Effects of Diverse Acrylates on the Electro-Optical Performance of Polymer-Dispersed Liquid Crystal Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
- (1)
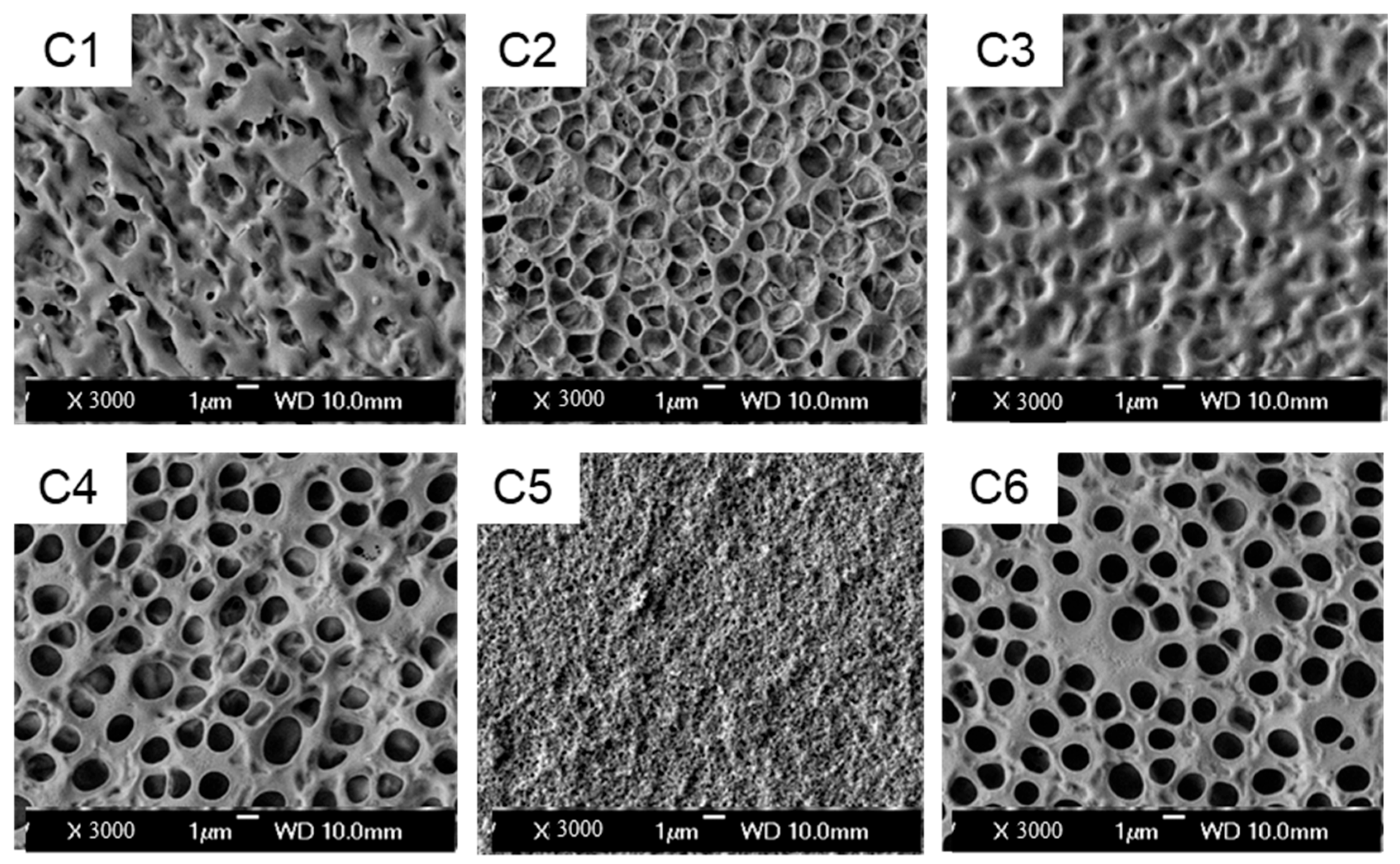
- Characterization of micro-morphology: The micro-morphology of the samples was inspected using a scanning electron microscope (SEM, Hitachi S-4800, Tokyo, Japan) operated at an accelerating voltage of 3.0 kV and a working distance (WD) of 10.0 mm. The preparation approach for the test sample was to cut the liquid crystal cell and then immerse it in cyclohexane for approximately ten days. After drying for one day, the sample was coated with gold and then characterized.
- (2)
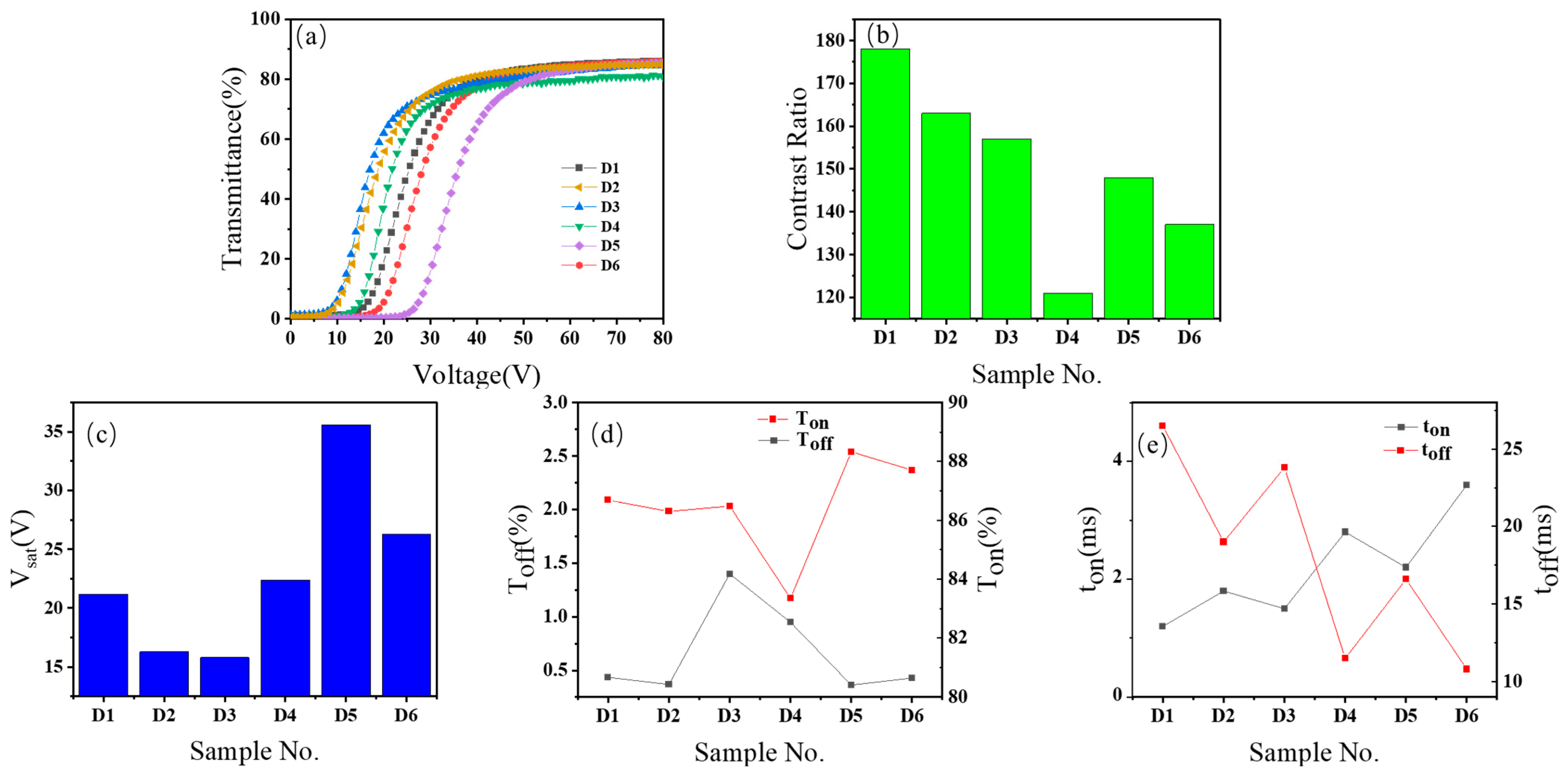
- Characterization of electro-optical performance: In the experiment, electro-optical performance testing was carried out using a liquid crystal parameter tester (LCT-5016C, Changchun Liancheng Instrument Co., Ltd., Changchun, China). A collimated light beam passed through the sample, and the optical characterization was performed using a standard white light source (halogen lamp, 300–800 nm spectral range) with controlled intensity of 100 mW/cm2. The transmitted light was collected by an electro-optical detector. Then, the light intensity was converted into an electrical signal and processed by software. Finally, the transmittance data were output by the instrument. During transmittance measurement, a square-wave modulated alternating current (AC) voltage pulse was applied across the sample, and the frequency was set to 1 kilohertz. The transmittance values when measured in air and when blocked (or in a blocked state) were normalized to 100% and 0%, respectively. Subsequently, relevant parameters were calculated, including the on-state transmittance (Ton), off-state transmittance (Toff), threshold voltage (Vth) and saturation voltage (Vsat), contrast ratio (CR), and rise (ton) and fall (toff) times. Ton and Toff denote the maximum and minimum transmissivity, respectively. Vth and Vsat are defined as the voltages required for the transmissivity to reach 10% and 90% of the maximum transmissivity Ton, respectively. The contrast ratio (CR) is defined as the ratio of on-state transmittance (Ton) to off-state transmittance (Toff), i.e., CR = Ton/Toff. ton and toff are the response times required for the transmissivity to ascend from 10% to 90% under the influence of an external voltage and the time needed to descend from 90% of Ton to 10% after a voltage pulse, respectively.
3. Results and Discussion
3.1. Influence of Acrylates with Different Functional Groups on the Properties of PDLC Films
- Electron-donating amines induce viscosity-mediated diffusion limitations;
- Halogen electronegativity modulates polymerization–LC diffusion competition;
- Hydrogen bonding directs rapid network consolidation.
3.2. Influence of Diverse Acrylates with Hydroxyl Moieties on the Performance of PDLC Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hikmet, R.A.M.; Kemperman, H. Electrically switchable mirrors and optical components made from liquid crystal gels. Nature 1998, 392, 476–479. [Google Scholar] [CrossRef]
- Doane, J.W.; Vaz, N.A.; Wu, B.G.; Žumer, S. Field controlled light scattering from nematic microdroplets. Appl. Phys. Lett. 1986, 48, 269–271. [Google Scholar] [CrossRef]
- He, Z.; Yin, K.; Hsiang, E.L.; Wu, S.T. Volumetric light-shaping polymer-dispersed liquid crystal films for mini-LED backlights. Liq. Cryst. 2020, 47, 1458–1463. [Google Scholar] [CrossRef]
- Halder, S.; Shin, Y.; Zhou, Z.; Zhang, X.; Hu, L.; Yang, D.K. Aligned polymer dispersed liquid crystal film for light enhancement of quantum dot backlight. Opt. Express 2021, 29, 43241. [Google Scholar] [CrossRef]
- Oh, S.W.; Baek, J.M.; Heo, J.; Yoon, T.H. Dye-doped cholesteric liquid crystal light shutter with a polymer-dispersed liquid crystal film. Dye. Pigment. 2016, 134, 36–40. [Google Scholar] [CrossRef]
- Wu, B.G.; Erdmann, J.H.; Doane, J.W. Response times and voltages for PDLC light shutters. Liq. Cryst. 1989, 5, 1453–1465. [Google Scholar] [CrossRef]
- Doane, J.W.; Golemme, A.; West, J.L.; Whitehead, J.B.; Wu, B.-G. Polymer dispersed liquid crystals for display application. Mol. Cryst. Liq. Cryst. 1988, 165, 511–532. [Google Scholar] [CrossRef]
- Drzaic, P.S. Reorientation dynamics of polymer dispersed nematic liquid crystal films. Liq. Cryst. 1988, 3, 1543–1559. [Google Scholar] [CrossRef]
- Xu, J.; Yu, M.; Chen, G.; Wang, X.; Hu, J.; Zou, C.; Wang, Q.; Xiao, J.; Gao, Y.; Zhu, S.; et al. Study on the preparation and performance of an electrically controlled dimming film with wide working temperature range. J. Mol. Liq. 2022, 367, 120408. [Google Scholar] [CrossRef]
- Wang, L.; Luo, S.; Liu, S.; He, Z.; Miao, Z. Mechanism of the effect of gel doping on the electro-optical properties of PDLC. Liq. Cryst. 2024, 51, 1980–1994. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Matsukizono, H.; Okumura, Y.; Kikuchi, H. Nanostructured Polymer-Dispersed Liquid Crystals Using a Ferroelectric Smectic A Liquid Crystal. Molecules 2024, 29, 4837. [Google Scholar] [CrossRef]
- Selvaraj, P.; Cheng, W.F.; Kuo, H.M.; Liu, C.K.; Wu, C.H.; Lai, C.K.; Lai, J.C.; Fang Jie, S.T.; Cheng, K.T. Self-Assembled Multistable Scattering Mode for Versatile Energy-Saving Smart Windows. Laser Photonics Rev. 2024, 18, 2301001. [Google Scholar] [CrossRef]
- Ben Salah, M.; Saadaoui, L.; Soltani, T.; Ben Hamadi, N.; Guesmi, A.; Maschke, U. New Series of Hydrogen-Bonded Liquid Crystal with High Birefringence and Conductivity. Molecules 2024, 29, 3422. [Google Scholar] [CrossRef]
- Yang, P.; Wu, Y.; Wang, K.; Lu, S.; Zhang, Y.; Shi, J.; Wu, K.; Wan, J. Enhanced Intrinsic Thermal Conductivity of Liquid Crystalline Polyester Through Monomer Structure Optimization in Main Chains. J. Mater. Chem. C 2025, 13, 9601–9610. Available online: https://pubs.rsc.org/en/content/articlelanding/2025/tc/d5tc00061k/unauth (accessed on 13 April 2025). [CrossRef]
- Zhang, H.; Cao, H.; Chen, M.; Zhang, L.; Jiang, T.; Chen, H.; Li, F.; Zhu, S.; Yang, H. Effects of the fluorinated liquid crystal molecules on the electro-optical properties of polymer dispersed liquid crystal films. Liq. Cryst. 2017, 44, 2301–2310. [Google Scholar] [CrossRef]
- Zhang, H.; Li, F.; Li, K.; Zhang, Y.; Peng, Y.; Lu, Y.; Liu, J.; Diao, K.; Zhao, Y.; Miao, Z. The effect of the LC mixtures with the different clearing point on temperature dependence of the electro-optical properties of polymer dispersed liquid crystals. Mol. Cryst. Liq. Cryst. 2021, 726, 27–40. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, S.; Saeed, M.H.; Chen, G.; Lin, H.; Huang, J.; Zhang, L.; Wang, Q.; Cao, H. Study on the morphologies and electro-optical properties of cyano-phenyl-ester liquid crystals/polymer composite films prepared by a stepwise polymerization. Liq. Cryst. 2020, 47, 1497–1506. [Google Scholar] [CrossRef]
- Mouquinho, A.; Barros, M.T.; Sotomayor, J. Pre-Polymer Chain Length: Influence on Permanent Memory Effect of PDLC Devices. Crystals 2024, 14, 249. [Google Scholar] [CrossRef]
- Wang, Q.; Park, J.O.; Srinivasarao, M.; Qiu, L.; Kumar, S. Control of polymer structures in phase-separated liquid crystal-polymer composite systems. Jpn. J. Appl. Phys. 2005, 44, 3115–3120. [Google Scholar] [CrossRef]
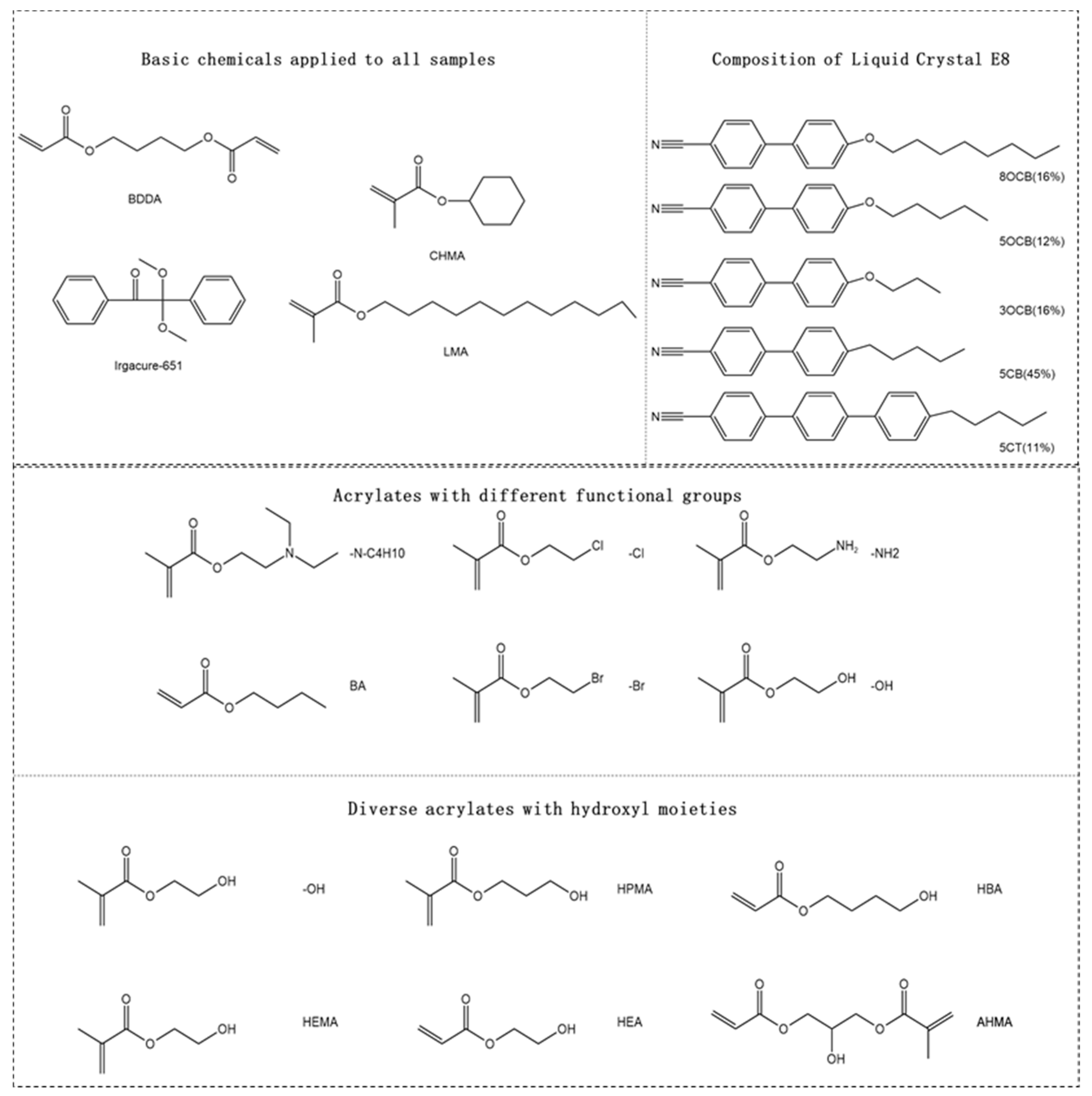


| Weight Ratio | Weight, g | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|---|
| 6% | 0.018 | -N-C4H10 | -Cl | -NH2 | BA | -Br | -OH |
| Weight Ratio | Weight, g | D1 | D2 | D3 | D4 | D5 | D6 |
|---|---|---|---|---|---|---|---|
| 6% | 0.018 | -OH | HEMA | HPMA | HEA | HBA | AHMA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, N.; Zhang, Z.; Yang, H. Effects of Diverse Acrylates on the Electro-Optical Performance of Polymer-Dispersed Liquid Crystal Films. Molecules 2025, 30, 2284. https://doi.org/10.3390/molecules30112284
Sun N, Zhang Z, Yang H. Effects of Diverse Acrylates on the Electro-Optical Performance of Polymer-Dispersed Liquid Crystal Films. Molecules. 2025; 30(11):2284. https://doi.org/10.3390/molecules30112284
Chicago/Turabian StyleSun, Nan, Zuowei Zhang, and Huai Yang. 2025. "Effects of Diverse Acrylates on the Electro-Optical Performance of Polymer-Dispersed Liquid Crystal Films" Molecules 30, no. 11: 2284. https://doi.org/10.3390/molecules30112284
APA StyleSun, N., Zhang, Z., & Yang, H. (2025). Effects of Diverse Acrylates on the Electro-Optical Performance of Polymer-Dispersed Liquid Crystal Films. Molecules, 30(11), 2284. https://doi.org/10.3390/molecules30112284





