Bioactive Compounds from Dodonaea viscosa Flowers: Potent Antibacterial and Antiproliferative Effects in Breast Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
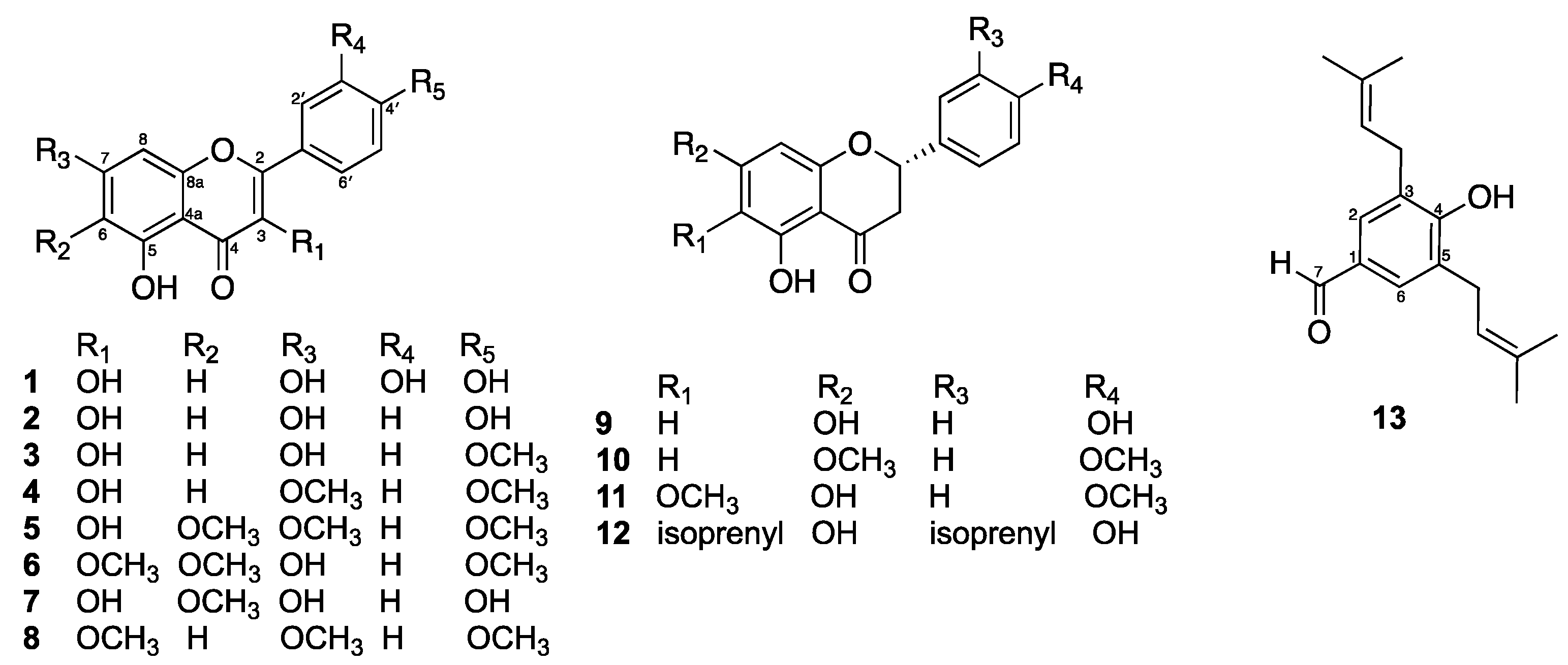
2.1. Isolated Compounds from D. viscosa Flowers
2.2. Antibacterial Activity
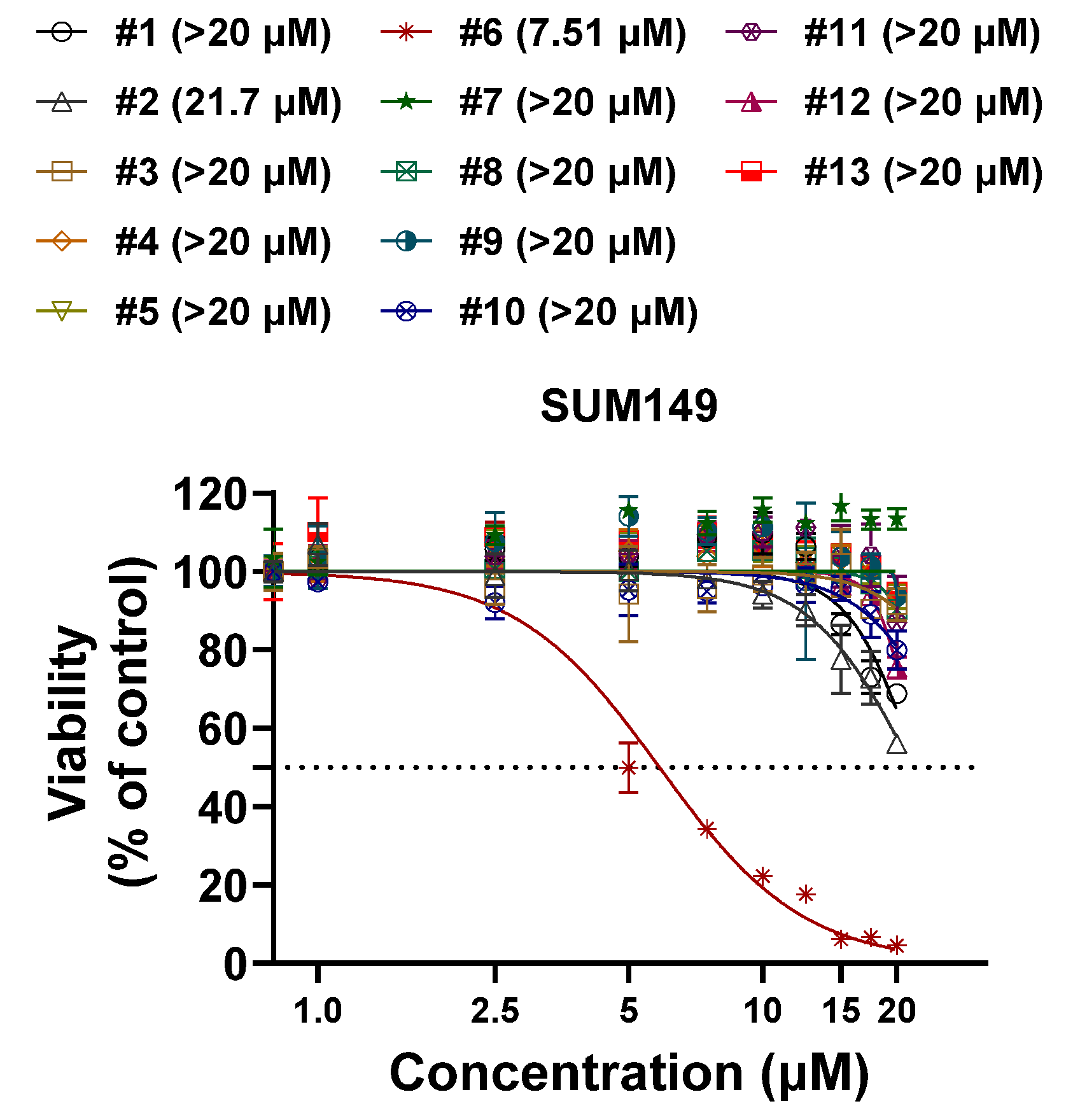
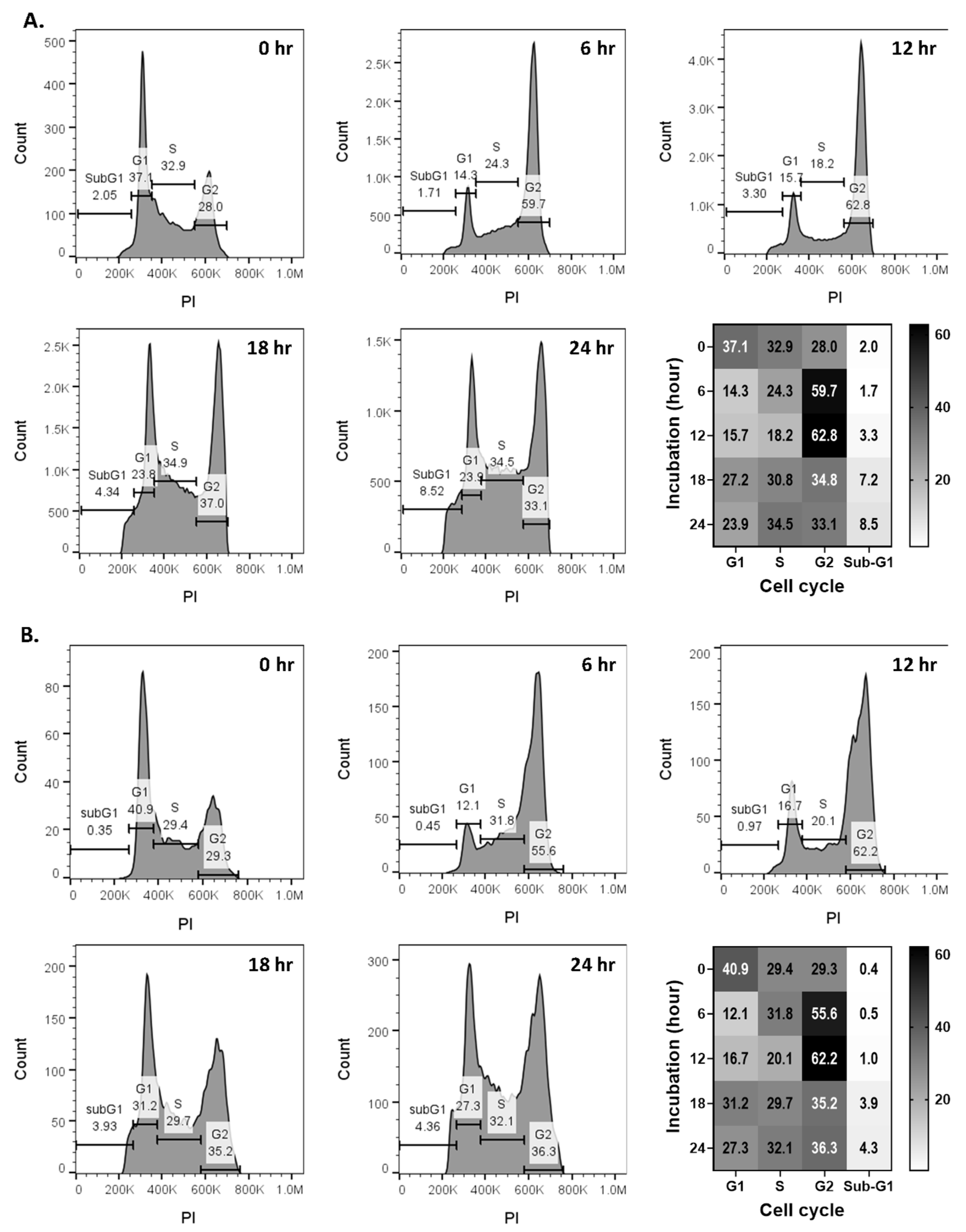
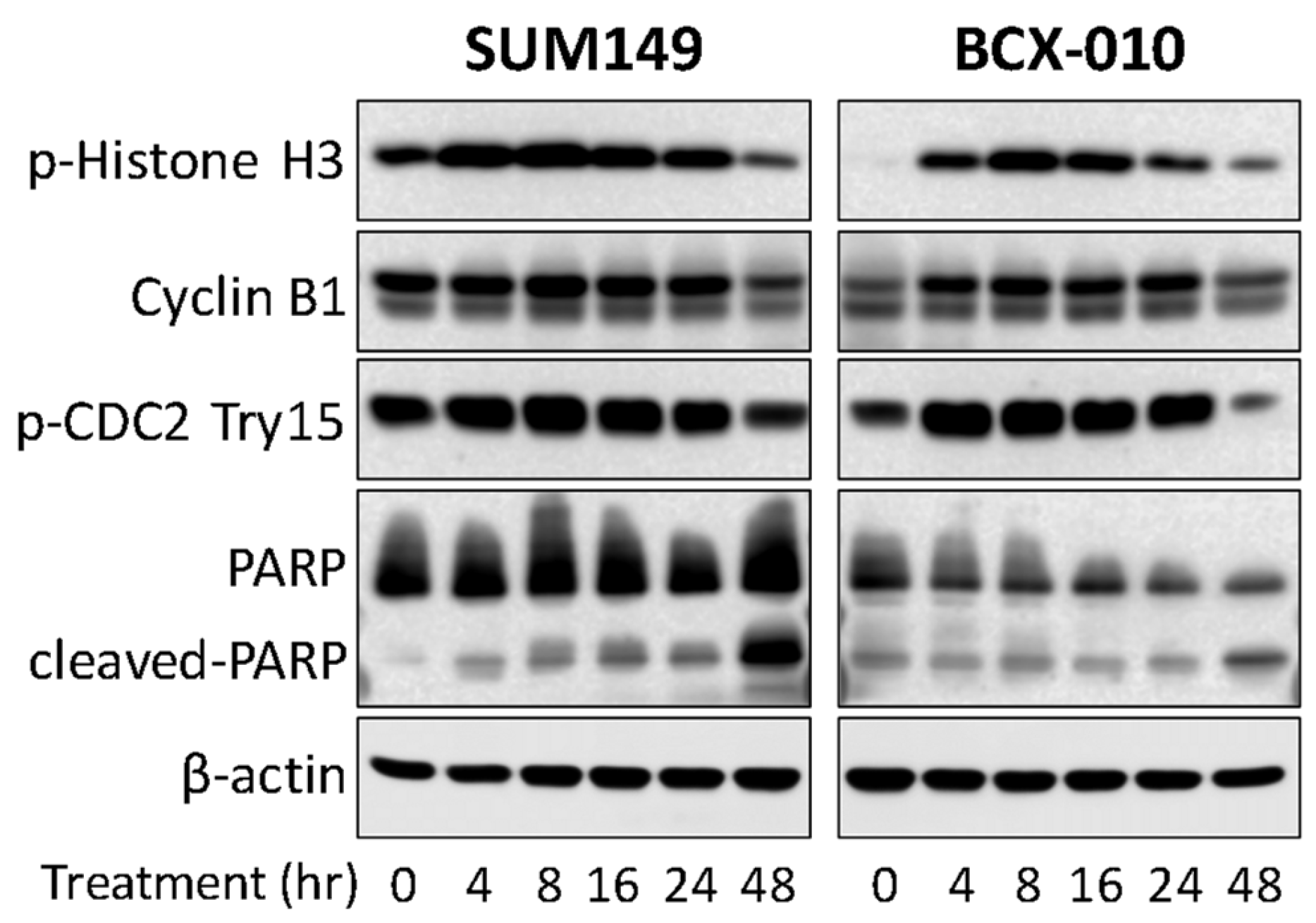
2.3. Compound 6 (Santin) from D. viscosa Flowers Inhibits Breast Cancer Cell Growth via G2-M Cell Cycle Arrest
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
- Quercetin (1): yellow solids, mp 315–316 °C; 1H NMR (acetone-d6, 400 MHz) δ 12.18 (1H, s, 5-OH), 7.80 (1H, d, J = 2.0 Hz, H-2′), 7.68 (1H, dd, J = 8.4, 2.0 Hz, H-6′), 6.97 (1H, d, J = 8.4 Hz, H-5′), 6.52 (1H, d, J = 2.0 Hz, H-8), and 6.25 (1H, d, J = 2.0 Hz, H-6).
- Kaempferol (2): yellow solids, mp 277–278 °C; 1H NMR (MeOD, 400 MHz) δ 8.08 (2H, d, J = 8.9 Hz, H-2′ and H-6′), 6.91 (2H, d, J = 8.9 Hz, H-3′ and H-5′), 6.38 (1H, d, J = 2.0 Hz, H-8), and 6.17 (1H, d, J = 2.0 Hz, H-6).
- Kaempferide (3): yellow solids, mp 228–229 °C; 1H NMR (acetone-d6, 400 MHz) δ 12.16 (1H, br s, 5-OH), 8.21 (2H, d, J = 8.8 Hz, H-2′ and H-6′), 7.10 (2H, d, J = 8.8 Hz, H-3′ and H-5′), 6.53 (1H, d, J = 2.0 Hz, H-8), 6.26 (1H, d, J = 2.0 Hz, H-6), and 3.89 (3H, s, CH3-4′).
- 3,5-Dihydroxy-7,4′-dimethoxyflavone (4): yellow solids, mp 158–159 °C; 1H NMR (CDCl3, 400 MHz) δ 11.74 (1H, br s, 5-OH), 8.28 (2H, d, J = 8.8 Hz, H-2′ and H-6′), 7.04 (2H, d, J = 8.8 Hz, H-3′ and H-5′), 6.48 (1H, d, J = 2.0 Hz, H-8), 6.37 (1H, d, J = 2.0 Hz, H-6), and 3.89 (6H, s, CH3-7 and CH3-4′).
- Mikanin (5): yellow solids, 1H NMR (CDCl3, 400 MHz) δ 11.83 (1H, br s, 5-OH), 8.19 (2H, d, J = 8.7 Hz, H-2′ and H-6′), 7.03 (2H, d, J = 8.7 Hz, H-3′ and H-5′), 6.55 (1H, s, H-8), 3.97 (3H, s, CH3-7), 3.93 (3H, s, CH3-6), and 3.89 (3H, s, CH3-4′).
- Santin (6): yellow solids, mp 157–158 °C; 1H NMR (CDCl3, 400 MHz) δ 12.90 (1H, br s, 5-OH), 8.08 (2H, d, J = 9.0 Hz, H-2′ and H-6′), 7.04 (2H, d, J = 9.0 Hz, H-3′ and H-5′), 6.58 (1H, s, H-8), 4.07 (3H, s, CH3-6), 3.92 (3H, s, CH3-4′), and 3.88 (3H, s, CH3-3).
- 6-Methoxykaempferol (7): yellow solids, mp 239–240 °C; 1H NMR (MeOD, 400 MHz) δ 8.09 (2H, d, J = 9.0 Hz, H-2′ and H-6′), 6.91 (2H, d, J = 9.0 Hz, H-3′ and H-5′), 6.52 (1H, s, H-8), and 3.90 (3H, s, CH3-6).
- 5-Hydroxy-3,7,4′-trimethoxyflavone (8): yellow solids, mp 140–141 °C; 1H NMR (CDCl3, 400 MHz) δ 12.67 (1H, br s, 5-OH), 8.11 (2H, d, J = 9.0 Hz, H-2′ and H-6′), 7.05 (2H, d, J = 9.0 Hz, H-3′ and H-5′), 6.46 (1H, d, J = 2.0 Hz, H-8), 6.37 (1H, d, J = 2.0 Hz, H-6), 3.92 (3H, s, CH3-7), 3.90 (3H, s, CH3-4′), and 3.88 (3H, s, CH3-3).
- Naringenin (9): pale-yellow solids, mp 250–251 °C; 1H NMR (MeOD, 400 MHz) δ 7.29 (2H, d, J = 8.5 Hz, H-2′ and H-6′), 6.82 (2H, d, J = 8.5 Hz, H-3′ and H-5′), 5.89 (1H, d, J = 2.0 Hz, H-8), 5.88 (1H, d, J = 2.0 Hz, H-6), 5.30 (1H, dd, J = 13.0, 3.0 Hz, H-2), 3.08 (1H, dd, J = 16.8, 13.0 Hz, H-3b), 2.65 (1H, dd, J = 16.8, 3.0 Hz, H-3a).
- 5-Hydroxy-7,4′-dimethoxyflavone (10): pale-yellow solids, mp 164–165 °C; 1H NMR (CDCl3, 400 MHz) δ 12.06 (1H, s, 5-OH), 7.42 (2H, d, J = 8.5 Hz, H-2′ and H-6′), 6.97 (2H, d, J = 8.5 Hz, H-3′ and H-5′), 6.09 (1H, d, J = 2.0 Hz, H-8), 6.07 (1H, d, J = 2.0 Hz, H-6), 5.40 (1H, dd, J = 13.0, 3.0 Hz, H-2), 3.86 (3H, s, CH3-7), 3.83 (3H, s, CH3-4′), 3.09 (1H, dd, J = 16.8, 13.0 Hz, H-3b), and 2.79 (1H, dd, J = 16.8, 3.0 Hz, H-3a).
- 2,3-Dihydro-5,7-dihydroxy-6-methoxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one (11): pale-yellow solids, mp 98–99 °C; 1H NMR (MeOD, 400 MHz) δ 7.26 (2H, d, J = 8.5 Hz, H-2′ and H-6′), 6.79 (2H, d, J = 8.5 Hz, H-3′ and H-5′), 6.10 (1H, d, J = 2.0 Hz, H-8), 5.27 (1H, dd, J = 13.0, 3.0 Hz, H-2), 3.83 (3H, s, CH3-6), 3.72 (3H, s, CH3-4′), 3.05 (1H, dd, J = 16.8, 13.0 Hz, H-3b), and 2.64 (1H, dd, J = 16.8, 3.0 Hz, H-3a).
- Macarangaflavanone B (12): pale-yellow solids, mp 159–160 °C; 1H NMR (MeOD, 400 MHz) δ 7.14 (1H, br s, H-2′), 7.09 (1H, br d, J = 8.0 Hz, H-6′), 6.77 (1H, d, J = 8.0 Hz, H-5′), 5.93 (1H, s, H-8), 5.16 (1H, dd, J = 13.0, 3.0 Hz, H-2), 3.02 (1H, dd, J = 16.8, 13.0 Hz, H-3b), 2.60 (1H, dd, J = 16.8, 3.0 Hz, H-3a), isoprenyl unit at C-6 [δH 5.19 (1H, t, J = 7.2 Hz), 3.21 (2H, d, J = 7.2 Hz), 1.75 (3H, s), and 1.70 (3H, s)], and isoprenyl unit at C-3′ [δH 5.33 (1H, t, J = 7.2 Hz), 3.31 (2H, d, J = 7.2 Hz), 1.72 (3H, s), and 1.63 (3H, s)].
- 3,5-Diprenyl-4-hydroxybenzaldehyde (13): white solid, mp 84–85 °C; 1H NMR (CDCl3, 400 MHz) δ 9.82 (1H, s, H-7), 7.56 (2H, s, H-2 and H-6), 6.07 (1H, s, 4-OH), 2× isoprenyl unit [δH 5.34 (2H, t, J = 7.2 Hz), 3.43 (4H, d, J = 7.2 Hz), 1.82 (6H, s), and 1.80 (6H, s)].
3.4. Minimum Inhibition Concentration (MIC)
3.5. Cell Lines and Reagents
3.6. Sulforhodamine B Cell Proliferation Assay: Anticancer Activity
3.7. Cell Cycle Analyses
3.8. Western Blotting
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nanea Valeros, Public Affairs Specialist, U.S. Fish and Wildlife Service. 17 September 2021. Available online: https://medium.com/usfwspacificislands/l%C4%81%CA%BBau-lapa%CA%BBau-reclaiming-traditional-practices-of-medicine-9d5ebfe281c1 (accessed on 26 October 2024).
- Almarfadi, O.M.; Siddiqui, N.A.; Shahat, A.A.; Fantoukh, O.I.; El Gamal, A.A.; Raish, M.; Bari, A.; Iqbal, M.; Alqahtani, A.S. Isolation of a novel isoprenylated phenolic compound and neuroprotective evaluation of Dodonaea viscosa extract against cerebral ischaemia–reperfusion injury in rats. Saudi Pharm. J. 2024, 32, 101898. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Irshad, S.; Khan, I.; Mohammad, A.; Anis, I.; Shah, M.R.; Khan, I.; Chebib, M. GABA(A) receptor modulation and neuropharmacological activities of viscosine isolated from Dodonaea viscosa (Linn). Pharmacol. Biochem. Behav. 2015, 136, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wang, X.H.; Liu, Y.N.; Zhao, T.; Hu, Z.; Li, J.Y.; Hou, A.J. Clerodane diterpenoids from Dodonaea viscosa and their inhibitory effects on ATP citrate lyase. Phytochemistry 2021, 183, 112614. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.T.; Lei, C.; You, J.Q.; Zhao, T.; Yu, M.H.; Shi, X.L.; Hu, X.; Hou, A.J. Dimeric clerodane diterpenoids and antiviral constituents of Dodonaea viscosa. Bioorg. Chem. 2021, 112, 104916. [Google Scholar] [CrossRef]
- Siddiqui, N.A.; Almarfadi, O.M.; Shahat, A.A.; Alqahtani, A.S.; El Gamal, A.A.; Raish, M.; Iqbal, M. Isolation of new compound and neuroprotective studies from Dodonaea viscosa. J. King Saud Univ. Sci. 2023, 35, 102704. [Google Scholar] [CrossRef]
- Yu, H.; Liu, B.; Zhao, Y.; Li, J.; Wu, G.; Ma, J.; Gui, F.; Tao, F.; Hao, X.; Ding, X.; et al. Combined activity of saponin B isolated from Dodonaea viscosa seeds with pesticide azadirachtin against the pest Spodoptera litura. Metabolites 2023, 14, 15. [Google Scholar] [CrossRef]
- Liang, W.; Yu, Q.; Zheng, Z.; Liu, J.; Cai, Q.; Liu, S.; Lin, S. Design and synthesis of phenyl sulfide-based cationic amphiphiles as membrane-targeting antimicrobial agents against Gram-positive pathogens. J. Med. Chem. 2022, 65, 14221–14236. [Google Scholar] [CrossRef]
- Li, F.; Arnsberger, P.; Miller, F.D. Profile of methicillin-resistant Staphylococcus aureus among nursing home residents in Hawai ‘i. Hawaii Med. J. 2010, 6, 126. [Google Scholar]
- Estivariz, C.F.; Park, S.Y.; Hageman, J.C.; Dvorin, J.; Melish, M.M.; Arpon, R.; Coon, P.; Slavish, S.; Kim, M.; McDougal, L.K. Emergence of community-associated methicillin-resistant Staphylococcus aureus in Hawaii, 2001–2003. J. Infect. 2007, 54, 349–357. [Google Scholar] [CrossRef]
- Li, F.; Park, S.Y.; Ayers, T.L.; Miller, F.D.; MacFadden, R.; Nakata, M.; Lee, M.C.; Effler, P.V. Methicillin-resistant Staphylococcus aureus, Hawaii, 2000–2002. Emerg. Infect. Dis. 2005, 11, 1205–1210. [Google Scholar] [CrossRef]
- Khurram, M.; Lawton, L.A.; Edwards, C.; Iriti, M.; Hameed, A.; Khan, M.A.; Khan, F.A.; Rahman, S.U. Rapid bioassay-guided isolation of antibacterial clerodane type diterpenoid from Dodonaea viscosa (L.) Jaeq. Int. J. Mol. Sci. 2015, 16, 20290–20307. [Google Scholar] [CrossRef] [PubMed]
- Rivenbark, A.G.; O’Connor, S.M.; Coleman, W.B. Molecular and cellular heterogeneity in breast cancer: Challenges for personalized medicine. Am. J. Pathol. 2013, 183, 1113–1124. [Google Scholar] [CrossRef]
- Luond, F.; Tiede, S.; Christofori, G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.; Sendi, M.A.; Kelly, C.M. Overview of recent advances in metastatic triple negative breast cancer. World J. Clin. Oncol. 2021, 12, 164–182. [Google Scholar] [CrossRef]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and triple-negative breast cancer: Challenges and treatment options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef]
- Elfgen, C.; Bjelic-Radisic, V. Targeted therapy in HR+ HER2- metastatic breast cancer: Current clinical trials and their implications for CDK4/6 inhibitor therapy and beyond treatment options. Cancers 2021, 13, 5994. [Google Scholar] [CrossRef]
- Lim, B.; Woodward, W.A.; Wang, X.; Reuben, J.M.; Ueno, N.T. Inflammatory breast cancer biology: The tumour microenvironment is key. Nat. Rev. Cancer 2018, 18, 485–499. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Herrera-Ramírez, A.; Cardona-G, W.; Melgar-Merino, E.J.; Chávez, H.; Pari-Olarte, J.B.; Loyola-Gonzales, E.; Kong-Chirinos, J.F.; Almeida-Galindo, J.S.; Peña-Rojas, G.; et al. Dodonaea viscosa Jacq. induces cytotoxicity, antiproliferative activity, and cell death in colorectal cancer cells via regulation of caspase 3 and p53. Front. Pharmacol. 2023, 14, 1197569. [Google Scholar] [CrossRef]
- Anglana, C.; Rojas, M.; Girelli, C.R.; Barozzi, F.; Quiroz-Troncoso, J.; Alegría-Aravena, N.; Montefusco, A.; Durante, M.; Fanizzi, F.P.; Ramírez-Castillejo, C.; et al. Methanolic extracts of D. viscosa specifically affect the cytoskeleton and exert an antiproliferative effect on human colorectal cancer cell lines, according to their proliferation rate. Int. J. Mol. Sci. 2023, 24, 14920. [Google Scholar] [CrossRef]
- Kheyar-Kraouche, N.; Boucheffa, S.; Bellik, Y.; Farida, K.; Brahmi-Chendouh, N. Exploring the potential of Inula viscosa extracts for antioxidant, antiproliferative and apoptotic effects on human liver cancer cells and a molecular docking study. BioTechnologia 2023, 104, 183–198. [Google Scholar] [CrossRef]
- Ren, G.; Hou, J.; Fang, Q.; Sun, H.; Liu, X.; Zhang, L.; Wang, P.G. Synthesis of flavonol 3-O-glycoside by UGT78D1. Glycoconj. J. 2012, 29, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Willuhn, G. Sesquiterpene lactone and flavonoid variability of the Arnica angustifolia aggregate (Asteraceae). Biochem. Syst. Ecol. 2000, 28, 133–142. [Google Scholar] [CrossRef]
- Lien, T.T.P.; Razumovskaya, R.G.; Spiridovich, E.V.; Tan, L.Q.; Hoa, N.T.; Hue, C.T. Chemical Composition and Biological Activity of Vietnamese Amaranthus spinosus. Chem. Nat. Compd. 2019, 55, 1164–1166. [Google Scholar] [CrossRef]
- El-Sayed, N.H.; Norris, J.A.; Ahmed, A.A.; Mabry, T.J. Flavonoids of Brickellia longifolia. Phytochemistry 1990, 29, 2364–2365. [Google Scholar] [CrossRef]
- Martinez, J.; Silván, A.M.; Abad, M.J.; Bermejo, P.; Villar, A.; Söllhuber, M. Isolation of two flavonoids from Tanacetum microphyllum as PMA-induced ear edema inhibitors. J. Nat. Prod. 1997, 60, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Poonam, V.; Kumar, G.; Reddy, L.C.S.; Jain, R.; Sharma, S.K.; Prasad, A.K.; Parmar, V.S. Chemical constituents of the genus Prunus and their medicinal properties. Curr. Med. Chem. 2011, 18, 3758–3824. [Google Scholar] [CrossRef]
- Yenjai, C.; Prasanphen, K.; Daodee, S.; Wongpanich, V.; Kittakoop, P. Bioactive flavonoids from Kaempferia parviflora. Fitoterapia 2004, 75, 89–92. [Google Scholar] [CrossRef]
- Nagarajan, G.R.; Parmar, V.S. Chemical examination of the heartwood of Prunus domestica. Planta Med. 1977, 31, 146–150. [Google Scholar] [CrossRef]
- Kamperdick, C.; Van, N.H.; Van Sung, T. Constituents from Miliusa balansae (Annonaceae). Phytochemistry 2002, 61, 991–994. [Google Scholar] [CrossRef]
- Pisutthanan, N.; Liawruangrath, B.; Liawruangrath, S.; Bremner, J.B. A new flavonoid from Chromolaena odorata. Nat. Prod. Res. 2006, 20, 1192–1198. [Google Scholar] [CrossRef]
- Shirataki, Y.; Yokoe, I.; Endo, M.; Komatsu, M. Determination of C-6 or C-8 substituted flavanone using 13C-1H long range coupling and the revised structures of some flavanones. Chem. Pharm. Bull. 1985, 33, 444–447. [Google Scholar] [CrossRef]
- Tip-Pyang, S.; Veerachato, G.; Phuwapraisirisan, P.; Sathanasaowapak, S. Antibacterial component from Arfeuillea arborescens Pierre. Chem. Res. Commun. 1999, 9, 15–19. [Google Scholar]
- Ali, H.; Kabir, N.; Muhammad, A.; Shah, M.R.; Musharraf, S.G.; Iqbal, N.; Nadeem, S. Hautriwaic acid as one of the hepatoprotective constituents of Dodonaea viscosa. Phytomedicine 2014, 21, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Sastry, K.N.S.; Nayudamma, Y. Leucocyanidin from Dodonaea viscosa bark. Leather Sci. 1966, 13, 174–176. [Google Scholar]
- Tong, Z.W.; Gul, H.; Awais, M.; Saddick, S.; Khan, F.S.; Gulfraz, M.; Afzal, U.; Nazir, K.; Malik, M.Y.; Khan, S.U.; et al. Determination of in vivo biological activities of Dodonaea viscosa flowers against CCL4 toxicity in albino mice with bioactive compound detection. Sci. Rep. 2021, 11, 13336. [Google Scholar] [CrossRef]
- Teffo, L.S.; Aderogba, M.A.; Eloff, J.N. Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from Dodonaea viscosa Jacq. var. angustifolia leaf extracts. S. Afr. J. Bot. 2010, 76, 25–29. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xiaoyun, X. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Schütz, B.A.; Wright, A.D.; Rali, T.; Sticher, O. Prenylated flavanones from leaves of Macaranga pleiostemona. Phytochemistry 1995, 40, 1273–1277. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Peter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Gupta, T.; Kataria, R.; Sardana, S. A Comprehensive Review on Current Perspectives of Flavonoids as Antimicrobial Agent. Curr. Top. Med. Chem. 2022, 22, 425–434. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Plochmann, K.; Korte, G.; Koutsilieri, E.; Richling, E.; Riederer, P.; Rethwilm, A.; Schreier, P.; Scheller, C. Structure–activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch. Biochem. Biophys. 2007, 460, 1–9. [Google Scholar] [CrossRef]
- Touil, Y.S.; Fellous, A.; Scherman, D.; Chabot, G.G. Flavanoid-induced morphological modifications of endothelial cells through microtubule stabilization. Nutr. Cancer 2009, 61, 310–321. [Google Scholar] [CrossRef]
- Gerken, T.J.; Roberts, M.C.; Dykema, P.; Melly, G.; Lucas, D.; De Los Santos, V.; Gonzalez, J.; Butaye, P.; Wiegner, T.N. Environmental surveillance and characterization of antibiotic-resistant Staphylococcus aureus at coastal beaches and rivers on the island of Hawaiʻi. Antibiotics 2021, 10, 980. [Google Scholar] [CrossRef]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar]
- Elshikh, M.; Ahmed, S.; Funston, S.; Dunlop, P.; McGaw, M.; Marchant, R.; Banat, I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016, 38, 1015–1019. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]

| Compounds | Gram-Positive | Gram-Negative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRSA USA-300 | MSSA LUU7 | MSSA 8325-4 | S. aureus 8384 | MSSA ONE6 | MSSA RI27 | MSSA LUE1 | MRSA N315 | MSSA Newman | E. coli | K. pneumoniae | P. aeruginosa | |
| D. viscosa flowers extract | 640 | 320 | 640 | 640 | 320 | 320 | 320 | 320 | 320 | 1280 | 1280 | 1280 |
| 1 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | 128 |
| 2 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 128 | 128 | 128 |
| 3 | - | - | - | - | - | - | - | - | - | - | 128 | - |
| 4 | - | - | - | - | - | - | - | - | - | - | 128 | 128 |
| 5 | - | - | - | - | - | - | - | - | - | - | 128 | - |
| 6 | - | - | - | - | - | - | - | - | - | - | 128 | - |
| 7 | - | - | - | - | - | - | - | - | - | - | 128 | 128 |
| 8 | - | - | - | - | - | - | - | - | - | - | - | 128 |
| 9 | - | - | - | - | - | - | - | - | - | - | - | 128 |
| 10 | - | - | - | - | - | - | - | - | - | - | 128 | 128 |
| 11 | - | - | - | - | - | - | - | - | - | 128 | 128 | 128 |
| 12 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 128 | 128 | 128 |
| 13 | - | - | - | - | - | - | - | - | - | - | 128 | 128 |
| Vancomycin | 0.5 | 1 | 0.5 | 0.5 | 0.5 | 1 | 0.25 | 0.5 | 0.5 | nt | nt | nt |
| Gentamicin | nt | nt | nt | nt | nt | nt | nt | nt | nt | 0.25 | 1 | 0.25 |
| Cell Lines | Subtype | IC50 ± S.D (µM) | Cell Lines | Tumor Type | IC50 ± S.D (µM) | Cell Lines | Tumor Type | IC50 ± S.D (µM) |
|---|---|---|---|---|---|---|---|---|
| SUM149 | TNBC | 7.73 ± 0.24 | HCC1806 | TNBC | 21.96 ± 1.29 | SUM159 | TNBC | 14.23 ± 0.48 |
| IBC3 | HER2+ | 25.61 ± 3.36 | HCC1937 | TNBC | 26.28 ± 3.43 | MDA-MB-468 | TNBC | 12.3 ± 0.30 |
| FC-IBC02 | TNBC | 17.81 ± 1.96 | MDA-MB-175 VII | HR+ BC | 21.48 ± 1.48 | MDA-MB-231 | TNBC | 4.96 ± 0.44 |
| A3250 | TNBC | 17.63 ± 1.84 | HCC3153 | TNBC | 15.46 ± 0.66 | A549 | NSCLC | 10.73 ± 0.81 |
| BCX-010 | TNBC | 4.22 ± 0.37 | ZR-75-1 | HR+ BC | 14.71 ± 0.58 | HT29 | Colon | 27.88 ± 5.43 |
| SUM190 | HER2+ | 6.74 ± 0.32 | CAL51 | TNBC | 9.16 ± 0.40 | HT116 | Colon | 17.03 ± 0.81 |
| KPL4 | HER2+ | 11.69 ± 0.36 | HCC1428 | HR+ BC | 24.35 ± 1.52 | SNU398 | HCC | 7.39 ± 0.54 |
| BT20 | TNBC | 26.71 ± 3.56 | MDA-MB-436 | TNBC | 23.48 ± 1.16 | MCF-10A | Normal | >20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raksat, A.; Yee, D.; Gi, Y.J.; Wongwiwatthananukit, S.; Chang, L.K.; Kaawaloa, K.P.; Wall, M.M.; Lee, J.; Chang, L.C. Bioactive Compounds from Dodonaea viscosa Flowers: Potent Antibacterial and Antiproliferative Effects in Breast Cancer Cells. Molecules 2025, 30, 2274. https://doi.org/10.3390/molecules30112274
Raksat A, Yee D, Gi YJ, Wongwiwatthananukit S, Chang LK, Kaawaloa KP, Wall MM, Lee J, Chang LC. Bioactive Compounds from Dodonaea viscosa Flowers: Potent Antibacterial and Antiproliferative Effects in Breast Cancer Cells. Molecules. 2025; 30(11):2274. https://doi.org/10.3390/molecules30112274
Chicago/Turabian StyleRaksat, Achara, Daniel Yee, Young Jin Gi, Supakit Wongwiwatthananukit, Leng Kar Chang, Kumu Piilani Kaawaloa, Marisa M. Wall, Jangsoon Lee, and Leng Chee Chang. 2025. "Bioactive Compounds from Dodonaea viscosa Flowers: Potent Antibacterial and Antiproliferative Effects in Breast Cancer Cells" Molecules 30, no. 11: 2274. https://doi.org/10.3390/molecules30112274
APA StyleRaksat, A., Yee, D., Gi, Y. J., Wongwiwatthananukit, S., Chang, L. K., Kaawaloa, K. P., Wall, M. M., Lee, J., & Chang, L. C. (2025). Bioactive Compounds from Dodonaea viscosa Flowers: Potent Antibacterial and Antiproliferative Effects in Breast Cancer Cells. Molecules, 30(11), 2274. https://doi.org/10.3390/molecules30112274








