Magnetic Biochar Derived from Waste Bamboo as a Peroxymonosulfate Activator for Tetracycline Hydrochloride Degradation
Abstract
1. Introduction
2. Results and Discussion
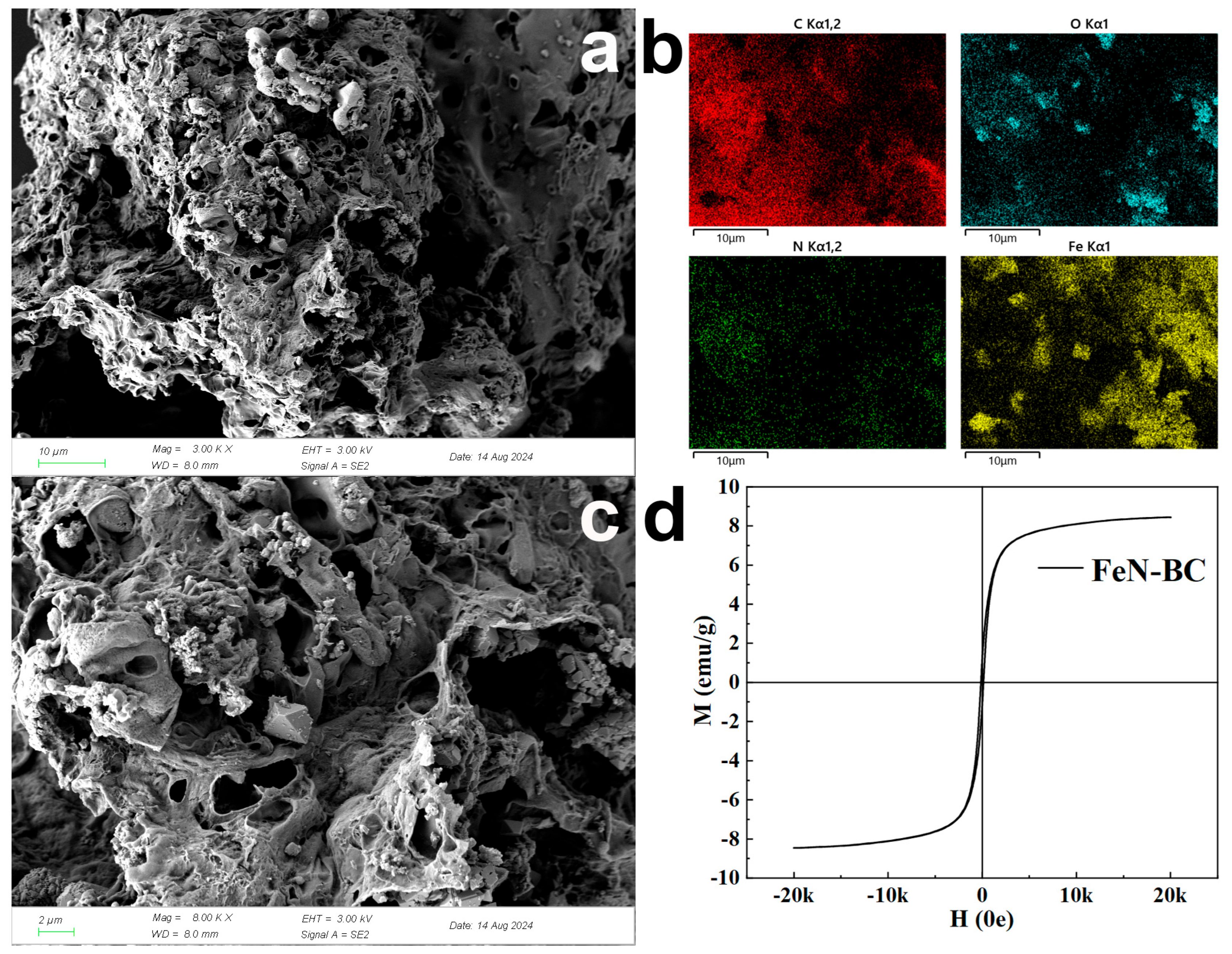
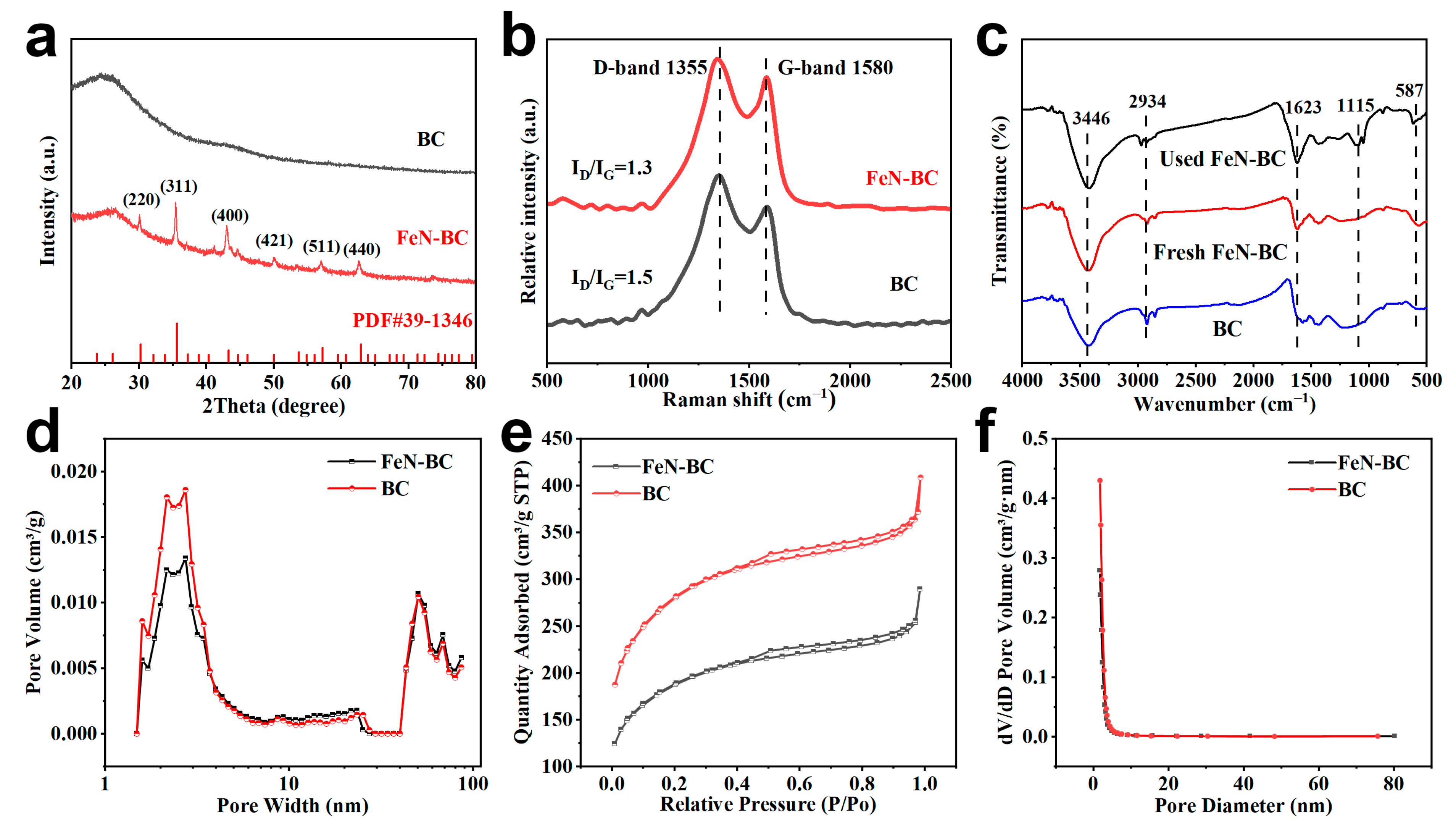
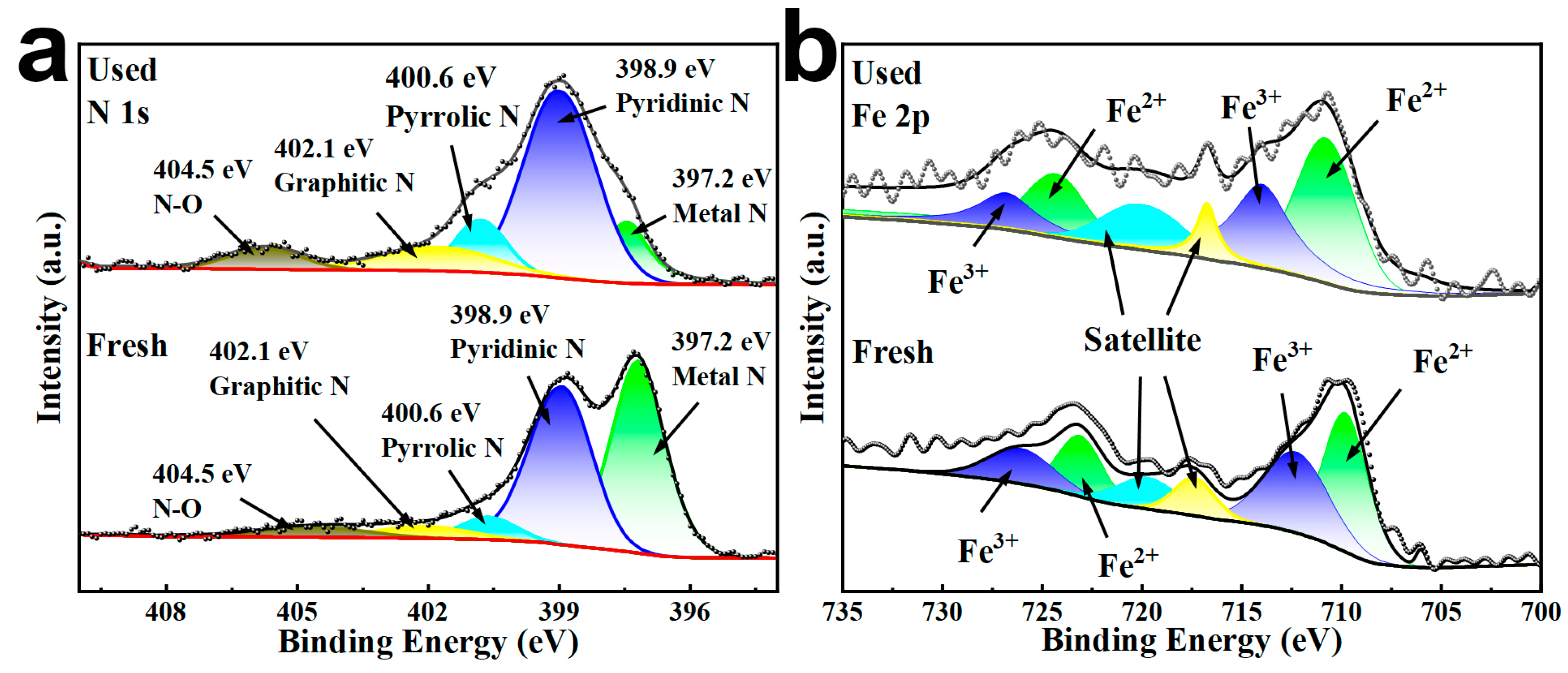
2.1. Characterizations
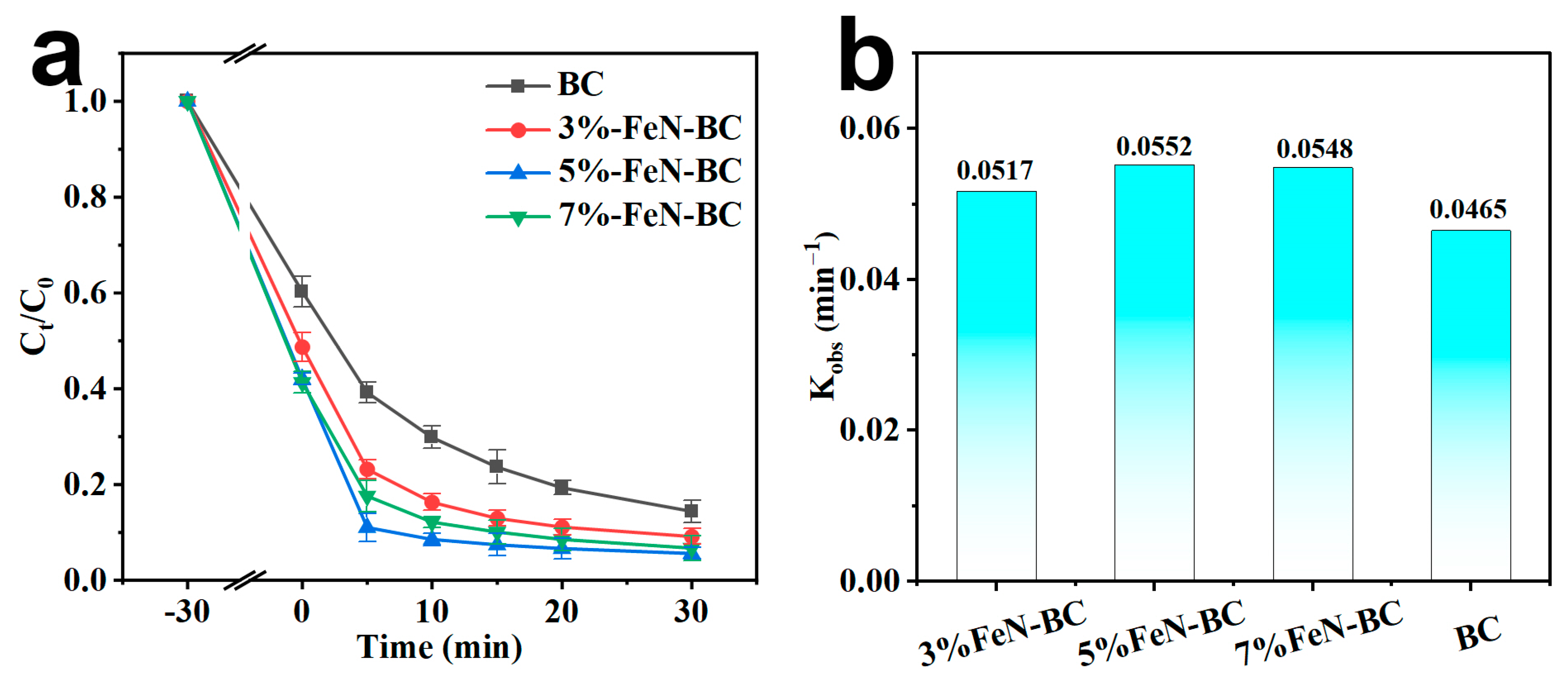
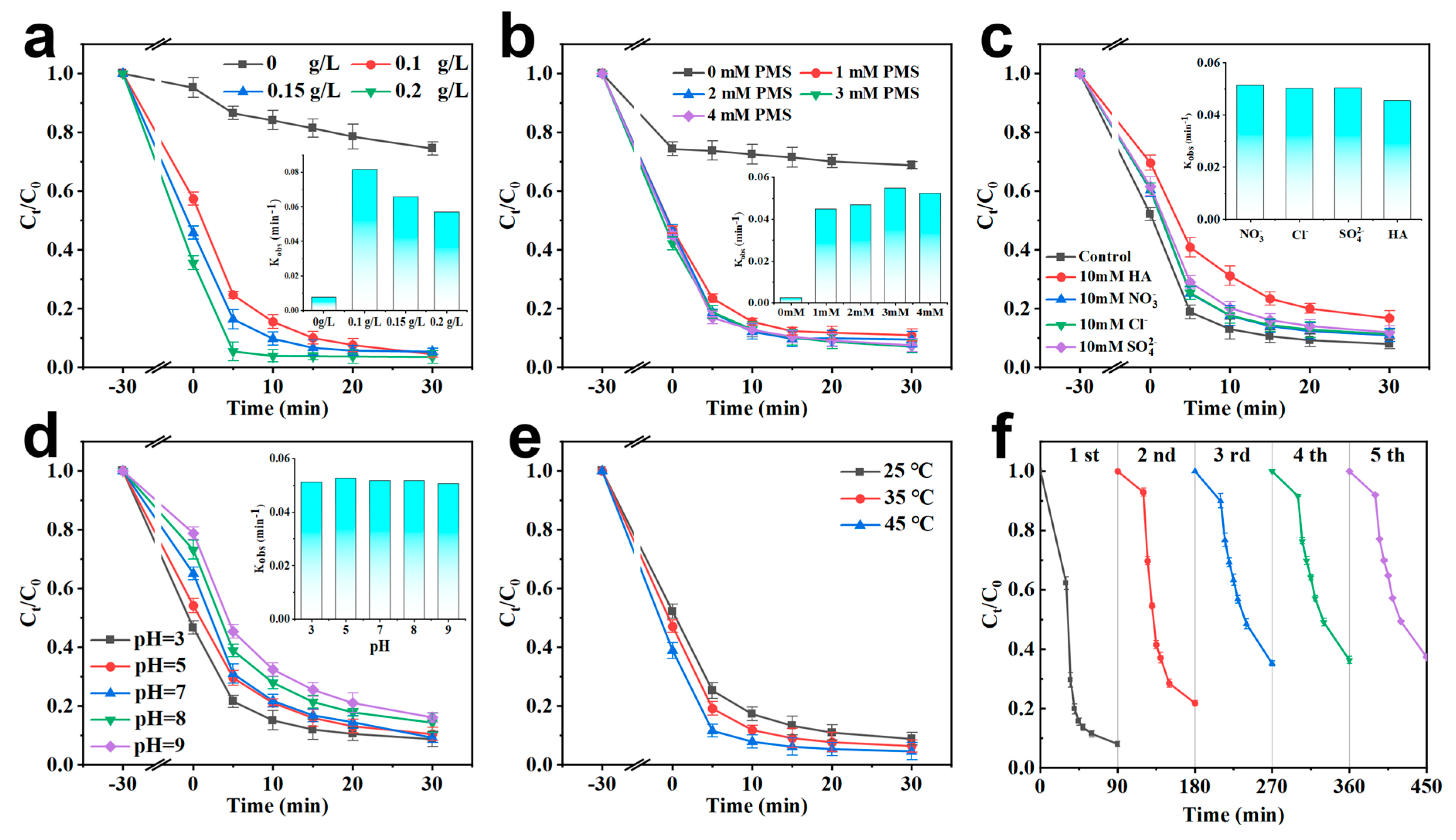
2.2. Catalytic Degradation Performance
2.3. Degradation Mechanism
3. Experimental
3.1. Materials
3.2. Preparation of FeN-BC
3.3. Characterization of FeN-BC
3.4. Catalytic Degradation of TC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Xu, X.-R.; Liu, S.-S.; Zhou, G.-J.; Sun, K.-F.; Zhao, J.-L.; Ying, G.-G. Antibiotics in Typical Marine Aquaculture Farms Surrounding Hailing Island, South China: Occurrence, Bioaccumulation and Human Dietary Exposure. Mar. Pollut. Bull. 2015, 90, 181–187. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Zhao, Y.; Li, B.; Huang, C.-L.; Zhang, S.-Y.; Yu, S.; Chen, Y.-S.; Zhang, T.; Gillings, M.R.; Su, J.-Q. Continental-Scale Pollution of Estuaries with Antibiotic Resistance Genes. Nat. Microbiol. 2017, 2, 16270. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, T.; Zhang, S.; Hou, J.; Cheng, L.; Chen, W.; Zhang, Q. Contamination Distribution and Non-Biological Removal Pathways of Typical Tetracycline Antibiotics in the Environment: A Review. J. Hazard. Mater. 2024, 463, 132862. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of Persulfate (PS) and Peroxymonosulfate (PMS) and Application for the Degradation of Emerging Contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated Persulfate for Organic Chemical Degradation: A Review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Das, U.; Dalai, A.K. In-Situ Chemical Oxidation: Principle and Applications of Peroxide and Persulfate Treatments in Wastewater Systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of Sulfate Radical through Heterogeneous Catalysis for Organic Contaminants Removal: Current Development, Challenges and Prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, S. Metal-Free Carbocatalysis in Advanced Oxidation Reactions. Acc. Chem. Res. 2018, 51, 678–687. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-Photochemical Production of Singlet Oxygen via Activation of Persulfate by Carbon Nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Xia, Y.; Liu, H.; Liu, Z.; Huang, Y. Preparation of Activated Carbon with High Nitrogen Content from Agro-Industrial Waste for Efficient Treatment of Chromium (VI) in Water. Ind. Crops Prod. 2023, 194, 116403. [Google Scholar] [CrossRef]
- Song, T.; Tian, W.; Qiao, K.; Zhao, J.; Chu, M.; Du, Z.; Wang, L.; Xie, W. Adsorption Behaviors of Polycyclic Aromatic Hydrocarbons and Oxygen Derivatives in Wastewater on N-Doped Reduced Graphene Oxide. Sep. Purif. Technol. 2021, 254, 117565. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Kang, J.; Wang, Y.; Indrawirawan, S.; Wang, S. Insights into Heterogeneous Catalysis of Persulfate Activation on Dimensional-Structured Nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Chen, W.; Xu, G.; Zhu, G.; Xie, G.; Xu, L.; Dong, C.; Gao, S.; Chen, Y.; et al. Co-Production of Porous N-Doped Biochar and Hydrogen-Rich Gas Production from Simultaneous Pyrolysis-Activation-Nitrogen Doping of Biomass: Synergistic Mechanism of KOH and NH3. Renew. Energy 2024, 229, 120777. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe–N Modification on the Properties of Biochars and Their Adsorption Behavior on Tetracycline Removal from Aqueous Solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-Doped Biochars as Adsorbents for Mitigation of Heavy Metals and Organics from Water: A Review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Hung, C.M.; Chen, C.W.; Huang, C.P.; Dong, C.D. N-Doped Metal-Free Biochar Activation of Peroxymonosulfate for Enhancing the Degradation of Antibiotics Sulfadiazine from Aquaculture Water and Its Associated Bacterial Community Composition. J. Environ. Chem. Eng. 2022, 10, 107172. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, C.; Liu, K.; Zhang, W.; Tsang, D.C.; Yang, Z.; Yang, X.; Yan, B.; Morel, J.L.; Qiu, R. Experimental and DFT Investigation on N-Functionalized Biochars for Enhanced Removal of Cr(VI). Environ. Pollut. 2021, 291, 118244. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, W.; Bi, F.; Yan, S.; Miao, X.; Zhang, B.; Wang, Y.; Ge, C.; Zhang, Y. Two-Step Ball Milling-Assisted Synthesis of N-Doped Biochar Loaded with Ferrous Sulfide for Enhanced Adsorptive Removal of Cr(VI) and Tetracycline from Water. Environ. Pollut. 2022, 206, 119398. [Google Scholar] [CrossRef]
- Luo, L.; Cheng, S.; Yue, L.; You, Z.; Cai, J. N-Doped Biochar from Chitosan Gel-like Solution: Effect of Hydrothermal Temperature and Superior Aqueous Cr (VI) Removal Performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128426. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, Y.; Liu, W.; Yang, D.; Liu, J.; Lu, Y.; Lin, H. Enhanced Adsorption of Phosphate from Pickling Wastewater by Fe-N Co-Pyrolysis Biochar: Performance, Mechanism and Reusability. Bioresour. Technol. 2023, 369, 128263. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Chai, C.; Cao, S.; Bai, X.; Ma, K.; Jin, X.; Shi, X.; Jin, P. Adsorption of Micropollutants from Wastewater Using Iron and Nitrogen Co-Doped Biochar: Performance, Kinetics and Mechanism Studies. J. Hazard. Mater. 2022, 424, 127606. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, J.; Peng, H.; Leng, S.; Li, H.; Qu, W.; Hu, Y.; Li, H.; Jiang, S.; Zhou, W.; et al. Effect of Biomass Type and Pyrolysis Temperature on Nitrogen in Biochar, and the Comparison with Hydrochar. Fuel 2021, 291, 120128. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Ma, F.; You, Y. FeOx@graphitic Carbon Core–Shell Embedded in Microporous N-Doped Biochar Activated Peroxydisulfate for Removal of Bisphenol a: Multiple Active Sites Induced Non-Radical/Radical Mechanism. Chem. Eng. J. 2022, 438, 135552. [Google Scholar] [CrossRef]
- Xu, L.; Peng, Y.; Fang, Z. Molybdate-Loaded Magnetic Biochar Activates Persulfate for Efficient Degradation of Sulfamethazine. Sep. Purif. Technol. 2025, 362, 131911. [Google Scholar] [CrossRef]
- Pang, Y.; Luo, K.; Tang, L.; Li, X.; Song, Y.; Li, C.; Wang, L. Preparation and Application of Magnetic Nitrogen-Doped rGO for Persulfate Activation. Environ. Sci. Pollut. Res. 2018, 25, 30575–30584. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Lian, C.; Wei, F.; Zhang, D.; Wu, G.; Chen, B.; Wang, S. Fe, Co, Ni Nanocrystals Encapsulated in Nitrogen-Doped Carbon Nanotubes as Fenton-like Catalysts for Organic Pollutant Removal. J. Hazard. Mater. 2016, 314, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.S.; Silva, A.M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Catalytic Wet Peroxide Oxidation: A Route towards the Application of Hybrid Magnetic Carbon Nanocomposites for the Degradation of Organic Pollutants. A Review. Appl. Catal. B Environ. 2016, 187, 428–460. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and Application of Magnetic Biochar in Water Treatment: A Critical Review. Sci. Total Environ. 2020, 711, 134847. [Google Scholar] [CrossRef]
- Huang, X.; Li, F.; Zhang, X.; Xu, S.; Liu, H.; Qiu, C.; He, Y.; Li, M.; Jiang, Y.; Jia, S.; et al. One-Step High-Efficiency Microwave Synthesis of N-Doped Bamboo Biochar for Tetracycline Degradation. Sep. Purif. Technol. 2025, 354, 129003. [Google Scholar] [CrossRef]
- Rong, X.; Xie, M.; Kong, L.; Natarajan, V.; Ma, L.; Zhan, J. The Magnetic Biochar Derived from Banana Peels as a Persulfate Activator for Organic Contaminants Degradation. Chem. Eng. J. 2019, 372, 294–303. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, P.; Faye, M.C.A.S.; Zhang, Y. Characterization of Biochar Derived from Rice Husks and Its Potential in Chlorobenzene Degradation. Carbon 2018, 130, 730–740. [Google Scholar] [CrossRef]
- Fan, X.; Cao, Q.; Meng, F.; Song, B.; Bai, Z.; Zhao, Y.; Chen, D.; Zhou, Y.; Song, M. A Fenton-like System of Biochar Loading Fe–al Layered Double Hydroxides (FeAl-LDH@BC)/H2O2 for Phenol Removal. Chemosphere 2021, 266, 128992. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhai, X.; Shao, W.; Bo, H.; Xu, L.; Guo, H.; Zhang, M.; Qiao, W. Activation of Peroxymonosulfate by Biochar-Supported Fe3O4 Derived from Oily Sludge to Enhance the Oxidative Degradation of Tetracycline Hydrochloride. J. Environ. Manag. 2023, 347, 119187. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Tang, J.; Cui, M.; Gao, B.; Shen, B. Biochar/Iron (BC/Fe) Composites for Soil and Groundwater Remediation: Synthesis, Applications, and Mechanisms. Chemosphere 2020, 246, 125609. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lu, T.; Tang, J.; Li, P.; Mašek, O.; Yang, L.; Wu, L.; He, L.; Ding, Y.; Gao, F.; et al. One-Pot Hydrothermal Synthesis of Magnetic N-Doped Sludge Biochar for Efficient Removal of Tetracycline from Various Environmental Waters. SSRN Electron. J. 2022, 297, 121426. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lua, S.-K.; Dong, Z.; Lim, T.-T. Performance of Magnetic Activated Carbon Composite as Peroxymonosulfate Activator and Regenerable Adsorbent via Sulfate Radical-Mediated Oxidation Processes. J. Hazard. Mater. 2015, 284, 1–9. [Google Scholar] [CrossRef]
- Feng, X.; Sun, D. Enhanced Naproxen Adsorption by a Novel B-Cyclodextrin Immobilized the Three-Dimensional Macrostructure of Reduced Graphene Oxide and Multiwall Carbon Nanotubes. SSRN Electron. J. 2022, 290, 120837. [Google Scholar] [CrossRef]
- Das, K.C.; Dhar, S.S. Rapid Catalytic Degradation of Malachite Green by MgFe2O4 Nanoparticles in Presence of H2O2. J. Alloys Compd. 2020, 828, 154462. [Google Scholar] [CrossRef]
- Wang, L.; Lu, X.; Chen, G.; Zhao, Y.; Wang, S. Synergy between MgFe2O4 and Biochar Derived from Banana Pseudo-Stem Promotes Persulfate Activation for Efficient Tetracycline Degradation. Chem. Eng. J. 2023, 468, 143773. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, D.; Zhang, X.; Gu, L.; Yuan, Y. Ascorbic Acid-Assisted Hydrothermal Route to Create Mesopores in Polymeric Carbon Nitride for Increased Photocatalytic Hydrogen Generation. Int. J. Hydrogen Energy 2021, 46, 38310–38318. [Google Scholar] [CrossRef]
- Deng, Y.; Xiao, T.; She, A.; Li, X.; Chen, W.; Ao, T.; Ni, F. One-Step Synthesis of Iron and Nitrogen Co-Doped Porous Biochar for Efficient Removal of Tetracycline from Water: Adsorption Performance and Fixed-Bed Column. J. Environ. Manag. 2024, 352, 119984. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Mao, Y.; Wang, H.; Xu, N. A Comparative Study of Arsenic(V), Tetracycline and Nitrate Ions Adsorption onto Magnetic Biochars and Activated Carbon. Chem. Eng. Res. Des. 2020, 159, 582–591. [Google Scholar] [CrossRef]
- Lei, J.; Han, Y.; Zhao, C.; Zhang, S.; Han, F.; Li, Z.; Hao, J.; Zhou, W. Activation Behavior of Cu0/FeS/N-Graphene Derived from Waste Soybean Residue for Peroxymonosulfate: Performance and Mechanism. Sep. Purif. Technol. 2023, 324, 124591. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Z.; Wang, S.; Wang, Z.; Jia, J.; Li, H.; Cao, Y.; Chen, Y.; Qin, Y.; Cui, F. Rapid Synthesis of Amorphous CoO Nanosheets: Highly Efficient Catalyst for Parachlorophenol Degradation by Peroxymonosulfate Activation. Sep. Purif. Technol. 2021, 263, 118369. [Google Scholar] [CrossRef]
- Jiang, S.-F.; Ling, L.-L.; Chen, W.-J.; Liu, W.-J.; Li, D.-C.; Jiang, H. High Efficient Removal of Bisphenol a in a Peroxymonosulfate/Iron Functionalized Biochar System: Mechanistic Elucidation and Quantification of the Contributors. Chem. Eng. J. 2019, 359, 572–583. [Google Scholar] [CrossRef]
- Lu, S.; Wang, G.; Chen, S.; Yu, H.; Ye, F.; Quan, X. Heterogeneous activation of peroxymonosulfate by LaCo1-xCuxO3 perovskites for degradation of organic pollutants. J. Hazard. Mater. 2018, 353, 401–409. [Google Scholar] [CrossRef]
- Meng, H.; Nie, C.; Li, W.; Duan, X.; Lai, B.; Ao, Z.; Wang, S.; An, T. Insight into the Effect of Lignocellulosic Biomass Source on the Performance of Biochar as Persulfate Activator for Aqueous Organic Pollutants Remediation: Epicarp and Mesocarp of Citrus Peels as Examples. J. Hazard. Mater. 2020, 399, 123043. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Fan, Y.; Tong, T.; Han, X.; Du, Y. Non-Radical-Dominated Catalytic Degradation of Bisphenol a by ZIF-67 Derived Nitrogen-Doped Carbon Nanotubes Frameworks in the Presence of Peroxymonosulfate. Chem. Eng. J. 2018, 336, 721–731. [Google Scholar] [CrossRef]
- Feng, Y.; Lee, P.-H.; Wu, D.; Shih, K. Rapid Selective Circumneutral Degradation of Phenolic Pollutants Using Peroxymonosulfate–Iodide Metal-Free Oxidation: Role of Iodine Atoms. Environ. Sci. Technol. 2017, 51, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, K.; Ma, S.; Dang, B.; Li, Y.; Fu, H.; Du, J.; Meng, Q. Activation of Peroxymonosulfate by Iron-Biochar Composites: Comparison of Nanoscale Fe with Single-Atom Fe. J. Colloid Interface Sci. 2021, 582, 598–609. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Zhou, L.; Sun, H.; Wang, G.; Wang, S. Occurrence of Radical and Nonradical Pathways from Carbocatalysts for Aqueous and Nonaqueous Catalytic Oxidation. Appl. Catal. B Environ. 2016, 188, 98–105. [Google Scholar] [CrossRef]
- Luo, X.; Shen, M.; Liu, J.; Ma, Y.; Gong, B.; Liu, H.; Huang, Z. Resource Utilization of Piggery Sludge to Prepare Recyclable Magnetic Biochar for Highly Efficient Degradation of Tetracycline through Peroxymonosulfate Activation. J. Clean. Prod. 2021, 294, 126372. [Google Scholar] [CrossRef]
- Wu, L.; Sun, Z.; Zhen, Y.; Zhu, S.; Yang, C.; Lu, J.; Tian, Y.; Zhong, D.; Ma, J. Oxygen Vacancy-Induced Nonradical Degradation of Organics: Critical Trigger of Oxygen (O2) in the Fe–Co LDH/Peroxymonosulfate System. Environ. Sci. Technol. 2021, 55, 15400–15411. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, F.; Guo, H.; Su, D. Activate Hydrogen Peroxide for Efficient Tetracycline Degradation via a Facile Assembled Carbon-Based Composite: Synergism of Powdered Activated Carbon and Ferroferric Oxide Nanocatalyst. Appl. Catal. B Environ. 2020, 269, 118784. [Google Scholar] [CrossRef]
- Ren, W.; Nie, G.; Zhou, P.; Zhang, H.; Duan, X.; Wang, S. The Intrinsic Nature of Persulfate Activation and N-Doping in Carbocatalysis. Environ. Sci. Technol. 2020, 54, 6438–6447. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, S.; Zhang, X.; Pan, Z.; Xu, R.; Wang, P.; Sun, T.; Fan, X.; Song, C.; Wang, T. N-Doped Coal-Based Carbon Membrane Coupling Peroxymonosulfate Activation for Bisphenol a Degradation: The Role of Micro-Carbon Structure and Nitrogen Species. J. Clean. Prod. 2023, 423, 138713. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Z.; Zhou, Z.; Li, X.; Lin, Y.; Cheng, J.J.; Yang, C. Catalytic Activity and Reaction Mechanisms of Single-Atom Metals Anchored on Nitrogen-Doped Carbons for Peroxymonosulfate Activation. J. Hazard. Mater. 2023, 459, 132133. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Chen, Y.; Zhang, Y.; Li, H.; Xu, S.; Fu, X.; Zhao, A.; Huang, X.; Lai, J. Magnetic Biochar Derived from Waste Bamboo as a Peroxymonosulfate Activator for Tetracycline Hydrochloride Degradation. Molecules 2025, 30, 2283. https://doi.org/10.3390/molecules30112283
Huang X, Chen Y, Zhang Y, Li H, Xu S, Fu X, Zhao A, Huang X, Lai J. Magnetic Biochar Derived from Waste Bamboo as a Peroxymonosulfate Activator for Tetracycline Hydrochloride Degradation. Molecules. 2025; 30(11):2283. https://doi.org/10.3390/molecules30112283
Chicago/Turabian StyleHuang, Xingyan, Yuanlong Chen, Yujia Zhang, Hongpeng Li, Shihao Xu, Xinhong Fu, Anjiu Zhao, Xiaobo Huang, and Jiaming Lai. 2025. "Magnetic Biochar Derived from Waste Bamboo as a Peroxymonosulfate Activator for Tetracycline Hydrochloride Degradation" Molecules 30, no. 11: 2283. https://doi.org/10.3390/molecules30112283
APA StyleHuang, X., Chen, Y., Zhang, Y., Li, H., Xu, S., Fu, X., Zhao, A., Huang, X., & Lai, J. (2025). Magnetic Biochar Derived from Waste Bamboo as a Peroxymonosulfate Activator for Tetracycline Hydrochloride Degradation. Molecules, 30(11), 2283. https://doi.org/10.3390/molecules30112283





