Abstract
Acospectoside A (1) and acovenoside B (2), two cytotoxic cardenolides extracted from the venomous South African bush Acokanthera oppositifolia, are distinguished by their unique structural motifs of the l-acovenose moiety at C-3 and a 1β-O-acetylated cardenolide aglycone. Here, we report the synthesis of these cardiac glycosides featuring delicate introductions of the 1-O-acetyl group under acid-catalyzed conditions, 14β-OH by Mukaiyama hydration, and a C17-butenolide moiety by Stille coupling.
1. Introduction
Cardiac glycosides, a structurally diverse group of natural products, are characterized by a steroid skeleton adorned with a sugar moiety at the C3 position and a lactone moiety at the C17 position [1,2]. For over two centuries, members of this family have been used in clinics for the treatment of heart failure and cardiac rhythm disorders [3,4,5]. Recent investigations have unveiled that diminished levels of specific endogenous cardiotonic steroids could promote tumorigenesis [6], thereby sparking significant interest in a novel hypothesis positing cardiac glycosides as potential anticancer agents [5]. In 1950, avenosides A and B, both sharing the distinctive l-acovenose moiety, were isolated from the South African poisonous bush Acokanthera oppositifolia [7]. Subsequent phytochemistry research paved the way for the isolation and characterization of acospectoside A and acobioside A (Figure 1) [8,9]. Notably, acospectoside A (1) could be metabolically converted to acovenoside B (2), acobioside A, and acovenoside A through enzymatic treatment with a snail (Helix pomatia) enzyme (Figure 1) [10,11]. These cardiac glycosides were further evaluated for their cardiotoxicity in cats and cytotoxicity against human carcinoma of the nasopharynx cells [10]. These findings indicated that the C-1 acetoxylated glycosides exhibited reduced activity compared to their hydroxylated counterparts, while monosides demonstrated greater cytotoxicity than the corresponding biosides. Amongst these cardiac glycosides, acovenoside A emerged as a potent inhibitor of the proliferation of human non-small-cell lung cancer (NSCLC) A-549 cells with an IC50 value of 68 nM, outperforming the anticancer drug doxorubicin (IC50 = 426 nM) [12]. However, the limited accessibility of these heterogeneous metabolites from natural sources has impeded in-depth investigations into their pharmaceutical potential and therapeutic applications. The chemical synthesis of these highly intricate cardiac glycosides presents a highly effective and transformative approach, offering a scalable and reproducible avenue for accessing these bioactive natural products and their structurally optimized derivatives [13,14,15,16]. Very recently, we developed a convenient approach to the first chemical synthesis of acovenoside A, oppofrioside, euonymuside A, and their congeners [17]. Here, we disclose the first total synthesis of acospectoside A (1) and acovensoide B (2), with 1-O-Ac-acovenosigenin A (3) as a common aglycone.
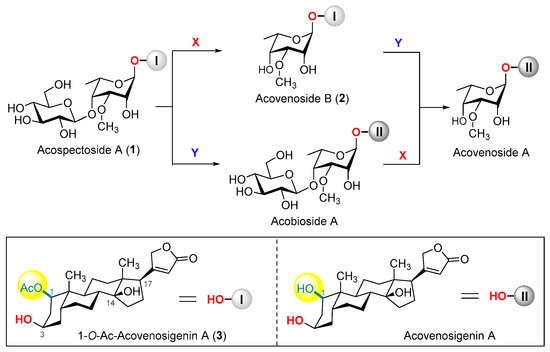
Figure 1.
Chemical structures of acospectoside A (1), acovenoside B (2), acobioside A, acovenoside A, and their conversions. (X = removal of d-glucose with β-glucosidase; Y = deacetylation with esterase).
2. Results
Building upon our prior investigations into the synthesis of steroid glycosides [17,18,19], we envisioned the assembly of acovenosides (1, 2) through late-stage glycosylation, employing 1-O-Ac-acovenosigenin A (3) and glycosyl imidate donors (Scheme 1). However, our initial endeavors to achieve the selective acetylation of acovenosigenin A were unsuccessful in yielding the desired aglycone 3. Consequently, a retrosynthetic analysis was undertaken, revealing that aglycone 3 could be elaborated on from the known intermediate (6) [17] via the introduction of the 1-O-acetyl group, modulating oxidation states at C-14, and installing the butenolide ring at C-17. A reduction of the 3-ketone group, followed by selective benzylation, led to the formation of intermediate 5. This intermediate was then transformed into 17-ketone 4 through a sequence involving Saegusa–Ito oxidation and Mukaiyama hydration. Finally, the introduction of the C-17 butenolide moiety and selective debenzylation afforded 1-OAc-acovenosigenin A (3). The timing of introducing the 1-O-acetyl group is of strategic importance, and a successful synthetic route could be determined by trial-and-error.
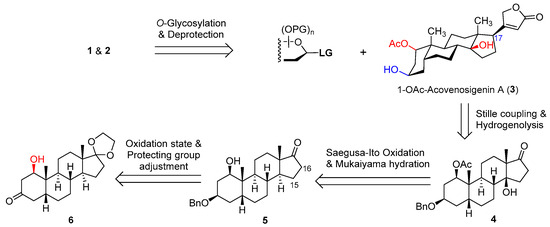
Scheme 1.
Retrosynthetic plan for acospectoside A (1) and acovenoside B (2).
The selective reduction of 3-ketone 6 with l-selectride [20], followed by oxidative cleavage of the resulting boronic ester with a sodium hydroxide solution and hydrogen peroxide afforded 1β,3β-diol 7 (96%) (Scheme 2). Selective 3-O-benzylation with benzyl bromide and sodium hydride delivered the desired 1-ol 8 (76%), alongside 3-ol isomer 9 (15%). Acidic hydrolysis of the C17-ethylene glycol ketal in 8 furnished 17-ketone 5 (82%). The treatment of ketone 5 with TMSOTf and triethylamine generated the corresponding trimethylsilylenol ether. Subsequent palladium-mediated Saegusa–Ito oxidation, utilizing O2 as a stoichiometric oxidant to form the 15,16-unsaturated enone 10, required extensive optimization. The conventional Saegusa–Ito oxidation protocol typically necessitates a superstoichiometric quantity of palladium(II) acetate to achieve satisfactory yields [21]. In 1995, Larock and co-workers pioneered a catalytic variant of Saegusa–Ito oxidation using palladium(II) acetate under an oxygen atmosphere [22]. Applying Larock’s protocol to the cyclic silylenol ether, with catalytic palladium(II) acetate (0.1 equiv.) and O2 as the reoxidant in DMSO, afforded the desired 15,16-unsaturated enone 10 in a moderate 45% yield, alongside 12% recycled 5 (attributed to the hydrolysis of the trimethylsilyl enol ether). Concurrently, lactone 11 was isolated as a side product (26%), arising from a Baeyer–Villiger-type rearrangement of the 17-ketone followed by the unsaturation of the α,β-position of carbonyl via β-hydride elimination of palladium in one-pot [23]. Given that elevating the temperature could mitigate the formation of lactone byproducts, the Saegusa–Ito oxidation was conducted at 60 °C, yielding enone 10 (33%) and lactone 11 (5%), though nearly half of 17-ketone 5 was recycled. To enhance the conversion rate, the amount of palladium(II) acetate was further increased to 0.3 equivalents. Optimal results were achieved within 1 h, delivering enone 10 in a good 74% yield, with 15% of 17-ketone 5 recycled. Extending the reaction time to 6 h only marginally improved the conversion rate, with 7% of 17-ketone 5 recycled, while enone 10 (33%), lactone 11 (5%), and unconjugated Δ14,15 12 (7%) were also obtained.
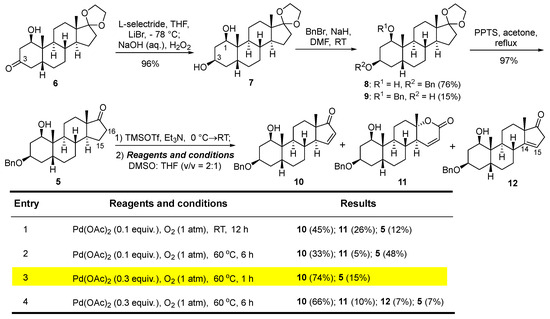
Scheme 2.
Synthesis of enone 10.
The isomerization of 10 was carried out in the presence of N,N-diisopropylethylamine and silicon dioxide at 90 °C, yielding unconjugated Δ14,15 derivative 12 (82%) (Scheme 3). The strategic introduction of the 1-O-acetyl group was successfully achieved by employing isopropenyl acetate and p-toluenesulfonic acid monohydrate, resulting in the formation of the fully protected intermediate 13 (81%). Mukaiyama hydration of Δ14,15 13, utilizing Mn(acac)2 and PhSiH3 under aerobic conditions, furnished tertiary alcohols 4β (68%) and 4α (32%) on a gram scale, showcasing the efficiency and scalability of the reaction [24]. 17-Ketone 4β was subsequently converted to vinyl iodide 14 (81%) with hydrazine hydrate and iodine. The Stille coupling reaction of vinyl iodide 14 with the known stannylated butanolide 15 catalyzed by Pd(PPh3)4 proceeded effectively, affording the desired cardiac steroid 16 in an excellent 89% yield [19]. The direct hydrogenation of the Δ16,17 double bond in 16 posed a risk of generating C-17 isomers. To circumvent this, the 14β-OH group in 16 was protected with TMSCl and imidazole, yielding intermediate 17 (83%). The subsequent hydrogenation of the Δ16,17 double bond in 17 proceeded smoothly on a gram scale, leading to the fully protected aglycone 18 (68%). Finally, the desired aglycone 3 was obtained through Pd/C-catalyzed debenzylation under atmospheric hydrogen in a mixed solvent of chloroform and methanol (78%). This sequence of reactions highlights the meticulous planning and optimization required to achieve the targeted cardiac steroid with high efficiency and selectivity.
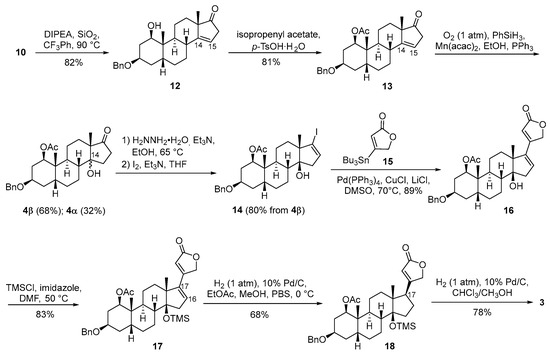
Scheme 3.
Synthesis of 1-O-Ac-acovenosigenin A (3).
In 1969, Kapadia speculated that the observed difficulty of the saponification of 3-O-acetyl groups in acospectoside A (1) and acovenoside B (2) could likely be attributed to the steric hindrance imposed by an axially bound acetoxyl group [10]. The subsequent experimental transformations revealed that acetyl groups situated within the equatorial positions of the carbohydrate moieties could be selectively removed through mild saponification using potassium bicarbonate, without any discernible impact on the 3-O-acetyl groups in the aglycone moieties [10]. With the desired aglycone derivative 3 and monosaccharide building blocks in hand, we embarked on the assembly of the target cardiac glycosides (Scheme 4). The known lactol 19 was transformed into an imidate donor with trichloroacetonitrile and cesium carbonate. The resulting reactive intermediate was promptly subjected to glycosylation with aglycone 3, promoted by TMSOTf, to afford glycoside 20 in a 50% yield over a two-step sequence. Finally, hydrogenative debenzylation and selective debenzoylation of the sugar residue were executed, culminating in the successful synthesis of acovenoside B (2) in a satisfactory 60% yield.
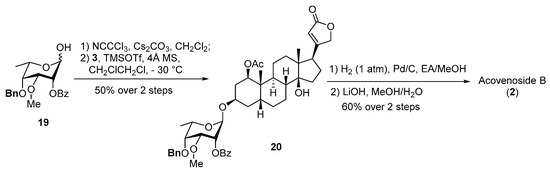
Scheme 4.
Synthesis of acovenoside B (2).
The known thioglycoside 21 underwent efficient debenzylation upon treatment with 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ), yielding 4-ol 22 (90%). The condensation of 22 with imidate 23 was complete within 2 h catalyzed by TMSOTf at −78 °C, and the desired disaccharide 24 was obtained in a good 76% yield (Scheme 5). Recognizing that debenzoylation posed a greater synthetic challenge than deacetylation, we strategically converted the benzoyl protecting groups in 24 to acetyl groups through a meticulous two-step sequence involving saponification and subsequent acetylation with isopropenyl acetate, affording disaccharide 26. The selective hydrolysis of the anomeric thio group was achieved using N-bromosuccinimide (NBS), furnishing lactol 27 in an excellent 93% yield. Lactol 27 was then condensed with trichloroacetonitrile in the presence of cesium carbonate to generate the disaccharide imidate donor, which was immediately coupled with aglycone 3 to produce bioside 28 (51% over two steps). Finally, hydrogenative debenzylation and selective deacetylation of the disaccharide residue furnished acospectoside A (1), with no influence on the 1-O-acetyl group. The NMR data of the synthetic acospectoside A (1) and acovenoside B (2) were identical to those reported for the natural products (see Tables S1 and S2 for details) [25].
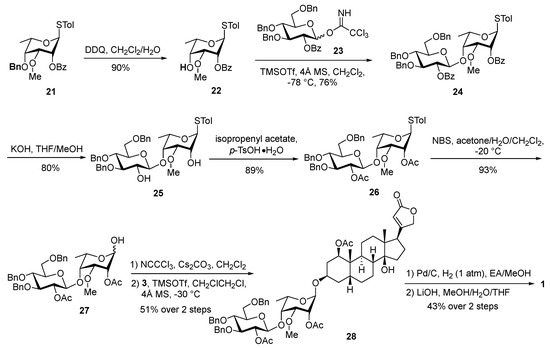
Scheme 5.
Synthesis of acospectoside A (1).
3. Experimental Section
3.1. General Information
Commercial reagents were used without further purification and made in China unless specified. Crushed 4 Å molecular sieves (MSs) were activated through flame-drying under high vacuum conditions immediately prior to use. Dry CH2Cl2, DMF, and toluene were obtained by drying with activated MSs. Dry pyridine and NEt3 were obtained by drying with anhydrous KOH. Anhydrous THF was obtained by refluxing with Na under an argon atmosphere. Thin layer chromatography (TLC) was performed on TLC silica gel 60 F254 (Merck, Darmstadt, Germany). The TLC plates were visualized with UV light and/or by staining with EtOH/H2SO4 (10%, v/v). Flash column chromatography was performed on silica gel, SiliaFlash P60 (40–63 μm, Silicycle, Quebec, QC, Canada). The NMR spectra were measured on a Bruker AM 400 (Zurich, Switzerland), Agilent 500 MHz (Santa Clara, CA, USA) NMR spectrometer at 25 °C. 1H and 13C NMR signals were calibrated to the residual proton and carbon resonance of the solvent (CDCl3: δH = 7.26 ppm, δC = 77.16 ppm; CD3OD: δH = 3.31 ppm, δC = 49.00 ppm; C5D5N: δH =8.74 ppm, δC = 155.35 ppm). High-resolution mass spectra were recorded with Shimadzu Biotech Axima Performance FTMS (Kyoto, Japan), maXis 4G FTMS, Thermo Scientific Q Exactive HF Orbitrap-FTMS (Waltham, MA, USA), or Agilent-TOF/LC-MS 1260-6230 FTMS. Optical rotations were measured on an Anton Paar MCP5500 polarimeter (Graz, Austria).
3.2. Synthesis of Compounds 1–28
3.2.1. Synthesis of Compound 7
To a mixture of compound 6 (5.2 g, 14.9 mmol, 1.0 equiv.) and LiBr (7.8 g, 89.5 mmol, 6.0 equiv.), dry THF (150 mL) was added under an atmosphere of Ar. l-selectride (22.4 mL, 1 M, 1.5 equiv., 0.5 mL/min) was added dropwise at −78 °C to the resulting mixture under an Ar atmosphere and stirred for 2 h at the same temperature. TLC indicated the reaction was complete (petroleum ether/EtOAc = 2/1). The reaction mixture was quenched with a 2 N NaOH aqueous solution (75 mL) and 30% H2O2 (7.5 mL). The mixture was extracted with EtOAc (150 mL × 3), and the organic phase was washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc/CH2Cl2 = 2/1/0.6) to give compound 7 (5.0 g, 96%) as a white amorphous solid. [α = −19.6 (c 1.0, CHCl3); 1H NMR (600 MHz, CDCl3) δ 4.13 (s, 1H), 3.95–3.79 (m, 5H), 3.42 (s, 2H), 2.09–1.89 (m, 4H), 1.86–1.69 (m, 3H), 1.68–1.60 (m, 1H), 1.53–1.31 (m, 7H), 1.28–1.18 (m, 4H), 1.15–1.10 (m, 1H), 1.09 (s, 3H), 0.82 (d, J = 1.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 119.6, 74.0, 68.5, 65.3, 64.6, 50.5, 45.9, 41.9, 39.8, 36.2, 34.2, 33.7, 32.3, 31.0, 30.7, 26.0, 25.3, 22.7, 20.5, 19.0, 14.6; HR-ESI-MS (m/z) calcd for C21H34O4Na [M+Na]+: 373.2349, found: 373.2347.
3.2.2. Synthesis of Compound 8 and 9
To a stirring solution of compound 7 (5.0 g, 14.4 mmol, 1.0 equiv.) and BnBr (1.7 mL, 14.4 mmol, 1.0 equiv.) in dry DMF (140 mL), 60% NaH (1.4 g, 36.0 mmol, 2.5 equiv.) was added in portions at room temperature. And the reaction mixture was stirred for 2 h at the same temperature. TLC indicated the reaction was complete. The reaction mixture was quenched with a saturated NH4Cl solution (20 mL). H2O (100 mL) was added to the mixture; the mixture extracted with EtOAc (300 mL), and the organic phase was washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 10/1 → 4/1) to give compound 8 (4.8 g, 76%) and to give compound 9 (923 mg, 15%) as white amorphous solids.
8: [α = −19.0 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.37–7.26 (m, 5H), 4.56–4.48 (m, 2H), 3.99 (d, J = 9.7 Hz, 1H), 3.95–3.82 (m, 6H), 3.71 (dt, J = 9.7, 3.0 Hz, 1H), 2.10 (dq, J = 14.8, 2.8 Hz, 1H), 2.02–1.92 (m, 2H), 1.91–1.86 (m, 1H), 1.86–1.74 (m, 2H), 1.70 (dt, J = 15.2, 3.1 Hz, 1H), 1.67–1.59 (m, 2H), 1.55–1.36 (m, 6H), 1.34–1.19 (m, 4H), 1.10 (s, 3H), 0.84 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 138.1, 128.6, 127.8, 127.7, 119.6, 76.0, 73.1, 70.7, 65.3, 64.6, 50.6, 45.9, 42.1, 40.0, 36.2, 34.2, 31.1, 31.0, 30.1, 29.9, 26.2, 25.3, 22.8, 20.4, 18.9, 14.6; HR-ESI-MS (m/z) calcd for C28H40O4Na [M+Na]+: 463.2819, found: 463.2823.
9: [α = −42.1 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.36–7.31 (m, 4H), 7.31–7.27 (m, 1H), 4.69 (d, J = 11.0 Hz, 1H), 4.40 (d, J = 11.0 Hz, 1H), 4.33 (d, J = 10.4 Hz, 1H), 3.98 (dt, J = 10.6, 3.0 Hz, 1H), 3.94–3.81 (m, 4H), 3.60 (s, 1H), 2.16–2.04 (m, 2H), 2.02–1.91 (m, 2H), 1.86–1.73 (m, 2H), 1.70–1.59 (m, 2H), 1.55–1.35 (m, 6H), 1.32–1.20 (m, 5H), 1.11 (s, 3H), 0.84 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 138.0, 128.6, 128.1, 127.9, 119.5, 82.8, 73.0, 68.0, 65.3, 64.6, 50.6, 45.9, 41.6, 40.2, 36.0, 34.2, 33.9, 31.3, 30.9, 28.6, 25.9, 25.2, 22.7, 20.7, 18.9, 14.6; HR-ESI-MS (m/z) calcd for C28H40O4Na [M+Na]+: 463.2819, found: 463.2822.
3.2.3. Synthesis of Compound 5
To a stirring solution of 8 (4.7 g, 10.7 mmol, 1.0 equiv.) in acetone (11 mL), pyridinium p-toluenesulfonate (4.0 g, 16.1 mmol, 1.5 equiv.) was added. The resulting mixture was heated to reflux for 3 h. TLC indicated the reaction was complete. The reaction mixture was concentrated under reduced pressure and added to a 1 N HCl aqueous solution (100 mL). The resulting mixture was extracted with CH2Cl2 (100 mL × 3); the combined organic phase was washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 10/1) to give 5 (4.1 g, 97%) as a white amorphous solid. [α = +55.6 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.36–7.26 (m, 5H), 4.58–4.49 (m, 2H), 4.03 (d, J = 9.7 Hz, 1H), 3.93–3.87 (m, 1H), 3.71 (dt, J = 9.8, 3.1 Hz, 1H), 2.43 (ddd, J = 19.1, 8.9, 1.1 Hz, 1H), 2.12 (dq, J = 14.9, 2.9 Hz, 1H), 2.07–2.00 (m, 2H), 1.96–1.85 (m, 3H), 1.81 (dt, J = 12.8, 3.0 Hz, 1H), 1.73–1.62 (m, 3H), 1.60–1.55 (m, 1H), 1.51 (ddd, J = 12.5, 9.1, 3.4 Hz, 1H), 1.48–1.42 (m, 1H), 1.40–1.29 (m, 3H), 1.28–1.15 (m, 3H), 1.13 (s, 3H), 0.86 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 221.3, 138.0, 128.6, 128.6, 127.9, 127.7, 75.8, 72.9, 70.8, 51.5, 47.7, 42.4, 40.2, 35.9, 35.6, 31.9, 31.1, 30.0, 26.1, 25.0, 21.9, 20.2, 18.9, 14.0; HR-ESI-MS (m/z) calcd for C26H36O3Na [M+Na]+: 419.2557, found: 419.2553.
3.2.4. Synthesis of Compounds 10 and 11
To a stirring solution of 5 (115 mg, 0.290 mmol, 1.0 equiv.) in dry CH2Cl2 (6 mL), NEt3 (0.161 mL, 1.02 mmol, 4.0 equiv.), and TMSOTf (0.185 mL, 0.185 mmol, 3.5 equiv.) were added successively at 0 °C under an Ar atmosphere. The mixture was gradually warmed up to room temperature and stirred for 1 h. TLC indicated the reaction was complete. The reaction mixture was quenched with a saturated NaHCO3 solution (15 mL) at 0 °C and extracted with CH2Cl2 (15 mL × 3); the combined organic phase was washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The resulting residue was dissolved in dry DMSO (4 mL) and THF (2 mL). Pd(OAc)2 (6.5 mg, 0.029 mmol, 0.1 equiv.) was added to the solution. The mixture was heated to 60 °C for 12 h under an O2 atmosphere. TLC indicated the reaction was complete (petroleum ether/EtOAc = 2/1). The reaction mixture was added to a saturated NH4Cl solution (20 mL) and extracted with EtOAc (20 mL × 3); the combined organic phase was washed with an aqueous NH4Cl, brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was dissolved with acetonitrile (20 mL). To the solution, FeCl3 (49 mg, 0.304 mmol, 1.05 equiv.) was added at 0 °C and stirred for 10 min. The mixture was neutralized by K2CO3 (120 mg, 0.870 mmol, 3.0 equiv.) and filtered; the filtrate was concentrated under reduced pressure and purified by silica gel column chromatography (petroleum ether/EtOAc = 6/1 → 4/1) to give 10 (51 mg, 45%), 11 (31 mg, 26%), and 5 (14 mg, 12%) as white amorphous solids.
10: [α = −55.2 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.52 (dd, J = 6.0, 1.8 Hz, 1H), 7.38–7.27 (m, 5H), 6.02 (dd, J = 5.9, 3.1 Hz, 1H), 4.59–4.50 (m, 2H), 4.09 (d, J = 9.6 Hz, 1H), 3.91 (q, J = 3.0 Hz, 1H), 3.72 (dt, J = 9.7, 3.0 Hz, 1H), 2.32 (ddd, J = 11.4, 3.1, 1.9 Hz, 1H), 2.15 (dq, J = 14.9, 2.9 Hz, 1H), 2.11–2.03 (m, 1H), 1.99–1.85 (m, 4H), 1.81–1.73 (m, 1H), 1.71–1.64 (m, 2H), 1.57–1.45 (m, 3H), 1.42–1.24 (m, 3H), 1.18 (s, 3H), 1.07 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 213.3, 158.6, 137.9, 131.8, 128.6, 127.9, 127.7, 75.7, 72.7, 70.8, 57.0, 51.0, 43.7, 40.4, 33.0, 31.0, 29.9, 29.8, 29.4, 26.0, 25.1, 20.8, 19.9, 18.8; HR-ESI-MS (m/z) calcd for C26H34O3Na [M+Na]+: 417.2400, found: 417.2387.
11: [α = −24.2 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 6.81 (dd, J = 9.9, 2.2 Hz, 1H), 6.02 (dd, J = 9.8, 3.0 Hz, 1H), 4.59–4.49 (m, 2H), 3.95–3.90 (m, 1H), 3.72 (d, J = 3.1 Hz, 1H), 2.33 (dt, J = 11.4, 2.5 Hz, 1H), 2.18–2.12 (m, 1H), 2.09–2.02 (m, 1H), 1.99 (dt, J = 12.6, 3.5 Hz, 1H), 1.93–1.82 (m, 3H), 1.76 (dd, J = 13.2, 4.4 Hz, 1H), 1.72 (s, 2H), 1.64–1.58 (m, 1H), 1.59–1.48 (m, 1H), 1.49–1.40 (m, 1H), 1.41–1.35 (m, 1H), 1.32 (s, 3H), 1.28–1.21 (m, 1H), 1.18 (dd, J = 12.2, 4.0 Hz, 1H), 1.09 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 164.0, 145.4, 137.9, 128.6, 127.9, 127.7, 121.8, 83.3, 75.6, 72.5, 70.9, 48.6, 41.9, 40.2, 38.4, 35.8, 30.6, 30.0, 29.8, 25.9, 24.6, 21.7, 18.7, 18.6; HR-ESI-MS (m/z) calcd for C26H35O4 [M+H]+: 411.2530, found: 411.2523.
To a stirring solution of 5 (15.0 g, 37.8 mmol, 1.0 equiv.) in dry CH2Cl2 (120 mL), NEt3 (21.0 mL, 151 mmol, 4.0 equiv.), and TMSOTf (23.9 mL, 132 mmol, 3.5 equiv.) were added successively at 0 °C under an Ar atmosphere. The mixture was gradually warmed up to room temperature and stirred for 1 h. TLC indicated the reaction was complete. The reaction mixture was quenched with a saturated NaHCO3 solution (100 mL) at 0 °C and the aqueous phase was extracted with CH2Cl2 (100 mL × 2). The combined organic phase was washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The resulting residue was dissolved in dry DMSO (280 mL) and THF (140 mL). Pd(OAc)2 (2.5 g, 11.3 mmol, 0.3 equiv.) was added to the solution. The mixture was heated to 60 °C for 12 h under an O2 atmosphere. TLC indicated the reaction was complete (petroleum ether/EtOAc = 2/1). FeCl3 (6.4 g, 39.7 mmol, 1.05 equiv.) was added to the reaction mixture at 0 °C and stirred for 10 min. The mixture was neutralized by K2CO3 (16.4 g, 119.0 mmol) and filtered; the filtrate was concentrated under reduced pressure and purified by silica gel column chromatography (petroleum ether/EtOAc/CH2Cl2 = 8/1/0.9 → 4/1/0.9) to give 10 (11.0 g, 74%) and 5 (2.3 g, 15%) as white amorphous solids.
3.2.5. Synthesis of Compound 12
To a mixture of 10 (2.9 g, 7.40 mmol, 1.0 equiv.) and SiO2 (8.0 g), benzotrifluoride (80 mL) and DIPEA (67.3 mL, 407 mmol, 55.0 equiv.) were added successively under an atmosphere of Ar. The resulting mixture was stirred for 2 h at 90 °C under an Ar atmosphere. TLC indicated the reaction was complete (petroleum ether/EtOA = 2/1). The reaction mixture was filtered, and the filter cake was washed with EtOA (150 mL). The filtrate was concentrated under reduced pressure. The residue was dissolved with CH2Cl2 (100 mL), and the organic phase was washed with an aqueous 5 wt% HCl solution, brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 10:1) to give 12 (2.4 g, 82%) as a white amorphous solid. [α = +84.3 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.39–7.27 (m, 5H), 5.50 (q, J = 2.1 Hz, 1H), 4.59–4.48 (m, 2H), 4.03 (d, J = 9.7 Hz, 1H), 3.89 (t, J = 2.9 Hz, 1H), 3.73 (d, J = 9.3 Hz, 1H), 3.00 (ddd, J = 23.1, 3.9, 1.9 Hz, 1H), 2.82 (dt, J = 23.1, 2.3 Hz, 1H), 2.26 (ddd, J = 13.6, 9.0, 4.9 Hz, 1H), 2.16–2.09 (m, 1H), 2.07–2.01 (m, 1H), 1.99–1.83 (m, 2H), 1.78 (dt, J = 12.9, 3.2 Hz, 1H), 1.70–1.58 (m, 3H), 1.55–1.48 (m, 1H), 1.48–1.42 (m, 1H), 1.41–1.34 (m, 1H), 1.29–1.19 (m, 2H), 1.15 (s, 3H), 1.11 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 153.6, 138.0, 128.6, 127.9, 127.7, 113.1, 75.7, 72.7, 70.8, 50.9, 43.0, 41.5, 40.4, 35.8, 33.5, 31.1, 30.3, 29.8, 25.8, 22.7, 20.8, 20.1, 18.8; HR-ESI-MS (m/z) calcd for C26H34O3Na [M+Na]+: 417.2400, found: 417.2411.
3.2.6. Synthesis of Compound 13
To a stirring solution of compound 12 (2.4 g, 6.03 mmol, 1.0 equiv.) in isopropenyl acetate (12 mL), TsOH·H2O (4.6 g, 24.1 mmol, 4.0 equiv.) was added at room temperature. After, the reaction mixture was stirred for 10 min at the same temperature. TLC indicated the reaction was complete. CH2Cl2 (100 mL) was added, and the reaction mixture was quenched with a saturated NaHCO3 solution (100 mL) at 0 °C. The organic phase was washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 10/1) to give compound 13 (2.14 g, 81%) as a white amorphous solid. [α = +51.9 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.34–7.29 (m, 4H), 7.26–7.22 (m, 1H), 5.52 (q, J = 2.1 Hz, 1H), 4.97 (t, J = 3.0 Hz, 1H), 4.47–4.39 (m, 2H), 3.73 (q, J = 3.0 Hz, 1H), 3.00 (ddd, J = 23.0, 3.8, 1.8 Hz, 1H), 2.82 (dt, J = 23.1, 2.2 Hz, 1H), 2.32–2.19 (m, 3H), 1.99–1.95 (m, 1H), 1.94 (s, 3H), 1.93–1.87 (m, 2H), 1.80 (dt, J = 13.1, 3.1 Hz, 1H), 1.67–1.61 (m, 3H), 1.55 (dt, J = 15.7, 3.3 Hz, 1H), 1.50–1.31 (m, 3H), 1.23 (td, J = 13.1, 3.9 Hz, 1H), 1.11 (s, 3H), 1.05 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 222.3, 171.5, 153.4, 139.2, 128.4, 127.4, 127.3, 113.4, 74.2, 72.6, 69.9, 50.9, 42.3, 41.5, 39.0, 35.7, 33.4, 32.1, 30.5, 27.4, 25.6, 22.3, 21.4, 21.3, 20.2, 18.4; HR-ESI-MS (m/z) calcd for C28H36O4Na [M+Na]+: 459.2506, found: 459.2512.
3.2.7. Synthesis of Compound 4α and 4β
To the solution of 13 (5.0 g, 11.5 mmol, 1.0 equiv.) in EtOH (230 mL), PPh3 (6.03 g, 23.0 mmol, 2.0 equiv.), and Mn(acac)2 (1.46 g, 5.75 mmol, 0.5 equiv.) were added. The reaction mixture was bubbled with O2 for 40 min. Next, to the solution, PhSiH3 (4.25 mL, 34.5 mmol, 3.0 equiv.) was added. The resulting mixture was stirred for 3 h at room temperature under an O2 atmosphere. TLC indicated the reaction was complete (petroleum ether/acetone = 4/1). The reaction mixture was quenched with a saturated Na2S2O3 solution (25 mL) at 0 °C. H2O (400 mL) was carefully added to the reaction mixture at room temperature. The resulting mixture was extracted with EtOAc (400 mL × 2); the combined organic phase was washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/acetone = 8/1) to give 4β (3.6 g, 68%) and 4α (1.7 g, 32%) as white amorphous solids.
4β: [α = −4.7 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.35–7.30 (m, 4H), 7.26–7.23 (m, 1H), 4.95 (t, J = 3.1 Hz, 1H), 4.48–4.41 (m, 2H), 3.79–3.74 (m, 1H), 2.44–2.39 (m, 2H), 2.30–2.24 (m, 1H), 2.23–2.10 (m, 3H), 1.94 (s, 2H), 1.93–1.80 (m, 3H), 1.78–1.66 (m, 3H), 1.64–1.50 (m, 4H), 1.44–1.34 (m, 3H), 1.30–1.21 (m, 2H), 1.05 (s, 2H), 1.00 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 221.0, 171.5, 139.1, 128.4, 127.4, 127.3, 82.5, 74.2, 72.4, 70.0, 41.6, 41.5, 38.8, 37.4, 33.1, 32.0, 31.7, 30.5, 27.5, 27.5, 25.8, 21.4, 20.4, 18.9, 18.5, 13.0; HR-ESI-MS (m/z) calcd for C28H42O5N [M+NH4]+: 472.3057, found: 472.3050.
4α: [α = +11.2 (c 1.0, CHCl3); 1H NMR (600 MHz, CDCl3) δ 7.32–7.30 (m, 3H), 7.27–7.22 (m, 2H), 4.98–4.94 (m, 1H), 4.51–4.34 (m, 2H), 3.77–3.73 (m, 1H), 2.41 (ddd, J = 18.7, 9.5, 2.2 Hz, 1H), 2.33 (dt, J = 18.6, 8.6 Hz, 1H), 2.29–2.22 (m, 1H), 2.22–2.17 (m, 1H), 2.02–1.94 (m, 2H), 1.94 (s, 3H), 1.92–1.82 (m, 4H), 1.76 (td, J = 13.4, 4.5 Hz, 1H), 1.68–1.62 (m, 2H), 1.60–1.49 (m, 2H), 1.48–1.26 (m, 4H), 1.03 (s, 3H), 0.99 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 218.7, 171.5, 139.2, 128.4, 127.4, 127.3, 81.4, 74.6, 72.6, 69.9, 52.6, 38.8, 38.2, 34.6, 33.1, 32.0, 30.5, 30.5, 27.2, 25.6, 25.1, 21.4, 19.5, 19.3, 18.5, 18.2; HR-ESI-MS (m/z) calcd for C28H38O5Na [M+Na]+: 477.2611, found: 477.2612.
3.2.8. Synthesis of Compound 14
To a stirring solution of 4β (4.9 g, 10.7 mmol, 1.0 equiv.) in EtOH (110 mL), Et3N (4.5 mL, 32.1 mmol, 3.0 equiv.) and H2NNH2·H2O (1.6 mL, 32.1 mmol, 3.0 equiv.) were added. The mixture was stirred at 65 °C for 5 h. TLC indicated the reaction was complete (CH2Cl2/MeOH = 20/1). The reaction mixture was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 60/1 → 20/1) to give intermediate hydrazone (4.4 g, 88%) as a white amorphous solid.
To the intermediate hydrazone (4.4 g, 9.5 mmol, 1.0 equiv.) in dry THF (100 mL), Et3N (13.1 mL, 94.5 mmol, 10.0 equiv.) and a solution of I2 (6.0 g, 23.6 mmol, 2.5 equiv.) in THF (100 mL) were added successively. The resulting mixture was stirred at 25 °C for 30 min under an Ar atmosphere. TLC indicated the reaction was complete (CH2Cl2/MeOH = 20/1). The reaction mixture was quenched with a saturated NaHCO3 solution (100 mL) and a Na2SO3 solution (100 mL) at 0 °C, and EtOAc (200 mL) was added. The mixture was extracted with EtOAc (100 mL × 2); the combined organic phase was washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 8/1) to give 14 (4.9 g, 91%) as a white foamy solid. [α = −9.54 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) 1H NMR (500 MHz, CDCl3) δ 7.34–7.29 (m, 4H), 7.26–7.22 (m, 1H), 6.11 (dd, J = 3.2, 1.8 Hz, 1H), 5.00–4.96 (m, 1H), 4.49–4.39 (m, 2H), 3.78–3.72 (m, 1H), 2.54 (dd, J = 16.5, 1.9 Hz, 1H), 2.25 (ddd, J = 16.5, 7.1, 3.0 Hz, 2H), 2.18 (dd, J = 13.4, 3.6 Hz, 1H), 1.95 (s, 3H), 1.91 (dd, J = 13.8, 3.6 Hz, 1H), 1.88–1.70 (m, 4H), 1.67–1.59 (m, 2H), 1.51–1.39 (m, 2H), 1.38–1.24 (m, 2H), 1.14–1.07 (m, 1H)), 1.05 (s, 3H), 0.99 (s, 3H), 0.97–0.90 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 171.5, 139.2, 133.7, 128.4, 127.4, 127.3, 111.4, 82.4, 74.3, 72.5, 69.9, 54.8, 42.8, 41.3, 38.8, 37.9, 37.5, 31.7, 30.5, 27.6, 25.8, 21.5, 20.4, 20.2, 18.7, 18.1; HR-ESI-MS (m/z) calcd for C28H37O4INa [M+Na]+: 587.1629, found: 587.1633.
3.2.9. Synthesis of Compound 16
A mixture of 14 (4.0 g, 7.1 mmol, 1.0 equiv.) and 16 (8.0 g, 21.3 mmol, 3.0 equiv.) was co-evaporated with toluene twice and recharged with Ar. CuCl (10.5 g, 106.0 mmol, 15.0 equiv.), LiCl (6.02 g, 142 mmol, 20.0 equiv.), and Pd(Ph3P)4 (819 mg, 0.709 mmol, 0.1 equiv.) were added. The resulting mixture was recharged with Ar and dry DMSO (150 mL) was added. The mixture was stirred for 2 h at 70 °C under an Ar atmosphere after Freeze–Pump–Thaw Degassing was performed twice. TLC indicated the reaction was complete (PE/EA = 2/1). The reaction mixture was quenched with a PBS (350 mL) at 0 °C. The mixture was filtered. The filter cake was washed with EtOAc (200 mL × 5). The aqueous phase was extracted with EtOAc (300 mL × 2); the combined organic phase was washed with a saturated NH4Cl solution (800 mL × 2) and brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (containing 10% K2CO3, and petroleum ether/EtOAc/CH2Cl2 = 2/1/0.2) to give compound 16 (3.3 g, 89%) as a pale yellow amorphous solid. [α = +37.5 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.36–7.29 (m, 4H), 7.24 (d, J = 6.1 Hz, 1H), 6.10 (d, J = 2.8 Hz, 1H), 5.96 (s, 1H), 5.02–4.89 (m, 3H), 4.49–4.39 (m, 2H), 3.75 (s, 1H), 2.73–2.64 (m, 1H), 2.39 (dd, J = 18.4, 3.4 Hz, 1H), 2.26 (dd, J = 15.9, 2.8 Hz, 1H), 2.20 (d, J = 13.3 Hz, 1H), 2.06–1.98 (m, 1H), 1.95 (s, 3H), 1.91–1.72 (m, 3H), 1.69–1.59 (m, 3H), 1.51–1.33 (m, 4H), 1.28 (s, 3H), 1.18–1.05 (m, 1H), 1.01 (s, 3H), 0.95–0.88 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 174.5, 171.5, 158.3, 144.0, 139.1, 132.2, 128.4, 127.4, 127.2, 112.6, 85.5, 74.2, 72.4, 71.8, 69.9, 52.2, 41.0, 40.6, 38.7, 38.4, 37.8, 31.6, 30.5, 27.6, 25.8, 21.4, 20.4, 20.3, 18.7, 16.7; HR-ESI-MS (m/z) calcd for C32H40O6Na [M+Na]+: 543.2717, found: 543.2715.
3.2.10. Synthesis of Compound 17
To a mixture of 16 (2.0 g, 3.8 mmol, 1.0 equiv.) and imidazole (1.6 g, 23.0 mmol, 6.0 equiv.), dry DMF (40 mL) was added under an atmosphere of Ar. TMSCl (1.5 mL, 11.5 mmol, 3.0 equiv.) was added to the resulting mixture under an Ar atmosphere and stirred for 12 h at 50 °C. TLC indicated the reaction was complete (petroleum ether/EtOAc = 1/1.5). The reaction mixture was quenched with MeOH (5 mL) at 0 °C. The mixture was extracted with CH2Cl2 (200 mL), washed with brine (200 mL × 3), and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 3/1) to give 17 (1.9 g, 83%) as a white foamy solid. [α = +39.6 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.34–7.26 (m, 5H), 6.03 (d, J = 2.8 Hz, 1H), 5.96 (s, 1H), 5.03–4.86 (m, 3H), 4.49–4.39 (m, 2H), 3.74 (d, J = 3.9 Hz, 1H), 2.59 (d, J = 18.5 Hz, 1H), 2.44 (dd, J = 18.6, 3.4 Hz, 1H), 2.25 (dd, J = 15.8, 2.8 Hz, 1H), 2.19 (d, J = 14.2 Hz, 1H), 1.98 (d, J = 3.6 Hz, 1H), 1.94 (s, 3H), 1.92–1.79 (m, 2H), 1.78–1.68 (m, 2H), 1.67–1.55 (m, 3H), 1.42–1.34 (m, 2H), 1.28–1.23 (m, 1H), 1.20 (s, 3H), 1.17–1.11 (m, 1H), 1.05 (d, J = 13.7 Hz, 1H), 1.00 (s, 3H), 0.00 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 174.5, 171.5, 158.4, 145.0, 139.2, 132.0, 128.4, 127.4, 127.3, 112.6, 89.8, 74.3, 72.5, 71.7, 69.9, 52.9, 42.0, 39.4, 38.7, 38.7, 37.8, 31.7, 30.5, 27.6, 26.0, 21.5, 20.7, 20.3, 18.8, 17.4, 2.8; HR-ESI-MS (m/z) calcd for C35H48O6SiNa [M+Na]+: 615.3112, found: 615.3120.
3.2.11. Synthesis of Compound 18
To a solution of 17 (1.9 g, 3.2 mmol, 1.0 equiv.) in a mixture of EtOAc/MeOH/PBS (60 mL/30 mL/60 mL), Pd/C (1.0 g) was added. The reaction mixture was recharged with H2 thrice, and then stirred for 3 h at 0 °C under an atmosphere of H2. TLC indicated the reaction was complete (petroleum ether/EtOAc = 2/1). The reaction mixture was filtered, and the filter cake was washed with EtOAc (100 mL × 3). The filtrate was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 2/1 → 1/1.5) to give 18 (1.3 g, 68%) as a colorless amorphous solid. [α = −4.35 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.34–7.29 (m, 4H), 7.26–7.24 (m, 1H), 5.84 (t, J = 1.5 Hz, 1H), 4.95–4.90 (m, 1H), 4.79–4.67 (m, 2H), 4.49–4.39 (m, 2H), 3.76 (t, J = 3.1 Hz, 1H), 2.56 (t, J = 7.6 Hz, 1H), 2.30–2.22 (m, 1H), 2.17 (d, J = 13.4 Hz, 1H), 2.10–2.02 (m, 1H), 1.98–1.95 (m, 1H), 1.94 (s, 3H), 1.93–1.85 (m, 2H), 1.84–1.69 (m, 4H), 1.67–1.55 (m, 3H), 1.50 (td, J = 11.8, 3.5 Hz, 1H), 1.43–1.33 (m, 3H), 1.32–1.18 (m, 2H), 0.97 (s, 3H), 0.88 (s, 3H), 0.13 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 174.4, 173.9, 171.5, 139.1, 128.4, 127.4, 127.3, 117.2, 91.3, 74.3, 74.0, 72.5, 70.0, 50.9, 50.6, 41.5, 40.9, 39.2, 38.2, 34.1, 31.7, 30.7, 27.4(2C), 26.1, 22.5, 21.5, 21.2, 18.4, 18.3, 3.1; HR-ESI-MS (m/z) calcd for C35H50O6SiNa [M+Na]+: 617.3269, found: 617.3275.
3.2.12. Synthesis of Compound 3
To a solution of 18 (1.5 g, 2.6 mmol, 1.0 equiv.) in a mixture of CHCl3/MeOH (60 mL/60 mL, Pd/C (0.8 g) was added. The reaction mixture was recharged with H2 thrice, and then stirred for 2 h at room temperature under an atmosphere of H2. TLC indicated the reaction was complete (petroleum ether/EtOAc = 1/1.5). The reaction mixture was filtered. The filtrate was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (CH2Cl2/MeOH = 30/1) to give 3 (869 mg, 78%) as a colorless amorphous solid. [α = +9.4 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 5.88 (s, 1H), 5.26 (d, J = 2.9 Hz, 1H), 4.97 (d, J = 18.0 Hz, 1H), 4.80 (d, J = 17.6 Hz, 1H), 4.04 (s, 1H), 2.80–2.75 (m, 1H), 2.23–2.11 (m, 1H), 2.09 (s, 3H), 2.06–1.79 (m, 6H), 1.77–1.68 (m, 2H), 1.66–1.59 (m, 2H), 1.58–1.49 (m, 2H), 1.47–1.26 (m, 6H), 0.96 (s, 3H), 0.87 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 174.6, 174.4, 169.9, 118.0, 85.4, 75.8, 73.6, 66.9, 50.8, 49.5, 41.8, 39.8, 39.1, 37.1, 33.3(2 C), 31.5, 31.1, 27.0, 25.8, 21.6, 21.4, 20.8, 18.5, 15.9; HR-ESI-MS (m/z) calcd for C25H36O6Na [M+Na]+: 455.2404, found: 455.2411.
3.2.13. Synthesis of Compound 20
To a stirring solution of 19 (10.0 mg, 0.027 mmol, 1.0 equiv.) in dry CH2Cl2 (7 mL), NCCCl3 (11 μL, 0.108 mmol, 4.0 equiv.) and Cs2CO3 (0.87 mg, 2.69 μmol, 0.1 equiv.) were added. The resulting mixture was stirred for 3 h at room temperature. TLC indicated the reaction was complete. The reaction mixture was filtered. The filtrate was concentrated in vacuo. The residue and aglycone 3 (9.7 mg, 0.022 mmol, 1.0 equiv.) were co-evaporated with toluene three times and dissolved in dry CH2Cl2 (1.5 mL); a 4Å molecular sieve (150 mg) was added. The mixture was stirred for 15 min at 25 °C under an Ar atmosphere. Next, TMSOTf (0.81 μL, 4.48 μmol, 0.2 equiv.) was added at −78 °C. The resulting mixture was stirred for 10 min at −78 °C. TLC indicated the reaction was complete (petroleum ether/EtOAc = 1/2). The reaction mixture was quenched with a saturated NaHCO3 aqueous solution (2 mL) and filtered. The filtrate was added to the saturated NaHCO3 solution (20 mL). The mixture was extracted with CH2Cl2 (20 mL × 3), washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 4/1) to give compound 20 (8.9 mg, 50%) as a white amorphous solid. [α = −34.5 (c 1.0, CHCl3); 1H NMR (600 MHz, CDCl3) δ 8.01–7.98 (m, 2H), 7.46–7.38 (m, 3H), 7.34–7.27 (m, 3H), 7.17–7.11 (m, 2H), 5.87 (t, J = 1.9 Hz, 1H), 5.43–5.34 (m, 1H), 5.00–4.94 (m, 3H), 4.91 (t, J = 3.0 Hz, 1H), 4.80 (dd, J = 18.1, 1.8 Hz, 1H), 4.62 (d, J = 11.6 Hz, 1H), 4.04 (s, 1H), 3.95–3.90 (m, 1H), 3.68–3.63 (m, 2H), 3.43 (s, 3H), 2.77 (dd, J = 9.5, 5.5 Hz, 1H), 2.19–2.10 (m, 2H), 2.08 (s, 3H), 2.07–1.99 (m, 2H), 1.91–1.76 (m, 4H), 1.74–1.65 (m, 3H), 1.64–1.57 (m, 3H), 1.57–1.50 (m, 2H), 1.48–1.42 (m, 1H), 1.39 (d, J = 15.3 Hz, 1H), 1.35 (d, J = 6.5 Hz, 3H), 1.30 (dt, J = 13.1, 3.4 Hz, 1H), 0.99 (s, 3H), 0.88 (s, 3H); δ 13C NMR (150 MHz, CDCl3) δ 174.5, 174.3, 171.0, 166.7, 139.3, 133.0, 130.4, 129.9, 128.3, 128.3, 128.0, 127.4, 118.0, 95.7, 85.5, 77.9, 76.4, 74.7, 74.3, 73.5, 69.3, 67.9, 67.3, 57.1, 50.9, 49.6, 41.7, 39.9, 38.8, 37.0, 33.3, 31.2, 30.0, 29.8, 28.0, 27.0, 25.8, 21.7, 20.7, 18.6, 17.1, 15.9; HR-ESI-MS (m/z) calcd for C46H58O11Na [M+Na]+: 809.3871, found: 809.3875.
3.2.14. Synthesis of Compound 2
To a solution of 20 (8.9 mg, 11.3 μmol, 1.0 equiv.) in EtOAc/MeOH (1.0 mL/1.0 mL), 10% Pd/C (8.9 mg) was added. The reaction mixture was recharged with H2 3 times, and then stirred for 18 h at 25 °C under an atmosphere of H2. TLC indicated the reaction was complete (petroleum ether/EtOAc = 1/2). The reaction mixture was filtered. The filtrate was concentrated under reduced pressure. The residue was dissolved in MeOH/H2O/THF (1.5 mL/0.5 mL/0.5 mL); LiOH (2.0 mg, 83.5 μmol, 7.4 equiv.) was added and then the mixture was stirred for 0.5 h at 25 °C. TLC indicated the reaction was complete (petroleum ether/EtOAc = 1/2). The reaction mixture was quenched with HOAc (4.8 μL, 83.5 μmol, 7.4 equiv.) and concentrated in vacuo. The residue was purified by reverse-phase columns (H2O/MeOH = 1/2) to afford acovensoide B (2) (4.0 mg, 60% over 2 steps) as a white amorphous solid. [α = −53.0 (c 0.4, CHCl3); 1H NMR (400 MHz, CDCl3) δ 5.88 (s, 1H), 5.01–4.93 (m, 2H), 4.88 (t, J = 3.2 Hz, 1H), 4.80 (d, J = 18.1 Hz, 1H), 4.04 (s, 1H), 3.86 (s, 1H), 3.84–3.76 (m, 2H), 3.47 (s, 3H), 3.34 (t, J = 3.2 Hz, 1H), 2.78 (dd, J = 9.3, 5.6 Hz, 1H), 2.22–2.03 (m, 3H), 2.01 (s, 3H), 1.97 (s, 1H), 1.88–1.68 (m, 7H), 1.58–1.49 (m, 4H), 1.46–1.39 (m, 1H), 1.38–1.32 (m, 2H), 1.29 (d, J = 6.5 Hz, 3H), 1.25–1.22 (m, 1H), 0.97 (s, 3H), 0.87 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 174.5, 174.3, 170.8, 117.9, 97.4, 85.4, 75.4, 74.2, 73.5, 70.1, 68.7, 68.6, 66.2, 55.6, 50.8, 49.5, 41.7, 39.8, 38.8, 36.9, 33.2, 31.1, 29.9, 27.8, 26.9, 25.8, 21.6, 21.5, 20.6, 18.5, 16.6, 15.8; HR-ESI-MS (m/z) calcd for C32H48O10Na [M+Na]+: 615.3140, found: 615.3142.
3.2.15. Synthesis of Compound 22
To a stirring solution of 21 (200 mg, 0.418 mmol, 1.0 equiv.) in a mixture of CH2Cl2/H2O (4 mL/0.4 mL), DDQ (285 mg, 1.3 mmol, 3.0 equiv.) was added. The resulting mixture was stirred for 12 h at room temperature. TLC indicated the reaction was complete (petroleum ether/EtOAc = 4/1). The reaction mixture was quenched with a saturated NaHCO3 solution (20 mL). The mixture was extracted with CH2Cl2 (20 mL × 3), washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 4/1) to give compound 22 (146 mg, 90%) as a pale yellow amorphous solid. [α = −106.8 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 8.02–7.99 (m, 2H), 7.59–7.54 (m, 1H), 7.44 (t, J = 7.8 Hz, 2H), 7.40–7.36 (m, 2H), 7.16–7.10 (m, 2H), 5.70 (dt, J = 3.5, 1.3 Hz, 1H), 5.56 (d, J = 1.4 Hz, 1H), 4.50–4.45 (m, 1H), 3.92 (ddt, J = 7.9, 3.1, 1.3 Hz, 1H), 3.66 (t, J = 3.5 Hz, 1H), 3.48 (s, 3H), 2.55 (d, J = 7.9 Hz, 1H), 2.33 (s, 3H), 1.40 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 165.6, 138.2, 133.6, 132.5, 130.1, 129.9, 129.8, 129.5, 128.7, 87.0, 75.2, 70.2, 69.6, 68.2, 56.5, 21.3, 16.5; HR-ESI-MS (m/z) calcd for C21H24O5SNa [M+Na]+: 411.1237, found: 411.1242.
3.2.16. Synthesis of Compound 24
To a stirring solution of 2-O-benzoyl-3,4,6-tri-O-benzyl-d-glucopyranose (223 mg, 0.402 mmol, 1.0 equiv.) in dry CH2Cl2 (4 mL), NCCCl3 (0.164 mL, 1.6 mmol, 4.0 equiv.) and Cs2CO3 (13.1 mg, 40.2 μmol, 0.1 equiv.) were added. The resulting mixture was stirred for 4 h at room temperature. TLC indicated the reaction was complete (petroleum ether/EtOAc = 4/1). The reaction mixture was filtered. The filtrate was concentrated in vacuo.
The residue and acceptor 22 (130 mg, 0.335 mmol, 1.0 equiv.) were co-evaporated with toluene thrice and dissolved in dry CH2Cl2 (3.5 mL); a 4 Å molecular sieve (350 mg) was added. The mixture was stirred for 15 min at 25 °C under an Ar atmosphere. Next, TMSOTf (0.81 μL, 4.48 μmol, 0.2 equiv.) was added at −78 °C. The resulting mixture was stirred for 2 h at −78 °C. TLC indicated the reaction was complete (petroleum ether/EtOAc = 3/1). The reaction mixture was quenched with a saturated NaHCO3 solution (2 mL) and filtered. The filtrate was added to the saturated NaHCO3 solution (20 mL). The mixture was extracted with CH2Cl2 (20 mL × 3), washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 6/1) to give compound 24 (234 mg, 76%) as a pale yellow foamy solid. [α = −57.0 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 8.31–8.25 (m, 2H), 8.17–8.11 (m, 2H), 7.75–7.65 (m, 4H), 7.55 (t, J = 7.8 Hz, 2H), 7.49–7.37 (m, 11H), 7.34 (dd, J = 7.3, 2.1 Hz, 2H), 7.26 (d, J = 3.0 Hz, 4H), 7.18 (d, J = 7.9 Hz, 2H), 5.58 (dd, J = 9.4, 8.0 Hz, 1H), 5.49–5.42 (m, 2H), 4.95 (d, J = 10.9 Hz, 1H), 4.88 (d, J = 11.1 Hz, 1H), 4.78 (d, J = 9.2 Hz, 1H), 4.76 (d, J = 6.0 Hz, 1H), 4.71 (d, J = 11.0 Hz, 1H), 4.62 (s, 2H), 4.42–4.36 (m, 1H), 4.09 (t, J = 3.4 Hz, 1H), 3.95 (t, J = 9.1 Hz, 1H), 3.91 (s, 1H), 3.86–3.76 (m, 3H), 3.74–3.69 (m, 1H), 3.58 (s, 3H), 2.42 (s, 3H), 1.38 (d, J = 6.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 166.2, 165.0, 138.1, 137.9(2), 137.9(0), 137.8, 133.2, 133.1, 132.7, 130.5, 130.1, 129.9(2), 129.8(6), 129.8, 129.5, 128.6(4), 128.5(9), 128.5(1), 128.4(8), 128.4, 128.3, 128.2, 128.0, 127.9, 127.8(0), 127.7(7), 102.2, 83.2, 78.2, 76.0, 75.6, 75.2, 75.1, 74.0, 73.7, 69.9, 69.2, 60.5, 58.8, 21.2, 15.4; HR-ESI-MS (m/z) calcd for C55H56O11SNa [M+Na]+: 947.3436, found: 947.3429.
3.2.17. Synthesis of Compound 25
To a stirring solution of 24 (60.0 mg, 64.9 μmol, 1.0 equiv.) in THF/MeOH (1 mL/1 mL), KOH (10.0 mg, 0.178 mmol, 2.7 equiv.) was added at room temperature. After, the reaction mixture was stirred for 12 h at the same temperature. TLC indicated the reaction was complete (petroleum ether/EtOAc = 2/1). The reaction mixture was quenched with HOAc (10.5 μL, 183 μmol, 2.7 equiv.). H2O (30 mL) was added to the mixture; the mixture was extracted with CH2Cl2 (30 mL × 3), washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 2/1) to give compound 25 (37.4 mg, 80%) as a white amorphous solid. [α = −128.8 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.41–7.27 (m, 15H), 7.22–7.19 (m, 2H), 7.13 (dt, J = 8.4, 1.3 Hz, 2H), 5.55 (d, J = 1.4 Hz, 1H), 5.03 (d, J = 11.2 Hz, 1H), 4.86 (d, J = 10.9 Hz, 1H), 4.83 (d, J = 11.2 Hz, 1H), 4.57 (d, J = 10.8 Hz, 1H), 4.53 (d, J = 12.0 Hz, 1H), 4.51–4.47 (m, 2H), 4.36 (q, J = 6.5 Hz, 1H), 4.17 (d, J = 6.4 Hz, 1H), 4.12 (dt, J = 2.7, 1.2 Hz, 1H), 4.03 (s, 1H), 3.71–3.66 (m, 2H), 3.65–3.55 (m, 3H), 3.52–3.49 (m, 1H), 3.52–3.49 (m, 1H), 3.51 (s, 3H), 2.34 (s, 3H), 1.31 (d, J = 6.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 138.9, 138.2, 138.2, 137.8, 132.1, 130.3, 130.0, 128.5, 128.5, 128.5, 128.1, 128.1, 127.9, 127.8, 127.8, 127.7, 102.0, 90.0, 84.4, 77.3, 76.0, 75.5, 75.4, 75.1, 73.8, 73.6, 73.5, 69.3, 69.2, 67.9, 56.3, 21.2, 17.3; HR-ESI-MS (m/z) calcd for C41H48O9SNa [M+Na]+: 739.2911, found: 739.2918.
3.2.18. Synthesis of Compound 26
To a stirring solution of compound 25 (37.4 mg, 52.2 μmol, 1.0 equiv.) in isopropenyl acetate (1 mL), TsOH·H2O (30 mg, 158 μmol, 3.0 equiv.) was added at room temperature. After, the reaction mixture was stirred for 1 h at the same temperature. TLC indicated the reaction was complete (petroleum ether/EtOAc = 2/1). CH2Cl2 (30 mL) was added, and the reaction mixture was quenched with a saturated NaHCO3 solution (30 mL) and extracted with CH2Cl2 (30 mL × 2). The organic phase was washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 4/1) to give compound 26 (37.3 mg, 89%) as a white amorphous solid. [α = −49.0 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.37–7.26 (m, 15H), 7.19 (dd, J = 7.3, 2.2 Hz, 2H), 7.11 (d, J = 7.9 Hz, 2H), 5.31 (d, J = 3.8 Hz, 1H), 5.15–5.10 (m, 1H), 5.10–5.06 (m, 1H), 4.79 (dd, J = 11.2, 2.4 Hz, 2H), 4.67 (d, J = 11.4 Hz, 1H), 4.57–4.48 (m, 3H), 4.42 (d, J = 7.9 Hz, 1H), 4.28 (qd, J = 6.7, 2.6 Hz, 1H), 3.91 (t, J = 3.0 Hz, 1H), 3.72 (dd, J = 10.6, 2.0 Hz, 1H), 3.68–3.62 (m, 3H), 3.61–3.57 (m, 1H), 3.53–3.47 (m, 1H), 3.44 (s, 3H), 2.32 (s, 3H), 2.14 (s, 3H), 1.95 (s, 3H), 1.31 (d, J = 6.7 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.2, 169.3, 138.3, 138.0, 138.0, 137.9, 132.7, 129.9, 129.7, 128.6, 128.5, 128.2, 128.0, 128.0, 127.9, 127.9, 127.8, 101.2, 83.3, 78.1, 76.6, 75.2, 75.1, 75.1, 74.4, 73.7, 73.5, 68.8, 58.1, 21.2(2C), 21.1, 15.9; HR-ESI-MS (m/z) calcd for C45H52O11SNa [M+Na]+: 823.3125, found: 823.3123.
3.2.19. Synthesis of Compound 27
To a stirring solution of 26 (37.3 mg, 0.047 mmol, 1.0 equiv.) in a mixture of acetone/H2O/CH2Cl2 (1 mL/0.1 mL/1 mL), NBS (24.9 mg, 0.140 mmol, 3.0 equiv.) was added at −20 °C. The resulting mixture was stirred for 0.5 h at the same temperature. TLC indicated the reaction was complete (petroleum ether/EtOAc = 3/1). The reaction mixture was quenched with NEt3 (0.5 mL). A saturated NaHCO3 solution (30 mL) was added. The mixture was extracted with CH2Cl2 (30 mL × 3), washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 2/1) to give compound 27 (30.0 mg, 93%) as a white foamy solid. The solid was used in the next step without further characterization.
3.2.20. Synthesis of Compound 28
To a stirring solution of 27 (30.0 mg, 0.043 mmol, 1.0 equiv.) in dry CH2Cl2 (2 mL), NCCCl3 (17.7 μL, 0.173 mmol, 4.0 equiv.) and Cs2CO3 (1.4 mg, 2.69 μmol, 0.1 equiv.) were added. The resulting mixture was stirred for 2 h at room temperature. TLC indicated the reaction was complete (petroleum ether/EtOAc = 1/2). The reaction mixture was filtered. The filtrate was concentrated in vacuo.
The residue and aglycone 3 (12.5 mg, 0.029 mmol, 1.5 equiv.) were co-evaporated with toluene three times and dissolved in dry CH2Cl2 (2.5 mL); a 4 Å molecular sieve (250 mg) was added. The mixture was stirred for 10 min at 25 °C under an Ar atmosphere. Next, TMSOTf (1.1 μL, 5.76 μmol, 0.2 equiv.) was added at −78 °C. The resulting mixture was stirred for 1 h at −78 °C. TLC indicated the reaction was complete (petroleum ether/EtOAc = 1/2). The reaction mixture was quenched with a saturated NaHCO3 solution (2 mL) and filtered. The filtrate was added to the saturated NaHCO3 solution (20 mL). The mixture was extracted with CH2Cl2 (20 mL × 3), and the organic phase was washed with brine, and dried with Na2SO4. After filtration, the solution was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (petroleum ether/EtOAc = 1/1) to give compound 28 (16.4 mg, 51% over 2 steps) as a pale yellow foamy solid. [α = −34.5 (c 1.0, CHCl3); 1H NMR (400 MHz, CDCl3) δ 7.34–7.27 (m, 13H), 7.20–7.15 (m, 2H), 5.87 (t, J = 1.8 Hz, 1H), 5.14–5.07 (m, 1H), 4.97 (dd, J = 18.3, 1.8 Hz, 1H), 4.91 (t, J = 3.0 Hz, 1H), 4.87 (t, J = 2.9 Hz, 1H), 4.83–4.74 (m, 4H), 4.66 (d, J = 11.4 Hz, 1H), 4.56–4.48 (m, 3H), 4.39 (d, J = 7.9 Hz, 1H), 3.95 (s, 1H), 3.86–3.83 (m, 1H), 3.81 (d, J = 6.7 Hz, 1H), 3.72–3.58 (m, 5H), 3.52–3.46 (m, 2H), 3.38 (s, 3H), 2.77 (dd, J = 9.5, 5.5 Hz, 1H), 2.17 (s, 1H), 2.13 (s, 3H), 2.11–2.06 (m, 2H), 2.01 (s, 3H), 1.98–1.96 (m, 3H), 1.94 (d, J = 1.4 Hz, 1H), 1.87–1.66 (m, 10H), 1.60–1.50 (m, 2H), 1.43–1.34 (m, 3H), 1.25 (d, J = 6.7 Hz, 3H), 0.96 (s, 3H), 0.87 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 174.5, 174.3, 171.7, 171.1, 169.3, 138.3, 138.0, 137.9, 128.6, 128.6, 128.5, 128.3, 128.1, 128.0, 127.9, 127.9, 118.0, 101.4, 95.0, 85.5, 83.4, 78.2, 75.2, 75.1, 75.1, 74.3, 74.1, 73.6, 73.6, 73.5, 70.0, 69.4, 68.9, 67.1, 60.5, 57.5, 50.9, 49.6, 41.7, 39.9, 38.8, 37.0, 33.3, 31.1, 29.9, 28.5, 28.4, 27.0, 25.8, 21.7, 21.6, 21.3, 21.1, 20.6, 18.5, 16.5, 15.9; HR-ESI-MS (m/z) calcd for C63H80O17Na [M+Na]+: 1131.5288, found: 1131.5281.
3.2.21. Synthesis of Compound 1
To a solution of 28 (8.6 mg, 7.8 μmol, 1.0 equiv.) in EtOAc/MeOH (1.0 mL/1.0 mL), 10% Pd/C (8.6 mg) was added. The reaction mixture was recharged with H2 thrice, and then stirred for 5 h at 25 °C under an atmosphere of H2. TLC indicated the reaction was complete (CH2Cl2/MeOH = 20/1). The reaction mixture was filtered. The filtrate was concentrated under reduced pressure.
The residue was dissolved in MeOH/H2O/THF (1.5 mL/0.5 mL/0.5 mL); LiOH (2.0 mg, 83.5 μmol, 10.8 equiv.) was added and then the mixture was stirred for 2 h at 25 °C. TLC indicated the reaction was complete (CH2Cl2 / MeOH = 10/1). The reaction mixture was quenched with HOAc (4.8 μL, 83.5 μmol, 10.8 equiv.) and concentrated in vacuo. The residue was purified by reverse-phase columns (H2O/MeOH = 2/1 → 1/2) to afford acospectoside A (1) (2.5 mg, 43% over 2 steps) as a white amorphous solid. [α = −27.0 (c 0.3, CH3OH); 1H NMR (400 MHz, C5D5N) δ 6.16 (d, J = 1.9 Hz, 1H), 5.50 (s, 1H), 5.35 (d, J = 18.4 Hz, 1H), 5.31 (d, J = 1.7 Hz, 1H), 5.18 (s, 1H), 5.09 (s, 1H), 4.57 (dd, J = 11.5, 2.6 Hz, 1H), 4.49 (d, J = 2.6 Hz, 1H), 4.44–4.37 (m, 1H), 4.29–4.18 (m, 3H), 4.14 (s, 1H), 4.10 (d, J = 6.6 Hz, 1H), 3.99 (q, J = 8.5, 7.2 Hz, 2H), 3.78 (t, J = 3.1 Hz, 1H), 3.70 (s, 3H), 2.86–2.76 (m, 1H), 2.29–2.22 (m, 1H), 2.21 (s, 3H), 2.17–2.06 (m, 4H), 2.02–1.89 (m, 3H), 1.87–1.79 (m, 2H), 1.74 (d, J = 6.6 Hz, 3H), 1.68 (d, J = 12.5 Hz, 1H), 1.64–1.54 (m, 1H), 1.47–1.19 (m, 8H), 1.04 (s, 3H), 1.00 (s, 3H); 13C NMR (150 MHz, C5D5N) δ 175.7, 174.4, 170.6, 117.6, 105.1, 99.0, 84.3, 78.4, 78.3, 76.5, 76.4, 75.3, 74.3, 73.5, 71.3, 69.8, 69.4, 67.3, 62.6, 55.9, 51.1, 49.8, 41.5, 39.4, 38.9, 36.7, 32.9, 31.4, 29.9, 28.1, 27.1, 26.1, 21.7, 21.3, 21.0, 18.3, 17.2, 16.0; HR-ESI-MS (m/z) calcd for C38H58O15Na [M+Na]+: 777.3668, found: 777.3676.
4. Conclusions
Here, we report the total synthesis of cytotoxic cardenolides acospectoside A (1) and acovensoide B (2), which were isolated from the South African poisonous bush Acokanthera oppositifolia and relevant plants. The synthesis features the selective introductions of a 15,16-unsaturated enone via the Larock protocol of Saegusa–Ito oxidation, 14β-OH by Mukaiyama hydration, and a C17-butenolide moiety by Stille coupling. Leveraging the strategic 1-O-acetylation of the aglycone moiety, glycosylation with imidate donors proceeds with remarkable selectivity, yielding glycosylated products in satisfactory amounts. Given the structural conservation inherent in the acovenoside family, the present synthetic approach should offer a promising gateway to accessing a broader array of the acovenoside congeners. This advancement stands to facilitate comprehensive investigations into the biological and pharmacological activities of these components, which have long been esteemed in traditional medicinal practices.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30112297/s1: Copies of the NMR spectra of compounds 1–28; Figure S1: 1H NMR spectrum of compound 7 (CDCl3, 500 MHz); and Table S1: Comparison of the Spectroscopic Data of Natural and Synthetic Acovenoside B (2).
Author Contributions
Conceptualization, P.X. and B.Y.; methodology, B.L.; formal analysis, B.L.; investigation, B.L.; resources, B.Y.; data curation, B.L.; writing—original draft preparation, B.L. and P.X.; writing—review and editing, B.Y.; visualization, B.L.; supervision, P.X. and B.Y.; project administration, P.X. and B.Y.; funding acquisition, B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (22031011), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB1060000), the Shanghai Municipal Science and Technology Major Project, and the Hangzhou leading innovation and entrepreneurship team project (TD2022002).
Institutional Review Board Statement
This study does not involve humans or animals.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Radford, D.J.; Gillies, A.D.; Hinds, J.A.; Duffy, P. Naturally occurring cardiac glycosides. Med. J. Aust. 1986, 144, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, H.P. Cardiac glycosides. In Naturally Occurring Glycosides; Ikan, R., Ed.; John Wiley & Sons Ltd.: New York, NY, USA, 1999; p. 83. [Google Scholar]
- Prassas, I.; Diamandis, E.P. Novel therapeutic applications of cardiac glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Shang, C.; Meng, T.; Lou, W. Anticancer potential of cardiac glycosides and steroid-azole hybrids. Steroids 2021, 171, 108852. [Google Scholar] [CrossRef]
- Diederich, M.; Muller, F.; Cerella, C. Cardiac glycosides: From molecular targets to immunogenic cell death. Biochem. Pharmacol. 2017, 125, 1–11. [Google Scholar] [CrossRef]
- von Euw, J.; Reichstein, T. Acovenosid A und acovensoid B, zwei glykoside aus den samen von acokanthera venenata G. Don. Erste Mitteilung. Glykoside und aglykone, 53. Mitteilung. Helv. Chim. Acta 1950, 33, 485–502. [Google Scholar]
- Kapadia, G.J. Acospectoside A, a new cardenolide glycoside. J. Pharm. Sci. 1965, 54, 1834–1835. [Google Scholar] [CrossRef]
- Kapadia, G.J. Structural elucidation of acospectoside A. Lloydia 1964, 27, 272. [Google Scholar]
- Kapadia, G.J. Acospectoside A II: The structure of the cardenolide glycoside. J. Pharm. Sci. 1969, 58, 1555–1557. [Google Scholar] [CrossRef]
- Kapadia, G.J. Acospectoside A III: Selective conversion into acovenoside B using snail enzyme with inhibited esterase activity. J. Pharm. Sci. 1970, 59, 723–724. [Google Scholar] [CrossRef]
- Gaafary, M.E.; Ezzat, S.M.; Sayed, A.M.E.; Sabry, O.M.; Hafner, S.; Lang, S.; Schmiech, M.; Syrovets, T.; Simmet, T. Acovenoside A induces mitotic catastrophe followed by apoptosis in non-small-cell lung cancer cells. J. Nat. Prod. 2017, 80, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Heasley, B. Chemical synthesis of the cardiotonic steroid glycosides and related natural products. Chem. Eur. J. 2012, 18, 3092–3120. [Google Scholar] [CrossRef] [PubMed]
- Urabe, D.; Nakagawa, Y.; Mukai, K.; Fukushima, K.-i.; Aoki, N.; Itoh, H.; Nagatomo, M.; Inoue, M. Total synthesis and biological evaluation of 19-hydroxysarmentogenin-3-O-α-l-rhamnoside, trewianin, and their aglycons. J. Org. Chem. 2018, 83, 13888–13910. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, B.; Nagorny, P. Enantioselective Total synthesis of cannogenol-3-O-α-l-rhamnoside via sequential Cu(II)-catalyzed michael addition/intramolecular aldol cyclization reactions. Org. Lett. 2018, 20, 154–157. [Google Scholar] [CrossRef]
- Carney, N.; Perry, N.; Garabedian, J.; Nagorny, P. Development of α-selective glycosylation with l-oleandral and its application to the total synthesis of oleandrin. Org. Lett. 2023, 25, 966–971. [Google Scholar] [CrossRef]
- Liu, B.; Bi, S.; Wang, J.; Xu, P.; Yu, B. Synthesis of acovenosides: Cardiac glycosides with potent antitumor activities. Org. Lett. 2024, 26, 8725–8729. [Google Scholar] [CrossRef]
- Wang, R.; Xiao, L.; Pan, J.; Bao, G.; Zhu, Y.; Zhu, D.; Wang, J.; Pei, C.; Ma, Q.; Fu, X.; et al. Natural product P57 induces hypothermia through targeting pyridoxal kinase. Nat. Commun. 2023, 14, 5984. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, R.; Cao, S.; Li, J.; Huang, G.; Wang, H.; Yang, T.; Tang, W.; Xu, P.; Yu, B. Merging total synthesis and NMR technology for deciphering the realistic structure of natural 2,6-dideoxyglycosides. Sci. Adv. 2024, 10, eadn1305. [Google Scholar] [CrossRef]
- Tückmantel, W.; Kozikowski, A.P.; Romanczyk, L.J. Studies in polyphenol chemistry and bioactivity. 1. Preparation of building blocks from (+)-Catechin. Procyanidin formation. Synthesis of the cancer cell growth inhibitor, 3-O-galloyl-(2R,3R)-epicatechin-4β,8-[3-O-galloyl-(2R,3R)-epicatechin]. J. Am. Chem. Soc. 1999, 121, 12073–12081. [Google Scholar] [CrossRef]
- Ito, Y.; Hirao, T.; Saegusa, T. Synthesis of α,β-unsaturated carbonyl compounds by palladium(II)-catalyzed dehydrosilylation of silyl enol ethers. J. Org. Chem. 1978, 43, 1011–1013. [Google Scholar] [CrossRef]
- Larock, R.C.; Hightower, T.R.; Kraus, G.A.; Hahn, P.; Zhang, D. A simple, effective, new, palladium-catalyzed conversion of enol silanes to enones and enals. Tetrahedron Lett. 1995, 36, 2423–2426. [Google Scholar] [CrossRef]
- Tamiya, M.; Takada, F.; Isaka, N.; Iimura, N.; Ishiguro, M. Modification of D-ring moiety of steroids—A novel palladium catalyzed Bayer–Villiger type rearrangement of cyclic silylenol ether derivatives. Hetrocycles 2011, 82, 1119–1125. [Google Scholar]
- Isayama, S.; Mukaiyama, T. A new method for preparation of alcohols from olefins with molecular oxygen and phenylsilane by the use of bis(acetylacetonato)cobalt(II). Chem. Lett. 1989, 18, 1071–1074. [Google Scholar] [CrossRef]
- Hanna, A.G.; Elgamal, M.H.A.; Hassan, A.Z.; Duddeck, H.; Simon, A.; Kovács, J.; Tóth, G. Complete 1H and 13C signal assignments of 5β-cardenolides isolated from Acokanthera spectabilis Hook F. Magn. Reson. Chem. 1998, 36, 936–942. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).