Activation of Perovskite Nanocrystals for Volumetric Displays Using Near-Infrared Photon Upconversion by Triplet Fusion
Abstract
1. Introduction
2. Results
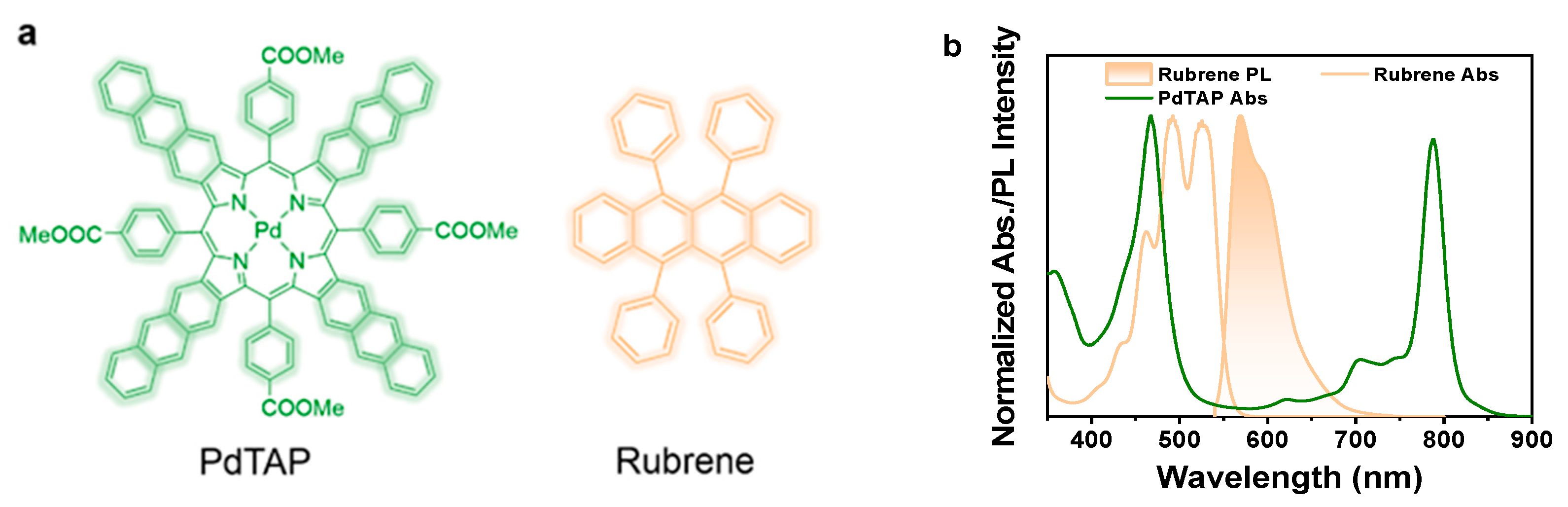
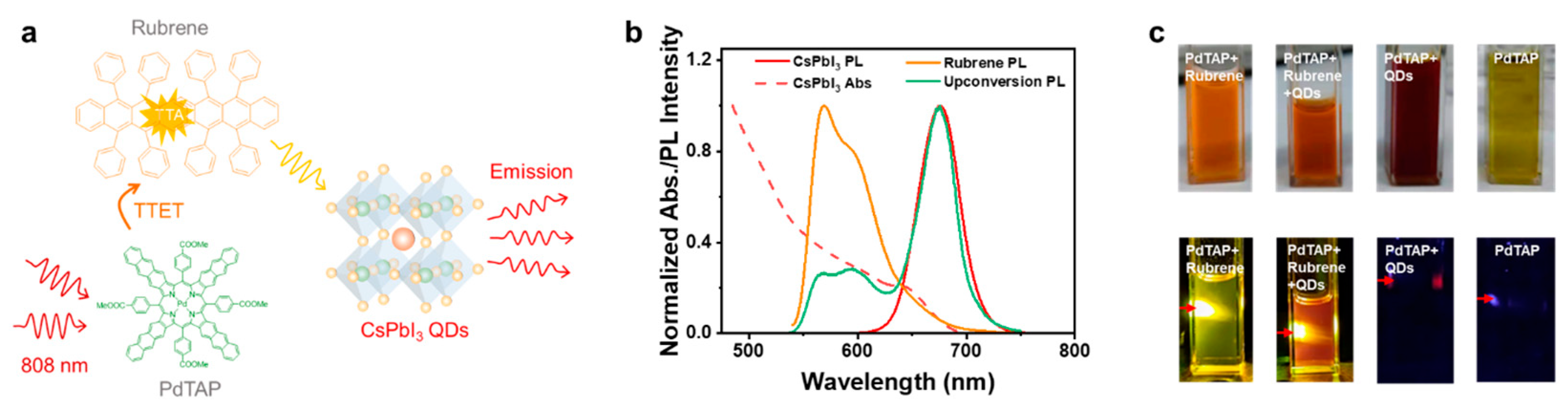
2.1. Characterization of Photophysical Properties
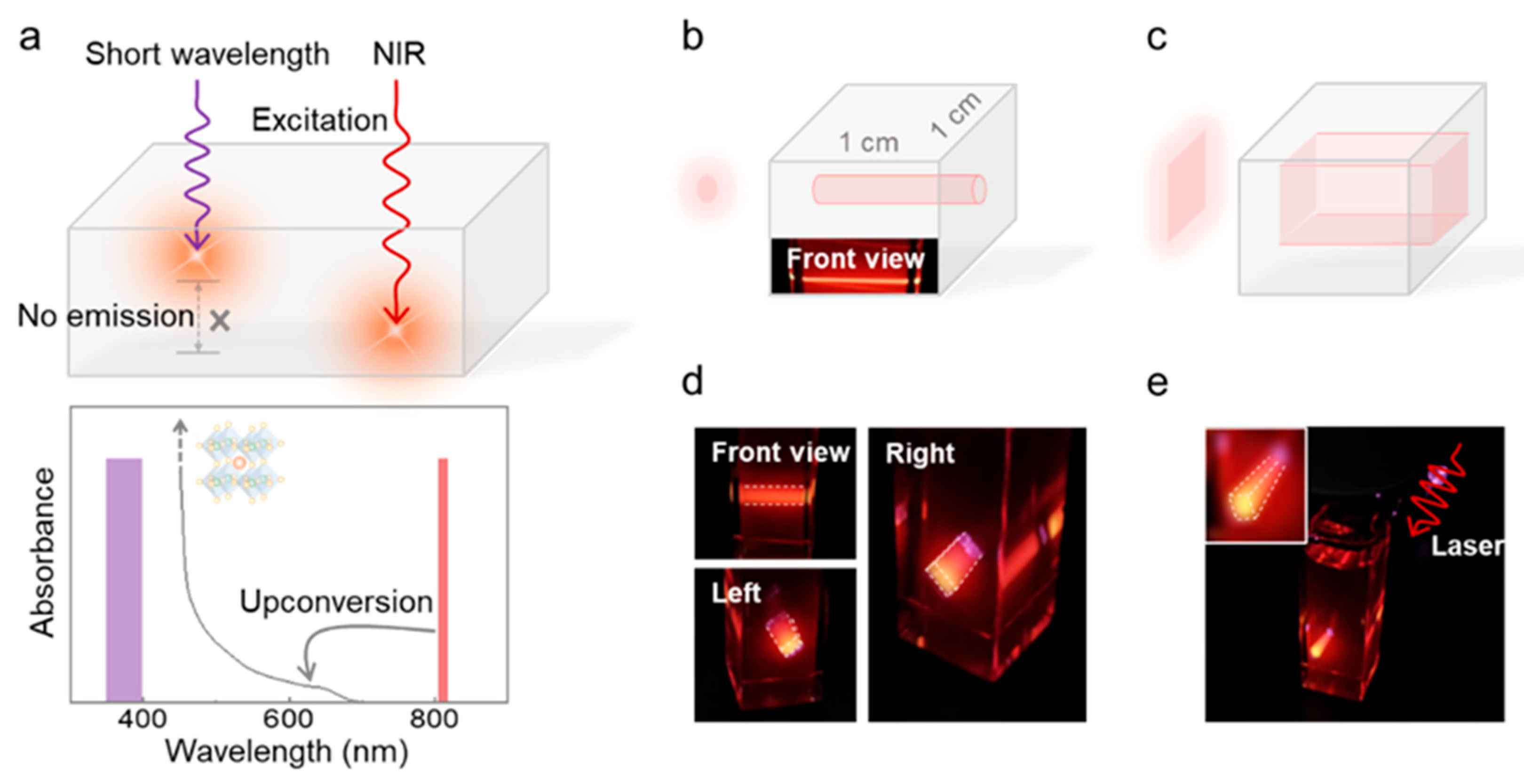
2.2. Volumetric Displays
3. Materials and Methods
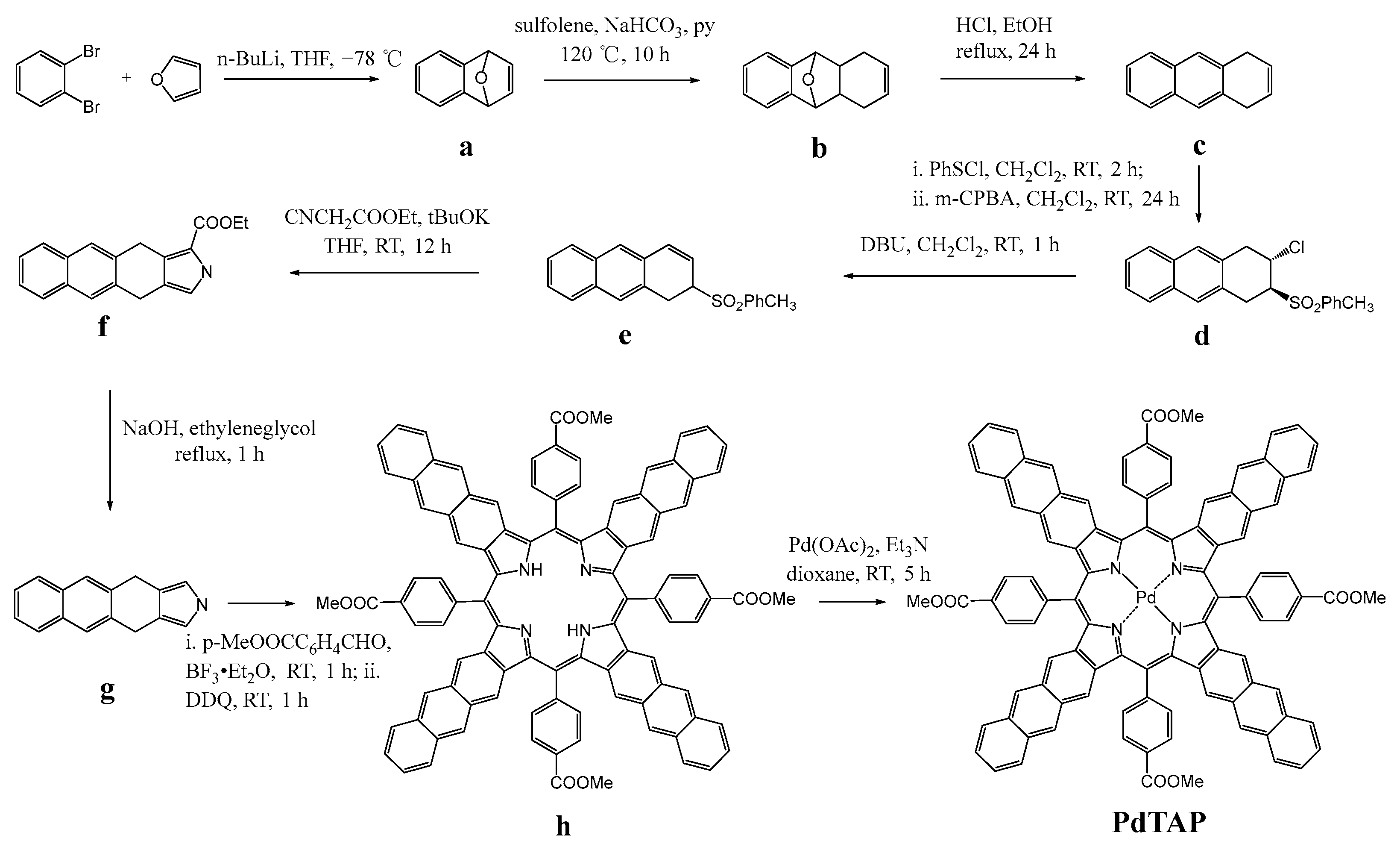
3.1. Synthesis of Pd-tetrakis-5,10,15,20-(p-methoxycarbonylphenyl)-tetraanthraporphyrin (PdTAP)
3.2. Synthesis of CsPbI3 QDs
3.3. Instrumentation
3.4. Measurement
3.4.1. Upconversion
3.4.2. Calculation of Spectral Overlap Integral
3.4.3. Volumetric Display
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef] [PubMed]
- Fakharuddin, A.; Gangishetty, M.K.; Abdi-Jalebi, M.; Chin, S.-H.; bin Mohd Yusoff, A.R.; Congreve, D.N.; Tress, W.; Deschler, F.; Vasilopoulou, M.; Bolink, H.J. Perovskite light-emitting diodes. Nat. Electron. 2022, 5, 203–216. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef] [PubMed]
- VanOrman, Z.A.; Drozdick, H.K.; Wieghold, S.; Nienhaus, L. Bulk halide perovskites as triplet sensitizers: Progress and prospects in photon upconversion. J. Mater. Chem. C 2021, 9, 2685–2694. [Google Scholar] [CrossRef]
- Moller, G.; Sullivan, C.M.; Cantrell, A.P.; Mardani, M.; Bieber, A.S.; Siegrist, T.; Nienhaus, L. Upconversion at Solid/Liquid Interfaces Using Perovskite Single Crystal Triplet Sensitizers. Chem. Mater. 2024, 36, 1941–1946. [Google Scholar] [CrossRef]
- Zhang, J.; Hodes, G.; Jin, Z.; Liu, S. All-Inorganic CsPbX3 Perovskite Solar Cells: Progress and Prospects. Angew. Chem. Int. Ed. 2019, 58, 15596–15618. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Luo, X.; Lai, R.; Li, Y.; Liang, G.; Wu, K. Visible-Light-Driven Sensitization of Naphthalene Triplets Using Quantum-Confined CsPbBr3 Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 1457–1463. [Google Scholar] [CrossRef]
- Luo, X.; Liang, G.; Han, Y.; Li, Y.; Ding, T.; He, S.; Liu, X.; Wu, K. Triplet Energy Transfer from Perovskite Nanocrystals Mediated by Electron Transfer. J. Am. Chem. Soc. 2020, 142, 11270–11278. [Google Scholar] [CrossRef] [PubMed]
- DuBose, J.T.; Kamat, P.V. Energy Versus Electron Transfer: Managing Excited-State Interactions in Perovskite Nanocrystal–Molecular Hybrids. Chem. Rev. 2022, 122, 12475–12494. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, W.; Huang, P.; Yang, D.; Shao, Z.; Chen, X. The marriage of perovskite nanocrystals with lanthanide-doped upconversion nanoparticles for advanced optoelectronic applications. Aggregate 2024, 5, e558. [Google Scholar] [CrossRef]
- Bharmoria, P.; Bildirir, H.; Moth-Poulsen, K. Triplet–triplet annihilation based near infrared to visible molecular photon upconversion. Chem. Soc. Rev. 2020, 49, 6529–6554. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Hazarika, A.; Zhao, Q.; Ling, X.; Moot, T.; Ma, W.; Luther, J.M. Metal Halide Perovskites in Quantum Dot Solar Cells: Progress and Prospects. Joule 2020, 4, 1160–1185. [Google Scholar] [CrossRef]
- Richards, B.S.; Hudry, D.; Busko, D.; Turshatov, A.; Howard, I.A. Photon Upconversion for Photovoltaics and Photocatalysis: A Critical Review. Chem. Rev. 2021, 121, 9165–9195. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, J.C.; Fischer, S. Upconversion for Photovoltaics—A Review of Materials, Devices and Concepts for Performance Enhancement. Adv. Opt. Mater. 2015, 3, 510–535. [Google Scholar] [CrossRef]
- Schulze, T.F.; Schmidt, T.W. Photochemical upconversion: Present status and prospects for its application to solar energy conversion. Energy Environ. Sci. 2015, 8, 103–125. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, P.; Gong, Z.; Tu, D.; Xu, J.; Zou, Q.; Li, R.; You, W.; Bünzli, J.-C.G.; Chen, X. Near-infrared-triggered photon upconversion tuning in all-inorganic cesium lead halide perovskite quantum dots. Nat. Commun. 2018, 9, 3462. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhou, J.; Schuck, P.J.; Suh, Y.D.; Schmidt, T.W.; Jin, D. Future and challenges for hybrid upconversion nanosystems. Nat. Photonics 2019, 13, 828–838. [Google Scholar] [CrossRef]
- Patel, S.K.; Cao, J.; Lippert, A.R. A volumetric three-dimensional digital light photoactivatable dye display. Nat. Commun. 2017, 8, 15239. [Google Scholar] [CrossRef]
- Wan, S.; Zhou, H.; Lin, J.; Lu, W. A Prototype of a Volumetric Three-Dimensional Display Based on Programmable Photo-Activated Phosphorescence. Angew. Chem. Int. Ed. 2020, 59, 8416–8420. [Google Scholar] [CrossRef]
- Singh-Rachford, T.N.; Castellano, F.N. Photon upconversion based on sensitized triplet–triplet annihilation. Coord. Chem. Rev. 2010, 254, 2560–2573. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zhao, X.; Chen, X.; Zhao, J. Heavy Atom-Free Triplet Photosensitizers: Molecular Structure Design, Photophysical Properties and Application in Photodynamic Therapy. Molecules 2023, 28, 2170. [Google Scholar] [CrossRef]
- Gao, M.; Zeng, L.; Jiang, L.; Zhang, M.; Chen, Y.; Huang, L. Bodipy Dimer for Enhancing Triplet-Triplet Annihilation Upconversion Performance. Molecules 2023, 28, 5474. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wei, L.; He, C.; Yang, C.; Wu, W. Supramolecular Annihilator with DPA Parallelly Arranged by Multiple Hydrogen-Bonding Interactions for Enhanced Triplet–Triplet Annihilation Upconversion. Molecules 2024, 29, 2203. [Google Scholar] [CrossRef]
- Gray, V.; Allardice, J.R.; Zhang, Z.; Rao, A. Organic-quantum dot hybrid interfaces and their role in photon fission/fusion applications. Chem. Phys. Rev. 2021, 2, 031305. [Google Scholar] [CrossRef]
- Gao, C.; Shukla, A.; Gao, H.; Miao, Z.; Zhang, Y.; Wang, P.; Luo, G.; Zeng, Y.; Wong, W.W.H.; Smith, T.A.; et al. Harvesting Triplet Excitons in High Mobility Emissive Organic Semiconductor for Efficiency Enhancement of Light-Emitting Transistors. Adv. Mater. 2023, 35, 2208389. [Google Scholar] [CrossRef]
- Antoniou, G.; Yuan, P.; Koutsokeras, L.; Athanasopoulos, S.; Fazzi, D.; Panidi, J.; Georgiadou, D.G.; Prodromakis, T.; Keivanidis, P.E. Low-power supralinear photocurrent generation via excited state fusion in single-component nanostructured organic photodetectors. J. Mater. Chem. C 2022, 10, 7575–7585. [Google Scholar] [CrossRef]
- Huang, L.; Han, G. Triplet–triplet annihilation photon upconversion-mediated photochemical reactions. Nat. Rev. Chem. 2024, 8, 238–255. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, J.; Yu, T.; Yang, G.; Li, Y. Molecular–Supramolecular Light Harvesting for Photochemical Energy Conversion: Making Every Photon Count. Acs Energy Lett. 2017, 2, 357–363. [Google Scholar] [CrossRef]
- Ravetz, B.D.; Pun, A.B.; Churchill, E.M.; Congreve, D.N.; Rovis, T.; Campos, L.M. Photoredox catalysis using infrared light via triplet fusion upconversion. Nature 2019, 565, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.N.; Schloemer, T.H.; Gangishetty, M.K.; Anderson, D.; Seitz, M.; Gallegos, A.O.; Stokes, R.C.; Congreve, D.N. Triplet fusion upconversion nanocapsules for volumetric 3D printing. Nature 2022, 604, 474–478. [Google Scholar] [CrossRef]
- Schloemer, T.; Narayanan, P.; Zhou, Q.; Belliveau, E.; Seitz, M.; Congreve, D.N. Nanoengineering Triplet–Triplet Annihilation Upconversion: From Materials to Real-World Applications. ACS Nano 2023, 17, 3259–3288. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, J.; Zhao, T.; Liu, M.; Duan, P. Photoswitchable Photon Upconversion from Turn-on Mode Fluorescent Diarylethenes. CCS Chem. 2021, 3, 665–674. [Google Scholar] [CrossRef]
- Sun, W.; Ronchi, A.; Zhao, T.; Han, J.; Monguzzi, A.; Duan, P. Highly efficient photon upconversion based on triplet–triplet annihilation from bichromophoric annihilators. J. Mater. Chem. C 2021, 9, 14201–14208. [Google Scholar] [CrossRef]
- Isokuortti, J.; Kuntze, K.; Virkki, M.; Ahmed, Z.; Vuorimaa-Laukkanen, E.; Filatov, M.A.; Turshatov, A.; Laaksonen, T.; Priimagi, A.; Durandin, N.A. Expanding excitation wavelengths for azobenzene photoswitching into the near-infrared range via endothermic triplet energy transfer. Chem. Sci. 2021, 12, 7504–7509. [Google Scholar] [CrossRef]
- Wellauer, J.; Ziereisen, F.; Sinha, N.; Prescimone, A.; Velić, A.; Meyer, F.; Wenger, O.S. Iron(III) Carbene Complexes with Tunable Excited State Energies for Photoredox and Upconversion. J. Am. Chem. Soc. 2024, 146, 11299–11318. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Hosoyamada, M.; Yurash, B.; Nguyen, T.-Q.; Yanai, N.; Kimizuka, N. Donor–Acceptor–Collector Ternary Crystalline Films for Efficient Solid-State Photon Upconversion. J. Am. Chem. Soc. 2018, 140, 8788–8796. [Google Scholar] [CrossRef]
- Raišys, S.; Juršėnas, S.; Simon, Y.C.; Weder, C.; Kazlauskas, K. Enhancement of triplet-sensitized upconversion in rigid polymers via singlet exciton sink approach. Chem. Sci. 2018, 9, 6796–6802. [Google Scholar] [CrossRef]
- Lin, T.-A.; Perkinson, C.F.; Baldo, M.A. Strategies for High-Performance Solid-State Triplet–Triplet-Annihilation-Based Photon Upconversion. Adv. Mater. 2020, 32, 1908175. [Google Scholar] [CrossRef]
- Luo, G.; Liu, Y.; Zeng, Y.; Yu, T.; Chen, J.; Hu, R.; Yang, G.; Li, Y. Enhancing photon upconversion with thermally activated sensitization and singlet energy collection. J. Mater. Chem. C 2022, 10, 8596–8601. [Google Scholar] [CrossRef]
- Luo, G.; Chen, Y.; Zeng, Y.; Yu, T.; Chen, J.; Hu, R.; Yang, G.; Li, Y. Funneling and Enhancing Upconversion Emission by Light-Harvesting Molecular Wires. J. Phys. Chem. Lett. 2021, 12, 9525–9530. [Google Scholar] [CrossRef] [PubMed]
- Abulikemu, A.; Sakagami, Y.; Heck, C.; Kamada, K.; Sotome, H.; Miyasaka, H.; Kuzuhara, D.; Yamada, H. Solid-State, Near-Infrared to Visible Photon Upconversion via Triplet–Triplet Annihilation of a Binary System Fabricated by Solution Casting. ACS Appl. Mater. Interfaces 2019, 11, 20812–20819. [Google Scholar] [CrossRef] [PubMed]
- Monguzzi, A.; Mezyk, J.; Scotognella, F.; Tubino, R.; Meinardi, F. Upconversion-induced fluorescence in multicomponent systems: Steady-state excitation power threshold. Phys. Rev. B 2008, 78, 195112. [Google Scholar] [CrossRef]
- Schmidt, T.W.; Castellano, F.N. Photochemical Upconversion: The Primacy of Kinetics. J. Phys. Chem. Lett. 2014, 5, 4062–4072. [Google Scholar] [CrossRef]
- Yakutkin, V.; Aleshchenkov, S.; Chernov, S.; Miteva, T.; Nelles, G.; Cheprakov, A.; Baluschev, S. Towards the IR Limit of the Triplet–Triplet Annihilation-Supported Up-Conversion: Tetraanthraporphyrin. Chem.—Eur. J. 2008, 14, 9846–9850. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef]
- Roy, P.; Sury, A.S.; Pillai, P.P. Electrostatics enable resonance energy transfer in all-InP quantum dot containing donor–acceptor assembly. Appl. Phys. Lett. 2024, 124, 222104. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Wei, T.; Pan, Y.; Zhou, E.; Yuan, Z.; Han, Y.; Li, M.; Ling, X.; Yin, L.; et al. Solution-Processable Near-Infrared–Responsive Composite of Perovskite Nanowires and Photon-Upconversion Nanoparticles. Adv. Funct. Mater. 2018, 28, 1801782. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, M.; Wang, Y.; Duan, P. Electric-field-regulated energy transfer in chiral liquid crystals for enhancing upconverted circularly polarized luminescence through steering the photonic bandgap. Adv. Mater. 2020, 32, 2000820. [Google Scholar] [CrossRef]
- Rao, M.; Fu, J.; Wen, X.; Sun, B.; Wu, J.; Liu, X.; Dong, X. Near-infrared-excitable perovskite quantum dots via coupling with upconversion nanoparticles for dual-model anti-counterfeiting. New J. Chem. 2018, 42, 12353. [Google Scholar] [CrossRef]
- Ma, J.; Wu, H.; Qiu, J.; Wang, J.; Wang, Q.; Yang, Y.; Zhou, D.; Han, J. NIR-excited all-inorganic perovskite quantum dots (CsPbBr 3) for a white light-emitting device. J. Mater. Chem. C 2019, 7, 3751–3755. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, B.; Wang, X.; Lu, L.; Lu, Q.; Sun, M.; Wu, T.; Ma, T.; Xu, J.; Xu, Y.; et al. Multimodal luminescent Yb3+/Er3+/Bi3+-doped perovskite single crystals for X-ray detection and anti-counterfeiting. Adv. Mater. 2020, 32, 2004506. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, Y.; Kim, K.; Huang, W.T.; Liu, R.S.; Hyun, J.K.; Kim, D.H. Gap surface plasmon-enhanced photoluminescence from upconversion nanoparticle-sensitized perovskite quantum dots in a metal–insulator–metal configuration under NIR excitation. J. Mater. Chem. C 2022, 10, 532–541. [Google Scholar] [CrossRef]
- Lin, J.; Yang, C.; Huang, P.; Wang, S.; Liu, M.; Jiang, N.; Chen, D. Photoluminescence tuning from glass-stabilized CsPbX3 (X= Cl, Br, I) perovskite nanocrystals triggered by upconverting Tm: KYb2F7 nanoparticles for high-level anti-counterfeiting. Chem. Eng. J. 2020, 395, 125214. [Google Scholar] [CrossRef]
- Ruan, L.; Zhang, Y. NIR-excitable heterostructured upconversion perovskite nanodots with improved stability. Nat. Commun. 2021, 12, 219. [Google Scholar] [CrossRef]
- Xie, L.; Hong, Z.; Zan, J.; Wu, Q.; Yang, Z.; Chen, X.; Ou, X.; Song, X.; He, Y.; Li, J.; et al. Broadband detection of X-ray, ultraviolet, and near-infrared photons using solution-processed perovskite–lanthanide nanotransducers. Adv. Mater. 2021, 33, 2101852. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Luo, G.; Niu, P.; Zhang, L.; Yu, T.; Chen, J.; Li, Y.; Zeng, Y. Activation of Perovskite Nanocrystals for Volumetric Displays Using Near-Infrared Photon Upconversion by Triplet Fusion. Molecules 2025, 30, 2273. https://doi.org/10.3390/molecules30112273
Hu Y, Luo G, Niu P, Zhang L, Yu T, Chen J, Li Y, Zeng Y. Activation of Perovskite Nanocrystals for Volumetric Displays Using Near-Infrared Photon Upconversion by Triplet Fusion. Molecules. 2025; 30(11):2273. https://doi.org/10.3390/molecules30112273
Chicago/Turabian StyleHu, Yu, Guiwen Luo, Pengfei Niu, Ling Zhang, Tianjun Yu, Jinping Chen, Yi Li, and Yi Zeng. 2025. "Activation of Perovskite Nanocrystals for Volumetric Displays Using Near-Infrared Photon Upconversion by Triplet Fusion" Molecules 30, no. 11: 2273. https://doi.org/10.3390/molecules30112273
APA StyleHu, Y., Luo, G., Niu, P., Zhang, L., Yu, T., Chen, J., Li, Y., & Zeng, Y. (2025). Activation of Perovskite Nanocrystals for Volumetric Displays Using Near-Infrared Photon Upconversion by Triplet Fusion. Molecules, 30(11), 2273. https://doi.org/10.3390/molecules30112273