Flavanones as Modulators of Gut Microbiota and Cognitive Function
Abstract
1. Introduction
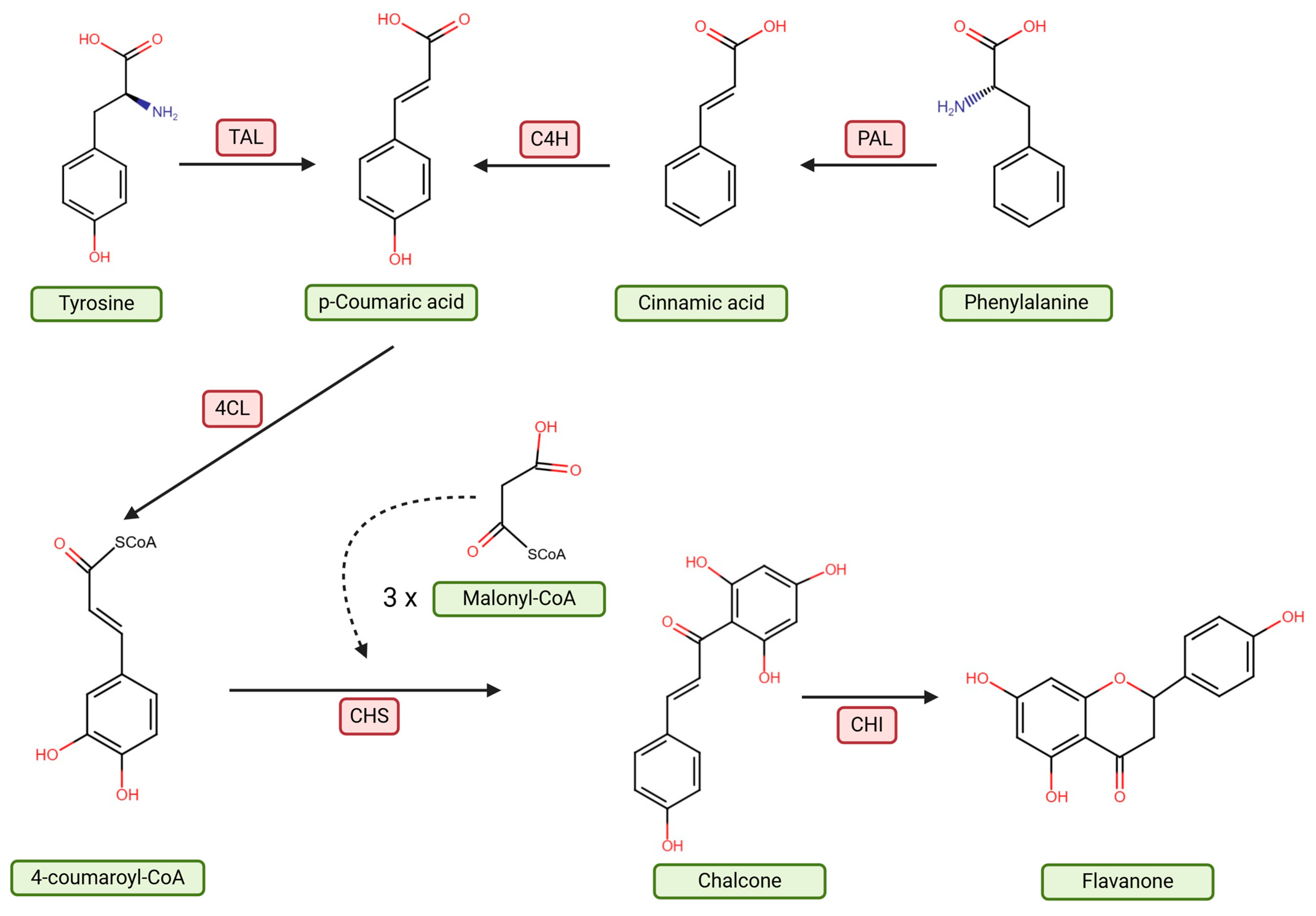
2. Characteristics of Flavanones and Their Metabolism in the Body
2.1. Naringenin
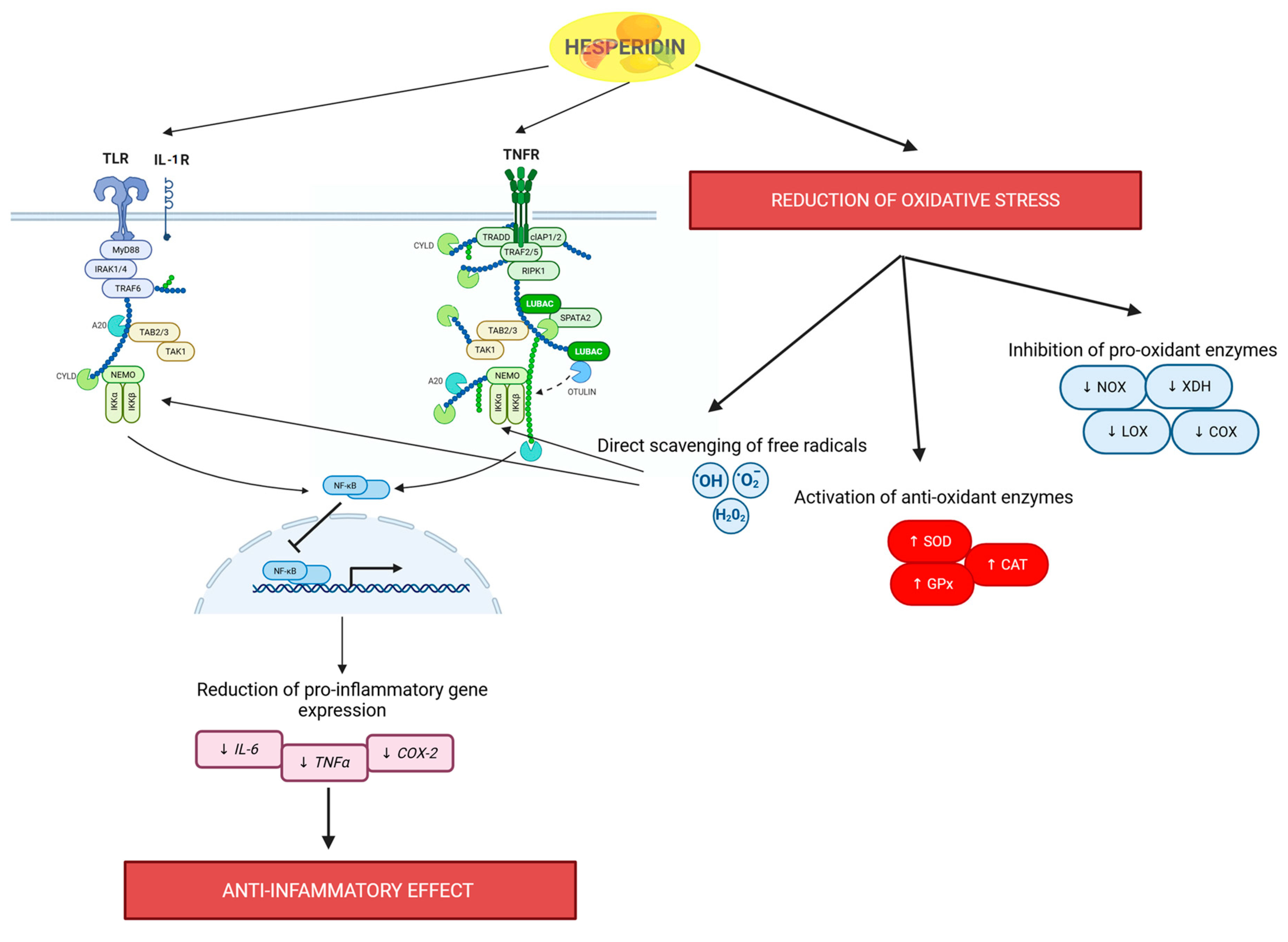
2.2. Hesperidin
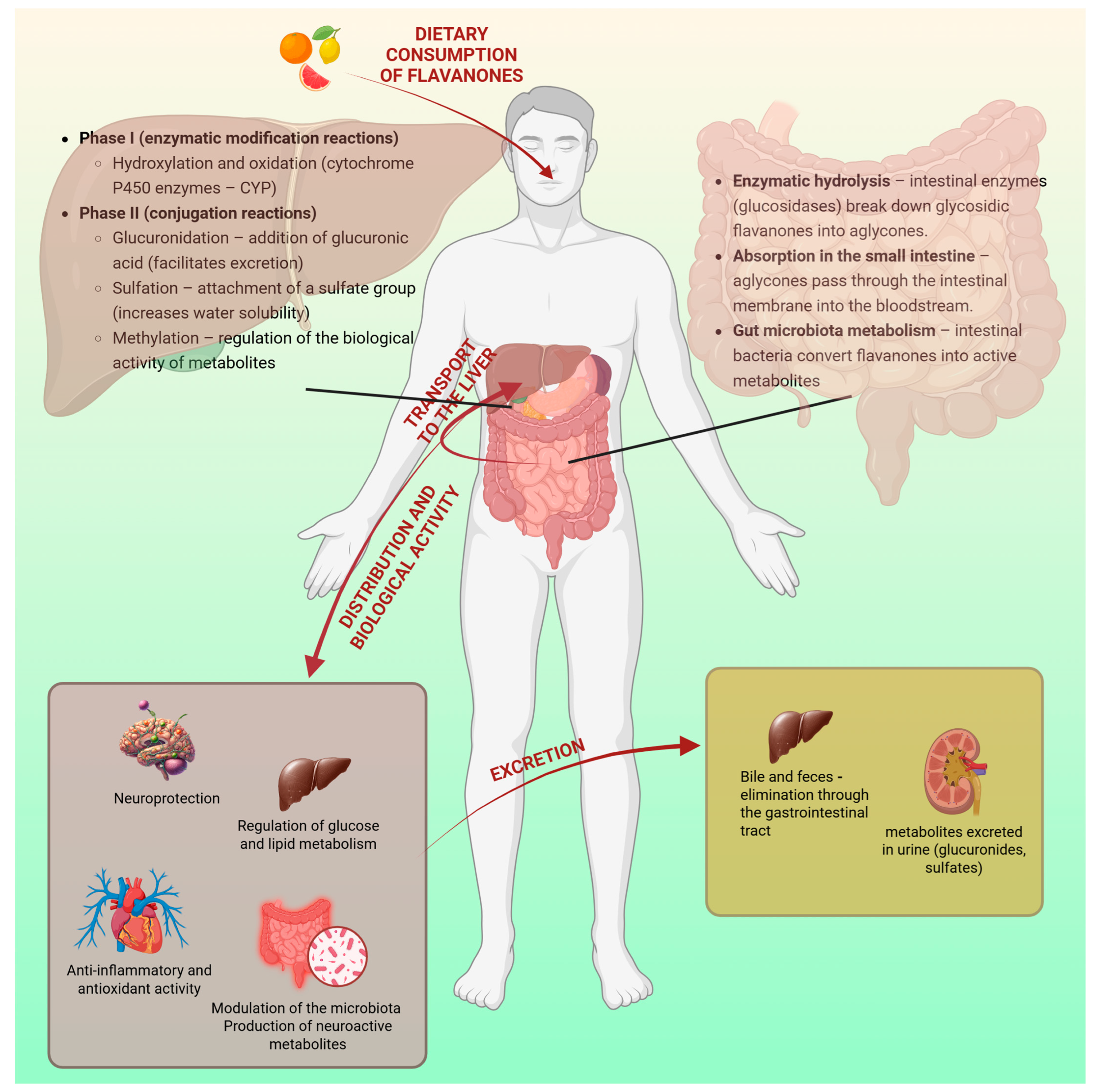
2.3. Flavanone Metabolism in the Gastrointestinal Tract
3. Intestinal Microbiota and Flavanone Impact
Flavanones Change the Composition in Gut Microbiota
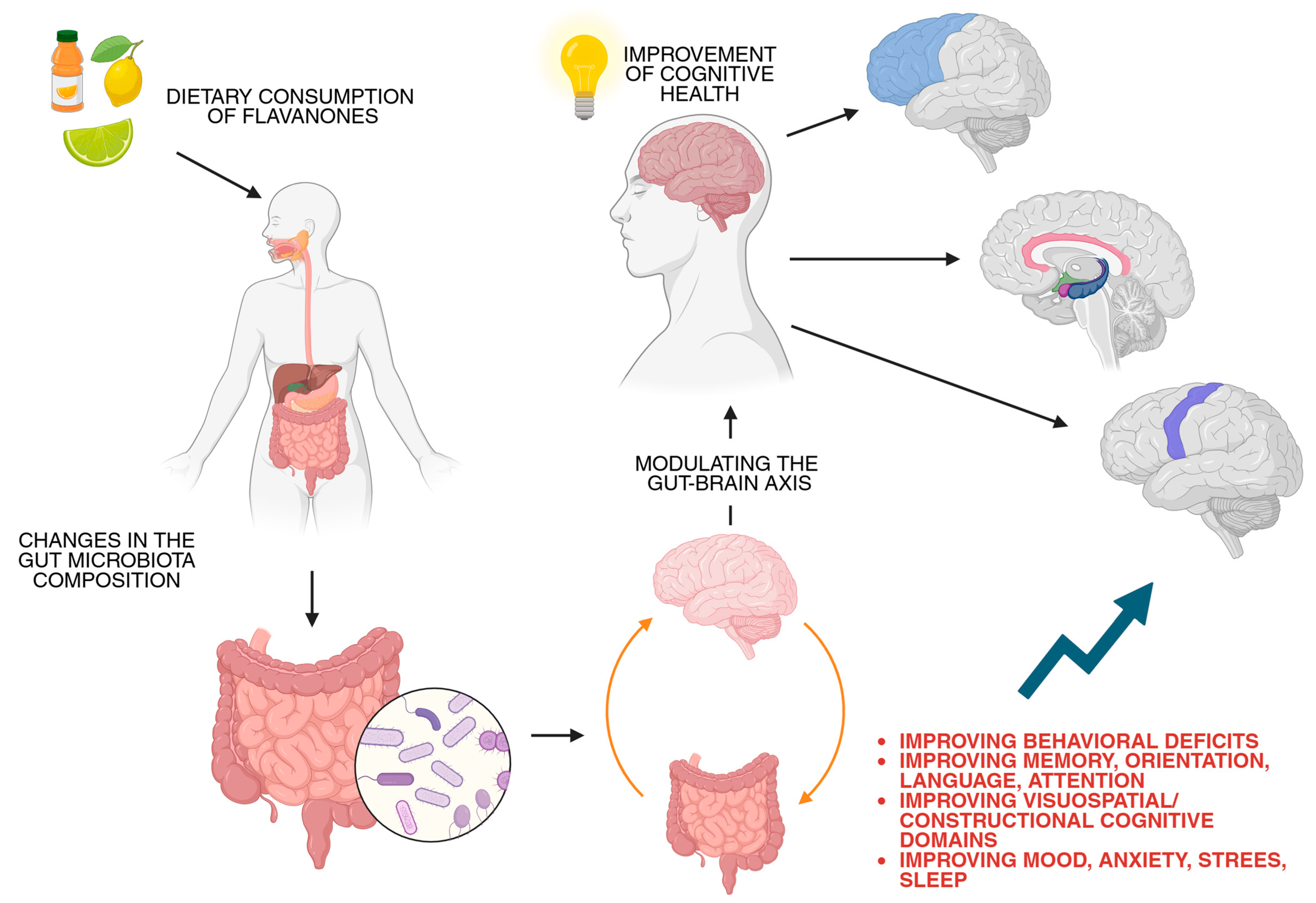
4. The Gut–Brain Axis and Cognitive Health
4.1. Significance of GBA in Cognitive Health
4.2. GBA and Neurotransmitters
4.3. Immune System
5. Potential Applications for Cognitive Health
6. Limitations and Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Zill, E.H.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef]
- Jez, J.M.; Bowman, M.E.; Noel, J.P. Role of Hydrogen Bonds in the Reaction Mechanism of Chalcone Isomerase. Biochemistry 2002, 41, 5168–5176. [Google Scholar] [CrossRef]
- Fowler, Z.L.; Koffas, M.A.G. Biosynthesis and biotechnological production of flavanones: Current state and perspectives. Appl. Microbiol. Biotechnol. 2009, 83, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef]
- Uçar, K.; Göktaş, Z. Biological activities of naringenin: A narrative review based on in vitro and in vivo studies. Nutr. Res. 2023, 119, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, Z.; Yuan, J.; Li, P.; Wang, Q.; Cho, N.; Wang, Y.; Lin, Z. The neuroprotective mechanisms of naringenin: Inhibition of apoptosis through the PI3K/AKT pathway after hypoxic-ischemic brain damage. J. Ethnopharmacol. 2024, 318, 116941. [Google Scholar] [CrossRef] [PubMed]
- Miler, M.; Živanović, J.; Ajdžanović, V.; Šošić-Jurjević, B.; Marković, Z.; Milošević, V. The effects of naringenin on NRF2 and antioxidant enzymes expressions in the thyroids of the old-aged Wistar rats. Endocr. Abstr. 2020, 70, 919. [Google Scholar] [CrossRef]
- Yoshida, H.; Takamura, N.; Shuto, T.; Ogata, K.; Tokunaga, J.; Kawai, K.; Kai, H. The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-α in mouse adipocytes. Biochem. Biophys. Res. Commun. 2010, 394, 728–732. [Google Scholar] [CrossRef]
- Yang, J.; Liu, L.; Li, M.; Huang, X.; Yang, H.; Li, K. Naringenin inhibits pro-inflammatory cytokine production in macrophages through inducing MT1G to suppress the activation of NF-κB. Mol. Immunol. 2021, 137, 155–162. [Google Scholar] [CrossRef]
- Liu, X.; Wang, N.; Fan, S.; Zheng, X.; Yang, Y.; Zhu, Y.; Lu, Y.; Chen, Q.; Zhou, H.; Zheng, J. The citrus flavonoid naringenin confers protection in a murine endotoxaemia model through AMPK-ATF3-dependent negative regulation of the TLR4 signalling pathway. Sci. Rep. 2016, 6, 39735. [Google Scholar] [CrossRef]
- Lim, Y.J.; Kim, J.H.; Pan, J.H.; Kim, J.K.; Park, T.S.; Kim, Y.J.; Lee, J.H. Naringin Protects Pancreatic β-Cells Against Oxidative Stress-Induced Apoptosis by Inhibiting Both Intrinsic and Extrinsic Pathways in Insulin-Deficient Diabetic Mice. Mol. Nutr. Food Res. 2018, 62, 1700810. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef]
- Kaźmierczak, T.; Cyboran-Mikołajczyk, S.; Trochanowska-Pauk, N.; Walski, T.; Nowicka, P.; Bonarska-Kujawa, D. Insights on the Mechanisms of the Protective Action of Naringenin, Naringin and Naringin Dihydrochalcone on Blood Cells in Terms of Their Potential Anti-Atherosclerotic Activity. Molecules 2025, 30, 547. [Google Scholar] [CrossRef]
- Bawazeer, N.A.; Choudary, H.; Zamzami, M.A.; Abdulaal, W.H.; Zeyadi, M.; Albukhari, A.; Middleton, B.; Moselhy, S.S. POSSIBLE REGULATION OF LDL-RECEPTOR BY NARINGENIN IN HEPG2 HEPATOMA CELL LINE. Afr. J. Tradit. Complement Altern. Med. 2017, 14, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Madureira, M.B.; Concato, V.M.; Cruz, E.M.S.; Bitencourt de Morais, J.M.; Inoue, F.S.R.; Concimo Santos, N.; Gonçalves, M.D.; Cremer de Souza, M.; Basso Scandolara, T.; Fontana Mezoni, M.; et al. Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer. Antioxidants 2023, 12, 586. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Hao, J.; Liu, T.; Zhang, D.; Lv, H.; Song, E.; Zhu, C. Hesperetin Suppresses Inflammatory Responses in Lipopolysaccharide-Induced RAW 264.7 Cells via the Inhibition of NF-κB and Activation of Nrf2/HO-1 Pathways. Inflammation 2016, 39, 964–973. [Google Scholar] [CrossRef]
- Kang, S.R.; Park, K.I.; Park, H.S.; Lee, D.H.; A Kim, J.; Nagappan, A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Park, M.K.; et al. Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chem. 2011, 129, 1721–1728. [Google Scholar] [CrossRef]
- Hosawi, S. Current Update on Role of Hesperidin in Inflammatory Lung Diseases: Chemistry, Pharmacology, and Drug Delivery Approaches. Life 2023, 13, 937. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Gao, G.; Ding, H.; Zhuang, C.; Fan, W. Effects of Hesperidin on H2O2-Treated Chondrocytes and Cartilage in a Rat Osteoarthritis Model. Med. Sci. Monit. 2018, 24, 9177–9186. [Google Scholar] [CrossRef]
- Abuelsaad, A.S.; Allam, G.; Al-Solumani, A.A. Hesperidin inhibits inflammatory response induced by Aeromonas hydrophila infection and alters CD4+/CD8+ T cell ratio. Mediat. Inflamm. 2014, 2014, 393217. [Google Scholar] [CrossRef]
- Amiri, H.; Javid, H.; Hashemi, S.F.; Reihani, A.; Esparham, A.; Hashemy, S.I. The protective effects of hesperidin as an antioxidant against quinolinic acid-induced toxicity on oligodendroglia cells: An in vitro study. Mult. Scler. Relat. Disord. 2024, 82, 105401. [Google Scholar] [CrossRef]
- Imperatrice, M.; Cuijpers, I.; Troost, F.J.; Sthijns, M. Hesperidin Functions as an Ergogenic Aid by Increasing Endothelial Function and Decreasing Exercise-Induced Oxidative Stress and Inflammation, Thereby Contributing to Improved Exercise Performance. Nutrients 2022, 14, 2955. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef] [PubMed]
- Lorzadeh, E.; Ramezani-Jolfaie, N.; Mohammadi, M.; Khoshbakht, Y.; Salehi-Abargouei, A. The effect of hesperidin supplementation on inflammatory markers in human adults: A systematic review and meta-analysis of randomized controlled clinical trials. Chem. Biol. Interact. 2019, 307, 8–15. [Google Scholar] [CrossRef]
- Shi, X.; Liao, S.; Mi, H.; Guo, C.; Qi, D.; Li, F.; Zhang, C.; Yang, Z. Hesperidin Prevents Retinal and Plasma Abnormalities in Streptozotocin-Induced Diabetic Rats. Molecules 2012, 17, 12868–12881. [Google Scholar] [CrossRef] [PubMed]
- Demonty, I.; Lin, Y.; Zebregs, Y.E.; Vermeer, M.A.; van der Knaap, H.C.; Jäkel, M.; Trautwein, E.A. The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J. Nutr. 2010, 140, 1615–1620. [Google Scholar] [CrossRef]
- Kowalczyk, A. Hesperidin, a Potential Antiviral Agent against SARS-CoV-2: The Influence of Citrus Consumption on COVID-19 Incidence and Severity in China. Medicina 2024, 60, 892. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Pharmacological Significance of Hesperidin and Hesperetin, Two Citrus Flavonoids, as Promising Antiviral Compounds for Prophylaxis Against and Combating COVID-19. Nat. Prod. Commun. 2021, 16, 1934578X211042540. [Google Scholar] [CrossRef]
- Ding, Z.; Sun, G.; Zhu, Z. Hesperidin attenuates influenza A virus (H1N1) induced lung injury in rats through its anti-inflammatory effect. Antivir. Ther. 2018, 23, 611–615. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Thakral, F.; Singhal, P.; Aggarwal, D.; Srivastava, S.; Pandey, A.; Sak, K.; Varol, M.; Khan, M.A.; et al. Molecular mechanisms of action of hesperidin in cancer: Recent trends and advancements. Exp. Biol. Med. 2020, 245, 486–497. [Google Scholar] [CrossRef]
- Kamaraj, S.; Anandakumar, P.; Jagan, S.; Ramakrishnan, G.; Periyasamy, P.; Asokkumar, S.; Subramanian, R.; Devaki, T. Hesperidin inhibits cell proliferation and induces mitochondrial-mediated apoptosis in human lung cancer cells through down regulation of β-catenin/c-myc. Biocatal. Agric. Biotechnol. 2019, 18, 101065. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Hollman, P.C.; Katan, M.B. Absorption, metabolism and health effects of dietary flavonoids in man. Pharm. Biol. 1997, 51, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.Q.; Cesar, T.B.; Manthey, J.A.; Baldwin, E.A.; Bai, J.; Raithore, S. Pharmacokinetics of flavanone glycosides after ingestion of single doses of fresh-squeezed orange juice versus commercially processed orange juice in healthy humans. J. Agric. Food Chem. 2014, 62, 12576–12584. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Leone, V.; Chang, E.B. Regional Diversity of the Gastrointestinal Microbiome. Cell Host Microbe 2019, 26, 314–324. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Yang, X.-Y.; Han, B.-S.; Hu, Y.-Y.; An, T.; Lv, B.-H.; Lian, J.; Wang, T.-Y.; Bao, X.-L.; Gao, L.; et al. Naringenin regulates gut microbiota and SIRT1/ PGC-1ɑ signaling pathway in rats with letrozole-induced polycystic ovary syndrome. Biomed. Pharmacother. 2022, 153, 113286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, M.; Jin, C.; Sun, X.; Feng, F.; Niu, X.; Wang, B.; Zhang, Y.; Wang, J. Naringenin confers protection against experimental autoimmune encephalomyelitis through modulating the gut-brain axis: A multiomics analysis. J. Nutr. Biochem. 2023, 122, 109448. [Google Scholar] [CrossRef]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Firrman, J.; Liu, L.; Argoty, G.A.; Zhang, L.; Tomasula, P.; Wang, M.; Pontious, S.; Kobori, M.; Xiao, W. Analysis of Temporal Changes in Growth and Gene Expression for Commensal Gut Microbes in Response to the Polyphenol Naringenin. Microbiol. Insights 2018, 11, 1178636118775100. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Han, M.J.; Kim, D.H. In vitro anti-Helicobacter pylori activity of some flavonoids and their metabolites. Planta Med. 1999, 65, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Unno, T.; Hisada, T.; Takahashi, S. Hesperetin Modifies the Composition of Fecal Microbiota and Increases Cecal Levels of Short-Chain Fatty Acids in Rats. J. Agric. Food Chem. 2015, 63, 7952–7957. [Google Scholar] [CrossRef] [PubMed]
- Fidélix, M.; Milenkovic, D.; Sivieri, K.; Cesar, T. Microbiota modulation and effects on metabolic biomarkers by orange juice: A controlled clinical trial. Food Funct. 2020, 11, 1599–1610. [Google Scholar] [CrossRef]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Duque, A.L.R.F.; Monteiro, M.; Adorno, M.A.T.; Sakamoto, I.K.; Sivieri, K. An exploratory study on the influence of orange juice on gut microbiota using a dynamic colonic model. Food Res. Int. 2016, 84, 160–169. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.; Wang, D.; Yao, Q.; Ma, G.-L.; Fan, X. Simulator of the Human Intestinal Microbial Ecosystem (SHIME®): Current Developments, Applications, and Future Prospects. Pharmaceuticals 2024, 17, 1639. [Google Scholar] [CrossRef]
- Mitsuoka, T. Bifidobacteria and their role in human health. J. Ind. Microbiol. 1990, 6, 263–267. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, Y.; Zhong, H.; Liu, Z.; Geng, J.; Wang, H.; Wang, W. Gut microbes in central nervous system development and related disorders. Front. Immunol. 2023, 14, 1288256. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.J.; Wu, X.L.; Chen, W.Q.; Li, Y.F.; Zhang, G.F.; Chao, L.M.; Zhou, J.H.; Guo, A.; Liu, C.; Guo, S.N. The Gut Microbiome Modulates the Changes in Liver Metabolism and in Inflammatory Processes in the Brain of Chronic Unpredictable Mild Stress Rats. Oxid. Med. Cell Longev. 2019, 2019, 7902874. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Tyler, K.; MacEachern, S.J.; Balemba, O.B.; Johnson, A.C.; Brooks, E.M.; Zhao, H.; Swain, G.M.; Moses, P.L.; Galligan, J.J.; et al. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology 2012, 142, 844–854.E4. [Google Scholar] [CrossRef]
- Baganz, N.L.; Blakely, R.D. A dialogue between the immune system and brain, spoken in the language of serotonin. ACS Chem. Neurosci. 2013, 4, 48–63. [Google Scholar] [CrossRef]
- Cloutier, N.; Allaeys, I.; Marcoux, G.; Machlus, K.R.; Mailhot, B.; Zufferey, A.; Levesque, T.; Becker, Y.; Tessandier, N.; Melki, I.; et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Natl. Acad. Sci. USA 2018, 115, E1550–E1559. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Ben Shaul, T.; Frenkel, D.; Gurevich, T. The Interplay of Stress, Inflammation, and Metabolic Factors in the Course of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 12409. [Google Scholar] [CrossRef] [PubMed]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Ostendorf, F.; Metzdorf, J.; Gold, R.; Haghikia, A.; Tönges, L. Propionic Acid and Fasudil as Treatment Against Rotenone Toxicity in an In Vitro Model of Parkinson’s Disease. Molecules 2020, 25, 2502. [Google Scholar] [CrossRef]
- Yunes, R.A.; Poluektova, E.U.; Dyachkova, M.S.; Klimina, K.M.; Kovtun, A.S.; Averina, O.V.; Orlova, V.S.; Danilenko, V.N. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe 2016, 42, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of Gamma-Aminobutyric Acid from Lactic Acid Bacteria: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. Gamma-aminobutyric acid as a potential postbiotic mediator in the gut-brain axis. NPJ Sci. Food 2024, 8, 16. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Liu, J.; Jin, Y.; Ye, Y.; Tang, Y.; Dai, S.; Li, M.; Zhao, G.; Hong, G.; Lu, Z.Q. The Neuroprotective Effect of Short Chain Fatty Acids Against Sepsis-Associated Encephalopathy in Mice. Front. Immunol. 2021, 12, 626894. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Peixoto, L.; Abel, T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 2013, 38, 62–76. [Google Scholar] [CrossRef]
- Sarubbo, F.; Cavallucci, V.; Pani, G. The Influence of Gut Microbiota on Neurogenesis: Evidence and Hopes. Cells 2022, 11, 382. [Google Scholar] [CrossRef]
- Li, Y.; Liu, A.; Chen, K.; Li, L.; Zhang, X.; Zou, F.; Zhang, X.; Meng, X. Sodium butyrate alleviates lead-induced neuroinflammation and improves cognitive and memory impairment through the ACSS2/H3K9ac/BDNF pathway. Environ. Int. 2024, 184, 108479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lu, J.; Jin, Z.; Xu, H.; Zhang, D.; Chen, J.; Wang, J. Gut microbiota metabolites: Potential therapeutic targets for Alzheimer’s disease? Front. Pharmacol. 2024, 15, 1459655. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, T.; Hu, X.; Luo, J.; Li, W.; Wu, X.; Duan, Y.; Jin, F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 2015, 310, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Sushma, G.; Vaidya, B.; Sharma, S.; Devabattula, G.; Bishnoi, M.; Kondepudi, K.K.; Sharma, S.S. Bifidobacterium breve Bif11 supplementation improves depression-related neurobehavioural and neuroinflammatory changes in the mouse. Neuropharmacology 2023, 229, 109480. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Mo, R.; Jiang, M.; Xu, H.; Jia, R. Effect of probiotics on cognitive function in adults with mild cognitive impairment or Alzheimer’s disease: A meta-analysis of randomized controlled trials. Med. Clin. 2024, 162, 565–573. [Google Scholar] [CrossRef]
- Camberos-Barraza, J.; Guadrón-Llanos, A.M.; De la Herrán-Arita, A.K. The Gut Microbiome-Neuroglia Axis: Implications for Brain Health, Inflammation, and Disease. Neuroglia 2024, 5, 254–273. [Google Scholar] [CrossRef]
- de Paiva, I.H.R.; da Silva, R.S.; Mendonça, I.P.; Duarte-Silva, E.; Botelho de Souza, J.R.; Peixoto, C.A. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Improve Neuroinflammation and Cognition By Up-regulating IRS/PI3K/AKT Signaling Pathway in Diet-induced Obese Mice. J. Neuroimmune Pharmacol. 2023, 18, 427–447. [Google Scholar] [CrossRef]
- Lochlainn, M.N.; Bowyer, R.; Whelan, K.; Steves, C.J. 66 The PROMOTe study: Prebiotic supplementation improves cognition versus placebo in healthy older twins. Age Ageing 2023, 52, afad156.006. [Google Scholar] [CrossRef]
- Capitão, L.P.; Baião, R.; Baek, H.K.; Kappelmann, N.; Sharman, R.; Harvey, C.J.; Montgomery, P.; Burnet, P.W. Prebiotic supplementation does not affect reading and cognitive performance in children: A randomised placebo-controlled study. J. Psychopharmacol 2020, 34, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Freijy, T.M.; Cribb, L.; Oliver, G.; Metri, N.J.; Opie, R.S.; Jacka, F.N.; Hawrelak, J.A.; Rucklidge, J.J.; Ng, C.H.; Sarris, J. Effects of a high-prebiotic diet versus probiotic supplements versus synbiotics on adult mental health: The "Gut Feelings" randomised controlled trial. Front. Neurosci 2022, 16, 1097278. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Hu, A.; Shu, X.; Huang, W.; Liu, J.; Wang, B.; Zhang, R.; Yue, M.; Yang, C. Lactobacillus plantarum-derived postbiotics prevent Salmonella-induced neurological dysfunctions by modulating gut-brain axis in mice. Front Nutr. 2022, 9, 946096. [Google Scholar] [CrossRef] [PubMed]
- Naghibi, M.; Pont-Beltran, A.; Lamelas, A.; Llobregat, L.; Martinez-Blanch, J.F.; Rojas, A.; Álvarez, B.; López Plaza, B.; Arcos Castellanos, L.; Chenoll, E.; et al. Effect of Postbiotic Bifidobacterium longum CECT 7347 on Gastrointestinal Symptoms, Serum Biochemistry, and Intestinal Microbiota in Healthy Adults: A Randomised, Parallel, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2024, 16, 3952. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, Y.; Sun, H.; Dang, Y.; Yin, M.; Xiao, M.; Wu, T. Fecal Microbiota Transplantation Improves Cognitive Function of a Mouse Model of Alzheimer’s Disease. CNS Neurosci. Ther. 2025, 31, e70259. [Google Scholar] [CrossRef]
- Cerna, C.; Vidal-Herrera, N.; Silva-Olivares, F.; Álvarez, D.; González-Arancibia, C.; Hidalgo, M.; Aguirre, P.; González-Urra, J.; Astudillo-Guerrero, C.; Jara, M.; et al. Fecal Microbiota Transplantation from Young-Trained Donors Improves Cognitive Function in Old Mice Through Modulation of the Gut-Brain Axis. Aging Dis. 2025. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, J.H.; Kim, J.S.; Kim, T.J.; Shin, J.; Im, J.H.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; et al. Fecal microbiota transplantation can improve cognition in patients with cognitive decline and Clostridioides difficile infection. Aging 2022, 14, 6449–6466. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Lin, Z.; Zheng, C.; Chen, S.; Zhou, H.; Liu, Z. Preliminary evidence for developing safe and efficient fecal microbiota transplantation as potential treatment for aged related cognitive impairments. Front. Cell. Infect. Microbiol. 2023, 13, 1103189. [Google Scholar] [CrossRef]
- Abdi Syahputra, R.; Dalimunthe, A.; Utari, Z.D.; Halim, P.; Sukarno, M.A.; Zainalabidin, S.; Salim, E.; Gunawan, M.; Nurkolis, F.; Park, M.N.; et al. Nanotechnology and flavonoids: Current research and future perspectives on cardiovascular health. J. Funct. Foods 2024, 120, 106355. [Google Scholar] [CrossRef]
| Study | Flavanone | Species | Studied Groups | Increased/Decreased After Flavanone Consumption | Reference |
|---|---|---|---|---|---|
| Wu et al. | Naringenin | Helicobacter, Dorea, Lachnospira, Butyricimonas, Roseburia, Streptococcus, Parabacteroides, Phascolarctobacterium, Blauria, Butyricicoccus, Paraprevotella, Coprococcus, Bosea, Coprobacillus | Sprague–Dawley rats | Increased | [48] |
| Akkermansia, Clostridium, Dehalobacterium, Pseudoxanthomonas, Bacillus, Desulfovibrio, Fusobacterium | Decreased | [48] | |||
| Blautia, Helicobacter, Ruminococcus, Lactobacillus, Coprococcus, Faecalibacterium, Parabacteroides, Streptococcus, Roseburia, Paraprevotella, Butyricicoccus | Sprague–Dawley rats with induced PCOS | Increased | [48] | ||
| Gemella, Prevotella, Fusobacterium, Veillonella | Decreased | [48] | |||
| Liu et al. | Naringenin | Burkholderiales, Methylophilales, Turicibacterales | Naïve mice fed with naringenin | Increased | [49] |
| Enterobacteriales, RF32, Sphingomonadales, Fusobacteriales, Rhizobiales, Rickettsiales | Naïve mice | [49] | |||
| Paraprevotellaceae, Alistipes, Chlorobi | EAE mice | Increased | [49] | ||
| Bacteroidetes, Akkermansia | Decreased | [49] | |||
| Parkar et al. | Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Lactobacillus rhamnosus | In vitro study | Decreased | [50] | |
| Duda-Chodak et al. | Naringenin | Bacteroides galacturonicus, Escherichia coli, Bifidobacterium catenulatum, Lactobacillus spp., Enterococcus caccae, Ruminococcus gauvreauii | In vitro study | Decreased | [51] |
| Hesperetin | Bacteroides galacturonicus, Escherichia coli, Bifidobacterium catenulatum, Enterococcus caccae, Ruminococcus gauvreauii | Decreased | [51] | ||
| Lactobacillus spp. | Increased | [51] | |||
| Firrman et al. | Naringenin | Bifidobacterium catenulatum | In vitro study | Increased | [52] |
| Enterococcus caccae | Decreased | [52] | |||
| Bae et al. | Naringenin and hesperitin | Helicobacter pylori | In vitro study | Decreased | [53] |
| Unno et al. | Hesperetin | Clostridium spp. | Wistar rats | Decreased | [54] |
| Lactobacilliales, Bifidobacterium, Bacteroides | Increased | [54] | |||
| Fidélix et al. | Orange Juice | Actinobacteria, Bifidobacteriaceaem Atopobiaceae, Coriobacteriaceae, Eggerthellaceae, Lactobacilliaceae, Leuconostocaceae, Clostridiaceae 1, Lachnospiraceae, Peptococcaceae, Ruminococcaceae, Akkermansia munciphila | Women with the Orange Juice Diet | Increased | [55] |
| Bacteroidaceae, Barnesiellaceae, Muribaculaceae, Prevotellaceae, Rikenellaceae, Tannerellaceae | Decreased | [55] | |||
| Lima et al. | Orange Juice | For Lactobacillus, Bifidobacterium, Clostridium, total anaerobic bacteria population | Women with the Orange Juice Diet | Increased | [56] |
| Duque et al. | Orange Juice | Lactobacillus Enterococcus, Bifidobacetrium, Clostridium | SHIME® study | Increased | [57] |
| Enterobacteria population in ascending colon | Decreased | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichon, N.; Szelenberger, R.; Stela, M.; Podogrocki, M.; Gorniak, L.; Bijak, M. Flavanones as Modulators of Gut Microbiota and Cognitive Function. Molecules 2025, 30, 2203. https://doi.org/10.3390/molecules30102203
Cichon N, Szelenberger R, Stela M, Podogrocki M, Gorniak L, Bijak M. Flavanones as Modulators of Gut Microbiota and Cognitive Function. Molecules. 2025; 30(10):2203. https://doi.org/10.3390/molecules30102203
Chicago/Turabian StyleCichon, Natalia, Rafał Szelenberger, Maksymilian Stela, Marcin Podogrocki, Leslaw Gorniak, and Michal Bijak. 2025. "Flavanones as Modulators of Gut Microbiota and Cognitive Function" Molecules 30, no. 10: 2203. https://doi.org/10.3390/molecules30102203
APA StyleCichon, N., Szelenberger, R., Stela, M., Podogrocki, M., Gorniak, L., & Bijak, M. (2025). Flavanones as Modulators of Gut Microbiota and Cognitive Function. Molecules, 30(10), 2203. https://doi.org/10.3390/molecules30102203