Bio-Based Polyurethane Asphalt Binder with Continuous Polymer-Phase Structure: Critical Role of Isocyanate Index in Governing Thermomechanical Performance and Phase Morphology
Abstract
1. Introduction
2. Results and Discussion
2.1. Rotational Viscosity–Time Characteristics
- Lower limit (time to reach 1 Pa·s): The minimum time for viscosity to reach 1 Pa·s, which is necessary for thermosetting polymer-modified asphalt materials;
- Upper limit (time to reach 3 Pa·s): The maximum allowable construction time for effective compaction.
2.2. Structural Characterization
2.3. Cure Kinetics
2.4. Glass Transition Temperature (Tg)
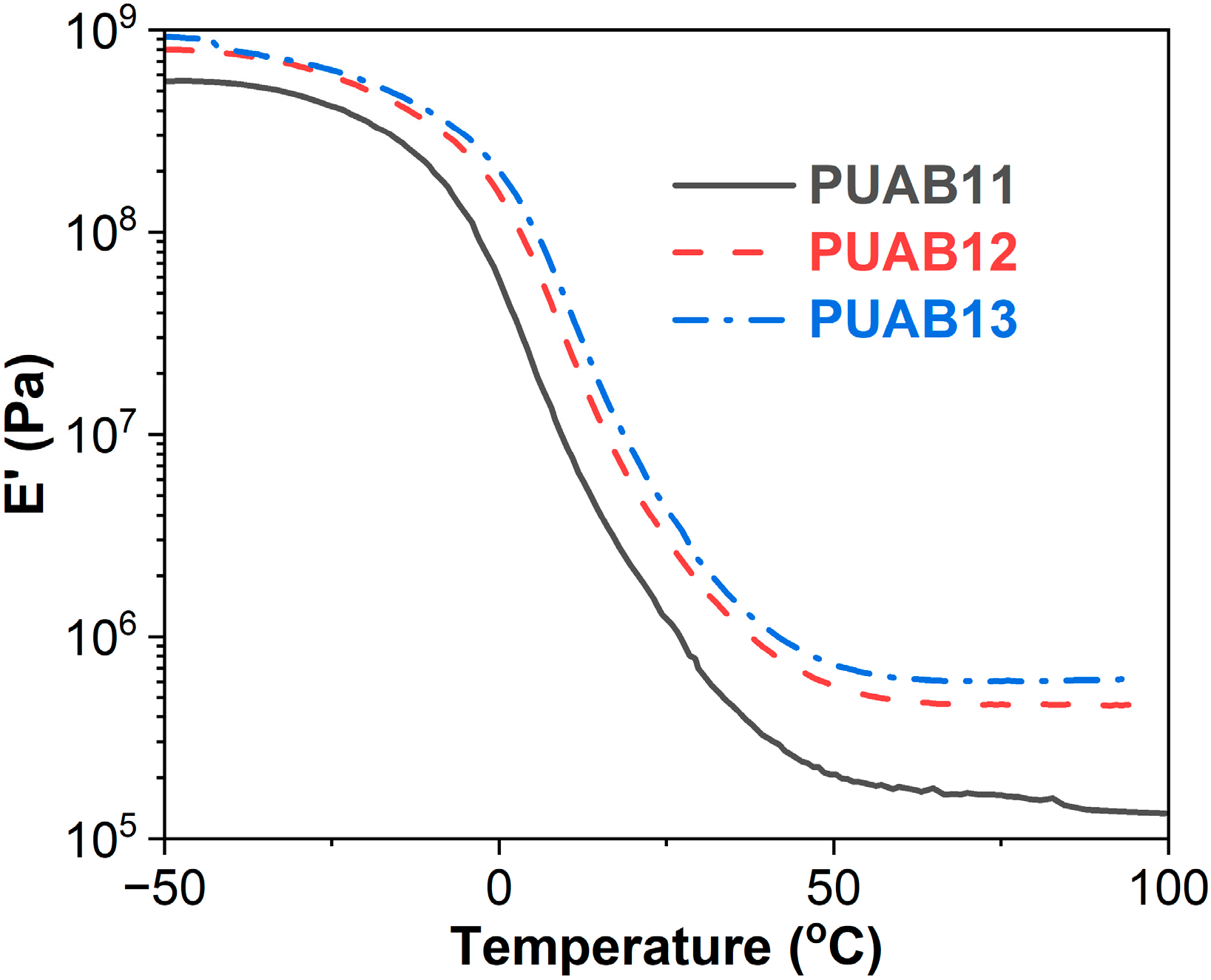
2.5. Storage Modulus
2.6. Damping Properties
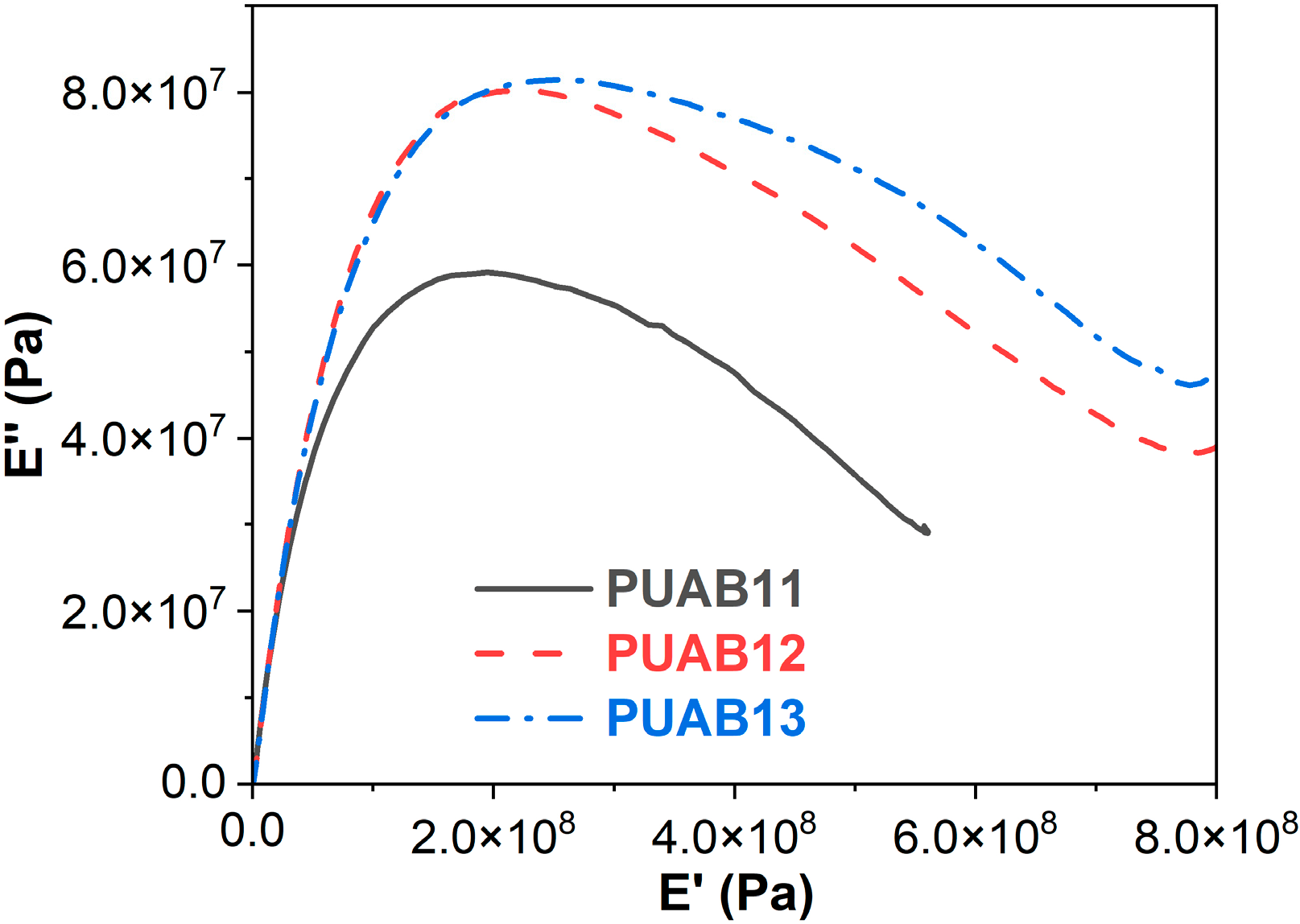
2.7. Cole–Cole Plots
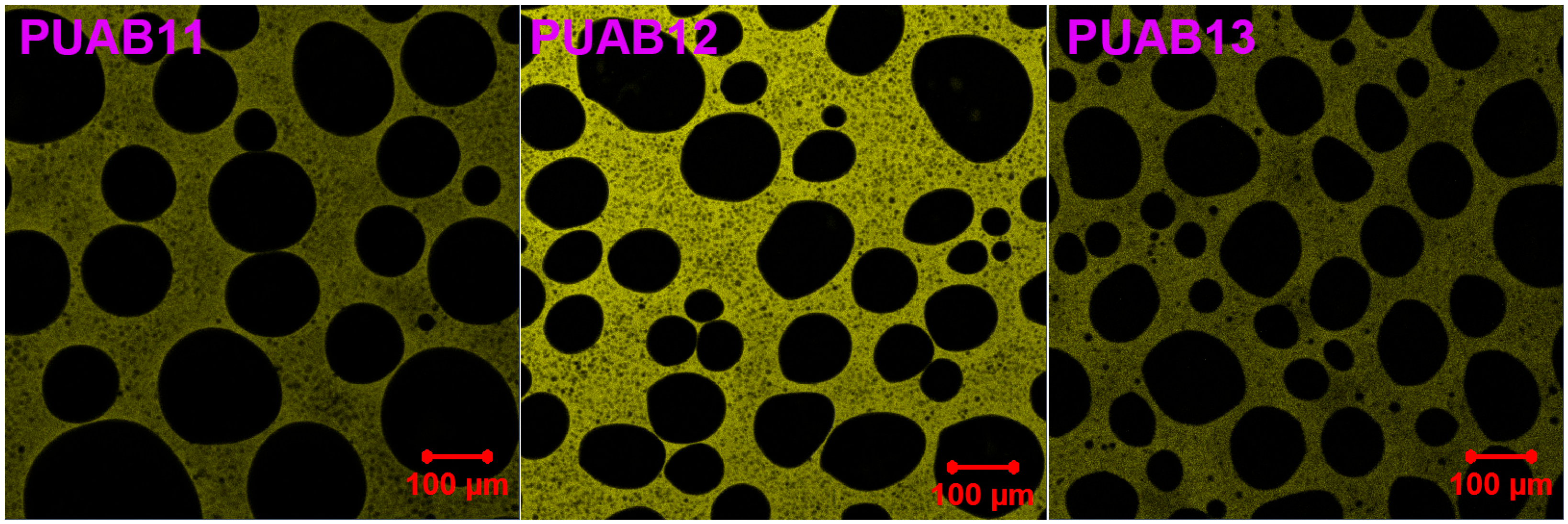
2.8. Morphology
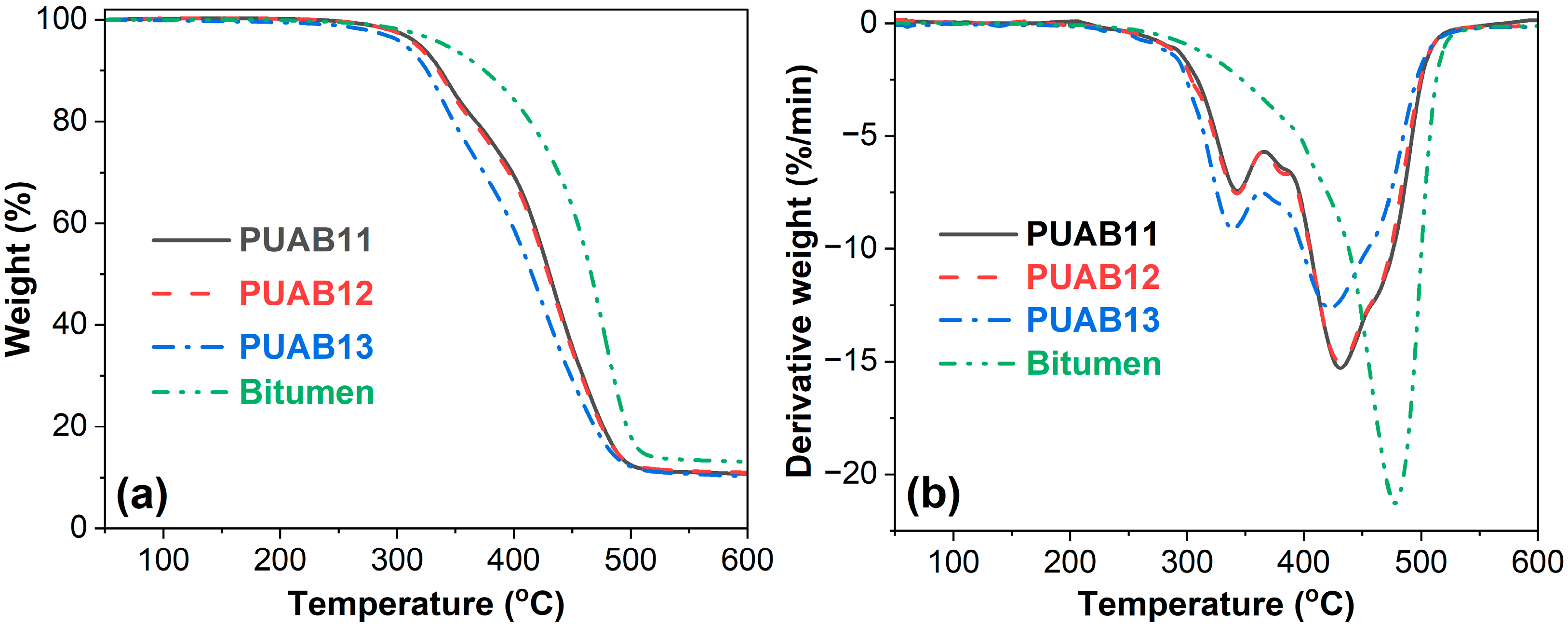
2.9. Thermal Stability
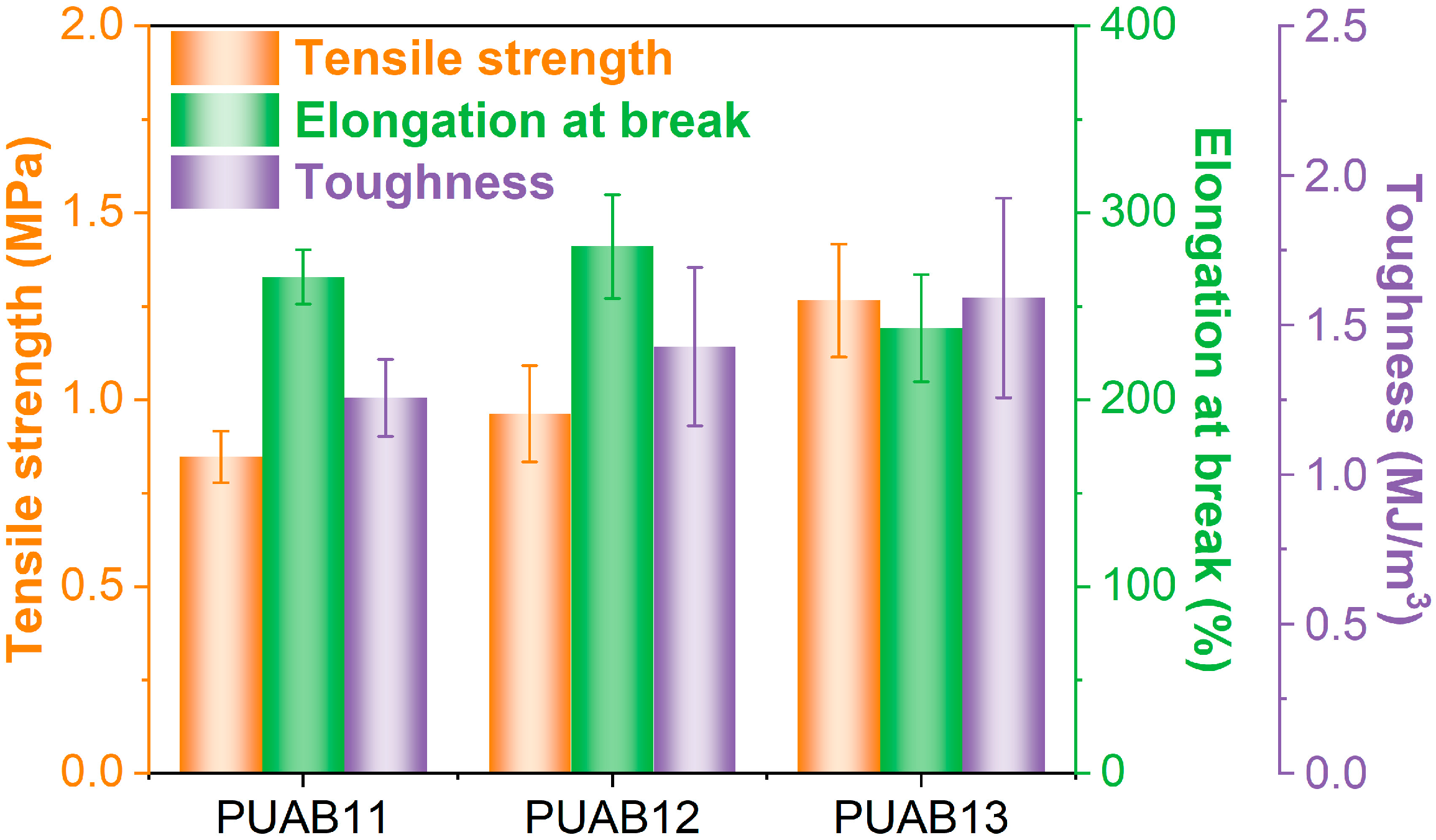
2.10. Mechanical Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of Bio-Based PUABs
3.3. Methods
3.3.1. ATR-FTIR Spectroscopy
3.3.2. Rheological Behavior
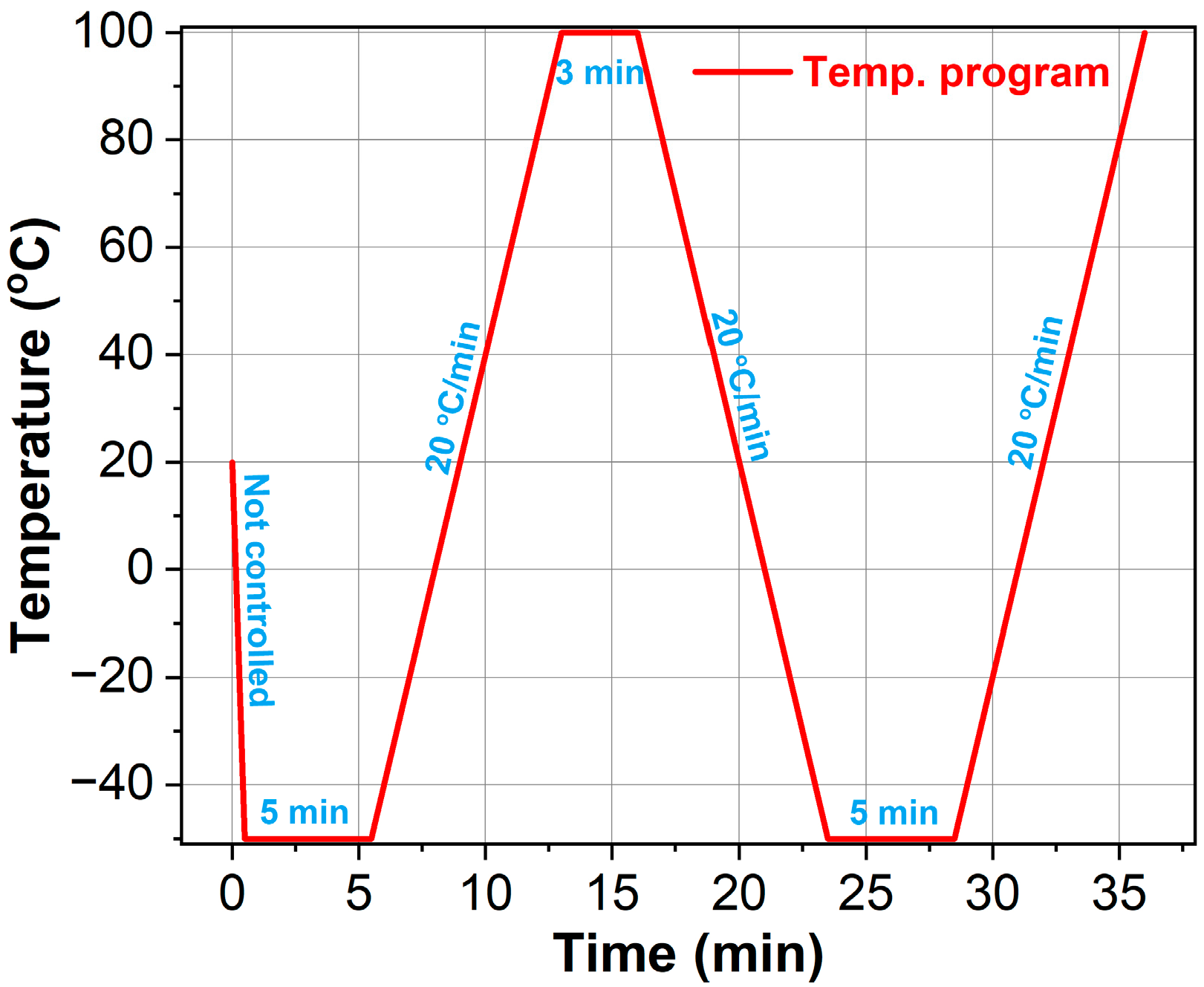
3.3.3. DSC
- (1)
- Initial heating and cooling: Samples were rapidly cooled to −50 °C (maximum instrument rate) followed by a 5 min isothermal hold to erase thermal history (chemical/physical aging effects).
- (2)
- Equilibration: Temperature stabilization was achieved prior to the second heating phase.
- (3)
- Second heating: Glass transition temperatures were determined via heat flow analysis.
3.3.4. DMA
3.3.5. TGA
3.3.6. UTM
3.3.7. LCM
4. Conclusions
- (1)
- The rotational viscosity of PUABs increases proportionally with the isocyanate index. Although PUABs demonstrate longer allowable construction time compared to WEABs, elevated NCO/OH ratios progressively reduce these processing windows.
- (2)
- PUABs exhibit a single glass transition temperature, confirming superior compatibility between bitumen and bio-based polyurethane. Notably, the Tg values of PUABs show a positive correlation with the isocyanate index, consistently exceeding that of neat bitumen.
- (3)
- While increasing the isocyanate index negatively impacts the final conversion of the cure reaction, thermal stability, and damping properties, it significantly enhances the storage modulus and mechanical performance.
- (4)
- Phase separation analysis reveals that higher NCO/OH ratios reduce the average diameters of dispersed bitumen domains (Dn from 59.9 μm to 45.0 μm and Dw from 85.6 μm to 58.3 μm) but concurrently increase domain size uniformity.
- (5)
- The rotational viscosity–time profile and elongation at break (>230%) meet the requirements for the thermosetting binder in steel deck bridge pavements. However, suboptimal tensile strength (1.27 MPa versus the required 1.50 MPa) necessitates further formulation.
- (6)
- Compared to conventional petroleum-based polyurethane asphalts, the biomass content of PU used for the production of bio-based PUAB containing 60 wt% bitumen is more than 70%, indicating that these PUABs are environmentally friendly and cost-efficient.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- You, Z.; Mills-Beale, J.; Foley, J.M.; Roy, S.; Odegard, G.M.; Dai, Q.; Goh, S.W. Nanoclay-modified asphalt materials: Preparation and characterization. Constr. Build. Mater. 2011, 25, 1072–1078. [Google Scholar] [CrossRef]
- Polacco, G.; Stastna, J.; Biondi, D.; Zanzotto, L. Relation between polymer architecture and nonlinear viscoelastic behavior of modified asphalts. Curr. Opin. Colloid Interface Sci. 2006, 11, 230–245. [Google Scholar] [CrossRef]
- Behnood, A.; Gharehveran, M.M. Morphology, rheology, and physical properties of polymer-modified asphalt binders. Eur. Polym. J. 2019, 112, 766–791. [Google Scholar] [CrossRef]
- Zhu, J.; Birgisson, B.; Kringos, N. Polymer modification of bitumen: Advances and challenges. Eur. Polym. J. 2014, 54, 18–38. [Google Scholar] [CrossRef]
- Yang, S.; Li, R.; Zhu, H.; Qin, Y.; Huang, C. Review of the state-of-the-art techniques for enhancing the toughness of thermosetting epoxy asphalt. Constr. Build. Mater. 2024, 449, 137660. [Google Scholar] [CrossRef]
- Zhou, D.; Liang, R.; Kang, Y. A review of chemo-rheological and thermo-rheological investigations on epoxy asphalt cementitious materials. Constr. Build. Mater. 2023, 395, 132309. [Google Scholar] [CrossRef]
- Huang, G.; Yang, T.; He, Z.; Yu, L.; Xiao, H. Polyurethane as a modifier for road asphalt: A literature review. Constr. Build. Mater. 2022, 356, 129058. [Google Scholar] [CrossRef]
- Xie, H.; Li, C.; Wang, Q. Thermosetting polymer modified asphalts: Current status and challenges. Polym. Rev. 2024, 64, 690–759. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Ban, X.; Liu, X.; Guo, Y.; Sun, J.; Liu, Y.; Zhang, S.; Lei, J. Thermosetting resin modified asphalt: A comprehensive review. J. Traffic Transp. Eng. Engl. Ed. 2023, 10, 1001–1036. [Google Scholar] [CrossRef]
- Cong, P.; Liu, C.; Han, Z.; Zhao, Y. A comprehensive review on polyurethane modified asphalt: Mechanism, characterization and prospect. J. Road Eng. 2023, 3, 315–335. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Panchireddy, S.; Grignard, B.; Calvo, I.; Jerome, C.; Detrembleur, C.; Sardon, H. Poly(hydroxyurethane) adhesives and coatings: State-of-the-art and future directions. ACS Sustain. Chem. Eng. 2021, 9, 9541–9562. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Berto, F.; Zhang, D. Pyrolysis kinetics and flammability evaluation of rigid polyurethane with different isocyanate content. Molecules 2021, 26, 2386. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Zhu, Y.; Sun, J.; Wang, L.; Huang, T.; Chen, L. Modification of asphalt using polyurethanes synthesized with different isocyanates. Constr. Build. Mater. 2022, 327, 126959. [Google Scholar] [CrossRef]
- Ban, X.; Zhang, Z.; Chang, P.; Zhang, S.; Liu, H.; Liang, Y.; Chen, Y. The performance and distribution of polyurethane-modified asphalt that exhibits different molecular weights. Sustainability 2023, 15, 6627. [Google Scholar] [CrossRef]
- Jin, X.; Sun, S.; Guo, N.; Huang, S.; You, Z.; Tan, Y. Influence on polyurethane synthesis parameters upon the performance of base asphalt. Front. Mater. 2021, 8, 656261. [Google Scholar] [CrossRef]
- Cuadri, A.A.; García-Morales, M.; Navarro, F.J.; Partal, P. Processing of bitumens modified by a bio-oil-derived polyurethane. Fuel 2014, 118, 83–90. [Google Scholar] [CrossRef]
- Kazemi, M.; Mohammadi, A.; Goli, A.; Fini, E. Introducing a sustainable bio-based polyurethane to enhance the healing capacity of bitumen. J. Mater. Civ. Eng. 2022, 34, 04021465. [Google Scholar] [CrossRef]
- Wei, K.; Cao, X.; Tang, B.; Wu, Y.; Jiang, T. Castor oil-based polyurethane Vitrimer modified asphalts regulated by dual dynamic reversible reactions and crosslinking density: Preparation, basic assessment and self-healing behavior. Constr. Build. Mater. 2024, 429, 136430. [Google Scholar] [CrossRef]
- Wei, K.; Cao, X.; Wu, Y.; Cheng, Z.; Tang, B.; Shan, B. Dynamic chemistry approach for self-healing of polymer-modified asphalt: A state-of-the-art review. Constr. Build. Mater. 2023, 403, 133128. [Google Scholar] [CrossRef]
- Peng, C.; Huang, S.; You, Z.; Xu, F.; You, L.; Ouyang, H.; Li, T.; Guo, C.; Ma, H.; Chen, P.; et al. Effect of a lignin-based polyurethane on adhesion properties of asphalt binder during UV aging process. Constr. Build. Mater. 2020, 247, 118547. [Google Scholar] [CrossRef]
- Vural Kök, B.; Aydoğmuş, E.; Yilmaz, M.; Akpolat, M. Investigation on the properties of new palm-oil-based polyurethane modified bitumen. Constr. Build. Mater. 2021, 289, 123152. [Google Scholar] [CrossRef]
- Gong, J.; Jing, F.; Zhao, R.; Li, C.; Cai, J.; Wang, Q.; Xie, H. Waste cooking oil-modified epoxy asphalt rubber binders with improved compatibility and extended allowable construction time. Molecules 2022, 27, 7061. [Google Scholar] [CrossRef] [PubMed]
- Asphalt Institute. Performance Graded Asphalt Binder Specification and Testing Superpave; Asphalt Institute: Lexington, KY, USA, 2003. [Google Scholar]
- GB/T 30598; General Specifications of Epoxy Asphalt Materials for Paving Roads and Bridges. The Standardization Administration of the People’s Republic of China: Beijing, China, 2014.
- Zhao, R.; Jing, F.; Li, C.; Wang, R.; Xi, Z.; Cai, J.; Wang, Q.; Xie, H. Viscosity-curing time behavior, viscoelastic properties, and phase separation of graphene oxide/epoxy asphalt composites. Polym. Compos. 2022, 43, 5454–5464. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Jia, M.; Qi, B.; Zhang, H.; Lv, W.; Mao, Z.; Chang, P.; Peng, J.; Liu, Y. Study on a thermosetting polyurethane modified asphalt suitable for bridge deck pavements: Formula and properties. Constr. Build. Mater. 2020, 241, 118122. [Google Scholar] [CrossRef]
- Meng, Y.; Zhan, L.; Hu, C.; Tang, Y.; Großegger, D.; Ye, X. Research on modification mechanism and performance of an innovative bio-based polyurethane modified asphalt: A sustainable way to reducing dependence on petroleum asphalt. Constr. Build. Mater. 2022, 350, 128830. [Google Scholar] [CrossRef]
- Sardari, A.; Sabbagh Alvani, A.A.; Ghaffarian, S.R. Castor oil-derived water-based polyurethane coatings: Structure manipulation for property enhancement. Prog. Org. Coat. 2019, 133, 198–205. [Google Scholar] [CrossRef]
- Feng, Z.-G.; Bian, H.-J.; Li, X.-J.; Yu, J.-Y. FTIR analysis of UV aging on bitumen and its fractions. Mater. Struct. 2016, 49, 1381–1389. [Google Scholar] [CrossRef]
- Duan, S.; Hu, J.; Cui, J.; Chen, Y.; Ma, T.; Wu, X. Acrylate composite polyurethane binder for steel bridge deck pavements: Process optimization by response surface methodology and microanalysis. J. Appl. Polym. Sci. 2024, 141, e55228. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate terminated castor oil-based polyurethane prepolymer: Synthesis and characterization. Prog. Org. Coat. 2015, 80, 39–48. [Google Scholar] [CrossRef]
- Su, Q.; Wei, D.; Dai, W.; Zhang, Y.; Xia, Z. Designing a castor oil-based polyurethane as bioadhesive. Colloids Surf. B Biointerfaces 2019, 181, 740–748. [Google Scholar] [CrossRef]
- Min, K.-E.; Hwang, Y.-G.; Choi, G.-Y.; Kim, H.-G.; Kim, W.-S.; Lee, D.-H.; Park, L.-S.; Seo, K.-H.; Kang, I.-K.; Jun, I.-R.; et al. Effect of reactive polyurethane on toughness of unsaturated polyester resin. J. Appl. Polym. Sci. 2002, 84, 735–740. [Google Scholar] [CrossRef]
- Boulkadid, M.K.; Touidjine, S.; Trache, D.; Belkhiri, S. Analytical methods for the assessment of curing kinetics of polyurethane binders for high-energy composites. Crit. Rev. Anal. Chem. 2022, 52, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z.; Luo, Y.; Sun, J.; Tian, P.; Liu, H.; Mao, Z.; Kan, S.; Yu, X. Curing characteristics and performance of a bio-based polyurethane engineered sealant with high bonding strength: Effect of R value and chain extender content. Constr. Build. Mater. 2024, 432, 136684. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Achilias, D.; Fernandez-Francos, X.; Galukhin, A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of thermal polymerization kinetics. Thermochim. Acta 2022, 714, 179243. [Google Scholar] [CrossRef]
- Menczel, J.D.; Judovits, L.; Prime, R.B.; Bair, H.E.; Reading, M.; Swier, S. Differential scanning calorimetry (DSC). In Thermal Analysis of Polymers: Fundamentals and Applications; Menczel, J.D., Prime, R.B., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 241–317. [Google Scholar]
- Memon, G.M.; Chollar, B.H. Glass transition measurements of asphalts by DSC. J. Therm. Anal. 1997, 49, 601–607. [Google Scholar] [CrossRef]
- Tabatabaee, H.A.; Velasquez, R.; Bahia, H.U. Predicting low temperature physical hardening in asphalt binders. Constr. Build. Mater. 2012, 34, 162–169. [Google Scholar] [CrossRef]
- Liu, H.; Su, B.; Ding, H.; Lei, Y.; Rahman, A.; Peng, Y.; Qiu, Y. Revealing time-dependent behavior of asphalt binder at low-temperature by crystallization kinetics. Constr. Build. Mater. 2024, 411, 134700. [Google Scholar] [CrossRef]
- Duncan, J. Principles and applications of mechanical thermal analysis. In Principles and Applications of Thermal Analysis; Gabbott, P., Ed.; Blackwell Publishing: Oxford, UK, 2008; pp. 119–163. [Google Scholar]
- Meng, Y.; Chen, K.; Yang, Y.; Jiang, T.; Hao, T.; Lu, X.; Zhang, Q. Synthesis and characterization of crosslinked castor oil-based polyurethane nanocomposites based on novel silane-modified isocyanate and their potential application in heat insulating coating. Polymers 2022, 14, 1880. [Google Scholar] [CrossRef]
- Xie, H.; Li, C.; Wang, Q. A critical review on performance and phase separation of thermosetting epoxy asphalt binders and bond coats. Constr. Build. Mater. 2022, 326, 126792. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef]
- Carreño Gómez, N.H.; Oeser, M.; Fleischel, O. Chemical modification of bitumen with novel isocyanate-based additive to enhance asphalt performance. Constr. Build. Mater. 2021, 301, 124128. [Google Scholar] [CrossRef]
- Cuadri, A.A.; García-Morales, M.; Navarro, F.J.; Partal, P. Isocyanate-functionalized castor oil as a novel bitumen modifier. Chem. Eng. Sci. 2013, 97, 320–327. [Google Scholar] [CrossRef]
- Nakamura, M.; Aoki, Y.; Enna, G.; Oguro, K.; Wada, H. Polyurethane damping material. J. Elastomers Plast. 2014, 47, 515–522. [Google Scholar] [CrossRef]
- Rao, M.D. Recent applications of viscoelastic damping for noise control in automobiles and commercial airplanes. J. Sound Vib. 2003, 262, 457–474. [Google Scholar] [CrossRef]
- Chang, M.C.O.; Thomas, D.A.; Sperling, L.H. Characterization of the area under loss modulus and tan δ–temperature curves: Acrylic polymers and their sequential interpenetrating polymer networks. J. Appl. Polym. Sci. 1987, 34, 409–422. [Google Scholar] [CrossRef]
- Keskkula, H.; Turley, S.G.; Boyer, R.F. The significance of the rubber damping peak in rubber-modified polymers. J. Appl. Polym. Sci. 1971, 15, 351–367. [Google Scholar] [CrossRef]
- Jing, F.; Wang, R.; Zhao, R.; Li, C.; Cai, J.; Ding, G.; Wang, Q.; Xie, H. Enhancement of bonding and mechanical performance of epoxy asphalt bond coats with graphene nanoplatelets. Polymers 2023, 15, 412. [Google Scholar] [CrossRef]
- Su, W.; Han, X.; Gong, J.; Xi, Z.; Zhang, J.; Wang, Q.; Xie, H. Toughening epoxy asphalt binder using core-shell rubber nanoparticles. Constr. Build. Mater. 2020, 258, 119716. [Google Scholar] [CrossRef]
- Wu, C.; Yang, H.; Cui, X.; Cai, J.; Yuan, Z.; Zhang, J.; Xie, H. Thermo-mechanical properties and phase-separated morphology of warm-mix epoxy asphalt binders with different epoxy resin concentrations. Molecules 2024, 29, 3251. [Google Scholar] [CrossRef]
- Tian, J.; Luo, S.; Lu, Q.; Liu, S. Effects of epoxy resin content on properties of hot mixing epoxy asphalt binders. J. Mater. Civ. Eng. 2022, 34, 04022145. [Google Scholar] [CrossRef]
- Liu, M.; Hu, J.; Sun, J.; Li, Y.; Luo, S. Characterization of roadway epoxy asphalt binder with different epoxy contents. J. Mater. Civ. Eng. 2023, 35, 04023144. [Google Scholar] [CrossRef]
- Pan, P.; Chen, Y.; Hu, X.; Dai, B.; Hu, X.; Wang, N. Preparation and performance evaluation of castor oil-based asphalt regeneration agent. Materials 2024, 17, 2078. [Google Scholar] [CrossRef] [PubMed]
- Hablot, E.; Zheng, D.; Bouquey, M.; Avérous, L. Polyurethanes based on castor oil: Kinetics, chemical, mechanical and thermal properties. Macromol. Mater. Eng. 2008, 293, 922–929. [Google Scholar] [CrossRef]
- Bicerano, J.; Seitz, J.T. Molecular Origins of Toughness in Polymers. In Polymer Toughening; Arends, C.B., Ed.; Marcel Dekker: New York, NY, USA, 1996; pp. 1–60. [Google Scholar]
- ASTM D5-06; Standard Test Method for Penetration of Bituminous Materials. iTeh, Inc.: Newark, DE, USA, 2006.
- ASTM D113-07; Standard Test Method for Ductility of Asphalt Materials. iTeh, Inc.: Newark, DE, USA, 2023.
- ASTM D36-06; Standard Test Method for Softening Point of Bitumen (Ring-and-Ball Apparatus). iTeh, Inc.: Newark, DE, USA, 2010.
- ASTM D4402-06; Standard Test Method for Viscosity Determination of Asphalt at Elevated Temperatures Using a Rotational Viscometer. iTeh, Inc.: Newark, DE, USA, 2012.
- ASTM D3344-90; Standard Test Method for Total Wax Content of Corrugated Paperboard. iTeh, Inc.: Newark, DE, USA, 2023.
- ASTM D4124-09; Standard Test Method for Separation of Asphalt into Four Fractions. iTeh, Inc.: Newark, DE, USA, 2018.
- ASTM D638; Standard Test Method for Tensile Properties of Plastics. iTeh, Inc.: Newark, DE, USA, 2022.
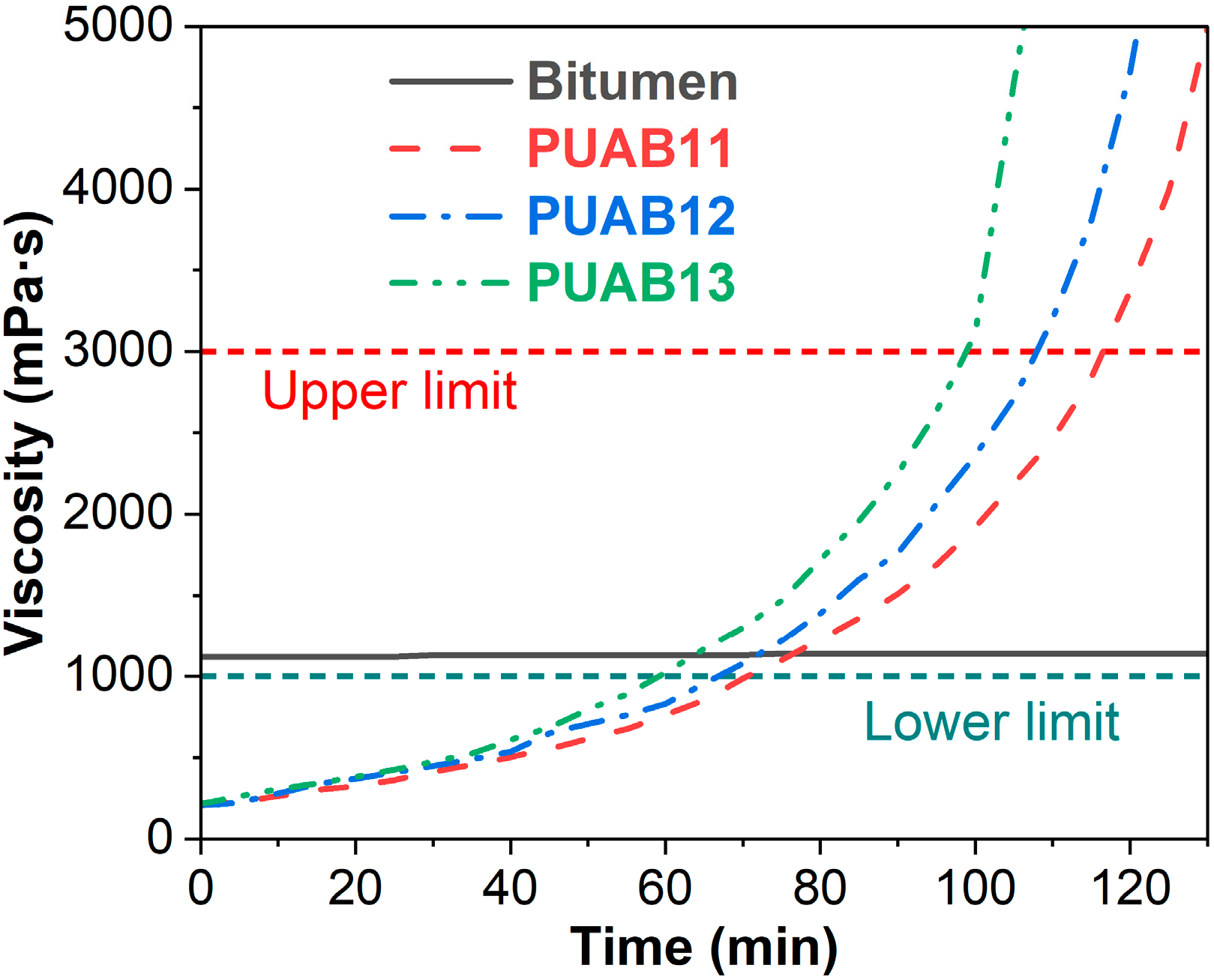
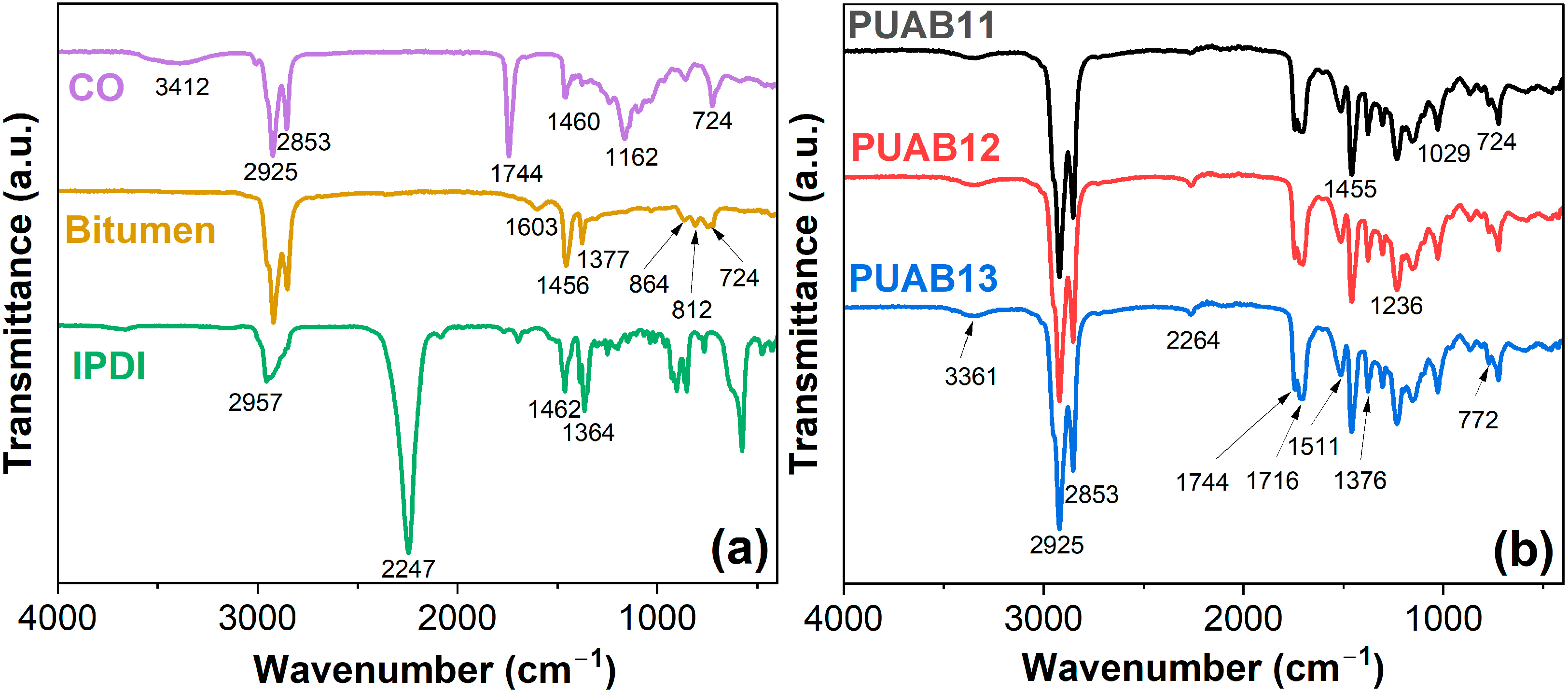


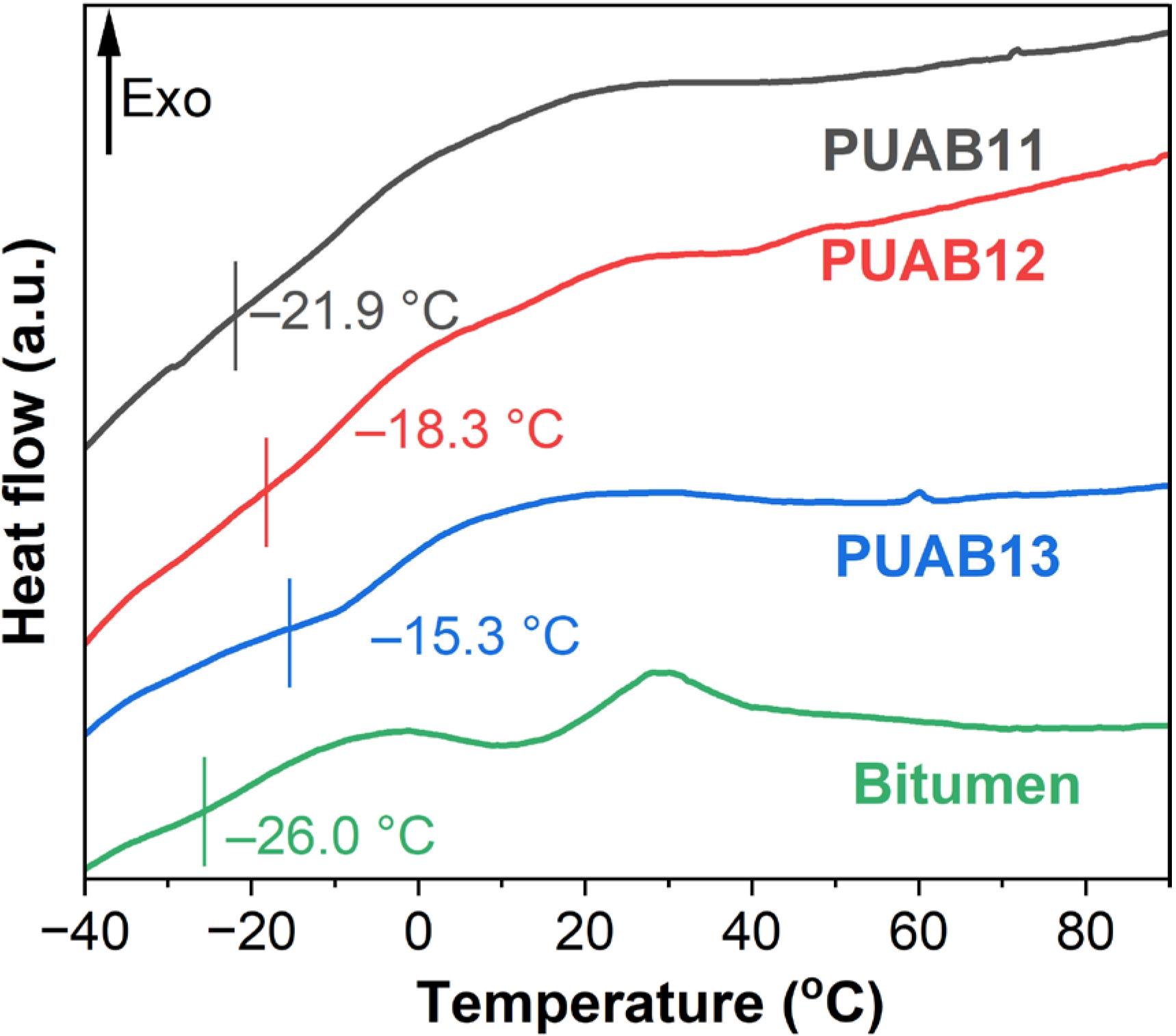
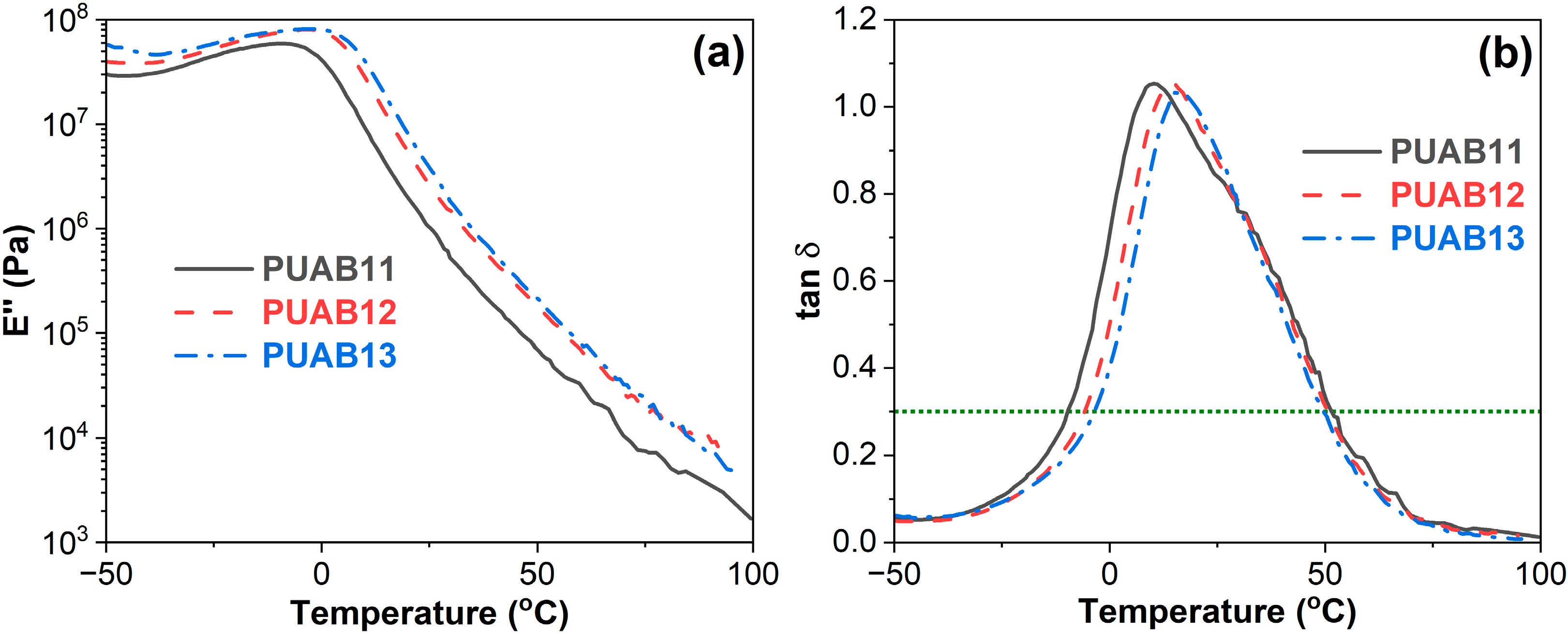


| Sample | Time to Reach 1 Pa⸱s (min) | Time to Reach 3 Pa⸱s (min) |
|---|---|---|
| PUAB11 | 77 | 117 |
| PUAB12 | 72 | 108 |
| PUAB13 | 64 | 99 |
| Peak Position (cm−1) | Characteristic Absorption Bands |
|---|---|
| 3412 | –OH group |
| 3361 | Stretching vibration of –NH |
| 2957, 2925 | Stretching –CH vibration of –CH2 |
| 2853 | Symmetric stretching of –CH2 |
| 2247, 2264 | –NCO group |
| 1744 | C=O group for ester |
| 1716 | Amide I: C=O stretching vibrations |
| 1603, 854, 812 | Vibration of aromatic rings |
| 1511 | –N–H in-plane bending |
| 1462 | Deforming vibrations of –CH– |
| 1456 | Scissoring vibration of –CH2– |
| 1377 | Umbrella vibration of –CH3 |
| 1364 | Bending vibration of –CH2 |
| 1162 | C–O–C group |
| 772 | N–H out-of-plane bending |
| 724 | Sympathetic vibration of [–CH2–]n, n ≥ 4 |
| Sample | Tg (°C) | υe (mol/m3) | ||
|---|---|---|---|---|
| DSC | E″-T | tan δ-T | ||
| PUAB11 | −21.9 | −8.5 | 10.2 | 25.7 |
| PUAB12 | −18.3 | −2.6 | 14.6 | 63.3 |
| PUAB13 | −15.3 | −1.7 | 16.1 | 78.9 |
| Sample | (tan δ)max | ΔT (°C) | Atanδ-T (K) |
|---|---|---|---|
| PUAB11 | 1.05 | 61.5 (−9.9~51.6) | 52.7 |
| PUAB12 | 1.05 | 56.6 (−5.9~50.7) | 48.5 |
| PUAB13 | 1.03 | 53.4 (−3.6~49.8) | 45.7 |
| Sample | Dn (μm) | Dw (μm) | Ɖ |
|---|---|---|---|
| PUAB11 | 59.9 ± 2.6 | 85.6 ± 5.6 | 1.43 |
| PUAB12 | 56.4 ± 4.7 | 74.5 ± 6.5 | 1.32 |
| PUAB13 | 45.0 ± 6.7 | 58.3 ± 7.2 | 1.29 |
| Sample | Ti (°C) | T1dmax (°C) | T2dmax (°C) | Char Residue at 600 °C (%) |
|---|---|---|---|---|
| PUAB11 | 319.6 | 344.1 | 431.6 | 10.7 |
| PUAB12 | 316.9 | 343.0 | 431.0 | 11.0 |
| PUAB13 | 306.9 | 339.1 | 423.1 | 10.3 |
| Bitumen | 341.8 | 477.3 | - | 13.1 |
| Properties | Standard | Value |
|---|---|---|
| Physical properties | ||
| Penetration (25 °C, 0.1 mm) | ASTM D5-06 [60] | 73.0 |
| Ductility (10 °C, cm) | ASTM D113-07 [61] | 15.8 |
| Softening point (°C) | ASTM D36-06 [62] | 48.2 |
| Viscosity (60 °C, Pa⸱s) | ASTM D4402-06 [63] | 173.0 |
| Wax content (%) | ASTM D3344-90 [64] | 1.83 |
| Chemical components | ||
| Saturates (%) | ASTM D4124-09 [65] | 20.0 |
| Aromatics (%) | 31.5 | |
| Resins (%) | 37.1 | |
| Asphaltenes (%) | 6.8 |
| Sample | CO (g) | IPDI (g) | Bitumen (g) | Biomass Contents in Bio-PUs (%) |
| PUAB11 | 100 | 35.0 | 202.5 | 74.1 |
| PUAB12 | 100 | 38.7 | 208.0 | 72.1 |
| PUAB13 | 100 | 41.9 | 212.9 | 70.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Cao, S.; Wu, C.; Xi, Z.; Cai, J.; Yuan, Z.; Zhang, J.; Xie, H. Bio-Based Polyurethane Asphalt Binder with Continuous Polymer-Phase Structure: Critical Role of Isocyanate Index in Governing Thermomechanical Performance and Phase Morphology. Molecules 2025, 30, 2466. https://doi.org/10.3390/molecules30112466
Yang H, Cao S, Wu C, Xi Z, Cai J, Yuan Z, Zhang J, Xie H. Bio-Based Polyurethane Asphalt Binder with Continuous Polymer-Phase Structure: Critical Role of Isocyanate Index in Governing Thermomechanical Performance and Phase Morphology. Molecules. 2025; 30(11):2466. https://doi.org/10.3390/molecules30112466
Chicago/Turabian StyleYang, Haocheng, Suzhou Cao, Chengwei Wu, Zhonghua Xi, Jun Cai, Zuanru Yuan, Junsheng Zhang, and Hongfeng Xie. 2025. "Bio-Based Polyurethane Asphalt Binder with Continuous Polymer-Phase Structure: Critical Role of Isocyanate Index in Governing Thermomechanical Performance and Phase Morphology" Molecules 30, no. 11: 2466. https://doi.org/10.3390/molecules30112466
APA StyleYang, H., Cao, S., Wu, C., Xi, Z., Cai, J., Yuan, Z., Zhang, J., & Xie, H. (2025). Bio-Based Polyurethane Asphalt Binder with Continuous Polymer-Phase Structure: Critical Role of Isocyanate Index in Governing Thermomechanical Performance and Phase Morphology. Molecules, 30(11), 2466. https://doi.org/10.3390/molecules30112466






