Exploration of a Novel Catalytic Approach for Synthesizing Glycolide and ε-Caprolactone Copolymers and Their Application as Carriers for Paclitaxel
Abstract
1. Introduction
2. Results and Discussion
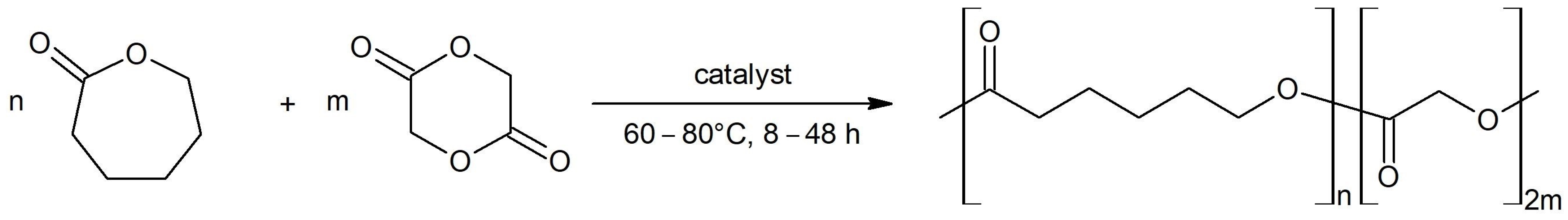
2.1. Synthesis and Characterization of PGCL Copolymers
2.2. Differential Scanning Calorimetry
2.3. Toxicity Studies
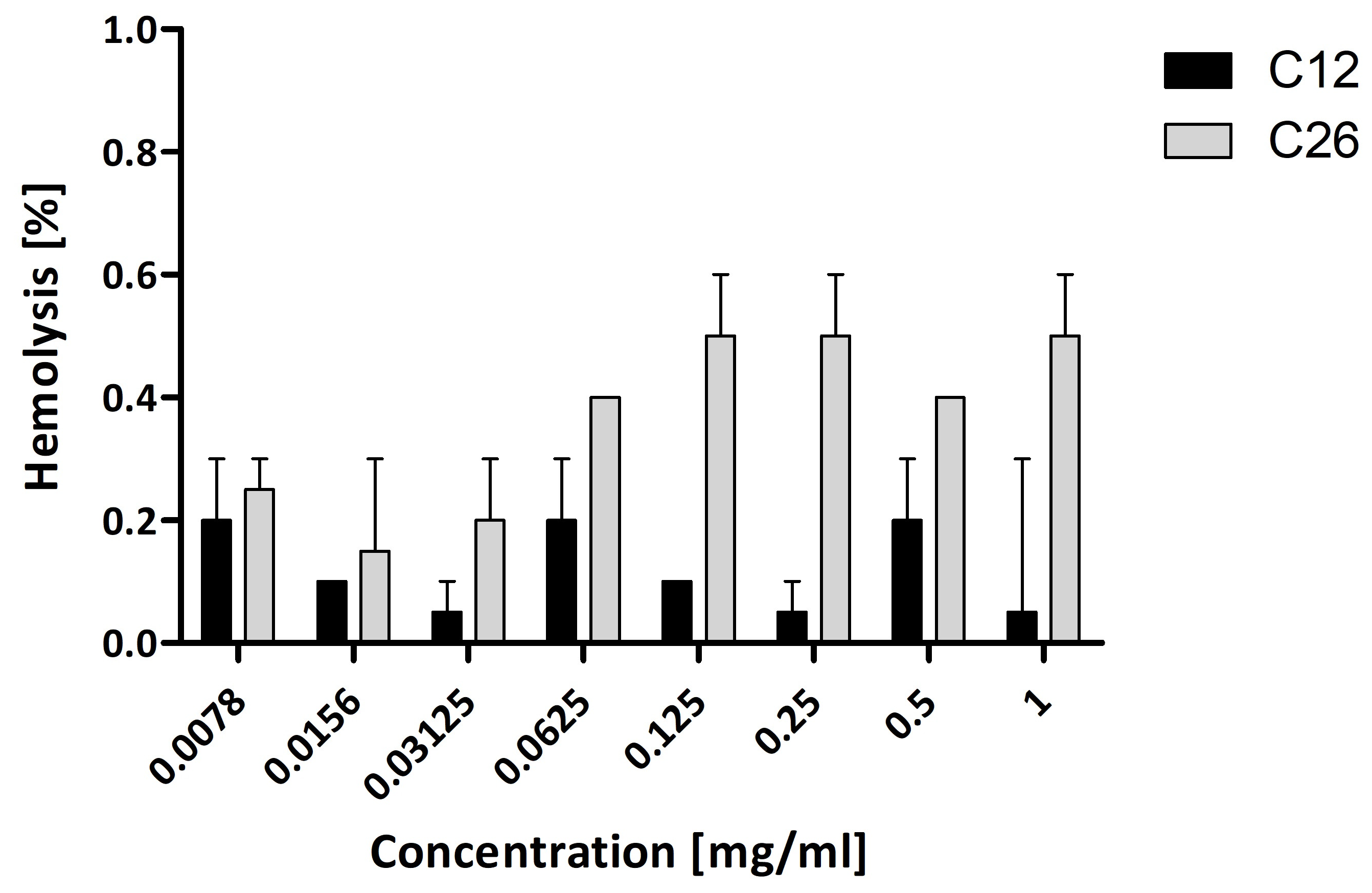
2.4. Hemolysis Assay
2.5. PACL Release Kinetics Studies
3. Materials and Methods
3.1. Chemicals
3.2. Polymerization Procedure
3.3. PACL Delivery System Preparation
3.4. PACL Release Kinetics Studies
3.5. HPLC Measurements
3.6. Toxicity Studies
3.7. Hemolysis Assay
3.8. GPC Measurements
3.9. NMR Measurements
3.10. DSC Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jastrzembski, B.G.; Shah, A.S. Microparticle Drug Delivery in Ophthalmology. Int. Ophthalmol. Clin. 2017, 57, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, F.; Mohanty, A.K.; Misra, M. Sustainable Biodegradable Coatings for Food Packaging: Challenges and Opportunities. Green Chem. 2024, 26, 4934–4974. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, R.; Zhu, Y.; Lin, Q. Applications of Biodegradable Materials in Food Packaging: A Review. Alex. Eng. J. 2024, 91, 70–83. [Google Scholar] [CrossRef]
- Lekic, N.; Dodds, S.D. Suture Materials, Needles, and Methods of Skin Closure: What Every Hand Surgeon Should Know. J. Hand Surg. 2022, 47, 160–171.e1. [Google Scholar] [CrossRef] [PubMed]
- Bialik, M.; Kuras, M.; Sobczak, M.; Oledzka, E. Biodegradable Synthetic Polyesters in the Technology of Controlled Dosage Forms of Antihypertensive Drugs—The Overview. Expert Opin. Drug Deliv. 2019, 16, 953–967. [Google Scholar] [CrossRef]
- Zhang, S.; Li, P.; Li, Z.-H. Toxicity of Organotin Compounds and the Ecological Risk of Organic Tin with Co-Existing Contaminants in Aquatic Organisms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 246, 109054. [Google Scholar] [CrossRef]
- Bhadran, A.; Shah, T.; Babanyinah, G.K.; Polara, H.; Taslimy, S.; Biewer, M.C.; Stefan, M.C. Recent Advances in Polycaprolactones for Anticancer Drug Delivery. Pharmaceutics 2023, 15, 1977. [Google Scholar] [CrossRef]
- Wu, D.; Lv, Y.; Guo, R.; Li, J.; Habadati, A.; Lu, B.; Wang, H.; Wei, Z. Kinetics of Sn(Oct)2-Catalyzed Ring Opening Polymerization of ε-Caprolactone. Macromol. Res. 2017, 25, 1070–1075. [Google Scholar] [CrossRef]
- Dobrzynski, P.; Li, S.; Kasperczyk, J.; Bero, M.; Gasc, F.; Vert, M. Structure−Property Relationships of Copolymers Obtained by Ring-Opening Polymerization of Glycolide and ε-Caprolactone. Part 1. Synthesis and Characterization. Biomacromolecules 2005, 6, 483–488. [Google Scholar] [CrossRef]
- Maciejowska, J.; Kasperczyk, J.; Dobrzyñski, P.; Bero, M. The Influence of Chain Microstructure on Hydrolytic Degradation of Glycolide/Lactide Copolymers Used in Drug Delivery Systems. J. Control. Release 2006, 116, e6–e8. [Google Scholar] [CrossRef]
- Chu, C.C. Materials for Absorbable and Nonabsorbable Surgical Sutures. In Biotextiles as Medical Implants; Elsevier: Amsterdam, The Netherlands, 2013; pp. 275–334. ISBN 978-1-84569-439-5. [Google Scholar]
- Jaworska, J.; Orchel, A.; Kaps, A.; Jaworska-Kik, M.; Hercog, A.; Stojko, M.; Włodarczyk, J.; Musiał-Kulik, M.; Pastusiak, M.; Bochenek, M.; et al. Bioresorbable Nonwoven Patches as Taxane Delivery Systems for Prostate Cancer Treatment. Pharmaceutics 2022, 14, 2835. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H. Enzymatic Ring-opening Polymerization (ROP) of Polylactones: Roles of Non-aqueous Solvents. J. Chem. Technol. Biotechnol. 2018, 93, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, J.; Sarkar, A.; Panda, T.K. Alkali and Alkaline Earth Metal Complexes as Versatile Catalysts for Ring-Opening Polymerization of Cyclic Esters. Chem. Rec. 2021, 21, 1898–1911. [Google Scholar] [CrossRef]
- Buchard, A.; Davidson, M.G.; Gobius Du Sart, G.; Jones, M.D.; Kociok-Köhn, G.; McCormick, S.N.; McKeown, P. Unexpected Periodicity in Cationic Group 5 Initiators for the Ring-Opening Polymerization of Lactones. Inorg. Chem. 2024, 63, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Hardouin Duparc, V.; Shakaroun, R.M.; Slawinski, M.; Carpentier, J.-F.; Guillaume, S.M. Ring-Opening (Co)Polymerization of Six-Membered Substituted δ-Valerolactones with Alkali Metal Alkoxides. Eur. Polym. J. 2020, 134, 109858. [Google Scholar] [CrossRef]
- Bandelli, D.; Weber, C.; Schubert, U.S. Strontium Isopropoxide: A Highly Active Catalyst for the Ring-Opening Polymerization of Lactide and Various Lactones. Macromol. Rapid Commun. 2019, 40, 1900306. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Polymerization of l,l-Lactide Initiated by Aluminum Isopropoxide Trimer or Tetramer. Macromolecules 1998, 31, 2114–2122. [Google Scholar] [CrossRef]
- Jelonek, K.; Musiał-Kulik, M.; Pastusiak, M.; Foryś, A.; Zięba, A.; Kasperczyk, J. Exploring Micelles and Nanospheres as Delivery Systems for Phenothiazine Derivatives in Cancer Therapy. Pharmaceutics 2024, 16, 1597. [Google Scholar] [CrossRef]
- Kularatne, R.N.; Taslimy, S.; Bhadran, A.; Cue, J.M.O.; Bulumulla, C.; Calubaquib, E.L.; Gunawardhana, R.; Biewer, M.C.; Stefan, M.C. A Binary Neodymium Catalyst for the Polymerization of Lactones. Polym. Chem. 2023, 14, 3962–3970. [Google Scholar] [CrossRef]
- Ghosh, S.; Glöckler, E.; Wölper, C.; Tjaberings, A.; Gröschel, A.H.; Schulz, S. Active Ga-Catalysts for the Ring Opening Homo- and Copolymerization of Cyclic Esters, and Copolymerization of Epoxide and Anhydrides. Dalton Trans. 2020, 49, 13475–13486. [Google Scholar] [CrossRef]
- Horeglad, P.; Rola-Noworyta, A.; Tuszyński, D.; Fabianowska, I.; Marek, N.A.; Gładysz, P.; Wielgus, I.; Dąbrowska, A.M. Enhancing the Stereoselectivity of Me2 GaOR(NHC) Species in the Ring-Opening Polymerization of Rac -Lactide, with the Help of the Chelation Effect. RSC Adv. 2024, 14, 28638–28647. [Google Scholar] [CrossRef]
- Wang, W. Recent Advances in the Titanium-Based Catalysts for Ring-Opening Polymerization. ACS Omega 2024, 9, 29983–29993. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, O.S.; Rawat, V.; Zhang, H.; Chibh, S.; Rencus-Lazar, S.; Diesendruck, C.E.; Gazit, E. Ring-opening Polymerization of Lactide Catalyzed Using Metal-coordinated Enzyme-like Amino Acid Assemblies. J. Pept. Sci. 2024, 30, e3626. [Google Scholar] [CrossRef] [PubMed]
- Rittinghaus, R.D.; Schäfer, P.M.; Albrecht, P.; Conrads, C.; Hoffmann, A.; Ksiazkiewicz, A.N.; Bienemann, O.; Pich, A.; Herres-Pawlis, S. New Kids in Lactide Polymerization: Highly Active and Robust Iron Guanidine Complexes as Superior Catalysts. ChemSusChem 2019, 12, 2161–2165. [Google Scholar] [CrossRef]
- Naranjo, J.; Castro-Osma, J.A.; De La Cruz-Martínez, F.; Lara-Sánchez, A. Recent Progress in Calcium-Catalyzed Polyester Synthesis. Dalton Trans. 2025, 54, 5640–5649. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Karp, J. Biology and Engineering of Stem Cell Niches; Academic Press: London, UK, 2017; ISBN 978-0-12-802734-9. [Google Scholar]
- Leuprostin—Summary of Product Characteristics. Available online: https://rejestry.ezdrowie.gov.pl/rpl/search/public (accessed on 1 March 2025).
- Reseligo—Summary of Product Characteristics. Available online: https://rejestry.ezdrowie.gov.pl/rpl/search/public (accessed on 1 March 2025).
- Rispolept Consta—Summary of Product Characteristics. Available online: https://rejestry.ezdrowie.gov.pl/rpl/search/public (accessed on 1 March 2025).
- Jaworska, J.; Smolarczyk, R.; Musiał-Kulik, M.; Cichoń, T.; Karpeta-Jarząbek, P.; Włodarczyk, J.; Stojko, M.; Janeczek, H.; Kordyka, A.; Kaczmarczyk, B.; et al. Electrospun Paclitaxel Delivery System Based on PGCL/PLGA in Local Therapy Combined with Brachytherapy. Int. J. Pharm. 2021, 602, 120596. [Google Scholar] [CrossRef]
- Jaworska, J.; Włodarczyk, J.; Karpeta-Jarząbek, P.; Janeczek, H.; Stojko, M.; Kasperczyk, J. Electrospun, Drug-Enriched Bioresorbable Nonwovens Based on Poly(Glycolide-ɛ-Caprolactone) and Poly(d,l-Lactide-Glycolide) for Urological Applications. Polym. Degrad. Stab. 2019, 167, 94–101. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Jo Lewis, P.; Chu, C.-C. Fabrication and Characterization of a Smart Drug Delivery System: Microsphere in Hydrogel. Biomaterials 2005, 26, 3299–3309. [Google Scholar] [CrossRef] [PubMed]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Ma, S.; Feng, X.; Liu, F.; Wang, B.; Zhang, H.; Niu, X. The Pro-inflammatory Response of Macrophages Regulated by Acid Degradation Products of Poly(Lactide-co-glycolide) Nanoparticles. Eng. Life Sci. 2021, 21, 709–720. [Google Scholar] [CrossRef]
- Abraxane—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/abraxane (accessed on 1 March 2025).
- Paclitaxel Kabi—Summary of Product Characteristic. Available online: https://rejestry.ezdrowie.gov.pl/rpl/search/public (accessed on 1 March 2025).
- Żółtowska, K.; Sobczak, M.; Olędzka, E. Novel Zinc-Catalytic Systems for Ring-Opening Polymerization of ε-Caprolactone. Molecules 2015, 20, 2816–2827. [Google Scholar] [CrossRef]
- Wyrębiak, R.; Oledzka, E.; Figat, R.; Sobczak, M. Application of Diethylzinc/Propyl Gallate Catalytic System for Ring-Opening Copolymerization of Rac-Lactide and ε-Caprolactone. Molecules 2019, 24, 4168. [Google Scholar] [CrossRef] [PubMed]
- Rittinghaus, R.D.; Zenner, J.; Pich, A.; Kol, M.; Herres-Pawlis, S. Master of Chaos and Order: Opposite Microstructures of PCL-Co-PGA-Co-PLA Accessible by a Single Catalyst. Angew. Chem. Int. Ed. 2022, 61, e202112853. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, M.; Shi, X.; Ma, J.; Wang, S.; Wu, S.; Yan, W.; Wu, F.; Zhang, P. Synergistically Promoting Bone Regeneration by Icariin-Incorporated Porous Microcarriers and Decellularized Extracellular Matrix Derived from Bone Marrow Mesenchymal Stem Cells. Front. Bioeng. Biotechnol. 2022, 10, 824025. [Google Scholar] [CrossRef]
- Zurita, R.; Puiggalí, J.; Franco, L.; Rodríguez-Galán, A. Copolymerization of Glycolide and Trimethylene Carbonate. J. Polym. Sci. Part Polym. Chem. 2006, 44, 993–1013. [Google Scholar] [CrossRef]
- ASTM F756; Practice for Assessment of Hemolytic Properties of Materials. F04 Committee ASTM International: West Conshohocken, PA, USA, 2013. [CrossRef]
- Goyal, T.; Schmotzer, C.L. Validation of Hemolysis Index Thresholds Optimizes Detection of Clinically Significant Hemolysis. Am. J. Clin. Pathol. 2015, 143, 579–583. [Google Scholar] [CrossRef]
- Li, S.; Dobrzynski, P.; Kasperczyk, J.; Bero, M.; Braud, C.; Vert, M. Structure−Property Relationships of Copolymers Obtained by Ring-Opening Polymerization of Glycolide and ε-Caprolactone. Part 2. Influence of Composition and Chain Microstructure on the Hydrolytic Degradation. Biomacromolecules 2005, 6, 489–497. [Google Scholar] [CrossRef]
- Domańska, I.M.; Figat, R.; Zalewska, A.; Cieśla, K.; Kowalczyk, S.; Kędra, K.; Sobczak, M. The Influence of Ionizing Radiation on Paclitaxel-Loaded Nanoparticles Based on PLGA. Appl. Sci. 2023, 13, 11052. [Google Scholar] [CrossRef]
- Stefanowicz, Z.; Sobczak, M.; Piętniewicz, A.; Kołodziejski, W.L. Macromolecular Conjugates of Paclitaxel: Synthesis, Characterization, and In Vitro Paclitaxel Release Studies Based on HPLC Validated Method. Acta Chromatogr. 2016, 28, 99–117. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 13829:2000; Water quality — Determination of the Genotoxicity of Water and Waste Water Using the umu-Test. International Organization for Standardization: Geneva, Switzerland, 2000.



| Entry | Reaction Time [h] | Reaction Temperature [°C] | Molar Ratio Zn/Monomers [%] | Molar Ratio GL/CL [%] | CL Conversion [%] | Đ a | TII b [%] | LC c | LGG d | Mn e [kDa] | R f [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 8 | 80 | 1.0 | 8.0 | 89 | 1.92 | 99 | 8.18 | 0.59 | 45.0 | 100 |
| C2 | 16 | 80 | 1.0 | 8.0 | 98 | 2.05 | 84 | 8.93 | 0.64 | 43.2 | 100 |
| C3 | 24 | 80 | 1.0 | 8.0 | 97 | 1.48 | 97 | 7.87 | 0.59 | 31.8 | 100 |
| C4 | 48 | 80 | 1.0 | 8.0 | 99 | 1.84 | 92 | 7.92 | 0.61 | 41.5 | 100 |
| C5 | 8 | 80 | 2.0 | 8.0 | 97 | 1.92 | 100 | 9.42 | 0.57 | 38.9 | 100 |
| C6 | 16 | 80 | 2.0 | 8.0 | 98 | 1.73 | 90 | 10.18 | 0.61 | 48.2 | 100 |
| C7 | 24 | 80 | 2.0 | 8.0 | 99 | 1.85 | 86 | 10.30 | 0.63 | 42.5 | 100 |
| C8 | 48 | 80 | 2.0 | 8.0 | 99 | 1.97 | 88 | 10.53 | 0.62 | 36.4 | 98 |
| C9 | 8 | 80 | 1.0 | 15.0 | 86 | 1.56 | 100 | 3.74 | 0.58 | 36.2 | 100 |
| C10 | 16 | 80 | 1.0 | 15.0 | 96 | 2.20 | 100 | 6.11 | 0.59 | 38.5 | 100 |
| C11 | 24 | 80 | 1.0 | 15.0 | 91 | 2.04 | 100 | 6.50 | 0.59 | 48.8 | 100 |
| C12 | 48 | 80 | 1.0 | 15.0 | 99 | 1.56 | 100 | 5.92 | 0.61 | 62.9 | 100 |
| C13 | 8 | 80 | 2.0 | 15.0 | 97 | 1.73 | 94 | 8.96 | 0.61 | 38.2 | 100 |
| C14 | 16 | 80 | 2.0 | 15.0 | 98 | 1.76 | 100 | 5.83 | 0.59 | 35.1 | 100 |
| C15 | 24 | 80 | 2.0 | 15.0 | 99 | 1.48 | 79 | 9.11 | 0.66 | 38.0 | 100 |
| C16 | 48 | 80 | 2.0 | 15.0 | 99 | 1.78 | 80 | 7.96 | 0.68 | 41.2 | 100 |
| C17 | 24 | 60 | 1.0 | 8.0 | 98 | 1.99 | 97 | 8.99 | 0.61 | 32.9 | 99 |
| C18 | 48 | 60 | 1.0 | 8.0 | 98 | 1.79 | 100 | 1.91 | 0.66 | 43.0 | 100 |
| C19 | 24 | 60 | 2.0 | 8.0 | 98 | 1.67 | 90 | 9.40 | 0.64 | 42.1 | 100 |
| C20 | 48 | 60 | 2.0 | 8.0 | 98 | 1.71 | 77 | 10.36 | 0.67 | 27.7 | 100 |
| C21 | 24 | 60 | 1.0 | 15.0 | 96 | 2.99 | 57 | 7.63 | 1.05 | 38.1 | 100 |
| C22 | 48 | 60 | 1.0 | 15.0 | 96 | 1.84 | 48 | 17.14 | 0.85 | 42.2 | 100 |
| C23 | 24 | 60 | 2.0 | 15.0 | 97 | 2.25 | 100 | 6.52 | 0.60 | 33.6 | 100 |
| C24 | 48 | 60 | 2.0 | 15.0 | 97 | 1.74 | 100 | 6.64 | 0.60 | 39.8 | 100 |
| Sample | Tm, onset (°C) | ΔH (J/g) |
|---|---|---|
| C7 | 43.1 | −506.9 |
| C12 | 50.7 | −334.0 |
| C14 | 47.1 | −548.0 |
| C18 | 47.1 | −222.2 |
| Sample | Genotoxicity Assay | Cytotoxicity Assay | |||
|---|---|---|---|---|---|
| −S9 a | +S9 b | ||||
| G ± SD | IR ± SD | G ± SD | IR ± SD | Cells Viability ± SD [%] | |
| C7 | 1.10 ± 0.11 | 1.09 ± 0.07 | 1.05 ± 0.04 | 0.94 ± 0.06 | 103 ± 4 |
| C12 | 1.16 ± 0.16 | 0.92 ± 0.27 | 1.09 ± 0.01 | 0.91 ± 0.08 | 93 ± 1 |
| C14 | 1.14 ± 0.05 | 0.85 ± 0.13 | 1.08 ± 0.03 | 0.83 ± 0.14 | 116 ± 3 |
| C18 | 1.07 ± 0.02 | 0.89 ± 0.12 | 1.11 ± 0.03 | 0.86 ± 0.14 | 113 ± 7 |
| C20 | 1.01 ± 0.02 | 0.95 ± 0.12 | 1.01 ± 0.02 | 0.91 ± 0.14 | 114 ± 1 |
| PC c | 1.04 ± 0.01 | 3.30 ± 0.28 | 0.87 ± 0.04 | 2.53 ± 0.41 | 1 ± 1 |
| NC d | 1.00 ± 0.01 | 1.00 ± 0.05 | 1.00 ± 0.04 | 1.00 ± 0.08 | 111 ± 1 |
| Model Type | R2 | n (Transport Mechanism) |
|---|---|---|
| Zero-order model | 0.831 | - |
| First-order model | 0.852 | - |
| Higuchi model | 0.969 | - |
| Korsmeyer-Peppas model | 0.993 | 0.329 (Fickian diffusion) |
| Proton a | δ [ppm] |
|---|---|
| –C(O)CH2O– (comonomeric) | 4.85–4.68 |
| –C(O)CH2O– (transesterified) | 4.60 |
| –C(O)CH2CH2CH2CH2CH2O– (comonomeric) | 4.17–4.13 |
| –C(O)CH2CH2CH2CH2CH2O– (homomonomeric) | 4.06–4.03 |
| –C(O)CH2CH2CH2CH2CH2O– (comonomeric) | 2.45–2.40 |
| –C(O)CH2CH2CH2CH2CH2O– (homomonomeric) | 2.32–2.27 |
| –C(O)CH2CH2CH2CH2CH2O– | 1.71–1.60 |
| –C(O)CH2CH2CH2CH2CH2O– | 1.44–1.33 |
| Carbon a | δ [ppm] |
|---|---|
| –C(O)CH2CH2CH2CH2CH2O– | 174.0–173.2 |
| –C(O)CH2O– | 168.0 |
| –C(O)CH2CH2CH2CH2CH2O– | 65.6–64.6 |
| –C(O)CH2O– | 61.0 |
| –C(O)CH2CH2CH2CH2CH2O– | 34.5–34.1 |
| –C(O)CH2CH2CH2CH2CH2O– | 28.8–28.6 |
| –C(O)CH2CH2CH2CH2CH2O– | 26.0–25.8 |
| –C(O)CH2CH2CH2CH2CH2O– | 25.0–24.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyrębiak, R.; Figat, R.; Oledzka, E.; Kasiński, A.; Kędra, K.; Laskowska, A.; Sobczak, M. Exploration of a Novel Catalytic Approach for Synthesizing Glycolide and ε-Caprolactone Copolymers and Their Application as Carriers for Paclitaxel. Molecules 2025, 30, 2318. https://doi.org/10.3390/molecules30112318
Wyrębiak R, Figat R, Oledzka E, Kasiński A, Kędra K, Laskowska A, Sobczak M. Exploration of a Novel Catalytic Approach for Synthesizing Glycolide and ε-Caprolactone Copolymers and Their Application as Carriers for Paclitaxel. Molecules. 2025; 30(11):2318. https://doi.org/10.3390/molecules30112318
Chicago/Turabian StyleWyrębiak, Rafał, Ramona Figat, Ewa Oledzka, Adam Kasiński, Karolina Kędra, Anna Laskowska, and Marcin Sobczak. 2025. "Exploration of a Novel Catalytic Approach for Synthesizing Glycolide and ε-Caprolactone Copolymers and Their Application as Carriers for Paclitaxel" Molecules 30, no. 11: 2318. https://doi.org/10.3390/molecules30112318
APA StyleWyrębiak, R., Figat, R., Oledzka, E., Kasiński, A., Kędra, K., Laskowska, A., & Sobczak, M. (2025). Exploration of a Novel Catalytic Approach for Synthesizing Glycolide and ε-Caprolactone Copolymers and Their Application as Carriers for Paclitaxel. Molecules, 30(11), 2318. https://doi.org/10.3390/molecules30112318








