Cell-Specific Vulnerability of Human Glioblastoma and Astrocytoma Cells to Mephedrone—An In Vitro Study
Abstract
1. Introduction
2. Results
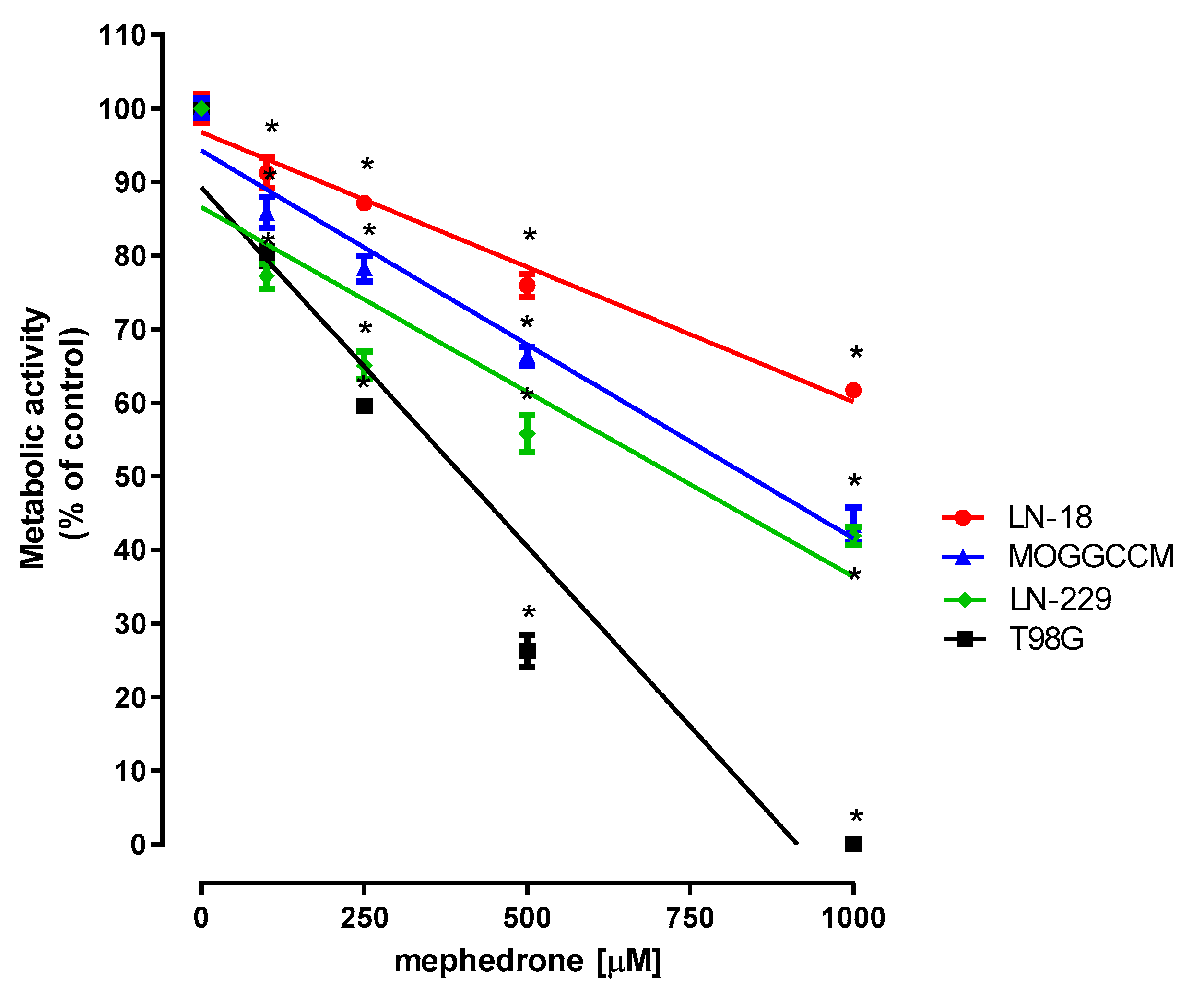
2.1. Effect of Mephedrone on the Metabolic Activity of Selected Astrocytoma and Glioblastoma Cells
2.2. Microscopic Detection of Apoptosis, Autophagy, Necrosis and Cytoplasmic Vacuolization
2.3. Effect of Mephedrone on the Proliferation of LN-18 and T98G Cells
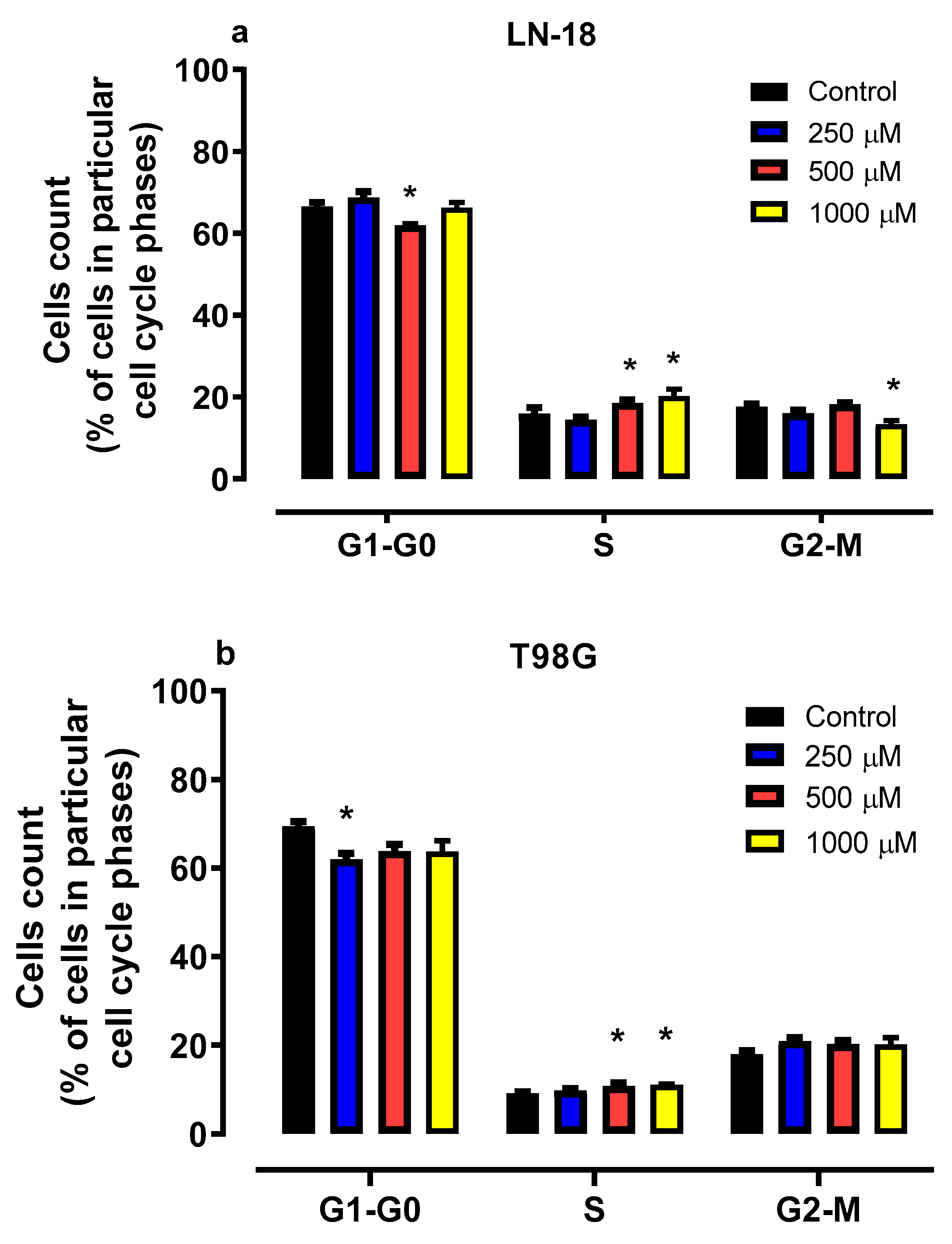
2.4. Effect of Mephedrone on Cell Cycle Progression in LN-18 and T98G Cells
2.5. Effect of Mephedrone on LN-18 and T98G Cell Morphology
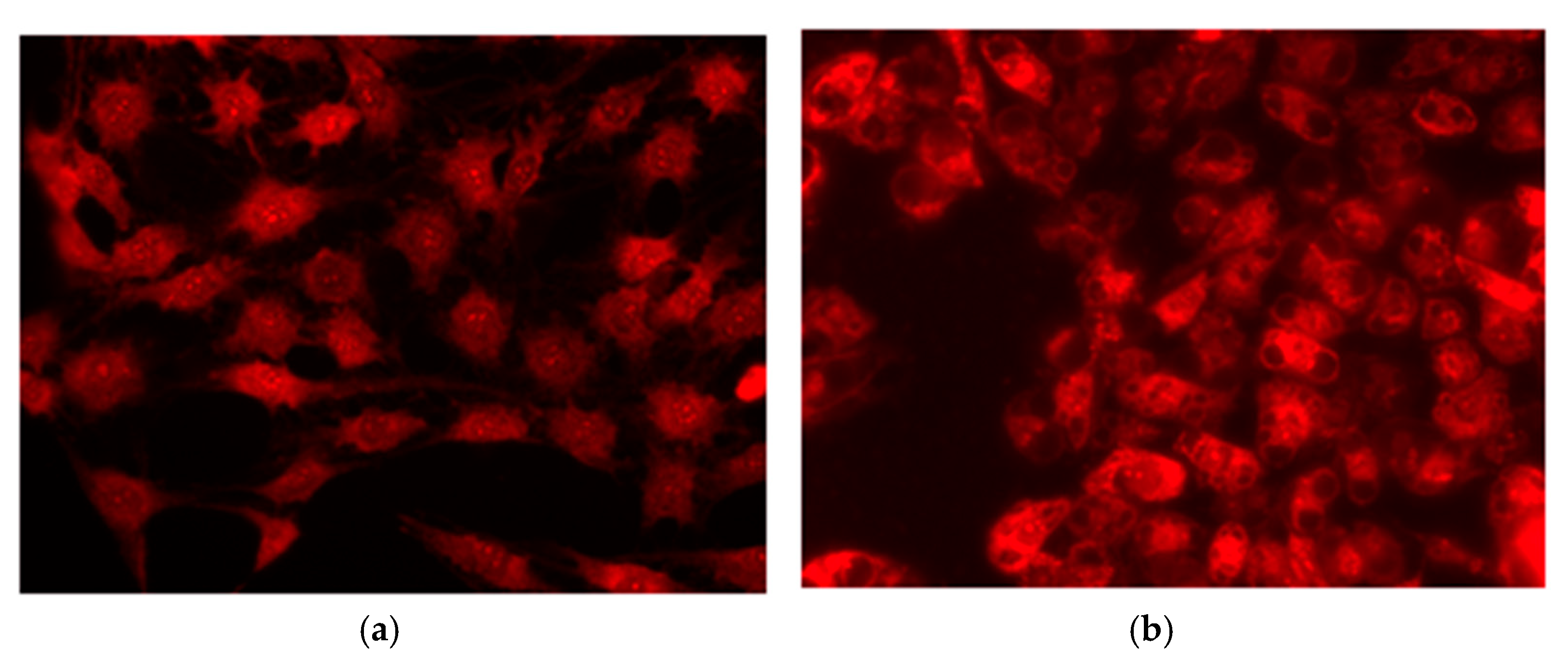
2.6. Fluorescence Microscopic and Electron Microscopic Evidence for Vacuolization of LN-18 Cells Exposed to Mephedrone
2.7. The Cytotoxic Effect of Mephedrone in LN-18 and T98G Cells
3. Discussion
4. Materials and Method
4.1. Reagents
4.2. Cell Lines
4.3. MTT Assay—Assessment of Cell Metabolic Activity
4.4. Microscopic Detection of Apoptosis, Autophagy, Necrosis and Cytoplasmic Vacuolization with Fluorochromes
4.5. BrdU Assay—Cell Proliferation Assessment
4.6. Flow Cytometry—Evaluation of Cell Cycle Progression
4.7. May–Grünwald–Giemsa Staining—Examination of Cell Morphology
4.8. Transmission Electron Microscopy
4.9. LDH Assay—Cytotoxicity Assessment
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef]
- Schonthal, A.H. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem. Pharmacol. 2013, 85, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, L.N.; Chow, E.K. Mechanisms of chemoresistance in cancer stem cells. Clin. Transl. Med. 2013, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Nie, C.; Lin, Y. The efficacy of targeted therapy combined with radiotherapy and temozolomide-based chemotherapy in the treatment of glioma: A systemic review and meta-analysis of phase II/III randomized controlled trials. Front. Oncol. 2023, 13, 1082539. [Google Scholar] [CrossRef]
- Ortiz, R.; Perazzoli, G.; Cabeza, L.; Jiménez-Luna, C.; Luque, R.; Prados, J.; Melguizo, C. Temozolomide: An updated overview of resistance mechanisms, nanotechnology advances and clinical applications. Curr. Neuropharmacol. 2021, 19, 513–537. [Google Scholar] [CrossRef]
- Silantyev, A.S.; Falzone, L.; Libra, M.; Gurina, O.I.; Kardashova, K.S.; Nikolouzakis, T.K.; Nosyrev, A.E.; Sutton, C.W.; Mitsias, P.D.; Tsatsakis, A. Current and future trends on diagnosis and prognosis of glioblastoma: From molecular biology to proteomics. Cells 2019, 8, 863. [Google Scholar] [CrossRef]
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef]
- Wang, X.; Hua, P.; He, C.; Chen, M. Non-apoptotic cell death-based cancer therapy: Molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharm. Sin. B 2022, 12, 3567–3593. [Google Scholar] [CrossRef]
- Button, R.W.; Roberts, S.L.; Willis, T.L.; Hanemann, C.O.; Luo, S. Accumulation of autophagosomes confers cytotoxicity. J. Biol. Chem. 2017, 292, 13599–13614. [Google Scholar] [CrossRef] [PubMed]
- Overmeyer, J.H.; Kaul, A.; Johnson, E.E.; Maltese, W.A. Active ras triggers death in glioblastoma cells through hyperstimulation of macropinocytosis. Mol. Cancer Res. 2008, 6, 965–977. [Google Scholar] [CrossRef]
- Overmeyer, J.H.; Young, A.M.; Bhanot, H.; Maltese, W.A. A chalcone-related small molecule that induces methuosis, a novel form of non-apoptotic cell death, in glioblastoma cells. Mol. Cancer 2011, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.W.; Overmeyer, J.H.; Young, A.M.; Erhardt, P.W.; Maltese, W.A. Synthesis and evaluation of indole-based chalcones as inducers of methuosis, a novel type of nonapoptotic cell death. J. Med. Chem. 2012, 55, 1940–1956. [Google Scholar] [CrossRef]
- Marszalek-Grabska, M.; Zakrocka, I.; Budzynska, B.; Marciniak, S.; Kaszubska, K.; Lemieszek, M.K.; Winiarczyk, S.; Kotlinska, J.H.; Rzeski, W.; Turski, W.A. Binge-like mephedrone treatment induces memory impairment concomitant with brain kynurenic acid reduction in mice. Toxicol. Appl. Pharmacol. 2022, 454, 116216. [Google Scholar] [CrossRef]
- den Hollander, B.; Sundström, M.; Pelander, A.; Ojanperä, I.; Mervaala, E.; Korpi, E.R.; Kankuri, E. Keto amphetamine toxicity—Focus on the redox reactivity of the cathinone designer drug mephedrone. Toxicol. Sci. 2014, 141, 120–131. [Google Scholar] [CrossRef]
- Available online: https://www.atcc.org/ (accessed on 20 March 2025).
- Available online: https://www.culturecollections.org.uk/ (accessed on 20 March 2025).
- Available online: https://www.cellosaurus.org/index.html (accessed on 20 March 2025).
- Available online: https://ckb.jax.org/ (accessed on 20 March 2025).
- Jakubowicz-Gil, J.; Bądziul, D.; Langner, E.; Wertel, I.; Zając, A.; Rzeski, W. Temozolomide and sorafenib as programmed cell death inducers of human glioma cells. Pharmacol. Rep. 2017, 69, 779–787. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Bądziul, D.; Langner, E.; Skalicka-Woźniak, K.; Maciejczyk, A.; Wertel, I.; Rzeski, W.; Jakubowicz-Gil, J. Coumarins modulate the anti-glioma properties of temozolomide. Eur. J. Pharmacol. 2020, 881, 173207. [Google Scholar] [CrossRef]
- Lee, S.Y.; Liu, S.; Mitchell, R.M.; Slagle-Webb, B.; Hong, Y.S.; Sheehan, J.M.; Connor, J.R. HFE polymorphisms influence the response to chemotherapeutic agents via induction of p16INK4A. Int. J. Cancer 2011, 129, 2104–2114. [Google Scholar] [CrossRef]
- Hermisson, M.; Klumpp, A.; Wick, W.; Wischhusen, J.; Nagel, G.; Roos, W.; Kaina, B.; Weller, M. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J. Neurochem. 2006, 96, 766–776. [Google Scholar] [CrossRef]
- St-Coeur, P.D.; Poitras, J.J.; Cuperlovic-Culf, M.; Touaibia, M.; Morin, P., Jr. Investigating a signature of temozolomide resistance in GBM cell lines using metabolomics. J. Neuro-Oncol. 2015, 125, 91–102. [Google Scholar] [CrossRef]
- Schnöller, L.E.; Albrecht, V.; Brix, N.; Nieto, A.E.; Fleischmann, D.F.; Niyazi, M.; Hess, J.; Belka, C.; Unger, K.; Lauber, K.; et al. Integrative analysis of therapy resistance and transcriptomic profiling data in glioblastoma cells identifies sensitization vulnerabilities for combined modality radiochemotherapy. Radiat. Oncol. 2022, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Avellaneda Matteo, D.; Grunseth, A.J.; Gonzalez, E.R.; Anselmo, S.L.; Kennedy, M.A.; Moman, P.; Scott, D.A.; Hoang, A.; Sohl, C.D. Molecular mechanisms of isocitrate dehydrogenase 1 (IDH1) mutations identified in tumors: The role of size and hydrophobicity at residue 132 on catalytic efficiency. J. Biol. Chem. 2017, 292, 7971–7983. [Google Scholar] [CrossRef]
- Alanazi, I.M.; Alzahrani, A.R.; Alsaad, M.A.; Moqeem, A.L.; Hamdi, A.M.; Taher, M.M.; Watson, D.G.; Grant, M.H. The effect of mephedrone on human neuroblastoma and astrocytoma cells. Saudi Pharm. J. 2024, 32, 102011. [Google Scholar] [CrossRef]
- Naseri, G.; Fazel, A.; Golalipour, M.J.; Haghir, H.; Sadeghian, H.; Mojarrad, M.; Hosseini, M.; Sabzevar, S.S.; Beheshti, F.; Ghorbani, A. Exposure to mephedrone during gestation increases the risk of stillbirth and induces hippocampal neurotoxicity in mice offspring. Neurotoxicol. Teratol. 2018, 67, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.; Costa, V.M.; Gaspar, H.; Santos, S.; Bastos, M.L.; Carvalho, F.; Capela, J.P. Adverse outcome pathways induced by 3,4-dimethylmethcathinone and 4-methylmethcathinone in differentiated human SH-SY5Y neuronal cells. Arch. Toxicol. 2020, 94, 2481–2503. [Google Scholar] [CrossRef] [PubMed]
- Slovackova, J.; Smarda, J.; Smardova, J. Roscovitine-induced apoptosis of H1299 cells depends on functional status of p53. Neoplasma 2012, 59, 606–612. [Google Scholar] [CrossRef]
- Menendez, D.; Inga, A.; Resnick, M.A. Estrogen receptor acting in cis enhances WT and mutant p53 transactivation at canonical and noncanonical p53 target sequences. Proc. Natl. Acad. Sci. USA 2010, 107, 1500–1505. [Google Scholar] [CrossRef]
- Boettcher, S.; Miller, P.G.; Sharma, R.; McConkey, M.; Leventhal, M.; Krivtsov, A.V.; Giacomelli, A.O.; Wong, W.; Kim, J.; Chao, S.; et al. A dominant-negative effect drives selection of TP53 missense mutations in myeloid malignancies. Science 2019, 365, 599–604. [Google Scholar] [CrossRef]
- Park, J.W.; Kang, J.; Lim, K.Y.; Kim, H.; Kim, S.I.; Won, J.K.; Park, C.-K.; Park, S.-H. The prognostic significance of p16 expression pattern in diffuse gliomas. J. Pathol. Transl. Med. 2021, 55, 102–111. [Google Scholar] [CrossRef]
- Whale, A.D.; Colman, L.; Lensun, L.; Rogers, H.L.; Shuttleworth, S.J. Functional characterization of a novel somatic oncogenic mutation of PIK3CB. Signal Transduct. Target. Ther. 2017, 2, 17063. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Hill, V.K.; Kim, J.S.; James, C.D.; Waldman, T. Correction of PTEN mutations in glioblastoma cell lines via AAV-mediated gene editing. PLoS ONE 2017, 12, e0176683. [Google Scholar] [CrossRef] [PubMed]
- Della Monica, R.; Cuomo, M.; Buonaiuto, M.; Costabile, D.; Franca, R.A.; Del Basso De Caro, M.; Catapano, G.; Chiariotti, L.; Visconti, R. MGMT and whole-genome DNA methylation impacts on diagnosis, prognosis and therapy of glioblastoma multiforme. Int. J. Mol. Sci. 2022, 23, 7148. [Google Scholar] [CrossRef]
- Schnöller, L.E.; Piehlmaier, D.; Weber, P.; Brix, N.; Fleischmann, D.F.; Nieto, A.E.; Selmansberger, M.; Heider, T.; Hess, J.; Niyazi, M.; et al. Systematic in vitro analysis of therapy resistance in glioblastoma cell lines by integration of clonogenic survival data with multi-level molecular data. Radiat. Oncol. 2023, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Aki, T.; Nara, A.; Uemura, K. Cytoplasmic vacuolization during exposure to drugs and other substances. Cell Biol. Toxicol. 2012, 28, 125–131. [Google Scholar] [CrossRef]
- Morissette, G.; Lodge, R.; Marceau, F. Intense pseudotransport of a cationic drug mediated by vacuolar ATPase: Procainamide-induced autophagic cell vacuolization. Toxicol. Appl. Pharmacol. 2008, 228, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863–55889. [Google Scholar] [CrossRef]
- Sperandio, S.; Poksay, K.; de Belle, I.; Lafuente, M.J.; Liu, B.; Nasir, J.; Bredesen, D.E. Paraptosis: Mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 2004, 11, 1066–1075. [Google Scholar] [CrossRef]
- Christofferson, D.E.; Yuan, J. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 2010, 22, 263–268. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Ding, T.; Ji, N.; Zhao, H. The dual role of macropinocytosis in cancers: Promoting growth and inducing methuosis to participate in anticancer therapies as targets. Front. Oncol. 2021, 10, 570108. [Google Scholar] [CrossRef] [PubMed]
- Dekker, N.J.; Laraia, L. Making bubbles: Targeting VPS41 induces vacuolization and methuosis. Cell Chem. Biol. 2023, 30, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ruan, X.; Ji, H.; Peng, L.; Qiu, Y.; Yang, D.; Song, X.; Ji, C.; Guo, D.; Jiang, S. Maduramicin triggers methuosis-like cell death in primary chicken myocardial cells. Toxicol. Lett. 2020, 333, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Schifano, F.; Albanese, A.; Fergus, S.; Stair, J.L.; Deluca, P.; Corazza, O.; Davey, Z.; Corkery, J.; Siemann, H.; Scherbaum, N.; et al. Mephedrone (4-methylmethcathinone; ‘meow meow’): Chemical, pharmacological and clinical issues. Psychopharmacology 2011, 214, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Ayestas, M.A., Jr.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; E Ruoho, A.; Cozzi, N.V. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef]
- Kolanos, R.; Sakloth, F.; Jain, A.D.; Partilla, J.S.; Baumann, M.H.; Glennon, R.A. Structural modification of the designer stimulant α-pyrrolidinovalerophenone (α-PVP) influences potency at dopamine transporters. ACS Chem. Neurosci. 2015, 6, 1726–1731. [Google Scholar] [CrossRef]
- Winstock, A.; Mitcheson, L.; Ramsey, J.; Davies, S.; Puchnarewicz, M.; Marsden, J. Mephedrone: Use, subjective effects and health risks. Addiction 2011, 106, 1991–1996. [Google Scholar] [CrossRef]
- Green, A.R.; King, M.V.; Shortall, S.E.; Fone, K.F.C. The preclinical pharmacology of mephedrone; not just MDMA by another name. Br. J. Pharmacol. 2014, 171, 2251–2268. [Google Scholar] [CrossRef]
- James, D.; Adams, R.D.; Spears, R.; Cooper, G.; Lupton, D.J.; Thompson, J.P.; Thomas, S.H.L. Clinical characteristics of mephedrone toxicity reported to the UK National Prisons Information Service. Emerg. Med. J. 2011, 28, 686–689. [Google Scholar] [CrossRef]
- Herzig, D.A.; Brooks, R.; Mohr, C. Inferring about individual drug and schizotypy effects on cognitive functioning in polydrug using mephedrone users before and after clubbing. Hum. Psychopharmacol. 2013, 28, 168–182. [Google Scholar] [CrossRef]
- Grochecki, P.; Smaga, I.; Lopatynska-Mazurek, M.; Gibula-Tarlowska, E.; Kedzierska, E.; Listos, J.; Talarek, S.; Marszalek-Grabska, M.; Hubalewska-Mazgaj, M.; Korga-Plewko, A.; et al. Effects of mephedrone and amphetamine exposure during adolescence on spatial memory in adulthood: Behavioral and neurochemical analysis. Int. J. Mol. Sci. 2021, 22, 589. [Google Scholar] [CrossRef]
- Motbey, C.P.; Karanges, E.; Li, K.M.; Wilkinson, S.; Winstock, A.R.; Ramsay, J.; Hicks, C.; Kendig, M.D.; Wyatt, N.; Callaghan, P.D.; et al. Mephedrone in adolescent rats: Residual memory impairment and acute but not lasting 5-HT depletion. PLoS ONE 2012, 7, e45473. [Google Scholar] [CrossRef]
- López-Arnau, R.; Martínez-Clemente, J.; Rodrigo, T.; Pubill, D.; Camarasa, J.; Escubedo, E. Neuronal changes and oxidative stress in adolescent rats after repeated exposure to mephedrone. Toxicol. Appl. Pharmacol. 2015, 286, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Krantz, M.J.; Mehler, P.S. Treating opioid dependence. Growing implications for primary care. Arch. Intern. Med. 2004, 164, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.L.A.; Kallweit, U.; Vignatelli, L.; Plazzi, G.; Lecendreux, M.; Baldin, E.; Dolenc-Groselj, L.; Jennum, P.; Khatami, R.; Manconi, M.; et al. European guideline and expert statements on the management of narcolepsy in adults and children. Eur. J. Neurol. 2021, 28, 2815–2830. [Google Scholar] [CrossRef]
- Cortese, S.; Newcorn, J.H.; Coghill, D. A practical, evidence-informed approach to managing stimulant-refractory attention deficit hyperactivity disorder (ADHD). CNS Drugs 2021, 35, 1035–1051. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Friesen, C.; Hormann, I.; Roscher, M.; Fichtner, I.; Alt, A.; Hilger, R.; Debatin, K.-M.; Miltner, E. Opioid receptor activation triggering downregulation of cAMP improves effectiveness of anti-cancer drugs in treatment of glioblastoma. Cell Cycle 2014, 13, 1560–1570. [Google Scholar] [CrossRef]
- Lal, S.; Shekher, A.; Puneet Narula, A.S.; Abrahamse, H.; Gupta, S.C. Cannabis and its constituents for cancer: History, biogenesis, chemistry and pharmacological activities. Pharmacol. Res. 2021, 163, 105302. [Google Scholar] [CrossRef]
- Yoshino, A.; Ogino, A.; Yachi, K.; Ohta, T.; Fukushima, T.; Watanabe, T.; Katayama, Y.; Okamoto, Y.; Naruse, N.; Sano, E.; et al. Gene expression profiling predicts response to temozolomide in malignant gliomas. Int. J. Oncol. 2010, 36, 1367–1377. [Google Scholar] [CrossRef]
- Shao, H.; Chung, J.; Balaj, L.; Charest, A.; Bigner, D.D.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Weissleder, R.; Lee, H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med. 2012, 18, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Harrabi, S.; Combs, S.E.; Brons, S.; Haberer, T.; Debus, J.; Weber, K.J. Temozolomide in combination with carbon ion or photon irradiation in glioblastoma multiforme cell lines—Does scheduling matter? Int. J. Radiat. Biol. 2013, 89, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [CrossRef]
- Jakubowicz-Gil, J.; Langner, E.; Badziul, D.; Wertel, I.; Rzeski, W. Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumor Biol. 2013, 34, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Sumorek-Wiadro, J.; Langner, E.; Wertel, I.; Maciejczyk, A.; Pawlikowska-Pawlęga, B.; Pawelec, J.; Wasiak, M.; Hułas-Stasiak, M.; Bądziul, D.; et al. Involvement of PI3K pathway in glioma cell resistance to temozolomide treatment. Int. J. Mol. Sci. 2021, 22, 5155. [Google Scholar] [CrossRef]
- Lemieszek, M.K.; Nunes, F.M.; Cardoso, C.; Marques, G.; Rzeski, W. Neuroprotective properties of Cantharellus cibarius polysaccharide fractions in different in vitro models of neurodegeneration. Carbohydr. Polym. 2018, 197, 598–607. [Google Scholar] [CrossRef]
- Ho, J.; Tumkaya, T.; Aryal, S.; Choi, H.; Claridge-Chang, A. Moving beyond P values: Data analysis with estimation graphics. Nat. Methods 2019, 16, 565–566. [Google Scholar] [CrossRef]



| Cell Line | Mephedrone [µM] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 100 | 250 | 500 | 1000 | 0 | 100 | 250 | 500 | 1000 | |
| Incubation Time 24 h | Incubation Time 48 h | |||||||||
| LN-18 | 0 | 3 | 14 | 72 | 87 | 0.5 | 12 | 24 | 81 | 86 |
| LN-229 | 0 | 0 | 0 | 0 | 2.7 | 0 | 0 | 0 | 0.2 | 0.9 |
| T98G | 0 | 0 | 0.5 | 2.1 | 3.7 | 0 | 0 | 0.7 | 2.7 | 2.9 |
| MOGGCCM | 0 | 0 | 0.5 | 1.9 | 2.7 | 0.1 | 0 | 0.9 | 1.2 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marszalek-Grabska, M.; Lemieszek, M.K.; Chojnacki, M.; Winiarczyk, S.; Jakubowicz-Gil, J.; Zarzyka, B.; Pawelec, J.; Kotlinska, J.H.; Rzeski, W.; Turski, W.A. Cell-Specific Vulnerability of Human Glioblastoma and Astrocytoma Cells to Mephedrone—An In Vitro Study. Molecules 2025, 30, 2277. https://doi.org/10.3390/molecules30112277
Marszalek-Grabska M, Lemieszek MK, Chojnacki M, Winiarczyk S, Jakubowicz-Gil J, Zarzyka B, Pawelec J, Kotlinska JH, Rzeski W, Turski WA. Cell-Specific Vulnerability of Human Glioblastoma and Astrocytoma Cells to Mephedrone—An In Vitro Study. Molecules. 2025; 30(11):2277. https://doi.org/10.3390/molecules30112277
Chicago/Turabian StyleMarszalek-Grabska, Marta, Marta Kinga Lemieszek, Michal Chojnacki, Sylwia Winiarczyk, Joanna Jakubowicz-Gil, Barbara Zarzyka, Jarosław Pawelec, Jolanta H. Kotlinska, Wojciech Rzeski, and Waldemar A. Turski. 2025. "Cell-Specific Vulnerability of Human Glioblastoma and Astrocytoma Cells to Mephedrone—An In Vitro Study" Molecules 30, no. 11: 2277. https://doi.org/10.3390/molecules30112277
APA StyleMarszalek-Grabska, M., Lemieszek, M. K., Chojnacki, M., Winiarczyk, S., Jakubowicz-Gil, J., Zarzyka, B., Pawelec, J., Kotlinska, J. H., Rzeski, W., & Turski, W. A. (2025). Cell-Specific Vulnerability of Human Glioblastoma and Astrocytoma Cells to Mephedrone—An In Vitro Study. Molecules, 30(11), 2277. https://doi.org/10.3390/molecules30112277







