Immunomodulatory Properties of Sweet Whey-Derived Peptides in THP-1 Macrophages
Abstract
1. Introduction
2. Results and Discussion
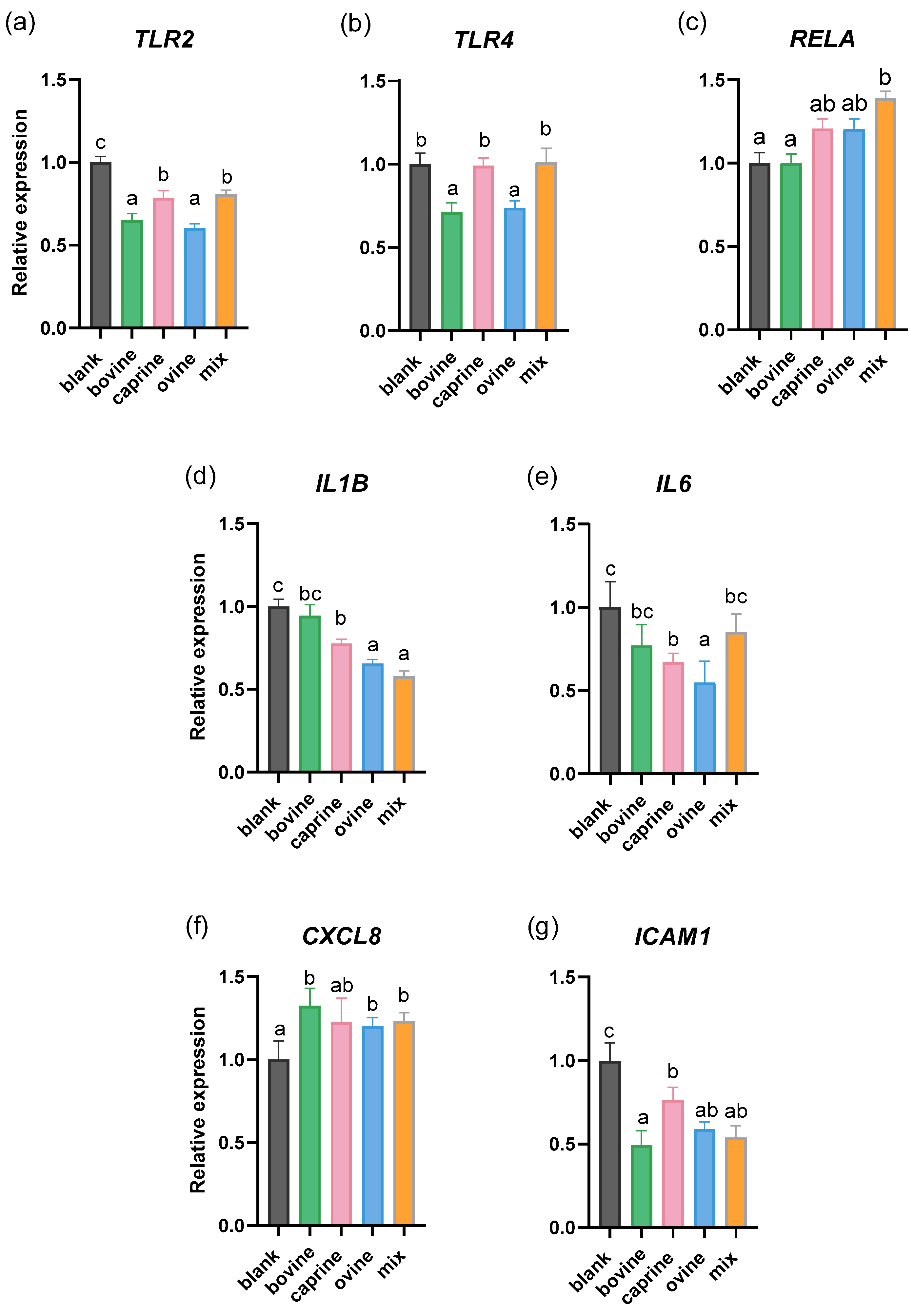
2.1. Effect of SW-D-P3 on mRNA Expression of Inflammation-Related Genes in Non-Challenged PMA-Induced THP-1-Derived Macrophages
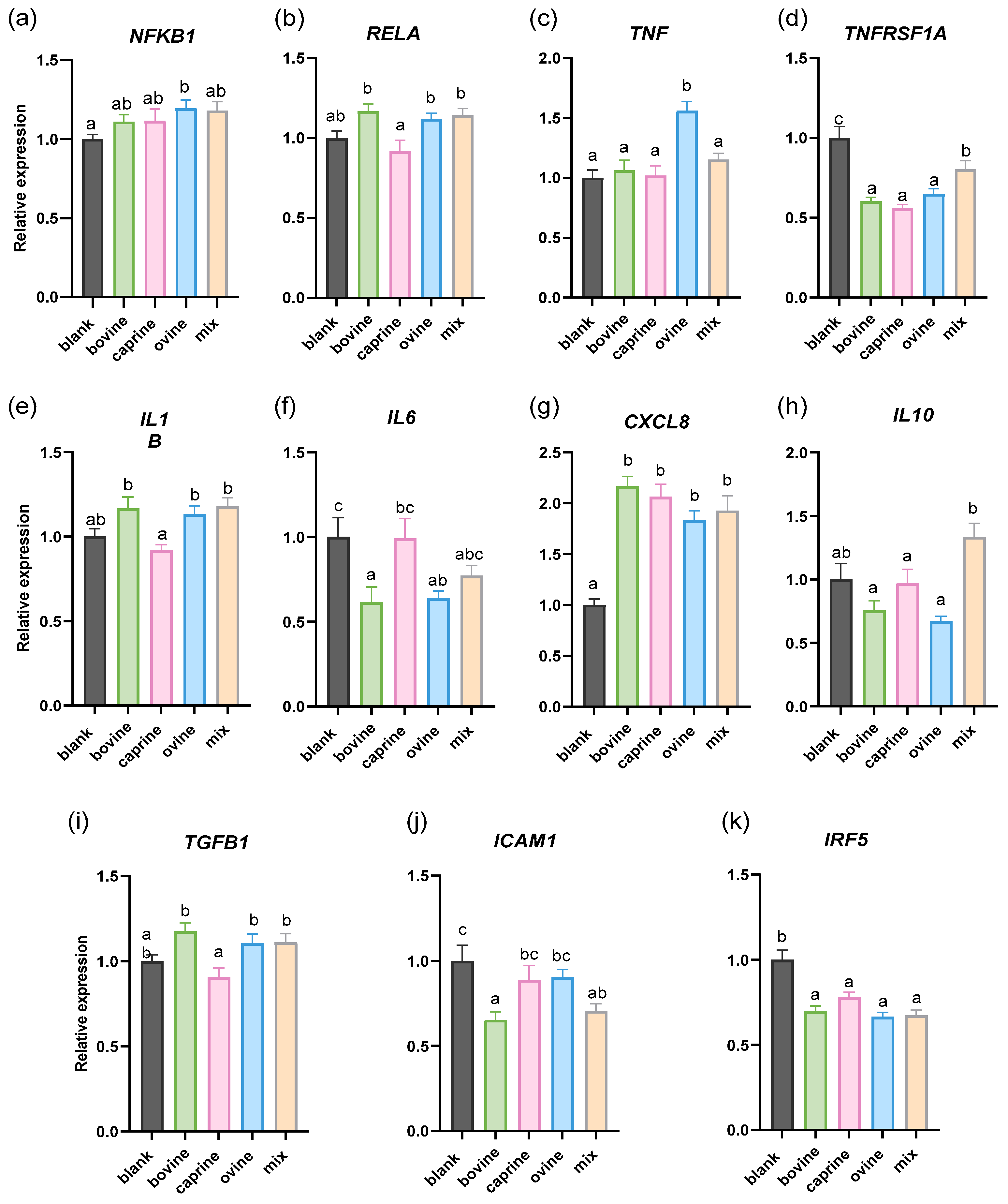
2.2. Effect of SW-D-P3 on mRNA Expression of Inflammation-Related Genes in LPS-Challenged PMA-Induced THP-1-Derived Macrophages
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Collection and Preparation of Sweet Whey
3.3. Simulated Gastrointestinal Digestion and Digestates’ Fractionation
3.4. THP-1 Cell Culture, Cell Viability, Differentiation and Activation
3.5. Quantification of Gene Expression in THP-1 Cells
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.M.; Díaz-Castro, J.; López-Aliaga, I. New Perspectives in Fermented Dairy Products and Their Health Relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Karimidastjerd, A.; Gulsunoglu-Konuskan, Z. Biological, Functional and Nutritional Properties of Caseinomacropeptide from Sweet Whey. Crit. Rev. Food Sci. Nutr. 2023, 63, 4261–4273. [Google Scholar] [CrossRef] [PubMed]
- Panesar, P.S.; Kennedy, J.F.; Gandhi, D.N.; Bunko, K. Bioutilisation of Whey for Lactic Acid Production. Food Chem. 2007, 105, 1–14. [Google Scholar] [CrossRef]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef]
- Ali, A.; Ain, Q.; Saeed, A.; Khalid, W.; Ahmed, M.; Bostani, A.; Ali, A.; Ain, Q.; Saeed, A.; Khalid, W.; et al. Bio-Molecular Characteristics of Whey Proteins with Relation to Inflammation. In New Advances in the Dairy Industry; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese Whey Wastewater: Characterization and Treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese Whey Management: A Review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a Source of Peptides with Remarkable Biological Activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Mehra, R.; Kumar, H.; Kumar, N.; Ranvir, S.; Jana, A.; Buttar, H.S.; Telessy, I.G.; Awuchi, C.G.; Okpala, C.O.R.; Korzeniowska, M.; et al. Whey Proteins Processing and Emergent Derivatives: An Insight Perspective from Constituents, Bioactivities, Functionalities to Therapeutic Applications. J. Funct. Foods 2021, 87, 104760. [Google Scholar] [CrossRef]
- Lbrahim El-Sayed, M.; Awad, S.; El-Sayed, M.; El, M. Milk Bioactive Peptides: Antioxidant, Antimicrobial and Anti-Diabetic Activities-Review Article Milk Bioactive Peptides: Antioxidant, Antimicrobial and Anti-Diabetic Activities. Adv. Biochem. 2019, 7, 22–23. [Google Scholar] [CrossRef]
- Mann, B.; Athira, S.; Sharma, R.; Kumar, R.; Sarkar, P. Bioactive Peptides from Whey Proteins. In Whey Proteins; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128121245. [Google Scholar]
- Korhonen, H. Milk-Derived Bioactive Peptides: From Science to Applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk Derived Bioactive Peptides and Their Impact on Human Health—A Review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.J.T.; Pihlanto-Leppälä, A.; Rantamäki, P.; Tupasela, T. The Functional and Biological Properties of Whey Proteins: Prospects for the Development of Functional Foods. Agric. Food Sci. 1998, 7, 283–296. [Google Scholar] [CrossRef]
- Pihlanto-Leppaïa, A.; Rokka, T.; Korhonen, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Bovine Milk Proteins. Int. Dairy J. 1998, 8, 325–331. [Google Scholar] [CrossRef]
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey Protein Hydrolysates as a Source of Bioactive Peptides for Functional Foods—Biotechnological Facilitation of Industrial Scale-Up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Ha, E.; Zemel, M.B. Functional Properties of Whey, Whey Components, and Essential Amino Acids: Mechanisms Underlying Health Benefits for Active People (Review). J. Nutr. Biochem. 2003, 14, 251–258. [Google Scholar] [CrossRef]
- Sánchez-Moya, T.; López-Nicolás, R.; Planes, D.; González-Bermúdez, C.A.; Ros-Berruezo, G.; Frontela-Saseta, C. In Vitro Modulation of Gut Microbiota by Whey Protein to Preserve Intestinal Health. Food Funct. 2017, 8, 3053–3063. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, T.; Liang, X.; Yuan, Q.; Zeng, X.; Wu, Z.; Pan, D.; Tao, M.; Guo, Y. Production and Transepithelial Transportation of Casein-Derived Peptides and Identification a Novel Antioxidant Peptide LHSMK. LWT 2021, 151, 112194. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive Peptides: Production and Functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Santiago-López, L.; Peres, C.M.; Peres, C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Assessment of Multifunctional Activity of Bioactive Peptides Derived from Fermented Milk by Specific Lactobacillus Plantarum Strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; García-Garibay, J.M.; Gómez-Ruíz, L.C.; Contreras-López, E.; Guzmán-Rodríguez, F.; González-Olivares, L.G. Bioactive Peptides of Whey: Obtaining, Activity, Mechanism of Action, and Further Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 10351–10381. [Google Scholar] [CrossRef]
- Gauthier, S.F.; Pouliot, Y.; Saint-Sauveur, D. Immunomodulatory Peptides Obtained by the Enzymatic Hydrolysis of Whey Proteins. Int. Dairy J. 2006, 16, 1315–1323. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In Vitro Human Digestion Models for Food Applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Jose, F.; Ramal-sanchez, M.; Bravo-trippetta, C.; Antonio, V.D.; Corvaglia, E.; Kämpfer, A.A.M.; Schins, R.P.F.; Sera, M.; Angelino, D. Development and Assessment of an Intestinal Tri-Cellular Model to Investigate the pro/Anti-inflammatory Potential of Digested Foods. Front. Immunol. 2025, 16, 1545261. [Google Scholar] [CrossRef]
- Alimenti, C.; Lianza, M.; Antognoni, F.; Giusti, L.; Bistoni, O.; Liotta, L.; Angeloni, C.; Lupidi, G.; Beghelli, D. Characterization and Biological Activities of In Vitro Digested Olive Pomace Polyphenols Evaluated on Ex Vivo Human Immune Blood Cells. Molecules 2023, 28, 2122. [Google Scholar] [CrossRef] [PubMed]
- García-Gurrola, A.; Wall-Medrano, A.; Olivas-Aguirre, M.A.; Olivas-Aguirre, F.J.; Escobar-Puentes, A.A. Immunomodulatory Properties of Nutraceuticals and Functional Foods. In Nutraceuticals and Functional Foods in Immunomodulators; Springer: Berlin/Heidelberg, Germany, 2023; pp. 21–72. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The Crucial Roles of Inflammatory Mediators in Inflammation: A Review. Vet. World 2018, 11, 627. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Li, H.; Wang, H.; Zhang, T.; Hutchinson, M.R.; Yin, H.; Wang, X. Small-Molecule Modulators of Toll-like Receptors. Acc. Chem. Res. 2020, 53, 1046–1055. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- De Nardo, D. Toll-like Receptors: Activation, Signalling and Transcriptional Modulation. Cytokine 2015, 74, 181–189. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR Signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Rusu, D.; Drouin, R.; Pouliot, Y.; Gauthier, S.; Poubelle, P.E. A Bovine Whey Protein Extract Stimulates Human Neutrophils to Generate Bioactive IL-1Ra through a NF-ΚB- and MAPK-Dependent Mechanism. J. Nutr. 2010, 140, 382–391. [Google Scholar] [CrossRef]
- Olsen, W.; Liang, N.; Dallas, D.C. Macrophage-Immunomodulatory Actions of Bovine Whey Protein Isolate, Glycomacropeptide, and Their In Vitro and In Vivo Digests. Nutrients 2023, 15, 4942. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.B.; Mark, M.R.; Gray, A.; Huang, A.; Xie, M.H.; Zhang, M.; Goddard, A.; Wood, W.I.; Gurney, A.L.; Godowski, P.J. Toll-like Receptor-2 Mediates Lipopolysaccharide-Induced Cellular Signalling. Nature 1998, 395, 284–288. [Google Scholar] [CrossRef]
- Lingappan, K. NF-ΚB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional Regulation of Macrophage Polarization: Enabling Diversity with Identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular Mechanisms That Influence the Macrophage M1-M2 Polarization Balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Schopohl, P.; Melzig, M.F. The Influence of Toll-like Receptor (TLR-) Agonists on Lysozyme Activity, TNF-Alpha Secretion and Intercellular Adhesion in THP-1 Cells. Pharmazie 2014, 69, 602–609. [Google Scholar] [CrossRef]
- Schopohl, P.; Grüneberg, P.; Melzig, M.F. The Influence of Harpagoside and Harpagide on TNFα-Secretion and Cell Adhesion Molecule MRNA-Expression in IFNγ/LPS-Stimulated THP-1 Cells. Fitoterapia 2016, 110, 157–165. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An Evolving Chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Egusquiaguirre, S.P.; Yeh, J.E.; Walker, S.R.; Liu, S.; Frank, D.A. The STAT3 Target Gene TNFRSF1A Modulates the NF-ΚB Pathway in Breast Cancer Cells. Neoplasia 2018, 20, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Choi, E.K.; Seo, K.W.; Bae, J.U.; Park, S.Y.; Kim, C.D. TLR4-Mediated Expression of Mac-1 in Monocytes Plays a Pivotal Role in Monocyte Adhesion to Vascular Endothelium. PLoS ONE 2014, 9, e104588. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Tabibian, J.; Venugopal, S.K.; Devaraj, S.; Jialal, I. Development of an In Vitro Screening Assay to Test the Antiinflammatory Properties of Dietary Supplements and Pharmacologic Agents. Clin. Chem. 2005, 51, 2252–2256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mao, X.Y.; Cheng, X.; Wang, X.; Wu, S.J. Free-Radical-Scavenging and Anti-Inflammatory Effect of Yak Milk Casein before and after Enzymatic Hydrolysis. Food Chem. 2011, 126, 484–490. [Google Scholar] [CrossRef]
- Ma, J.; Chen, T.; Mandelin, J.; Ceponis, A.; Miller, N.E.; Hukkanen, M.; Ma, G.F.; Konttinen, Y.T. Regulation of Macrophage Activation. Cell. Mol. Life Sci. 2003, 60, 2334–2346. [Google Scholar] [CrossRef]
- Tsukada, S.; Parsons, C.J.; Rippe, R.A. Mechanisms of Liver Fibrosis. Clin. Chim. Acta 2006, 364, 33–60. [Google Scholar] [CrossRef]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.B.; Dockrell, D.H. The Identification of Markers of Macrophage Differentiation in PMA-Stimulated THP-1 Cells and Monocyte-Derived Macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 116283. [Google Scholar] [CrossRef]
- Stout, R.D.; Suttles, J. Functional Plasticity of Macrophages: Reversible Adaptation to Changing Microenvironments. J. Leukoc. Biol. 2004, 76, 509–513. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Shi, H.; Yu, L. Isolation and Characterization of Anti-Inflammatory Peptides Derived from Whey Protein. J. Dairy Sci. 2016, 99, 6902–6912. [Google Scholar] [CrossRef]
- Benoit, M.; Desnues, B.; Mege, J.-L. Macrophage Polarization in Bacterial Infections. J. Immunol. 2008, 181, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-Derived Bioactive Peptides and Their Health Promoting Effects: A Potential Role in Atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Paik, Y.H.; Schwabe, R.F.; Bataller, R.; Russo, M.P.; Jobin, C.; Brenner, D.A. Toll-Like Receptor 4 Mediates Inflammatory Signaling by Bacterial Lipopolysaccharide in Human Hepatic Stellate Cells. Hepatology 2003, 37, 1043–1055. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Dekkers, R.; Gros, M.; Van Neerven, R.J.J.; Groeneveld, A.; De Vos, P.; Faas, M.M. Toll-like Receptor Mediated Activation Is Possibly Involved in Immunoregulating Properties of Cow’s Milk Hydrolysates. PLoS ONE 2017, 12, e0178191. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Sangild, P.T.; Li, Y.; Bering, S.B.; Chatterton, D.E.W. Processing of Whey Modulates Proliferative and Immune Functions in Intestinal Epithelial Cells. J. Dairy Sci. 2016, 99, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Fekete, A.A.; Giromini, C.; Chatzidiakou, Y.; Givens, D.I.; Lovegrove, J.A. Whey Protein Lowers Blood Pressure and Improves Endothelial Function and Lipid Biomarkers in Adults with Prehypertension and Mild Hypertension: Results from the Chronic Whey2Go Randomized Controlled Trial1,2. Am. J. Clin. Nutr. 2016, 104, 1534–1544. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Saint-Sauveur, D.; Gauthier, S.F.; Boutin, Y.; Montoni, A. Immunomodulating Properties of a Whey Protein Isolate, Its Enzymatic Digest and Peptide Fractions. Int. Dairy J. 2008, 18, 260–270. [Google Scholar] [CrossRef]
- Da Silva, M.S.; Bigo, C.; Barbier, O.; Rudkowska, I. Whey Protein Hydrolysate and Branched-Chain Amino Acids Downregulate Inflammation-Related Genes in Vascular Endothelial Cells. Nutr. Res. 2017, 38, 43–51. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Kanwar, R.K. Gut Health Immunomodulatory and Anti-Inflammatory Functions of Gut Enzyme Digested High Protein Micro-Nutrient Dietary Supplement-Enprocal. BMC Immunol. 2009, 10, 7. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Dekkers, R.; Ulfman, L.H.; Groeneveld, A.; De Vos, P.; Faas, M.M. Immunomodulating Protein Aggregates in Soy and Whey Hydrolysates and Their Resistance to Digestion in an: In Vitro Infant Gastrointestinal Model: New Insights in the Mechanism of Immunomodulatory Hydrolysates. Food Funct. 2018, 9, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.; Matsubara, T.; Koyama, T.; Iwamoto, H.; Miyaji, K. Whey Protein Hydrolysate Mitigates Both Inflammation and Endotoxin Tolerance in THP-1 Human Monocytic Leukemia Cells. Immun. Inflamm. Dis. 2022, 10, e737. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Hernández Álvarez, A.J.; Maycock, J.; Murray, B.S.; Boesch, C. Differential Effects of Oilseed Protein Hydrolysates in Attenuating Inflammation in Murine Macrophages. Food Biosci. 2022, 49, 101860. [Google Scholar] [CrossRef]
- Gjevestad, G.O.; Ottestad, I.; Biong, A.S.; Iversen, P.O.; Retterstøl, K.; Raastad, T.; Skålhegg, B.S.; Ulven, S.M.; Holven, K.B. Consumption of Protein-Enriched Milk Has Minor Effects on Inflammation in Older Adults—A 12-Week Double-Blind Randomized Controlled Trial. Mech. Ageing Dev. 2017, 162, 1–8. [Google Scholar] [CrossRef]
- Li, M.; Li, Q.; Abdlla, R.; Chen, J.; Yue, X.; Quek, S.Y. Donkey Whey Proteins Ameliorate Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice by Downregulating the S100A8-TRAF6-NF-ΚB Axis-Mediated Inflammatory Response. Food Sci. Hum. Wellness 2023, 12, 1809–1819. [Google Scholar] [CrossRef]
- Buey, B.; Bellés, A.; Latorre, E.; Abad, I.; Pérez, M.D.; Grasa, L.; Mesonero, J.E.; Sánchez, L. Comparative Effect of Bovine Buttermilk, Whey, and Lactoferrin on the Innate Immunity Receptors and Oxidative Status of Intestinal Epithelial Cells. Biochem. Cell Biol. 2021, 99, 54–60. [Google Scholar] [CrossRef]
- Arbizu, S.; Chew, B.; Mertens-Talcott, S.U.; Noratto, G. Commercial Whey Products Promote Intestinal Barrier Function with Glycomacropeptide Enhanced Activity in Downregulating Bacterial Endotoxin Lipopolysaccharides (LPS)-Induced Inflammation In Vitro. Food Funct. 2020, 11, 5842–5852. [Google Scholar] [CrossRef]
- Mukhopadhya, A.; Noronha, N.; Bahar, B.; Ryan, M.T.; Murray, B.A.; Kelly, P.M.; O’Loughlin, I.B.; O’Doherty, J.V.; Sweeney, T. Anti-Inflammatory Effects of a Casein Hydrolysate and Its Peptide-Enriched Fractions on TNFα-Challenged Caco-2 Cells and LPS-Challenged Porcine Colonic Explants. Food Sci. Nutr. 2014, 2, 712–723. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, W.; Yuan, Z.; Wang, X.; Huang, A. Anti-Inflammatory Effect of Two Novel Peptides Derived from Binglangjiang Buffalo Whey Protein in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. Food Chem. 2023, 429, 136804. [Google Scholar] [CrossRef]
- Zheng, W.; Li, Y.; Wang, Y.; He, J.; Zhao, Q.; Huang, A. Identification of a Novel Peptide with Anti-Inflammatory Activity from Binglangjiang Buffalo Fermented Milk and Its Potential Inhibitory Mechanism in Lipopolysaccharide-Stimulated RAW264.7 Cells. Food Chem. 2025, 468, 142451. [Google Scholar] [CrossRef]
- Iskandar, M.M.; Dauletbaev, N.; Kubow, S.; Mawji, N.; Lands, L.C. Whey Protein Hydrolysates Decrease IL-8 Secretion in Lipopolysaccharide (LPS)-Stimulated Respiratory Epithelial Cells by Affecting LPS Binding to Toll-like Receptor 4. Br. J. Nutr. 2013, 110, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Beverly, R.L.; Scottoline, B.P.; Dallas, D.C. Peptides Derived from In Vitro and In Vivo Digestion of Human Milk Are Immunomodulatory in THP-1 Human Macrophages. J. Nutr. 2022, 152, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Menahem, C.; Foist, M.; Mansour, Y.; Shtaif, B.; Bar-Maisels, M.; Phillip, M.; Gat-Yablonski, G. A Whey-Based Diet Can Ameliorate the Effects of LPS-Induced Growth Attenuation in Young Rats. Nutrients 2023, 15, 1823. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Ellis, V. Acute Effects of Whey Protein Isolate on Blood Pressure, Vascular Function and Inflammatory Markers in Overweight Postmenopausal Women. Br. J. Nutr. 2011, 105, 1512–1519. [Google Scholar] [CrossRef]
- Ballard, K.D.; Bruno, R.S.; Seip, R.L.; Quann, E.E.; Volk, B.M.; Freidenreich, D.J.; Kawiecki, D.M.; Kupchak, B.R.; Chung, M.Y.; Kraemer, W.J.; et al. Acute Ingestion of a Novel Whey-Derived Peptide Improves Vascular Endothelial Responses in Healthy Individuals: A Randomized, Placebo Controlled Trial. Nutr. J. 2009, 8, 34. [Google Scholar] [CrossRef]
- Kaur, H.; Gupta, T.; Kapila, S.; Kapila, R. Protective Effects of Potential Probiotic Lactobacillus Rhamnosus (MTCC-5897) Fermented Whey on Reinforcement of Intestinal Epithelial Barrier Function in a Colitis-Induced Murine Model. Food Funct. 2021, 12, 6102–6116. [Google Scholar] [CrossRef]
- Amoroso, M.; Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-Induced Cytokine Production in Human Monocytes and Macrophages. Crit. Rev. Immunol. 2012, 31, 379–446. [Google Scholar]
- Williams, S. Official Methods of Analysis of the Association of Official Analytical Chemists. Soil Sci. Soc. Am. J. 1971, 35, iv. [Google Scholar] [CrossRef]
- Dalaka, E.; Politis, I.; Theodorou, G. Antioxidant Activity of Sweet Whey Derived from Bovine, Ovine and Caprine Milk Obtained from Various Small-Scale Cheese Plants in Greece before and after In Vitro Simulated Gastrointestinal Digestion. Antioxidants 2023, 12, 1676. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food-an International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Dalaka, E.; Stefos, G.C.; Politis, I.; Theodorou, G. Evaluation of In Vitro Antihypertensive and Anti-Inflammatory Properties of Dairy By-Products. Appl. Sci. 2024, 14, 6885. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.; Vreeburg, R.A.M.M.; Savelkoul, F.J.; Wichers, H.J.; Savelkoul, H.F.J.; Wichers, H.J. Transcription Profiles of LPS-Stimulated THP-1 Monocytes and Macrophages: A Tool to Study Inflammation Modulating Effects of Food-Derived Compounds. Food Funct. 2010, 1, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 Cell Line: An in Vitro Cell Model for Immune Modulation Approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Walker, S.E.; Lorsch, J. RNA Purification—Precipitation Methods, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 530, ISBN 9780124200371. [Google Scholar]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Templeton, G.F.; Templeton, G.F. A Two-Step Approach for Transforming Continuous Variables to Normal: Implications and Recommendations for IS Research. Commun. Assoc. Inf. Syst. 2011, 28, 41–58. [Google Scholar] [CrossRef]
| Gene (Accession Number) | Primer Direction | Sequence (5′-3′) | Amplicon Size | Reaction Efficiency |
|---|---|---|---|---|
| TLR2 (NM_001318793.2) | Forward | ATCAGCAGGAACAGAGCACA | 173 | 102 |
| Reverse | ACTCAGGAGCAGCAAGCAC | |||
| TLR4 (NM_003266.4) | Forward | GATTTATCCAGGTGTGAAATCCAG | 174 | 105 |
| Reverse | TAGAGATGCTAGATTTGTCTCCAC | |||
| NFKB1 (NM_001382627.1) | Forward | GATCTGCCAACTACTCCCA | 137 | 92 |
| Reverse | CCCAGAGACCTCATAGTTGTC | |||
| RELA (NM_001145138) | Forward | GGACTACGACCTGAATGCTG | 228 | 105 |
| Reverse | ACCTCAATGTCCTCTTTCTGC | |||
| TNF (NM_000594.4) | Forward | TTCCTCAGCCTCTTCTCCT | 196 | 100 |
| Reverse | GAGGGTTTGCTACAACATGG | |||
| TNFRSF1A (NM_001065.4) | Forward | GTTCCACCTTCACCTCCAG | 199 | 99 |
| Reverse | GGGTCATCAGTGTCTAGGC | |||
| IL1B (NM_000576.3) | Forward | CAGATGAAGTGCTCCTTCCAG | 244 | 99 |
| Reverse | CCTCGTTATCCCATGTGTCG | |||
| IL6 (NM_000600.5) | Forward | GGATTCAATGAGGAGACTTGC | 205 | 95 |
| Reverse | CATTTGTGGTTGGGTCAGG | |||
| CXCL8 (NM_000584.4) | Forward | GCTAAAGAACTTAGATGTCAGTGC | 191 | 97 |
| Reverse | AACTTCTCCACAACCCTCTG | |||
| IL10 (NM_000572.3) | Forward | CATGCTTCGAGATCTCCGAG | 122 | 103 |
| Reverse | AACCCAGGTAACCCTTAAAGTC | |||
| TGFB1 (NM_000660.7) | Forward | TGAACCCGTGTTGCTCTC | 287 | 94 |
| Reverse | TAGTGAACCCGTTGATGTCC | |||
| ICAM1 (NM_000201.3) | Forward | CAGACCTTTGTCCTGCCA TCGTTGCCATAGGTGACTG | 176 | 95 |
| Reverse | ||||
| IRF5 (NM_032643.5) | Forward | GGAAATACACCGAAGGCGT ATCCTCTGCAGCTCTTCCT | 244 | 108 |
| Reverse | ||||
| B2M (NM_004048) | Forward | GCTATCCAGCGTACTCCA CTTAACTATCTTGGGCTGTGAC | 285 | 103 |
| Reverse | ||||
| RPL37A (NM_000998) | Forward | AGTACACTTGCTCTTTCTGTGG GGAAGTGGTATTGTACGTCCAG | 119 | 106 |
| Reverse | ||||
| RPS18 (NM_022551) | Forward | CTGAGGATGAGGTGGAACG | 240 | 98 |
| Reverse | CAGTGGTCTTGGTGTGCT | |||
| HPRT1 (NM_000194) | Forward | CTTTGCTTTCCTTGGTCAGG | 111 | 99 |
| Reverse | CAAATCCAACAAAGTCTGGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalaka, E.; Stefos, G.C.; Politis, I.; Theodorou, G. Immunomodulatory Properties of Sweet Whey-Derived Peptides in THP-1 Macrophages. Molecules 2025, 30, 1261. https://doi.org/10.3390/molecules30061261
Dalaka E, Stefos GC, Politis I, Theodorou G. Immunomodulatory Properties of Sweet Whey-Derived Peptides in THP-1 Macrophages. Molecules. 2025; 30(6):1261. https://doi.org/10.3390/molecules30061261
Chicago/Turabian StyleDalaka, Eleni, Georgios C. Stefos, Ioannis Politis, and Georgios Theodorou. 2025. "Immunomodulatory Properties of Sweet Whey-Derived Peptides in THP-1 Macrophages" Molecules 30, no. 6: 1261. https://doi.org/10.3390/molecules30061261
APA StyleDalaka, E., Stefos, G. C., Politis, I., & Theodorou, G. (2025). Immunomodulatory Properties of Sweet Whey-Derived Peptides in THP-1 Macrophages. Molecules, 30(6), 1261. https://doi.org/10.3390/molecules30061261









