NaOH/Urea-Compatible Chitosan/Carboxymethylcellulose Films: Orthogonal Optimization of Packaging Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Thickness and Apparent Analysis of (CS/CMC) Composite Film
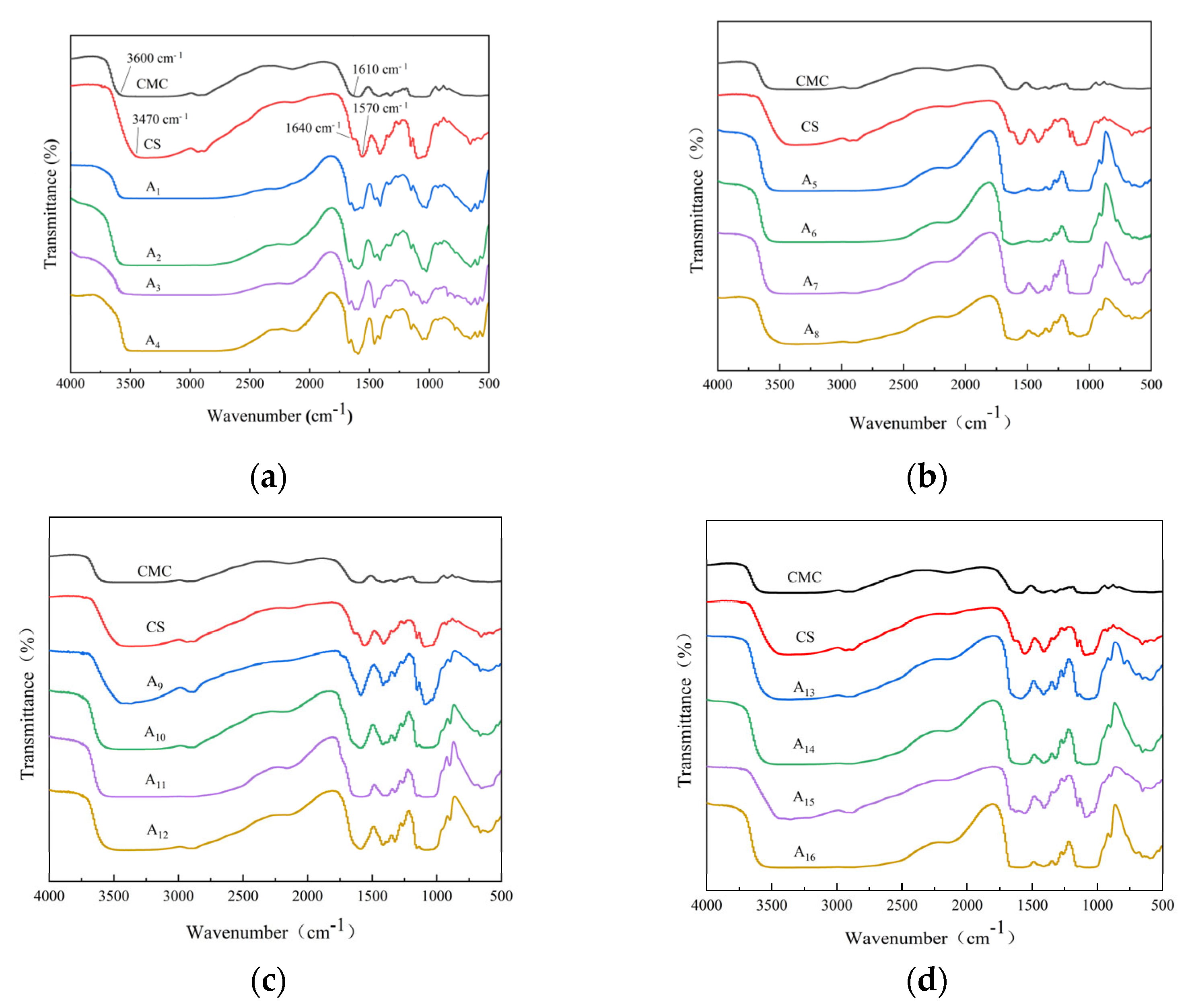
2.2. FTIR Analysis of CS/CMC Composite Films
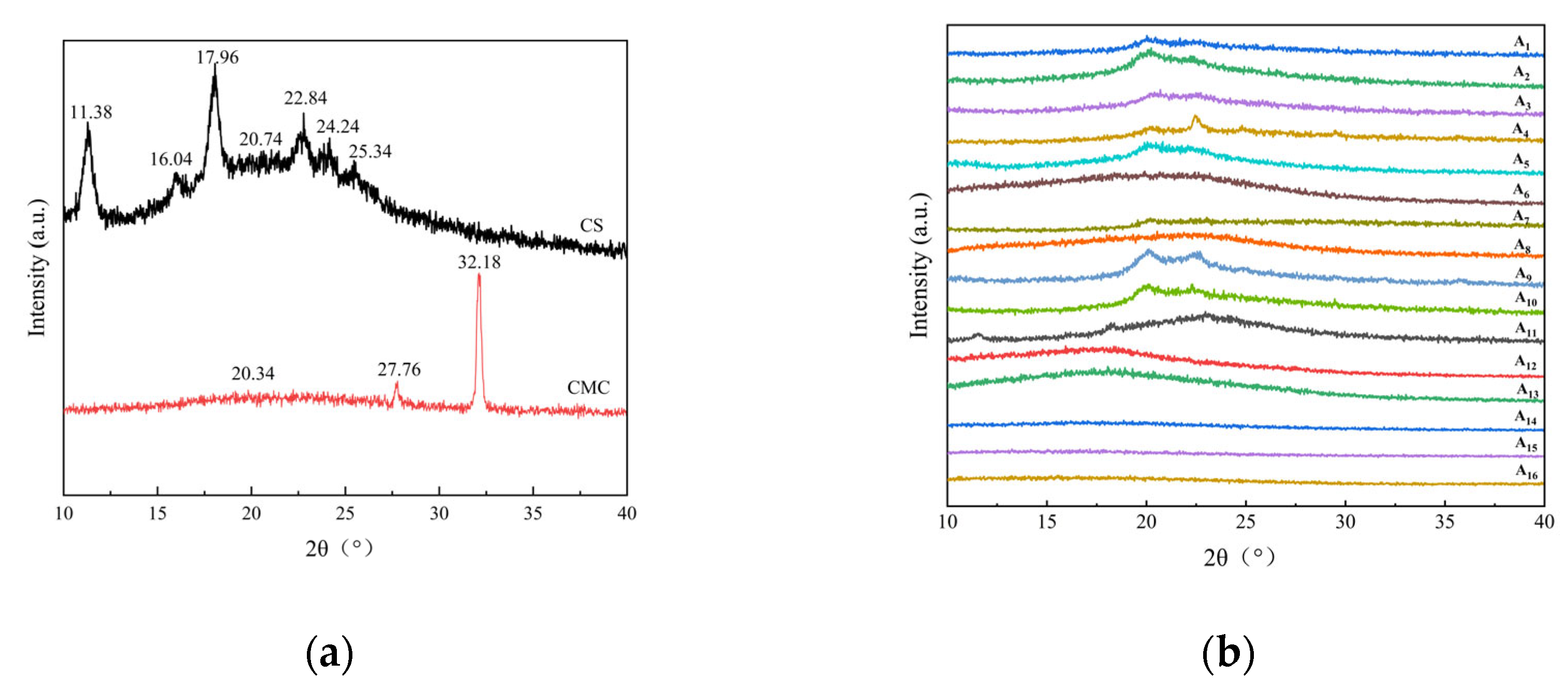
2.3. XRD Analysis of CS/CMC Composite Films
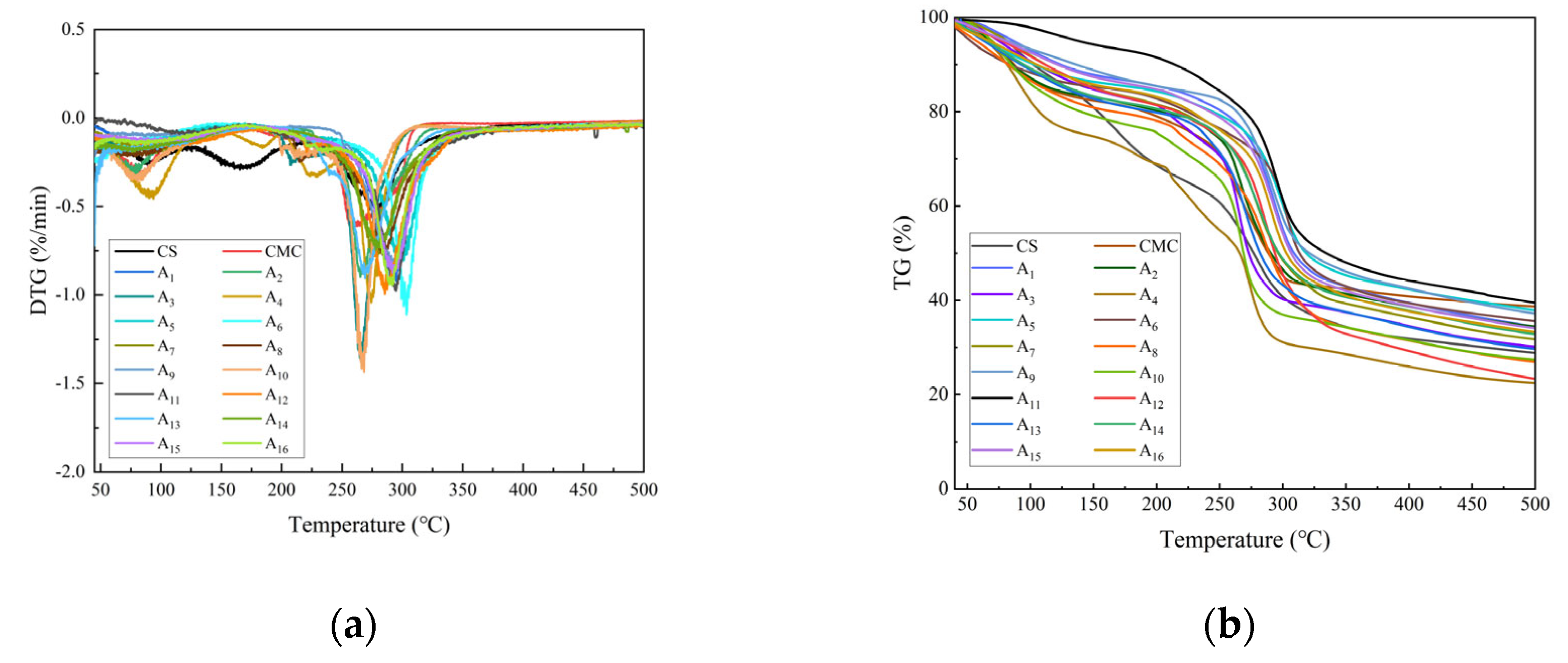
2.4. Thermogravimetric Analysis of CS/CMC Composite Films
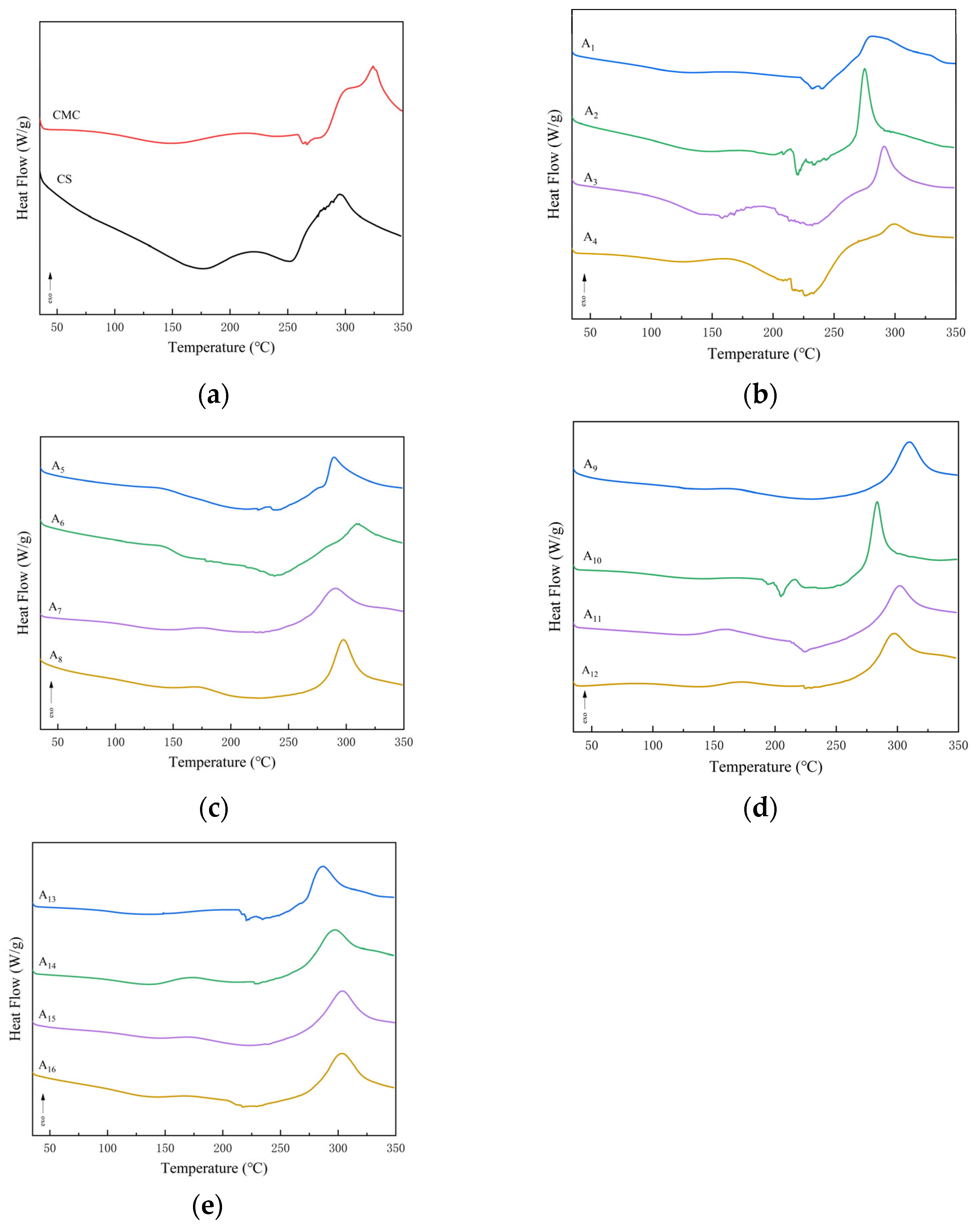
2.5. DSC Analysis of CS/CMC Composite Film
2.6. Mechanical Properties of CS/CMC Composite Films
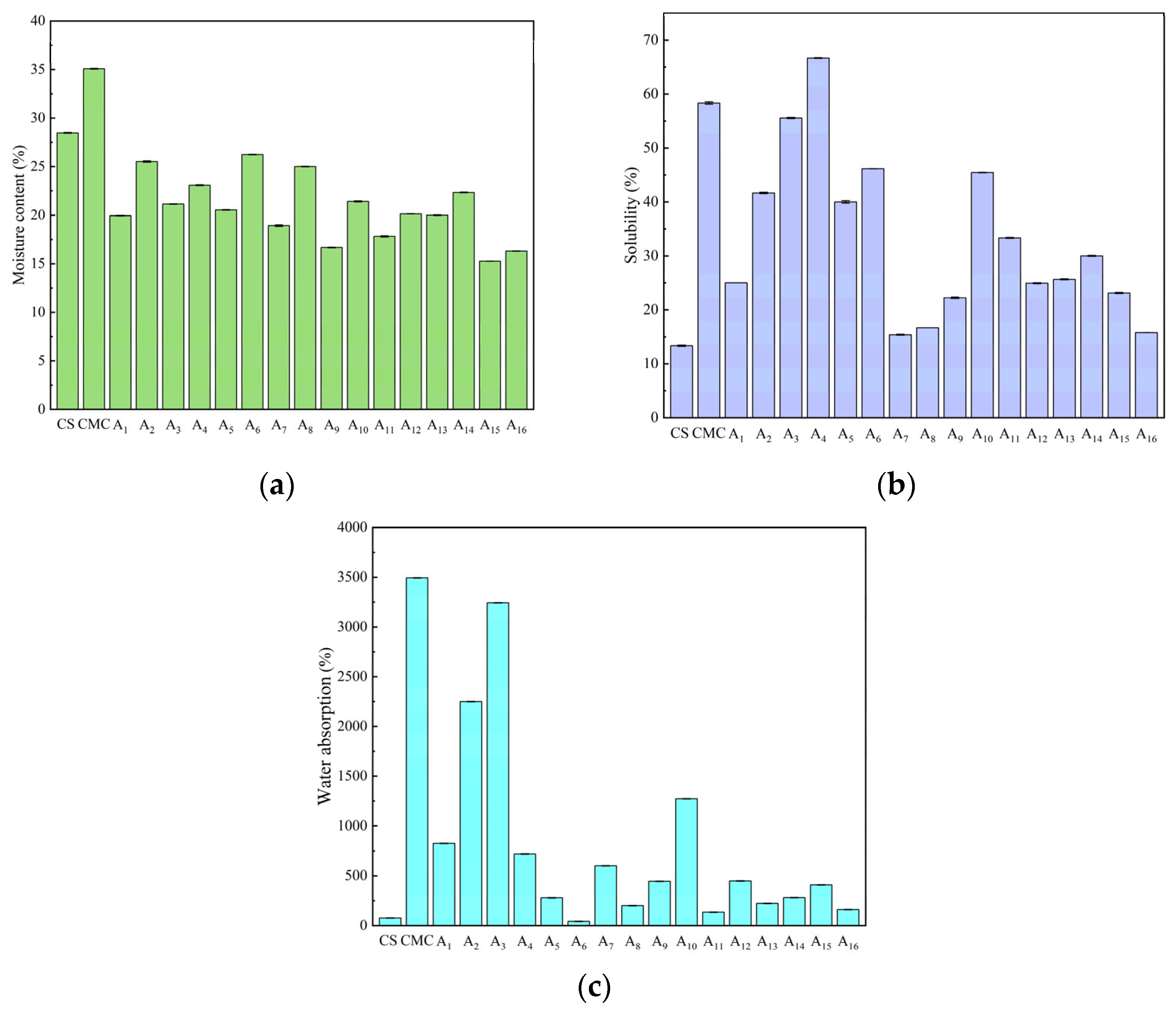
2.7. Moisture Content, Water Absorption, and Solubility of CS/CMC Composite Films
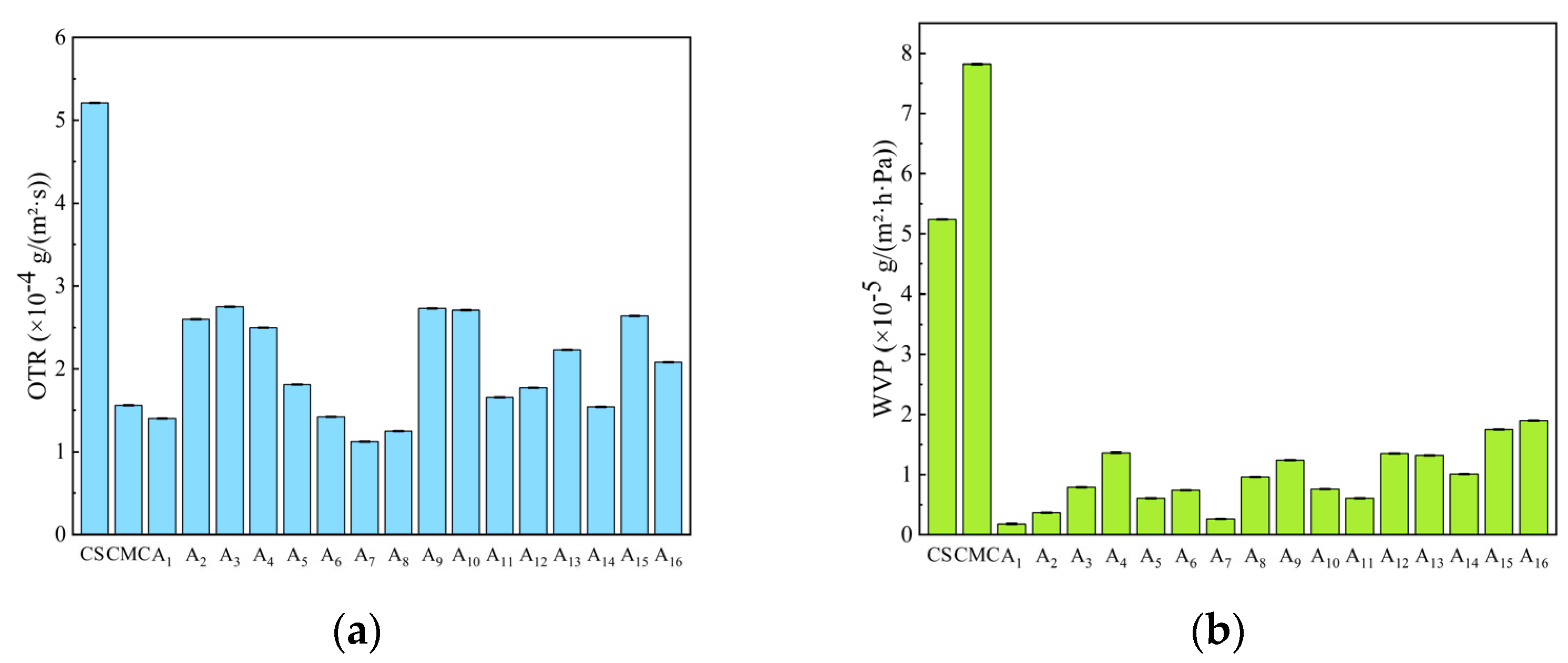
2.8. Water Vapor Permeability (WVP) and Oxygen Transmission Rate (OTR) of CS/CMC Composite Films
2.9. UV-Blocking Properties
2.10. Statistical Analysis of Orthogonal Experiment
3. Materials and Methods
3.1. Materials
3.2. Film Preparation
3.3. Orthogonal Experimental Design
3.3.1. Factors and Levels
3.3.2. Experimental Matrix
3.4. Characterization and Testing
3.4.1. Thickness and Appearance of CS/CMC Composite Film
3.4.2. FTIR Analysis
3.4.3. X-Ray Diffraction (XRD)
3.4.4. Thermogravimetric Analysis
3.4.5. Differential Scanning Calorimetry (DSC)
3.4.6. Mechanical Properties
3.4.7. Moisture Content, Water Absorption and Solubility
3.4.8. Water Vapor Transmission Rate (WVP)
3.4.9. Oxygen Permeability (OTR)
3.4.10. Ultraviolet–Visible Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leite, L.S.F.; Le Gars, M.; Azeredo, H.M.; Moreira, F.K.; Mattoso, L.H.; Bras, J. Insights into the effect of carboxylated cellulose nanocrystals on mechanical and barrier properties of gelatin films for flexible packaging applications. Int. J. Biol. Macromol. 2024, 280, 135726. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Preparation and applications of chitosan and cellulose composite materials. J. Environ. Manag. 2022, 301, 113850. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, T.; Qi, J.; Liu, K.; Zhang, M.; Si, C. Nano/micro flexible fiber and paper-based advanced functional packaging materials. Food Chem. 2024, 458, 140329. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Y.; Tang, Z.; Chen, K.; Wang, Z.; Cheng, G.; Chi, H.; Soteyome, T. A pH-sensitive film based on chitosan/gelatin and anthocyanin from Zingiber striolatum Diels for monitoring fish freshness. Food Chem.-X 2024, 23, 101639. [Google Scholar] [CrossRef]
- Nunes, C.; Coimbra, M.A.; Ferreira, P. Tailoring Functional Chitosan-Based Composites for Food Applications. Chem. Rec. 2018, 18, 1138–1149. [Google Scholar] [CrossRef]
- Mujtaba, M.; Salaberria, A.M.; Andres, M.A.; Kaya, M.; Gunyakti, A.; Labidi, J. Utilization of flax (Linum usitatissimum) cellulose nanocrystals as reinforcing material for chitosan films. Int. J. Biol. Macromol. 2017, 104, 944–952. [Google Scholar] [CrossRef]
- He, X.; Zhang, Z.; Xuan, X.; Tan, T.; Sun, J.; Wang, B.; Tian, Y.; Chen, H. Structure and properties of chitosan plasticized with hydrophobic short-chain fatty acid cosolvent. Int. J. Biol. Macromol. 2025, 300, 140250. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Zhang, C.; Liu, K.; Duan, G. Source of Nanocellulose and Its Application in Nanocomposite Packaging Material: A Review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef]
- Revathi, S.; Amanullah, M.; Al-Samghan, A.S.; Joseph, J.J.; Pazhanisamy, P.; Addich, M.; Gomathi, T. Sustainable heavy metal (Cr(VI) ion) remediation: Ternary blend approach with chitosan, carboxymethyl cellulose, and bioactive glass. Int. J. Biol. Macromol. 2024, 278, 134769. [Google Scholar] [CrossRef]
- Shi, X.; Kang, M.; Zhang, H.; Xu, J.; Wang, C.; Xu, Y.; Chang, C.; Ge, B.; Gao, S. Hydrogen Bond Cross-Linked Montmorillonite-Enhanced Biodegradable Carboxymethyl Cellulose/Guar Gum Composite Films. Acs Appl. Polym. Mater. 2024, 6, 10788–10797. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Liu, B.; Wang, K.; Li, H.; Peng, L. Carboxymethyl cellulose-based multifunctional film integrated with polyphenol-rich extract and carbon dots from coffee husk waste for active food packaging applications. Food Chem. 2024, 448, 139143. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Wang, M.; Zhou, Z.; You, Y.; Wang, G.; Wu, D. Cellulose-based intelligent packaging films with antibacterial, UV-blocking, and biodegradable properties for shrimp freshness monitoring. Chem. Eng. J. 2024, 488, 150975. [Google Scholar] [CrossRef]
- Chen, H.; Meng, X.; Zhang, F.; Chen, J.; Ding, X.; Jian, T.; Niu, G.; Tong, B.; Gai, Y.; Zhao, H.; et al. Development of chitosan-carboxymethyl cellulose edible films loaded with blackberry anthocyanins and tea polyphenols and their application in beef preservation. Food Hydrocoll. 2025, 164, 111198. [Google Scholar] [CrossRef]
- Lan, W.; He, L.; Liu, Y. Preparation and Properties of Sodium Carboxymethyl Cellulose/Sodium Alginate/Chitosan Composite Film. Coatings 2018, 8, 291. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent Advances in Applications of Cellulose Derivatives-Based Composite Films with Hydroxyapatite. Materials 2020, 13, 2481. [Google Scholar] [CrossRef]
- Hu, X.; Du, Y.; Tang, Y.; Wang, Q.; Feng, T.; Yang, J.; Kennedy, J.F. Solubility and property of chitin in NaOH/urea aqueous solution. Carbohydr. Polym. 2007, 70, 451–458. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid dissolution of cellulose in LiOH/Urea and NaOH/Urea aqueous solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Yu, Y.; Xie, C. Preparation of a PVA/Chitosan/Glass Fiber Composite Film and the Performance in CO2 Separation. Films 2023, 13, 36. [Google Scholar] [CrossRef]
- Abu El-Soad, A.M.; Lazzara, G.; El-Magied, M.O.A.; Cavallaro, G.; Al-Otaibi, J.S.; Sayyed, M.I.; Kovaleva, E.G. Chitosan Functionalized with Carboxyl Groups as a Recyclable Biomaterial for the Adsorption of Cu (II) and Zn (II) Ions in Aqueous Media. Int. J. Mol. Sci. 2022, 23, 2396. [Google Scholar] [CrossRef]
- Liu, S.; Li, L. Unique gelation of chitosan in an alkali/urea aqueous solution. Polymer 2018, 141, 124–131. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, J.; Yang, B.; Fu, F.; Tang, H.; Zhang, J.; Zhao, Y.; Zhang, Y.; Liu, L.; Zhu, Y.; et al. Self-assembly of chitosan and cellulose chains into a 3D porous polysaccharide alloy films: Co-dissolving, structure and biological properties. Appl. Surf. Sci. 2019, 493, 1032–1041. [Google Scholar] [CrossRef]
- Duan, J.; Liang, X.; Cao, Y.; Wang, S.; Zhang, L. High Strength Chitosan Hydrogels with Biocompatibility via New Avenue Based on Constructing Nanofibrous Architecture. Macromolecules 2015, 48, 2706–2714. [Google Scholar] [CrossRef]
- Zeng, J.; Ren, X.; Zhu, S.; Gao, Y. Cellulose nanocrystals from pomegranate peel: Isolation, characterization, and its reinforcement for chitosan film. J. Mater. Sci. 2022, 57, 11062–11076. [Google Scholar] [CrossRef]
- Chen, C.; Deng, S.; Tian, H.; Yu, H.; Huang, J.; Lou, X.; Yuan, H. Enhanced fresh walnut preservation using chitosan films reinforced with cinnamon essential oil and bacterial cellulose pickering emulsion. Food Hydrocoll. 2025, 165, 111267. [Google Scholar] [CrossRef]
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. Mechanical and Water-Resistant Properties of Eco-Friendly Chitosan Film Reinforced with Cellulose Nanocrystals. Polymers 2019, 11, 166. [Google Scholar] [CrossRef]
- Almeida, E.V.R.; Frollini, E.; Castellan, A.; Coma, V. Chitosan, sisal cellulose, and biocomposite chitosan/sisal cellulose films prepared from thiourea/NaOH aqueous solution. Carbohydr. Polym. 2010, 80, 655–664. [Google Scholar] [CrossRef]
- Morgado, D.L.; Frollini, E.; Castellan, A.; Rosa, D.S.; Coma, V. Biobased films prepared from NaOH/thiourea aqueous solution of chitosan and linter cellulose. Cellulose 2011, 18, 699–712. [Google Scholar] [CrossRef]
- Tu, G.; Liu, X.; Li, Z.; Li, S.; Liu, J.; Li, W. Characterizing gelation kinetics of chitosan dissolved in an alkali/urea aqueous solution: Mechanisms accounting for the morphological development. J. Membr. Sci. 2021, 635, 119516. [Google Scholar] [CrossRef]
- Pimonpan, K.; Chalalai, J.; Saroat, R.; Wirongrong, T.; Warinporn, K. Carboxymethyl cellulose from Young Palmyra palm fruit husk: Synthesis, characterization, and film properties. Food Hydrocoll. 2022, 124 Pt A, 107277. [Google Scholar] [CrossRef]
- He, M.; Chen, H.; Zhang, X.; Wang, C.; Xu, C.; Xue, Y.; Wang, J.; Zhou, P.; Zhao, Q. Construction of novel cellulose/chitosan composite hydrogels and films and their applications. Cellulose 2018, 25, 1987–1996. [Google Scholar] [CrossRef]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, J.; Zhang, R.; Yan, C.; Cui, L.; Zhu, J. A pH-responsive carboxymethyl cellulose/chitosan hydrogel for adsorption and desorption of anionic and cationic dyes. Cellulose 2021, 28, 897–909. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.-W. Biopolymer-based UV protection functional films for food packaging. Food Hydrocoll. 2023, 142, 108771. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Ye, L.; Zhi, C.; Zhang, T.; Miao, M. Development of starch-cellulose composite films with antimicrobial potential. Int. J. Biol. Macromol. 2024, 276, 133836. [Google Scholar] [CrossRef]
- ASTM F2103-18; Standard Guide for Characterization and Testing of Chitosan Salts as Starting Materials Intended for Use in Biomedical and Tissue-Engineered Medical Product Applications. ASTM International: West Conshohocken, PA, USA, 2018.
- GB/T 13022-1991; Plastics—Determination of Tensile Properties of Films. Standards Press of China: Beijing, China, 1991.
- Muhammad Shahar, Y.; Isaiah Henry, I.; Bako, H.K.; Alnadari, F.; Bilal, M.; Rehman, F.; Zhu, J.; Zhou, T.; Zhao, Z.; Li, C. A novel carboxymethyl cellulose/gum xanthan and citric acid-based film that enhances the precision of blackcurrant anthocyanin-induced color detection for beef spoilage tracking. Food Chem. 2024, 461, 140905. [Google Scholar] [CrossRef]
- Manjushree Nagaraj, G.; Saraswati, P.M.; D’Souza, O.J.; Eelager, M.P.; Kurabetta, L.K.; Chougale, R.B.; Kadapure, A.J.; Kumar, S.P. Fabrication of CuO nanoparticles embedded novel chitosan/hydroxypropyl cellulose bio-nanocomposites for active packaging of jamun fruit. Food Hydrocoll. 2024, 152, 109937. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Li, Y.-C.; Xiao, H.; Wang, X. Novel chitosan films with laponite immobilized Ag nanoparticles for active food packaging. Carbohydr. Polym. 2018, 199, 210–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; Huang, K.; Xu, Z.; Feng, S.; Li, H.; Zou, Z. Developing strong and tough cellulose acetate/ZIF67 intelligent active films for shrimp freshness monitoring. Carbohydr. Polym. 2023, 302, 120375. [Google Scholar] [CrossRef]

| Run No. | Thickness (mm) | Apparent |
|---|---|---|
| CS | 0.11 ± 0.01 |  |
| CMC | 0.11 ± 0.01 |  |
| A1 | 0.10 ± 0.01 |  |
| A2 | 0.15 ± 0.01 |  |
| A3 | 0.19 ± 0.02 |  |
| A4 | 0.22 ± 0.01 |  |
| A5 | 0.16 ± 0.02 |  |
| A6 | 0.24 ± 0.02 |  |
| A7 | 0.30 ± 0.02 |  |
| A8 | 0.36 ± 0.03 |  |
| A9 | 0.38 ± 0.03 |  |
| A10 | 0.32 ± 0.02 |  |
| A11 | 0.27 ± 0.02 |  |
| A12 | 0.40 ± 0.03 |  |
| A13 | 0.33 ± 0.03 |  |
| A14 | 0.38 ± 0.01 |  |
| A15 | 0.43 ± 0.02 |  |
| A16 | 0.46 ± 0.02 |  |
| Run No. | Experimental Design | Response Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A: CS % (w/v) | B: CMC % (w/v) | C: NaOH % (w/v) | D: Urea % (w/v) | Tensile Strength (MPa) | Elongation at Break (%) | T280 (%) | T320 (%) | WVP (×10−5 g/(m2·h·Pa)) | OTR (×10−4 g/(m2·s)) | |
| A1 | 1 (1.0%) | 1 (1.0%) | 1 (1.0%) | 1 (4.0%) | 46.08 | 4.88 | 2.44 | 3.80 | 0.18 | 1.40 |
| A2 | 1 | 2 (1.5%) | 2 (1.5%) | 2 (8.0%) | 11.77 | 30.06 | 14.48 | 19.19 | 0.37 | 2.60 |
| A3 | 1 | 3 (2.0%) | 3 (2.0%) | 3 (12.0%) | 3.54 | 46.27 | 4.07 | 7.21 | 0.79 | 2.75 |
| A4 | 1 | 4 (2.5%) | 4 (2.5%) | 4 (16.0%) | 1.76 | 52.11 | 0.73 | 1.29 | 1.36 | 2.50 |
| A5 | 2 (1.5%) | 1 | 2 | 3 | 4.06 | 42.22 | 4.30 | 5.73 | 0.61 | 1.81 |
| A6 | 2 | 2 | 1 | 4 | 2.45 | 58.04 | 6.67 | 8.40 | 0.74 | 1.42 |
| A7 | 2 | 3 | 4 | 1 | 3.47 | 68.00 | 1.34 | 2.14 | 0.26 | 1.12 |
| A8 | 2 | 4 | 3 | 2 | 11.57 | 26.64 | 4.72 | 6.72 | 0.96 | 1.25 |
| A9 | 3 (2.0%) | 1 | 3 | 4 | 1.62 | 35.67 | 0.77 | 1.43 | 1.24 | 2.73 |
| A10 | 3 | 2 | 4 | 3 | 1.74 | 39.51 | 2.54 | 4.87 | 0.76 | 2.71 |
| A11 | 3 | 3 | 1 | 2 | 13.3 | 29.77 | 4.36 | 6.50 | 0.61 | 1.66 |
| A12 | 3 | 4 | 2 | 1 | 7.84 | 36.68 | 0.36 | 0.61 | 1.35 | 1.77 |
| A13 | 4 (2.5%) | 1 | 4 | 2 | 1.64 | 41.34 | 0.83 | 1.33 | 1.32 | 2.23 |
| A14 | 4 | 2 | 3 | 1 | 5.73 | 37.30 | 0.62 | 1.08 | 1.01 | 1.54 |
| A15 | 4 | 3 | 2 | 4 | 3.72 | 24.25 | 0.18 | 0.40 | 1.75 | 2.64 |
| A16 | 4 | 4 | 1 | 3 | 12.14 | 26.73 | 1.27 | 2.35 | 1.90 | 2.08 |
| Response Variable | Experimental Factors | ||||
|---|---|---|---|---|---|
| A: CS | B: CMC | C: NaOH | D: Urea | ||
| Tensile strength (MPa) | k1 | 15.79 | 13.35 | 18.49 | 15.78 |
| k2 | 5.39 | 5.42 | 6.85 | 9.57 | |
| k3 | 6.13 | 6.01 | 5.62 | 5.37 | |
| k4 | 5.81 | 8.33 | 2.15 | 2.39 | |
| R | 10.40 | 7.93 | 16.34 | 13.39 | |
| Order of priority | C > D > A > B | ||||
| Optimization | A1 | B1 | C1 | D1 | |
| Elongation at break (%) | k1 | 33.33 | 31.03 | 29.86 | 36.71 |
| k2 | 48.72 | 41.23 | 33.30 | 31.95 | |
| k3 | 35.41 | 42.07 | 36.47 | 38.68 | |
| k4 | 32.41 | 35.54 | 50.24 | 42.52 | |
| R | 16.32 | 11.04 | 20.38 | 10.57 | |
| Order of priority | C > A > B > D | ||||
| Optimization | A2 | B3 | C4 | D4 | |
| T280 (%) | k1 | 5.43 | 2.09 | 3.69 | 1.09 |
| k2 | 4.16 | 6.08 | 4.83 | 6.10 | |
| k3 | 2.01 | 2.39 | 2.55 | 3.05 | |
| k4 | 0.73 | 1.77 | 1.26 | 2.09 | |
| R | 4.71 | 4.31 | 3.57 | 5.01 | |
| Order of priority | D > A > B > C | ||||
| Optimization | A4 | B4 | C4 | D1 | |
| T320 (%) | k1 | 7.87 | 3.07 | 5.26 | 5.26 |
| k2 | 5.60 | 8.39 | 6.48 | 8.44 | |
| k3 | 3.35 | 3.91 | 4.11 | 5.04 | |
| k4 | 1.29 | 2.74 | 2.26 | 2.88 | |
| R | 6.58 | 5.64 | 4.23 | 5.56 | |
| Order of priority | A > B > D > C | ||||
| Optimization | A4 | B4 | C4 | D4 | |
| WVP (×10−5 g/(m2·h·Pa)) | k1 | 0.68 | 0.84 | 0.86 | 0.70 |
| k2 | 0.64 | 0.72 | 1.02 | 0.82 | |
| k3 | 0.99 | 0.85 | 1.00 | 1.02 | |
| k4 | 1.50 | 1.39 | 0.93 | 1.27 | |
| R | 0.85 | 0.67 | 0.16 | 0.57 | |
| Order of priority | A > B > D > C | ||||
| Optimization | A2 | B2 | C1 | D1 | |
| OTR (×10−4 g/(m2·s)) | k1 | 2.31 | 2.04 | 1.64 | 1.46 |
| k2 | 1.40 | 2.07 | 2.20 | 1.93 | |
| k3 | 2.22 | 2.04 | 2.07 | 2.34 | |
| k4 | 2.12 | 1.90 | 2.14 | 2.32 | |
| R | 0.91 | 0.17 | 0.56 | 0.88 | |
| Order of priority | A > D > C > B | ||||
| Optimization | A2 | B4 | C1 | D1 | |
| Source of Variance | Implicit Variable | Sum of Squared Deviations | Degree of Freedom | Mean Square | F | p * |
|---|---|---|---|---|---|---|
| A: CS | Tensile strength | 301.945 | 3 | 100.648 | 0.932 | 0.522 |
| Elongation at break | 694.891 | 3 | 231.63 | 0.650 | 0.634 | |
| T280 | 54.411 | 3 | 18.137 | 5.171 | 0.105 | |
| T320 | 98.135 | 3 | 32.712 | 5.866 | 0.090 | |
| WVP | 1.875 | 3 | 0.625 | 32.975 | 0.008 | |
| OTR | 2.077 | 3 | 0.692 | 15.418 | 0.025 | |
| B: CMC | Tensile strength | 156.147 | 3 | 52.049 | 0.482 | 0.718 |
| Elongation at break | 322.13 | 3 | 107.377 | 0.301 | 0.825 | |
| T280 | 48.159 | 3 | 16.053 | 4.576 | 0.122 | |
| T320 | 81.576 | 3 | 27.192 | 4.876 | 0.113 | |
| WVP | 1.083 | 3 | 0.361 | 19.052 | 0.019 | |
| OTR | 0.070 | 3 | 0.023 | 0.519 | 0.698 | |
| C: NaOH | Tensile strength | 603.983 | 3 | 201.328 | 1.864 | 0.311 |
| Elongation at break | 957.717 | 3 | 319.239 | 0.895 | 0.535 | |
| T280 | 26.683 | 3 | 8.894 | 2.536 | 0.232 | |
| T320 | 36.101 | 3 | 12.034 | 2.158 | 0.272 | |
| WVP | 0.066 | 3 | 0.022 | 1.166 | 0.451 | |
| OP | 0.780 | 3 | 0.260 | 5.793 | 0.092 | |
| D: Urea | Tensile strength | 404.415 | 3 | 134.805 | 1.248 | 0.430 |
| Elongation at break | 231.841 | 3 | 77.28 | 0.217 | 0.879 | |
| T280 | 54.645 | 3 | 18.215 | 5.193 | 0.105 | |
| T320 | 100.416 | 3 | 33.472 | 6.002 | 0.088 | |
| WVP1 | 0.756 | 3 | 0.252 | 13.291 | 0.031 | |
| OTR | 2.063 | 3 | 0.688 | 15.314 | 0.025 |
| Compose | A: CS | B: CMC | C: NaOH | D: Urea |
|---|---|---|---|---|
| Dry film mass percentage (w/w%) | 4.3–22.3 | 4.8–22.6 | 4.8–22.6 | 4.3–20.9 |
| Level | Factor | |||
|---|---|---|---|---|
| A: CS % (w/v) | B: CMC %(w/v) | C: NaOH %(w/v) | D: Urea % (w/v) | |
| 1 | 1.0 | 1.0 | 1.0 | 4.0 |
| 2 | 1.5 | 1.5 | 1.5 | 8.0 |
| 3 | 2.0 | 2.0 | 2.0 | 12.0 |
| 4 | 2.5 | 2.5 | 2.5 | 16.0 |
| Run No. | A: CS %(w/v) | B: CMC %(w/v) | C: NaOH %(w/v) | D: Urea %(w/v) |
|---|---|---|---|---|
| A1 | 1.0 | 1.0 | 1.0 | 4.0 |
| A2 | 1.0 | 1.5 | 1.5 | 8.0 |
| A3 | 1.0 | 2.0 | 1.0 | 12.0 |
| A4 | 1.0 | 2.5 | 2.5 | 16.0 |
| A5 | 1.5 | 1.0 | 1.5 | 12.0 |
| A6 | 1.5 | 1.5 | 1.0 | 16.0 |
| A7 | 1.5 | 2.0 | 2.5 | 4.0 |
| A8 | 1.5 | 2.5 | 2.0 | 8.0 |
| A9 | 2.0 | 1.0 | 2.0 | 16.0 |
| A10 | 2.0 | 1.5 | 2.5 | 12.0 |
| A11 | 2.0 | 2.0 | 1.0 | 8.0 |
| A12 | 2.0 | 2.5 | 1.5 | 4.0 |
| A13 | 2.5 | 1.0 | 2.5 | 8.0 |
| A14 | 2.5 | 1.5 | 2.0 | 4.0 |
| A15 | 2.5 | 2.0 | 1.5 | 16.0 |
| A16 | 2.5 | 2.5 | 1.0 | 12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Sun, H.; Yao, L.; Weng, Y. NaOH/Urea-Compatible Chitosan/Carboxymethylcellulose Films: Orthogonal Optimization of Packaging Properties. Molecules 2025, 30, 2279. https://doi.org/10.3390/molecules30112279
Yu C, Sun H, Yao L, Weng Y. NaOH/Urea-Compatible Chitosan/Carboxymethylcellulose Films: Orthogonal Optimization of Packaging Properties. Molecules. 2025; 30(11):2279. https://doi.org/10.3390/molecules30112279
Chicago/Turabian StyleYu, Chang, Hui Sun, Lin Yao, and Yunxuan Weng. 2025. "NaOH/Urea-Compatible Chitosan/Carboxymethylcellulose Films: Orthogonal Optimization of Packaging Properties" Molecules 30, no. 11: 2279. https://doi.org/10.3390/molecules30112279
APA StyleYu, C., Sun, H., Yao, L., & Weng, Y. (2025). NaOH/Urea-Compatible Chitosan/Carboxymethylcellulose Films: Orthogonal Optimization of Packaging Properties. Molecules, 30(11), 2279. https://doi.org/10.3390/molecules30112279








