Purification of Flavonoids from an Aqueous Cocoa (Theobroma cocoa L.) Extract Using Macroporous Adsorption Resins
Abstract
1. Introduction
2. Results
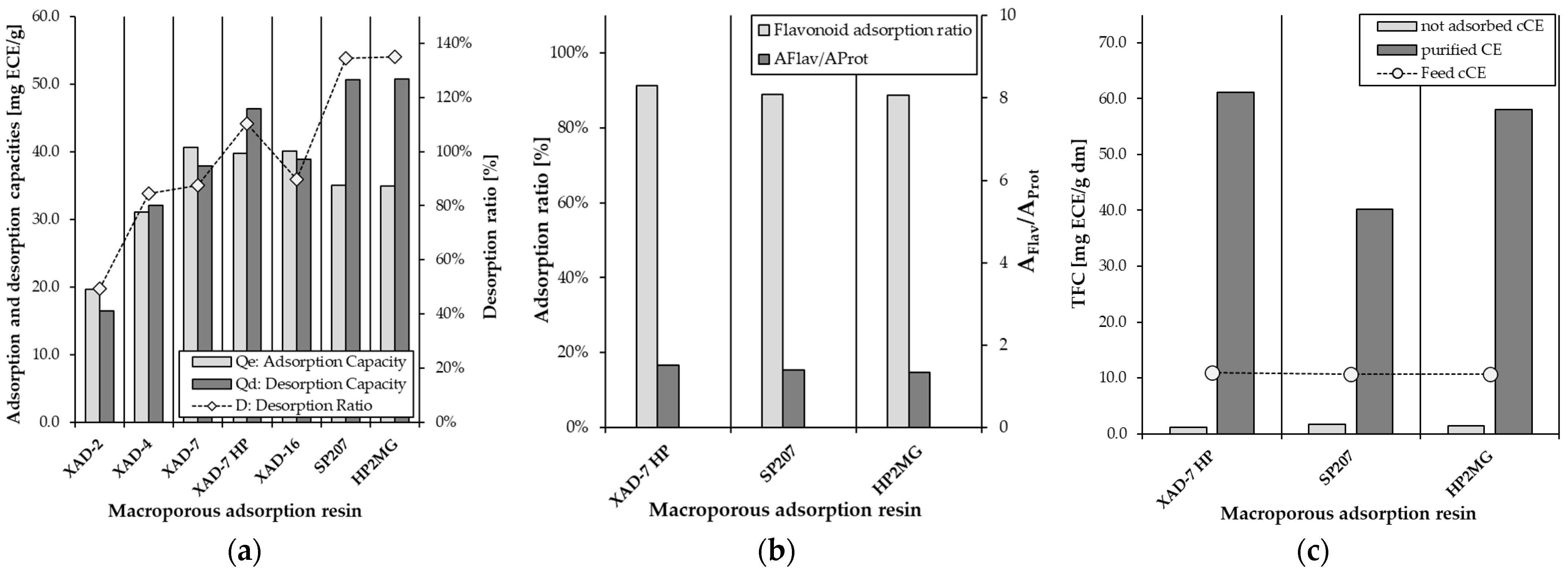
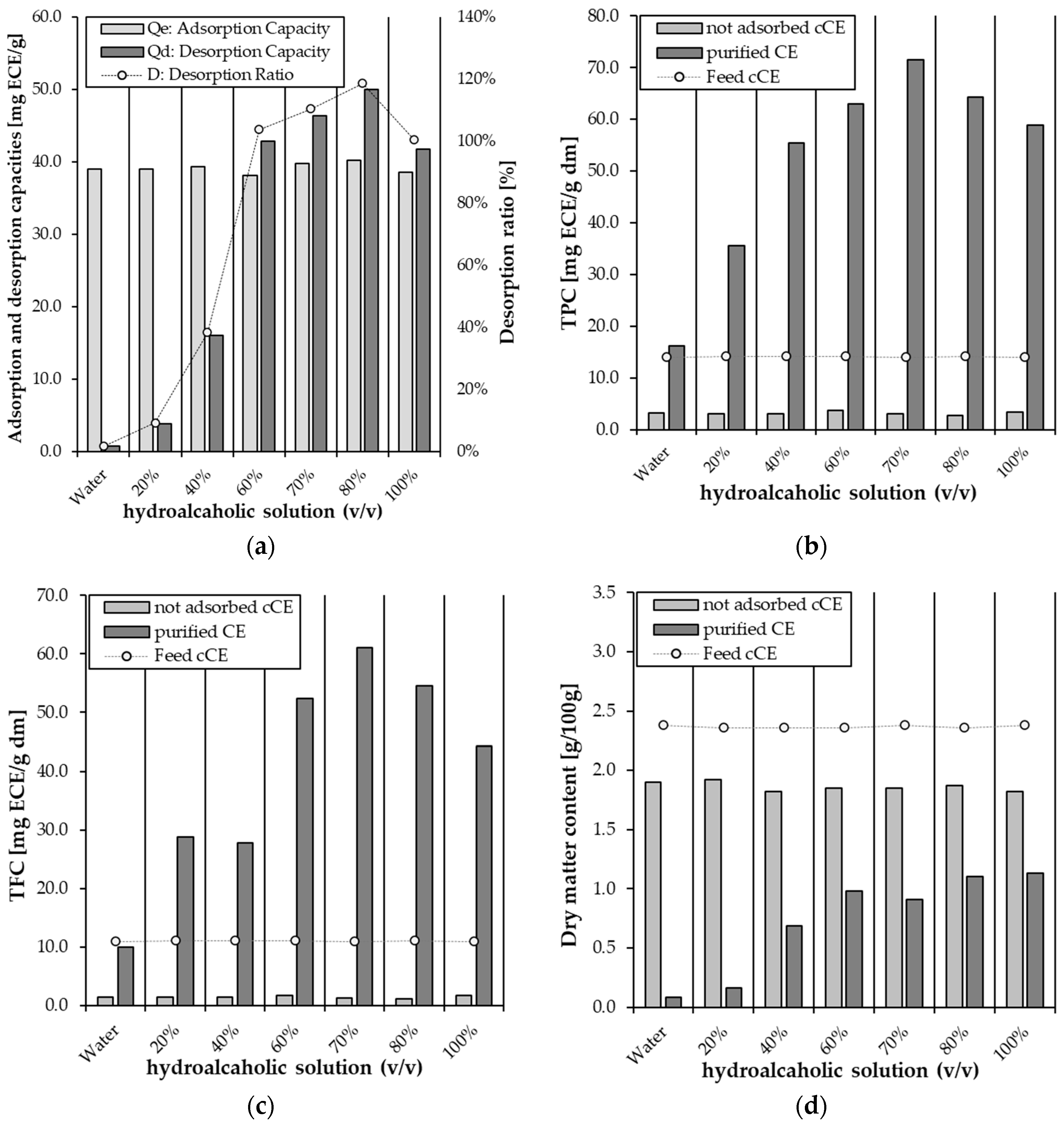
2.1. Screening of Macroporous Adsorption Resins for the Purification of Cocoa Flavonoids
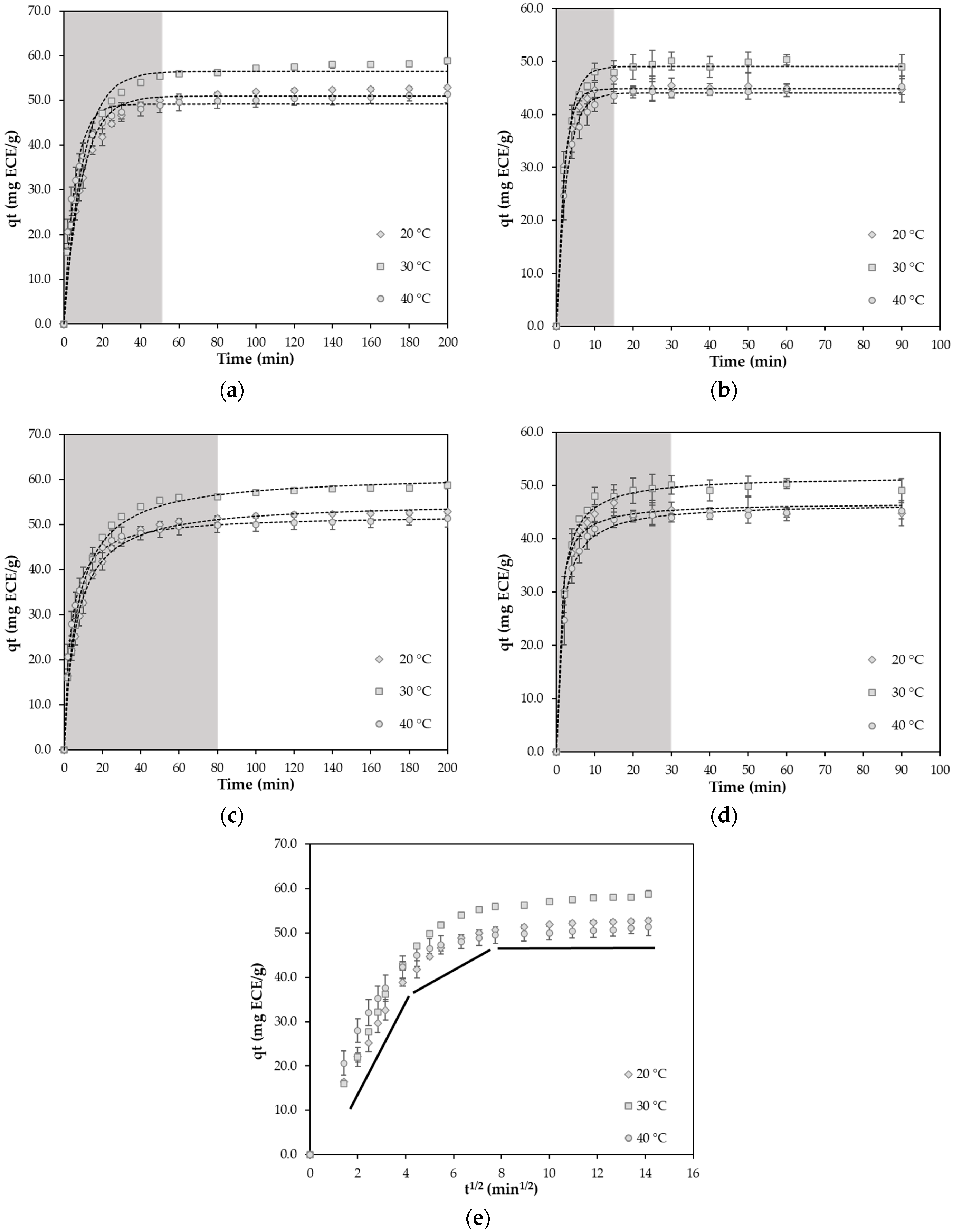
2.2. Static Adsorption and Desorption Kinetics on the XAD-7HP Resin
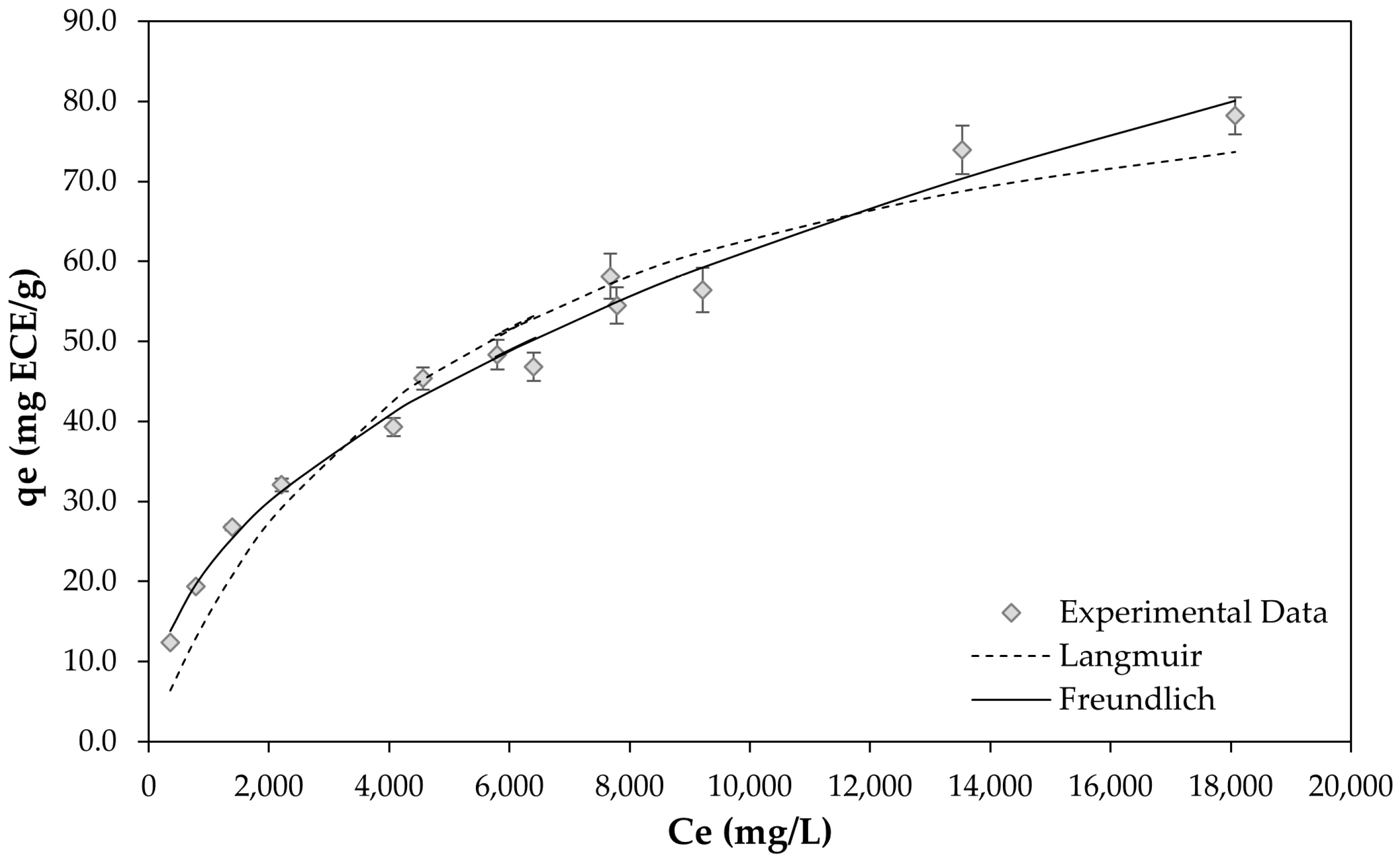
2.3. Static Adsorption Isotherms on the XAD-7HP Resin
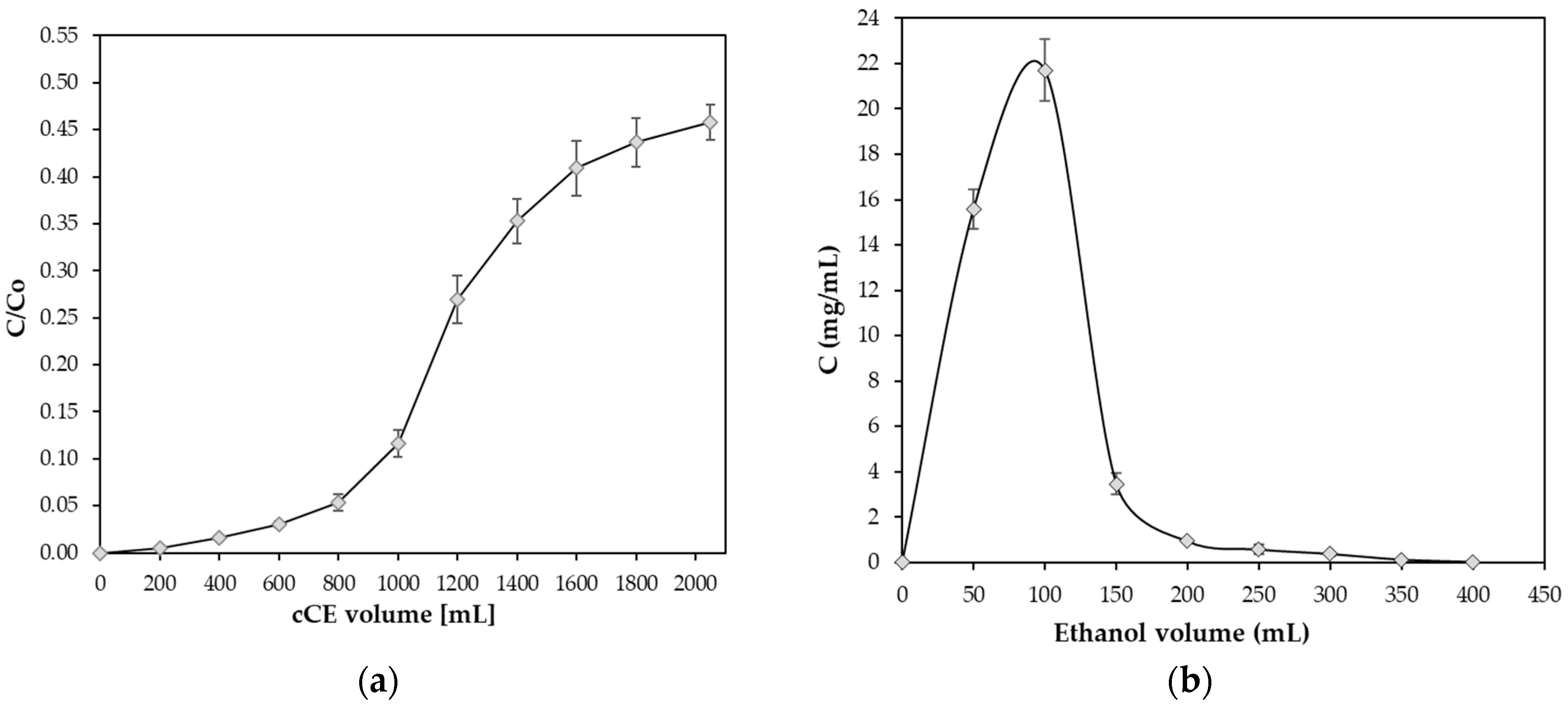
2.4. Dynamic Adsorption and Desorption of cCE Flavanols on the XAD-7HP Resin
3. Materials and Methods
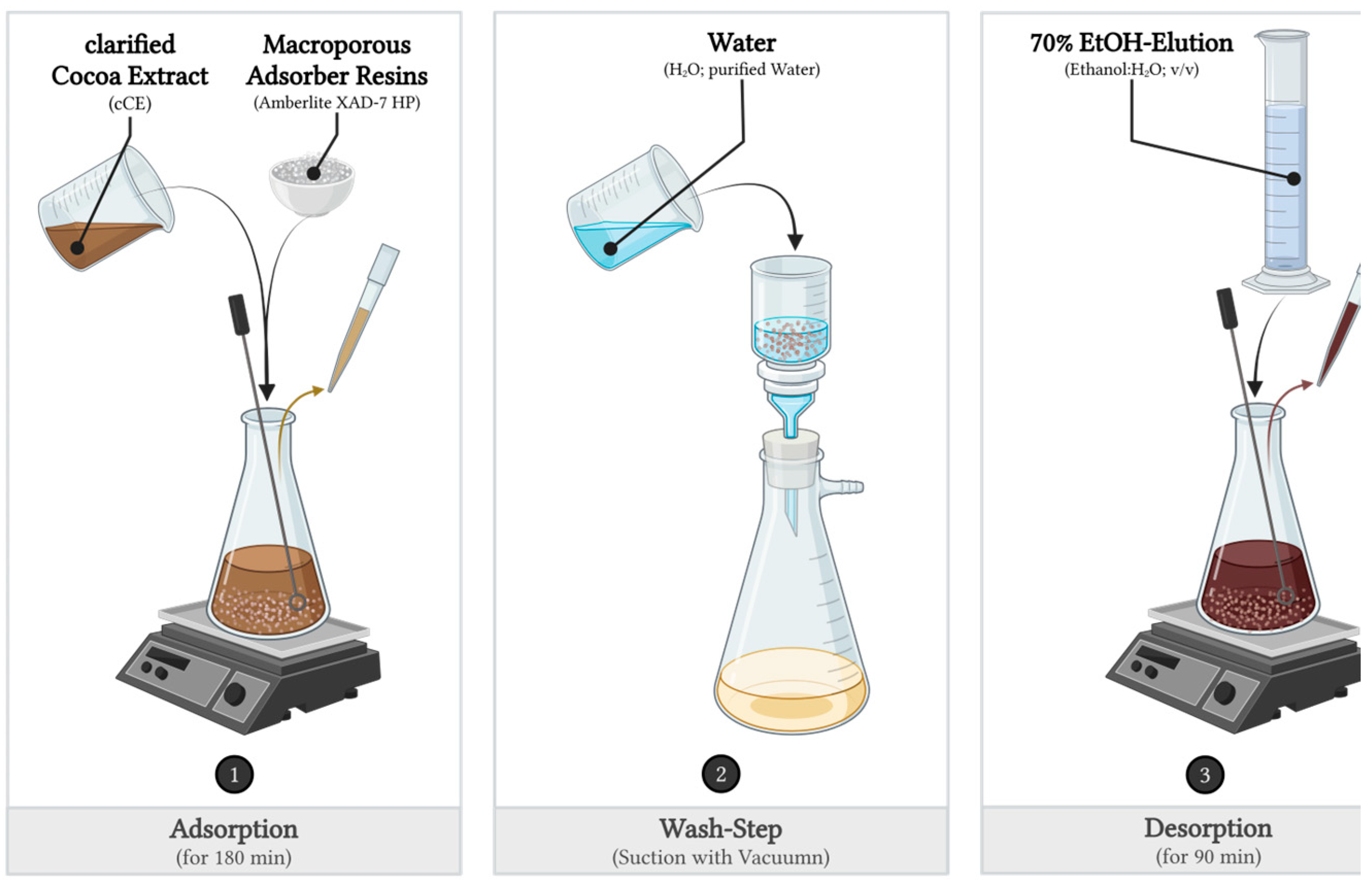
3.1. Adsorption and Desorption Experimental Set-Up
3.1.1. Sample Preparation
3.1.2. Adsorbents and Pretreatment
3.1.3. Dynamic Adsorption and Desorption Experiments with a Glass Column
3.1.4. Static Adsorption and Desorption Kinetics Experiments
3.1.5. Static Adsorption Isotherms Experiments
3.1.6. Dynamic Adsorption and Desorption Experiments
3.2. Chemicals and Reagents
3.3. Physicochemical Analysis of the Extract, Supernatant, and Filtrate
3.3.1. Dry Matter Content Based on a Halogen Dryer Method
3.3.2. Protein Content Using the Bradford Method
3.3.3. Total Phenolic Content (TPC) Using the Folin–Ciocalteu Assay
3.3.4. Total Flavonoid Content (TFC) Using the Aluminum Chloride Assay
3.3.5. Content of Individual Flavanols Using UPLC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassano, A.; De Luca, G.; Conidi, C.; Drioli, E. Effect of Polyphenols-Membrane Interactions on the Performance of Membrane-Based Processes. A Review. Coord. Chem. Rev. 2017, 351, 45–75. [Google Scholar] [CrossRef]
- Andújar, I.; Recio, M.C.; Giner, R.M.; Ríos, J.L. Cocoa Polyphenols and Their Potential Benefits for Human Health. Oxid. Med. Cell Longev. 2012, 2012, 906252. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Pan, Z.J.; Liao, W.; Li, J.; Gruget, P.; Kitts, D.D.; Lu, X. Determination of Antioxidant Capacity and Phenolic Content of Chocolate by Attenuated Total Reflectance-Fourier Transformed-Infrared Spectroscopy. Food Chem. 2016, 202, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Bu, F.; Cheng, X.; Zhao, C.; Yin, Y.; Liu, P. Purification of Phenolics from Plantago Depressa by Macroporous Resin: Adsorption/Desorption Characteristics, Chromatographic Process Development and UPLC-TQ-MS/MS Quantitative Analysis. LWT 2024, 203, 116405. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A Critical Analysis of Extraction Techniques Used for Botanicals: Trends, Priorities, Industrial Uses and Optimization Strategies. TrAc Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, M.; Sun-Waterhouse, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and Desorption Behaviour of the Flavonoids from Glycyrrhiza glabra L. Leaf on Macroporous Adsorption Resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef]
- Scordino, M.; Di Mauro, A.; Passerini, A.; Maccarone, E. Adsorption of Flavonoids on Resins: Cyanidin 3-Glucoside. J. Agric. Food Chem. 2004, 52, 1965–1972. [Google Scholar] [CrossRef]
- Du, H.; Wang, H.; Yu, J.; Liang, C.; Ye, W.; Li, P. Enrichment and Purification of Total Flavonoid C-Glycosides from Abrus mollis Extracts with Macroporous Resins. Ind. Eng. Chem. Res. 2012, 51, 7349–7354. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Zhang, R. Purification and Antioxidant Properties of Triterpenic Acids from Blackened Jujube (Ziziphus jujuba Mill.) by Macroporous Resins. Food Sci. Nutr. 2021, 9, 5070–5082. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; González-López, N.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery of Antioxidants from Industrial Waste Liquors Using Membranes and Polymeric Resins. J. Food Eng. 2010, 96, 127–133. [Google Scholar] [CrossRef]
- Chang, X.-L.; Wang, D.; Chen, B.-Y.; Feng, Y.-M.; Wen, S.-H.; Zhan, P.-Y. Adsorption and Desorption Properties of Macroporous Resins for Anthocyanins from the Calyx Extract of Roselle (Hibiscus sabdariffa L.). J. Agric. Food Chem. 2012, 60, 2368–2376. [Google Scholar] [CrossRef]
- Kammerer, J.; Boschet, J.; Kammerer, D.R.; Carle, R. Enrichment and Fractionation of Major Apple Flavonoids, Phenolic Acids and Dihydrochalcones Using Anion Exchange Resins. LWT—Food Sci. Technol. 2011, 44, 1079–1087. [Google Scholar] [CrossRef]
- Kammerer, D.; Gajdos Kljusuric, J.; Carle, R.; Schieber, A. Recovery of Anthocyanins from Grape Pomace Extracts (Vitis vinifera L. Cv. Cabernet Mitos) Using a Polymeric Adsorber Resin. Eur. Food Res. Technol. 2005, 220, 431–437. [Google Scholar] [CrossRef]
- Cardona, J.A.; Lee, J.-H.; Talcott, S.T. Color and Polyphenolic Stability in Extracts Produced from Muscadine Grape (Vitis rotundifolia) Pomace. J. Agric. Food Chem. 2009, 57, 8421–8425. [Google Scholar] [CrossRef]
- Soto, M.L.; Conde, E.; González-López, N.; Conde, M.J.; Moure, A.; Sineiro, J.; Falqué, E.; Domínguez, H.; Núñez, M.J.; Parajó, J.C. Recovery and Concentration of Antioxidants from Winery Wastes. Molecules 2012, 17, 3008–3024. [Google Scholar] [CrossRef] [PubMed]
- Che Zain, M.S.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption and Desorption Properties of Total Flavonoids from Oil Palm (Elaeis guineensis Jacq.) Mature Leaf on Macroporous Adsorption Resins. Molecules 2020, 25, 778. [Google Scholar] [CrossRef]
- Brune, B.J.; Koehler, J.A.; Smith, P.J.; Payne, G.F. Correlation between Adsorption and Small Molecule Hydrogen Bonding. Langmuir 1999, 15, 3987–3992. [Google Scholar] [CrossRef]
- Mardis, K.L.; Glemza, A.J.; Brune, B.J.; Payne, G.F.; Gilson, M.K. Differential Adsorption of Phenol Derivatives onto a Polymeric Sorbent: A Combined Molecular Modeling and Experimental Study. J. Phys. Chem. B 1999, 103, 9879–9887. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Wang, L.-L.; Chen, L.-C.; Liu, T.; Sha, R.-Y.; Mao, J.-W. Enrichment and Separation of Steroidal Saponins from the Fibrous Roots of Ophiopogon japonicus Using Macroporous Adsorption Resins. RSC Adv. 2019, 9, 6689–6698. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D. Adsorption Kinetics, Isotherm and Thermodynamics Studies of Flavones from Vaccinium Bracteatum Thunb Leaves on NKA-2 Resin. Chem. Eng. J. 2014, 254, 579–585. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, M.; Lin, L. Adsorption and Desorption Characteristics of Adlay Bran Free Phenolics on Macroporous Resins. Food Chem. 2016, 194, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Tao, G.; Tang, J.; Lou, Z.; Wang, H.; Gu, X.; Hu, L.; Yin, M. Preparative Separation and Purification of Rosavin in Rhodiola Rosea by Macroporous Adsorption Resins. Sep. Purif. Technol. 2009, 69, 22–28. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, M.; Yang, H.; Jo, J.; Han, D.; Jeon, Y.-J.; Cho, S. Enrichment and Purification of Marine Polyphenol Phlorotannins Using Macroporous Adsorption Resins. Food Chem. 2014, 162, 135–142. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, H.; Dong, Y.; Yang, B.; Zhao, M. Macroporous Resin Purification Behavior of Phenolics and Rosmarinic Acid from Rabdosia serra (MAXIM.) HARA Leaf. Food Chem. 2012, 130, 417–424. [Google Scholar] [CrossRef]
- Hui, Z.; Jun, W.; Jing, J.; Ji, L.; Xiuquan, L.; Dingqiang, L. Enrichment and Purification of Total Chlorogenic Acids from Tobacco Waste Extract with Macroporous Resins. Sep. Sci. Technol. 2010, 45, 794–800. [Google Scholar] [CrossRef]
- Li, C.; Liang, H.; Yuan, Q.; Hou, X. Optimization of Sulforaphane Separation from Broccoli Seeds by Macroporous Resins. Sep. Sci. Technol. 2008, 43, 609–623. [Google Scholar] [CrossRef]
- Buran, T.J.; Sandhu, A.K.; Li, Z.; Rock, C.R.; Yang, W.W.; Gu, L. Adsorption/Desorption Characteristics and Separation of Anthocyanins and Polyphenols from Blueberries Using Macroporous Adsorbent Resins. J. Food Eng. 2014, 128, 167–173. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Carle, R.; Stanley, R.A.; Saleh, Z.S. Pilot-Scale Resin Adsorption as a Means to Recover and Fractionate Apple Polyphenols. J. Agric. Food Chem. 2010, 58, 6787–6796. [Google Scholar] [CrossRef]
- Fu, B.; Liu, J.; Li, H.; Li, L.; Lee, F.S.C.; Wang, X. The Application of Macroporous Resins in the Separation of Licorice Flavonoids and Glycyrrhizic Acid. J. Chromatogr. A 2005, 1089, 18–24. [Google Scholar] [CrossRef]
- Zhang, X.; Su, J.; Chu, X.; Wang, X. A Green Method of Extracting and Recovering Flavonoids from Acanthopanax Senticosus Using Deep Eutectic Solvents. Molecules 2022, 27, 923. [Google Scholar] [CrossRef]
- Monsanto, M.; Mestrom, R.; Zondervan, E.; Bongers, P.; Meuldijk, J. Solvent Swing Adsorption for the Recovery of Polyphenols from Black Tea. Ind. Eng. Chem. Res. 2015, 54, 434–442. [Google Scholar] [CrossRef]
- da R. Rodrigues, N.; Mohammad, S.S.; Gao, C.; Ivone M. J. Barbosa, M.; Gu, L.; Junior, J.L.B. Separation Process Characteristics of Phenolic Compounds from Organic Purple-fleshed Sweet Potatoes (Ipomoea batatas L.) Leaves Using Macroporous Resins. J. Food Process Eng. 2023, 46, e14365. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, H.; Zhang, X.; Chen, C.; Zhao, S.; Ge, F.; Liu, D. Recovery of Flavonoids from Walnuts De-Pellicle Wastewater with Macroporous Resins and Evaluation of Antioxidant Activities In Vitro. J. Food Process Eng. 2017, 40, e12275. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X.; et al. Adsorption Properties of Macroporous Adsorbent Resins for Separation of Anthocyanins from Mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Scordino, M.; Di Mauro, A.; Passerini, A.; Maccarone, E. Adsorption of Flavonoids on Resins: Hesperidin. J. Agric. Food Chem. 2003, 51, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Das, A.B.; Goud, V.V.; Das, C. Adsorption/Desorption, Diffusion, and Thermodynamic Properties of Anthocyanin from Purple Rice Bran Extract on Various Adsorbents. J. Food Process Eng. 2018, 41, e12834. [Google Scholar] [CrossRef]
- Zhou, X.; Fan, J.; Li, N.; Qian, W.; Lin, X.; Wu, J.; Xiong, J.; Bai, J.; Ying, H. Adsorption Thermodynamics and Kinetics of Uridine 5′-Monophosphate on a Gel-Type Anion Exchange Resin. Ind. Eng. Chem. Res. 2011, 50, 9270–9279. [Google Scholar] [CrossRef]
- Sun, P.-C.; Liu, Y.; Yi, Y.-T.; Li, H.-J.; Fan, P.; Xia, C.-H. Preliminary Enrichment and Separation of Chlorogenic Acid from Helianthus tuberosus L. Leaves Extract by Macroporous Resins. Food Chem. 2015, 168, 55–62. [Google Scholar] [CrossRef]
- Gan, J.; Zhang, X.; Ma, C.; Sun, L.; Feng, Y.; He, Z.; Zhang, H. Purification of Polyphenols from Phyllanthus emblica L. Pomace Using Macroporous Resins: Antioxidant Activity and Potential Anti-Alzheimer’s Effects. J. Food Sci. 2022, 87, 1244–1256. [Google Scholar] [CrossRef]
- Pan, B.; Pan, B.; Zhang, W.; Zhang, Q.; Zhang, Q.; Zheng, S. Adsorptive Removal of Phenol from Aqueous Phase by Using a Porous Acrylic Ester Polymer. J. Hazard. Mater. 2008, 157, 293–299. [Google Scholar] [CrossRef]
- Lou, S.; Chen, Z.; Liu, Y.; Ye, H.; Di, D. Synthesis of Functional Adsorption Resin and Its Adsorption Properties in Purification of Flavonoids from Hippophae rhamnoides L. Leaves. Ind. Eng. Chem. Res. 2012, 51, 2682–2696. [Google Scholar] [CrossRef]
- Wang, R.; Peng, X.; Wang, L.; Tan, B.; Liu, J.; Feng, Y.; Yang, S. Preparative Purification of Peoniflorin and Albiflorin from Peony Rhizome Using Macroporous Resin and Medium-pressure Liquid Chromatography. J. Sep. Sci. 2012, 35, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Hühn, T. Cocoa Extraction Methods and Techniques. European Patent Application EP3747275A1, 9 December 2020. [Google Scholar]
- Hühn, T.; Laux, R. Cocoa Bean Processing Methods and Techniques. European Patent Application EP2378892A2, 26 April 2017. [Google Scholar]
- Chetschik, I.; Pedan, V.; Chatelain, K.; Kneubühl, M.; Hühn, T. Characterization of the Flavor Properties of Dark Chocolates Produced by a Novel Technological Approach and Comparison with Traditionally Produced Dark Chocolates. J. Agric. Food Chem. 2019, 67, 3991–4001. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.; Yan, X.; Zhou, Z.; Zhao, J.; Xia, J.; Gao, X.; Ye, M.; Deng, L.; Zeng, Z.; et al. Enrichment of Polyphenols from Cinnamomum Camphora Seed Kernel by Macroporous Adsorption Resins and Evaluation of Its Antioxidant and Enzyme Inhibitory Activities. Ind. Crops Prod. 2024, 222, 119486. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for Pollutants Removal: A Review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Rivera-Tovar, P.R.; Pérez-Manríquez, J.; Mariotti-Celis, M.S.; Escalona, N.; Pérez-Correa, J.R. Adsorption of Low Molecular Weight Food Relevant Polyphenols on Cross-Linked Agarose Gel. J. Mol. Liq. 2022, 347, 117972. [Google Scholar] [CrossRef]
- Raposo, F.; De La Rubia, M.A.; Borja, R. Methylene Blue Number as Useful Indicator to Evaluate the Adsorptive Capacity of Granular Activated Carbon in Batch Mode: Influence of Adsorbate/Adsorbent Mass Ratio and Particle Size. J. Hazard. Mater. 2009, 165, 291–299. [Google Scholar] [CrossRef]
- Chen, T.; Da, T.; Ma, Y. Reasonable Calculation of the Thermodynamic Parameters from Adsorption Equilibrium Constant. J. Mol. Liq. 2021, 322, 114980. [Google Scholar] [CrossRef]
- Beeler, N.; Hühn, T.; Rohn, S.; Colombi, R. Development and Optimization of a Cocoa Extraction Treatment by Means of the Response Surface Methodology (RSM) and Artificial Neural Networks (ANN). Ind. Crops Prod. 2024, 222, 119610. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Pedan, V.; Fischer, N.; Rohn, S. Extraction of Cocoa Proanthocyanidins and Their Fractionation by Sequential Centrifugal Partition Chromatography and Gel Permeation Chromatography. Anal. Bioanal. Chem. 2016, 408, 5905–5914. [Google Scholar] [CrossRef] [PubMed]
- Zzaman, W.; Bhat, R.; Yang, T.A. Effect of Superheated Steam Roasting on the Phenolic Antioxidant Properties of Cocoa Beans. J. Food Process Preserv. 2014, 38, 1932–1938. [Google Scholar] [CrossRef]
| Process | Temp. (°C) | qe (exp) (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe (cal) (mg/g) | R2 | k2 (g/mg × min) | qe (cal) (mg/g) | R2 | |||
| Absorption | 20 | 52.84 | 0.108 | 50.97 | 0.959 | 0.003 | 55.13 | 0.992 |
| 30 | 58.81 | 0.104 | 56.52 | 0.977 | 0.002 | 61.32 | 0.994 | |
| 40 | 51.37 | 0.176 | 49.09 | 0.934 | 0.006 | 52.09 | 0.995 | |
| Desorption | 20 | 44.81 | 0.504 | 44.85 | 0.964 | 0.023 | 46.77 | 0.940 |
| 30 | 49.04 | 0.411 | 49.03 | 0.661 | 0.015 | 51.71 | 0.971 | |
| 40 | 45.25 | 0.370 | 44.07 | 0.974 | 0.014 | 46.72 | 0.977 | |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/g) | RL | R2 | 1/n | Kf | R2 |
| 93.61 | 2.05 × 10−4 | 0.524 | 0.944 | 0.448 | 0.996 | 0.987 |
| Sample | Amount [g] | Cocoa Flavanols (Sum of DP1–DP7) | Cocoa Flavanols (Sum of DP1–DP7) | Recovery Yield Flavanols | Yield Sample as Powder |
|---|---|---|---|---|---|
| [mg/mL] | [mg/mL dm] | [%] | [%] | ||
| cCE | 1900 | 1.71 | 90.72 | 100 | 100 |
| n.a. cCE | 1900 | 0.39 | 23.71 | 23 | 86 |
| Wash Water | 400 | 0.74 | 135.80 | 9 | 6 |
| pCE | 400 | 5.47 | 456.05 | 67 | 13 |
| Resins | XAD-2 | XAD-4 | XAD-7 | XAD-7HP | XAD-16 | SP207 | HP2-MG |
|---|---|---|---|---|---|---|---|
| Structure | PS-DVB | PS-DVB | Aliphatic ester | Aliphatic ester | PS-DVB | B-PS-DVB | Polymethacrylate |
| Polarity | Non-polar | Non-polar | Strongly polar | Strongly polar | Non-polar | Non-polar | Moderately polar |
| Surface area [m2/g] | 330 | 725 | 450 | 500 | 900 | 630 | 470 |
| Porosity [mL/g] | 0.65 | 0.98 | 1.14 | 1.08 | 1.82 | 1.1 | 1.2 |
| Pore radius [Å] | 90 | 50 | 90 | 550 | 100 | 120 | 170 |
| Particle size [mm] | 0.25–0.84 | 0.25–0.84 | 0.25–0.84 | 0.43–0.69 | 0.25–0.84 | 0.25–0.60 | 0.25–0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beeler, N.; Hühn, T.; Rohn, S.; Colombi, R. Purification of Flavonoids from an Aqueous Cocoa (Theobroma cocoa L.) Extract Using Macroporous Adsorption Resins. Molecules 2025, 30, 2336. https://doi.org/10.3390/molecules30112336
Beeler N, Hühn T, Rohn S, Colombi R. Purification of Flavonoids from an Aqueous Cocoa (Theobroma cocoa L.) Extract Using Macroporous Adsorption Resins. Molecules. 2025; 30(11):2336. https://doi.org/10.3390/molecules30112336
Chicago/Turabian StyleBeeler, Nicole, Tilo Hühn, Sascha Rohn, and Renato Colombi. 2025. "Purification of Flavonoids from an Aqueous Cocoa (Theobroma cocoa L.) Extract Using Macroporous Adsorption Resins" Molecules 30, no. 11: 2336. https://doi.org/10.3390/molecules30112336
APA StyleBeeler, N., Hühn, T., Rohn, S., & Colombi, R. (2025). Purification of Flavonoids from an Aqueous Cocoa (Theobroma cocoa L.) Extract Using Macroporous Adsorption Resins. Molecules, 30(11), 2336. https://doi.org/10.3390/molecules30112336







