y.Y.; Wang, L.; Feng, W. Azobenzene-based solar thermal fuels: Design, properties, and applications. Chem. Soc. Rev. 2018, 47, 7339–7369. [Google Scholar] [CrossRef] [PubMed]
- Kolpak, A.M.; Grossman, J.C. Azobenzene-functionalized carbon nanotubes as high-energy density solar thermal fuels. Nano Lett. 2011, 11, 3156–3162. [Google Scholar] [CrossRef]
- Kucharski, T.J.; Tian, Y.C.; Akbulatov, S.; Boulatov, R. Chemical solutions for the closed-cycle storage of solar energy. Energy Environ. Sci. 2011, 4, 4449–4472. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Tang, S.; Liu, X.; Han, Y.; Zhang, S.; Liu, K.; Feng, W. An innovative azobenzene-based photothermal fabric with excellent heat release performance for wearable thermal management device. Small 2024, 20, 2404310. [Google Scholar] [CrossRef]
- Xu, X.; Xing, Y.; Yin, Y.; Fang, W.; Wu, B.; Bei, P.; Feng, J.; Yu, H.; Wang, G.; Li, W.-Y. Flexible wearable fabrics for solar thermal energy storage and release in on-demand environments. Chem. Eng. J. 2023, 466, 143175. [Google Scholar] [CrossRef]
- Fei, L.; Zhang, Z.-Y.; Tan, Y.; Ye, T.; Dong, D.; Yin, Y.; Li, T.; Wang, C. Efficient and robust molecular solar thermal fabric for personal thermal management. Adv. Mater. 2023, 35, 2209768. [Google Scholar] [CrossRef]
- Fei, L.; Yu, W.; Tan, J.; Yin, Y.; Wang, C. High solar energy absorption and human body radiation reflection janus textile for personal thermal management. Adv. Fiber Mater. 2023, 5, 955–967. [Google Scholar] [CrossRef]
- Fei, L.; Yin, Y.; Yang, M.; Zhang, S.; Wang, C. Wearable solar energy management based on visible solar thermal energy storage for full solar spectrum utilization. Energy Storage Mater. 2021, 42, 636–644. [Google Scholar] [CrossRef]
- Fei, L.; Yin, Y.; Zhang, J.; Wang, C. A Visible energy management by photochromic solar thermal fuel using a color display. Sol. RRL 2020, 4, 2000499. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Gao, J.; Fang, W.; Ge, J.; Yang, X.; Zhai, F.; Yu, Y.; Feng, W. Metallic-ion controlled dynamic bonds to co-harvest isomerization energy and bond enthalpy for high-energy output of flexible self-heated textile. Adv. Sci. 2022, 9, 2201657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, Y.; Bai, J.; Mu, J.; Chen, L.; Zhang, N.; Han, J.; Liu, F.; Yan, S. Preparation of flexible photo-responsive film based on novel photo-liquefiable azobenzene derivative for solar thermal fuel application. Dye. Pigment. 2022, 202, 110277. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; He, Y.; Wang, Z.; Xu, J.; Xie, M.; Tao, P.; Ji, D.; Moth-Poulsen, K.; Li, T. Photochemical phase transitions enable coharvesting of photon energy and ambient heat for energetic molecular solar thermal batteries that upgrade thermal energy. J. Am. Chem. Soc. 2020, 142, 12256–12264. [Google Scholar] [CrossRef]
- Shangguan, Z.; Sun, W.; Zhang, Z.-Y.; Fang, D.; Wang, Z.; Wu, S.; Deng, C.; Huang, X.; He, Y.; Wang, R.; et al. A rechargeable molecular solar thermal system below 0 °C. Chem. Sci. 2022, 13, 6950–6958. [Google Scholar] [CrossRef]
- Ge, F.; Yu, W.; Yin, Y.; Wang, C. Solar-driven thermochromic fabric based on photothermal conversion for light intensity monitoring. J. Mater. Chem. A 2021, 9, 20565–20575. [Google Scholar] [CrossRef]
- Xu, X.; Wang, G. Molecular solar thermal systems towards phase change and visible light photon energy storage. Small 2022, 18, 2107473. [Google Scholar] [CrossRef] [PubMed]
- Kwaria, D.; McGehee, K.; Liu, S.; Kikkawa, Y.; Ito, S.; Norikane, Y. Visible-light-photomeltable azobenzenes as solar thermal fuels. ACS Appl. Opt. Mater. 2023, 1, 633–639. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, Y.; Feng, W. Azobenzene-based solar thermal fuels: A review. Nano-Micro Lett. 2022, 14, 138. [Google Scholar] [CrossRef]
- Kimizuka, N.; Yanai, N.; Morikawa, M.-a. Photon upconversion and molecular solar energy storage by maximizing the potential of molecular self-assembly. Langmuir 2016, 32, 12304–12322. [Google Scholar] [CrossRef]
- Naimovičius, L.; Bharmoria, P.; Moth-Poulsen, K. Triplet–triplet annihilation mediated photon upconversion solar energy systems. Mater. Chem. Front. 2023, 7, 2297–2315. [Google Scholar] [CrossRef]
- Wang, Z.; Jones, B.E.; Franca, L.G.; Lawson, T.; Jevric, M.; Moth-Poulsen, K.; Evans, R.C. Multilayer films for photon upconversion-driven photoswitching. J. Mater. Chem. C 2024, 12, 19030–19034. [Google Scholar] [CrossRef] [PubMed]
- Bharmoria, P.; Ghasemi, S.; Edhborg, F.; Losantos, R.; Wang, Z.; Mårtensson, A.; Morikawa, M.-A.; Kimizuka, N.; İşci, Ü.; Dumoulin, F.; et al. Far-red triplet sensitized Z-to-E photoswitching of azobenzene in bioplastics. Chem. Sci. 2022, 13, 11904–11911. [Google Scholar] [CrossRef]
- Wu, W.; Yao, L.; Yang, T.; Yin, R.; Li, F.; Yu, Y. NIR-Light-induced deformation of cross-linked liquid-crystal polymers using upconversion nanophosphors. J. Am. Chem. Soc. 2011, 133, 15810–15813. [Google Scholar] [CrossRef]
- Siewertsen, R.; Neumann, H.; Buchheim-Stehn, B.; Herges, R.; Näther, C.; Renth, F.; Temps, F. Highly Efficient Reversible Z−E Photoisomerization of a Bridged Azobenzene with Visible Light through Resolved S1(n-π*) Absorption Bands. J. Am. Chem. Soc. 2009, 131, 15594–15595. [Google Scholar] [CrossRef]
- Hammerich, M.; Schutt, C.; Stahler, C.; Lentes, P.; Rohricht, F.; Hoppner, R.; Herges, R. Heterodiazocines: Synthesis and photochromic properties, trans to cis switching within the bio-optical window. J. Am. Chem. Soc. 2016, 138, 13111–13114. [Google Scholar] [CrossRef] [PubMed]
- Rullo, A.; Reiner, A.; Reiter, A.; Trauner, D.; Isacoff, E.Y.; Woolley, G.A. Long wavelength optical control of glutamate receptor ion channels using a tetra-ortho-substituted azobenzene derivative. Chem. Commun. 2014, 50, 14613–14615. [Google Scholar] [CrossRef]
- Bleger, D.; Schwarz, J.; Brouwer, A.M.; Hecht, S. o-Fluoroazobenzenes as readily synthesized photoswitches offering nearly quantitative two-way isomerization with visible light. J. Am. Chem. Soc. 2012, 134, 20597–20600. [Google Scholar] [CrossRef] [PubMed]
- Knie, C.; Utecht, M.; Zhao, F.; Kulla, H.; Kovalenko, S.; Brouwer, A.M.; Saalfrank, P.; Hecht, S.; Bléger, D. ortho-Fluoroazobenzenes: Visible light switches with very long-lived Z isomers. Chem.—Eur. J. 2014, 20, 16492–16501. [Google Scholar] [CrossRef]
- Konrad, D.B.; Savasci, G.; Allmendinger, L.; Trauner, D.; Ochsenfeld, C.; Ali, A.M. Computational design and synthesis of a deeply red-shifted and bistable azobenzene. J. Am. Chem. Soc. 2020, 142, 6538–6547. [Google Scholar] [CrossRef]
- Shi, Y.; Gerkman, M.A.; Qiu, Q.; Zhang, S.; Han, G.G.D. Sunlight-activated phase change materials for controlled heat storage and triggered release. J. Mater. Chem. A 2021, 9, 9798–9808. [Google Scholar] [CrossRef]
- Samanta, S.; Beharry, A.A.; Sadovski, O.; McCormick, T.M.; Babalhavaeji, A.; Tropepe, V.; Woolley, G.A. Photoswitching azo compounds in vivo with red light. J. Am. Chem. Soc. 2013, 135, 9777–9784. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, B.; Zhang, P.; Xing, Y.; Shi, K.; Fang, W.; Yu, H.; Wang, G. Arylazopyrazole-based dendrimer solar thermal fuels: Stable visible light storage and controllable heat release. ACS Appl. Mater. Interfaces 2021, 13, 22655–22663. [Google Scholar] [CrossRef]
- Huang, X.; Shangguan, Z.; Zhang, Z.-Y.; Yu, C.; He, Y.; Fang, D.; Sun, W.; Li, Y.-C.; Yuan, C.; Wu, S.; et al. Visible-light-induced reversible photochemical crystal–liquid transitions of azo-switches for smart and robust adhesives. Chem. Mater. 2022, 34, 2636–2644. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Feng, X. Theoretical study on the isomerization mechanism of visible light-driven azobenzene-based materials. Comput. Theor. Chem. 2024, 1238, 114719. [Google Scholar] [CrossRef]
- Norikane, Y.; Hirai, Y.; Yoshida, M. Photoinduced isothermal phase transitions of liquid-crystalline macrocyclic azobenzenes. Chem. Commun. 2011, 47, 1770–1772. [Google Scholar] [CrossRef]
- Qiu, Q.; Gerkman, M.A.; Shi, Y.; Han, G.G.D. Design of phase-transition molecular solar thermal energy storage compounds: Compact molecules with high energy densities. Chem. Commun. 2021, 57, 9458–9461. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cho, S.; Wan, J.; Han, G.G.D. Photoswitches and photochemical reactions for optically controlled phase transition and energy storage. Chem 2023, 9, 2378–2389. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Z.-Y.; Li, T.; Wang, R.; Li, T. Optically-controlled variable-temperature storage and upgrade of thermal energy by photoswitchable phase change materials. ACS Mater. Lett. 2023, 5, 2019–2027. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Luo, W.; Quan, X.; Li, H.; Huang, J.; Feng, W. High-energy and light-actuated phase change composite for solar energy storage and heat release. Surf. Interfaces 2021, 24, 101071. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, R.; Luo, W.; Hu, Y.; Wang, H.; Wang, C.; Li, X.; Huang, J. Photoguided AZO-phase change composite for high-energy solar storage and heat release at near ambient temperature. J. Energy Storage 2024, 101, 113974. [Google Scholar] [CrossRef]
- Merino, E. Synthesis of azobenzenes: The coloured pieces of molecular materials. Chem. Soc. Rev. 2011, 40, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, J.; Lawrence, J.; Yee, G.G. Photochemical storage potential of azobenzenes. Sol. Energy 1983, 30, 271–274. [Google Scholar] [CrossRef]
- Adamson, A.W.; Vogler, A.; Kunkely, H.; Wachter, R. Photocalorimetry. Enthalpies of photolysis of trans-azobenzene, ferrioxalate and cobaltioxalate ions, chromium hexacarbonyl, and dirhenium decarbonyl. J. Am. Chem. Soc. 1978, 100, 1298–1300. [Google Scholar] [CrossRef]
- Liang, R.; Yuan, B.; Zhang, F.; Feng, W. Azopyridine polymers in organic phase change materials for high energy density photothermal storage and controlled release. Angew. Chem. Int. Ed. 2025, 64, e202419165. [Google Scholar] [CrossRef]
- Ishiba, K.; Morikawa, M.; Chikara, C.; Yamada, T.; Iwase, K.; Kawakita, M.; Kimizuka, N. Photoliquefiable ionic crystals: A phase crossover approach for photon energy storage materials with functional multiplicity. Angew. Chem. Int. Ed. 2015, 54, 1532–1536. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).